
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 03 June 2021
Sec. Women's Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.636378
This article is part of the Research TopicBreast Cancer in Young WomenView all 18 articles
 Soojeong Choi1
Soojeong Choi1 Young Jae Lee2
Young Jae Lee2 Jae Ho Jeong3
Jae Ho Jeong3 Jinhong Jung4
Jinhong Jung4 Jong Won Lee1
Jong Won Lee1 Hee Jeong Kim1
Hee Jeong Kim1 Beom Seok Ko1
Beom Seok Ko1 Byung Ho Son1
Byung Ho Son1 Sei Hyun Ahn1
Sei Hyun Ahn1 Yura Lee1,5*
Yura Lee1,5* Il Yong Chung1*
Il Yong Chung1*Background: Although the guidelines recommend gynecological assessment and close monitoring for symptoms of endometrial cancer in postmenopausal breast cancer survivors taking tamoxifen (TAM), the risk of endometrial cancer in young breast cancer survivors has not yet been fully assessed. This study aimed to investigate the risk of developing endometrial cancer and the frequencies of gynecological examinations in young breast cancer survivors taking TAM in South Korea.
Methods: A nationwide retrospective cohort study was conducted using the Health Insurance Review and Assessment Service claims data. Kaplan–Meier analyses and log-rank tests were used to assess the probability of endometrial cancer, benign endometrial conditions, and the probability of invasive endometrial procedure. To analyze the risk of endometrial cancer and benign endometrial conditions, we used a multivariable Cox proportional hazards regression model.
Results: Between 2010 and 2015, 60,545 newly diagnosed female breast cancer survivors were included. The total person–years were 256,099 and 140 (0.23%) patients developed endometrial cancer during the study period. In breast cancer survivors aged ≥60 years [hazard ratio (HR), 5.037; 95% confidence interval (CI), 2.185–11.613], 50–59 years (HR, 4.343; 95% CI, 2.122–8.891), and 40–49 years (HR, 2.121; 95% CI, 1.068–4.213), TAM was associated with an increased risk of endometrial cancer. In subjects aged below 40 years, TAM did not significantly increase the risk of endometrial cancer. However, among the TAM subgroups, breast cancer survivors aged below 40 years [1.61 per 1,000 person–years (PY); HR, 12.460; 95% CI, 2.698–57.522] and aged 40–49 years (2.22 per 1,000 PY; HR, 9.667; 95% CI, 4.966–18.819) with TAM-related endometrial diseases showed significantly increased risks of endometrial cancer. Among the TAM subgroup with benign endometrial conditions, the ratios of the frequency of invasive diagnostic procedures to the incidence of endometrial cancer were higher in subjects under 40 than subjects aged 60 or more.
Conclusion: Young breast cancer survivors with TAM-related benign endometrial diseases are at a higher risk of developing endometrial cancer. Gynecological surveillance should be tailored to the risk of endometrial cancer in young breast cancer survivors to improve the early detection of endometrial cancer and avoid unnecessary invasive procedures.
In hormone receptor-positive breast cancer, use of tamoxifen (TAM) as an adjuvant antihormonal treatment is important (1, 2). TAM is associated with various adverse effects, including hot flashes, vaginal discharge, menstrual irregularities, sexual dysfunction, thromboembolic complications, hyperplasia, and endometrial cancer (3, 4). These adverse effects can reduce patients’ adherence to adjuvant endocrine treatment (5). Endometrial cancer is known as a serious adverse effect related to the use of TAM (6–8).
Most guidelines recommend gynecological assessment for endometrial cancer in postmenopausal breast cancer survivors receiving TAM (9–11). However, routine examinations are not warranted as such surveillance is not effective in enhancing the early detection of endometrial cancer and may lead to more invasive and costly diagnostic procedures in asymptomatic postmenopausal women. Gynecological assessments are recommended to be performed only in postmenopausal women with complaints of abnormal vaginal symptoms (9, 11).
No existing guidelines have recommended gynecological assessment for endometrial cancer in premenopausal women receiving TAM. This is because previous studies did not show a significantly increased risk of endometrial cancer in premenopausal women receiving TAM (1, 10). However, in these studies, the numbers of young breast cancer survivors were small, the incidence of endometrial cancer was low, and assessments for various TAM-related gynecological symptoms were not conducted. Therefore, the risk of endometrial cancer in premenopausal women were possibly underestimated.
Hence, this nationwide retrospective cohort study was conducted using claims data from the Health Insurance Review and Assessment Service (HIRA). This study aimed to investigate the incidence of endometrial cancer in young breast cancer survivors and to analyze the frequency of invasive endometrial procedures in breast cancer survivors who were treated with TAM.
Healthcare in South Korea is delivered through a single-payer healthcare system supported by the government. The HIRA has a major role in collecting data of all healthcare services delivered to patients and assessing the healthcare services for reimbursement decisions (12). The HIRA data include patients’ general information, diagnoses, and healthcare services provided such as medications and procedures.
