
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 25 February 2021
Sec. Pharmacology of Anti-Cancer Drugs
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.640656
This article is part of the Research TopicChemo-Radiation-Resistance in Cancer TherapyView all 35 articles
Betulin is a lupane-type pentacyclic triterpene, which is isolated from birch bark. It has a broad spectrum of biological and pharmacological properties, such as anti-inflammatory, anti-tumor, anti-viral, and anti-bacterial activity. Herein, we explored the factors that may result in betulin resistance, especially with respect to its interaction with ATP-binding cassette subfamily C member 1 (ABCC1). ABCC1 is an important member of the ATP-binding cassette (ABC) transporter family, which is central to mediating multidrug resistance (MDR) in naturally derived anticancer agents. An MTT-based cell viability assay showed that ABCC1 overexpression has the ability to desensitize both cancer cell line and gene-transfected cell line to betulin and that this betulin-induced resistance can be antagonized by a known ABCC1 inhibitor MK571 at 25 μM. Additionally, betulin upregulates the ABCC1 protein expression level in both concentration-dependent and time-dependent manners, also blocks the transport function mediated by ABCC1. Subsequently, a high affinity score of betulin was achieved in a computational docking analysis, demonstrating a strong interaction of betulin with ABCC1.
To date, many drugs have originated from natural products, including vincristine, vinblastine, doxorubicin, and paclitaxel all have the potential to inhibit tumor progression (1). However, the effectiveness of these drugs can be restricted by multidrug resistance (MDR)-associated ATP-binding cassette (ABC) transporters (2). Specifically, vincristine, vinblastine, doxorubicin, and paclitaxel can be transported by ABC sub-family B member 1 (ABCB1, multidrug resistance protein 1/MDR1, P-glycoprotein/P-gp) (3); whereas, vincristine, vinblastine, and doxorubicin are substrate drugs of ABC sub-family C member 1 (ABCC1, multidrug resistance protein 1/MRP1) (4).
The 190 kDa MRP1, located in human chromosome locus p13.11, was firstly isolated from doxorubicin-resistance small cell lung cancer line H69AR, and was found to be associated with MDR in 1992 (5, 6). The protein structure of MRP1 has three membrane-spanning domains (MSDs), and two nucleotide-binding domains (NBDs) (7). Functionally, the NBDs serve as the energy source to produce hydrolyzed ATP, while the MSDs provide support for drug binding, putative drug transport channel, dimerization, and trafficking (4). MRP1 has a wide distribution, for example throughout the adrenal gland, bladder, choroid plexus, helper T cells, and muscle cells (8).
Betulin, a lupane-type pentacyclic triterpene, is isolated from bark of birches. Due to its poor solubility in aqueous media, several more soluble derivatives and betulin nanoparticles were developed (9, 10). The chemical structure of betulin is presented in Figure 1A. Betulin and its derivatives have a broad spectrum of anti-cancer profile, such as against lung, breast, prostate, colon, colorectal, cervical, and pancreatic cancer, as well as melanoma and leukemia (11). The interaction between betulinic acid and ABCB1 or ABCG2 is conclusive (12, 13); however, the effect of ABCC1 on betulin efficacy needs to be elucidated. Interestingly, it has been documented that ABCC1 overexpression is correlated with lung, breast, prostate, and ovarian cancer, gastrointestinal carcinoma, melanoma, and leukemia (14, 15). Based on the overlapping cancer spectrum, we postulated that the overexpression of ABCC1 may attenuate the anticancer efficacy of betulin.
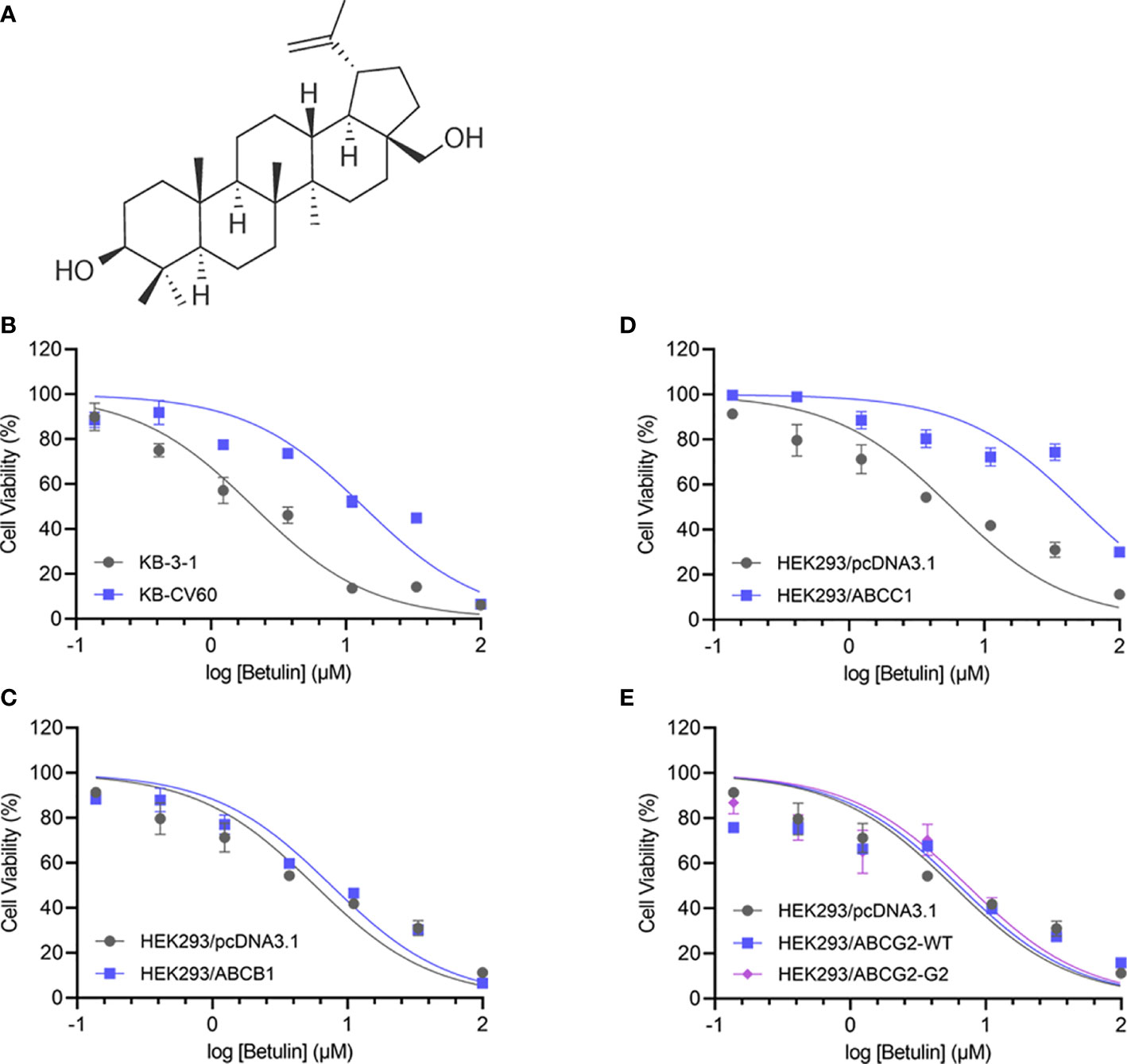
