- Department of Urology, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Purpose: We developed a nomogram to predict the possibility of lymph node metastasis in patients with squamous cell carcinoma of the penis.
Methods: Identifying patients with squamous cell carcinoma of the penis diagnosed between 2004 and 2015 in the Surveillance, Epidemiology, and End Results (SEER) database. Univariate and multivariate analyses were carried out by logistic regression to assess significant predictors associated with lymph node metastasis. A nomogram was established and validated by a calibration plot and receptor operating characteristic curve (ROC) analysis.
Results: A total of 1,016 patients with penile squamous cell carcinoma (SCCP) were enrolled in this study. One hundred and ninety-five patients (19%) had lymph node involvement (N1-3). Multivariate analysis showed that age, primary tumor site, grade, tumor size, and T stage were identified as being significantly (p < 0.05) associated with lymph node involvement. All the above factors that showed a statistically significant predictive capability were selected for building the nomogram. This model had a calibration slope of 0.9 and a c-index of 0.776, indicating the good discrimination and effectiveness of the nomogram in predicting lymph node status.
Conclusion: Although the prediction model has some limitations, the nomogram revealed the relationship between the clinicopathological characteristics of SCCP patients and the risk of lymph node metastasis. This tool will assist patients in counseling and guide treatment decisions for SCCP patients.
Introduction
Penile cancer is a rare malignant tumor of the genitourinary system, accounting for <0.1% of all malignancies in men living in the developed world, while its incidence rates are higher in parts of South America and Africa (1). Squamous cell carcinoma is the most common histology of penile cancer, accounting for more than 95% (2), and commonly occurs in men between 50–70 years old (3). Besides, 80% of the primary tumors are localized at the glans and prepuce (4).
Lymph node metastases of squamous cell carcinoma of the penis (SCCP) affects the selection of surgical therapy and is also a strong predictor of prognosis, patients with lymph node metastases were proven to have a worse prognosis (5). About 80% of men with low-grade penile cancer can achieve prolonged survival, but as the degree of lymph node metastasis increases, the survival rate decreases precipitously (6, 7). The 5-year survival of patients with inguinal lymph node (ILN) metastasis can be as high as 80%, while patients with pelvic lymph node (PLN) metastasis and distant metastases have a survival rate of 0–33% (8, 9). Early metastatic spread to regional lymph nodes can be life-threatening (10).
Because of the high possibility of lymph node dissection, it is very important to determine the appropriate surgical candidate. However, few studies to date have evaluated the risk factors or predictive models of lymph node metastases. Ficarra et al. (11) formed the first nomogram to predict lymph node involvement based on a cohort of 265 patients. The clinical stage of the inguinal lymph node, histological grade, and other tumors pathological features w included in the model, and multivariate analysis showed that only lymphovascular invasion and clinically palpable lymph nodes were significant predictors of lymph node status. Velazquez et al. (12) later developed a more specific nomogram to predict lymph node metastasis, found that perineural infiltration and grade were significant predictors. Also, Bhagat et al. (13) demonstrated that age, tumor grade, lymphatic vascular infiltration, and clinically palpable lymph nodes were predictors of lymph node involvement. However, the tumor stage had not proven to be significant which is analogous to some other research (12, 14). Recently, a cohort study including 380 penile cancer patients between 2000 and 2010 was implemented to identify predictors of lymph node involvement, multivariable analysis demonstrated that age, pathological stage, tumor grade were independently associated with lymph node involvement. Moreover, the accuracy tests of the risk stratification scheme suggested that there were no significant differences between different risk group systems (15). The result is still controversial. It is worth noting that in terms of demographics and clinicopathological information, there is great heterogeneity among SCCP patients, such as age, race, marital status pathological type, tumor size, and primary tumor site (16). Therefore, a well-designed predictive model for lymph node metastases in SCCP patients covering more factors is needed. This study aimed to identify clinical and pathology characters of SCCP, to predict lymph node metastases of non-metastatic (M0) squamous cell carcinoma of the penis, then construct and validate a novel nomogram for predicting lymph node metastases in M0 SCCP using a cohort from the Surveillance, Epidemiology, and End Results (SEER) database.
Methods
Patients and Selection Criteria
This retrospective study analyzed the data of patients with squamous cell carcinoma of the penis diagnosed between 2004 and 2015, extracted from the Surveillance, Epidemiology, and End Results (SEER) database (accession number is 15779-Nov2019). Incomplete records on primary tumor site, grade, TNM stage, marital status, tumor size were excluded from the study. and non-squamous cell carcinoma [According to the “International Classification of Diseases-Oncology, 3rd edition” (ICD-O-3), the code of squamous cell carcinoma of the penis was 8051–8052 and 8070–8075 (17)] and patients with distant metastasis were also not included. Patients were excluded if they underwent any type of neoadjuvant therapy (including radiation, chemotherapy, hormone, therapy, or other systemic therapy). All the patients we included underwent surgical treatment, including partial penectomy, total penectomy, and organ sparing surgery. And lymph node staging was identified through surgery. The demographic variables of marital status at diagnosis, age at diagnosis, race and primary tumor site, tumor characteristics of differentiation grade, histological type, T stage, N stage, and tumor size were collected from the SEER database using SEER-stat software.
TNM stages of the penile tumor were determined according to the American Joint Committee on Cancer (AJCC) 6th edition staging system using available clinical and pathologic data on tumor invasion, lymph nodes status, and distant metastasis, respectively. The definitions are as follows: T1 is defined as a tumor invading subepithelial connective tissue; T2 is defined as a tumor invading corpus spongiosum with or without invasion of the urethra; T3 is defined as a tumor invading corpus cavernosum with or without invasion of the urethra; T4 is defined as a tumor invading other adjacent structures; N0 means no palpable or visibly enlarged inguinal lymph nodes; N1 means palpable mobile unilateral inguinal lymph node; N2 means palpable mobile multiple or bilateral inguinal lymph nodes; N3 means fixed inguinal nodal mass or pelvic lymphadenopathy, unilateral or bilateral; M0 means no distant metastasis; and M1 means distant metastasis. The histopathological grading of penile carcinoma was determined according to the SEER cancer grade system. Data of marital status at diagnosis, age at diagnosis, race, and tumor size were divided into different groups after being processed.
