
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 17 March 2021
Sec. Cancer Molecular Targets and Therapeutics
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.638146
This article is part of the Research TopicRadiochemotherapy in Multimodal Cancer TherapyView all 20 articles
Cancer is one of the most common causes of death worldwide. Although the existing therapies have made great progress and significantly improved the prognosis of patients, it is undeniable that these treatment measures still cause some serious side effects. In this context, a new treatment method is needed to address these shortcomings. In recent years, the magnetic fields have been proposed as a novel treatment method with the advantages of less side effects, high efficiency, wide applications, and low costs without forming scars. Previous studies reported that static magnetic fields (SMFs) and low-frequency magnetic fields (LF-MFs, frequency below 300 Hz) exert anti-tumor function, independent of thermal effects. Magnetic fields (MFs) could inhibit cell growth and proliferation; induce cell cycle arrest, apoptosis, autophagy, and differentiation; regulate the immune system; and suppress angiogenesis and metastasis via various signaling pathways. In addition, they are effective in combination therapies: MFs not only promote the absorption of chemotherapy drugs by producing small holes on the surface of cell membrane but also enhance the inhibitory effects by regulating apoptosis and cell cycle related proteins. At present, MFs can be used as drug delivery systems to target magnetic nanoparticles (MNPs) to tumors. This review aims to summarize and analyze the current knowledge of the pre-clinical studies of anti-tumor effects and their underlying mechanisms and discuss the prospects of the application of MF therapy in cancer prevention and treatment.
Cancer is a serious threat to human health and one of the leading causes of death worldwide. According to estimates with regard to morbidity and mortality for 36 kinds of cancers in 185 countries, about 18.1 million new cancer cases plus 9.6 million cancer-associated deaths happened in 2018 (1). Among these cancers, the highest incidence types are lung (11.6%), breast (11.6%), prostate (7.1%), and colorectal (6.1%) cancers. At present, the primary options for advanced cancer treatments, namely chemotherapy and radiotherapy, always have some limitations such as severe side effects and drug resistance (2–4). It is necessary to develop new therapies to address these disadvantages. In this context, more attention was paid for alternative treatments involving some non-invasive approaches like light, heat, electrical field, magnetic field (MFs), and ultrasound therapies (5–9), which are of high efficiency and incur low costs without inducing infections or forming scars. Among them, the MF therapy has been studied a lot in recent years, as early as 1971, when Weber et al. (10) validated the inhibitory effects of MFs on tumor-bearing mice. Over the next few decades, many researchers have explored this phenomenon and put forward more evidence about the relevant mechanisms (11–13); at the same time, clinical trials demonstrated its advantage in relieving clinical symptoms, and improving the quality of life of patients with recurrent and rapidly progressing tumors (Table 1) (17). Early studies have shown that in the field of cancer treatment, MFs have potential application prospects with few side effects and wide applications. MFs could non-invasively induce the death of cancer cells, whereas lymphocytes showed little necrosis in vitro (18, 19). In other medical studies, the MF therapy has been reported to have beneficial results in peripheral nerve regeneration (20), osteo-necrosis (21), and injury-induced osteoporosis (22). MFs at frequencies above 100 kHz predominately show thermal effects; otherwise, they would exert non-thermal effects (23). Recently, non-thermal biological effects of MFs have been reported in many aspects, among which are studies on tumor treatment. The inhibitory effects of static magnetic fields (SMFs) and low-frequency magnetic fields (LF-MFs, with frequency below 300 Hz) have been studied against a wide variety of human cancer cell lines, such as leukemia (24–31), fibrosarcoma (32), colon carcinoma (32–34), and breast cancer (35–40). Furthermore, MFs suppress the growth of Lewis lung carcinoma (LLC) (41) and Ehrlich ascites carcinoma (42, 43) in vivo, and even prolong survival and improve the general symptoms of 21 patients with advanced gastric cancer (44). MFs have shown to exert anti-tumor action through various pathways and multiple molecular mechanisms, such as the inhibition of cell growth and proliferation; the induction of apoptosis, cell cycle arrest, and autophagy; participation in immune regulation as well as depression of angiogenesis, and metastasis; and promotion of differentiation. Of interest, they are effective in combination therapies with chemotherapeutic agents and magnetic nanoparticles (MNPs).
Two conditions of the molecular mechanism, namely thermal effects and non-thermal effects, are involved in MF-induced biological effects (23). According to IEEE C95.1-2019, thermal effects are defined as “changes associated with heating of the whole body or an affected region sufficient to induce a biological effect.” Electro-stimulation is the dominant effect at low frequencies and thermal effects dominate above radio frequencies. The International Commission on Non-Ionizing Radiation Protection (ICNIRP) gives a more detailed description of electro-magnetic fields at radio frequency (100 kHz−300 GHz), which could penetrate the body and cause a vibration of charged or polar molecules inside, resulting in friction and heat. Thermal effects lead to an increase in bulk temperature, which would thermally induce membrane depolarization, excitation, and breakdown and show a distinct side effect on the organisms (45). Non-thermal effects could be described as direct interactions of MF with biological cells that are not associated with any heating but are associated mainly with electro-stimulation (23, 46). Based on the physical mechanisms, extremely LF-MFs (<300 Hz) are regarded as non-thermal effects (47).
Magnetic fields are generally generated by permanent magnets or electric currents, and there are several classification methods for MFs. According to the mechanism of the generation of MFs, they are divided into permanent magnetic fields and electromagnetic fields. While the variation rate of the intensity of the MF with spatial displacement is equal to 0, it is a uniform MF; otherwise, it is a gradient magnetic field (GMF). Moreover, if the distribution of the MF changes with time, they are classified as SMFs and non-SMFs, such as alternating magnetic fields (AMFs), pulsed magnetic fields (PMFs), and rotating magnetic fields (RMFs). In consideration of their working frequency, the MF is classified into low frequency (LF) (<300 kHz), medium frequency (MF) (300 kHz−3 MHz), and high frequency (HF) (>3 MHz). According to the Regulations (2012) of the International Telecommunication Union (ITU), LF-MFs are further divided into tremendously LF(<3 Hz), extremely LF (3–30 Hz), super LF (30–300 Hz), ultra LF (300–3000 Hz), very LF (3–30 kHz), and LF (30–300 kHz) (48). Many studies have shown that SMFs and LF-MFs (f <300 Hz) exerted anti-tumor effects, in which the temperature was maintained at around 37°C for cell culture in vitro and excluded thermal effects (30, 49, 50). To comprehend the non-thermal effects of the MF therapy on cancers, we focus on the abovementioned MF types in this review, aiming at describing the state of the art of MF therapy, discussing the current understanding of the underlying anti-cancer mechanisms, and outlining future therapeutic perspectives in oncology. Common setups, types, exposure direction, and duration of the action are summarized in Table 2.