Figure 1 Chemical structure and cell viability-concentration curves. (A) The chemical structure of betulin. The cell viability-concentration curves for (B) KB-3-1 and KB-CV60 cells, (C) HEK293/pcDNA3.1 and ABCC1-tansfected HEK293 cells, (D) HEK293/pcDNA3.1 and ABCB1-tansfected HEK293 cells, (E) HEK293/pcDNA3.1 and HEK293 cells transfected with wild-type or R482G-mutant ABCG2 after treatment with serial concentrations of betulin. Each point is shown as mean ± SD, from the experiment that was repeated at least three times and performed independently.
Herein, we focused on the interaction of betulin with ABCC1, and found that ABCC1 overexpression confers drug resistance to betulin. This finding may provide a valuable foundation for future preclinical and clinical investigations of betulin.
Betulin was kindly provided as a free sample from MedChemExpress (Purity > 98.0%, Monmouth Junction, NJ). Dulbecco’s modified Eagle medium, trypsin-EDTA, penicillin/streptomycin, and fetal bovine serum were purchased from Corning (Corning, NY). Phosphate buffer saline (PBS) was obtained from VWR chemicals (Solon, OH). Vincristine, dimethyl sulfoxide (DMSO), and methylthiazolyldiphenyl-tetrazolium bromide (MTT) were obtained from Millipore-Sigma (Burlington, MA). MK571 and G418 were purchased from Enzo Life Sciences (Farmingdale, NY). Anti-MRP1/ABCC1 (D5C1X) antibody (product #72202), anti-GAPDH (D16H11) antibody (product #5174), and HRP-conjugated anti-rabbit IgG secondary antibody (product #7074) were obtained from Cell Signaling Technology (Danvers, MA). [3H]-Vincristine (0.7 Ci/mmol) was purchased from Moravek Biochemicals (Brea, CA). Liquid scintillation cocktail, and all other chemicals and reagents were purchased from ThermoFisher Scientific (Waltham, MA).
Human epidermoid carcinoma cell line KB-3-1 was used as the drug-sensitive cell line, and its ABCC1-overexpressing cell line KB-CV60 was maintained in the medium with 1 µg/ml of cepharanthine and 60 ng/ml of vincristine (16). HEK293/pcDNA3.1, HEK293/ABCC1, HEK293/ABCB1, HEK293/ABCG2-WT, and HEK293/ABCG2-G2 were transfected with either an empty vector pcDNA3.1 or a pcDNA3.1 vector containing a full length ABCC1, ABCB1, or ABCG2 encoding arginine (R) or glycine (G) at position 482 (17). All transfected cells were selected in the medium with 2 mg/ml G418. All cell lines were cultured in medium supplemented with 10% serum and 1% antibiotics at 37°C under 5% CO2. All drug resistant cell lines were cultured in drug-free medium for more than 2 weeks prior to further use.
An established protocol of MTT assay was used to determine the cell viability of betulin with or without an inhibitor (18). DMSO was used as a solvent to prepare a stock solution (10 mM) of all compounds. As the highest concentration in the cell viability assay was 100 μM, the final concentration of DMSO was 1% in the treatment medium. Also, all control groups in the experiment were treated with solvent only. Briefly, 5,000 to 7,000 cells/well were evenly seeded in a 96-well plate, and incubated at 37°C overnight prior to further experiment. On the next day, a serial dilution of substrate drugs (0–100 μM) with or without inhibitors at the indicated concentration was added to the designated well. The cells were further incubated for 72 h. On the last day of treatment period, MTT solution was added to each well, and incubated for 4 h protected from light. After incubation, the supernatant was removed, and 100 μl/well DMSO was added to dissolve the formazan crystals. Subsequently, absorbance at 570 nm was measured by an UV/Vis microplate spectrophotometer (Fisher Science, Fair Lawn, NJ). The log scale curves in GraphPad (log inhibitor vs. responses) were used to fit cell viability curves and to calculate the IC50 values. The values of resistance fold (RF) were calculated as previously stated (19).
