
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 19 April 2021
Sec. Women's Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.640268
 Niuniu Hou1†
Niuniu Hou1† Juliang Zhang1†
Juliang Zhang1† Lu Yang1†
Lu Yang1† Ying Wu1†
Ying Wu1† Zhe Wang1
Zhe Wang1 Mingkun Zhang1
Mingkun Zhang1 Li Yang2
Li Yang2 Guangdong Hou3
Guangdong Hou3 Jianfeng Wu2
Jianfeng Wu2 Yidi Wang1
Yidi Wang1 Bingyao Dong1
Bingyao Dong1 Lili Guo1
Lili Guo1 Mei Shi4*
Mei Shi4* Rui Ling1*
Rui Ling1*Background and Objectives: To establish a prognostic stratification nomogram for T1–2 breast cancer with 1–3 positive lymph nodes to determine which patients can benefit from postmastectomy radiotherapy (PMRT).
Methods: A population-based study was conducted utilizing data collected from the Surveillance, Epidemiology, and End Results database. Chi-square test or Fisher exact test was used to compare the distribution of characteristics. Cox analysis identified significant prognostic factors for survival. A prognostic stratification model was constructed by R software. Propensity score matching was applied to balance characteristics between PMRT cohort and control cohort. Kaplan-Meier method was performed to evaluate the performance of stratification and the benefits of PMRT in the total population and three risk groups.
Results: The overall performance of the nomogram was good (3-year, 5-year, 10-year AUC were 0.75, 0.72 and 0.67, respectively). The nomogram was performed to excellently distinguish low-risk, moderate-risk, and high-risk groups with 10-year overall survival (OS) of 86.9%, 73.7%, and 62.7%, respectively (P<0.001). In the high-risk group, PMRT can significantly better OS with 10-year all-cause mortality reduced by 6.7% (P = 0.027). However, there was no significant survival difference between PMRT cohort and control cohort in low-risk (P=0.49) and moderate-risk groups (P = 0.35).
Conclusion: The current study developed the first prognostic stratification nomogram for T1–2 breast cancer with 1–3 positive axillary lymph nodes and found that patients in the high-risk group may be easier to benefit from PMRT.
Breast cancer is the most common malignancy, and its mortality rate ranks second among all cancer-related deaths in females (1). Postmastectomy radiotherapy (PMRT) is an effective treatment for breast cancer, which was proposed to reduce local recurrence and prolong survival when rationally combined with systematic treatment (2, 3). PMRT has become an essential therapy for patients of breast cancer with at least four positive lymph nodes.
However, whether PMRT could improve outcomes for T1–2 breast cancer with 1–3 axillary lymph nodes remains controversial. The meta-analysis of the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) proved that PMRT could effectively decrease the local recurrence of T1–2 breast cancer with 1–3 positive axillary lymph nodes and improve the prognosis (4). Furthermore, the American Society of Clinical Oncology (ASCO) guidelines highly recommend PMRT for T1–2 breast cancer with 1–3 positive axillary lymph nodes (5). But studies have proposed that the 10-year recurrence rate of patients enrolled in clinical trials included in EBCTCG’ meta-analysis was significantly higher compared with T1–2 breast cancer with 1–3 positive axillary lymph nodes in the modern medicine era (20.3% versus 4–10%) (6). Additionally, several retrospective analyses found that PMRT did not decrease the recurrence rate or prolong OS among T1–2 breast cancer with 1–3 positive axillary lymph nodes (7–9). Moreover, the long-term side effects caused by radiotherapies, such as cardiovascular system damage, secondary cancer, and arm lymphedema, could not be ignored (10). These researchers considered that for patients with 1–3 positive axillary lymph nodes, the strong recommendation of PMRT might be unreasonable. Therefore, it is essential for clinicians to screen out the potential population who may benefit from PMRT.
Many studies have tried to identify prognostic factors of T1–2 breast cancer with 1–3 lymph nodes in recent years. Several variables, such as age, tumor size, grade, surgical margin status, prognostic scores, and the number of positive axillary lymph nodes, have been verified as correlative variables with survival (3, 11–24). Additionally, axillary lymph node dissection (ALND) is highly recommended for clinically node-positive breast cancer for its excellent regional control and better prognosis. A prospective single-institution study concluded that the number of positive nodes of ALND and tumor size were both associated with receipt of PMRT (25). Furthermore, several retrospective studies reported that T1–2 breast cancer with 1–3 positive axillary lymph nodes has a great heterogeneity, and the benefit from PMRT would vary significantly in this population (13, 24, 26). Hence, personalized PMRT may benefit the patient to the greatest extent in this heterogeneous population. Nomogram can incorporate all independent prognostic factors, individually predict each patient’s prognosis, and provide a very reliable reference for the treatment (27–29). However, few studies were conducted to establish a prognostic stratification model for T1–2 breast cancer with 1–3 positive axillary lymph nodes.
Based on the data of 11917 patients, the current study aimed to comprehensively analyze prognostic factors of T1–2 breast cancer with 1–3 positive axillary lymph nodes, to establish a prognostic stratification model for patients, and to identify the potential population who could benefit from PMRT.
Data in this study were extracted from the SEER (Surveillance, Epidemiology, and End Results database) 18 Registries, which collects cancer incidence data on patient’s demographics, tumor characteristics, therapy, and follow-up, covers nearly 30% of the population in the United States, and is updated annually. SEER ∗ Stat version 8.3.5 (username:11764-Nov2019) was performed to obtain patients diagnosed with breast cancer in 2000-2014.
The inclusion criteria were listed as follows: (a) female breast cancer was diagnosed as the first and only cancer in 2000-2014 according to the International Classification of Disease, 3rd edition code (ICD-O-3), (b) T1-2, N1, M0 according to the 6th American Joint Committee on Cancer (AJCC), (c) all patients with breast cancer were confirmed by positive histology, (d) ‘malignant’ in behavior code (ICD-O-3). 71008 patients with T1-2, N1 breast cancer were included in our study. Patients were excluded based on the following criteria: (a) incomplete demographic information, such as race and marital status, (b) those younger than 20 or older than 70, (c) bilateral breast cancer and unilateral breast cancer-side unspecified (d) histology types other than infiltrating duct carcinoma (ICD-O-3 code 8500/3) or lobular carcinoma (8520/3), (e) unknown the number of examined lymph nodes, (f) Positive lymph nodes other than 1-3, (g) not received breast mastectomy and underwent axillary surgery (RX Summ–Surg Prim Site: 00, 19, 20, 21, 22, 23, 24) (h) unknown tumor grade, unknown ER status, and PR status, (i) missing PMRT data, (j) incomplete follow-up (survival months = 0).
20170 T1–2 breast cancer with 1–3 positive axillary lymph nodes were finally included in this study. 18607 patients who were diagnosed of T1–2 breast cancer with 1–3 positive axillary lymph nodes between 2005 and 2014 were included for formal analysis. Among them, 6690 (36.0%) patients received PMRT were involved in the PMRT cohort, and 11917 (64.0%) patients did not receive PMRT were involved in the control cohort to construct a nomogram. An independent cohort of 1110 T1-2 breast cancer patients with 1-3 positive nodes in 2000-2004 was selected as the validation cohort. The detailed flowchart for this study was presented in Figure 1.