The SEER database is a public database and has patient anonymization, the use of a public database without patient identification information meets the requirements of the institutional review board and the ethics committee.
Statistical Analysis
Statistical analyses to identify prediction factors were performed using SPSS 15.0 for Windows (SPSS, Chicago, IL). The Chi-square-test was used to determine the significance of differences between categorical variables. Some variables such as tumor size were grouped based on the median of the overall data. Univariate and multivariate analyses were carried out by logistic regression, and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. All reported p-values were two-sided, and a p-value of <0.05 was considered statistically significant.
A mosaic plot was constructed to show the distribution and relationship of clinicopathological characteristics of SCCP patients by using the package of vcd in R version 2.14.1 (http://www.r-project.org/). Nomograms from multivariable logistic models are a popular visual plot to display the predicted probabilities of an event for decision support (18). A nomogram was formulated based on the results of multivariate analysis and by using the package of rms in R version 2.14.1 (http://www.r-project.org/), to predict lymph node metastases in M0 squamous cell carcinoma of the penis. To test the performance of the nomogram, it was subjected to 1000 bootstrap resamples for internal validation to calculate the corrected c-index. A calibration curve was created using the observed lymph node status and the predicted lymph node status. Moreover, the ROC curve was used to evaluate the effectiveness of the nomogram.
Results
Patient Characteristics
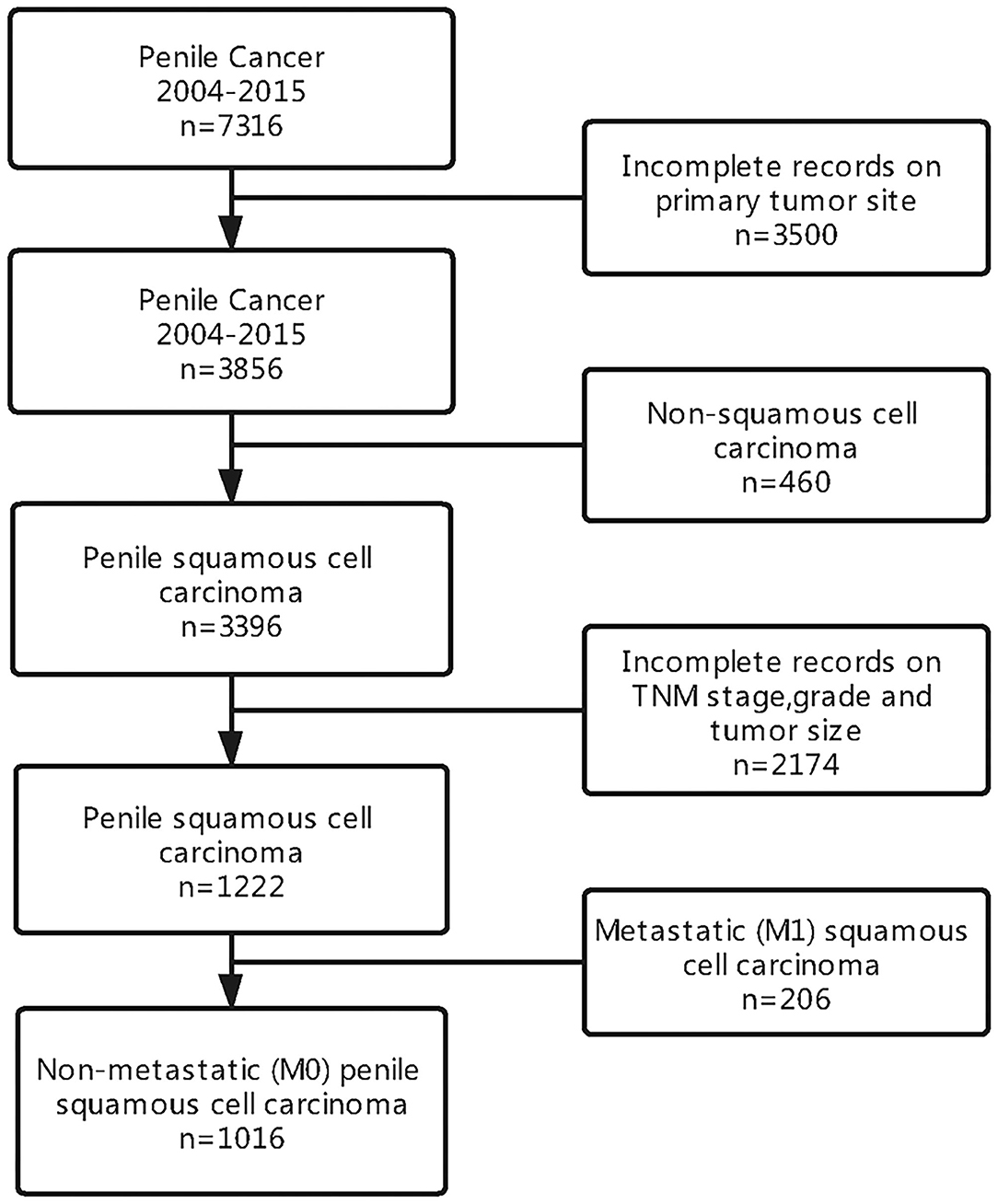
We identified a cohort of men with penile squamous cell carcinoma from the SEER database. Of the 7,316 patients diagnosed with penile cancer, a total of 1,016 men were included in our analysis. A summary of study selection criteria can be seen in Figure 1.
A total of 1,016 patients with penile non-metastatic (M0) squamous cell carcinoma were enrolled in this study, and the specific tumor site was identified in all patients. Among the 1,016 included patients, 195 (19%) had lymph node involvement (N1-3) and 821 (81%) had N0 status. The median age of patients was 60 years, and the mean tumor size was 3 cm. The majority of patients where white (855, 84.2%), with a significantly smaller percentage of men being black (96, 9.4%) or other (65, 6.4%). Most of the cases were married (703, 69.2%). Patients characteristics and the association of lymph node status with demographic characteristics and clinicopathological characteristics were presented in Table 1.