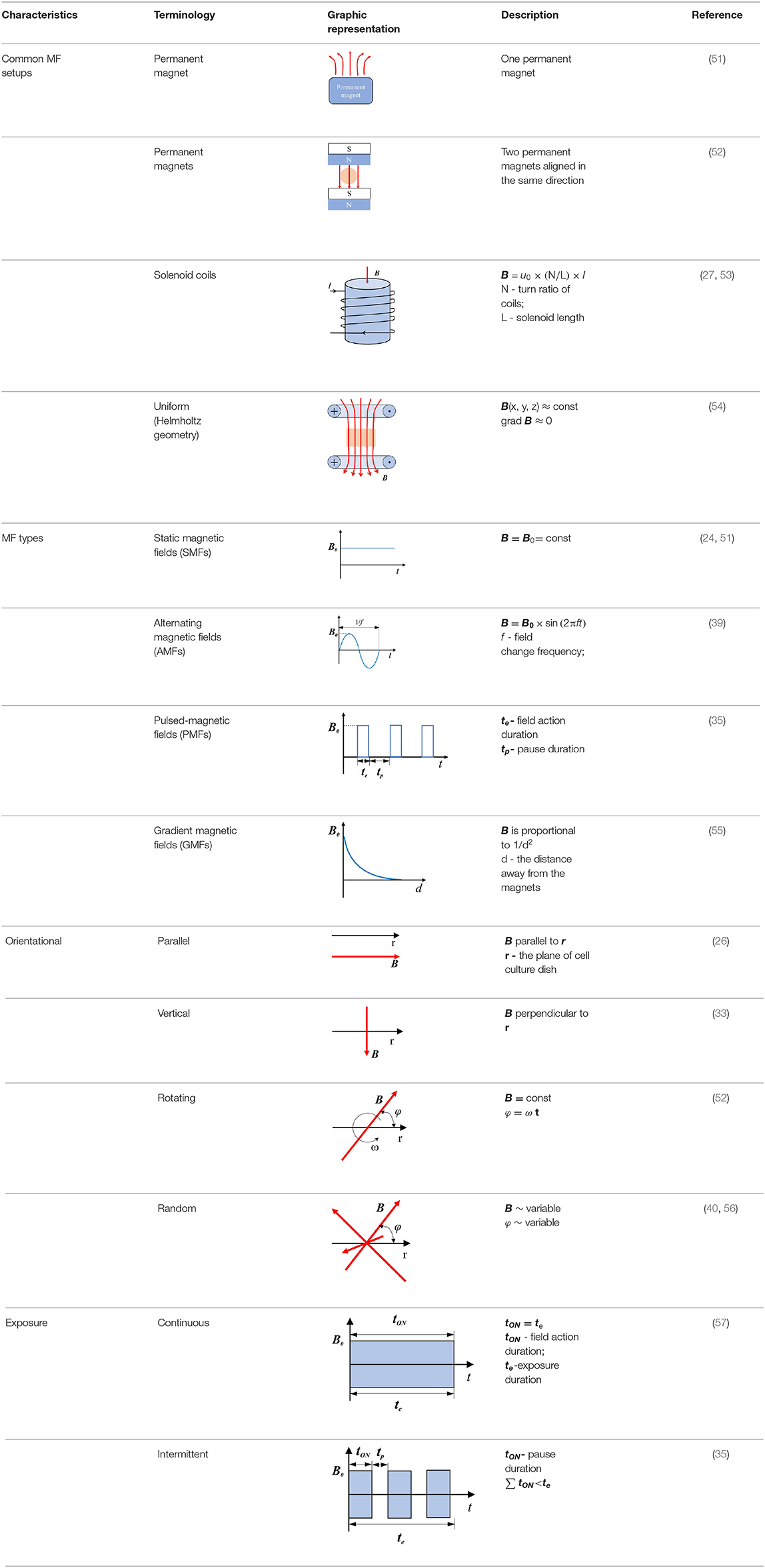
Table 2. Common setups, types, exposure direction, and duration of the action of MFs used in anti-tumor studies.
In this review, we focus on the non-thermal effects of SMFs and LF-MFs (<300 Hz) on cancer cells and their applications in cancer treatment. The review aims to highlight the critical areas regarding the uses of MF therapy, which are not fully understood and need to be investigated further.
The literature search was carried out with Scopus, Google Scholar, PubMed, Web of Sciences (ISI Web of Knowledge), Medline, and Wiley Online Library databases. Available publications (in English) in peer-reviewed journals on the biological effects of SMFs and LF-MFs between 2008 and 2019 were selected for analysis. We focus on SMF- and LF-MF-induced anti-tumor effects in in vivo and in vitro studies. The studies on the influence of SMFs and LF-MFs on other organs and systems were excluded from the literature. The keywords used for the literature research were “apoptosis,” “cell cycle arrest,” “autophagy,” “angiogenesis,” “immune,” “inflammation,” “differentiation” (as a combination with “low frequency electromagnetic fields” or “static magnetic fields,” and “tumor” or “cancer” or “oncology”).
Magnetic fields exert their function through various pathways and multiple targets. A large number of recent studies have shown that MFs have anti-tumor effects by inhibiting cell proliferation and inducing cell cycle arrest, apoptosis, and autophagy.
The cell cycle, which consists of the G1, S, G2, and M phases, is a very complex and delicate regulation process closely related to cell differentiation, growth, and death. Abnormal expressions of some cell cycle proteins could cause uncontrolled replication of cancer cells; so it is a promising therapy to target cyclins (58).
DNA integrity is critical to cells; common radiotherapy and most of the chemotherapies exert their function by damaging the cancer cells of DNA, which would inhibit proliferation at cell cycle checkpoints and lead to cell death (59). SMFs (8.8 mT, 12 h) enhanced the killing potency of cisplatin, adriamycin, and paclitaxel by triggering DNA damage, inducing cell ultrastructure alteration, and arresting K562 cells at the G2/M phase (27–29). RMFs (0.4 T, 7.5 Hz, 2 h/day) inhibited the growth of B16-F10 in vitro, elevated the survival rate, and inhibited the proliferation in the lung metastasis model mice, where an increase in the G2/M phase was detected (52). CDK1-cyclin B, also known as cell division control protein kinase 2-cyclin B (cdc2-cyclin B) functions at the G2/M phase of the cell cycle, to accelerate cell mitosis (60). SMFs (200 ± 60 mT, 48 ± 4 h) induced human malignant glioblastomata, such as U87 and U251, to arrest the G2/M phase by downregulating the expressions of cyclin B1 and CDK1 (61). The p53 protein is a critical participant in the signal transduction pathway which mediated apoptosis and G1 cell cycle arrest in mammalian cells (62). LF-MFs significantly inhibited tumor growth, induced cell senescence, inhibited iron metabolism of the LLC murine model, and the in vitro induced G0/G1 phase arrest of A549 lung cancer cells via stabilizing p53 protein and activation of the P53-miR-34a-E2F1/E2F3 pathway (41). In addition, earlier experiments with high risk BE(2)-C neuroblastomas continuously exposed in 50 Hz, 1mT LF-MF for 72 h led to an enhanced cell response to ATRA, along with an increase in the levels of p21, Cdk-5, and G0/G1 population (63). A 24-h exposure of 50 Hz, 100 uT LF-MF exposure slowed down the progression of the cell cycle, which is associated with the regulation of p21 in early response (64). These data indicate that MFs are found to arrest cells at different stages, thus leading to anti-proliferation effects on cells by modulating cell cycle regulatory proteins, as summarized in Figure 1.
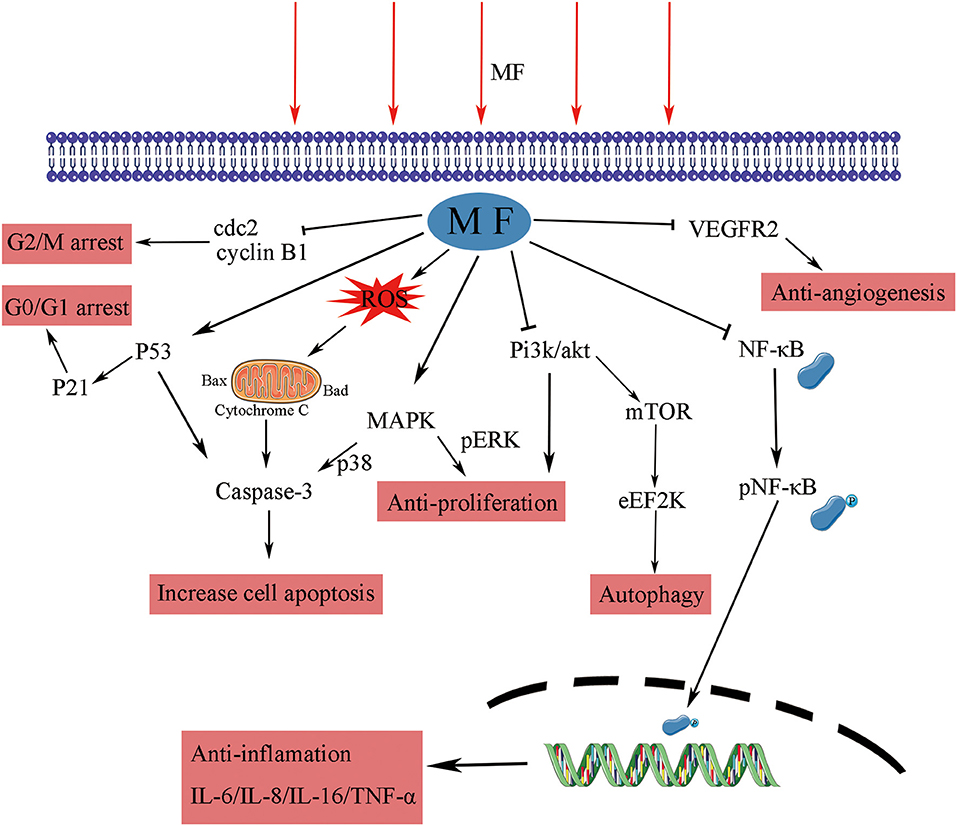
Figure 1. The effect of MFs on different signaling pathways and their molecular targets. MF, magnetic field; cdc, cell division control protein kinase; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; Akt, protein kinase B; Pi3k, phosphatidylinositol 3-kinase; mTOR, mechanistic target of rapamycin kinase; eEF2K, eukaryotic elongation factor 2 kinase; NF-κB, nuclear factor kappa B; IL, interleukin; TNF, tumor necrosis factor; VEGFR, vascular endothelial growth factor receptor.
