- 1Medical Oncology Unit, University Hospital and University of Cagliari, Cagliari, Italy
- 2Oncologia Medica, Università Vita-Salute, IRCCS Ospedale San Raffaele, Milano, Italy
Targeting tumor-driven angiogenesis is an effective strategy in the management of metastatic colorectal cancer (mCRC); however, the choice of second-line therapy is complicated by the availability of several drugs, the occurrence of resistance and the lack of validated prognostic and predictive biomarkers. This review examines the use of angiogenesis-targeted therapies for the second-line management of mCRC patients. Mechanisms of resistance and anti-placental growth factor agents are discussed, and the role of aflibercept, a recombinant fusion protein consisting of portions of human vascular endothelial growth factor receptor (VEGFR)-1 and VEGFR-2, is highlighted. The novel mechanism of action of aflibercept makes it a useful second-line agent in mCRC patients progressing after oxaliplatin-based chemotherapy, as well as in those with resistance after bevacizumab.
Introduction
Colorectal cancer (CRC) is the fourth most common tumor and it stands at the second place for cancer death worldwide (1). Almost 20% of CRC patients are diagnosed at an advanced stage and approximately a further 20% develop metastases later in life (2). Nowadays, it is well known that tumor-driven angiogenesis plays a crucial role in CRC growth and metastatic spread (3–15).
Tumor angiogenesis leads to the formation of new blood vessels through a very complex and coordinated process (16–19). Physiologic angiogenesis is a well-controlled event, regulated via the balance of pro- and antiangiogenic factors (19). Among these, the vascular endothelial growth factor (VEGF) signaling represents a key pathway and it comprises a family of five secreted proteins [VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF)] and three receptor tyrosine kinases (RTK): VEGF receptor (VEGFR)-1, VEGFR-2, and VEGFR-3 (19, 20). VEGFR-1 [also named Fms-like tyrosine kinase-1 (FLT1)] and VEGFR-3 [also named Fms-related tyrosine kinase 4 (FLT4)] have been shown to be involved in tumor progression and metastasis in CRC, and VEGFR-2 (also named fetal liver kinase FLK1/KDR) has been implicated in endothelial cell survival, proliferation and migration (21, 22). Furthermore, PlGF induces angiogenesis via several mechanisms, both directly and indirectly (23–25). Consequently, angiogenesis is one of the most important therapeutic targets for the treatment of metastatic CRC (mCRC) and a consistent number of agents targeting the angiogenesis pathway have been developed and are now available for mCRC patients across different lines of treatment (2, 26).
The aim of this review is to explore the role of anti-angiogenesis strategies in the second-line management of mCRC, including mechanisms of resistance and the use of anti-PlGF agents. In particular, individualized treatment is discussed. Moreover, the role of aflibercept, a recombinant fusion protein consisting of portions of human VEGFR-1 and -2 (27), is emphasized, in order to discuss the results and potential benefits showed by this innovative agent with a unique mechanism of action.
Management of mCRC: Targeting the Angiogenesis in the Second-Line Setting
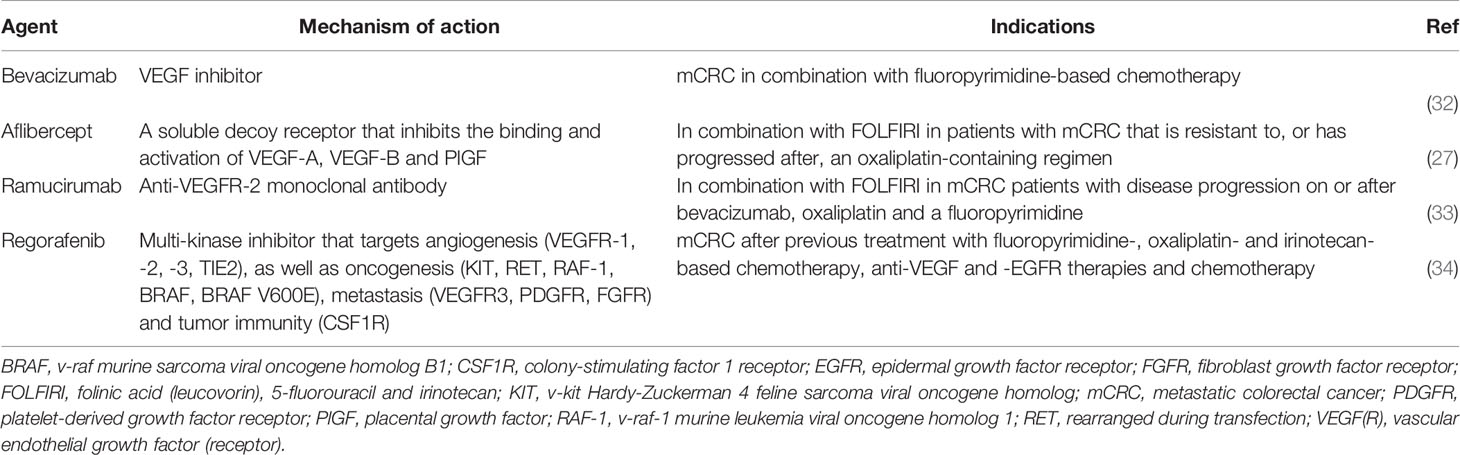
Medical treatment of mCRC is mainly palliative, aimed at slowing disease progression, prolonging survival and maintaining quality of life (QoL), even if in some specific cases the curative aim cannot be excluded (e.g. single resectable metastatic site) (28). Antiangiogenic agents are among the most effective drugs for the treatment of mCRC, both in first and second-line setting; they are recommended in combination with fluoropyrimidine-based chemotherapy plus oxaliplatin and/or irinotecan (29–31). Antiangiogenic agents currently approved for the treatment of mCRC in Europe are shown in Table 1.
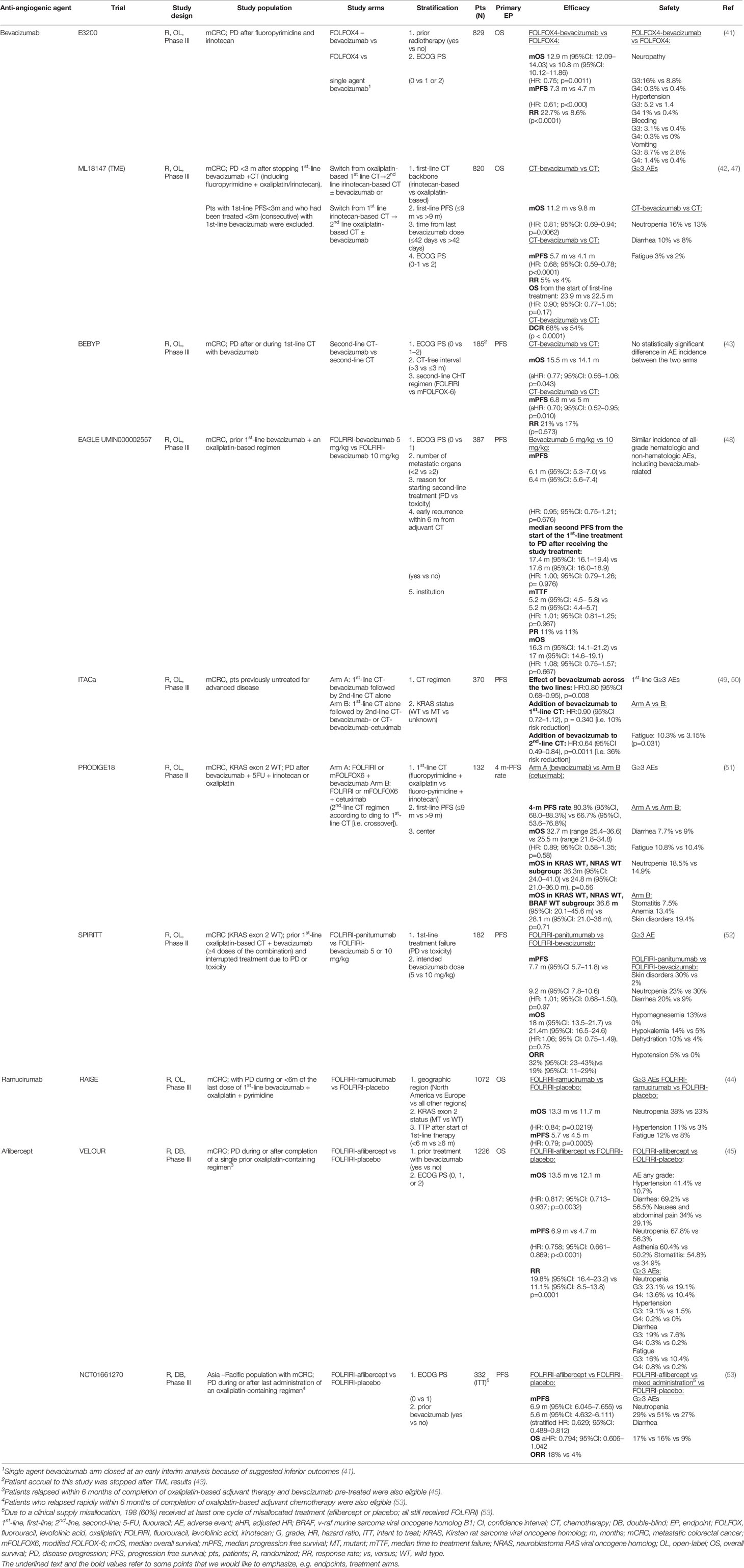
The choice of second-line treatment is individually tailored depending the therapeutic scheme received in the first-line setting (29, 31, 35–38) and its outcome and how well it was tolerated, patient fitness and clinical characteristics plus their tumor biologic and molecular features, especially rat sarcoma viral oncogene homolog (RAS)/v-raf murine sarcoma viral oncogene homolog B1 (BRAF) mutation status (2, 29, 31, 39, 40). Second-line approved angiogenesis-targeted options include bevacizumab, aflibercept and ramucirumab (41–46), which all demonstrated an increase in overall survival (OS) in the second-line setting in phase III trials (Table 2). Unfortunately, these trials included different patient populations and no head-to-head randomized phase III trials have been conducted to compare these three agents (54). Moreover, no randomized studies have been conducted to evaluate the best treatment sequence after first-line anti-EGFR and anti-VEGF (2, 39, 40).
Bevacizumab is a monoclonal antibody (mAb) targeting anti-VEGF-A; its role in the second-line setting evaluated both in the bevacizumab-naïve and bevacizumab-pretreated settings, and both in RAS wild type (WT) and mutant type (MT) patients. Treatment sequence strategies with bevacizumab as second-line treatment after first-line bevacizumab include maintenance therapy versus “stop and go” treatment, which were investigated in several studies (CAIRO3, AIO 0207, PRODIGE 9, OPTIMOX2 and SAKK 41/06 studies), a meta-analysis (including OPTIMOX2, CAIRO3 and AIO 0207 trials) (55), and a pooled analysis of randomized phase III trials (56). Maintenance therapy was associated with significantly improved time to failure (hazard ratio (HR) 0.79; 95% confidence interval (CI) 0.7–0.9, p=0.0005) (56) and progression-free survival (PFS) than “stop and go” [meta-analysis HR 0.53, 95%CI 0.40–0.69 (55); pooled analysis HR 0.56; 95%CI 0.44–0.71, p<0.00001 (56)]. Based on these data, the maintenance strategy appears appealing and aims to reduce the occurrence of oxaliplatin-induced neuropathy, although clinicians should expect to encounter other toxicities that are likely to eventually develop with such an approach, including hypertension and proteinuria.