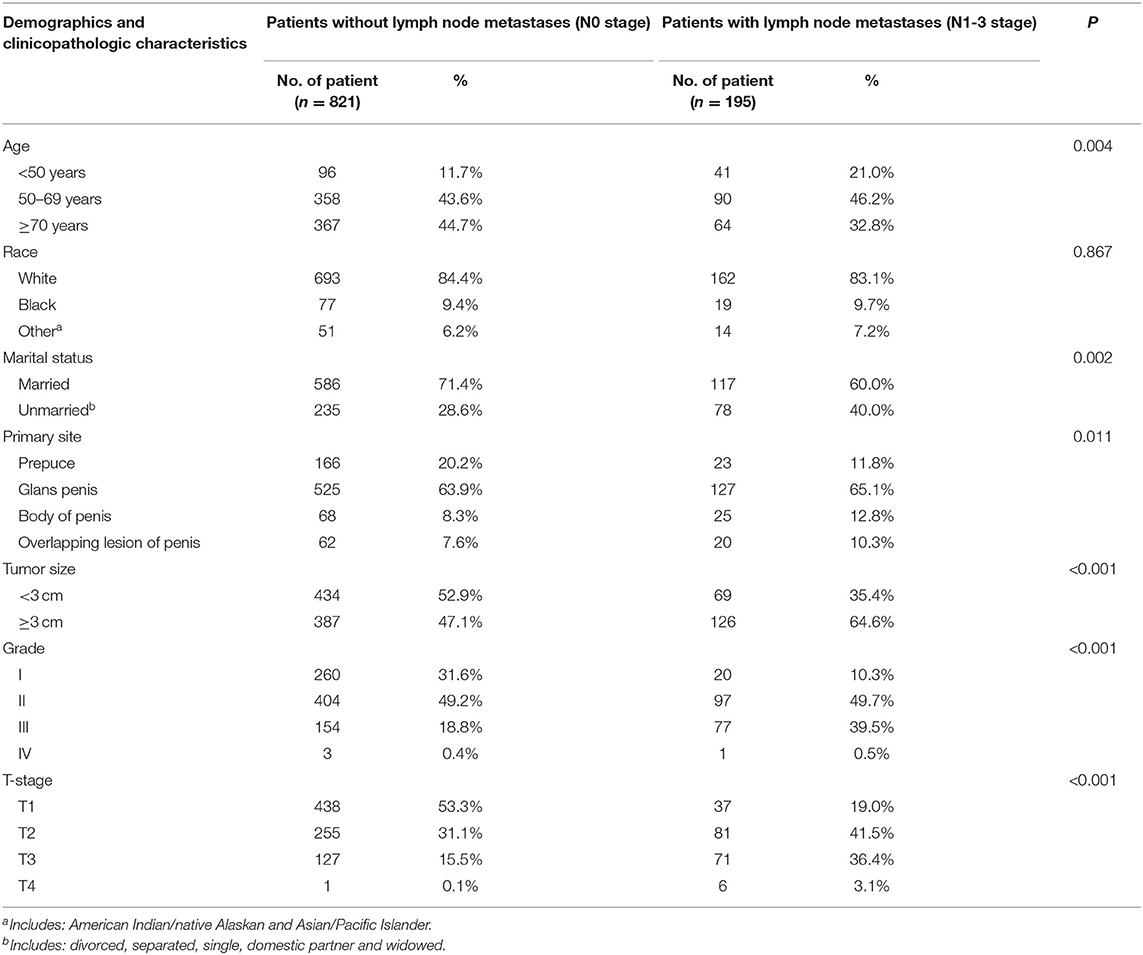
Table 1. Comparison of demographics and clinicopathological characteristics of patients with different lymph nodes status.
There were no significant differences in the race category by lymph node status. And there was a statistically significant difference in age, marital status, the primary site of the tumor, tumor size, different grade of differentiation, and T stage between the patients with lymph node involvement and those without (p < 0.05 for all).
Comparison of Oncology Features of Patients With Different Primary Tumor Site
In this group of patients, tumors occurred in the prepuce, glans, the body of the penis, and some were overlapping lesions, the numbers were 189 (18.6%), 652 (64.2%), 93 (9.1%), and 82 (8.1%), respectively. Patients were grouped concerning their primary tumor site and compare the oncology features of each group, the result was shown in Figure 2. T stage, N stage, tumor grade, and tumor size had a different distribution in patients with different primary tumor site (all p < 0.001). Most patients with primary tumors that localized at the prepuce were in T1 and T2 stage, and a higher proportion of tumors with overlapping lesions were in stage T3 compared with those localized at other sites (Figure 2A); Compared with primary tumors that localized at the prepuce, tumors that localized at the body of the penis had a higher probability of lymph node involvement (Figure 2B); Compared with tumors located in other sites, tumors with overlapping lesions or localized at the body of the penis had a worse differentiation grade (Figure 2C); Compared with tumors located in other sites, tumors with overlapping lesions had a larger tumor size (Figure 2D); In general, the primary tumor site was closely related to the pathological characteristics of the tumor.