Apoptosis, which is a form of programmed cell death as well as a target for anti-tumor therapies, plays an important role in cancer treatment (65). There are two main apoptosis pathways: one occurs through the mitochondrial pathway (intrinsic pathway) and another through the cell death receptor pathway (extrinsic pathway). The intracellular mitochondrial pathway is mainly regulated by B-cell lymphoma-2 family, which could promote the formation of channels in the extracellular membrane of mitochondria to change the permeability, release a variety of apoptosis-related proteins to activate caspase, and induce apoptosis (66, 67). Targeting some pro-apoptosis proteins, anti-apoptosis proteins, and mitochondrial membrane permeability are attractive for cancer therapy, by contributing to the occurrence of the intrinsic apoptosis pathway (68, 69).
Magnetic fields have been shown to induce apoptosis in human tumor cells studied in vitro. A 50-Hz LF-MF (5.1 mT, 2 h/day) inhibited proliferation of nephroblastoma and neuroblastoma cells, induced apoptosis in vitro, and promoted the efficacy of cisplatin in vivo (49). Reactive oxygen species (ROS) and mitochondria play an important role in the induction of apoptosis (70), and an increase in ROS levels can lead to cytochrome c release and mitochondrial apoptosis (54). The MF treatment has been shown to promote the generation of ROS in many studies (31, 71, 72), with exposure within a 60 Hz sinusoidal MF for 48 h in induced human prostate cancer for DU145, PC3, and LNCaP apoptoses, associated with the accumulation of ROS in an intensity-dependent manner (73). Generally, apoptosis provoked by genotoxins is largely due to DNA damage (74), while DNA double-strand breaks (DSBs) are one of the most severe types of DNA lesions (75). Repetitive exposure to LF-MFs induced DNA damage and accumulation of DSBs and triggered apoptosis in Hela and MCF7 cell lines (35, 76). As p53 is a tumor suppressor gene that plays a pivotal role in apoptosis, PMFs could trigger apoptosis cell death by upregulating the p53 level and through the mitochondrial-dependent pathway (57). LF-MFs (300 mT, 6 Hz, 24 h) also induced apoptosis by suppressing protein kinase B (Akt) signaling, activating p38 mitogen-activated protein kinase (MAPK) signaling, and caspase-9, which is the executor of the mitochondrial apoptosis pathway (77).
The findings of these studies have shown that MFs affect apoptosis in the cancer cell lines of various origins. However, at present, there are few studies in this area, and further studies are required for detailed mechanisms. The proposed mechanism involved in the effects of MFs on tumor cell apoptosis is shown in Figure 1.
Autophagy is thought to have a therapeutic potential to prevent cancer development, but whether to enhancing or inhibiting it will achieve the desired anti-tumor effects remains questionable (78). Autophagy could be ascertained by detecting LC3-II, a marker of autophagic vesicle accumulation (79). To date, miRNAs were proved to involve in the modulation of a wide range of biological processes, including apoptosis and autophagy (80). The expression of the autophagy marker, LC3-II, detected by Western blotting and GFP-LC3 puncta-formation assay examined by confocal microscopy, showed that RMFs (0.4T, 7.5 Hz, 4 h/day) induced autophagic cell death and suppressed cancer growth in vitro and in vivo. The main mechanism involved the upregulation of the expression level of miR-486, which was targeting BCAP, the inhibition of Akt/mechanistic target of rapamycin kinase (mTOR), and the induction of autophagy by RMF (81). These findings showed the potential of MF in triggering the autophagic cell death.
The immune function in an organism exerts an essential role in the occurrence and metastasis of tumors. The RMF (0.4T, 7.5 Hz, 2 h/day) has the capacity to elevate the survival rate of tumor-bearing by modulating the immune response and functions of innate immune cells and adaptive immune cells, such as regulating cytokine production in mice serum, promoting T-cell polarization in the spleen, preventing the differentiation of the regulatory cells (Tregs), and increasing the expression of CD40 in dendritic cells (52). Furthermore, analogous results were discovered in mouse H22 hepatocellular carcinoma, with an enhanced anti-tumor immune response; the inhibition of tumor growth; and the suppression of interleukin-6 (IL-6), granulocyte colony-stimulating factor (G-CSF), and keratinocyte-derived chemokine (KC). Meanwhile, the MF exposure was associated with the activation of macrophages and dendritic cells, enhancement of the profiles of CD4+T and CD8+T lymphocytes, the balance of Th17/Treg, and the reduction of the inhibitory function of Treg cells in vivo (82). A combination of SMF with AMF stimulated the production of tumor necrosis factor-α (TNF-α), interferon-gamma, IL-2, and IL-3 in healthy mouse cells, inhibited solid tumor growth, and enhanced the average lifespan, after daily exposure for 2 h within 14 days (83).
Inflammation is a key factor in the immune response to injury and infection; some studies have shown that the progression of various cancers may be closely related to chronic inflammation (84, 85). Exposure to PMF with an intensity of 40 Gauss and frequency below 30 Hz for 48 h decreased the production of the inflammation marker TNF-α and the transcription factor nuclear factor kappa B (NF-κB). In RAW 264.7 macrophage-like cells, induced by LPS, this regulation process could be appropriately applied to patients with sepsis (86). The upregulation of A2A and A3ARs adenosine receptor mRNA levels by the PMF (1.5 ± 0.2 mT, 75 Hz, 24 h) mediated the anti-inflammation effect, induced the decrease of NF-κB expression, upregulated p53, and induced apoptosis in tumor cells (57). The GMF (6.39–513.69 mT, 24 h) significantly inhibited the release of pro-inflammatory cytokines, IL-6, IL-8, and TNF-α, from macrophages and assisted the production of anti-inflammatory cytokine, IL-10, when treate alone for 24 h and then combined with LPS (87). A similar response was induced by the PMF in N9 microglial cells (88). An in vitro study found that SMF (0.4 T, 6 h) could attenuate LPS-induced neuro-inflammatory responses in BV-2 cells, and this effect was associated with increased microglial membrane rigidity and downregulation of IL-6 release (89). MFs have the ability to enhance the immune response of the body to tumors by modulating the functions of immune cells and inhibiting chronic inflammation (Figure 1); while the regulation of the immune system is complex, further research is needed to explain the relationship.
Angiogenesis is a critical physiological and pathological process in embryo development, tumor development, and metastasis. The formation of new blood vessels gradually has become an essential therapeutic target in cancer treatment, ischemic diseases, and chronic inflammation (90). Vascular endothelial cell migration is an important part of the angiogenesis process of tumors, and vascular endothelial growth factor (VEGF-A, VEGF) and its receptor-2 (VEGFR-2) play an important role in tumor angiogenesis, which gradually becomes a target in anti-tumor therapy (91). The SMF (600 mT, 10 days) has been shown to inhibit angiogenesis by reducing vessel diameters, the functional vessel density (FVD), and red blood cell velocity to retard vessel maturation by in vivo tests (92). After 24-h exposure in the GMF (0.2–0.4 T, 2.09 T/m), the proliferation ability of human umbilical vein endothelial cells (HUVECs) was significantly inhibited. In the chick embryo chorioallantoic membrane (CAM) model, vascular numbers of continuously exposure treatment group (7–11 days) are fewer than those in the control group, which is consistent with the results in matrigel plugs models (55). Sinusoidal MF (1 mT, 50 Hz,72 h) inhibited the formation of tubule-like structures and downregulated the process and migration of HUVECs by reducing the expression and activation levels of VEGFR2 (93). A combination therapy of MF (0.04 T,50 Hz, 1 h) and saffron had synergic effects on VEGFR2 gene expression; they reduced the VEGFR-2 level by 36%, while MF alone only induced a 20% decline in human breast cancer cells (37). A therapeutic MF device, which could generate a defined 120 Hz semi sine wave signal with variable intensity (10–20 mT), was tested for the optimal intensity and treatment period of MF therapy for breast cancer. Exposure to 20 mT for 10 min two times a day within 12 days was the most effective tumor suppressor; the MF treatment reduced the vascular (CD31 immuno-histochemically positive) volume fraction (94). These studies indicate that the MF theraphy is a promising therapy that may target tumor angiogenesis through the pathways showed in Figure 1.