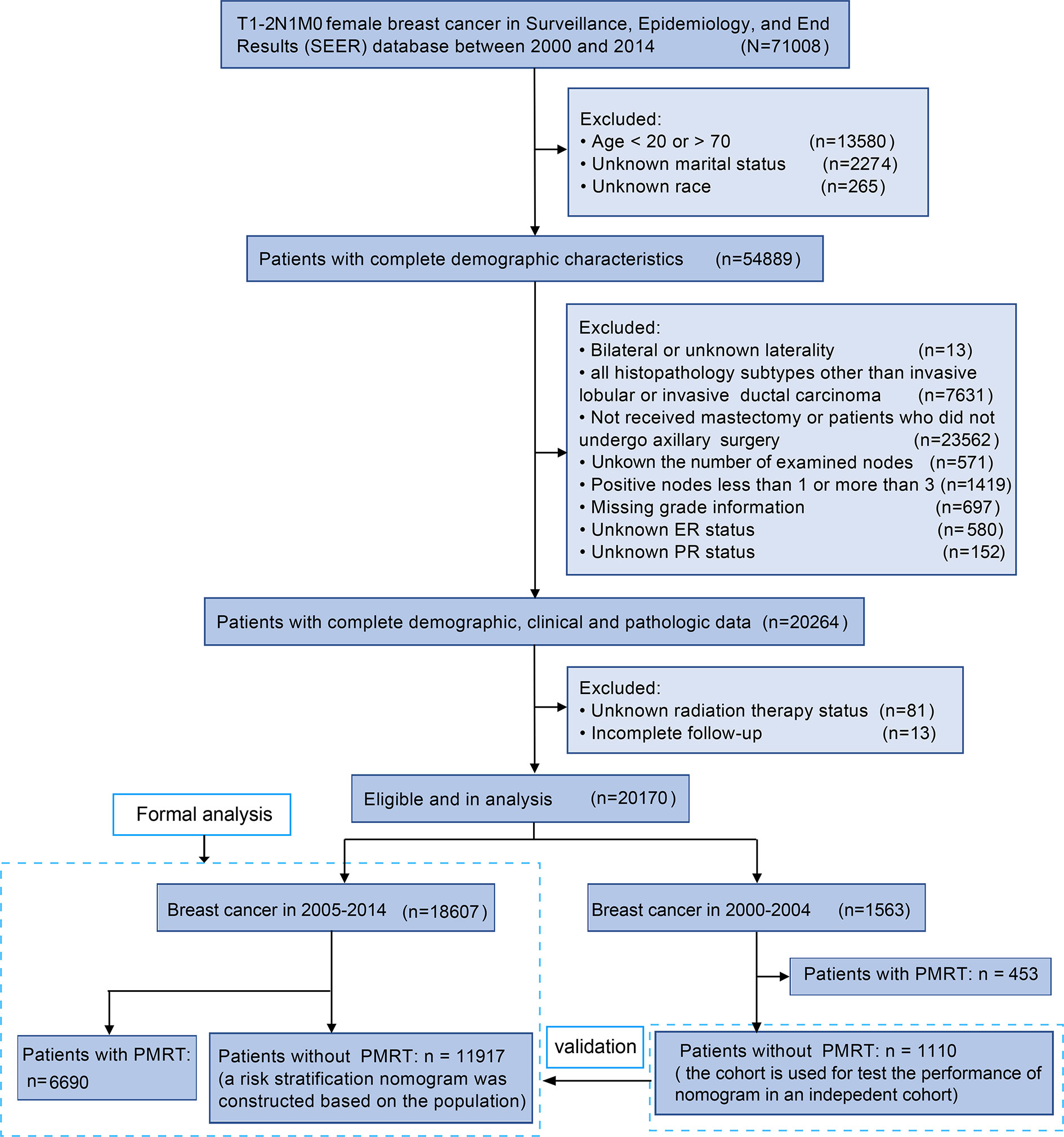
Figure 1 Flowchart of the study design. A total of 20170 female T1–2 breast cancer with 1–3 positive axillary lymph nodes were finally involved in our study.
The following clinical and pathological variables were obtained as variables: race, marital status, age at diagnosis, T stage, N stage, histology, grade, ER status, PR status, chemotherapy status, regional nodes examined, regional nodes positive, PMRT status, and follow-up information.
The main primary endpoint of this study was overall survival (OS) because it was more reliable and objective than recurrence-free survival and could comprehensively reflect the overall benefit of PMRT (the balance between benefit and toxicity of PMRT). OS were defined by survival months and survival status.
SPSS 22.0 statistical software (IBM Corp., Armonk, NY, USA) was used to analyze the data. The categorical variables were categorized based on previous research results. Chi-square test or Fisher’s exact test was used to compare the baseline characteristics between the PMRT cohort and control cohort, whereas quantitative variables were listed as median with interquartile range (IQR) and compared by Student’s t test or non-parametric Mann-Whitney U test. The Backward method was used for univariable and multivariable Cox analysis. Variables with P < 0.05 in univariable Cox analysis were incorporated into multivariable Cox analysis to determine independent prognostic factors of the control cohort. The Kaplan-Meier method was performed to calculate the survival rate, and the Log-rank test was applied to compare the differences between the curves.
A backward step-down selection identified a final model according to the Akaike information criterion (30). Based on the multivariable Cox regression results in the control cohort, a nomogram was constructed using R software 3.6.3 (rms package in http://www.r-project.org/) (31). In this study, the bootstrap method = 200 was used to verify the model’s performance. 3-year, 5-year, and 10-year ROC curves and calibration curves were plotted to evaluate the predictive performance of the model (32). The larger the area under the ROC curve (AUC), the higher predictive accuracy of the nomogram. The closer the calibration curve is to the ideal curve, the more unbiased prediction of the model. All T1–2 breast cancer with 1–3 positive axillary lymph nodes were divided into three risk groups (low-risk, moderate-risk, and high-risk group) according to optimal cutoffs of total points of the nomogram constructed in the control cohort. Decision curves were plotted to evaluate the net benefit of the nomogram and nomogram-assisted risk stratification (33). In order to reduce the effect of potential confounding factors on selection bias, the propensity score matching (PSM) without replacement was applied to compare using the nearest-neighbor method with a caliper = 0.02 (34). Standardized mean difference (SMD) was measured for the baseline variable of all independent predictors before and after PSM. SMD of < 0.10 for a given variable demonstrates a relatively small imbalance (35). MatchIt package was used to balance the baseline characteristics between the PMRT cohort and control cohort in different risk groups. By comparing the survival of the PMRT cohort and control cohort in each risk group, we identified a potential population that could benefit from PMRT. The ggplot2 package was performed to plot the Kaplan-Meier curves. Caret package and ggDCA package were applied to draw decision curves. Forestplot package was used to present hazard ratios of PMRT cohort and control cohort in different risk groups.
X-tile was applied to identify the optimal cutoff value of the model scores (36). A two-sided P < 0.05 was considered as statistically significant.
A total of 18607 T1–2 breast cancer with 1–3 positive axillary lymph nodes in 2005–2014 were finally selected. Among them, 6690 (36.0%) patients received PMRT were involved in the PMRT cohort, and 11917 (64.0%) patients did not receive PMRT were involved in the control cohort to construct a nomogram (Figure 1). The median follow-up for the total population was 69 months (IQR, 42–100 months), with 3-year, 5-year, and 10-year OS being 94.7%, 89.7%, and 79.2%, respectively. The median follow-ups for the PMRT cohort and control cohort were 63 months (IQR, 40–93 months) and 73 months (IQR, 44–103 months), respectively. 805 (12.0%) people died in the PMRT cohort, while 1635 (13.7%) people died in the control cohort.
Baseline characteristics between the two cohorts were shown in Table 1. Notably, compared with patients in the control cohort, patients in the PMRT cohort were often younger in age, had a significantly higher proportion of Black in race, grade 3, T2, received chemotherapy, performed ALND, fewer examined nodes (≤12), and three positive axillary lymph nodes, and a lower proportion of ER positive and PR positive.