Other treatment sequencing strategies investigated with bevacizumab include first-line chemotherapy plus bevacizumab followed by second-line chemotherapy alone (Arm A), which was compared with first-line chemotherapy alone followed by second-line chemotherapy plus bevacizumab (with or without cetuximab, according to KRAS status, Arm B) in the ITACa trial (49, 50) (Table 2). However, results from this study should be interpreted with caution because of significant study limitations, including slow and poor recruitment, a change in the primary objective (from OS to PFS), the high proportion of patient withdrawals and consequently a lack of patients entering into second-line treatment with or without bevacizumab, thus affecting the treatment duration. Indeed, patients in Arm A received a median of 12 chemotherapy cycles [range 1–43, interquartile range (IQR) 6–16] and among the 45 patients who received bevacizumab maintenance, treatment duration was restricted to a median of 6 cycles (range 1–30, IQR 3–13).
Ramucirumab is a fully human immunoglobulin (Ig) G-1 mAb which binds to the VEGFR-2 extracellular domain; it has been evaluated in combination with folinic acid, fluorouracil (5-FU), and irinotecan (FOLFIRI) as second-line treatment in patients pretreated with oxaliplatin, fluoropyrimidine, and bevacizumab (44) (Table 2).
Aflibercept, also known as VEGF-Trap, was evaluated in combination with FOLFIRI in the second-line setting in the prospective, international, randomized, double-blind, parallel-group phase III VELOUR study (45, 46) and in a phase III trial in an Asian patient population by Li and colleagues (53) (Table 2). Currently, aflibercept can be administered only in combination with a FOLFIRI backbone; mFOLFOX6 is an unsuitable backbone (no significant improvement in PFS, and very high toxicity, when administered in combination with mFOLFOX6 in the first-line setting in the AFFIRM trial) (57).
There is only evidence from phase I and II trials (58, 59) and no evidence from randomized studies for the use of aflibercept with capecitabine-based or 5-FU bolus-based treatment.
Resistance Mechanisms
There are couple of known mechanisms whereby resistance to VEGF-targeted therapies can develop. Indeed, in most patients, despite prolonged anti-angiogenic treatment and VEGF-A blockade, angiogenesis is re-established. Anti-angiogenic agents also act by normalizing the blood vessel texture, which is convoluted and dysfunctional in cancer, thus increasing blood flow and suggesting that the administration of an anti-angiogenic drug plus chemotherapy increases the delivery of the chemotherapeutic agent to the cancer tissue (60). Preclinical studies showed the tumor capability to develop compensatory mechanisms leading to restoration of high vessel density and consequently cancer growth; this phenomenon appears related to hypoxia-triggered upregulation of alternative pro-angiogenic factors (19). The biomarker landscape in mCRC is evolving (61), but unfortunately to date, no biomarkers to define which patients will respond to anti-angiogenic treatment and who will develop resistance have been validated yet, although several have been investigated (tissue-based or circulating).
Various angiogenesis-related single nucleotide polymorphisms (SNPs) in the VEGF gene have been studied in mCRC (62–64). In 46 patients receiving first-line bevacizumab-based therapy, the CC genotype of rs3025039 polymorphism of VEGF-Ac.*237C>T was significantly associated with time to treatment failure; patients with at least one T allele had worse OS and PFS (64). Conversely, VEGF-A rs699947 A/A allele was associated with increased PFS and OS and the ICAM-1 rs1799969 G/A allele was correlated with longer OS (64). Worse OS with bevacizumab regimens was observed in patients with CD133 CC genotype in the rs3130 (65). Shorter OS and PFS with chemotherapy plus bevacizumab were reported for VEGFR1 rs9513070/rs9554320/rs9582036 GCA haplotype* (66) and BMAL1 SNPs (rs7396943, rs7938307, rs2279287) (67). An interleukin (IL) 8 polymorphism (c.-251TA+AA) (39) and the IL-6 rs2069837 G allele were associated with worse PFS with bevacizumab-based therapies (40). In the TME trial (ML18147), SNP analysis found a correlation between OS and VEGFA, VEGFR2 and EGLN1 SNPs; moreover, a correlation between PFS and VEGFA, VEGFR1, VEGFR2, EGLN3 SNPs was observed (68). Five HIF2α SNPs were associated with bevacizumab treatment effect.
Angiogenesis genotyping by Giampieri and colleagues in 138 mCRC patients treated with regorafenib, found that the VEGF-A rs2010963 SNP maintained an independent correlation with PFS and OS (69). Recent evidence suggests micro-RNA(miRNA) might modulate tumor angiogenesis by targeting anti- and pro-angiogenic factors, including hypoxia inducible factor (HIF), VEGF, and EGF. miR-23a-3p was reported as a key regulator of IL-17C-induced tumor angiogenesis in CRC (70), and high expression of miRNA-126 is related to increased VEGF-A signaling in endothelial cells and might be a promising biomarker for anti-angiogenic therapies (71). Nevertheless, data on the predictive role of miRNA remain preliminary.
Resistance to bevacizumab can result from the development of alternative angiogenesis pathways (72, 73). This induced pro-angiogenic factor substitution via activation and/or upregulation of members of the fibroblast growth factor (FGF) family, notch signaling, ephrin, or angiopoietin-1 re-establishes tumor neovascularization, resulting in tumor relapse (72, 73). In some patients, the angiogenic factor profile is different before the occurrence of progressive disease (PD) than that observed at the time of radiographic progression (74). Indeed, elevated levels of pro-angiogenic cytokines [e.g., basic FGF (bFGF)] placental growth factor (PlGF), hepatocyte growth factor (HGF)], have been demonstrated in subsets of patients prior to radiographic PD, with the suggestion that compensatory angiogenic factors may be stimulating new vessel growth in preparation for clinically evident progression (74). In such patients, second-line treatment with alternative anti-angiogenic therapies, rather than bevacizumab continuation, would be more beneficial (74).
Several circulating biomarker studies have been performed to evaluate the role of angiogenic factors other than VEGF-A in patients treated with anti-angiogenic agents (Table 3) (43–45, 75–82). Recently, the role of PlGF emerged as another potential crucial factor involved in anti-angiogenic agent resistance. PlGF was initially considered only as an indirect actor of angiogenesis, more specifically as a competitor for VEGF-A to bind VEGFR-1 and soluble VEGFR-1 (sVEGFR-1) thus increasing the availability of VEGF-A to bind and activate VEGFR-2 (83). Conversely, PIGF can also directly induce angiogenesis through regulation of the crosstalk between VEGFR-1 and VEGFR-2, amplification of the VEGF-A signal through VEGFR-2 activation, enhancement of the angiogenic signal through the activation of the VEGFR-1/VEGFR-2 heterodimers via VEGF/PlGF heterodimers, impairment of dendritic cell maturation leading to immune suppression and promotion of the metastatic process by recruiting pro-angiogenic progenitor cells from the bone marrow to the tumor and the pre-metastatic niche, with consequent proliferation of metastatic cells (23–25). Various studies reported an increase of PlGF in the development of resistance, despite an initial decrease of VEGF-A levels (77, 79, 84), thus suggesting its potential role in tumor resistance. In a study by Lieu and colleagues, PlGF and VEGF-D were associated with resistance to bevacizumab-containing chemotherapy in mCRC (78). In mouse models targeting PIGF lead to a reduction of tumor growth (85, 86). Upregulation of PlGF appears as one of the main mechanism of resistance to angiogenesis-blockade and as a consequence, is a crucial potential therapeutic target for mCRC patients who have progressed on VEGF or VEGFR inhibitors (19). Bevacizumab targets only VEGF-A, preventing its interaction with VEGFR, so redundancy in angiogenesis pathways leads to treatment resistance via PlGF and VEGF-D. In contrast, aflibercept leads to trapping of VEGF-A, B, and PlGF-1 and PlGF-2, preventing VEGF and PlGF from binding to their native receptors, thus providing a more complete and efficient blockade of angiogenesis and its resistance strategies. Moreover, by inhibiting the upregulation of compensatory angiogenic factors, aflibercept may inhibit immune cell recruitment and further metastatic spread (28, 82). A post hoc biomarker analysis of the VELOUR trial assessed the impact of prior bevacizumab treatment and VEGF-A and PlGF levels on outcomes following second-line FOLFIRI-aflibercept treatment (Table 3) (82). In the FOLFIRI-aflibercept group, patients achieved prolonged OS and PFS irrespective of baseline VEGF-A and PlGF levels. So, aflibercept may provide benefit in patients with high VEGF-A or PlGF serum levels (82).
No definitive conclusions can be drawn on the plethora of data relating to putative biomarkers, and resistance mechanisms remain a multifactorial and challenging issue in mCRC.
Individualized Treatment
While selected biomarker testing is now standard practice in CRC, the usefulness of other potential predictive and prognostic markers in clinical practice is unclear and still under evaluation, prompting the need for clear evidence-based recommendations. Currently, treatment algorithms, such as those developed by European Society for Medical Oncology and the Italian Medical Oncology Association (29, 31), provide guidance for the management of patients with mCRC according to RAS/BRAF status [wild type (WT) or mutant (MT)] and prior treatment.
Second-Line Treatment for RAS MT mCRC Patients: Anti-Angiogenesis Beyond Progression
The continuation of anti-angiogenic blockade is now a standard option for mCRC patients who showed PD after first-line treatment with bevacizumab. Preclinical data suggested continuous expression of VEGF at PD occurrence and, as a consequence, a prolonged exposure to anti-angiogenic drugs might delay tumor growth (87). Some studies indicate that longer duration of anti-angiogenic treatment may lead to improved outcomes, whereas early discontinuation after first line chemotherapy could results in “tumor rebound” or the occurrence of more aggressive PD. Based on this rationale, anti-angiogenic blockade might continue to be effective even when tumor cells develop resistance to chemotherapy, while interruption of the anti-angiogenic inhibition could result in detrimental effects (39, 40). An exploratory analysis of the ML18147 trial assessed study outcomes according to Kirsten RAS oncogene (KRAS) status (47). Overall, 300/820 patients (49%) had KRAS MT tumors. In this group, mPFS was 5.5 months for patients receiving chemotherapy plus bevacizumab and 4.1 months for patients receiving chemotherapy only (HR: 0.70; 95% CI: 0.56–0.89; p = 0.0027), and median OS (mOS) was 10.4 versus 10.0 months, respectively (HR: 0.92; 95% CI: 0.71–1.18; p = 0.4969). In both analyses, no treatment interaction by KRAS status was observed (mPFS, p = 0.4436; mOS, p = 0.1266) suggesting that bevacizumab continuation might be an option beyond first progression, irrespective of KRAS status (47). Nevertheless, it is important to keep in mind that this trial excluded patients with aggressive disease (PD <3 months after the last bevacizumab administration, first-line PFS was <3 months, bevacizumab given for <3 months [consecutive] in the first-line setting) (42).
A post hoc analysis of the RAISE trial evaluated the association of RAS mutational status with outcomes. A favorable and comparable ramucirumab treatment effect was observed both for RAS MT (median OS 12.9 months with FOLFIRI-ramucirumab versus 11.5 months with FOLFIRI-placebo, HR: 0.86; 95% CI: 0.71–1.04, p = 0.1110; median PFS 5.7 months with FOLFIRI-ramucirumab versus 4.3 months with FOLFIRI-placebo, HR: 0.81; 95% CI: 0.68–0.97, p = 0.0209) and RAS/BRAF WT tumors (median OS 16.2 months with FOLFIRI-ramucirumab versus 15.5 months with FOLFIRI-placebo, HR: 0.86; 95% CI: 0.64–1.14, p = 0.2899; median PFS 5.7 months with FOLFIRI-ramucirumab and with FOLFIRI-placebo, HR: 0.78; 95% CI: 0.6–11.0, p = 0.0512). Treatment-by-mutation status interaction tests (OS, p = 0.523; PFS, p = 0.655) indicated that the ramucirumab benefit was not statistically different among the mutation sub-groups (88). As specified in the study design and the inclusion criteria, the RAISE trial enrolled patients progressing after first-line treatment with oxaliplatin and bevacizumab, so the efficacy and safety of the anti-angiogenic sequence bevacizumab-ramucirumab was established right in the phase III pivotal trial.