Tumor metastasis is the leading cause for death in patients with cancer, and up to 90% of cancer deaths occur due to metastasis. After intermittent treatment for several weeks, a therapeutic electromagnetic field (15 mT, 10 min/day) has proved to inhibit the metastatic spread in the nude mice injected with breast cancer cells, which might be associated with the decrease in volume density of blood vessels (95). Furthermore, the RMF (0.4 T, 7.5 Hz, 2 h) significantly suppressed the metastasis of melanoma and survival time of the mice injected with B16-F19 cells (52). Actin cytoskeleton plays a major role in the process of driving cellular protrusions, such as lamellipodia and filopodia, at the leading edge of the cell, which is necessary for cell migration (96). In the absence of the geomagnetic field, also known as hypomagnetic field environment, the SH-SY5Y neuroblastoma cell adhesion and migration ability were diminished. Geomagnetic field shielding decreased the irregularity and eccentricity of the cell shape; cells maintain a weakened adhesive morphology, thicker, smaller, and rounder, which may be associated with its negative regulation of actin assembly (97).
Moreover, the growth rate of tumors is closely related to the degree of tumor differentiation, which is an important reference index in cancer diagnosis and treatment. The LF-MF (5 mT, 50 Hz) was proved to cause an increase in 20% differentiation of hemin-induced K562 cells with a daily exposure of 1 h for 4 days (30). Another study found that the LF-MF (2 mT, 50 Hz, 96 h) exposure decreased the cellular proliferation potential and contributed to the ATRA-treated acute promyelocytic leukemia NB4 cell differentiation that varies with dose, where ROS and extracellular signal-regulated kinase (ERK) signaling pathways may be involved (31). These data suggested that MFs play promising roles as an assistant therapy in combination with other drugs to induce differentiation of leukemia cells. However, only a few studies have focused on the effects of MFs on the differentiation of cancer cells, and the mechanism involved might need a more detailed research.
Chemotherapy always meets with increased toxicity and side effects caused by high dosage and drug resistance triggered by prolonged treatment, while combination therapy has obvious advantages by avoiding these. The co-treatment of SMF and cisplatin (10 ug/ml) for 12 h substantially suppressed the growth of K562 cells and augmented the chemosensitivity to cisplatin. This effect was correlated with the enhanced level of DNA damage and the arrest of the S-phase (27). Exposure to SMF with the intensity of 10 mT for 48 h led to a marked decrease in the viability percentage of cisplatin-treated HeLa cells through ROS accumulation (72). Appropriate SMF therapy increased the sensitivity of ovarian cancer cells, such as A2780 and A2780-CP, to cisplatin depending upon dose and exposure time, via producing small holes and large verrucous structures on the surface of the cell membrane (27, 98). The expression of P-glycoprotein is associated with multidrug resistance (MDR) in cancer cells, which is one of the main mechanisms of drug resistance in cancer cells (99). A combination with the SMF (8.8 m T, 12 h) decreased the expression of P-glycoprotein (P-gp) in K562 cancer cells, while adriamycin itself induced an increase (28). PMF (2 mT, 75 Hz, 1 h/day) coupled with temozolomide could slow down the proliferation of chemo- and radio-resistant T98G glioblastoma cell line by epigenetically affecting the regulation of oncogenes and tumor suppressors (100). The LF-MF (10 mT, 100 Hz, 144 h) promoted the sensitization of human glioblastomata, namely U87 and T98G, to temozolomide, which led to an increased apoptosis rate, with the evidence of increasing the expression of p53 and Bax and decreasing the expression of Bcl-2 and cyclin D1 (54). Capsaicin is the major pungent ingredient of the hot chili peppers, which could bind to distinct cell surface receptors including transient receptor potential vanilloid 1 (TRPV1) ion channel to exert anti-tumor function. An increased apoptosis rate was realized through the mitochondria-dependent apoptosis pathway, and the conformational change of TRPV1 triggered by the SMF (0.5 T, 72 h) might be the reason for this enhancement effect (101). Pre-exposure to 50 Hz LF-MF for 12 h and treatement with 5-fluorouracil (5-FU) for 24 h significantly inhibited the proliferation of MCF7 cells. This phenomenon was explained by increased DNA synthesis and upregulated cyclin E and cyclin D1 by the the MF to accumulate cancer cells at the S phase, which was more sensitive to 5-FU (102). The MF also showed a potential to retard tumor growth, elevate survival improvement, and reduce side effects when combined with radiotherapy and bacteriolytic therapy (43, 103). Therefore, the results of these studies support the fact that the MFs can be used as an adjunctive treatment to enhance the effects of chemotherapeutic drugs by increasing the DNA damage, cell apoptosis, and arresting the cell cycle, as summarized in Figure 2.
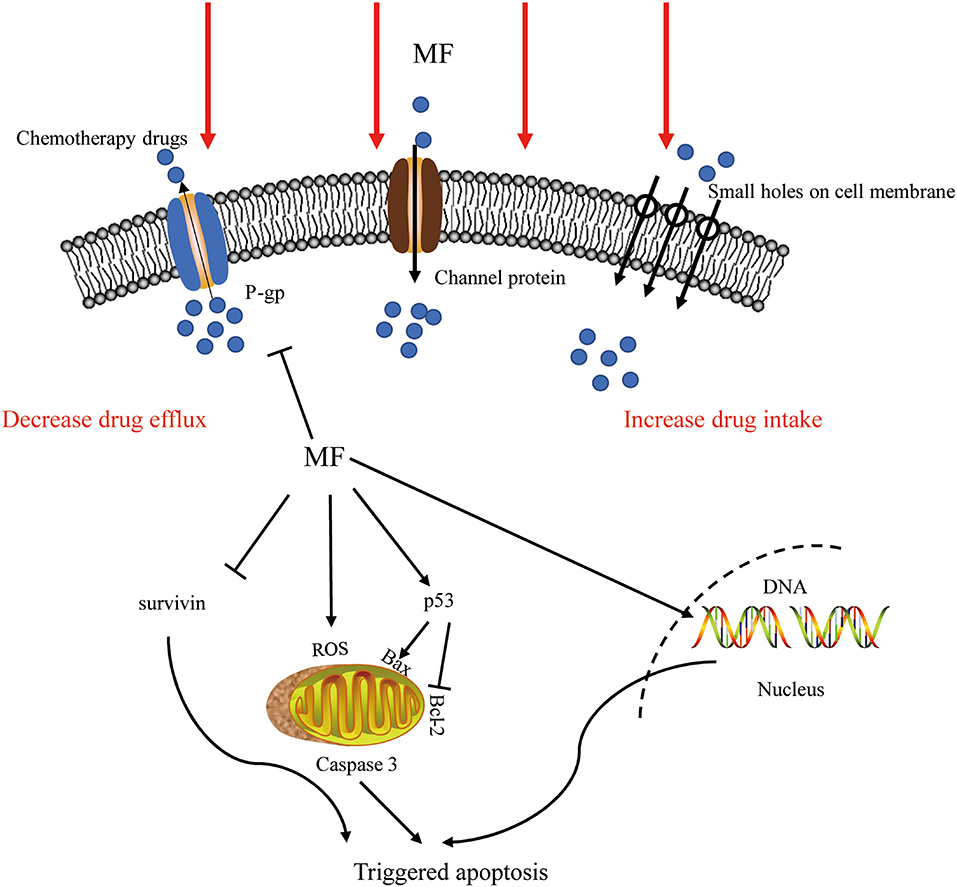
Figure 2. The effect of MF in combination therapy. The MFs not only increased the intake by producing small holes on the surface of the cell membrane and decreased the efflux of chemotherapeutic drugs by inhibiting ABC transporters but also affected ROS generation, DNA integrity, and apoptosis-related pathways to trigger apoptosis. P-gp, P-glycoprotein; MF, magnetic field; ROS, reactive oxygen species.