The data registered between January 2008 and December 2018 were extracted from the HIRA database. Patients who were newly diagnosed with breast cancer from January 2010 to December 2015 were included. To exclude prevalent breast cancer patients, we selected a 2-year washout period (from January 2008 to December 2009). Male breast cancer patients, patients with a history of in situ carcinoma, patients with presumed metastatic or recurrent breast cancer, and patients who did not undergo breast cancer surgery were excluded. Patients with previous or recent history of other cancer types including endometrial cancer, who underwent hysterectomy, or who had an oophorectomy were also excluded. Moreover, patients without follow-up claims data 1 year after the initiation of endocrine treatment were excluded. Newly diagnosed breast cancer was defined using the C50 code (invasive breast cancer) based on the 10th revision of the International Classification of Diseases (ICD-10) plus the V193 code, which is a claim code for reimbursement of cancer patients (13).
From January 2010 to December 2015, 203,956 breast cancer patients who were assigned with the C50 and V193 codes were identified. We excluded 861 male patients, 6,535 patients with a previous history of in situ carcinoma, 13,493 patients with metastatic or recurrent breast cancer, 17,142 patients who did not undergo breast cancer surgery, 16,036 patients with preexisting or who were recently diagnosed with other cancer types, 1,642 patients with previous hysterectomy, 99 patients who previously underwent oophorectomy, and 366 patients who did not have follow-up data 1 year after the initiation of endocrine treatment (Supplementary Figure 1).
Patients’ baseline characteristics and the Charlson Comorbidity Index (CCI) based on ICD-10 codes were analyzed (14). Hypertension (HT), diabetes mellitus (DM), and dyslipidemia were defined using ICD-10 codes (HT, I10–13, 15, and 16; DM, E10–14; and dyslipidemia, E78) and their related medications. Breast cancer treatments were evaluated based on claims data 1 year after the breast cancer diagnosis. Data on surgery, radiation, chemotherapy, endocrine therapy, and trastuzumab were reviewed.
Endometrial cancer was defined using the newly claimed endometrial cancer codes (C55, C54, and D07). Benign endometrial condition was defined using the newly claimed diagnoses such as vaginal bleeding (N93 and N90), endometrial hyperplasia (N851 and N850), and polyp of endometrium (N840). Procedures including endometrial evaluation (aspiration, biopsy, and polypectomy) and dilatation and curettage (D&C) were identified using electronic data interchange codes. In-hospital mortality was assessed.
For landmark analysis, the index date was defined as the date 1 year after the initiation of endocrine therapy or the date of surgery if antihormonal medications were not prescribed.
The incidence rates of endometrial cancer were compared between the TAM group and no TAM group. In the TAM subgroup stratified according to age at diagnosis (<40, 40–49, 50–59, and ≥60 years), the incidence of endometrial cancer and benign endometrial condition, total frequency of procedures, frequency of procedures per 1,000 person–years, and the ratio of the frequency of invasive diagnostic procedures to the incidence of endometrial cancer were calculated. Kaplan–Meier analysis and the log-rank test were used to assess the disease-free probability of patients with endometrial cancer and benign endometrial condition, and the procedure-free probability of the endometrial evaluation and D&C.
To analyze the risk of endometrial cancer and benign endometrial conditions, we used a Cox proportional hazards regression model adjusted for age at diagnosis, insurance, CCI, previous dyslipidemia, previous diabetes mellitus, previous hypertension, previous polycystic ovarian syndrome, chemotherapy, radiation, and trastuzumab.
Statistical analyses were conducted using R software (version 3.6.1, R Foundation for Statistical Computing, Vienna, Austria) and SAS (version 9.4, SAS Institute Inc., Cary, NC, USA). This study was approved by the Institutional Review Board of Asan Medical Center (IRB no. S2019-1702-0001).
A total of 60,545 breast cancer survivors were included in this analysis. The total person–years were 256,099, and the mean duration after cohort entry was 66 months. Of these patients, 27,034 (44.65%) received TAM, while 33,511 (55.35%) were not treated with TAM (Table 1). Of the total patients, 29,635 (48.9%) breast cancer survivors were aged below 50 years, and 7,519 (12.4%) young breast cancer survivors were aged below 40 years (Supplementary Table 1). The proportion of breast cancer survivors who received any type of chemotherapy was 60.74% (n = 36,776). During the study period, 68.66% (41,569) of these patients received radiation therapy. Trastuzumab was prescribed in 8,619 (14.23%) patients. All patients underwent breast cancer surgery.
Of the total study population, 140 (0.23%) patients developed endometrial cancer during the study period (Table 1). There were 98 (0.36%) and 42 (0.12%) endometrial cancer cases in the TAM group and non-TAM group, respectively.
In breast cancer survivors aged 40 or over, TAM significantly increased the risk of endometrial cancer (Table 2 and Figure 1). The incidence of endometrial cancer in subjects ≥60 years who were treated with TAM was the highest in all age subgroups [1.38 per 1,000 person–years (PY); hazard ratio (HR), 5.037; 95% confidence interval (CI), 2.185–11.613; P<0.001]. However, in subjects aged below 40 years, TAM did not significantly increase the risk of endometrial cancer, and the incidence rate of endometrial cancer was low (0.62 per 1,000 PY; HR, 2.048; 95% CI, 0.658–6.377; P = 0.216).