Wirapati and colleagues evaluated the impact of RAS, BRAF and sidedness on aflibercept activity in mCRC patients enrolled in the VELOUR study; next generation sequencing (NGS) data on molecular status were available for 482 of 1226 patients, and 264 patients had RAS MT disease (89). The treatment effects on OS for the 482 patients was confirmed significant (HR: 0.80; 95% CI: 0.65–0.99), and similar to the intention-to-treat (ITT) results (HR: 0.82; 95% CI: 0.71–0.93). RAS MT patients receiving FOLFIRI-aflibercept had an OS of 12.6 versus 11.2 months for those receiving FOLFIRI-placebo (HR: 0.93; 95% CI: 0.70–1.23 p = 0.13). None of the mutation subgroup results showed significant interaction, and sidedness didn’t influence efficacy (89).
Thus, aflibercept, bevacizumab and ramucirumab show potential benefit in the treatment of RAS MT mCRC patients. Bevacizumab and aflibercept have been compared in this setting in an Italian, real-world, retrospective, single-center, non-randomized study (90). Seventy-four RAS MT mCRC patients whose disease had progressed after first-line treatment with FOLFOX-bevacizumab received second-line FOLFIRI-bevacizumab (arm A) or FOLFIRI-aflibercept (arm B). The two regimens appeared equally effective; despite a longer mOS observed for the combination of FOLFIRI-aflibercept, statistical significance was not reached (12.1 vs 8.9 months; HR: 1.02; 95% CI: 0.57–1.84). The study presented several biases which might have influenced results: in the FOLFIRI-aflibercept arm patients had a more extensive disease (>2 metastatic sites), a significant shorter duration of first-line treatment, and no maintenance treatment was allowed (90). Also, given the retrospective nature of the research, these data should be interpreted with extreme caution.
Second-Line Treatment for RAS WT mCRC Patients – Focus on Sequence Anti-EGFR – Anti-Angiogenic Agents
All anti-angiogenic agents approved in second-line setting demonstrated their efficacy in RAS WT patients (47, 88, 89, 91), regardless of prior treatment with anti-angiogenic drugs. The administration of two consecutive lines including an anti-angiogenic agent has already been discussed in section 4.1 (both in RAS MT and WT patients); for these patients, the subsequent use of an anti-EGFR mAb in further lines remains an option.
Currently, second-line options for RAS WT mCRC patients progressing after first-line chemotherapy and an anti-EGFR mAb include bevacizumab and aflibercept (29–31, 92). To date, limited data are available on mCRC patients receiving aflibercept after an anti-EGFR based-treatment and no head-to-head comparative trials have been conducted to assess which is the best anti-angiogenic agent in this specific treatment setting. As a consequence, and clinical practice is essentially based on speculations deriving from first-line studies (93, 94). The retrospective SLAVE study evaluated the effectiveness of second-line bevacizumab-based or aflibercept-based regimens in 277 RAS WT mCRC patients progressing after a first-line anti-EGFR based treatment in a multicenter real-world cohort (95). No statistically significant difference between patients receiving bevacizumab-based and those receiving aflibercept-based regimens was observed in univariate analyses of objective response rate (ORR), PFS (HR: 1.34; 95% CI: 0.95–1.89; p = 0.0932) and OS (HR: 1.31; 95% CI: 0.89–1.93; p = 0.1600). As for multivariate analysis, after adjusting for the key covariates (age, gender, performance status, number of metastatic sites, and primary tumor side) bevacizumab-based regimens had slightly longer PFS than aflibercept-based regimens (HR: 1.44; 95% CI: 1.02–2.03; p = 0.0399), whereas no significant difference in OS was observed (HR: 1.47; 95% CI: 0.99–2.17; p = 0.0503). These data must be considered with caution due to the retrospective nature of the study, therefore no definitive conclusions can be drawn (95). In another retrospective study, Vera and colleagues analyzed the efficacy and safety of second-line FOLFIRI-aflibercept in RAS WT mCRC patients resistant to, or who had progressed after, an oxaliplatin plus anti-EGFR regimen (96). PFS was 6.9 months (95% CI: 6.1–7.8), the ORR was 33% and mOS was 14.5 months (95% CI: 9.7–19.3). As for safety, 37.5% of the patients reported grade 3–4 toxicities (hematologic 16.6%, hypertension 7.5%, asthenia 5.9%, and perforation 2.5%). Though retrospective, these results were consistent with the VELOUR trial, showing FOLFIRI-aflibercept efficacy was maintained irrespective of prior anti-EGFR treatment, thus suggesting a role for this regimen also in this population (96). The ongoing, prospectively stratified, biologically enriched, multicenter, phase II DISTINCTIVE study is assessing the efficacy of aflibercept in combination with FOLFIRI in the second-line treatment of RAS WT mCRC patients who received first-line oxaliplatin in combination with an anti-EGFR mAb (either panitumumab or cetuximab); one of the study aims is to prospectively validate VEGFR2 plasma levels as a predictive factor for efficacy of aflibercept in combination with FOLFIRI (97).
In the FIRE-3/AIO KRK0306 trial, evidence seemed to suggest that in WT patients the sequence anti-EGFR–anti-angiogenic might lead to more favorable results than the reverse sequence. Indeed, both PFS (6.5 vs 4.7 months; HR: 0.68; 95% CI: 0.54–0.85; p < 0.001) and OS (16.3 vs 13.2 months; HR: 0.70; 95% CI: 0.55–0.88; p = 0.0021) from start of second-line treatment were longer in patients treated with oxaliplatin-based chemotherapy plus bevacizumab after FOLFIRI plus cetuximab versus oxaliplatin-based chemotherapy plus cetuximab or panitumumab after FOLFIRI plus bevacizumab (HR: 0.95; 95% CI: 0.55–1.63; p = 0.841). In the CALGB/SWOG 80405 trial comparing first-line therapy with cetuximab vs bevacizumab plus mFOLFOX6 or FOLFIRI, 88% of patients received subsequent therapy, but no detailed or specific information on second-line treatment is available yet to determine the impact of treatment sequence on survival parameters. Further data from larger prospective trials in RAS WT mCRC patients focused on second-line treatment are needed to assess the best treatment option in this setting. Indeed, to date, the major of data are derived from patients enrolled in the CALGB/SWOG 80405 and FIRE-3 trials and who had received second-line treatment; no prospective specific second-line studies have been performed in this patient setting.
Second-Line Treatment for BRAF MT mCRC Patients
BRAF mutation is present in 5–10% of mCRC patients and its correlation with poor prognosis widely known (98, 99). Unfortunately, survival after disease progression on first-line chemotherapy is markedly shorter in BRAF MT than WT patients (4.2 vs 9.2 months, adjusted HR: 1.69; p < 0.001), and fewer MT patients go on to receive second-line therapy (33% vs 51%), either because they are ineligible (unfit) or because their disease progresses so rapidly (100). In the exploratory analysis of the ML18147 trial, only 14 patients had a BRAF mutation (7%, 6 patients in the bevacizumab plus chemotherapy group and 8 in the control group) and due to the very small number of BRAF MT subjects no correlative analysis could be carried out (47). In the post hoc analysis of the RAISE trial, 41 patients (4.5%) were BRAF MT. Ramucirumab-treated BRAF MT patients showed a promising trend in OS and PFS benefit with ramucirumab over placebo, with mOS and mPFS more than double that of placebo (mOS, 9.0 vs 4.2 months; HR: 0.54; 95% CI: 0.25–1.13; mPFS, 5.7 vs 2.7 months; HR: 0.55; 95% CI: 0.28–1.08). BRAF MT mCRC patients had worse survival than RAS/BRAF WT regardless of treatment, confirming BRAF as a negative prognostic factor also in the second-line setting. The sample size was too small to draw definitive conclusions on real difference of ramucirumab effect in the BRAF MT patients versus the RAS/BRAF WT or RAS MT, and requires further validation (88). Even if none of the mutation subgroups demonstrated significant interaction and though limited by the small sample size, the VELOUR analysis showed also a promising trend for better outcome with aflibercept and FOLFIRI for BRAF MT mCRC patients in OS, PFS and RR. Globally, 36 patients harboring BRAF MT were evaluated; mOS was 10.3 months in the FOLFIRI-aflibercept group versus 5.5 months in the control group (HR: 0.42; 95% CI: 0.16–1.09; p = 0.08) and mPFS was 5.5 versus 2.2 months (HR: 0.59; 95% CI: 0.22–1.58) (89, 96). Gelsomino and colleagues conducted a pooled analysis with the aim to assess the impact of anti-angiogenic drugs in patients with pre-treated BRAF MT mCRC. The analysis included patients enrolled in randomized, controlled trials who received second-line chemotherapy plus either antiangiogenic agents, namely ramucirumab or aflibercept, or placebo. The results were then pooled with the data and outcomes of BRAF MT patients enrolled in TRIBE and TRIBE-2 study who had received either second-line chemotherapy plus bevacizumab or chemotherapy alone. This analysis included 129 patients and confirmed a significant advantage of anti-angiogenic drugs compared to placebo in terms of OS (HR: 0.50; 95% CI: 0.29–0.85; p = 0.01) in pre-treated BRAF MT mCRC patients.
Recently, the randomized, phase III, open-label BEACON CRC trial assessed the superiority in OS, ORR and patient-reported QoL of encorafenib plus cetuximab with binimetinib (triplet arm) or without binimetinib (doublet arm) versus either cetuximab-irinotecan or cetuximab-FOLFIRI (control arm) in BRAF V600E MT mCRC patients with PD after one (65%) or two previous regimens. Respective ORRs were 26.8% (95% CI: 21.1–33.1), 19.5% (95% CI: 14.5–25.4), and 1.8% (95% CI: 0.5–4.6). mOS was 9.3 months (95% CI: 8.2–10.8) in the triplet arm and 5.9 months (95% CI: 5.1–7.1) in the control arm (HR: 0.60; 95% CI: 0.47–0.75). mOS in the doublet arm was 9.3 months (95% CI: 8.0–11.3; HR vs control: 0.61; 95% CI: 0.48–0.77) (101–103).
Targeted therapy with encorafenib plus cetuximab is now an established second-line strategy for this subgroup of patients. The combination of chemotherapy plus bevacizumab, ramucirumab or aflibercept represents an alternative option, even if none of the chemotherapy-based combinations have been formally compared with encorafenib plus cetuximab (104, 105).
In the era of precision medicine and target-tailored treatment, on the basis of these recent findings in BRAF MT mCRC patients, the anti-angiogenic second-line approach and its direct comparison with second-line target doublet or triplet in this specific population surely requires further research.
Focus on Aflibercept
Aflibercept, an innovative anti-angiogenic agent, is a recombinant fusion protein containing VEGF-binding portions from the extracellular domains of human VEGFR 1 and 2, fused to the Fc portion of human IgG1. Targeting VEGF-A, VEGF-B, and PlGF with high-affinity, aflibercept prevents these ligands from binding to their endogenous receptors and thus providing a wider pharmacologic blockade of the VEGF pathway (28, 45, 46). In this way, aflibercept is able to overcome biologic mechanisms of resistance occurring during previous angiogenesis blockade (28). In this section, we focus specifically on this innovative agent.