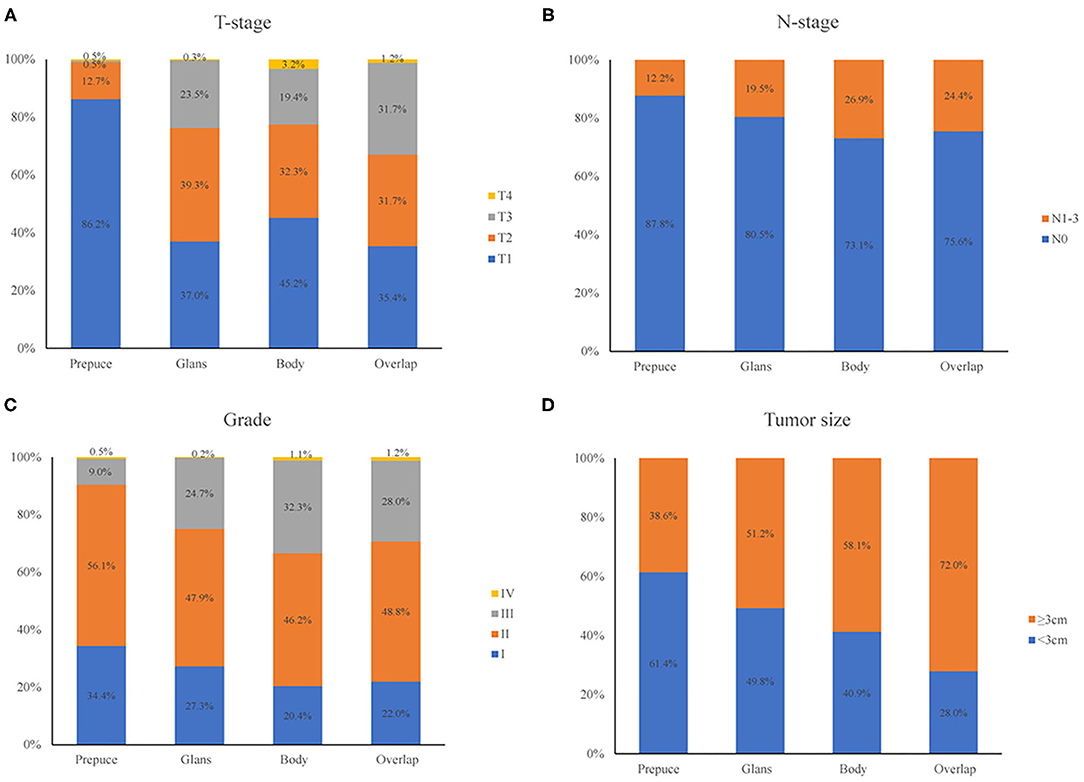
Figure 2. Comparison of oncology features of patients with different primary tumor site. T-stage (A), N-stage (B), tumor grade (C), and tumor size (D) had different distribution in patients with different primary tumor site (all p < 0.001).
Distribution and Relationship of Clinicopathological Characteristics in Patients With SCCP
A mosaic plot was applied to show the distribution and relationship of clinicopathological characteristics of SCCP patients. In the mosaic plot, the area of the nested matrix is proportional to the cell frequency, where the frequency is the frequency in the multi-dimensional contingency table. The color and shading can indicate the residual value of the fitted model. Patients with lymph node involvement (N1-3) had higher tumor grade, more advanced clinical tumor stage, larger tumor size, and its primary tumor site was also significantly different from patients without lymph node involvement (N0). The result was shown in Figure 3.
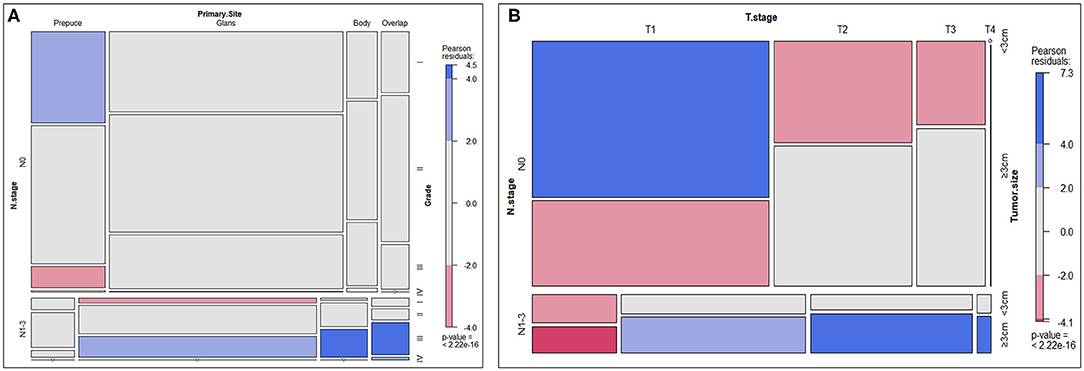
Figure 3. Mosaic plot. (A) Distribution and relationship of N-stage, primary site, and tumor grade. (B) Distribution and relationship of N-stage, T-stage, and tumor size.
Univariate and Multivariate Analyses and Identification of Predictive Factors
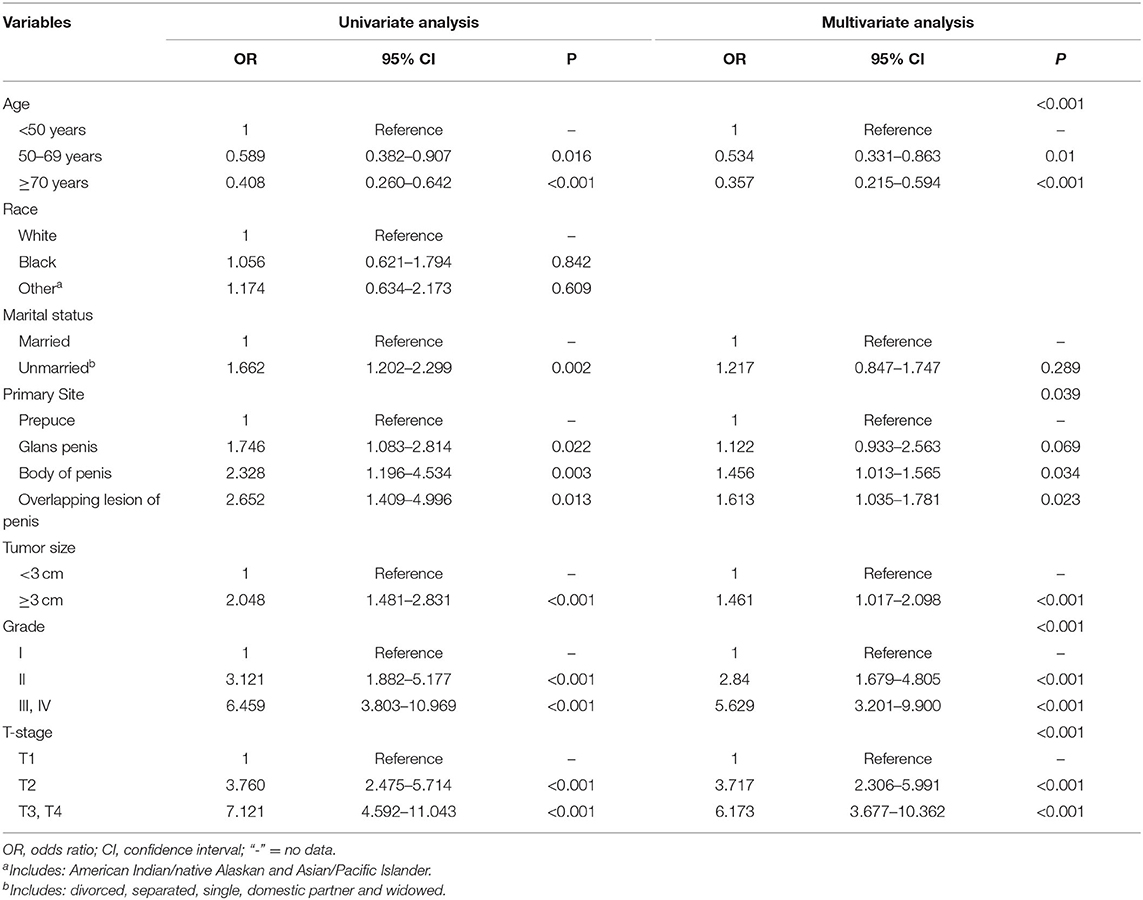
Binary classification logistic regression was applied to investigate the predictive factors associated with lymph node metastases in patients with M0 SCCP. The results of the univariate analysis showed that age, marital status, primary tumor site, grade, tumor size, and T stage were identified as being significantly (p < 0.05) associated with lymph node involvement (N1-3). Further multivariate analysis showed that younger age [(<50 years vs. 50–69 years, OR 0.534, 95% CI, 0.331–0.863), (<50 years vs. ≥70 years, p = 0.01 OR 0.357, 95% CI, 0.215–0.594), p < 0.0001], larger tumor (OR 1.461, 95% CI, 1.017–2.098, p < 0.0001), higher grade [(II vs. I, OR 2.84, 95% CI, 1.679–4.805), (III, IV vs. I, OR 5.629, 95% CI, 3.201–9.900), p < 0.0001] and T stage [(T2 vs. T1, OR 3.717, 95% CI, 2.306–5.991), (T3, T4 vs. T1, OR 6.173, 95% CI, 3.677–10.362), p < 0.0001] were high risk factors of lymph node involvement. Moreover, the primary tumor was localized in the body of the penis (OR 1.456, 95% CI, 1.013–1.565, p = 0.034), overlapping lesion of the penis (OR 1.613, 95% CI, 1.035–1.781, p = 0.023) were also predictors of lymph node metastasis in penile squamous cell carcinoma. A summary of the results was displayed in Table 2.
Construction and Validation of the Nomogram
All the above factors that showed a statistically significant predictive capability for lymph node metastasis were selected for building the nomogram. The factors included in the final nomogram were age, primary tumor site, grade, tumor size, and T stage. In the nomogram, tumor grade and T stage have a more significant impact on the lymph node metastasis. Besides, age, primary tumor site, and tumor size also had varying degrees of influence on lymph node involvement, shown in Figure 4.
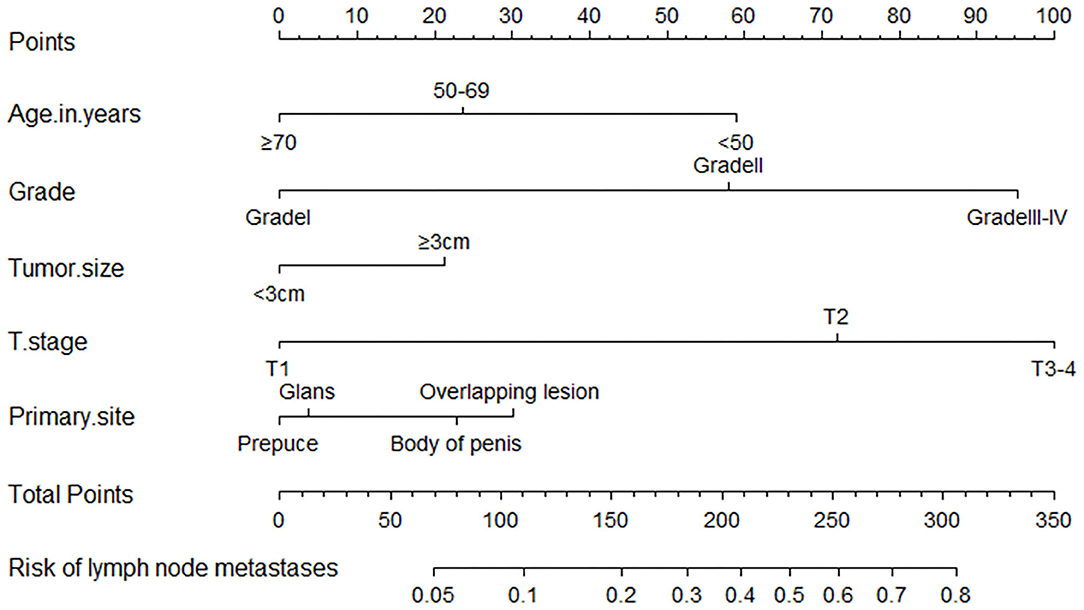
Figure 4. A nomogram for predicting the probability of lymph node metastases. To use the nomogram, the value for each predictor is determined by drawing a line upward to the point reference line, the points are summed, and a line is drawn downward from the total points line to find the predicted probability of lymph node metastases.
The process of using the nomogram model to individually predict the risk of lymph node involvement of a patient is as follows: (1) Determine the score of each predictor on the scale, (2) calculate the total score of 5 predictors, (3) draw a straight line from the total points line down to the bottom risk line to find the risk of lymph node involvement of a patient.