The transport function of MDR-associated ABC transporter was determined by tritium-labeled substrate accumulation assay (20). Briefly, 5×105 cells/well were seeded evenly in a 24-well plate, and incubated for 24 h. On the second day, cells were treated with betulin or MK571 at the indicated concentrations for 2 h. Then, [3H]-vincristine was added to the designated wells at a final concentration of 36 nM. After 2 h incubation, cells were washed with PBS twice, and harvested and transferred into scintillation fluid. Subsequently, the intracellular radioactivity was measured by a liquid scintillation analyzer (Packard Instrument, Downers Grove, IL).
As previously described, the protein expression level of ABCC1 was examined by a Western blot analysis (19). In short, cells were treated with or without betulin, and then the lysate was collected. This was followed by determining the protein concentration in the lysates, and equal amount of protein sample (12 μg) was separated by SDS-PAGE then transferred onto PVDF membrane. The membranes were blocked for 2 h with 5% non-fat milk at room temperature followed by incubation with primary antibody (anti-MRP1/ABCC1 and anti-GAPDH at 1:1000) overnight at 4°C. On the second day, after washing with TBST three times, the membranes were incubated with HPR-conjugated secondary antibody (at 1:1000) for 1 h at room temperature. Subsequently, the protein was visualized using an ECL substrate by a digital Western blot scanner (LI-COR, NE), and quantified and analyzed by Fiji software (NIH, Bethesda, MD).
The betulin 3D structure was constructed for docking simulation with an ABCC1 model as previously described (21). ABCC1 protein model 5UJA (LTC4 bound) was obtained from RCSB Protein Data Bank. The model is inward-facing ABCC1 with a resolution of 3.34 (22). Docking calculations were performed in AutoDock Vina (version 1.1.2) (23). Hydrogen atoms and partial charges were added using AutoDock Tools (ADT, version 1.5.4). Docking grid center coordinates were determined from the bound ligand LTC4 provided in 5UJA PDB files. Receptor/ligand preparation and docking simulation were performed using default settings. The top-scoring pose (sorted by affinity score: kcal/mol) was selected for further analysis and visualization.
All data were presented as mean ± SD, and evaluated using a one-way or two-way ANOVA as appropriate by GraphPad software (La Jolla, CA). Statistical significance was considered when p < 0.05.
An MTT assay was performed to examine the cell viability of substrate drugs with or without an inhibitor. Herein, the RF values were used to assess the degree of attenuated effectiveness resulting from ABCC1 overexpression. As shown in Figures 1B, C, the efficacy of betulin was restricted in cells expressing ABCC1 as evidenced by higher IC50 values in MDR cells mediated by ABCC1 compared to their corresponding drug-sensitive cell line counterparts. The RF value of betulin was significantly increased to 6.53-fold in KB-CV60 cells, and 8.93-fold in ABCC1-transfected HEK293 cells (Table 1). Importantly, the betulin-induced drug resistance can be antagonized by a known ABCC1 inhibitor MK571 at 25 μM. By contrast, vincristine serves as a reference substrate to compare the drug resistance conferred by ABCC1. Based on Table 1, vincristine had 19.33- and 23.79-fold resistance in drug-selected cancer cells and gene-transfected cells expressing ABCC1, and similarly, this resistance can be reversed by MK571 at 25 μM. Thus, betulin is less sensitive in ABCC1-overexpressing cells, and an established ABCC1 inhibitor could overcome the resistance effect induced by betulin.
As overexpression of ABCB1 and ABCG2 are also central to MDR, cell lines expressing either ABCB1 or ABCG2 were investigated. Based on Figures 1D, E, the IC50 values of betulin were 5.72, 7.56, 6.37, and 7.34 μM for HEK293/pcDNA3.1, HEK293/ABCB1, HEK293/ABCG2-WT, and HEK293/ABCG2-G2 cells, respectively. The RF values were 1.32, 1.11, and 1.28 in ABCB1-overexpressing cells, and wide-type or R482G-mutant ABCG2-overexpressing cell lines, respectively (Figures 1D, E). Given no significant difference in RF values between drug-sensitive and MDR cells mediated by ABCB1 or ABCG2, it is likely that ABCB1 and ABCG2 overexpression did not affect the effectiveness of betulin.
A tritium-labeled drug accumulation analysis was conducted to assess the interaction between betulin and MDR-associated ABC transporter. Our results in Figure 2A showed that betulin at 25 μM enhanced the intracellular vincristine accumulation from 44% to 52% compared with KB-CV60 cells without an inhibitor. Herein, 25 μM MK571 acted as a reference ABCC1 inhibitor to increase substrate drug accumulation in KB-CV60 cells (from 44% to 65%). Therefore, betulin at a high concentration has the ability to impede the ABCC1 transport function resulting in increased level of intracellular accumulation of substrate drug.
