
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 26 February 2021
Sec. Women's Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.634857
Background: The Cancer Genome Atlas (TCGA) project shed light on the vital role of tumor molecular features in predicting endometrial cancer patients’ prognosis. This study aims to investigate the survival impact of surgical approaches on patients with different genetic alterations.
Methods: 473 endometrial cancer patients from TCGA database were selected. To analyze the prognostic impact of surgical approach, survival analyses were conducted in patients with different molecular features. Finally, a simplified molecular stratification model was established to select patients suitable for open or minimally invasive surgery (MIS).
Results: In our cohort, 291 patients received open surgery and 182 received MIS. Molecular features influenced patients’ survival after different surgical approaches. Based on survival analyses, three molecular subtypes were generated, with subtype 1 harboring POLE mutation (POLEmt), microsatellite-instability high (MSI-H), homologous recombination repair (HRR) pathway mutation or MUC16 mutation (MUC16mt); subtype 3 carrying TP53 mutation; and subtype 2 without specific molecular feature. The survival influence of molecular subtypes depended on surgical approaches. In the open surgery cohort, three subtypes showed similar survival outcome, while in the MIS cohort, prognosis varied significantly among three subtypes, with subtype 1 the best and subtype 3 the worst. In stepwise Cox regression, molecular subtype was an independent predictor of recurrence-free survival in patients receiving MIS (p < 0.001).
Conclusion: The molecular features of endometrial cancer are associated with patients’ prognosis after different surgical approaches. MIS should be recommended in patients with POLEmt, MSI-H, HRR pathway mutation or MUC16mt, while for patients with TP53 mutation, open surgery is better concerning oncological safety.
Endometrial cancer is one of the most common gynecologic malignancies both in western countries and around the world (1, 2). During the past decades, some vital clinical trials, including LAP2 (3, 4), GOG 258 (5), the Post-Operative Radiation Therapy in Endometrial Carcinoma (PORTEC) serial trials (6–8), etc., have been carried out for refining endometrial cancer treatment, with a goal of designing individualized surgical and adjuvant therapy strategy. In particular, the advantages of minimally invasive surgery (MIS) over open surgery have been proved in two large trials, LAP2 and the Laparoscopic Approach to Cancer of the Endometrium (LACE) trial (3, 4, 9, 10). In a meta-analysis including 9 studies, the non-inferiority of total laparoscopic hysterectomy (TLH) versus total abdominal hysterectomy (TAH) regarding endometrial cancer patients’ long term survival was further demonstrated (11).
Until now, the treatment of endometrial cancer is basically determined by clinicopathological staging and patients’ risk stratification. But in recent years, increasingly more attention has been paid to tumor molecular features. In 2013, the Cancer Genome Atlas (TCGA) research network proposed four molecular subtypes of endometrial cancer based on multi-omics analysis (12), which linked molecular features to endometrial cancer patients’ survival outcomes. Besides, data about molecular targeted therapies and immunotherapies in patients with certain genetic alterations are accumulating. And in the currently ongoing PORTEC-4a trial, researchers are trying to decide radiotherapy strategy based on patients’ molecular risk profile in stage I endometrial cancer (13). These lead us to reconsider the possible survival influence of surgical approaches (open surgery vs. MIS) on patients with distinct molecular features.
Previously, our study proved that tumor microsatellite status influenced the recurrence of endometrial cancer after different surgical approaches (14). This retrospective study based on TCGA data aims to further group patients by certain genetic alterations and analyze their survival outcome after MIS or open surgeries.
In the study, the clinical and genetic data of TCGA Uterine Corpus Endometrial Carcinoma (UCEC) project were downloaded from the Genome Data Commons (GDC) Data Portal (https://portal.gdc.cancer.gov), the UCSC-Xena platform (15) and cBioPortal (16, 17). According to previous studies (12, 18–21), six vital genetic features related to patients’ prognosis (POLE, microsatellite status, homologous recombination repair [HRR] pathways, MUC16, CTNNB1, TP53) were selected for study. HRR mutation was defined as missense mutations, nonsense mutations, insertions, deletions or splice mutations in genes of HRR related pathways, as reported by the previous study (22) (see Table S1 for the entire gene list used for deciding HRR mutation in the study).
There were totally 548 patients in the database. No patient had a history of neoadjuvant therapy. Five patients with colorectal cancer history, 24 without information of surgical approach and 46 with incomplete genetic information were excluded, and finally 473 eligible patients were selected for further analysis. The study protocol was exempted by the Institutional Review Board of Peking University People’s Hospital since only deidentified data from a public database was used.