VELOUR Trial – Exploratory and Post Hoc Analysis
Further analyses of the VELOUR study have been conducted to assess the efficacy and safety of aflibercept in specified populations and according to the molecular profile of mCRC.
Survival
An integrated analysis of the time courses of both the efficacy and safety of aflibercept plus FOLFIRI confirmed a continued and persistent OS increase over time. Indeed, mOS improved progressively to 2.6 months at 18 months and 4.4 months at 24 months. The estimated probabilities of survival were 38.5% versus 30.9% at 18 months, 28.0% versus 18.7% at 24 months and 22.3% versus 12.0% at 30 months for patients receiving FOLFIRI-aflibercept versus those receiving FOLFIRI-placebo, respectively, with a consistent proportional improvement in the HR over time; survival at 24 months was improved by 50% and almost doubled at 30 months. Notably, survival results were not influenced by post-VELOUR anti-cancer treatments. As for safety, even if most chemotherapy- and anti-VEGF-related grade 3–4 adverse events (AEs) were more common in patients receiving FOLFIRI-aflibercept versus FOLFIRI-placebo, they were reported within the first four treatment cycles, were mostly reversible and of single occurrence and decreased over further cycles. This information provides useful data to anticipate and treat drug-related toxicities (106).
Van Cutsem and colleagues conducted a post hoc survival analysis after the exclusion of patients who had disease recurrence during or within 6 months of completing adjuvant oxaliplatin-based therapy, namely the adjuvant rapid relapsers (ARR) (10% of patients; n = 124, including 17 patients who also received bevacizumab in the adjuvant setting) (46). Results showed that OS in the ITT minus ARR (ITT-ARR) population (n = 1102) was longer in the experimental arm than in the control arm (HR: 0.78; 95% CI: 0.68–0.90; median survival difference: 1.87 months). Moreover, in the subgroup of patients assigned to the prior bevacizumab stratum at randomization, OS was numerically longer if they received aflibercept plus FOLFIRI than placebo plus FOLFIRI (HR: 0.81; 95% CI: 0.63–1.04; median survival difference: 2.14 months). The benefit observed from aflibercept plus second-line FOLFIRI was irrespective of the timing of first-line PD (<3 months, ≥3 to <6 months, ≥6 to<9 months and ≥9 months), suggesting efficacy also in patients who rapidly progressed on first-line treatment. No unexpected toxicity occurred. Even if no definitive conclusion for the “pure second line setting” can be established, the comparison of this post hoc analysis with the VELOUR primary analysis suggests that the inclusion of the ARR may have underestimated the aflibercept benefit both in bevacizumab-pretreated and bevacizumab-naïve patients, and that subjects developing rapid progression after first-line treatment are good candidates for FOLFIRI-aflibercept (46).
Subgroups and Specific Populations Analysis
Pre-specified subgroup analyses of the VELOUR trial are shown in Table 4. In the analysis by Tabernero and colleagues, the efficacy of FOLFIRI-aflibercept over FOLFIRI-placebo was confirmed irrespective of demographic and baseline characteristics and stratification factors (107). Notably, a significantly greater benefit was observed with aflibercept for patients with liver-only metastases than those with either no liver metastases or ‘liver and other sites’ metastases (OS, p = 0.090; PFS, p = 0.008), thus suggesting aflibercept plus FOLFIRI as an optimal treatment choice in patients with liver-limited disease. Moreover, aflibercept efficacy was not decreased, and toxicity was not worsened, by previous exposure to an anti-angiogenic drug, showing aflibercept as an optimal candidate in this setting of patients (107).
The pre-specified post hoc multivariate analysis of the VELOUR ITT population, by Chau and colleagues, suggests that patients with an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 0 and any number of metastatic sites and patients with ECOG PS of 1 and <2 metastatic sites might obtain a greater benefit from treatment with FOLFIRI-aflibercept, and this may help to improve the selection of patients (108).
An age-based analysis confirmed the efficacy of FOLFIRI-aflibercept in patients aged ≥65 and <65 years, despite an increase of AEs in older patients. However, through careful follow-up for toxicity and prompt management of AEs, mCRC patients with good PS may gain PFS and OS benefit from aflibercept in combination with FOLFIRI, irrespective of age, with previous evaluation and accurate selection of elderly patients (109).
Aflibercept in Real-Life Setting
Aflibercept has been studied in real-life and clinical practice settings.
The prospective, observational, non-comparative, post-authorization safety study (PASS) OZONE trial conducted in 12 European and North American countries evaluated the safety and effectiveness of aflibercept plus FOLFIRI in 766 mCRC patients treated in daily practice after PD on an oxaliplatin-based regimen. 58.6% had received bevacizumab. Grade ≥3 treatment emergent AEs (TEAEs) were reported in 68.3% of patients, with neutropenia (15.1%), hypertension (10.2%), diarrhea (9.5%), and asthenia (9.1%) the most frequent, whereas AEs typically related to anti-angiogenic treatment were uncommon. No difference in the safety profile was observed in subgroup analyses except a more frequent incidence of grade ≥3 hypertension in bevacizumab-naïve patients. mOS was 12.5 months, mPFS was 6.1 months and ORR was 16.3%. Multivariate analysis found no statistical and clinically meaningful differences between groups defined by age, ethnicity, baseline renal function, or number of prior lines, even if the subgroups of patients without hepatic impairment and no prior use of bevacizumab seemed to be favored (110).
Several retrospective and real-world studies in mCRC patients receiving FOLFIRI-aflibercept have been performed in Spain (111–117), France (118–120), the USA (121) and Asia (122–126). Its safety profile described in these registry and real-world studies was consistent with those reported in clinical trials; even grade ≥3 AE rates were lower in the real-life population (neutropenia: 7.7–16.2%, fatigue 6–18%, hypertension 5.6–8%), although this finding might be related to underreporting or to an improved management of patients and AEs (127). The Aflibercept Safety and health-related Quality-of-life Program (ASQoP) (NCT01571284) was a global, multicenter, single-arm, open-label study evaluating the safety and health-related QoL (HRQoL) of FOLFIRI-aflibercept in mCRC patients previously treated with an oxaliplatin-containing regimen. The primary objective was to evaluate the safety of this therapeutic association in pre-treated mCRC patients in a setting more similar to real-life and the secondary objective was to assess the impact of this combination on patient-reported HRQoL. Moreover, this trial provided access to aflibercept to mCRC patients before marketing authorization and commercial availability. ASQoP enrolled 779 patients; FOLFIRI-aflibercept was well tolerated and the most common TEAEs of any grade were diarrhea (61.6%), hypertension (48.4%), and nausea (43.3%), whereas the most common grade 3–4 TEAEs were hypertension (24.1%), neutropenia (23.1%), and diarrhea (15.3%). The incidence of TEAEs was similar in bevacizumab pre-treated and bevacizumab-naïve patients (except grade 3–4 hypertension and any-grade proteinuria, with a slightly lower incidence in bevacizumab pre-treated patients), and in patients aged <65 and ≥65 years (aside from dehydration, which was more common in elderly than younger patients). No new safety signals emerged (128, 129). Clinically meaningful improvements and/or maintenance of HRQoL was reported in most patients (129). Also, in a cohort of 200 Italian patients from the study, no negative effects on HRQoL were observed and rates of TEAEs were similar to those reported in the VELOUR trial (128). These results were confirmed by an interim analysis of the larger (n = 1500 patients) ongoing QoLiTrap (AIO-LQ-0113) study, in which no clinically relevant deterioration in global health status evaluated through the EORTC-QLQ C30 questionnaire was observed during study treatment (130).
Exploring Further Prognostic and Predictive Clinical and Translational Biomarkers
Montes and colleagues conducted an exploratory analysis of an observational, retrospective study in a real‐world population of 78 mCRC patients treated with FOLFIRI‐aflibercept as second‐line treatment or after rapid progression during adjuvant oxaliplatin with the aim to identify prognostic and predictive factors for survival outcomes. Regarding prognostic factors, metachronous versus synchronous metastasis and left versus right tumors were significantly related to survival. Patients who developed metachronous metastasis had significantly longer PFS (11.0 months; 95% CI: 4.1–17.9) compared with patients with synchronous metastasis (5.0 months; 95% CI: 3.0–7.0; p = 0.028); the same was observed for OS, which reached 17 months (95% CI: 7.8–26.2) in metachronous versus 10 months (95% CI: 8.2–11.8) in synchronous patients (p = 0.039). Moreover, mPFS was significantly longer in patients with left‐sided tumors (7 months; 95% CI: 5.2–8.8) versus 3 months (95% CI: 0.1–5.9) in patients with right‐sided tumors (p = 0.044); mOS was 12.0 months (95% CI: 9.9–14.9) in the left-sided group versus 8.0 months (CI 95%: 5.70–10.3) for the right-sided (p = 0.041). With regard to predictive factors, the occurrence of hypertension during treatment was related to significantly longer mPFS (10.6 months; 95% CI: 6.3–13.7 vs 4.0 months; 95% CI: 2.7–5.3) compared with patients who did not develop hypertension (p = 0.009) and OS (17.0 months; 95% CI: 0–35.5 vs 10.0 months; 95% CI: 7.2–12.8; p < 0.001). Furthermore, the study confirmed the efficacy and safety of FOLFIRI-aflibercept in a real-world population. This analysis is limited by the small number of patients enrolled and, therefore, the findings have to be interpreted with caution, particularly those of the timing of metastatic disease occurrence (113). The same authors developed and internally validated a prognostic nomogram in a multicenter sample of 250 patients from nine Spanish hospitals in order to stratify patients eligible for second-line FOLFIRI-aflibercept based on their probability of survival and to optimize treatment results. The prognostic nomogram for OS included six variables: ECOG PS, tumor location, number of metastatic sites, mutational status, better response to previous treatment, and carcinoembryonic antigen. The model was well calibrated and had acceptable discriminatory capacity (optimism-corrected c-index: 0.723; 95% CI: 0.666–0.778). mOS was 6.1 months (95% CI: 5.1–8.8), 12.4 months (95% CI: 9.36–14.8), and 22.9 months (95% CI: 16.6–not reached) for high-, intermediate-, and low-risk groups, respectively. Prognosis was not influenced by age, comorbidity, or use of modified FOLFIRI regimens (117).
Hamaguchi and colleagues conducted an ancillary exploratory analysis of the relationship between 78 potential prognostic biomarkers and efficacy endpoints following aflibercept plus FOLFIRI in 62 Japanese patients enrolled in a single arm, phase II study (125, 131). Baseline levels of extracellular newly identified receptor for advanced glycation end‐products binding protein (EN-RAGE), insulin‐like growth factor‐binding protein 1, IL-8, kallikrein 5, pulmonary surfactant‐associated protein D, tissue inhibitor of metalloproteinases 1 (TIMP-1), tenascin‐C, and tumor necrosis factor receptor 2 were correlated with OS in a univariate Cox regression analysis. The most significant OS differences were observed for TIMP-1, IL-8, and EN-RAGE (all p < 0.001); lower baseline concentrations of each of these were related to longer OS. Conversely, no correlation was found for PFS and maximum tumor shrinkage. Among the biomarkers having a ±30% change in plasma concentration from baseline to pre‐dose 3, P1GF was reported to have the most significant change (4716% change). In patients stratified by prior bevacizumab, baseline levels of log‐transformed VEGF, PlGF, and decorin were significantly higher in bevacizumab-pretreated patients; on the contrary, baseline levels of ANG‐2 were significantly lower in this population (131).