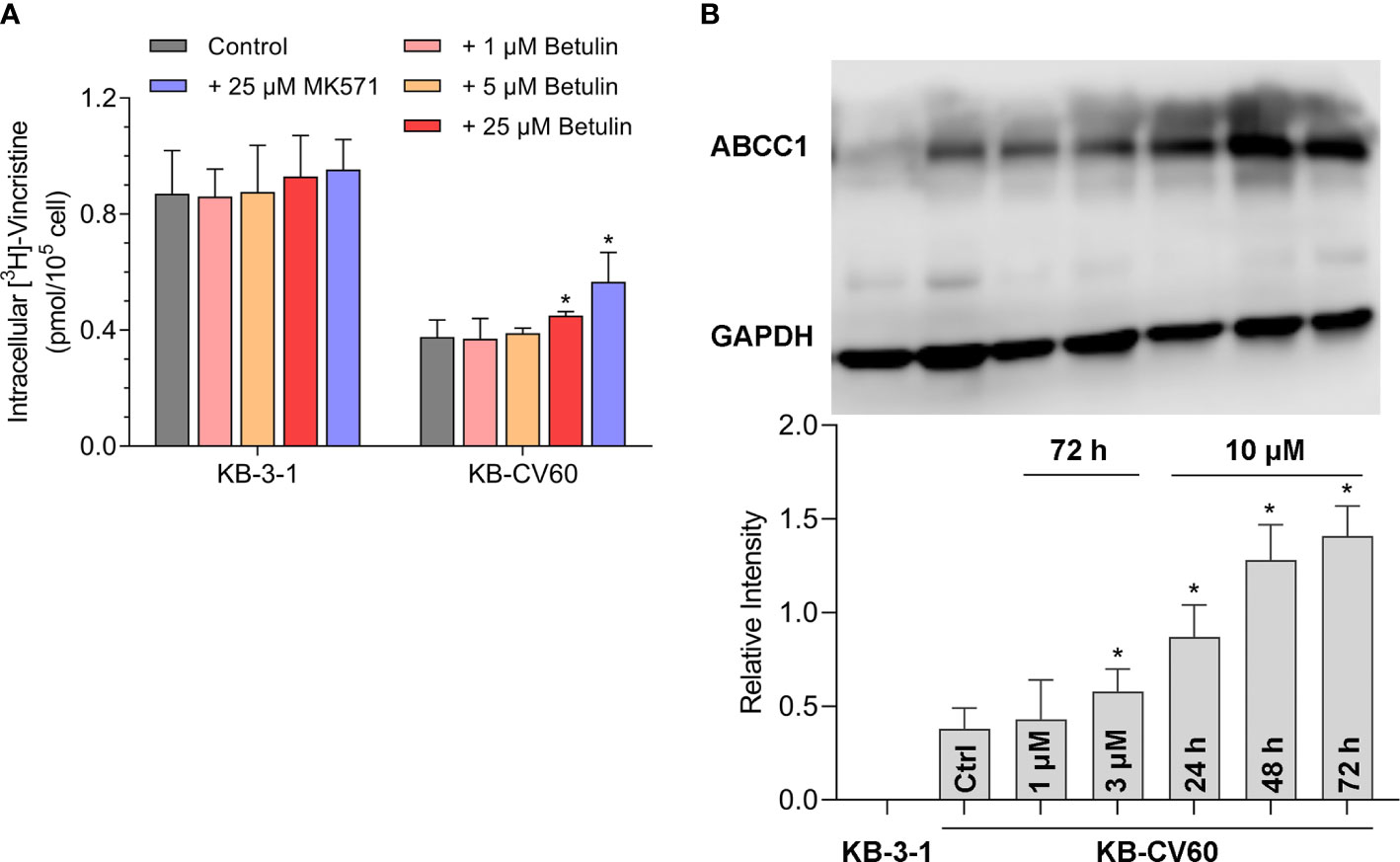
Figure 2 Effects of betulin on the transport function mediated by ABCC1 and the ABCC1 protein expression level. (A) [3H]-vincristine intracellular accumulation in KB-3-1 and KB-CV60 cells after betulin or MK571 treatment at indicated concentrations. (B) The ABCC1 protein expression level after incubated with 1 μM or 3 μM betulin for 72 h, or with 10 μM betulin up to 72 h. All data are presented as mean ± SD. *indicates p < 0.05 compared with its corresponding control group.
It is documented that upregulation of ABC transporter expression can induce MDR. A Western blot analysis was used to determine the ABCC1 protein expression level after betulin treatment. As shown in Figure 2B, 3 μM betulin had the ability to significantly induce ABCC1 expression after a 72 h incubation period. Also, the expression level of ABCC1 time-dependently increased following treatment with betulin at 10 μM up to 72 h. Betulin time- and concentration-dependently upregulated ABCC1 protein expression.
To further validate the interaction of betulin and ABCC1 protein, an in silico analysis was conducted. Our results showed that betulin docked into the drug-binding site of ABCC1 with an affinity score of −6.8 kcal/mol. Overall, betulin binds in the pocket surrounded by the transmembrane domains of ABCC1 protein (Figure 3A), partially overlapping the leukotriene C4 (LTC4)-binding site (Figure 3C). Details of the ligand-receptor interaction are displayed in Figure 3B. The primary factor contributing to the binding affinity of betulin to the ABCC1 protein is via hydrophobic interactions. According to Figures 3B, D, betulin is positioned and stabilized in the hydrophobic cavity formed by Leu381, Phe385, Phe389, Tyr440, Thr439, Ile598, Phe594, Met1092, Thr1241, Tyr1242, Asn1244, and Tpr1245. Betulin was stabilized by a hydrogen bond formed with Trp553. Together, these results demonstrated that betulin has an interaction with the ABCC1 protein.