Drug delivery systems (DDSs) were developed for targeting active biomolecules at the specific site of infection when treating patients with cancer, to improve the selectivity of the action sites of drugs, eliminate the side effects, and improve treatment efficiency. The MF targeting systems are always applied in combination with magnetic materials and anticancer drugs. Under the function of 100 Hz, 0.7 mT AMF, folic acid-modified magnetic nanoparticles (FA-MNPs) and alpha fetal protein monoclonal antibody-loaded MNPs (ATP-loaded MNPs) selectively induced the apoptosis of cancer cells and elevated the cellular iron uptake in a dose-dependent manner but had slight toxic effects on healthy cells (104, 105). The growth-inhibitory effects induced by SMFs and RMFs were enhanced by pretreating the cells with MNPs, while regulating the type and parameters of MFs could affect anti-tumor effects (106). The SMF along with low-intensity pulsed ultrasound (LIPUS) plus methotrexate (MT) prevented the growth of cancer cells better than bare drugs and single DDS, without any inhibition on the healthy cells (107). The detailed in vitro experiment results were subsequently validated via in vivo experiments, and the LIPUS+SMF DDS therapy improved at least 40% of the treatment efficacy, therapy reducing the natural activities of the cancer cells by changing the permeability, the potential of the cell membrane, and ROS generation (108). These results indicate that the MF could act as a DDS to target solid tumors in combination with MNPs to inhibit proliferation (Figure 3).
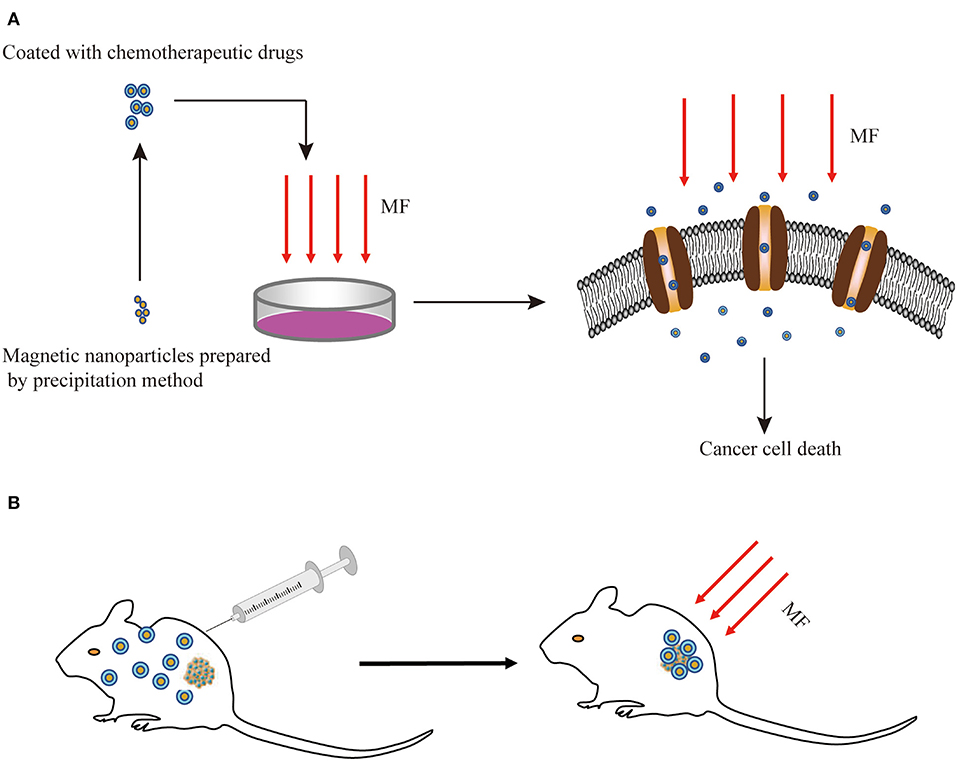
Figure 3. Schematic diagram of the combination of MFs with MNPs for cancer treatment in vitro (A) and in vivo (B). MF, magnetic field; MNPs, magnetic nanoparticles.
Numerous studies have shown that a wide range of types of MFs could affect the tumor cells at different degrees, while the dominant effects were associated with thermal or non-thermal mechanisms. The focus of this review is non-thermal effects, which were produced directly by the applied MFs themselves, instead of being produced indirectly as a result of heating. We summarized the performance, namely inhibiting cancer cell proliferation and inducing cell death in in vitro and in vivo models, of SMFs and LF-MFs in anti-tumor treatments. Also, co-treating with chemotherapy would achieve better therapeutic effects; meanwhile, the MF could serve as a DDS, targeting MNPs to the tumor, and the side effects are within the controllable range. Although various potential mechanisms of MFs against different cancer cell lines have been reported and discussed, few studies were performed on in vivo models. At present, most of these studies are confined to in vitro studies. Also, relevant clinical trials to test the safety and efficacy of MFs are not available. The limitations in these clinical studies might be due to their controversial roles in in vitro and in vivo studies, which are affected by some experimental variables such as the frequencies, intensities, or exposure duration of the MFs. Before clinical applications, there is still a demand for systematically exploring. Future studies should aim at finding optimum parameters at which these types of MFs will be most effective. Epidemiological studies have suggested that MFs at 50/60 Hz were also related to the development of depressive state anxiety, metabolic disturbance, poor sleep quality, and locomotor activity. However, according to ICNIRP, there is no sufficient scientific evidence for the association between MF exposure and these effects. Therefore, the most effective MF therapy should be tested further to guarantee its possible investigation in human. As for the MF devices, in consideration of the increasingly available clinical applications, the expectations should be portable and affordable. Future studies are expected to further determine the potential of the MF therapy in oncology.
TL and AX participated in the selection of papers and contributed to the writing of the paper. AX and QW collected, disposed, and analyzed the data. TL and XL helped to modify this manuscript. All authors contributed to the article and approved the submitted version.
This research was funded by the National Foundation of China (Grant Nos. 41722405 and 41874209) and the Ministerial Foundation of Jilin Province (20160414002GH, 20180201017GX, and 2017C046-1).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Baguley BC. Novel strategies for overcoming multidrug resistance in cancer. Biodrugs. (2002) 16:97–103. doi: 10.2165/00063030-200216020-00003
3. Wang J, Seebacher N, Shi H, Kan Q, Duan Z. Novel strategies to prevent the development of multidrug resistance (MDR) in cancer. Oncotarget. (2017) 8:84559–71. doi: 10.18632/oncotarget.19187
4. Christian Nicolaj A, Jan A. Genetic variants and normal tissue toxicity after radiotherapy: a systematic review. Radiother Oncol. (2009) 92:299–309. doi: 10.1016/j.radonc.2009.06.015
5. Dabrowski JM, Arnaut LG. Photodynamic therapy (PDT) of cancer: from local to systemic treatment. Photochem Photobiol Sci. (2015) 14:1765–80. doi: 10.1039/C5PP00132C
6. Zhang H, Liu K, Xue Z, Yin H, Dong H, Jin W, et al. High-voltage pulsed electric field plus photodynamic therapy kills breast cancer cells by triggering apoptosis. Am J Transl Res. (2018) 10:334–51.
7. Ibsen S, Schutt CE, Esener S. Microbubble-mediated ultrasound therapy: a review of its potential in cancer treatment. Drug Des Devel Ther. (2013) 7:375–88. doi: 10.2147/DDDT.S31564
8. Sengupta S, Balla VK. A review on the use of magnetic fields and ultrasound for non-invasive cancer treatment. J Adv Res. (2018) 14:97–111. doi: 10.1016/j.jare.2018.06.003
9. Macdonald IJ, Dougherty TJ. Basic principles of photodynamic therapy. J Porphyr Phthalocyanines. (2009) 05:105–29. doi: 10.1002/jpp.328
10. Weber T, Cerilli GJ. Inhibition of tumor growth by the use of non-homogeneous magnetic fields. Cancer. (1971) 28:340–3. doi: 10.1002/1097-0142(197108)28:2<340::AID-CNCR2820280213>3.0.CO;2-E
11. Mizushima Y, Akaoka I, Nishida Y. Effects of magnetic field on inflammation. Experientia. (1975) 31:1411–2. doi: 10.1007/BF01923216
12. Williams CD, Markov MS. Therapeutic electromagnetic field effects on angiogenesis during tumor growth: a pilot study in mice. Electro Magnetobiol. (2001) 20:323–9. doi: 10.1081/JBC-100108573
13. Tofani S, Barone D, Cintorino M, de Santi MM, Ferrara A, Orlassino R, et al. Static and ELF magnetic fields induce tumor growth inhibition and apoptosis. Bioelectromagnetics. (2001) 22:419–28. doi: 10.1002/bem.69
14. Mulay IL, Mulay LN. Effect of a magnetic field on sarcoma 37 ascites tumour cells. Nature. (1961) 190:1019. doi: 10.1038/1901019a0
15. Degen IL. Treatment of traumatic edema by a magnetic field. J Trauma. (1971) 11:979. doi: 10.1097/00005373-197111000-00015
16. Chakkalakal D, Mollner T Mr, Fritz E, Novak J, Mcguire M. Magnetic field induced inhibition of human osteosarcoma cells treated with adriamycin. Canc Biochem Biophys. (1999) 17:89–98.