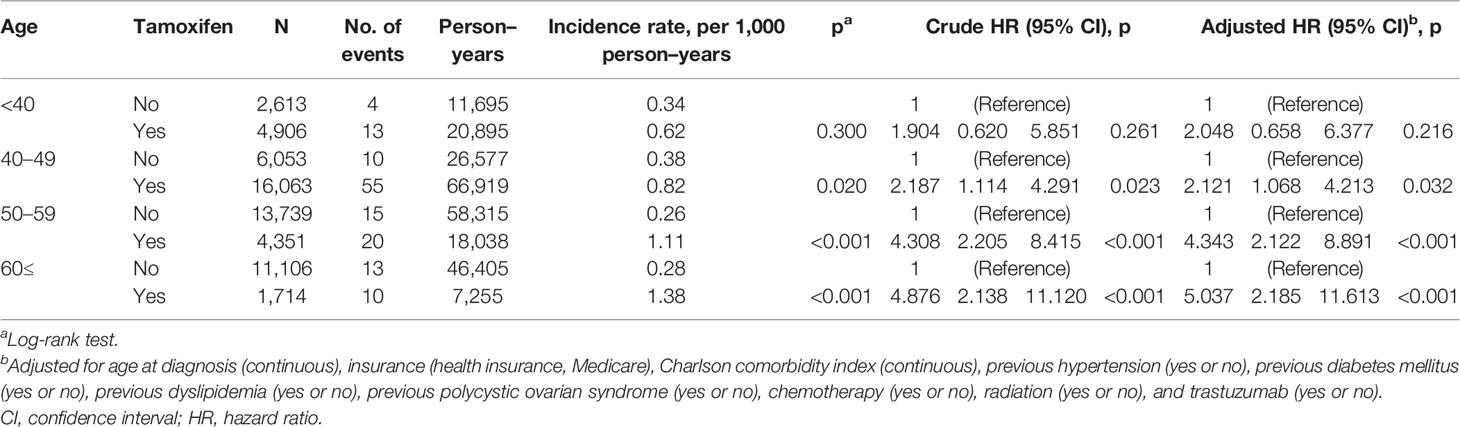
Table 2 Univariate analysis and multivariable Cox regression analysis of endometrial cancer risk related to tamoxifen by age at diagnosis.
Figure 1 Endometrial cancer-free probability in breast cancer survivors by tamoxifen and age at diagnosis.(A) Age<40, (B) Age 40-49, (C) Age 50-59, (D) Age <60.
TAM significantly increased the risk of benign endometrial conditions in all age subgroups (Supplementary Table 2 and Supplementary Figure 2). The incidence of benign endometrial conditions in subjects under 40 years who were treated with TAM (88.60 per 1,000 PY) was the highest in all age subgroups. The incidence of benign endometrial conditions in subjects 60 years or more who were not treated with TAM (10.80 per 1,000 PY) was the lowest in all age subgroups.
Among the TAM subgroups, benign endometrial conditions were significantly related to an increased risk of endometrial cancer in all age subgroups (Table 3). The incidence of endometrial cancer in subjects ≥60 years with benign endometrial conditions was the highest in all age subgroups (5.99 per 1,000 PY). In subjects without benign endometrial conditions, the incidence of endometrial cancer was low.
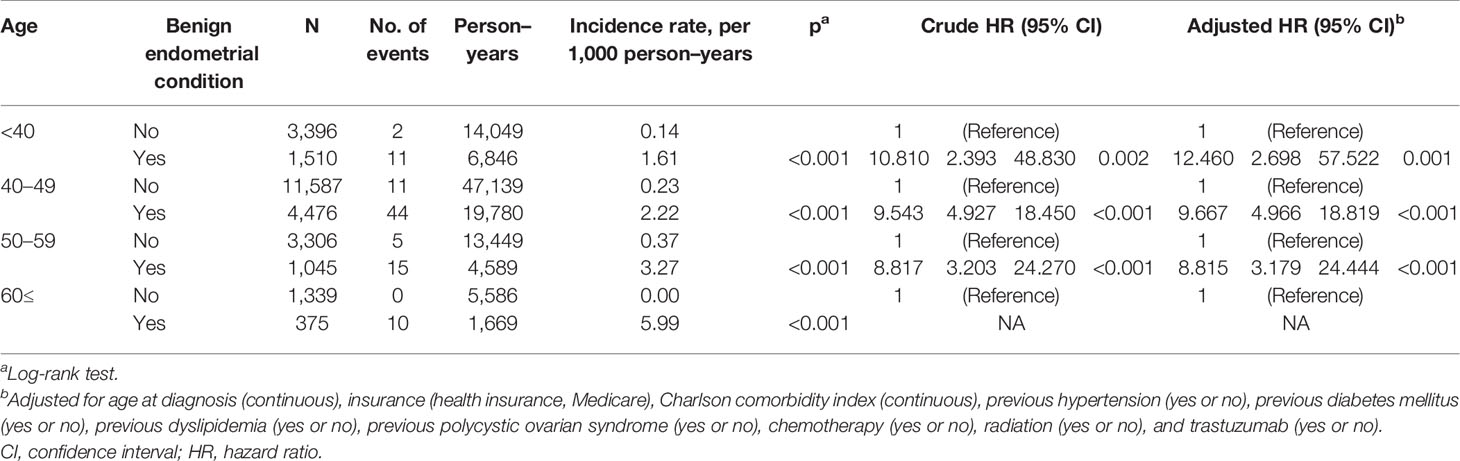
Table 3 Univariate analysis and multivariable Cox regression analysis of endometrial cancer risk related to benign endometrial condition in patients treated with tamoxifen.