Conclusions
Targeting angiogenesis is an effective strategy in the management of mCRC. Development of resistance and the discovery of various prognostic and predictive biomarkers require further considerations for the choice of second-line therapy.
In bevacizumab-naïve patients both bevacizumab or aflibercept (for patients progressing after an oxaliplatin-based regimen) represent second-line treatment options (29, 31), whereas bevacizumab, aflibercept or ramucirumab (in combination with FOLFIRI in patients who also received first-line oxaliplatin), might be an option in bevacizumab-pretreated patients (29, 31). Either aflibercept or ramucirumab, in combination with FOLFIRI, is specifically recommended in patients who progress quickly after first-line bevacizumab (29, 31). Ramucirumab is an option only after both oxaliplatin and bevacizumab. Aflibercept is effective and well tolerated, both in clinical trials and in real-life populations and represents a useful second-line strategy in combination with FOLFIRI in patients progressing after oxaliplatin-based chemotherapy, as well as in those with resistance after bevacizumab.
Each anti-angiogenic drug has its peculiar mechanism of action and demonstrated efficacy and safety in pivotal and real-world studies. Despite all the studies conducted so far, we do not yet have any validated biomarker which might guide our choice of second-line anti-angiogenic drug. The identification of pro-angiogenic plasma biomarkers would allow for selection of patients who would derive more benefit from second-line angiogenesis inhibition. Nonetheless, even if promising, preliminary findings cannot currently be applied to clinical practice. The lack of identification of a reliable biomarker might have various explanations. Firstly, angiogenesis is not a static but a dynamic process, with continuous changes and interactions among the different circulating angiogenic factors and the pathways involved, making identification of a single biomarker difficult. Moreover, defining and validating a quantitative biomarker threshold that is clearly associated with a benefit/resistance from available anti-angiogenic agents represents another challenge. Secondly, several factors contribute to the efficacy of a drug, so only angiogenesis by itself might not explain the global therapeutic results of an anti-angiogenic agent, thus making it difficult to identify a single robust factor as a biomarker.
While we await further data coming from clinical and translational studies that might guide biomarker-driven anti-angiogenic treatment, choice of second-line antiangiogenic drug currently has to be individualized for each patient according to their clinical features, outcomes and tolerability of prior treatments, and the tumor molecular profile.
Author Contributions
EL, SC, and MS have contributed to the manuscript preparation, and have read and approved all drafts. All authors contributed to the article and approved the submitted version.
Funding
Springer editorial assistance and publication cost were supported by an unrestricted grant from Sanofi Genzyme Italy. This medical writing and editorial assistance was funded by Sanofi Genzyme Italy. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
SC has received speaker fees and grants from Amgen, Sanofi, Servier outside this study. MS has received consultant, advisory board and speakers’ bureau fees from Amgen, Sanofi, MSD, Eisai, Merck, Bayer outside this study.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Andrea Bothwell and Tracy Harrison of Springer Healthcare Communications who helped to write the outline and first draft of this manuscript, and who provided editorial assistance post-submission, respectively.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Lai E, Liscia N, Donisi C, Mariani S, Tolu S, Pretta A, et al. Molecular-Biology-Driven Treatment for Metastatic Colorectal Cancer. Cancers (Basel) (2020) 12:1214. doi: 10.3390/cancers12051214
3. Mousa L, Salem ME, Mikhail S. Biomarkers of Angiogenesis in Colorectal Cancer. Biomark Cancer (2015) 7:13–9. doi: 10.4137/BIC.S25250
4. Folkman J. Role of Angiogenesis in Tumor Growth and Metastasis. Semin Oncol (2002) 29:15–8. doi: 10.1053/sonc.2002.37263
5. Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell (2000) 100:57–70. doi: 10.1016/s0092-8674(00)81683-9
6. Carmeliet P. Angiogenesis in Life, Disease and Medicine. Nature (2005) 438:932–6. doi: 10.1038/nature04478
8. Jürgensmeier JM, Schmoll HJ, Robertson JD, Brooks L, Taboada M, Morgan SR, et al. Prognostic and Predictive Value of VEGF, sVEGFR-2 and CEA in mCRC Studies Comparing Cediranib, Bevacizumab and Chemotherapy. Br J Cancer (2013) 108:1316–23. doi: 10.1038/bjc.2013.79
9. Larijani LV, Ghasemi M, Charati JY, Mehrabian-Fard M, Saravi NS. VEGF in Colorectal Cancer Evaluation of VEGF Immunohistochemical Expression and Correlation With Clinicopathologic Features in Colorectal Cancer. Govaresh (2015) 20:199–204.
10. Martins SF, Reis RM, Rodrigues AM, Baltazar F, Filho AL. Role of Endoglin and VEGF Family Expression in Colorectal Cancer Prognosis and Anti-Angiogenic Therapies. World J Clin Oncol (2011) 2:272–80. doi: 10.5306/wjco.v2.i6.272
11. De Vita F, Orditura M, Lieto E, Infusino S, Morgillo F, Martinelli E, et al. Elevated Perioperative Serum Vascular Endothelial Growth Factor Levels in Patients With Colon Carcinoma. Cancer (2004) 100:270–8. doi: 10.1002/cncr.11911
12. Tsai HL, Yang IP, Lin CH, Chai CY, Huang YH, Chen CF, et al. Predictive Value of Vascular Endothelial Growth Factor Overexpression in Early Relapse of Colorectal Cancer Patients After Curative Resection. Int J Colorectal Dis (2013) 28:415–24. doi: 10.1007/s00384-012-1570-z
13. Martins SF, Garcia EA, Luz MA, Pardal F, Rodrigues M, Filho AL. Clinicopathological Correlation and Prognostic Significance of VEGF-A, Vegf-C, VEGFR-2 and VEGFR-3 Expression in Colorectal Cancer. Cancer Genomics Proteomics (2013) 10:55–67.
14. Zheng S, Han MY, Xiao ZX, Peng JP, Dong Q. Clinical Significance of Vascular Endothelial Growth Factor Expression and Neovascularization in Colorectal Carcinoma. World J Gastroenterol (2003) 9:1227–30. doi: 10.3748/wjg.v9.i6.1227
15. Carmeliet P, Jain RK. Angiogenesis in Cancer and Other Diseases. Nature (2000) 407:249–57. doi: 10.1038/35025220
16. DiPietro LA. Angiogenesis and Wound Repair: When Enough is Enough. J Leukoc Biol (2016) 100:979–84. doi: 10.1189/jlb.4MR0316-102R
17. Folkman J. Fundamental Concepts of the Angiogenic Process. Curr Mol Med (2003) 3:643–51. doi: 10.2174/1566524033479465
18. Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
19. Macarulla T, Montagut C, Sánchez-Martin FJ, Granja M, Verdaguer H, Sastre J, et al. The Role of PIGF Blockade in the Treatment of Colorectal Cancer: Overcoming the Pitfalls. Expert Opin Biol Ther (2020) 20:15–22. doi: 10.1080/14712598.2020.1677603
20. Ferrara N, Gerber HP, LeCouter J. The Biology of VEGF and its Receptors. Nat Med (2003) 9:669–76. doi: 10.1038/nm0603-669
21. Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, Ardipradja K, et al. VEGF-D Promotes Tumor Metastasis by Regulating Prostaglandins Produced by the Collecting Lymphatic Endothelium. Cancer Cell (2012) 21:181–95. doi: 10.1016/j.ccr.2011.12.026
22. Khan K, Cunningham D, Chau I. Targeting Angiogenic Pathways in Colorectal Cancer: Complexities, Challenges and Future Directions. Curr Drug Targets (2017) 18:56–71. doi: 10.2174/1389450116666150325231555
23. Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, et al. Role of PlGF in the Intra- and Intermolecular Cross Talk Between the VEGF Receptors Flt1 and Flk1. Nat Med (2003) 9:936–43. doi: 10.1038/nm884
24. Carmeliet P, Jain RK. Molecular Mechanisms and Clinical Applications of Angiogenesis. Nature (2011) 473:298–307. doi: 10.1038/nature10144
25. Zoccoli A, Iuliani M, Pantano F, Imperatori M, Intagliata S, Vincenzi B, et al. Premetastatic Niche: Ready for New Therapeutic Interventions? Expert Opin Ther Targets (2012) 16 Suppl 2:S119–29. doi: 10.1517/14728222.2012.656092
26. Kanat O, Ertas H. Existing Anti-Angiogenic Therapeutic Strategies for Patients With Metastatic Colorectal Cancer Progressing Following First-Line Bevacizumab-Based Therapy. World J Clin Oncol (2019) 10:52–61. doi: 10.5306/wjco.v10.i2.52
27. sanofi-aventis groupe. ZALTRAP 25 Mg/Ml Concentrate for Solution for Infusion: Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/eylea-epar-product-information_en.pdf (Accessed 3 April 2020).
28. Scartozzi M, Vincent L, Chiron M, Cascinu S. Aflibercept, a New Way to Target Angiogenesis in the Second Line Treatment of Metastatic Colorectal Cancer (Mcrc). Target Oncol (2016) 11:489–500. doi: 10.1007/s11523-016-0447-4
29. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO Consensus Guidelines for the Management of Patients With Metastatic Colorectal Cancer. Ann Oncol (2016) 27:1386–422. doi: 10.1093/annonc/mdw235
30. National Comprehensive Cancer Network. Nccn Clinical Practice Guidelines in Oncology: Colon Cancer (Version 2.2021). Available at: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (Accessed 10 March 2021).
31. The Italian Association of Medical Oncology (AIOM). Linee Guida Tumori Del Colon 2020. Available at: https://www.aiom.it/wp-content/uploads/2020/10/2020_LG_AIOM_Colon.pdf (Accessed 8 March 2021).
32. Roche Registration GmbH. Avastin 25 Mg/Ml Concentrate for Solution for Infusion: Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/avastin-epar-product-information_en.pdf (Accessed 3 April 2020).
33. Eli Lilly Nederland BV. Cyramza 10 Mg/Ml Concentrate for Solution for Infusion: Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/cyramza-epar-product-information_en.pdf (Accessed 3 April 2020).
34. Bayer AG. Stivarga 40 Mg Film-Coated Tablets: Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/stivarga-epar-product-information_en.pdf (Accessed 3 April 2020).