17. Vasishta VG. Sequentially programmed magnetic field therapy in the management of recurrent anaplastic astrocytoma: a case report and literature review. Case Rep Oncol. (2010) 3:189–94. doi: 10.1159/000316358
18. Radeva M, Berg H. Differences in lethality between cancer cells and human lymphocytes caused by LF-electromagnetic fields. Bioelectromagnetics. (2004) 25:503. doi: 10.1002/bem.20023
19. Hisamitsu T, Narita K, Kasahara T, Seto A, Yu Y, Asano K. Induction of apoptosis in human leukemic cells by magnetic fields. Jpn J Physiol. (1997) 47:307–10. doi: 10.2170/jjphysiol.47.307
20. Suszyński K, Marcol W, Szajkowski S, Pietrucha-Dutczak M, Cieślar G, Sieroń A, et al. Variable spatial magnetic field influences peripheral nerves regeneration in rats. Electromagn Biol Med. (2014). doi: 10.3109/15368378.2013.801351
21. Ding S, Peng H, Fang HS, Zhou JL, Wang Z. Pulsed electromagnetic fields stimulation prevents steroid-induced osteonecrosis in rats. BMC Musculoskelet Disord. (2011) 12:215. doi: 10.1186/1471-2474-12-215
22. Manjhi J, Kumar S, Behari J, Mathur R. Effect of extremely low frequency magnetic field in prevention of spinal cord injury-induced osteoporosis. J Rehabil Res Dev. (2013) 50:17–30. doi: 10.1682/JRRD.2011.12.0248
23. Israel M, Zaryabova V, Ivanova M. Electromagnetic field occupational exposure: non-thermal vs. thermal effects. Electromagnetic Biol Med. (2013) 32:145–54. doi: 10.3109/15368378.2013.776349
24. Yang P, Hu L, Zhe W, Chong D, Wei Z, Qian A, et al. Inhibitory effects of moderate static magnetic field on leukemia. IEEE Trans Magn. (2009) 45:2136–9. doi: 10.1109/TMAG.2009.2012703
25. Gellrich D, Becker S, Strieth S. Static magnetic fields increase tumor microvessel leakiness and improve antitumoral efficacy in combination with paclitaxel. Cancer Lett. (2014) 343:107–14. doi: 10.1016/j.canlet.2013.09.021
26. Tian X, Wang D, Zha M, Yang X, Ji X, Zhang L, et al. Magnetic field direction differentially impacts the growth of different cell types. Electromagn Biol Med. (2018) 37:114–25. doi: 10.1080/15368378.2018.1458627
27. Chen WF, Qi H, Sun RG, Liu Y, Zhang K, Liu JQ. Static magnetic fields enhanced the potency of cisplatin on k562 cells. Cancer Biother Radiopharm. (2010) 25:401–8. doi: 10.1089/cbr.2009.0743
28. Hao Q, Wenfang C, Xia A, Qiang W, Ying L, Kun Z, et al. Effects of a moderate-intensity static magnetic field and adriamycin on K562 cells. Bioelectromagnetics. (2011) 32:191–9. doi: 10.1002/bem.20625
29. Sun RG, Chen WF, Qi H, Zhang K, Bu T, Liu Y, et al. Biologic effects of SMF and paclitaxel on K562 human leukemia cells. Gen Physiol Biophys. (2012) 31:1–10. doi: 10.4149/gpb_2012_002
30. Ayse IG, Zafer A, Sule O, Isil IT, Kalkan T. Differentiation of K562 cells under ELF-EMF applied at different time courses. Electromagn Biol Med. (2010) 29:122–30. doi: 10.3109/15368378.2010.502451
31. Errico Provenzano A, Amatori S, Nasoni MG, Persico G, Russo S, Mastrogiacomo AR, et al. Effects of fifty-hertz electromagnetic fields on granulocytic differentiation of atra-treated acute promyelocytic leukemia nb4 cells. Cell Physiol Biochem. (2018) 46:389–400. doi: 10.1159/000488473
32. Martino CF, Portelli L, McCabe K, Hernandez M, Barnes F. Reduction of the Earth's magnetic field inhibits growth rates of model cancer cell lines. Bioelectromagnetics. (2010) 31:649–55. doi: 10.1002/bem.20606
33. Destefanis M, Viano M, Leo C, Gervino G, Ponzetto A, Silvagno F. Extremely low frequency electromagnetic fields affect proliferation and mitochondrial activity of human cancer cell lines. Int J Radiat Biol. (2015) 91:964–72. doi: 10.3109/09553002.2015.1101648
34. Wang T, Nie Y, Zhao S, Han Y, Du Y, Hou Y. Involvement of midkine expression in the inhibitory effects of low-frequency magnetic fields on cancer cells. Bioelectromagnetics. (2011) 32:443–52. doi: 10.1002/bem.20654
35. Crocetti S, Beyer C, Schade G, Egli M, Frohlich J, Franco-Obregon A. Low intensity and frequency pulsed electromagnetic fields selectively impair breast cancer cell viability. PLoS ONE. (2013) 8:e72944. doi: 10.1371/journal.pone.0072944
36. Zhang L, Ji X, Yang X, Zhang X. Cell type- and density-dependent effect of 1 T static magnetic field on cell proliferation. Oncotarget. (2017) 8:13126–41. doi: 10.18632/oncotarget.14480
37. Marzieh M, Javad B, Khadijeh S. The synergic effects of Crocus Sativus L. and low frequency electromagnetic field on VEGFR2 gene expression in human breast cancer cells. Avicenna J Med Biotechnol. (2014) 6:123–7.
38. Buckner CA, Buckner AL, Koren SA, Persinger MA, Lafrenie RM. Inhibition of cancer cell growth by exposure to a specific time-varying electromagnetic field involves T-type calcium channels. PLoS ONE. (2015) 10:e0124136. doi: 10.1371/journal.pone.0124136
39. Filipovic N, Djukic T, Radovic M, Cvetkovic D, Curcic M, Markovic S, et al. Electromagnetic field investigation on different cancer cell lines. Cancer Cell Int. (2014) 14:84. doi: 10.1186/s12935-014-0084-x
40. Buckner CA, Buckner AL, Koren SA, Persinger MA, Lafrenie RM. Exposure to a specific time-varying electromagnetic field inhibits cell proliferation via cAMP and ERK signaling in cancer cells. Bioelectromagnetics. (2018) 39:217–30. doi: 10.1002/bem.22096
41. Ren J, Ding L, Xu Q, Shi G, Li X, Li X, et al. LF-MF inhibits iron metabolism and suppresses lung cancer through activation of P53-miR-34a-E2F1/E2F3 pathway. Sci Rep. (2017) 7:749. doi: 10.1038/s41598-017-00913-2
42. Novikov VV, Novikov GV, Fesenko EE. Effect of weak combined static and extremely low-frequency alternating magnetic fields on tumor growth in mice inoculated with the Ehrlich ascites carcinoma. Bioelectromagnetics. (2009) 30:343–51. doi: 10.1002/bem.20487
43. Ali FM, El-Gebaly RH, Hamad AM. Combination of bacteriolytic therapy with magnetic field for Ehrlich tumour treatment. Gen Physiol Biophys. (2017) 36:259–71. doi: 10.4149/gpb_2016051
44. Chen Z, Liu H, Wang H, Wu C, Feng H, Han J. Effect of low-frequency rotary magnetic fields on advanced gastric cancer: Survival and palliation of general symptoms. J Cancer Res Ther. (2018) 14:815–9. doi: 10.4103/jcrt.JCRT_991_17
45. Foster KR. Thermal and non-thermal mechanisms of interaction of radio-frequency energy with biological systems. IEEE Trans Plasma Sci. (2000) 28:15–23. doi: 10.1109/27.842819
46. Zakaria Z, Rahim RA, Lee PY, Mansor MSB, Muji SZM. Review on interaction between electromagnetic field and biological tissues. Sens Transducers. (2012) 143:60–70.