The definitions of all clinicopathological terms were based on those defined in the Common Data Element (CDE) Browser 5.3.5 (https://cdebrowser.nci.nih.gov). The American Joint Committee on Cancer (AJCC) Clinical Group Stage system was adopted for the staging of all patients, and the stage of each case was decided according to the version used when the diagnosis was made. For neoplasm grade, there were four different values in the original dataset (G1, G2, G3 and high grade). In accordance with the FIGO criteria (23), G1, G2, and G3 were used to describe well-differentiated, moderately-differentiated and poorly-differentiated tumors, respectively. Since high grade was used to describe tumor samples that exhibit poorly differentiated or undifferentiated cells, cases with a value of high grade were reclassified as G3 in the analysis. Besides, deep myometrial invasion was defined as invasion depth of the tumor ≥50% of the whole thickness of the myometrium.
The median follow-up time for eligible patients was 30.6 months (interquartile range: 18.0 - 56.1 months). We used overall survival (OS) and recurrence-free survival (RFS) as two measures of patients’ survival outcomes. For the cases in TCGA database, OS and RFS were determined from when initial pathological diagnosis of endometrial cancer was made to when death or disease recurrence occurred, respectively. In all survival analyses, cases without any endpoint events were censored at their last follow-up.
In the study, χ2 test and Fisher’s exact test were performed for comparing categorical variables, and student t test was used for comparing continuous variables. The Venn diagram of the distribution of genetic features among eligible patients was drawn using the jvenn online tool (24). Kendall correlation analysis was used to see the correlation between different features. To analyze the survival outcome of patients receiving different surgical approaches, Kaplan-Meier survival analyses (log rank test) were conducted. The results of Kaplan-Meier method were further verified by Cox proportional hazard models. Propensity score matching (PSM) was used for adjusting baseline characteristics. In PSM, a clipper width of 0.02 and a match ratio of 1:1 were adopted. Multivariate stepwise Cox regression (method: backward: conditional; entry criteria: p < 0.05; removal criteria: p > 0.10) was used to reveal independent survival risk factors for the open surgery cohort and the MIS cohort. In stepwise regression, variables with p < 0.05 in univariate analyses were included (lymph node metastasis was excluded for its overlap with disease stage, and postoperative adjuvant therapy was also excluded for collinearity with multiple clinicopathological factors and significant influence on the models). In all Cox regression models, the proportional hazard hypothesis was tested with time-dependent covariates. Statistical analyses were performed using Statistics Package for the Social Sciences (SPSS) software (version 22.0; IBM Corporation, Armonk, NY, USA) and R software version 3.5.3 (https://www.r-project.org/). In all analyses, two-sided p values were used, and p values less than 0.05 were considered statistically significant.
Among all the eligible patients, 291 (61.5%) received open surgery and 182 (38.5%) received MIS. Two surgical approaches did not show significant differences in lymph node resection rate and extent (Table S2). The distribution of six genetic features (POLE mutation [POLEmt], microsatellite-instability high [MSI-H], HRR mutation, MUC16 mutation [MUC16mt], CTNNB1 mutation [CTNNB1mt] and TP53 mutation [TP53mt]) were comparable between the two groups (Table 1). Besides, the distributions of POLEmt, MSI-H, HRR mutation, MUC16mt showed significant overlap, and were highly correlated with each other in the entire cohort (Figure S1).
We divided endometrial cancer cases according to the status of the six genetic features, respectively, and the survival influence of surgical approach in each subgroup was analyzed. In Kaplan-Meier survival analyses, minimally invasive surgery was associated with shorter RFS in POLE wild type (POLEwt), non MSI-H, HRR wild type, MUC16 wild type (MUC16wt), CTNNB1 wild type (CTNNB1wt), or TP53mt patients (p = 0.008, 0.015, 0.003, 0.008, 0.017 and 0.032) (Figure 1). But in the counterpart cohorts, the survival outcome after two surgeries was similar. Cox regressions validated the results above (Table S3).