In younger breast cancer survivors aged under 40 years (HR, 12.460; 95% CI, 2.698–57.522; P = 0.001) and 40–49 years (HR, 9.667; 95% CI, 4.966–18.819; P < 0.001), benign endometrial conditions significantly increased the risk of endometrial cancer, although the actual incidence rates of endometrial cancer were lower than those of their older counterparts (1.61 per 1,000 PY in subjects under 40 years; 2.22 per 1,000 PY in subjects aged 40–49 years).
The frequencies of invasive endometrial procedures were higher in the TAM groups than those in the non-TAM subgroups (Table 4 and Figure 2). Among the TAM group aged 60 years or more, invasive endometrial evaluations and D&C were performed about 18 and 23 times to detect one endometrial cancer, respectively. However, in the TAM group aged below 40 years, invasive endometrial evaluations and D&C were conducted more than 46 and 54 times to find one endometrial cancer, respectively.
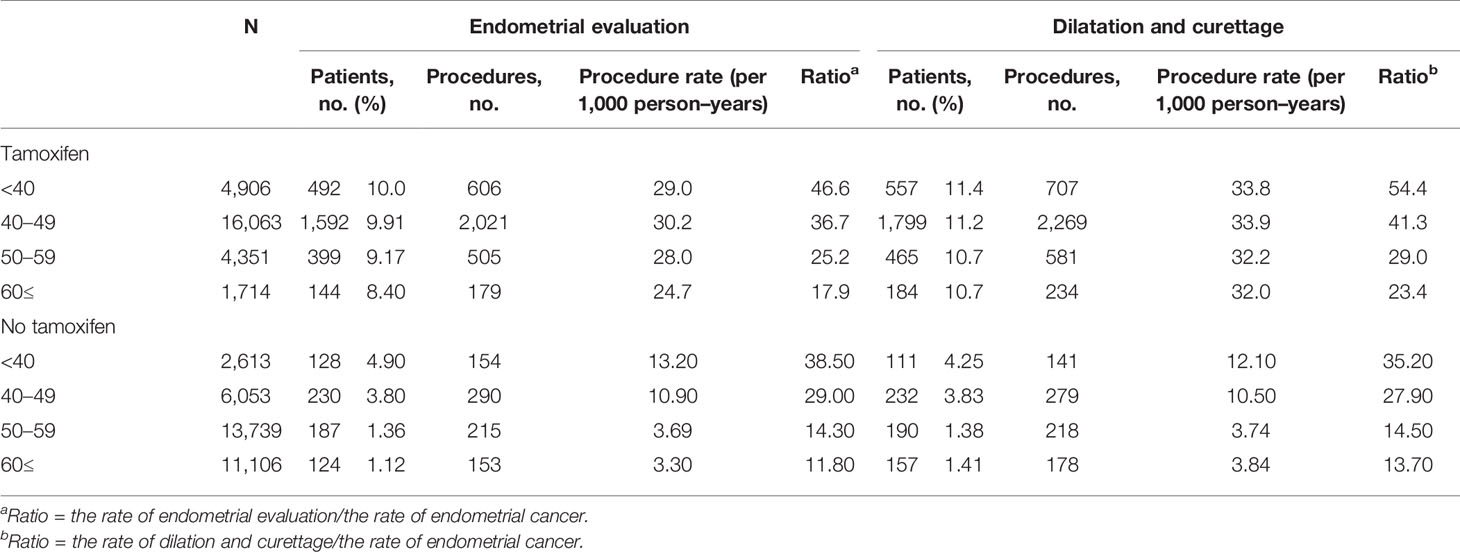
Table 4 Frequencies of endometrial procedures in breast cancer survivors 1 year after initiation of treatment.