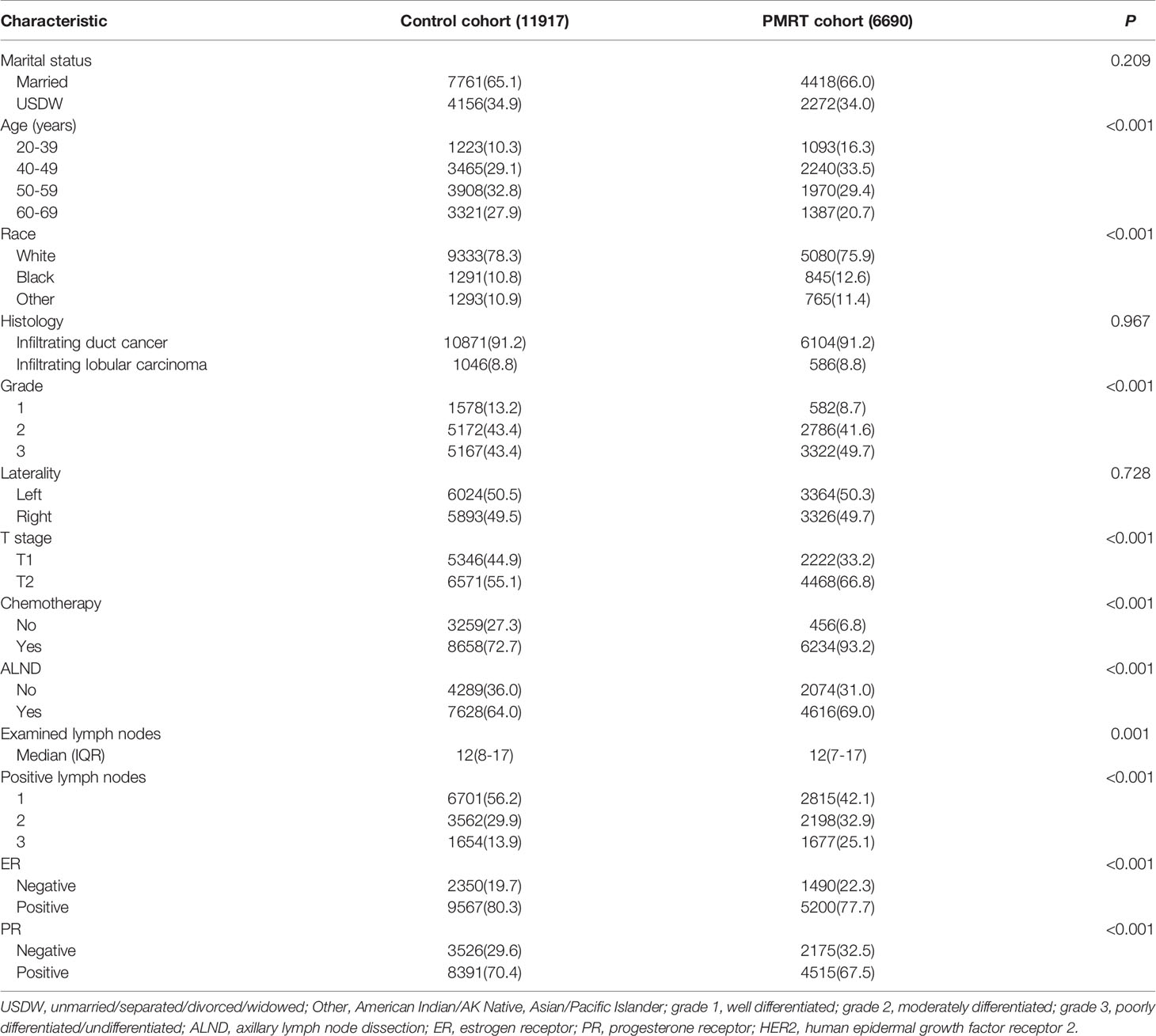
Table 1 Demographic and clinicopathologic features of patients between the control cohort and PMRT cohort in T-2 breast cancer with 1-3 positive lymph nodes.
The 3-year, 5-year, and 10-year OS of 11917 breast cancer in control cohort were 94.6%, 89.6%, and 78.8%, respectively. Table 2 presented the univariable and multivariable results. Married, other in race, 40-49 in age, grade 1, T1, examined nodes >12, one positive lymph nodes, ER positive, PR positive, and given chemotherapy were independent protective factors for T1–2 breast cancer with 1–3 positive axillary lymph nodes.
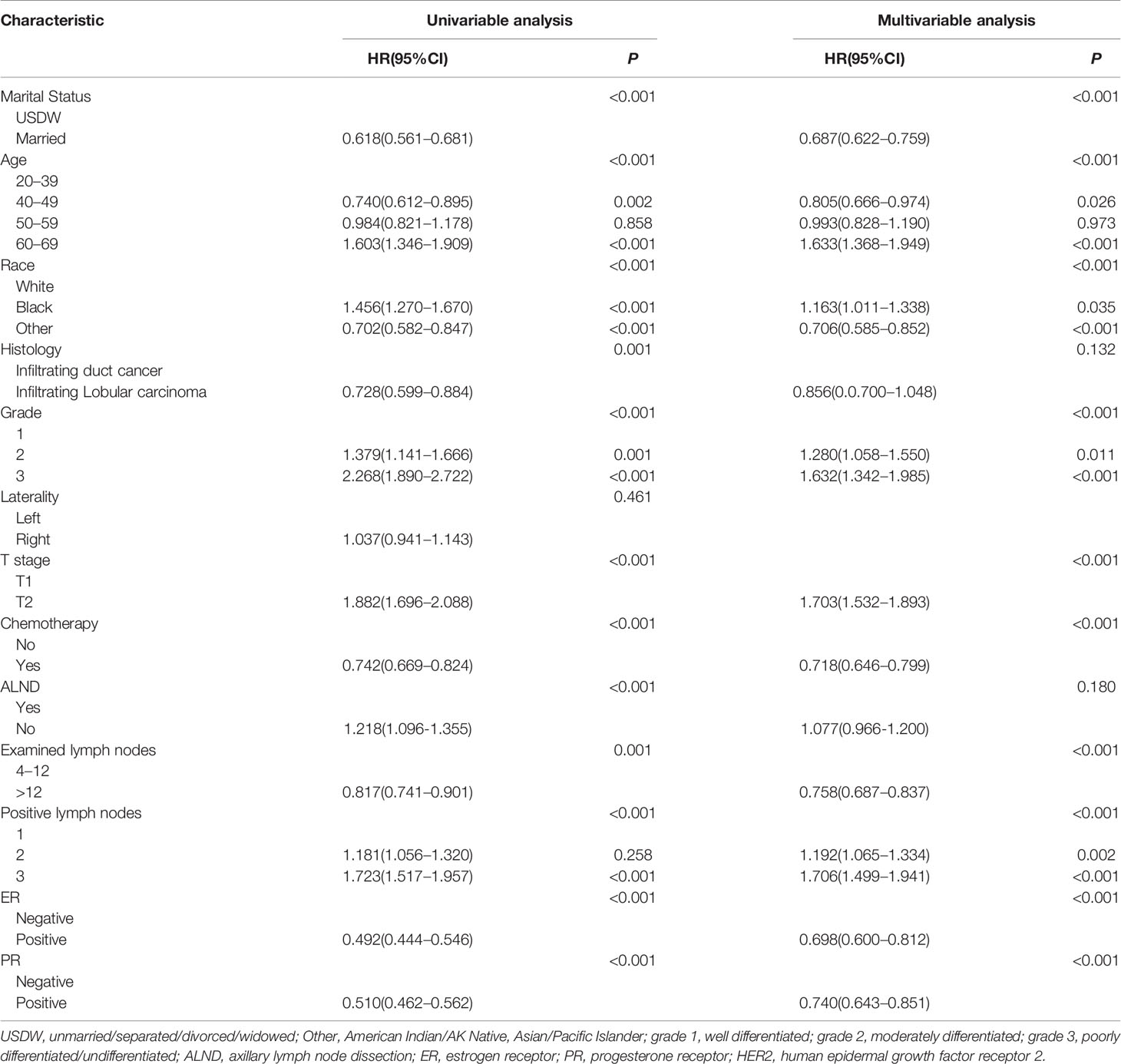
Table 2 Univariable and multivariable Cox analysis for predicting overall survival in T1–2 breast cancer with 1–3 positive lymph nodes in the control cohort.