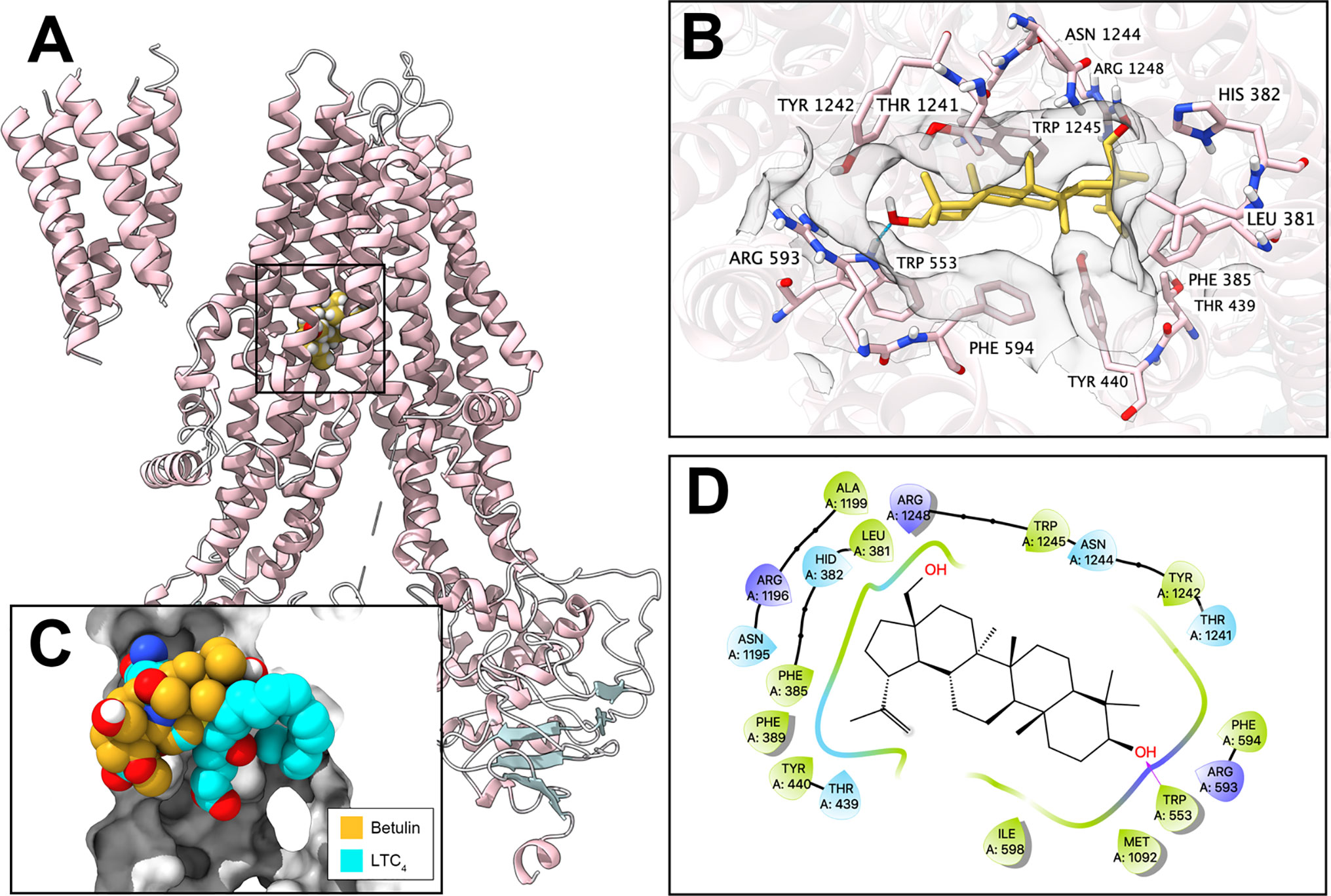
Figure 3 Interaction between betulin and human ABCC1 protein. (A) Overview of the best-scoring pose of betulin in the drug-binding pocket of ABCC1 protein (5UJA). ABCC1 was displayed as colored ribbons (helix: pink; strand: blue; coil: white). Betulin was displayed as colored balls. (B) Details of the interactions between betulin and ABCC1 (5UJA) binding pocket. ABCC1 helices were displayed as colored ribbons. Important residues were displayed as colored sticks (carbon: pink; oxygen: red; nitrogen: blue; hydrogen: white; fluoride: lime; chloride: light green). Surface formed by important residues were depicted as grey solid planes. Betulin was displayed as colored sticks (carbon: yellow; oxygen: red; nitrogen: blue; chloride: lime; fluoride: light green). Hydrogen bonds were displayed as blue dash lines. (C) Binding poses of betulin and ABCC1 substrate LTC4 in ABCC1 binding pocket. (D) 2D diagram of the interaction between betulin and ABCC1. Amino acids within 3 Å from betulin were displayed as colored bubbles (green: hydrophobic; blue: polar). Purple solid lines with arrow indicate hydrogen bonds.