The bootstrap method was applied to internally verify the prediction performance of the model, indicated that the model has better discrimination in predicting lymph node involvement in SCCP patients, and a calibration plot was forthput to assess the agreement between observed and predicted values, showed that this nomogram was well-calibrated (Figure 5A). Moreover, we evaluated the effectiveness of the nomogram in predicting lymph node metastasis by using the ROC curve (Figure 5B), according to Youden's method, optimal cutoff values of the nomogram were 0.189, and the sensitivity, specificity associated with the 0.189 cut-offs were 79.5 and 34.2%, respectively. According to the clinicopathological data of the studied cohort, we could assess the possible risk of lymph node involvement of patients with SCCP, and patients with the risk of lymph node invasion >0.189 were considered as a high-risk group, which it was recommended to perform a lymphadenectomy. In general, this model had a calibration slope of 0.9 and a c-index of 0.776, indicating the good discrimination and effectiveness of the nomogram in predicting lymph node status.
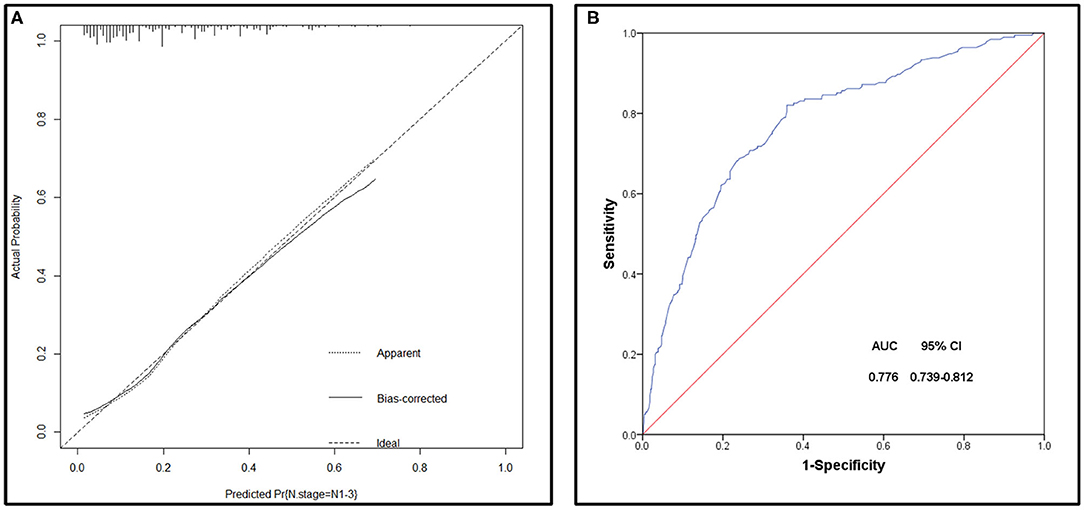
Figure 5. (A) Calibration plot of the nomogram for the probability of lymph node metastases (bootstrap 1,000 repetitions). (B) Receiving operating characteristic (ROC) curve of the prediction model.
Discussion
The lack of expertise of clinicians and the public disgrace among patients have created an environment where up to 25% of men have advanced disease at the time of diagnosis (19), and treatment is delayed for over 1 year in up to 50% of patients (20). Therefore, many patients with penile cancer have metastatic disease, the progression of metastasis follows a predictable gradual invasion pattern from the primary tumor to the inguinal lymphatic pool, then spread to the pelvic lymph nodes and systemic spread (21), which is a major prognostic factor for penile cancer survival and associated with poor prognosis (22). Approximately 70% of patients were metastatic lymph nodes among patients with at least one clinically palpable nodule (cN+) (22). In other cases, lymph node enlargement is caused by inflammation, usually secondary to infection of the primary tumor (10). In the present study, 19% of patients were positive for lymph node metastasis among 1016 included SCCP patients identified in the SEER database. Meanwhile, the Incidence of lymph node involvement can be upwards of 49% in intermediate-high risk tumors (pT1b, T2- T4) (23). Early metastatic spread to regional lymph nodes can be life-threatening. Guidelines had recommended lymphadenectomy because of concerns about the adverse effects of a delayed intervention on survival for penile cancer patients diagnosed with lymph node involvement. Therefore, the management of regional lymph nodes is very important for patient survival (24). Additionally, clinicopathological characteristics of tumors can be used to stratify patients and to prompt the inguinal lymph node dissection (ilND) performance (25). We constructed a predictive nomogram to evaluate the probability of lymph node metastasis in patients with M0 SCCP based on the SEER database.
Recently, A study established an NCDB-based nomogram to predict lymph node metastasis in penile cancer, showed that tumor grade, tumor lymphovascular invasion, and clinical lymph node status were all related to the increased incidence of lymph node metastases (26). Our research showed that the following five factors were independently associated with lymph node metastasis, including age, tumor grade, tumor size, T stage, and primary tumor site. All the above factors were selected for building the nomogram. Previous studies reported that model with AUC between 0.7 and 0.9 have moderate accuracy, indicating an acceptable degree of discrimination. In our study, this model had a calibration slope of 0.9 and a c-index of 0.776, indicating the good discrimination and effectiveness of the nomogram in predicting lymph node status. According to Youden's method, optimal cutoff values of the nomogram were 0.189, and the sensitivity, specificity associated with the 0.189 cut-offs were 79.5 and 34.2%, respectively. As we couldn't have both high sensitivity and high specificity, higher sensitivity is what we more needed considering that the purpose of our nomogram is to prevent false negativity. Historically, prophylactic inguinal lymph node dissection (ILND) has demonstrated a survival advantage in this population of patients, and failed detection of micrometastatic disease can have a significant impact on survival. However, contemporary philosophy dictates that subjecting all patients with the intermediate-risk disease to radical ILND carries an unacceptable risk of complications and long-term morbidity. By using the optimal cutoff value and the nomogram, we could predict the risk of lymph node invasion correctly in patients with different clinicopathological characteristics and determine the optimal management of lymph nodes in penile cancer. Regarding the five parameters included in this nomogram, the T stage had the highest discriminating power. Previous research showed that the incidence of lymph node involvement is 0–30% in patients with low-grade tumors (≤ T1a), while in patients with ≥T1b tumors or lymphovascular invasion, the incidence of lymph node metastasis is close to 50% (27). Among patients with advanced tumors, 50–70% of T2 tumor patients, and 50–100% of >T3 tumor patients have lymph node metastasis (28). Our study was consistent with previous research which reported that men with higher T stage were at higher risk of lymph node metastasis (29, 30). Consistent with previous studies (14, 31), we found that younger age was a high-risk factor of lymph node involvement, which may be related to human papillomavirus (HPV) infection. In pathogenic pathways involved in the development of penile carcinomas, about one-third of cases are associated with HPV infection (32), meanwhile, HPV infection has been found to have different age distribution characteristics (33). Our study corroborated those previous studies (12–14), with the addition of tumor size and primary tumor site as significant predictors. Tumor size ≥3 cm was significantly associated with an increased risk of lymph node involvement. Also, we analyzed the correlation between the primary site and clinicopathological characteristics of patients with penile squamous cell carcinoma and whether it is a risk factor for lymph node metastasis. Our research showed that tumors that occur in the body of the penis and overlapping lesions had a higher probability of lymph node metastasis.