A nomogram for predicting the OS was created by integrating all independent prognostic factors (Figure 2A).
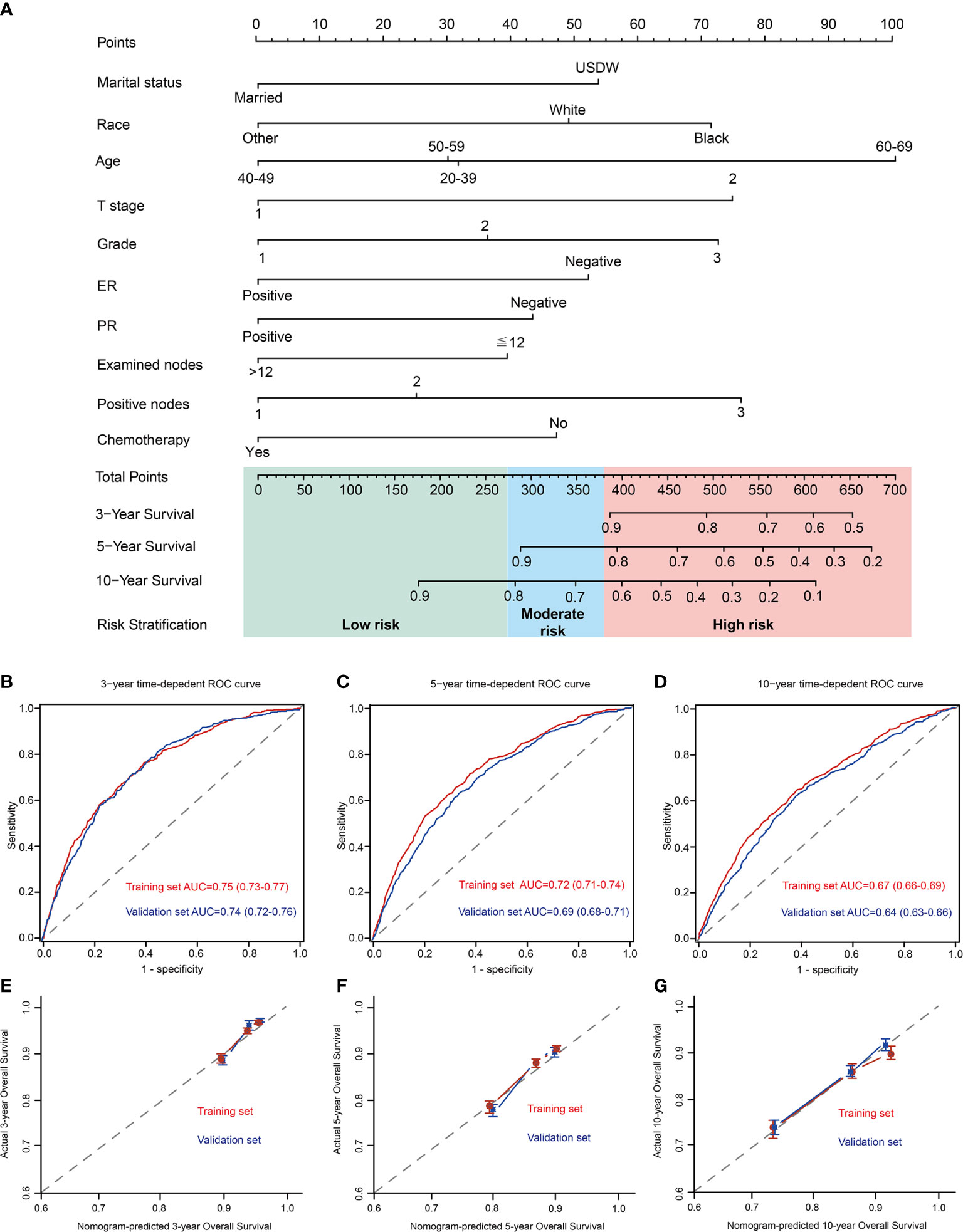
Figure 2 Development of a prognostic stratification nomogram and validation of the proposed nomogram. (A) a prognostic stratification nomogram to accurately predict overall survival for T1–2 breast cancer with 1–3 positive lymph nodes. (B–D) ROC curves for predicting the overall survival in the internal and external validation at 3-year, 5-year, and 10-year, respectively. The values in brackets of Figure 2B–D represent the area under the ROC curves (AUC). (E–G) the calibration curves for predicting patients’ overall survival in the internal and external validation at 3-year, 5-year, and 10-year, respectively. USDW, unmarried/separated/divorced/widowed; Other, American Indian/AK Native, Asian/Pacific Islander; grade 1, well differentiated; grade 2, moderately differentiated; grade 3, poorly differentiated/undifferentiated; ER, estrogen receptor; PR, progesterone receptor.
In control cohort (internal validation), the prognostic model predicts OS with excellent performance, with 3-year, 5-year, and 10-year AUC were 0.75 (95% CI, 0.73–0.77), 0.72 (95% CI, 0.71–0.74), and 0.67 (95%CI 0.66–0.69), respectively (Figures 2B–D). Moreover, the 3-year, 5-year, and 10-year calibration curves further presented excellent agreement between predictions and observation in the probability of 3-year, 5-year, and 10-year survival (Figures 2E–G).
An independent cohort of 1110 T1–2 breast cancer patients with 1–3 positive nodes in 2000-2004 were selected as the validation cohort to verify the performance of the nomogram. Baseline characteristics between control cohort and validation cohort were shown in Supplementary Table 1. Compared with population in control cohort, patients in validation set have a higher proportion of grade 3, received ALND, infiltrating duct cancer, ER negative and PR negative. In validation cohort (external validation), the prognostic model predicts OS with 3-year, 5-year, and 10-year AUC were 0.74 (95% CI, 0.72–0.76), 0.69 (95% CI, 0.68–0.71), and 0.64 (95%CI 0.63–0.66), respectively (Figures 2B–D). Furthermore, the 3-year, 5-year, and 10-year calibration curves further presented high agreement between predictions and observations in the probability of 3-year, 5-year, and 10-year survival in external validation (Figures 2E–G).
18607 patients were divided into three prognostic groups based on patients’ total scores using X-tile software (Supplementary Figure S1): low-risk group (9978 patients; total score ≤ 274); moderate-risk group (6283 patients; 274 < total score ≤ 380); high-risk group (2346 patients; total score > 380). The survival curves presented excellent discrimination at 10-year OS among the low-risk group, moderate-risk group, and high-risk group, with 10-year OS rates of 87.3%, 74.0%, and 60.9%, respectively (P < 0.001, Figure 3A). The baseline characteristics of the PMRT cohort and control cohort in different risk groups were shown in the Supplementary Table 2.