Nowadays, many natural-derived drugs serve as sources of novel drug discovery and are tested clinically (24, 25). Unfortunately, the efficacy of certain natural products could be compromised by MDR-associated ABC transporters (3, 4). ABCC1 (MRP1), ABCB1 (MDR1, P-glycoprotein/P-gp), and ABCG2 (BCRP/MXR) are extensively studies, and are commonly responsible for MDR (18, 19). Betulin and betulinic acid derived from birch bark have a broad spectrum of pharmacological activity (11, 26). Researchers reported that betulinic acid inhibits ABCB1-, ABCG2-, and ABCB5-mediated MDR with similar effectiveness as counterparts in parental cell lines (12). Also, Zhao et al. found that betulinic acid nanoparticle exerts its anticancer efficacy via downregulating ABCG1 oncogene expression level (13). These provide us a clue that betulin may interact with MDR-associated ABC transporters. However, the interaction of betulin and ABCC1 remains inconclusive and needs to be determined. Considering the similar cancer spectrum between ABCC1 and betulin as described in the introduction section, we herein focused on the effectiveness of betulin influenced by ABCC1 overexpression.
Our experiments started from an MTT-based cell viability assay to assess the cytotoxicity of betulin and a reference substrate drug vincristine with or without an inhibitor. The results indicated that ABCC1 overexpression can confer resistance to betulin in cancer cells expressing ABCC1. As KB-3-1 is a human epidermoid carcinoma cell line, it is possible that, to some extent, developing other mechanism induces drug resistance apart from ABCC1 overexpression in the KB-CV60 cell line. Hence, the cell viability of betulin was determined on ABCC1-transfected HEK293 cells. Similarly, betulin resistance was found in HEK293/ABCC1 cells as evidenced by the higher RF value in cells transfected with ABCC1 compared with its corresponding sensitive HEK293/pcDNA3.1 cells counterpart. Vincristine, a vinca alkaloid isolated from the Madagascar periwinkle Catharanthus roseus (27), served as a reference substrate of ABCC1 (2). Although betulin does not have as high of an RF value as vincristine, it is still rather comparable to vincristine. Importantly, betulin-conferred drug resistance can be antagonized by a known ABCC1 inhibitor, MK571. Overexpression of ABCB1 and ABCG2 is also central to MDR, therefore cell viability of cells overexpressing ABCB1 or ABCG2 was also examined after betulin treatment. Given different cellular context, it is possible that switching arginine to glycine (R > G) in the ABCG2 gene at amino acid 482 could affect substrate specificity and different resistance levels to substrate drugs (17). Our results showed that no significant difference in IC50 values was observed in drug-sensitive cells and corresponding MDR cells mediated by ABCB1, and wild-type or R482G-mutant ABCG2, which were consistent with results from Saeed et al (12).. Together, ABCC1 overexpression may promote betulin resistance, while overexpression of ABCB1 and ABCG2 could not confer resistance to betulin. Thus, we hypothesized that betulin is a, ABCC1 substrate.
Following mechanism-based studies, a Western blot analysis was conducted to assess the ABCC1 protein expression. Our results showed that within a 72 h incubation period, betulin upregulates ABCC1 expression level in time- and concentration-dependent manners. Interestingly, other researchers generated Pearson’s correlation coefficients (R-values) to correlate the expression level of different genes and the log10IC50 values for betulinic acid (12). Results from Saeed et al. demonstrated that there is no significant correlation between betulinic acid and ABCC1 gene expression. In the chemical structure, betulin and betulinic acid are different in hydroxymethyl and carboxyl groups, which may lead to differences in their pharmacological properties. We also postulated that this inconsistency may be the results from post-transcriptional and/or post-translational modification and regulation (28–31), which needs further validation.
Substrate drugs can occupy MDR-associated ABC transporters, and result in a competition with another drug substrate for transport function (20). As a result, a repurposed drug substrate acting as an inhibitor or a reversal agent has the ability to sensitize MDR-associated ABC transporter to another drug substrate (32). In our study, the [3H]-vincristine accumulation in cancer cells was measured. Results from an accumulation assay demonstrated that betulin at 25 μM inhibits MDR-mediated by ABCC1. However, the inhibitory effect of betulin to ABCC1 is not as strong as 25 μM MK571, which might be the result of high resistance level caused by ABCC1 in KB-CV60 cells and/or the lower RF value difference of betulin relative to vincristine between KB-3-1 cells and KB-CV60 cells. Notably, the concentrations of betulin used in the accumulation assay are higher than the IC50 values in corresponding cell lines, which can be toxic to the cells. However, the 4 h incubation of betulin with cells may not affect the cellular function. This is confirmed in the parental cells that no significant difference was observed between the vehicle group and the treatment groups. Of note, even the weaker inhibitory effect of betulin than MK571 counterpart, our findings do not warrant further investigation of betulin as a reversal agent or modulator to MDR mediated by ABCC1 overexpression.
The computational docking analysis serves as an efficient tool to predict the interaction of compound with protein models even though it indicates a virtual binding instead of an actual one (33, 34). Betulin received an affinity score of −6.8 kcal/mol with human ABCC1 protein model (5UJA). Also, it is known that leukotriene C4 (LTC4) can be pumped by ABCC1 (35). Our results revealed that betulin has binding interaction with human ABCC1 protein, and more importantly shares certain overlapping binding sites with LTC4. Overall, betulin may exhibit a similar substrate behavior.