In addition, recent research found that some biomarkers such as plasma C-reactive protein (CRP) and IGFBP2 levels were associated with higher tumor stages and lymph node metastasis (34, 35). Related predictive models can be further improved by including additional biomarkers.
Several limitations exist in our study. First, lymphovascular invasion (LVI) of the SEER database was recorded from 2010. Considering that the data in the database is incomplete, so we did not include it. As LVI was associated with increased rates of lymph node metastasis (13), including this predictive factor may improve the sensitivity and specificity of our nomogram. Besides, the SEER database was retrospectively collected and contains limited clinicopathologic data, central pathology review was not recorded, and there was no specific type of lymph node metastasis, so we cannot distinguish between the inguinal lymph node and pelvic lymph node metastasis. Generally, metastatic progression follows a predictable and stepwise pattern of invasion from the primary tumor to inguinal lymph basin before spreading to pelvic nodes and systemic dissemination, but distinguish pelvic from inguinal nodes is important to the identification of suitable surgical methods for nodal dissection. In addition, the predictive accuracy of nomograms should be tested through externally validated.
Conclusion
In short, through retrospective analysis of 1,016 SCCP patients, this study established a new nomogram based on five independent risk factors to predict lymph node metastasis. The nomogram demonstrated well discrimination and effectiveness in predicting lymph node status. Although the prediction model has some limitations, the nomogram revealed the relationship between the clinicopathological characteristics of SCCP patients and the risk of lymph node metastasis. This tool will assist patients in counseling and guide treatment decisions for SCCP patients.
Data Availability Statement
The original contributions generated in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
XZ designed the study. WZ, PG, and JG performed the literature research. Data were extracted by WZ, GL, and XW. WZ and XZ conducted the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. O'Sullivan B, Brierley J, Byrd D, Bosman F, Kehoe S, Kossary C, et al. The TNM classification of malignant tumours-towards common understanding and reasonable expectations. Lancet Oncol. (2017) 18:849–51. doi: 10.1016/S1470-2045(17)30438-2
2. Chipollini J, Tang DH, Sharma P, Spiess PE. National trends and predictors of organ-sparing for invasive penile tumors: expanding the therapeutic window. Clin Genitourin Cancer. (2018) 16:e383–9. doi: 10.1016/j.clgc.2017.09.004
3. Clark PE, Spiess PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, et al. Penile cancer: clinical practice guidelines in oncology. J Natl Compr Cancer Network. (2013) 11:594–615. doi: 10.6004/jnccn.2013.0076
4. Goodman MT, Hernandez BY, Shvetsov YB. Demographic and pathologic differences in the incidence of invasive penile cancer in the United States, 1995-2003. Cancer Epidemiol Biomarkers Prev. (2007) 16:1833–9. doi: 10.1158/1055-9965.EPI-07-0221
5. Wen S, Ren W, Xue B, Fan Y, Jiang Y, Zeng C, et al. Prognostic factors in patients with penile cancer after surgical management. World J Urol. (2018) 36:435–40. doi: 10.1007/s00345-017-2167-5
6. Hakenberg OW, Compérat EM, Minhas S, Necchi A, Protzel C, Watkin N. EAU guidelines on penile cancer: 2014 update. Eur Urol. (2015) 67:142–50. doi: 10.1016/j.eururo.2014.10.017
7. Ficarra V, Akduman B, Bouchot O, Palou J, Tobias-Machado M. Prognostic factors in penile cancer. Urology. (2010) 76(Suppl. 1):S66–73. doi: 10.1016/j.urology.2010.04.008
8. Srinivas V, Morse MJ, Herr HW, Sogani PC, Whitmore WF Jr. Penile cancer: relation of extent of nodal metastasis to survival. J Urol. (1987) 137:880–2. doi: 10.1016/S0022-5347(17)44281-9
9. Pandey D, Mahajan V, Kannan RR. Prognostic factors in node-positive carcinoma of the penis. J Surg Oncol. (2006) 93:133–8. doi: 10.1002/jso.20414
10. O'Brien JS, Perera M, Manning T, Bozin M, Cabarkapa S, Chen E, et al. Penile cancer: contemporary lymph node management. J Urol. (2017) 197:1387–95. doi: 10.1016/j.juro.2017.01.059
11. Ficarra V, Zattoni F, Artibani W, Fandella A, Martignoni G, Novara G, et al. Nomogram predictive of pathological inguinal lymph node involvement in patients with squamous cell carcinoma of the penis. J Urol. (2006) 175:1700–4; discussion 4-5. doi: 10.1016/S0022-5347(05)01003-7
12. Velazquez EF, Ayala G, Liu H, Chaux A, Zanotti M, Torres J, et al. Histologic grade and perineural invasion are more important than tumor thickness as predictor of nodal metastasis in penile squamous cell carcinoma invading 5 to 10 mm. Am J Surg Pathol. (2008) 32:974–9. doi: 10.1097/PAS.0b013e3181641365
13. Bhagat SK, Gopalakrishnan G, Kekre NS, Chacko NK, Kumar S, Manipadam MT, et al. Factors predicting inguinal node metastasis in squamous cell cancer of penis. World J Urol. (2010) 28:93–8. doi: 10.1007/s00345-009-0421-1
14. Lopes A, Hidalgo GS, Kowalski LP, Torloni H, Rossi BM, Fonseca FP. Prognostic factors in carcinoma of the penis: multivariate analysis of 145 patients treated with amputation and lymphadenectomy. J Urol. (1996) 156:1637–42. doi: 10.1016/S0022-5347(01)65471-5
15. Qu XM, Siemens DR, Louie AV, Yip D, Mahmud A. Validation of predictors for lymph node status in penile cancer: results from a population-based cohort. Can Urol Assoc J. (2018) 12:119–25. doi: 10.5489/cuaj.4711
16. Attalla K, Paulucci DJ, Blum K, Anastos H, Moses KA, Badani KK, et al. Demographic and socioeconomic predictors of treatment delays, pathologic stage, and survival among patients with penile cancer: a report from the National Cancer Database. Urol Oncol. (2018) 36:14.e7–e24. doi: 10.1016/j.urolonc.2017.09.014
17. Fritz April Percy Constance Jack Andrew. International Classification of Diseases for Oncology. 3rd ed. Geneva: World Health Organization (2000).
18. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. (2008) 26:1364–70. doi: 10.1200/JCO.2007.12.9791
19. Skeppner E, Andersson SO, Johansson JE, Windahl T. Initial symptoms and delay in patients with penile carcinoma. Scand J Urol Nephrol. (2012) 46:319–25. doi: 10.3109/00365599.2012.677473
20. Gursel EO, Georgountzos C, Uson AC, Melicow MM, Veenema RJ. Penile cancer. Urology. (1973) 1:569–78. doi: 10.1016/0090-4295(73)90517-7
21. Dewire D, Lepor H. Anatomic considerations of the penis and its lymphatic drainage. Urol Clin N Am. (1992) 19:211–9.
22. Marconnet L, Rigaud J, Bouchot O. Long-term followup of penile carcinoma with high risk for lymph node invasion treated with inguinal lymphadenectomy. J Urol. (2010) 183:2227–32. doi: 10.1016/j.juro.2010.02.025
23. Graafland NM, Lam W, Leijte JA, Yap T, Gallee MP, Corbishley C, et al. Prognostic factors for occult inguinal lymph node involvement inpenile carcinoma and assessment of the high-risk EAU subgroup: a two-institution analysis of 342 clinically node-negative patients. Eur Urol. (2010) 58:742–7. doi: 10.1016/j.eururo.2010.08.015
24. Kroon BK, Horenblas S, Lont AP, Tanis PJ, Gallee MP, Nieweg OE. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastases. J Urol. (2005) 173:816–9. doi: 10.1097/01.ju.0000154565.37397.4d
25. Marchioni M, Berardinelli F, De Nunzio C, Spiess P, Porpiglia F, Schips L, et al. New insight in penile cancer. Minerva urologica e nefrologica. (2018) 70:559–69. doi: 10.23736/S0393-2249.18.03215-0
26. Peak TC, Russell GB, Dutta R, Rothberg MB, Chapple AG, Hemal AK. A National Cancer Database-based nomogram to predict lymph node metastasis in penile cancer. BJU Int. (2019) 123:1005–10. doi: 10.1111/bju.14652
27. Protzel C, Alcaraz A, Horenblas S, Pizzocaro G, Zlotta A, Hakenberg OW. Lymphadenectomy in the surgical management of penile cancer. Eur Urol. (2009) 55:1075–88. doi: 10.1016/j.eururo.2009.02.021
28. Leone A, Diorio GJ, Pettaway C, Master V, Spiess PE. Contemporary management of patients with penile cancer and lymph node metastasis. Nat Rev Urol. (2017) 14:335–47. doi: 10.1038/nrurol.2017.47
29. Leijte JA, Gallee M, Antonini N, Horenblas S. Evaluation of current TNM classification of penile carcinoma. J Urol. (2008) 180:933–8; discussion 8. doi: 10.1016/j.juro.2008.05.011
30. Zhu Y, Zhang HL, Yao XD, Zhang SL, Dai B, Shen YJ, et al. Development and evaluation of a nomogram to predict inguinal lymph node metastasis in patients with penile cancer and clinically negative lymph nodes. J Urol. (2010) 184:539–45. doi: 10.1016/j.juro.2010.03.145
31. Ficarra V, Zattoni F, Cunico SC, Galetti TP, Luciani L, Fandella A, et al. Lymphatic and vascular embolizations are independent predictive variables of inguinal lymph node involvement in patients with squamous cell carcinoma of the penis: Gruppo Uro-Oncologico del Nord Est (Northeast Uro-Oncological Group) Penile Cancer data base data. Cancer. (2005) 103:2507–16. doi: 10.1002/cncr.21076
32. Hakenberg OW, Dräger DL, Erbersdobler A, Naumann CM, Jünemann KP, Protzel C. The diagnosis and treatment of penile cancer. Deutsches Arzteblatt Int. (2018) 115:646–52. doi: 10.3238/arztebl.2018.0646
33. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. (2017) 141:664–70. doi: 10.1002/ijc.30716
34. Al Ghazal A, Steffens S, Steinestel J, Lehmann R, Schnoeller TJ, Schulte-Hostede A, et al. Elevated C-reactive protein values predict nodal metastasis in patients with penile cancer. BMC Urol. (2013) 13:53. doi: 10.1186/1471-2490-13-53
Keywords: penis (MeSH), carcinoma, squamous cell, lymph nodes, nomograms
Citation: Zhang W, Gao P, Gao J, Wu X, Liu G and Zhang X (2021) A Clinical Nomogram for Predicting Lymph Node Metastasis in Penile Cancer: A SEER-Based Study. Front. Oncol. 11:640036. doi: 10.3389/fonc.2021.640036
Received: 10 December 2020; Accepted: 05 February 2021;
Published: 09 March 2021.
Edited by:
Marco Borghesi, University of Genoa, ItalyReviewed by:
Alessandro Tafuri, University of Verona, ItalyGuru Sonpavde, Dana–Farber Cancer Institute, United States
Copyright © 2021 Zhang, Gao, Gao, Wu, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiansheng Zhang, eGlhbnNoZW5nLXpoYW5nQDE2My5jb20=
 Wei Zhang
Wei Zhang Xiansheng Zhang
Xiansheng Zhang