35. Lenz H, Niedzwiecki D, Innocenti F, Blanke C, Mahony MR, O’Neil BH, et al. 501o - CALGB/SWOG 80405: Phase III Trial of Irinotecan/5-FU/leucovorin (FOLFIRI) or Oxaliplatin/5-FU/leucovorin (mFOLFOX6) With Bevacizumab (BV) or Cetuximab (CET) for Patients (Pts) With Expanded Ras Analyses Untreated Metastatic Adenocarcinoma of the Colon or Rectum (Mcrc). Ann Oncol (2014) 25(Suppl 4):v1–v41. doi: 10.1093/annonc/mdu438.13
36. Stintzing S, Modest DP, Rossius L, Lerch MM, von Weikersthal LF, Decker T, et al. FOLFIRI Plus Cetuximab Versus FOLFIRI Plus Bevacizumab for Metastatic Colorectal Cancer (FIRE-3): A Post-Hoc Analysis of Tumour Dynamics in the Final RAS Wild-Type Subgroup of This Randomised Open-Label Phase 3 Trial. Lancet Oncol (2016) 17:1426–34. doi: 10.1016/S1470-2045(16)30269-8
37. Rivera F, Karthaus M, Hecht JR, Sevilla I, Forget F, Fasola G, et al. Final Analysis of the Randomised PEAK Trial: Overall Survival and Tumour Responses During First-Line Treatment With mFOLFOX6 Plus Either Panitumumab or Bevacizumab in Patients With Metastatic Colorectal Carcinoma. Int J Colorectal Dis (2017) 32:1179–90. doi: 10.1007/s00384-017-2800-1
38. Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA (2017) 317:2392–401. doi: 10.1001/jama.2017.7105
39. Giampieri R, Scartozzi M, Del Prete M, Fulli A, Faloppi L, Bianconi M, et al. The “Angiogenetic Ladder”, Step-Wise Angiogenesis Inhibition in Metastatic Colorectal Cancer. Cancer Treat Rev (2014) 40:934–41. doi: 10.1016/j.ctrv.2014.06.004
40. Giampieri R, Caporale M, Pietrantonio F, De Braud F, Negri FV, Giuliani F, et al. Second-Line Angiogenesis Inhibition in Metastatic Colorectal Cancer Patients: Straightforward or Overcrowded? Crit Rev Oncol Hematol (2016) 100:99–106. doi: 10.1016/j.critrevonc.2016.02.005
41. Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in Combination With Oxaliplatin, Fluorouracil, and Leucovorin (FOLFOX4) for Previously Treated Metastatic Colorectal Cancer: Results From the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol (2007) 25:1539–44. doi: 10.1200/JCO.2006.09.6305
42. Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, et al. Continuation of Bevacizumab After First Progression in Metastatic Colorectal Cancer (ML18147): A Randomised Phase 3 Trial. Lancet Oncol (2013) 14:29–37. doi: 10.1016/S1470-2045(12)70477-1
43. Masi G, Salvatore L, Boni L, Loupakis F, Cremolini C, Fornaro L, et al. Continuation or Reintroduction of Bevacizumab Beyond Progression to First-Line Therapy in Metastatic Colorectal Cancer: Final Results of the Randomized BEBYP Trial. Ann Oncol (2015) 26:724–30. doi: 10.1093/annonc/mdv012
44. Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, et al. Ramucirumab Versus Placebo in Combination With Second-Line FOLFIRI in Patients With Metastatic Colorectal Carcinoma That Progressed During or After First-Line Therapy With Bevacizumab, Oxaliplatin, and a Fluoropyrimidine (RAISE): A Randomised, Double-Blind, Multicentre, Phase 3 Study. Lancet Oncol (2015) 16:499–508. doi: 10.1016/S1470-2045(15)70127-0
45. Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, et al. Addition of Aflibercept to Fluorouracil, Leucovorin, and Irinotecan Improves Survival in a Phase III Randomized Trial in Patients With Metastatic Colorectal Cancer Previously Treated With an Oxaliplatin-Based Regimen. J Clin Oncol (2012) 30:3499–506. doi: 10.1200/JCO.2012.42.8201
46. Van Cutsem E, Joulain F, Hoff PM, Mitchell E, Ruff P, Lakomý R, et al. Aflibercept Plus FOLFIRI vs. Placebo Plus FOLFIRI in Second-Line Metastatic Colorectal Cancer: A Post Hoc Analysis of Survival From the Phase III VELOUR Study Subsequent to Exclusion of Patients Who had Recurrence During or Within 6 Months of Completing Adjuvant Oxaliplatin-Based Therapy. Target Oncol (2016) 11:383–400. doi: 10.1007/s11523-015-0402-9
47. Kubicka S, Greil R, André T, Bennouna J, Sastre J, Van Cutsem E, et al. Bevacizumab Plus Chemotherapy Continued Beyond First Progression in Patients With Metastatic Colorectal Cancer Previously Treated With Bevacizumab Plus Chemotherapy: ML18147 Study KRAS Subgroup Findings. Ann Oncol (2013) 24:2342–9. doi: 10.1093/annonc/mdt231
48. Iwamoto S, Takahashi T, Tamagawa H, Nakamura M, Munemoto Y, Kato T, et al. FOLFIRI Plus Bevacizumab as Second-Line Therapy in Patients With Metastatic Colorectal Cancer After First-Line Bevacizumab Plus Oxaliplatin-Based Therapy: The Randomized Phase III EAGLE Study. Ann Oncol (2015) 26:1427–33. doi: 10.1093/annonc/mdv197
49. Petracci E, Scarpi E, Passardi A, Biggeri A, Milandri C, Vecchia S, et al. Effectiveness of Bevacizumab in First- and Second-Line Treatment for Metastatic Colorectal Cancer: ITACa Randomized Trial. Ther Adv Med Oncol (2020) 12:1758835920937427. doi: 10.1177/1758835920937427
50. Passardi A, Nanni O, Tassinari D, Turci D, Cavanna L, Fontana A, et al. Effectiveness of Bevacizumab Added to Standard Chemotherapy in Metastatic Colorectal Cancer: Final Results for First-Line Treatment From the ITACa Randomized Clinical Trial. Ann Oncol (2015) 26:1201–7. doi: 10.1093/annonc/mdv130
51. Bennouna J, Hiret S, Bertaut A, Bouche O, Deplanque G, Borel C, et al. Continuation of Bevacizumab vs Cetuximab Plus Chemotherapy After First Progression in KRAS Wild-Type Metastatic Colorectal Cancer: The UNICANCER Prodige18 Randomized Clinical Trial. JAMA Oncol (2019) 5:83–90. doi: 10.1001/jamaoncol.2018.4465
52. Hecht JR, Cohn A, Dakhil S, Saleh M, Piperdi B, Cline-Burkhardt M, et al. Spiritt: A Randomized, Multicenter, Phase II Study of Panitumumab With FOLFIRI and Bevacizumab With FOLFIRI as Second-Line Treatment in Patients With Unresectable Wild Type Kras Metastatic Colorectal Cancer. Clin Colorectal Cancer (2015) 14:72–80. doi: 10.1016/j.clcc.2014.12.009
53. Li J, Xu R, Qin S, Liu T, Pan H, Xu J, et al. Aflibercept Plus FOLFIRI in Asian Patients With Pretreated Metastatic Colorectal Cancer: A Randomized Phase III Study. Future Oncol (2018) 14:2031–44. doi: 10.2217/fon-2017-0669
54. Modest DP, Pant S, Sartore-Bianchi A. Treatment Sequencing in Metastatic Colorectal Cancer. Eur J Cancer (2019) 109:70–83. doi: 10.1016/j.ejca.2018.12.019
55. Zhao L, Wang J, Li H, Che J, Cao B. Meta-Analysis Comparing Maintenance Strategies With Continuous Therapy and Complete Chemotherapy-Free Interval Strategies in the Treatment of Metastatic Colorectal Cancer. Oncotarget (2016) 7:33418–28. doi: 10.18632/oncotarget.8644
56. Tamburini E, Rudnas B, Nicoletti S, Santelmo C, Fantini M, Ridolfi C, et al. Maintenance Bevacizumab Versus Stop and Go Therapy After Induction Therapy in First-Line Treatment of Stage IV Colorectal Cancer: Pooled Analysis of Randomized Clinical Trials. J Clin Oncol (2015) 33:e14622–2. doi: 10.1200/jco.2015.33.15_suppl.e14622
57. Folprecht G, Pericay C, Saunders MP, Thomas A, Lopez Lopez R, Roh JK, et al. Oxaliplatin and 5-FU/folinic Acid (Modified FOLFOX6) With or Without Aflibercept in First-Line Treatment of Patients With Metastatic Colorectal Cancer: The AFFIRM Study. Ann Oncol (2016) 27:1273–9. doi: 10.1093/annonc/mdw176
58. Camera S, Deleporte A, Bregni G, Trevisi E, Pretta A, Telli TA, et al. Momentum: A Phase I Trial Investigating 2 Schedules of Capecitabine With Aflibercept in Patients With Gastrointestinal and Breast Cancer. Clin Colorectal Cancer (2020) 19:311–318 e1. doi: 10.1016/j.clcc.2020.05.007
59. Strickler JH, Rushing CN, Niedzwiecki D, McLeod A, Altomare I, Uronis HE, et al. A Phase Ib Study of Capecitabine and Ziv-Aflibercept Followed by a Phase II Single-Arm Expansion Cohort in Chemotherapy Refractory Metastatic Colorectal Cancer. BMC Cancer (2019) 19:1032. doi: 10.1186/s12885-019-6234-8
60. Haibe Y, Kreidieh M, El Hajj H, Khalifeh I, Mukherji D, Temraz S, et al. Resistance Mechanisms to Anti-angiogenic Therapies in Cancer. Front Oncol (2020) 10:221. doi: 10.3389/fonc.2020.00221
61. Taieb J, Jung A, Sartore-Bianchi A, Peeters M, Seligmann J, Zaanan A, et al. The Evolving Biomarker Landscape for Treatment Selection in Metastatic Colorectal Cancer. Drugs (2019) 79:1375–94. doi: 10.1007/s40265-019-01165-2
62. Papachristos A, Kemos P, Katsila T, Panoilia E, Patrinos GP, Kalofonos H, et al. VEGF-a and ICAM-1 Gene Polymorphisms as Predictors of Clinical Outcome to First-Line Bevacizumab-Based Treatment in Metastatic Colorectal Cancer. Int J Mol Sci (2019) 20:5791. doi: 10.3390/ijms20225791
63. Paré-Brunet L, Sebio A, Salazar J, Berenguer-Llergo A, Río E, Barnadas A, et al. Genetic Variations in the VEGF Pathway as Prognostic Factors in Metastatic Colorectal Cancer Patients Treated With Oxaliplatin-Based Chemotherapy. Pharmacogenomics J (2015) 15:397–404. doi: 10.1038/tpj.2015.1
64. Sibertin-Blanc C, Mancini J, Fabre A, Lagarde A, Del Grande J, Levy N, et al. Vascular Endothelial Growth Factor A C.*237C>T Polymorphism is Associated With Bevacizumab Efficacy and Related Hypertension in Metastatic Colorectal Cancer. Dig Liver Dis (2015) 47:331–7. doi: 10.1016/j.dld.2014.12.013
65. Aravantinos G, Isaakidou A, Karantanos T, Sioziou A, Theodoropoulos GE, Pektasides D, et al. Association of CD133 Polymorphisms and Response to Bevacizumab in Patients With Metastatic Colorectal Cancer. Cancer Biomark (2015) 15:843–50. doi: 10.3233/CBM-150528
66. González-Vacarezza N, Alonso I, Arroyo G, Martínez J, De Andrés F, LLerena A, et al. Predictive Biomarkers Candidates for Patients With Metastatic Colorectal Cancer Treated With Bevacizumab-Containing Regimen. Drug Metab Pers Ther (2016) 31:83–90. doi: 10.1515/dmpt-2015-0027