Betulin is susceptible to drug resistance mediated by ABCC1 overexpression, and a known ABCC1 inhibitor, MK571, can sensitize the cells expressing ABCC1 to betulin. ABCC1-induced resistance to betulin can be explained by its upregulated protein expression of ABCC1. Additionally, betulin at high concentration has the ability to inhibit ABCC1 transport function, which may affect the pharmacokinetic profile of other ABCC1 drug substrates, such as vincristine. These findings may be a valuable foundation for follow-up clinical investigation on the potential use of betulin.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Conceptualization: X-YC. Methodology: YY, X-YC, and J-QW. Writing-original draft: YY and X-YC. Writing-review and copyediting: X-YC, YY, Z-XW, and Z-SC. Supervision: JL and Z-SC. All authors contributed to the article and approved the submitted version.
This research was funded by the National Natural Science Foundation of China (No.81973761).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authors are grateful to Dr. Shin-Ichi Akiyama (Kagoshima University, Kagoshima, Japan) for providing KB-3-1 and KB-CV60 cell lines. We thank Drs. Susan E. Bates (NCI, NIH, Bethesda, MD) for kindly providing gene-transfected cell lines. We thank Dr. Yangmin Chen for editing the article. We thank for the supported from the National Natural Science Foundation of China (No. 81973761).
1. Muniraj N, Siddharth S, Sharma D. Bioactive Compounds: Multi-Targeting Silver Bullets for Preventing and Treating Breast Cancer. Cancers (2019) 11(10):1563. doi: 10.3390/cancers11101563
2. Ji N, Yang Y, Cai CY, Lei ZN, Wang JQ, Gupta P, et al. Selonsertib (GS-4997), an ASK1 inhibitor, antagonizes multidrug resistance in ABCB1- and ABCG2-overexpressing cancer cells. Cancer Lett (2019) 440-441:82–93. doi: 10.1016/j.canlet.2018.10.007
3. Huang BY, Zeng Y, Li YJ, Huang XJ, Hu N, Yao N, et al. Uncaria alkaloids reverse ABCB1-mediated cancer multidrug resistance. Int J Oncol (2017) 51:257–68. doi: 10.3892/ijo.2017.4005
4. Yin JY, Huang Q, Yang Y, Zhang JT, Zhong MZ, Zhou HH, et al. Characterization and analyses of multidrug resistance-associated protein 1 (MRP1/ABCC1) polymorphisms in Chinese population. Pharmacogenetics Genomics (2009) 19:206–16. doi: 10.1097/FPC.0b013e328323f680
5. Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science (New York NY) (1992) 258:1650–4. doi: 10.1126/science.1360704
6. Perdu J, Germain DP. Identification of novel polymorphisms in the pM5 and MRP1 (ABCC1) genes at locus 16p13.1 and exclusion of both genes as responsible for pseudoxanthoma elasticum. Hum Mutat (2001) 17:74–5. doi: 10.1002/1098-1004(2001)17:1<74::AID-HUMU14>3.0.CO;2-F
7. Higgins CF, Linton KJ. The ATP switch model for ABC transporters. Nat Struct Mol Biol (2004) 11:918–26. doi: 10.1038/nsmb836
8. Eckford PD, Sharom FJ. ABC efflux pump-based resistance to chemotherapy drugs. Chem Rev (2009) 109:2989–3011. doi: 10.1021/cr9000226
9. Zhao X, Wang W, Zu Y, Zhang Y, Li Y, Sun W, et al. Preparation and characterization of betulin nanoparticles for oral hypoglycemic drug by antisolvent precipitation. Drug Deliv (2014) 21:467–79. doi: 10.3109/10717544.2014.881438
10. Drąg-Zalesińska M, Drąg M, Poręba M, Borska S, Kulbacka J, Saczko J. Anticancer properties of ester derivatives of betulin in human metastatic melanoma cells (Me-45). Cancer Cell Int (2017) 17:4. doi: 10.1186/s12935-016-0369-3
11. Amiri S, Dastghaib S, Ahmadi M, Mehrbod P, Khadem F, Behrouj H, et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol Adv (2020) 38:107409. doi: 10.1016/j.biotechadv.2019.06.008
12. Saeed MEM, Mahmoud N, Sugimoto Y, Efferth T, Abdel-Aziz H. Betulinic Acid Exerts Cytotoxic Activity Against Multidrug-Resistant Tumor Cells via Targeting Autocrine Motility Factor Receptor (AMFR). Front Pharmacol (2018) 9:841. doi: 10.3389/fphar.2018.00481
13. Zhao H, Mu X, Zhang X, You Q. Lung Cancer Inhibition by Betulinic Acid Nanoparticles via Adenosine 5’-Triphosphate (ATP)-Binding Cassette Transporter G1 Gene Downregulation. Med Sci Monit Int Med J Exp Clin Res (2020) 26:e922092. doi: 10.12659/MSM.922092
14. Hipfner DR, Deeley RG, Cole SP. Structural, mechanistic and clinical aspects of MRP1. Biochim Biophys Acta (1999) 1461:359–76. doi: 10.1016/S0005-2736(99)00168-6
15. Shukla S, Ohnuma S, Ambudkar SV. Improving cancer chemotherapy with modulators of ABC drug transporters. Curr Drug Targets (2011) 12:621–30. doi: 10.2174/138945011795378540