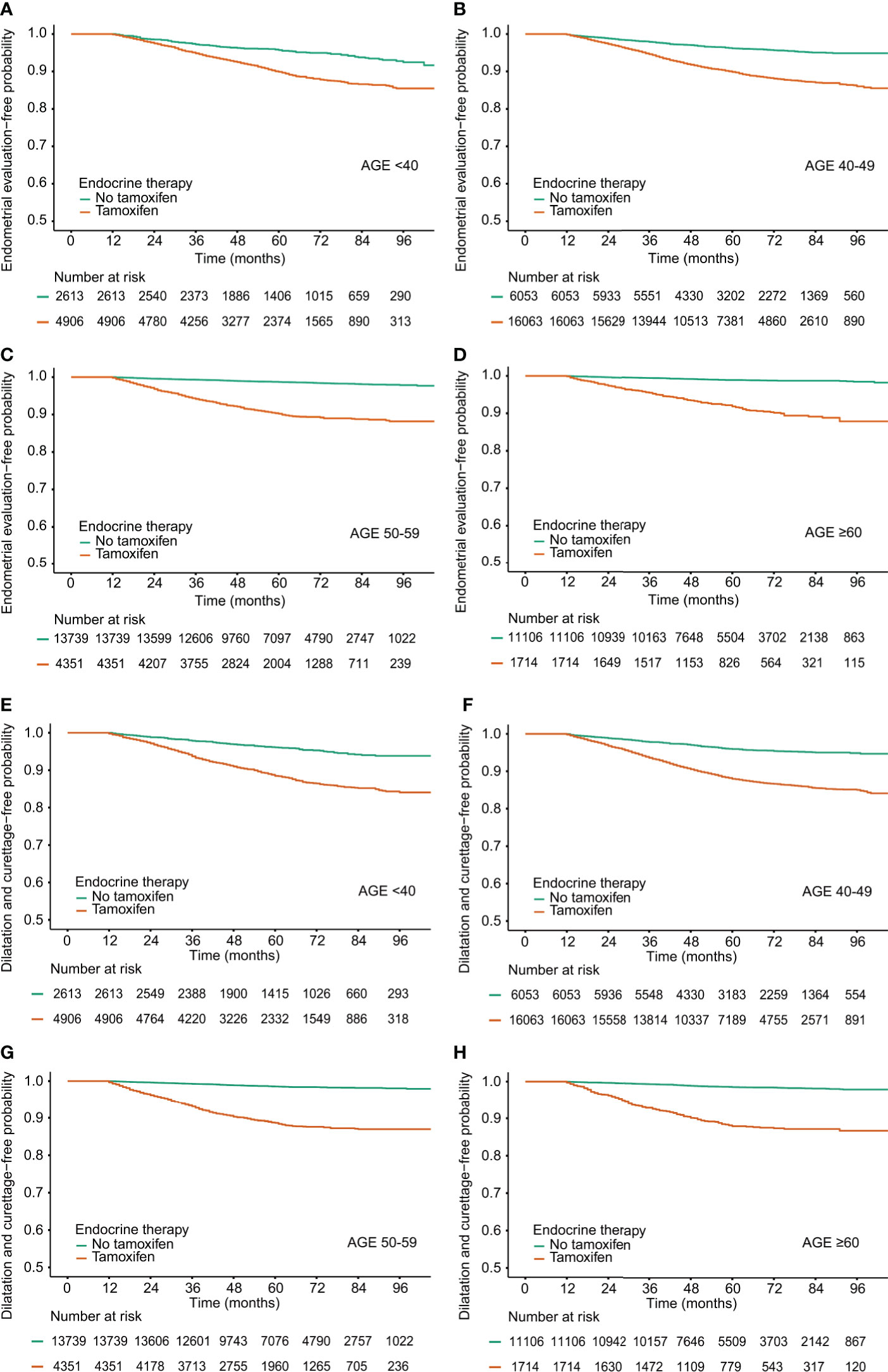
Figure 2 Procedure-free probability in breast cancer survivors by tamoxifen and age at diagnosis. (A–D) Endometrial evaluation, (E–H) Dilatation and Curettage.
Among breast cancer survivors treated with TAM, the rates of invasive endometrial procedures were higher in subjects with benign endometrial conditions (Supplementary Table 3). Among the TAM-treated subjects with benign endometrial conditions, the ratios of the frequency of invasive diagnostic procedures to the incidence of endometrial cancer were 49.0 (endometrial evaluation) and 59.5 (D&C) in subjects under 40 years. However, in subjects aged 60 or more, the ratios were 14.6 (endometrial evaluation) and 21.2 (D&C), respectively.
This study demonstrated that the risk of endometrial cancer increased in young breast cancer survivors on TAM. Even young breast cancer survivors aged below 40 years who had TAM-related benign endometrial conditions such as vaginal bleeding, endometrial hyperplasia, and endometrial polyp showed a significantly increased risk of endometrial cancer compared with those without benign endometrial conditions. Although the incidence rates of endometrial cancer in younger breast cancer survivors were lower than those of their older counterparts; the frequencies of invasive endometrial procedures were higher in younger breast cancer survivors than in their older counterparts.