67. Burgermeister E, Battaglin F, Eladly F, Wu W, Herweck F, Schulte N, et al. Aryl Hydrocarbon Receptor Nuclear Translocator-Like (ARNTL/BMAL1) is Associated With Bevacizumab Resistance in Colorectal Cancer Via Regulation of Vascular Endothelial Growth Factor a. EBioMedicine (2019) 45:139–54. doi: 10.1016/j.ebiom.2019.07.004
68. Kubicka S, Von Moos R, Greil R, Sastre J, Osterlund PJ, Arnold D, et al. Bevacizumab (BEV) Continued Beyond First Progression in Patients (Pts) With Metastatic Colorectal Cancer (mCRC) Previously Treated With BEV + Chemotherapy (CT): Biomarker Findings From ML18147. J Clin Oncol (2013) 31:452–2. doi: 10.1200/jco.2013.31.4_suppl.452
69. Giampieri R, Salvatore L, Del Prete M, Prochilo T, D’Anzeo M, Loretelli C, et al. Angiogenesis Genotyping and Clinical Outcome During Regorafenib Treatment in Metastatic Colorectal Cancer Patients. Sci Rep (2016) 6:25195. doi: 10.1038/srep25195
70. Lee Y, Kim SJ, Choo J, Heo G, Yoo JW, Jung Y, et al. miR-23a-3p is a Key Regulator of IL-17C-Induced Tumor Angiogenesis in Colorectal Cancer. Cells (2020) 9:1363. doi: 10.3390/cells9061363
71. Hansen TF, Kjaer-Frifeldt S, Christensen RD, Morgenthaler S, Blondal T, Lindebjerg J, et al. Redefining High-Risk Patients With Stage II Colon Cancer by Risk Index and microRNA-21: Results From a Population-Based Cohort. Br J Cancer (2014) 111:1285–92. doi: 10.1038/bjc.2014.409
72. Bergers G, Hanahan D. Modes of Resistance to Anti-Angiogenic Therapy. Nat Rev Cancer (2008) 8:592–603. doi: 10.1038/nrc2442
73. Ellis LM, Hicklin DJ. Pathways Mediating Resistance to Vascular Endothelial Growth Factor-Targeted Therapy. Clin Cancer Res (2008) 14:6371–5. doi: 10.1158/1078-0432.CCR-07-5287
74. Kopetz S, Hoff PM, Morris JS, Wolff RA, Eng C, Glover KY, et al. Phase II Trial of Infusional Fluorouracil, Irinotecan, and Bevacizumab for Metastatic Colorectal Cancer: Efficacy and Circulating Angiogenic Biomarkers Associated With Therapeutic Resistance. J Clin Oncol (2010) 28:453–9. doi: 10.1200/JCO.2009.24.8252
75. Giampieri R, Puzzoni M, Daniele B, Ferrari D, Lonardi S, Zaniboni A, et al. First-Line FOLFIRI and Bevacizumab in Patients With Advanced Colorectal Cancer Prospectively Stratified According to Serum LDH: Final Results of the GISCAD (Italian Group for the Study of Digestive Tract Cancers) Central (ColorEctalavastiNTRiAlLdh) Trial. Br J Cancer (2017) 117:1099–104. doi: 10.1038/bjc.2017.234
76. Giampieri R, Ziranu P, Daniele B, Zizzi A, Ferrari D, Lonardi S, et al. From CENTRAL to SENTRAL (Serum aNgiogenesis cenTRAL): Circulating Predictive Biomarkers to anti-VEGFR Therapy. Cancers (Basel) (2020) 12:1330. doi: 10.3390/cancers12051330
77. Loupakis F, Cremolini C, Fioravanti A, Orlandi P, Salvatore L, Masi G, et al. Pharmacodynamic and Pharmacogenetic Angiogenesis-Related Markers of First-Line FOLFOXIRI Plus Bevacizumab Schedule in Metastatic Colorectal Cancer. Br J Cancer (2011) 104:1262–9. doi: 10.1038/bjc.2011.85
78. Lieu CH, Tran H, Jiang ZQ, Mao M, Overman MJ, Lin E, et al. The Association of Alternate VEGF Ligands With Resistance to anti-VEGF Therapy in Metastatic Colorectal Cancer. PloS One (2013) 8:e77117. doi: 10.1371/journal.pone.0077117
79. Horita Y, Yamada Y, Kato K, Hirashima Y, Akiyoshi K, Nagashima K, et al. Phase II Clinical Trial of Second-Line FOLFIRI Plus Bevacizumab for Patients With Metastatic Colorectal Cancer: AVASIRI Trial. Int J Clin Oncol (2012) 17:604–9. doi: 10.1007/s10147-011-0331-2
80. Cremolini C, Loupakis F, Bocci G, Falcone A. Biomarkers and Response to Bevacizumab–Letter. Clin Cancer Res (2014) 20:1056–7. doi: 10.1158/1078-0432.CCR-13-2763
81. Tabernero J, Hozak RR, Yoshino T, Cohn AL, Obermannova R, Bodoky G, et al. Analysis of Angiogenesis Biomarkers for Ramucirumab Efficacy in Patients With Metastatic Colorectal Cancer From RAISE, a Global, Randomized, Double-Blind, Phase III Study. Ann Oncol (2018) 29:602–9. doi: 10.1093/annonc/mdx767
82. Van Cutsem E, Paccard C, Chiron M, Tabernero J. Impact of Prior Bevacizumab Treatment on VEGF-A and PlGF Levels and Outcome Following Second-Line Aflibercept Treatment: Biomarker. post hoc Anal VELOUR trial Clin Cancer Res (2020) 26:717–25. doi: 10.1158/1078-0432.CCR-19-1985
83. Cudmore MJ, Hewett PW, Ahmad S, Wang KQ, Cai M, Al-Ani B, et al. The Role of Heterodimerization Between VEGFR-1 and VEGFR-2 in the Regulation of Endothelial Cell Homeostasis. Nat Commun (2012) 3:972. doi: 10.1038/ncomms1977
84. Hayashi H, Arao T, Matsumoto K, Kimura H, Togashi Y, Hirashima Y, et al. Biomarkers of Reactive Resistance and Early Disease Progression During Chemotherapy Plus Bevacizumab Treatment for Colorectal Carcinoma. Oncotarget (2014) 5:2588–95. doi: 10.18632/oncotarget.1811
85. Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, et al. Synergism Between Vascular Endothelial Growth Factor and Placental Growth Factor Contributes to Angiogenesis and Plasma Extravasation in Pathological Conditions. Nat Med (2001) 7:575–83. doi: 10.1038/87904
86. Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, et al. Anti-PlGF Inhibits Growth of VEGF(R)-inhibitor-resistant Tumors Without Affecting Healthy Vessels. Cell (2007) 131:463–75. doi: 10.1016/j.cell.2007.08.038
87. Bergers G, Benjamin LE. Tumorigenesis and the Angiogenic Switch. Nat Rev Cancer (2003) 3:401–10. doi: 10.1038/nrc1093
88. Yoshino T, Portnoy DC, Obermannova R, Bodoky G, Prausová J, Garcia-Carbonero R, et al. Biomarker Analysis Beyond Angiogenesis: RAS/RAF Mutation Status, Tumour Sidedness, and Second-Line Ramucirumab Efficacy in Patients With Metastatic Colorectal Carcinoma From RAISE-a Global Phase III Study. Ann Oncol (2019) 30:124–31. doi: 10.1093/annonc/mdy461
89. Wirapati P, Pomella V, Vandenbosch B, Kerr P, Maiello E, Jeffery GM, et al. VELOUR Trial Biomarkers Update: Impact of RAS, BRAF, and Sidedness on Aflibercept Activity. J Clin Oncol (2017) 35(Suppl 15):3538. doi: 10.1200/JCO.2017.35.15_suppl.3538
90. Ottaiano A, Capozzi M, Tafuto S, De Stefano A, De Divitiis C, Romano C, et al. FOLFIRI-Aflibercept vs. FOLFIRI-bevacizumab as Second Line Treatment of RAS Mutated Metastatic Colorectal Cancer in Real Practice. Front Oncol (2019) 9:766. doi: 10.3389/fonc.2019.00766
91. Cremolini C, Antoniotti C, Rossini D, Lonardi S, Loupakis F, Pietrantonio F, et al. Upfront FOLFOXIRI Plus Bevacizumab and Reintroduction After Progression Versus mFOLFOX6 Plus Bevacizumab Followed by FOLFIRI Plus Bevacizumab in the Treatment of Patients With Metastatic Colorectal Cancer (TRIBE2): A Multicentre, Open-Label, Phase 3, Randomised, Controlled Trial. Lancet Oncol (2020) 21:497–507. doi: 10.1016/S1470-2045(19)30862-9
92. Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, et al. Pan-Asian Adapted ESMO Consensus Guidelines for the Management of Patients With Metastatic Colorectal Cancer: A JSMO-ESMO Initiative Endorsed by CSCO, Kaco, MOS, SSO and TOS. Ann Oncol (2018) 29:44–70. doi: 10.1093/annonc/mdx738
93. Chen D, Gu K, Wang H. Optimizing Sequential Treatment With anti-EGFR and VEGF mAb in Metastatic Colorectal Cancer: Current Results and Controversies. Cancer Manag Res (2019) 11:1705–16. doi: 10.2147/CMAR.S196170
94. Wainberg ZA, Drakaki A. The Importance of Optimal Drug Sequencing in Metastatic Colorectal Cancer: Biological Rationales for the Observed Survival Benefit Conferred by First-Line Treatment With EGFR Inhibitors. Expert Opin Biol Ther (2015) 15:1205–20. doi: 10.1517/14712598.2015.1050375
95. Parisi A, Cortellini A, Cannita K, Venditti O, Camarda F, Calegari MA, et al. Evaluation of Second-Line anti-VEGF After First-Line anti-EGFR Based Therapy in RAS Wild-Type Metastatic Colorectal Cancer: The Multicenter “Slave” Study. Cancers (Basel) (2020) 12:1259. doi: 10.3390/cancers12051259
96. Vera R, Mata E, Gonzalez E, Juez I, Alonso V, Iranzo P, et al. Is Aflibercept an Optimal Treatment for Wt RAS mCRC Patients After Progression to First Line Containing Anti-EGFR? Int J Colorectal Dis (2020) 35:739–46. doi: 10.1007/s00384-020-03509-x
97. Ziranu P, Demurtas L, Puzzoni M, Loupakis F, Daniele B, Rimassa L, et al. P-291 - The DISTINCTIVE Study: A Biologically Enriched Phase II Study of seconD-line Folfiri/aflIbercept in proSpecTIvely Stratified, anti-EGFR resistaNt, Metastatic coloreCTal Cancer patIents With RAS Validated Wild Type Status - Trial in Progress. Ann Oncol (2018) 29(Suppl 5):v82. doi: 10.1093/annonc/mdy151.290
98. Lai E, Pretta A, Impera V, Mariani S, Giampieri R, Casula L, et al. BRAF-Mutant Colorectal Cancer, a Different Breed Evolving. Expert Rev Mol Diagn (2018) 18:499–512. doi: 10.1080/14737159.2018.1470928
99. Scartozzi M, Giampieri R, Aprile G, Iacono D, Santini D, dell’Aquila E, et al. The Distinctive Molecular, Pathological and Clinical Characteristics of BRAF-mutant Colorectal Tumors. Expert Rev Mol Diagn (2015) 15:979–87. doi: 10.1586/14737159.2015.1047346
100. Seligmann JF, Fisher D, Smith CG, Richman SD, Elliott F, Brown S, et al. Investigating the Poor Outcomes of BRAF-mutant Advanced Colorectal Cancer: Analysis From 2530 Patients in Randomised Clinical Trials. Ann Oncol (2017) 28:562–8. doi: 10.1093/annonc/mdw645
101. Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N Engl J Med (2019) 381:1632–43. doi: 10.1056/NEJMoa1908075
102. Kopetz S, Grothey A, Van Cutsem E, Yaeger R, Wasan HS, Yoshino T, et al. Encorafenib Plus Cetuximab With or Without Binimetinib for BRAF V600E-Mutant Metastatic Colorectal Cancer: Quality-of-life Results From a Randomized, Three-Arm, Phase III Study Versus the Choice of Either Irinotecan or FOLFIRI Plus Cetuximab (BEACON CRC). J Clin Oncol (2020) 38(Suppl 15):4039. doi: 10.1200/JCO.2020.38.15_suppl.4039