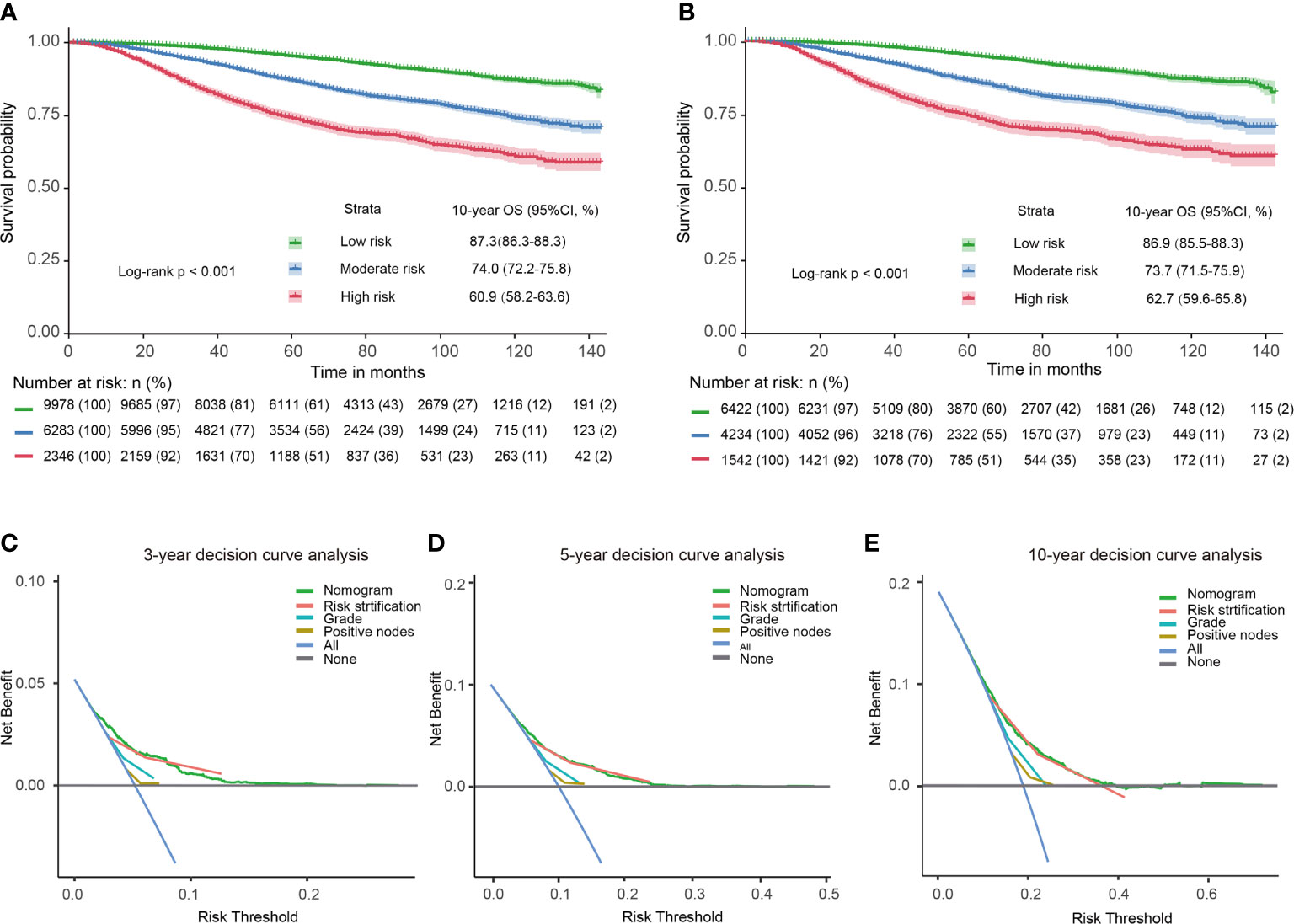
Figure 3 Kaplan-Meier survival curves and decision curves for T1–2 breast cancer with 1–3 positive lymph nodes. Survival curves in the entire cohort before PSM (A) and after PSM (B) stratified by the total score of the nomogram. 3-year (C), 5-year (D), 10-year (E) decision curves show that nomogram and its risk stratification have the highest net benefit almost across the entire threshold probabilities. Blue line: net benefit of a strategy of treating all T1–2 breast cancer with 1–3 positive lymph nodes. Gray line: net benefit of treating no patients of T1–2 breast cancer with 1–3 positive lymph nodes. Colored lines: net benefit of a strategy of treating patients according to the nomogram, risk stratification, grade, and positive nodes.
PSM was used to balance the independent prognostic factors (marital status, race, age, grade, T stage, examined lymph nodes, positive lymph nodes, ER status, PR status, and chemotherapy status) between the control cohort and PMRT cohort in different prognostic groups. There were 6422, 4234, and 1542 cases T1-2N1M0 breast cancer left in the low-risk group, moderate-risk group, and high-risk group, respectively. As shown in Table 3, SMDs of all variables were less than 0.1. The OS rates of the three risk groups after PSM were also significantly different(P < 0.001), with 10-year OS rates of 86.9%, 73.7%, and 62.7%, respectively (P < 0.001, Figure 3B). Compared with the low-risk group, hazard ratios (HRs) of the middle-risk group and the high-risk group were 2.465 (95% CI, 2.192–2.772) and 4.449 (95% CI, 3.904–5.070), respectively. The survival curves of breast cancer patients in the three groups were significantly different (P <0.001). Moreover, 3-year, 5-year and 10-year decision curves presented that the net benefit of risk stratification was close to the model’s net benefit and was superior to the net benefit of single factors almost across the entire range of threshold probabilities, further validating that the favorable performance of our risk stratification nomogram (Figures 3C–E).
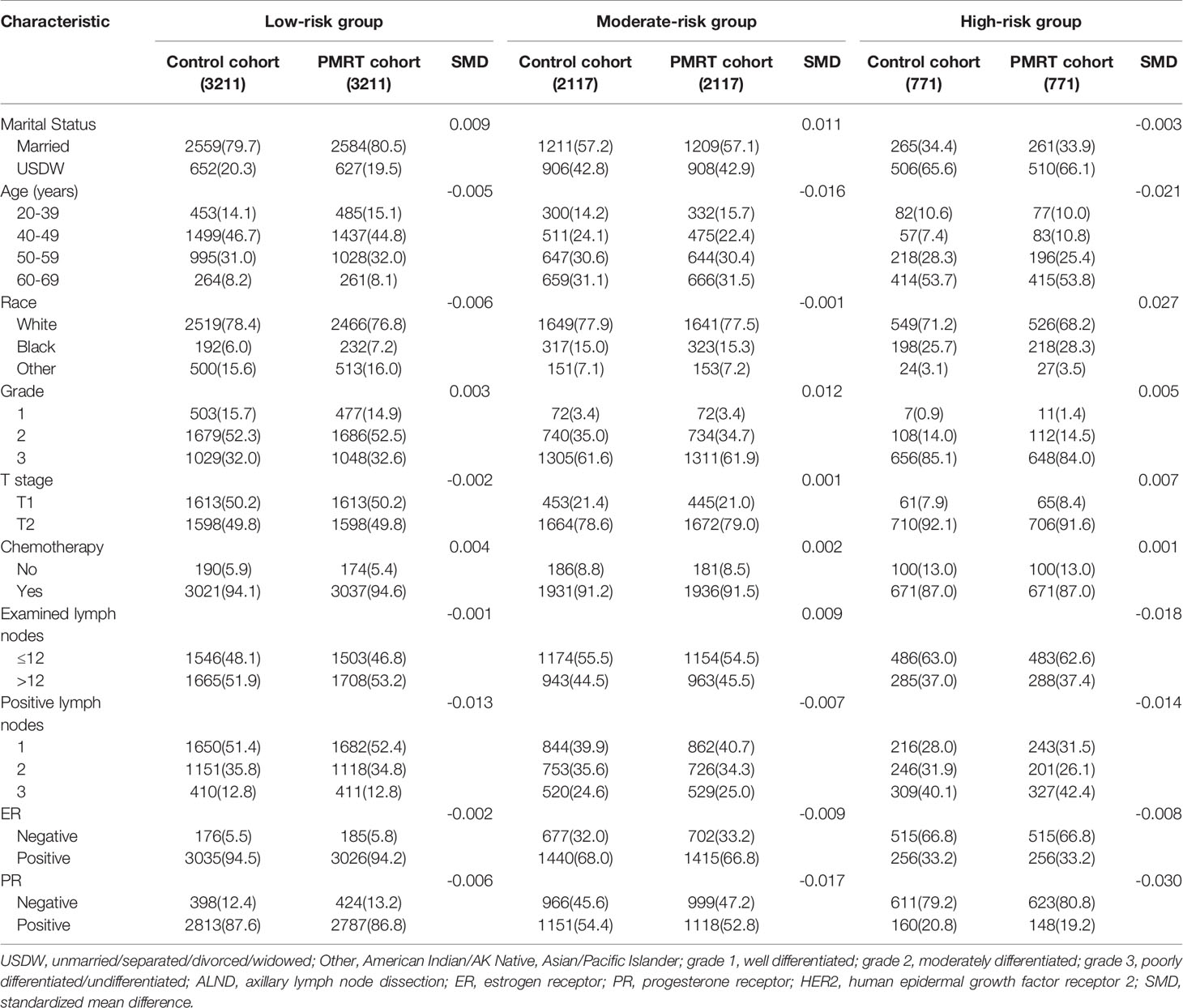
Table 3 The baseline characteristics of the patients with PMRT or observation in each risk group based on the PSM.