47. Engstrom S. Physical mechanisms of non-thermal extremely-low-frequency magnetic-field effects. Ursi Radio Sci Bull. (2004) 77:95–106. doi: 10.23919/URSIRSB.2004.7909638
48. Golovin YI, Klyachko NL, Majouga AG, Sokolsky M, Kabanov AV. Theranostic multimodal potential of magnetic nanoparticles actuated by non-heating low frequency magnetic field in the new-generation nanomedicine. J Nanopart Res. (2017) 19:63. doi: 10.1007/s11051-017-3746-5
49. Yuan LQ, Wang C, Zhu K, Li HM, Gu WZ, Zhou DM, et al. The antitumor effect of static and extremely low frequency magnetic fields against nephroblastoma and neuroblastoma. Bioelectromagnetics. (2018) 39:375–85. doi: 10.1002/bem.22124
50. Fan Z, Hu P, Xiang L, Liu Y, Lu T. A static magnetic field inhibits the migration and telomerase function of mouse breast cancer cells. BioMed Res Int. (2020) 2020:1–9. doi: 10.1155/2020/7472618
51. Zhang L, Yang X, Liu J, Luo Y, Li Z, Ji X, et al. 1T moderate intensity static magnetic field affects Akt/mTOR pathway and increases the antitumor efficacy of mTOR inhibitors in CNE-2Z cells. Sci Bull. (2015) 60:2120–8. doi: 10.1007/s11434-015-0950-5
52. Nie Y, Du L, Mou Y, Xu Z, Weng L, Du Y, et al. Effect of low frequency magnetic fields on melanoma: tumor inhibition and immune modulation. BMC Cancer. (2013) 13:582. doi: 10.1186/1471-2407-13-582
53. Ahmadianpour MR, Abdolmaleki P, Mowla SJ, Hosseinkhani S. Static magnetic field of 6 mT induces apoptosis and alters cell cycle in p53 mutant Jurkat cells. Electromagn Biol Med. (2013) 32:9–19. doi: 10.3109/15368378.2012.692748
54. Akbarnejad Z, Eskandary H, Dini L, Vergallo C, Nematollahi-Mahani SN, Farsinejad A, et al. Cytotoxicity of temozolomide on human glioblastoma cells is enhanced by the concomitant exposure to an extremely low-frequency electromagnetic field (100Hz, 100G). Biomed Pharmacother. (2017) 92:254–64. doi: 10.1016/j.biopha.2017.05.050
55. Wang Z, Yang P, Xu H, Qian A, Hu L, Shang P. Inhibitory effects of a gradient static magnetic field on normal angiogenesis. Bioelectromagnetics. (2009) 30:446–53. doi: 10.1002/bem.20501
56. Buckner CA, Buckner AL, Koren SA, Persinger MA, Lafrenie RM. The effects of electromagnetic fields on B16-BL6 cells are dependent on their spatial and temporal character. Bioelectromagnetics. (2017) 38:165–74. doi: 10.1002/bem.22031
57. Vincenzi F, Targa M, Corciulo C, Gessi S, Merighi S, Setti S, et al. The anti-tumor effect of A3 adenosine receptors is potentiated by pulsed electromagnetic fields in cultured neural cancer cells. PLoS ONE. (2012) 7:e39317. doi: 10.1371/journal.pone.0039317
58. Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. (2017) 17:93–115. doi: 10.1038/nrc.2016.138
59. Cheung-Ong K, Giaever G, Nislow C. DNA-damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem Biol. (2013) 20:648–59. doi: 10.1016/j.chembiol.2013.04.007
60. Liu B, Liu L, Zang A, Song Z, Yang H, Wang Z, et al. Tanshinone IIA inhibits proliferation and induces apoptosis of human nasopharyngeal carcinoma cells via p53-cyclin B1/CDC2. Oncol Lett. (2019) 18:3317–22. doi: 10.3892/ol.2019.10658
61. Kim SC, Im W, Shim JY, Kim SK, Kim BJ. Static magnetic field controls cell cycle in cultured human glioblastoma cells. Cytotechnology. (2016) 68:2745–51. doi: 10.1007/s10616-016-9973-2
62. Canman CE, Wolff AC, Chen CY, Fornace AJ, Kastan MB. The p53-dependent G1 cell cycle checkpoint pathway and ataxia-telangiectasia. Cancer Res. (1994) 54:5054–8.
63. Marcantonio P, Del Re B, Franceschini A, Capri M, Lukas S, Bersani F, et al. Synergic effect of retinoic acid and extremely low frequency magnetic field exposure on human neuroblastoma cell line BE(2)C. Bioelectromagnetics. (2010) 31:425–33. doi: 10.1002/bem.20581
64. Luukkonen J, Hoyto A, Sokka M, Liimatainen A, Syvaoja J, Juutilainen J, et al. Modification of p21 level and cell cycle distribution by 50 Hz magnetic fields in human SH-SY5Y neuroblastoma cells. Int J Radiat Biol. (2017) 93:240–8. doi: 10.1080/09553002.2017.1235298
65. Mohammad RM, Muqbil I, Lowe L, Yedjou C, Hsu HY, Lin LT, et al. Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol. (2015) 35:S78–103. doi: 10.1016/j.semcancer.2015.03.001
66. Reubold TF, Eschenburg S. A molecular view on signal transduction by the apoptosome. Cell Signal. (2012) 24:1420–5. doi: 10.1016/j.cellsig.2012.03.007
67. Adrain C, Martin SJ. The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem Sci. (2001) 26:390–7. doi: 10.1016/S0968-0004(01)01844-8
68. Liam P, Grant N, Caitlin C, Matthew G, Greenwood MT. Anti-apoptosis and cell survival: a review. Biochim Biophys Acta. (2011) 1813:238–59. doi: 10.1016/j.bbamcr.2010.10.010
69. Jae-Kyun K, Kyoung-Han C, Zui P, Peihui L, Noah W, Chul-Woo K, et al. The tail-anchoring domain of Bfl1 and HCCS1 targets mitochondrial membrane permeability to induce apoptosis. J Cell Sci. (2007) 120(Pt. 16):2912–23. doi: 10.1242/jcs.006197
70. Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. (2000) 5:415–8. doi: 10.1023/A:1009616228304
71. Storch K, Dickreuter E, Artati A, Adamski J, Cordes N. BEMER electromagnetic field therapy reduces cancer cell radioresistance by enhanced ROS formation and induced DNA damage. PLoS ONE. (2016) 11:e0167931. doi: 10.1371/journal.pone.0167931
72. Kamalipooya S, Abdolmaleki P, Salemi Z, Javani Jouni F, Zafari J, Soleimani H. Simultaneous application of cisplatin and static magnetic field enhances oxidative stress in HeLa cell line. In Vitro Cell Dev Biol Anim. (2017) 53:783–90. doi: 10.1007/s11626-017-0148-z
73. Koh EK, Ryu BK, Jeong DY, Bang IS, Nam MH, Chae KS. A 60-Hz sinusoidal magnetic field induces apoptosis of prostate cancer cells through reactive oxygen species. Int J Radiat Biol. (2008) 84:945–55. doi: 10.1080/09553000802460206
74. Roos WP, Bernd K. DNA damage-induced cell death by apoptosis. Trends Mol Med. (2006) 12:440–50. doi: 10.1016/j.molmed.2006.07.007
75. Elisabetta C. Fine-tuning the ubiquitin code at DNA double-strand breaks: deubiquitinating enzymes at work. Front Genet. (2015) 6:282. doi: 10.3389/fgene.2015.00282
76. Kim J, Ha CS, Lee HJ, Song K. Repetitive exposure to a 60-Hz time-varying magnetic field induces DNA double-strand breaks and apoptosis in human cells. Biochem Biophys Res Commun. (2010) 400:739–44. doi: 10.1016/j.bbrc.2010.08.140
77. Lei H, Xu Y, Guan R, Li M, Hui Y, Gao Z, et al. Effect of gyromagnetic fields on human prostatic adenocarcinoma cells. Onco Targets Ther. (2015) 8:3489–97. doi: 10.2147/OTT.S95306
78. Yasuko K, Takao K, Raymond S, Seiji K. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. (2005) 5:726–34. doi: 10.1038/nrc1692
79. Boone BA, Zeh HJ III, Bahary N. Autophagy inhibition in pancreatic adenocarcinoma. Clin Colorectal Cancer. (2018) 17:25–31. doi: 10.1016/j.clcc.2017.10.013