16. Aoki S, Chen ZS, Higasiyama K, Setiawan A, Akiyama S, Kobayashi M. Reversing effect of agosterol A, a spongean sterol acetate, on multidrug resistance in human carcinoma cells. Japanese J Cancer Research: Gann (2001) 92:886–95. doi: 10.1111/j.1349-7006.2001.tb01177.x
17. Robey RW, Honjo Y, Morisaki K, Nadjem TA, Runge S, Risbood M, et al. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer (2003) 89:1971–8. doi: 10.1038/sj.bjc.6601370
18. Yang Y, Ji N, Cai CY, Wang JQ, Lei ZN, Teng QX, et al. Modulating the function of ABCB1: in vitro and in vivo characterization of sitravatinib, a tyrosine kinase inhibitor. Cancer Commun (Lond Engl) (2020) 40:285–300. doi: 10.1002/cac2.12040
19. Yang Y, Ji N, Teng QX, Cai CY, Wang JQ, Wu ZX, et al. Sitravatinib, a Tyrosine Kinase Inhibitor, Inhibits the Transport Function of ABCG2 and Restores Sensitivity to Chemotherapy-Resistant Cancer Cells in vitro. Front Oncol (2020) 10:700. doi: 10.3389/fonc.2020.00700
20. Wu ZX, Yang Y, Teng QX, Wang JQ, Lei ZN, Wang JQ, et al. Tivantinib, A c-Met Inhibitor in Clinical Trials, Is Susceptible to ABCG2-Mediated Drug Resistance. Cancers (2020) 12(1):186. doi: 10.3390/cancers12010186
21. Wang JQ, Li JY, Teng QX, Lei ZN, Ji N, Cui Q, et al. Venetoclax, a BCL-2 Inhibitor, Enhances the Efficacy of Chemotherapeutic Agents in Wild-Type ABCG2-Overexpression-Mediated MDR Cancer Cells. Cancers (2020) 12(2):466. doi: 10.3390/cancers12020466
22. Johnson ZL, Chen J. Structural Basis of Substrate Recognition by the Multidrug Resistance Protein MRP1. Cell (2017) 168:1075–85.e9. doi: 10.1016/j.cell.2017.01.041
23. Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem (2010) 31:455–61. doi: 10.1002/jcc.21334
24. Gezici S, Şekeroğlu N. Current Perspectives in the Application of Medicinal Plants Against Cancer: Novel Therapeutic Agents. Anti Cancer Agents Medicinal Chem (2019) 19:101–11. doi: 10.2174/1871520619666181224121004
25. Tao H, Zuo L, Xu H, Li C, Qiao G, Guo M, et al. Alkaloids as Anticancer Agents: A Review of Chinese Patents in Recent 5 Years. Recent Pat Anticancer Drug Discov (2020) 15:2–13. doi: 10.2174/1574892815666200131120618
26. Scheffler A. The Wound Healing Properties of Betulin from Birch Bark from Bench to Bedside. Planta Med (2019) 85:524–7. doi: 10.1055/a-0850-0224
27. Qu Y, Safonova O, De Luca V. Completion of the canonical pathway for assembly of anticancer drugs vincristine/vinblastine in Catharanthus roseus. Plant J: Cell Mol Biol (2019) 97:257–66. doi: 10.1111/tpj.14111
28. Dykes IM, Emanueli C. Transcriptional and Post-transcriptional Gene Regulation by Long Non-coding RNA. Genomics Proteomics Bioinf (2017) 15:177–86. doi: 10.1016/j.gpb.2016.12.005
29. Corbett AH. Post-transcriptional regulation of gene expression and human disease. Curr Opin Cell Biol (2018) 52:96–104. doi: 10.1016/j.ceb.2018.02.011
30. Tolsma TO, Hansen JC. Post-translational modifications and chromatin dynamics. Essays Biochem (2019) 63:89–96. doi: 10.1042/EBC20180067
31. Ho EA, Piquette-Miller M. Regulation of multidrug resistance by pro-inflammatory cytokines. Curr Cancer Drug Targets (2006) 6:295–311. doi: 10.2174/156800906777441753
32. Levy ES, Samy KE, Lamson NG, Whitehead KA, Kroetz DL, Desai TA. Reversible inhibition of efflux transporters by hydrogel microdevices. Eur J Pharmaceutics Biopharmaceutics (2019) 145:76–84. doi: 10.1016/j.ejpb.2019.10.007
33. Ferreira RJ, Bonito CA, Cordeiro M, Ferreira MU, Dos Santos D. Structure-function relationships in ABCG2: insights from molecular dynamics simulations and molecular docking studies. Sci Rep (2017) 7:15534. doi: 10.1038/s41598-017-15452-z
34. Lionta E, Spyrou G, Vassilatis DK, Cournia Z. Structure-based virtual screening for drug discovery: principles, applications and recent advances. Curr Topics Medicinal Chem (2014) 14:1923–38. doi: 10.2174/1568026614666140929124445
Keywords: betulin, ATP-binding cassette sub-family C member 1 (ABCC1), multidrug resistance-associated protein 1 (MRP1), multidrug resistance (MDR), natural product
Citation: Chen X-Y, Yang Y, Wang J-Q, Wu Z-X, Li J and Chen Z-S (2021) Overexpression of ABCC1 Confers Drug Resistance to Betulin. Front. Oncol. 11:640656. doi: 10.3389/fonc.2021.640656
Received: 11 December 2020; Accepted: 13 January 2021;
Published: 25 February 2021.
Edited by:
Benyi Li, University of Kansas Medical Center, United StatesCopyright © 2021 Chen, Yang, Wang, Wu, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Li, bGlqaW5ndGlnZXJAMTYzLmNvbQ==; Zhe-Sheng Chen, Y2hlbnpAc3Rqb2hucy5lZHU=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.