All direct survival differences between the PMRT cohort and control cohort before the PSM were presented in Supplementary Figure 2. 10-year OS was 80.8% in the PMRT cohort and 77.8% in the control cohort after PSM (P = 0.05) (Figure 4A). PMRT improved the OS of patients in the high-risk group but did not better OS among those in the low-risk and moderate-risk groups. In the low-risk group, 10-year OS was nearly equivalent (P = 0.49), with 88.0% in the PMRT cohort and 86.3% in the control cohort (Figure 4B). In the moderate-risk group, 10-year OS rates of PMRT cohort and control cohort were 75.7% and 72.2%, respectively (P = 0.35) (Figure 4C). In the high-risk group, PMRT can significantly improve 10-year OS, with 66.3% in the PMRT cohort and 59.6% in the control cohort (Figure 4D). This study found that PMRT can significantly improve the OS of T1–2 breast cancer with 1–3 positive lymph nodes in the high-risk group (Figure 4E).
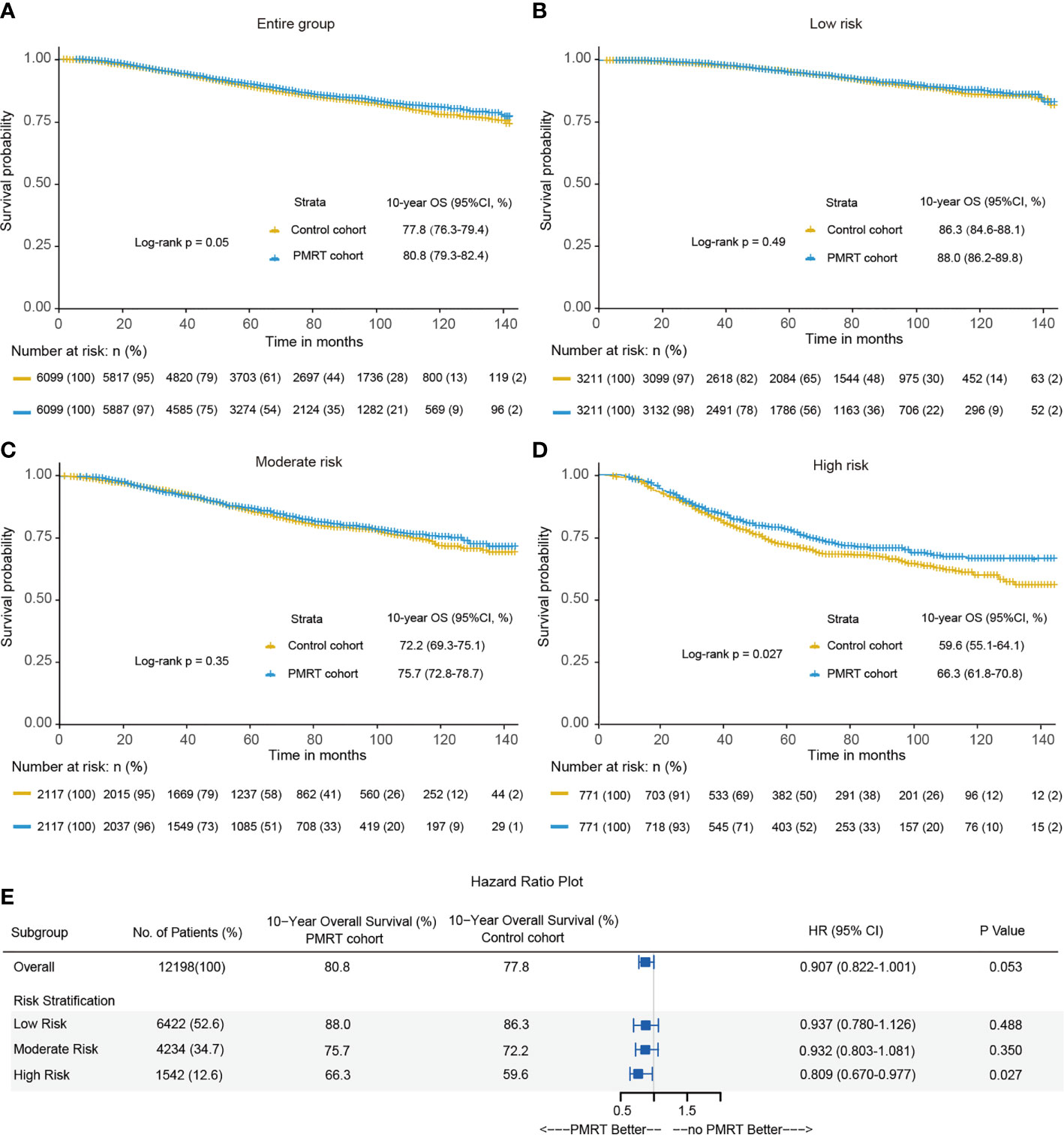
Figure 4 Kaplan-Meier curves of overall survival between the PMRT cohort and observation cohort for the entire group (A), low-risk group (B), moderate-risk group (C) and high-risk group (D) after PSM. (E) The forest plot for hazard ratio (HR) comparing 10-year overall survival between the control cohort and PMRT cohort in different risk groups after PSM.
Based on the data of 18607 T1–2 breast cancer with 1–3 positive axillary lymph nodes from the real-world, we determined its independent prognostic factors, developed a prognostic stratification model that can predict individual prognosis with favorable accuracy and discrimination, and applied the model to stratify the entire cohort into different risk groups to identify the optimal candidates benefiting from PMRT. In the entire cohort, PMRT did not improve 10-year OS of T1–2 breast cancer with 1–3 positive axillary lymph nodes significantly. In the low-risk group and moderate-risk group, 10-year OS rates of the PMRT cohort and control cohort were not significantly different, while PMRT can significantly improve the 10-year OS rate in the high-risk group. The innovation and advantage of this research lies in: (a) conducting the study based on a large sample size; (b) building up the first prognostic stratification nomogram specially for T1–2 breast cancer with 1–3 positive axillary lymph nodes based on multi-ethnic population; (c) validating excellent performance of risk stratification of nomogram by using Kaplan-Meier method and decision curve analysis; (d) applying PSM to balance the baseline characteristics between the PMRT cohort and control cohort in prognostic stratification groups to minimize the confounding factors of independent features; (e) finding that PMRT could effectively improve the OS of patients in the high-risk group after PSM, with 10-year OS absolute improvement by 6.7%.
In this study, marital status, age, race, grade, T stage, chemotherapy status, examined nodes, number of positive axillary lymph nodes, ER status, and PR status were independent prognostic factors for T1–2 breast cancer with 1–3 positive axillary lymph nodes. Consistent with the previous research results, grade 3, T2, no chemotherapy, fewer examined lymph nodes (4–13), more positive axillary lymph nodes, ER-negative, and PR-negative were independent risk factors for prognosis of T1–2 breast cancer with 1–3 positive axillary lymph nodes (11–24, 37). Also, our study indicated that patients with USDW (unmarried/separated/divorced/widowed) in marital status and black in race often have a high prognostic risk. Although such findings have not been reported in T1–2 breast cancer with 1–3 positive axillary lymph nodes, studies both breast cancer and metastatic breast cancer have reported similar results (38, 39). Interestingly, our analysis demonstrated that the 60–69 group was an independent risk factor for OS more than the 20-39 group, which seemed partly different from those studies reported that 40 years old or younger was a significant risk factor of T1–2 breast cancer with 1–3 positive axillary lymph nodes (12, 18–24). Consistent with the findings of Zhang et al. (40), this is mainly due to the fact that people aged 60-69 have more other serious diseases (such as diseases of heart, and chronic obstructive pulmonary disease and allied cond), and had a higher incidence of all-cause mortality compared with patients aged 20-39.