To our knowledge, this is the first nationwide study to demonstrate the increased risk of endometrial cancer in young breast cancer survivors treated with TAM. Previous studies were limited by the fact that the sample size was not enough to assess the endometrial cancer risk in premenopausal breast cancer survivors on TAM treatment. The National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 study, where the current guidelines are based, indicated that treatment with TAM did not increase the endometrial cancer risk in premenopausal women and that additional monitoring was not required (10). However, this study enrolled 5,077 patients aged below 50 years (2,596 in the placebo group and 2,581 in the TAM group), and the number of participants aged below 40 years was 244 (185 in the placebo group and 159 in the TAM group). Of the total sample aged below 50 years, 17 (eight in the placebo group and nine in the TAM group) patients developed endometrial cancer. Treatment with TAM did not significantly increase the risk of endometrial cancer in women aged below 50 years (risk ratio, 1.21; 95% CI, 0.41–3.60). From the NASBP B-14 study, the incidence of endometrial cancer in TAM-treated breast cancer survivors were investigated (1). This study enrolled 2,843 participations (1,424 in the placebo group and 1,419 in the TAM group), and only 62 patients were aged below 50 years. A total of 15 patients (zero in the placebo group and 15 in the TAM group) developed endometrial cancer; of them, only one patient in the TAM group was aged below 50 years. These results suggest that the previous studies may underestimate the risk of endometrial cancer in TAM-treated premenopausal breast cancer patients. In our study, 29,635 breast cancer survivors aged below 50 years were included, and 68 of them developed endometrial cancer. Meanwhile, 7,519 young breast cancer survivors aged below 40 years were investigated.
Because the current international guidelines are based on the previous studies, gynecological assessment is not recommended for premenopausal women taking TAM. According to the American Cancer Society/American Society of Clinical Oncology Survivorship Care Guidelines, clinicians should perform annual gynecological assessments in postmenopausal women taking TAM, and patients should inform their physicians if unexpected bleeding occurs (11). According to the 2014 American College of Obstetricians and Gynecologists guidelines, postmenopausal women taking TAM should monitor for symptoms of endometrial disease or cancer (9). In the European Society for Medical Oncology guidelines for postmenopausal breast cancer survivors, appropriate diagnostic tests are recommended to be carried out in those with symptoms of endometrial hyperplasia (15).
In our study, young breast cancer survivors aged below 50 years who had TAM-related endometrial diseases or symptoms showed a significantly increased risk of endometrial cancer. This suggests that gynecological assessments of endometrial cancer in these young breast cancer survivors on higher risk of developing endometrial cancer should be considered. However, the actual incidence rates of endometrial cancer in younger breast cancer survivors (1.61 per 1,000 PY in subjects under 40 years; 2.22 per 1,000 PY in subjects aged 40–49 years) were lower than those of their older counterparts (3.27 per 1,000 PY in subjects in their 50s; 5.99 per 1,000 PY in subjects ≥60 years), and therefore balanced decisions on screening for endometrial cancer should be made.
The guidelines indicated that more attention should be paid to the symptoms related to endometrial hyperplasia. In patients who did not develop any adverse effects after taking TAM, initial screening and regular Papanicolaou smear examinations are usually recommended because the benefits of routine endometrial surveillance in asymptomatic patients on TAM therapy remain unknown. There are no clear guidelines on which gynecological examination should be performed (16, 17). In previous studies, endometrial biopsy or transvaginal ultrasound was not used to screen asymptomatic women receiving TAM (18, 19). Although another previous study reported that there was no significant increase in the incidence of endometrial cancer in young breast cancer patients aged below 40 years, the study did not analyze the presence or absence of benign endometrial symptoms and disease (20). In our study, the risk of endometrial cancer increased only in young breast cancer survivors with TAM-related endometrial benign disease. Hence, it may be appropriate to assess for signs of endometrial hyperplasia like abnormal bleeding, including spotting and abnormal vaginal discharge in young breast cancer survivors on TAM.
In a large-scale randomized controlled trial of female breast cancer survivors, treatment with TAM for 10 years further reduced the recurrence and mortality rates, particularly after 10 years, compared with discontinuing this treatment after 5 years (21, 22). Extended adjuvant TAM therapy is associated with an increase in the risk of endometrial cancer (23). The longer the TAM treatment period is, the more important endometrial cancer screening through gynecological examination can become. Young breast cancer survivors undergoing extended endocrine therapy with TAM up to 10 years should be informed of the possible risk of developing endometrial cancer and to monitor for symptoms related to benign endometrial disease. TAM administered at dose of 5 mg/d for 3 years can reduce the recurrence of breast cancer by 50% with a limited toxicity and may be used as a new treatment option for these patients (24).
To the best of our knowledge, the frequencies of gynecological diagnostic procedures among young breast cancer survivors in the real-world setting have rarely been reported (25). Invasive procedure can cause harm to these young patients. In this study, young breast cancer survivors with TAM-related endometrial conditions showed a significantly increased risk of endometrial cancer. However, the ratios of endometrial evaluation and D&C to the incidence of endometrial cancer in breast cancer survivors aged below 50 years were higher than those in breast cancer survivors aged 50 years and older. Considering the actual incidence of endometrial cancer by age, these invasive procedures should be cautiously opted to avoid causing unnecessary harm.