80. Yu Y, Cao L, Yang L, Kang R, Lotze M, Tang D. microRNA 30A promotes autophagy in response to cancer therapy. Autophagy. (2012) 8:853–5. doi: 10.4161/auto.20053
81. Xu Y, Wang Y, Yao A, Xu Z, Dou H, Shen S, et al. Low frequency magnetic fields induce autophagy-associated cell death in lung cancer through miR-486-mediated inhibition of Akt/mTOR signaling pathway. Sci Rep. (2017) 7:11776. doi: 10.1038/s41598-017-10407-w
82. Nie Y, Chen Y, Mou Y, Weng L, Xu Z, Du Y, et al. Low frequency magnetic fields enhance antitumor immune response against mouse H22 hepatocellular carcinoma. PLoS ONE. (2013) 8:e72411. doi: 10.1371/journal.pone.0072411
83. Novoselova EG, Novikov VV, Lunin SM, Glushkova OV, Novoselova TV, Parfenyuk SB, et al. Effects of low-level combined static and weak low-frequency alternating magnetic fields on cytokine production and tumor development in mice. Electromagn Biol Med. (2019) 38:74–83. doi: 10.1080/15368378.2018.1545667
84. Zaalberg A, Moradi Tuchayi S, Ameri AH, Ngo KH, Cunningham TJ, Eliane JP, et al. Chronic inflammation promotes skin carcinogenesis in cancer-prone discoid lupus erythematosus. J Invest Dermatol. (2019) 139:62–70. doi: 10.1016/j.jid.2018.06.185
85. Demb J, Wei EK, Izano M, Kritchevsky S, Swede H, Newman AB, et al. Chronic inflammation and risk of lung cancer in older adults in the health, aging and body composition cohort study. J Geriatr Oncol. (2019) 10:265–71. doi: 10.1016/j.jgo.2018.07.008
86. Ross CL, Harrison BS. Effect of pulsed electromagnetic field on inflammatory pathway markers in RAW 264.7 murine macrophages. J Inflamm Res. (2013) 6:45–51. doi: 10.2147/JIR.S40269
87. Vergallo C, Dini L, Szamosvolgyi Z, Tenuzzo BA, Carata E, Panzarini E, et al. In vitro analysis of the anti-inflammatory effect of inhomogeneous static magnetic field-exposure on human macrophages and lymphocytes. PLoS ONE. (2013) 8:e72374. doi: 10.1371/journal.pone.0072374
88. Vincenzi F, Ravani A, Pasquini S, Merighi S, Gessi S, Setti S, et al. Pulsed electromagnetic field exposure reduces hypoxia and inflammation damage in neuron-like and microglial cells. J Cell Physiol. (2016) 232:1200–8. doi: 10.1002/jcp.25606
89. Shen LK, Huang HM, Yang PC, Huang YK, Wang PD, Leung TK, et al. A static magnetic field attenuates lipopolysaccharide-induced neuro-inflammatory response via IL-6-mediated pathway. Electromagn Biol Med. (2014) 33:132–8. doi: 10.3109/15368378.2013.794734
90. Harlozinska A. Progress in molecular mechanisms of tumor metastasis and angiogenesis. Anticancer Res. (2005) 25:3327–33.
91. Ferrara N, Gerber HJ. The biology of VEGF and its receptors. Nat Med. (2003) 9:669–76. doi: 10.1038/nm0603-669
92. Strelczyk D, Eichhorn ME, Luedemann S, Brix G, Dellian M, Berghaus A, et al. Static magnetic fields impair angiogenesis and growth of solid tumors in vivo. Cancer Biol Ther. (2014) 8:1756–62. doi: 10.4161/cbt.8.18.9294
93. Delle Monache S, Angelucci A, Sanita P, Iorio R, Bennato F, Mancini F, et al. Inhibition of angiogenesis mediated by extremely low-frequency magnetic fields (ELF-MFs). PLoS ONE. (2013) 8:e79309. doi: 10.1371/journal.pone.0079309
94. Cameron IL, Markov MS, Hardman WE. Optimization of a therapeutic electromagnetic field (EMF) to retard breast cancer tumor growth and vascularity. Cancer cell Int. (2014) 14:1–10. doi: 10.1186/s12935-014-0125-5
95. Cameron IL, Sun LZ, Short N, Hardman WE, Williams CD. Therapeutic electromagnetic field (TEMF) and gamma irradiation on human breast cancer xenograft growth, angiogenesis and metastasis. Cancer Cell Int. (2005) 5:23. doi: 10.1186/1475-2867-5-23
96. Le CC, Carlier MF. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev. (2008) 88:489–513. doi: 10.1152/physrev.00021.2007
97. Mo WC, Zhang ZJ, Wang DL, Liu Y, Bartlett PF, He RQ. Shielding of the geomagnetic field alters actin assembly and inhibits cell motility in human neuroblastoma cells. Sci Rep. (2016) 6:22624. doi: 10.1038/srep32055
98. Jalali A, Zafari J, Jouni FJ, Abdolmaleki P, Shirazi FH, Khodayar MJ. Combination of static magnetic field and cisplatin in order to reduce drug resistance in cancer cell lines. Int J Radiat Biol. (2019) 95:1194–201. doi: 10.1080/09553002.2019.1589012
99. Zhang K, Chen W, Bu T, Qi H, Sun R, He X. Decreased P-glycoprotein is associated with the inhibitory effects of static magnetic fields and cisplatin on K562 cells. Bioelectromagnetics. (2014) 35:437–43. doi: 10.1002/bem.21863
100. Pasi F, Fassina L, Mognaschi ME, Lupo G, Corbella F, Nano R, et al. Pulsed electromagnetic field with temozolomide can elicit an epigenetic pro-apoptotic effect on glioblastoma T98G cells. Anticancer Res. (2016) 36:5821–6. doi: 10.21873/anticanres.11166
101. Chen WT, Lin GB, Lin SH, Lu CH, Hsieh CH, Ma BL, et al. Static magnetic field enhances the anticancer efficacy of capsaicin on HepG2 cells via capsaicin receptor TRPV1. PLoS ONE. (2018) 13:e0191078. doi: 10.1371/journal.pone.0191078
102. Han Q, Chen R, Wang F, Chen S, Sun X, Guan X, et al. Pre-exposure to 50 Hz-electromagnetic fields enhanced the antiproliferative efficacy of 5-fluorouracil in breast cancer MCF-7 cells. PLoS ONE. (2018) 13:e0192888. doi: 10.1371/journal.pone.0192888
103. Wen J, Jiang S, Chen B. The effect of 100 Hz magnetic field combined with X-ray on hepatoma-implanted mice. Bioelectromagnetics. (2011) 32:322–4. doi: 10.1002/bem.20646
104. Wen J, Jiang S, Chen Z, Zhao W, Yi Y, Yang R, et al. Apoptosis selectively induced in BEL-7402 cells by folic acid-modified magnetic nanoparticles combined with 100 Hz magnetic field. Int J Nanomed. (2014) 9:2043–50. doi: 10.2147/IJN.S60457
105. Ju H, Cui Y, Chen Z, Fu Q, Sun M, Zhou Y. Effects of combined delivery of extremely low frequency electromagnetic field and magnetic Fe3O4 nanoparticles on hepatic cell lines. Am J Transl Res. (2016) 8:1838–47.
106. Spyridopoulou K, Makridis A, Maniotis N, Karypidou N, Myrovali E, Samaras T, et al. Effect of low frequency magnetic fields on the growth of MNP-treated HT29 colon cancer cells. Nanotechnology. (2018) 29:175101. doi: 10.1088/1361-6528/aaaea9
107. Sengupta S, Khatua C, Balla VK. In vitro carcinoma treatment using magnetic nanocarriers under ultrasound and magnetic fields. ACS Omega. (2018) 3:5459–69. doi: 10.1021/acsomega.8b00105
Keywords: magnetic fields, anti-tumor, molecular mechanism, static magnetic fields, low-frequency magnetic fields
Citation: Xu A, Wang Q, Lv X and Lin T (2021) Progressive Study on the Non-thermal Effects of Magnetic Field Therapy in Oncology. Front. Oncol. 11:638146. doi: 10.3389/fonc.2021.638146
Received: 05 December 2020; Accepted: 08 February 2021;
Published: 17 March 2021.
Edited by:
Benjamin Frey, University Hospital Erlangen, GermanyReviewed by:
Michael Hader, University Hospital Erlangen, GermanyCopyright © 2021 Xu, Wang, Lv and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingting Lin, dHRsaW5Aamx1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.