Considering that T1–2 breast cancer with 1–3 positive axillary lymph nodes had several independent prognostic factors, we incorporated all factors into the prognostic stratification model. The prognostic model can stratify the prognosis of patients into three risk groups. Our analysis proposed that patients in the high-risk group may be the potential population that could benefit from PMRT. Patients from the high-risk group have more independent risk factors and possibly higher all-cause mortality than those in the low-risk and moderate-risk groups. The escalation of treatment provided patients with better local control and prolongs survival to patients with higher death risk. Recently, several studies have tried to identify potential candidates who could benefit from PMRT in T1–2 breast cancer with 1–3 positive axillary lymph nodes. Chen et al. and Dai et al. reported that PMRT could only improve the prognosis of patients with three positive axillary lymph nodes (41, 42). A study conducted in the University of Chicago revealed that PMRT could reduce the all-cause mortality of patients with two positive lymph nodes and tumors 2–5 cm in size or three positive nodes by 14% (26). A clinical study in Korea revealed that PMRT could significantly better the prognosis for patients with an intermediate ratio of positive lymph nodes to total nodes dissected (23). Almost all these studies only took tumor burden (tumor size and number of positive lymph nodes) into consideration and did not take other critical factors seriously. Our study comprehensively incorporated all independent factors into prognostic nomogram and compared the net benefit between the risk stratification nomogram-assisted decision and single factor-assisted decisions. Notably, the net benefit of decisions based on the model and its risk stratification were significantly higher than those of the number of positive lymph nodes and grade proposed by previous researchers (41, 42). Although a multi-center study in China had established the first risk stratification nomogram for T1-2 breast cancer with 1-3 positive lymph nodes, it only included the Chinese population. Due to differences of race and other factors, the model applied in other races may have great limitations (43).
The current study built up the novel risk stratification model of T1–2 breast cancer with 1–3 positive axillary lymph nodes, which could accurately stratify the prognosis of patients into different risk groups and determine which patients were optimal candidates who may benefit from PMRT. In principle, our nomogram could identify whether T1–2 breast cancer with 1–3 positive axillary lymph nodes were potential population benefiting from PMRT. Although our study had many advantages, there were still several limitations: (a) the SEER database lacked detailed information on chemotherapy, endocrine therapy, and the dose, area, and complications of radiotherapy, so our study may have confounding factors; (b) there was no data on recurrence after surgery in the database, so we cannot identify the impact of PMRT on recurrence; (c) this study was limited by its retrospective design, but the PSM method was performed to reduce the confounding factors of independent features; (d) our prognostic stratification model had not been verified by other centers or databases (e.g., National Cancer Database, SEER-Medicare, etc.).
This study constructed a novel prognostic stratification model for T1–2 breast cancer with 1–3 positive axillary lymph nodes, which could help determine the potential population who could benefit from PMRT. The prognostic stratification model was expected to promote individual treatment of PMRT for T1–2 breast cancer with 1–3 positive axillary lymph nodes so that patients would benefit most from PMRT.
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/data/.
This study was approved by the ethics committee of the local hospital (Xijing hospital), and because it was a retrospective study, permission for the exemption of informed consent was obtained.
RL and MS designed the study. NH, JZ, LuY, and YW wrote the primary manuscript. NH, ZW, MZ, LiY, and GH extracted the data from the SEER database. NH, JW, YDW, BD, and LG performed the statistical analysis. All authors read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (Nos. 81572917).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.640268/full#supplementary-material
1. DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding SA, et al. Breast cancer statistics, 2019. CA Cancer J Clin (2019) 69:438–51. doi: 10.3322/caac.21583
2. Borm KJ, Oechsner M, Combs SE, Duma MN. Deep-Inspiration Breath-Hold Radiation Therapy in Breast Cancer: A Word of Caution on the Dose to the Axillary Lymph Node Levels. Int J Radiat Oncol Biol Phys (2018) 100:263–9. doi: 10.1016/j.ijrobp.2017.09.026
3. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med (2002) 347:1233–41. doi: 10.1056/NEJMoa022152
4. McGale P, Taylor C, Correa C, Cutter D, Duane F, Ewertz M, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet (2014) 383:2127–35. doi: 10.1016/S0140-6736(14)60488-8
5. Recht A, Comen EA, Fine RE, Fleming GF, Hardenbergh PH, Ho AY, et al. Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. J Clin Oncol (2016) 34:4431–42. doi: 10.1200/JCO.2016.69.1188
6. McBride A, Allen P, Woodward W, Kim M, Kuerer HM, Drinka EK, et al. Locoregional recurrence risk for patients with T1,2 breast cancer with 1-3 positive lymph nodes treated with mastectomy and systemic treatment. Int J Radiat Oncol Biol Phys (2014) 9:392–8. doi: 10.1016/j.ijrobp.2014.02.013
7. Muhsen S, Moo TA, Patil S, Stempel M, Powell S, Morrow M, et al. Most Breast Cancer Patients with T1-2 Tumors and One to Three Positive Lymph Nodes Do Not Need Postmastectomy Radiotherapy. Ann Surg Oncol (2018) 25:1912–20. doi: 10.1245/s10434-018-6422-9
8. Wu SP, Tam M, Shaikh F, Lee A, Chun J, Schnabel F, et al. Post-mastectomy Radiation Therapy in Breast Cancer Patients with Nodal Micrometastases. Ann Surg Oncol (2018) 25:2620–31. doi: 10.1245/s10434-018-6632-1
9. Jia MM, Liang ZJ, Chen Q, Zheng Y, Li LM, Cao XC, et al. Effects of postmastectomy radiotherapy on prognosis in different tumor stages of breast cancer patients with positive axillary lymph nodes. Cancer Biol Med (2014) 11:123–29. doi: 10.7497/j.issn.2095-3941.2014.02.007
10. Schafer R, Strnad V, Polgar C, Uter W, Hildebrandt G, Ott OJ, et al. Quality-of-life results for accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation in early breast cancer after breast-conserving surgery (GEC-ESTRO): 5-year results of a randomised, phase 3 trial. Lancet Oncol (2018) 19:834–44. doi: 10.1016/S1470-2045(18)30195-5
11. Woodward WA, Strom EA, Tucker SL, Katz A, McNeese MD, Perkins GH, et al. Locoregional recurrence after doxorubicin-based chemotherapy and postmastectomy: Implications for breast cancer patients with early-stage disease and predictors for recurrence after postmastectomy radiation. Int J Radiat Oncol Biol Phys (2003) 57:336–44. doi: 10.1016/s0360-3016(03)00593-5
12. Taghian A, Jeong JH, Mamounas E, Anderson S, Bryant J, Deutsch M, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol (2004) 22:4247–54. doi: 10.1200/JCO.2004.01.042