This study has some limitations. First, the HIRA data lacked information on laboratory examinations, imaging studies, family history, and pathological outcomes such as cancer stage, hormone receptor status, and HER2 overexpression. Second, patients’ adherence to individual treatments could not be specified. Third, vaginal ultrasound exams were not able to be analyzed because information about ultrasound is not archived in the HIRA database. Fourth, clinical results such as recurrence, metastasis, or the cause of death were not available. Only in-hospital mortality was assessed. Lastly, although pregnancy is known to reduce the risk of endometrial cancer, the multivariate analyses in this study were not adjusted by this factor due to insufficient data.
In conclusion, young breast cancer survivors with TAM-related endometrial benign disease are at a higher risk of developing endometrial cancer. Clinicians should assess for benign endometrial conditions in premenopausal breast cancer survivors who are taking TAM. Gynecological surveillance should be tailored to the risk of endometrial cancer in young breast cancer survivors to improve the early detection of endometrial cancer and avoid unnecessary invasive procedures.
The datasets generated for this study is not publicly available. These datasets are from the Korean national database, and are not allowed to be extracted from the server by laws. Requests to access the datasets should be directed to the Health Insurance Review and Assessment Service.
The studies involving human participants were reviewed and approved by the Institutional Review Board of Asan Medical Center (IRB no. S2019-1702-0001). Written informed consent from the participants’ legal guardian/next of kin was not required in accordance with the national legislation and the institutional requirements.
SC: planned, wrote, and revised the article. JHJ, YJL, JJ, JL, HK, BK, BS, and SA reviewed and edited the article. YL: planned, analyzed the data, and performed the statistics. IC: planned, wrote, revised, supervised, and designed the concept of the article. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We extend our gratitude to the Health Insurance Review and Assessment Service for providing the nationwide data.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.636378/full#supplementary-material
Supplementary Figure 1 | Study population.
Supplementary Figure 2 | Benign endometrial condition-free probability in breast cancer survivors by tamoxifen and age at diagnosis.
1. Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial Cancer in Tamoxifen-Treated Breast Cancer Patients: Findings From the National Surgical Adjuvant Breast and Bowel Project (Nsabp) B-14. J Natl Cancer Inst (1994) 86(7):527–37. doi: 10.1093/jnci/86.7.527
2. Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update. J Clin Oncol (2014) 32(21):2255–69. doi: 10.1200/JCO.2013.54.2258
3. Gallicchio L, Lord G, Tkaczuk K, Danton M, Lewis LM, Lim CK, et al. Association of Tamoxifen (TAM) and TAM Metabolite Concentrations With Self-Reported Side Effects of TAM in Women With Breast Cancer. Breast Cancer Res Treat (2004) 85(1):89–97. doi: 10.1023/B:BREA.0000021050.92539.b0
4. Ganz PA. Impact of Tamoxifen Adjuvant Therapy on Symptoms, Functioning, and Quality of Life. J Natl Cancer Inst Monogr (2001) 2001(30):130–4. doi: 10.1093/oxfordjournals.jncimonographs.a003450
5. Chlebowski RT, Kim J, Haque R. Adherence to Endocrine Therapy in Breast Cancer Adjuvant and Prevention Settings. Cancer Prev Res (Phila) (2014) 7(4):378–87. doi: 10.1158/1940-6207.CAPR-13-0389
6. Jones ME, van Leeuwen FE, Hoogendoorn WE, Mourits MJ, Hollema H, van Boven H, et al. Endometrial Cancer Survival After Breast Cancer in Relation to Tamoxifen Treatment: Pooled Results From Three Countries. Breast Cancer Res (2012) 14(3):R91. doi: 10.1186/bcr3206
7. Seoud M, Shamseddine A, Khalil A, Salem Z, Saghir N, Bikhazi K, et al. Tamoxifen and Endometrial Pathologies: A Prospective Study. Gynecol Oncol (1999) 75(1):15–9. doi: 10.1006/gyno.1999.5519
8. Swerdlow AJ, Jones ME, British Tamoxifen Second Cancer Study Group. Tamoxifen Treatment for Breast Cancer and Risk of Endometrial Cancer: A Case-Control Study. J Natl Cancer Inst (2005) 97(5):375–84. doi: 10.1093/jnci/dji057
9. Committee opinion. No. 601: Tamoxifen and Uterine Cancer. Obstet Gynecol (2014) 123(6):1394–7. doi: 10.1097/01.AOG.0000450757.18294.cf
10. Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for Prevention of Breast Cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst (1998) 90(18):1371–88. doi: 10.1093/jnci/90.18.1371
11. Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol (2016) 34(6):611–35. doi: 10.1200/JCO.2015.64.3809
12. Kim JA, Yoon S, Kim LY, Kim DS. Towards Actualizing the Value Potential of Korea Health Insurance Review and Assessment (HIRA) Data as a Resource for Health Research: Strengths, Limitations, Applications, and Strategies for Optimal Use of HIRA Data. J Korean Med Sci (2017) 32(5):718–28. doi: 10.3346/jkms.2017.32.5.718
13. Chung IY, Lee J, Park S, Lee JW, Youn HJ, Hong JH, et al. Nationwide Analysis of Treatment Patterns for Korean Breast Cancer Survivors Using National Health Insurance Service Data. J Korean Med Sci (2018) 33(44):e276. doi: 10.3346/jkms.2018.33.e276
14. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med Care (2005) 43(11):1130–9. doi: 10.1097/01.mlr.0000182534.19832.83
15. Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up†. Ann Oncol (2019) 30(8):1194–220. doi: 10.1093/annonc/mdz173
16. Neven P, Vernaeve H. Guidelines for Monitoring Patients Taking Tamoxifen Treatment. Drug Saf (2000) 22(1):1–11. doi: 10.2165/00002018-200022010-00001
17. Goldstein SR. Controversy About Uterine Effects and Safety of SERMs: The Saga Continues. Menopause (2002) 9(5):381–4. doi: 10.1097/00042192-200209000-00012
18. Barakat RR, Gilewski TA, Almadrones L, Saigo PE, Venkatraman E, Hudis C, et al. Effect of Adjuvant Tamoxifen on the Endometrium in Women With Breast Cancer: A Prospective Study Using Office Endometrial Biopsy. J Clin Oncol (2000) 18(20):3459–63. doi: 10.1200/JCO.2000.18.20.3459
19. Gerber B, Krause A, Muller H, Reimer T, Külz T, Makovitzky J, et al. Effects of Adjuvant Tamoxifen on the Endometrium in Postmenopausal Women With Breast Cancer: A Prospective Long-Term Study Using Transvaginal Ultrasound. J Clin Oncol (2000) 18(20):3464–70. doi: 10.1200/JCO.2000.18.20.3464
20. Chu SC, Hsieh CJ, Wang TF, Hong MK, Chu TY. Younger Tamoxifen-Treated Breast Cancer Patients Also had Higher Risk of Endometrial Cancer and the Risk Could be Reduced by Sequenced Aromatase Inhibitor Use: A Population-Based Study in Taiwan. Ci Ji Yi Xue Za Zhi (2020) 32(2):175–80. doi: 10.4103/tcmj.tcmj_17_19
21. Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-Term Effects of Continuing Adjuvant Tamoxifen to 10 Years Versus Stopping at 5 Years After Diagnosis of Oestrogen Receptor-Positive Breast Cancer: ATLAS, a Randomised Trial. Lancet (2013) 381(9869):805–16. doi: 10.1016/S0140-6736(12)61963-1
22. Gray RG, Rea D, Handley K, Bowden SJ, Perry P, Earl HM, et al. Attom: Long-term Effects of Continuing Adjuvant Tamoxifen to 10 Years Versus Stopping at 5 Years in 6,953 Women With Early Breast Cancer. J Clin Oncol (2013) 31(18 suppl):5–. doi: 10.1200/jco.2013.31.18_suppl.5
23. Fleming CA, Heneghan HM, O’Brien D, McCartan DP, McDermott EW, Prichard RS. Meta-Analysis of the Cumulative Risk of Endometrial Malignancy and Systematic Review of Endometrial Surveillance in Extended Tamoxifen Therapy. Br J Surg (2018) 105(9):1098–106. doi: 10.1002/bjs.10899
24. DeCensi A, Puntoni M, Guerrieri-Gonzaga A, Caviglia S, Avino F, Cortesi L, et al. Randomized Placebo Controlled Trial of Low-Dose Tamoxifen to Prevent Local and Contralateral Recurrence in Breast Intraepithelial Neoplasia. J Clin Oncol (2019) 37(19):1629–37. doi: 10.1200/JCO.18.01779
Keywords: breast neoplasms, dilatation and curettage, gynecological examination, tamoxifen, endometrial neoplasms
Citation: Choi S, Lee YJ, Jeong JH, Jung J, Lee JW, Kim HJ, Ko BS, Son BH, Ahn SH, Lee Y and Chung IY (2021) Risk of Endometrial Cancer and Frequencies of Invasive Endometrial Procedures in Young Breast Cancer Survivors Treated With Tamoxifen: A Nationwide Study. Front. Oncol. 11:636378. doi: 10.3389/fonc.2021.636378
Received: 01 December 2020; Accepted: 06 May 2021;
Published: 03 June 2021.
Edited by:
Seema Singh, Mitchell Cancer Institute, United StatesReviewed by:
Shereen Elazzazy, Hamad Medical Corporation, QatarCopyright © 2021 Choi, Lee, Jeong, Jung, Lee, Kim, Ko, Son, Ahn, Lee and Chung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Il Yong Chung, ZG9vcmtlZXBlcjFAZ21haWwuY29t; Yura Lee, aGFlcGFyeUBuYXZlci5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.