103. Kopetz S, Grothey A, Van Cutsem E, Yaeger R, Wasan HS, Yoshino T, et al. Encorafenib Plus Cetuximab With or Without Binimetinib for BRAF V600E Metastatic Colorectal Cancer: Updated Survival Results From a Randomized, Three-Arm, Phase III Study Versus Choice of Either Irinotecan or FOLFIRI Plus Cetuximab (BEACON CRC). J Clin Oncol (2020) 38(Suppl 15):4001. doi: 10.1200/JCO.2020.38.15_suppl.4001
104. Gelsomino F, Casadei-Gardini A, Rossini D, Boccaccino A, Masi G, Cremolini C, et al. The Role of Anti-Angiogenics in Pre-Treated Metastatic BRAF-mutant Colorectal Cancer: A Pooled Analysis. Cancers (Basel) (2020) 12:1022. doi: 10.3390/cancers12041022
105. Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI Plus Bevacizumab Versus FOLFIRI Plus Bevacizumab as First-Line Treatment of Patients With Metastatic Colorectal Cancer: Updated Overall Survival and Molecular Subgroup Analyses of the Open-Label, Phase 3 TRIBE Study. Lancet Oncol (2015) 16:1306–15. doi: 10.1016/S1470-2045(15)00122-9
106. Ruff P, Ferry DR, Lakomy R, Prausova J, Van Hazel GA, Hoff PM, et al. Time Course of Safety and Efficacy of Aflibercept in Combination With FOLFIRI in Patients With Metastatic Colorectal Cancer Who Progressed on Previous Oxaliplatin-Based Therapy. Eur J Cancer (2015) 51:18–26. doi: 10.1016/j.ejca.2014.10.019
107. Tabernero J, Van Cutsem E, Lakomý R, Prausová J, Ruff P, van Hazel GA, et al. Aflibercept Versus Placebo in Combination With Fluorouracil, Leucovorin and Irinotecan in the Treatment of Previously Treated Metastatic Colorectal Cancer: Prespecified Subgroup Analyses From the VELOUR Trial. Eur J Cancer (2014) 50:320–31. doi: 10.1016/j.ejca.2013.09.013
108. Chau I, Joulain F, Iqbal SU, Bridgewater J. A VELOUR Post Hoc Subset Analysis: Prognostic Groups and Treatment Outcomes in Patients With Metastatic Colorectal Cancer Treated With Aflibercept and FOLFIRI. BMC Cancer (2014) 14:605. doi: 10.1186/1471-2407-14-605
109. Ruff P, Van Cutsem E, Lakomy R, Prausova J, van Hazel GA, Moiseyenko VM, et al. Observed Benefit and Safety of Aflibercept in Elderly Patients With Metastatic Colorectal Cancer: An Age-Based Analysis From the Randomized Placebo-Controlled Phase III VELOUR Trial. J Geriatr Oncol (2018) 9:32–9. doi: 10.1016/j.jgo.2017.07.010
110. Chau I, Fakih M, Garcia-Alfonso P, Linke Z, Ruiz Casado A, Marques EP, et al. Safety and Effectiveness of Aflibercept + Fluorouracil, Leucovorin, and Irinotecan (FOLFIRI) for the Treatment of Patients With Metastatic Colorectal Cancer (mCRC) in Current Clinical Practice: OZONE Study. Cancers (Basel) (2020) 12:657. doi: 10.3390/cancers12030657
111. Fernández Montes A, López López C, Carmona A, Páez D, Lopez Muñoz AM, Gutiérrez Abad D, et al. Efficacy and Toxicity Results of FOLFIRI-aflibercept in Advanced Colorectal Cancer (Crcm) in “Real Life”, Retrospective Study. Prognostic Factors and Specific Populations. Ann Oncol (2017) 28(Suppl 3):iii103. doi: 10.1093/annonc/mdx261.294
112. González-Flores E, González-Astorga B, Delgado Ureña Ma T, González Cebrián I, Sánchez-Toro C, García-García J, et al. P-317 - Experience With Aflibercept as a Second Line Chemotherapy in Metastatic Colorectal Cancer: Safety and Efficacy in a Real-Life Population. Ann Oncol (2017) 28(Suppl 3):iii110–1. doi: 10.1093/annonc/mdx261.314
113. Fernández Montes A, Martínez Lago N, Covela Rúa M, de la Cámara Gómez J, González Villaroel P, Méndez Méndez JC, et al. Efficacy and Safety of FOLFIRI/aflibercept in Second-Line Treatment of Metastatic Colorectal Cancer in a Real-World Population: Prognostic and Predictive Markers. Cancer Med (2019) 8:882–9. doi: 10.1002/cam4.1903
114. Salgado Fernández M, Pérez Hoyos MT, Díaz de Corcuera I, Vidal Arbués A. And García De La Torre M, Aflibercept for Metastatic Colorectal Cancer: Safety Data From the Spanish Named Patient Program. Expert Opin Drug Saf (2015) 14:1171–9. doi: 10.1517/14740338.2015.1057495
115. Feliu J, Díez de Corcuera I, Manzano JL, Valladares-Ayerbes M, Alcaide J, García García T, et al. Effectiveness and Safety of Aflibercept for Metastatic Colorectal Cancer: Retrospective Review Within an Early Access Program in Spain. Clin Transl Oncol (2017) 19:498–507. doi: 10.1007/s12094-016-1556-3
116. Martínez-Lago N, Carmona Campos M, Gonzalez Villarroel P, Salgado Fernandez M, De la Cámara Gómez J, Romero Reinoso C, et al. P-157 - Efficacy and Safety of FOLFIRI/aflibercept (FA) in an Elderly Population With Metastatic Colorectal Cancer (mCRC) After the Failure of an Oxaliplatin-Based Regimen. Ann Oncol (2019) 30(Suppl 4):iv42–3. doi: 10.1093/annonc/mdz155.156
117. Fernández Montes A, López López C, Argilés Martínez G, Páez López D, López Muñoz AM, García Paredes B, et al. Prognostic Nomogram and Patterns of Use of FOLFIRI-aflibercept in Advanced Colorectal Cancer: A Real-World Data Analysis. Oncologist (2019) 24:e687–95. doi: 10.1634/theoncologist.2018-0824
118. Carola C, Ghiringhelli F, Kim S, André T, Barlet J, Bengrine-Lefevre L, et al. FOLFIRI3-Aflibercept in Previously Treated Patients With Metastatic Colorectal Cancer. World J Clin Oncol (2018) 9:110–8. doi: 10.5306/wjco.v9.i5.110
119. Devaux M, Gerard L, Richard C, Bengrine-Lefevre L, Vincent J, Schmitt A, et al. Retrospective Evaluation of FOLFIRI3 Alone or in Combination With Bevacizumab or Aflibercept in Metastatic Colorectal Cancer. World J Clin Oncol (2019) 10:75–85. doi: 10.5306/wjco.v10.i2.75
120. Auvray M, Tougeron D, Auclin E, Moulin V, Artru P, Hautefeuille V, et al. Efficacy and Safety of Aflibercept in Combination With Chemotherapy Beyond Second-Line Therapy in Metastatic Colorectal Carcinoma Patients: An AGEO Multicenter Study. Clin Colorectal Cancer (2020) 19:39–47.e5. doi: 10.1016/j.clcc.2019.08.003
121. Ivanova JI, Saverno KR, Sung J, Duh MS, Zhao C, Cai S, et al. Real-World Treatment Patterns and Effectiveness Among Patients With Metastatic Colorectal Cancer Treated With Ziv-Aflibercept in Community Oncology Practices in the USA. Med Oncol (2017) 34:193. doi: 10.1007/s12032-017-1049-4
122. Chong DQ, Manalo M, Imperial M, Teo P, Yong G, Ng M, et al. Safety and Efficacy of Aflibercept in Combination With Fluorouracil, Leucovorin and Irinotecan in the Treatment of Asian Patients With Metastatic Colorectal Cancer. Asia Pac J Clin Oncol (2016) 12:275–83. doi: 10.1111/ajco.12496
123. Yusof MM, Abdullah NM, Sharial MM, Zaatar A. Safety and Management of Toxicity Related to Aflibercept in Combination With Fluorouracil, Leucovorin and Irinotecan in Malaysian Patients With Metastatic Colorectal Cancer. Asian Pac J Cancer Prev (2016) 17:973–8. doi: 10.7314/apjcp.2016.17.3.973
124. Lu CS, Lin JK, Chen WS, Lin TC, Jiang JK, Yang SH, et al. Intraperitoneal Ziv-Aflibercept Effectively Manages Refractory Ascites in Colorectal Cancer Patients. Oncotarget (2017) 8:36707–15. doi: 10.18632/oncotarget.13543
125. Denda T, Sakai D, Hamaguchi T, Sugimoto N, Ura T, Yamazaki K, et al. Phase II Trial of Aflibercept With FOLFIRI as a Second-Line Treatment for Japanese Patients With Metastatic Colorectal Cancer. Cancer Sci (2019) 110:1032–43. doi: 10.1111/cas.13943
126. Yoshino T, Yamazaki K, Yamaguchi K, Doi T, Boku N, Machida N, et al. A Phase I Study of Intravenous Aflibercept With FOLFIRI in Japanese Patients With Previously Treated Metastatic Colorectal Cancer. Invest New Drugs (2013) 31:910–7. doi: 10.1007/s10637-012-9895-6
127. Muro K, Salinardi T, Singh AR, Macarulla T. Safety of Aflibercept in Metastatic Colorectal Cancer: A Literature Review and Expert Perspective on Clinical and Real-World Data. Cancers (Basel) (2020) 12:844. doi: 10.3390/cancers12040844
128. Pastorino A, Di Bartolomeo M, Maiello E, Iaffaioli V, Ciuffreda L, Fasola G, et al. Aflibercept Plus FOLFIRI in the Real-Life Setting: Safety and Quality of Life Data From the Italian Patient Cohort of the Aflibercept Safety and Quality-of-Life Program Study. Clin Colorectal Cancer (2018) 17:e457–70. doi: 10.1016/j.clcc.2018.03.002
129. Riechelmann RP, Srimuninnimit V, Bordonaro R, Kavan P, Di Bartolomeo M, Maiello E, et al. Aflibercept Plus FOLFIRI for Second-Line Treatment of Metastatic Colorectal Cancer: Observations From the Global Aflibercept Safety and Health-Related Quality-of-life Program (Asqop). Clin Colorectal Cancer (2019) 18:183–91.e3. doi: 10.1016/j.clcc.2019.05.003
130. Hofheinz R, Scholten F, Derigs H, Thaler J, von Moos R. Quality of Life in Patients Treated With Aflibercept and FOLFIRI for Metastatic Colorectal Cancer: Interim Analysis With Focus on Therapy Lines of the non-Interventional Study QoLiTrap (Aio-Lq-0113). Ann Oncol (2019) 30(Suppl 4):iv118. doi: 10.1093/annonc/mdz156.025
Keywords: aflibercept, angiogenesis, biomarkers, metastatic colorectal cancer, second-line therapy, anti-VEGF
Citation: Lai E, Cascinu S and Scartozzi M (2021) Are All Anti-Angiogenic Drugs the Same in the Treatment of Second-Line Metastatic Colorectal Cancer? Expert Opinion on Clinical Practice. Front. Oncol. 11:637823. doi: 10.3389/fonc.2021.637823
Received: 04 December 2020; Accepted: 19 April 2021;
Published: 10 May 2021.
Edited by:
Carlotta Antoniotti, University of Pisa, ItalyReviewed by:
Mitsukuni Suenaga, Tokyo Medical and Dental University, JapanPia Osterlund, Tampere University Hospital, Finland
Copyright © 2021 Lai, Cascinu and Scartozzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mario Scartozzi, bWFyaW9zY2FydG96emlAdW5pY2EuaXQ=
 Eleonora Lai
Eleonora Lai Stefano Cascinu2
Stefano Cascinu2 Mario Scartozzi
Mario Scartozzi