13. Truong PT, Olivotto IA, Kader HA, Panades M, Speers CH, Berthelet E. Selecting breast cancer patients with T1-T2 tumors and one to three positive axillary nodes at high postmastectomy locoregional recurrence risk for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys (2005) 61:1337–47. doi: 10.1016/j.ijrobp.2004.08.009
14. Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol (2007) 82:247–53. doi: 10.1016/j.radonc.2007.02.001
15. Tendulkar RD, Rehman S, Shukla ME, Reddy CA, Moore H, Budd GT, et al. Impact of postmastectomy radiation on locoregional recurrence in breast cancer patients with 1-3 positive lymph nodes treated with modern systemic therapy. Int J Radiat Oncol Biol Phys (2012) 83:e577–81. doi: 10.1016/j.ijrobp.2012.01.076
16. Moo TA, McMillan R, Lee M, Stempel M, Patil S, Ho A, et al. Selection criteria for postmastectomy radiotherapy in t1-t2 tumors with 1 to 3 positive lymph nodes. Ann Surg Oncol (2013) 20:3169–74. doi: 10.1245/s10434-013-3117-0
17. Kong M, Hong SE. Which patients might benefit from postmastectomy radiotherapy in breast cancer patients with t1-2 tumor and 1-3 axillary lymph nodes metastasis? Cancer Res Treat (2013) 45:103–11. doi: 10.4143/crt.2013.45.2.103
18. Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med (1997) 337:949–55. doi: 10.1056/NEJM199710023371401
19. Jwa E, Shin KH, Lim HW, Jung SY, Lee S, Kang HS, et al. Identification of Risk Factors for Locoregional Recurrence in Breast Cancer Patients with Nodal Stage N0 and N1: Who Could Benefit from Post-Mastectomy Radiotherapy? PloS One (2015) 10:e0145463. doi: 10.1371/journal.pone.0145463
20. Lai SF, Chen YH, Kuo WH, Lien HC, Wang MY, Lu YS, et al. Locoregional Recurrence Risk for Postmastectomy Breast Cancer Patients With T1-2 and One to Three Positive Lymph Nodes Receiving Modern Systemic Treatment Without Radiotherapy. Ann Surg Oncol (2016) 23:3860–9. doi: 10.1245/s10434-016-5435-5
21. Yin H, Qu Y, Wang X, Ma T, Zhang H, Zhang Y, et al. Impact of postmastectomy radiation therapy in T1-2 breast cancer patients with 1-3 positive axillary lymph nodes. Oncotarget (2017) 8:49564–73. doi: 10.18632/oncotarget.17318
22. Park HJ, Shin KH, Kim JH, Ahn SD, Kim JY, Park W, et al. Incorporating Risk Factors to Identify the Indication of Post-mastectomy Radiotherapy in N1 Breast Cancer Treated with Optimal Systemic Therapy: A Multicenter Analysis in Korea (KROG 14-23). Cancer Res Treat (2017) 49:739–47. doi: 10.4143/crt.2016.405
23. Kim SI, Cho SH, Lee JS, Moon HG, Noh WC, Youn HJ, et al. Clinical relevance of lymph node ratio in breast cancer patients with one to three positive lymph nodes. Br J Cancer (2013) 109:1165–71. doi: 10.1038/bjc.2013.465
24. Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol (2017) 28:1700–12. doi: 10.1093/annonc/mdx308
25. Grossmith S, Nguyen A, Hu J, Plichta JK, Nakhlis F, Cutone L, et al. Multidisciplinary Management of the Axilla in Patients with cT1-T2 N0 Breast Cancer Undergoing Primary Mastectomy: Results from a Prospective Single-Institution Series. Ann Surg Oncol (2018) 25:3527–34. doi: 10.1245/s10434-018-6525-3
26. Huo D, Hou N, Jaskowiak N, Winchester DJ, Winchester DP, Yao K. Use of Postmastectomy Radiotherapy and Survival Rates for Breast Cancer Patients with T1-T2 and One to Three Positive Lymph Nodes. Ann Surg Oncol (2015) 22:4295–304. doi: 10.1245/s10434-015-4528-x
27. Wang Q, Xia D, Bai W, Wang E, Sun J, Huang M, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: A multicentre observational study. J Hepatol (2019) 70:893–903. doi: 10.1016/j.jhep.2019.01.013
28. Li J, Wang Q, Lei Z, Wu D, Si A, Wang K, et al. Adjuvant Transarterial Chemoembolization Following Liver Resection for Intrahepatic Cholangiocarcinoma Based on Survival Risk Stratification. Oncologist (2015) 20:640–7. doi: 10.1634/theoncologist.2014-0470
29. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol (2013) 31:1188–95. doi: 10.1200/JCO.2012.41.5984
30. Harrell FJ, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med (1996) 15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4
31. Frank E, Harrell JR. Rms: Regression Modeling Strategies. R Package version 3.4-0. Available at: http://CRAN.Rproject.org/packagerms.
32. Blanche P, Dartigues JF, Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med (2013) 32:5381–97. doi: 10.1002/sim.5958
33. Vickers AJ, Elkin EB. Decision Curve Analysis: A Novel Method for Evaluating Prediction Models. Med Decis Making (2006) 26:565–74. doi: 10.1177/0272989X06295361
34. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat (2011) 10:150–61. doi: 10.1002/pst.433
35. Bangalore S, Guo Y, Samadashvili Z, Blecker S, Xu J, Hannan EL, et al. Everolimus-eluting stents or bypass surgery for multivessel coronary disease. N Engl J Med (2015) 372:1213–22. doi: 10.1056/NEJMoa1412168
36. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res (2004) 10:7252–59. doi: 10.1158/1078-0432
37. Krag DN, Single RM. Breast Cancer Survival According to Number of Nodes Removed. Ann Surg Oncol (2003) 10:1152–59. doi: 10.1245/aso.2003.03.073
38. Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital status and survival in patients with cancer. J Clin Oncol (2013) 31:3869–76. doi: 10.1200/JCO.2013.49.6489
39. Ji P, Gong Y, Jiang CC, Hu X, Di GH, Shao ZM. Association between socioeconomic factors at diagnosis and survival in breast cancer: A population-based study. Cancer Med (2020) 9:1922–36. doi: 10.1002/cam4.2842
40. Zhang N, Zhang J, Zhang H, Liu Y, Zhao W, Wang L, et al. Individualized Prediction of Survival Benefit from Postmastectomy Radiotherapy for Patients with Breast Cancer with One to Three Positive Axillary Lymph Nodes. Oncologist (2019) 24:e1286–93. doi: 10.1634/theoncologist.2019-0124
41. Chen M, Huang Y, Leng Z, Yang G, Li F, Yang H, et al. Post-mastectomy Radiotherapy in T1-2 Breast Cancer Patients With One to Three Lymph Node Metastases: A Propensity Score Matching Analysis. Front Oncol (2019) 9:1551. doi: 10.3389/fonc.2019.01551
42. Dai KC, Mongoue-Tchokote S. Prognostic significance of the number of positive lymph nodes in women with T1-2N1 breast cancer treated with mastectomy: should patients with 1, 2, and 3 positive lymph nodes be grouped together? Int J Radiat Oncol Biol Phys (2013) 85:1200–5. doi: 10.1016/j.ijrobp.2012.11.005
Keywords: breast cancer, lymph nodes, SEER, nomogram, prognosis, postmastectomy radiotherapy
Citation: Hou N, Zhang J, Yang L, Wu Y, Wang Z, Zhang M, Yang L, Hou G, Wu J, Wang Y, Dong B, Guo L, Shi M and Ling R (2021) A Prognostic Risk Stratification Model to Identify Potential Population Benefiting From Postmastectomy Radiotherapy in T1–2 Breast Cancer With 1–3 Positive Axillary Lymph Nodes. Front. Oncol. 11:640268. doi: 10.3389/fonc.2021.640268
Received: 11 December 2020; Accepted: 30 March 2021;
Published: 19 April 2021.
Edited by:
Hee Jeong Kim, Asan Medical Center, South KoreaReviewed by:
Lingmi Hou, Affiliated Hospital of North Sichuan Medical College, ChinaCopyright © 2021 Hou, Zhang, Yang, Wu, Wang, Zhang, Yang, Hou, Wu, Wang, Dong, Guo, Shi and Ling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Ling, bGluZ3J1aTAxMDVAMTYzLmNvbQ==; Mei Shi, bXNoaTgyQGhvdG1haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.