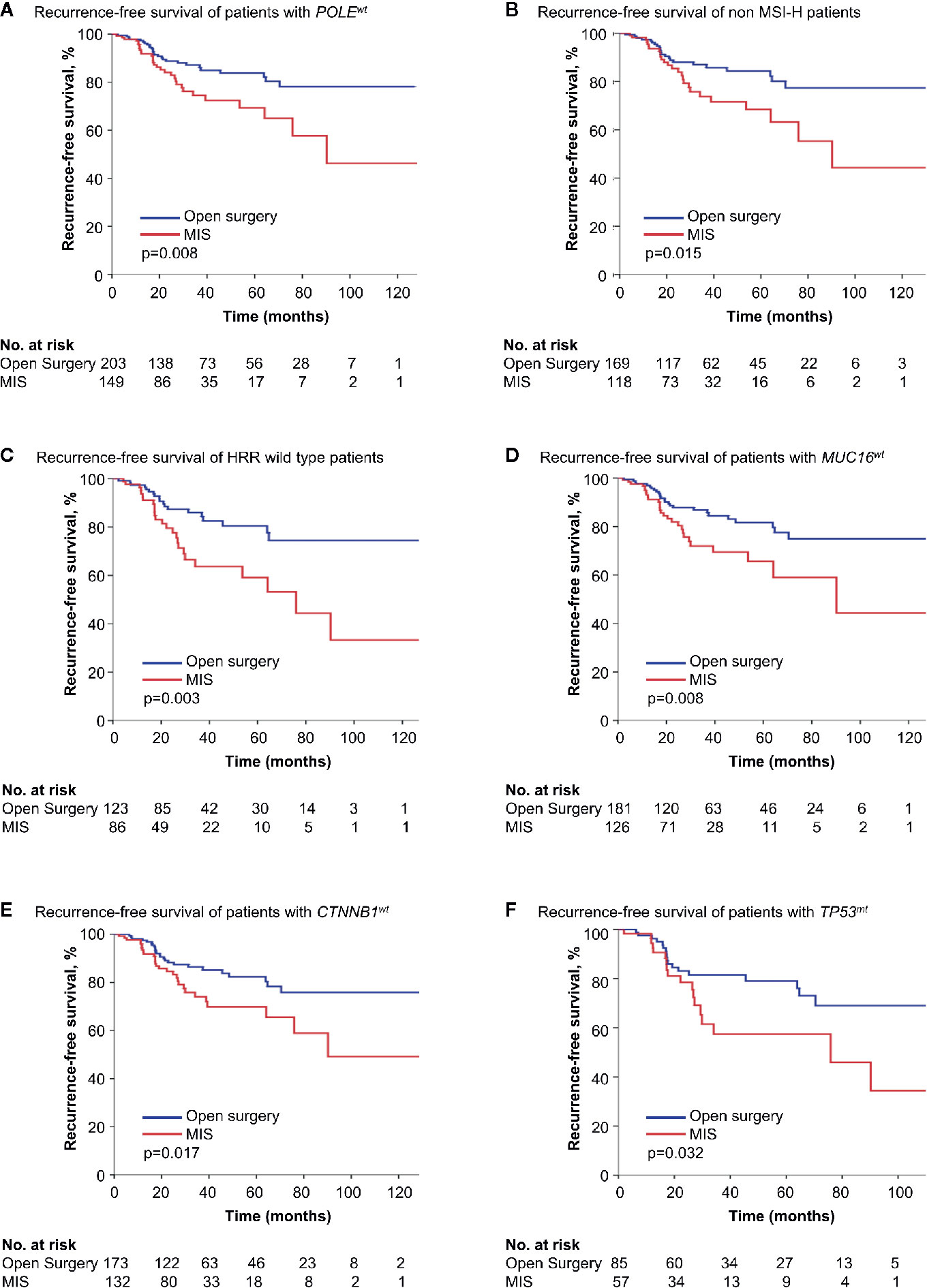
Figure 1 Kaplan-Meier survival curves of patients with different genetic features by surgical approach. (A) Recurrence-free survival of patients with POLEwt. (B) Recurrence-free survival of non MSI-H patients. (C) Recurrence-free survival of HRR with wild type patients. (D) Recurrence-free survival of patients with MUC16wt. (E) Recurrence-free survival of patients with CTNBB1wt. (F) Recurrence-free survival of patients with TP53wt. MIS, minimally invasive surgery; POLEwt, POLE wild type; MSI-H, microsatellite-instability high; HRR, homologous recombination repair; MUC16wt, MUC16 wild type; CTNNB1wt, CTNNB1 wild type; TP53mt, TP53 mutation.
To further reveal the impact of surgical approach on different patients, four TCGA molecular subtypes of endometrial cancer (12) were analyzed separately. In patients of POLE ultramutated and MSI hypermutated type, where one or more of the four alterations (POLEmt, MSI-H, HRR mutation and MUC16mt) presented in most cases, surgical approaches showed no influence on survival. For copy-number low and copy-number high type, still the survival difference between the two surgery groups was not significant enough (p = 0.075 and 0.073 for RFS in Kaplan-Meier analysis) (Figure 2, Table S4). But when further stratification was done based on CTNNB1 and TP53 status, MIS was found to be associated with shorter RFS in copy-number low type with CTNNB1wt and copy-number high type with TP53mt (p = 0.048 and 0.037 in Kaplan-Meier analysis) (Figure S2).
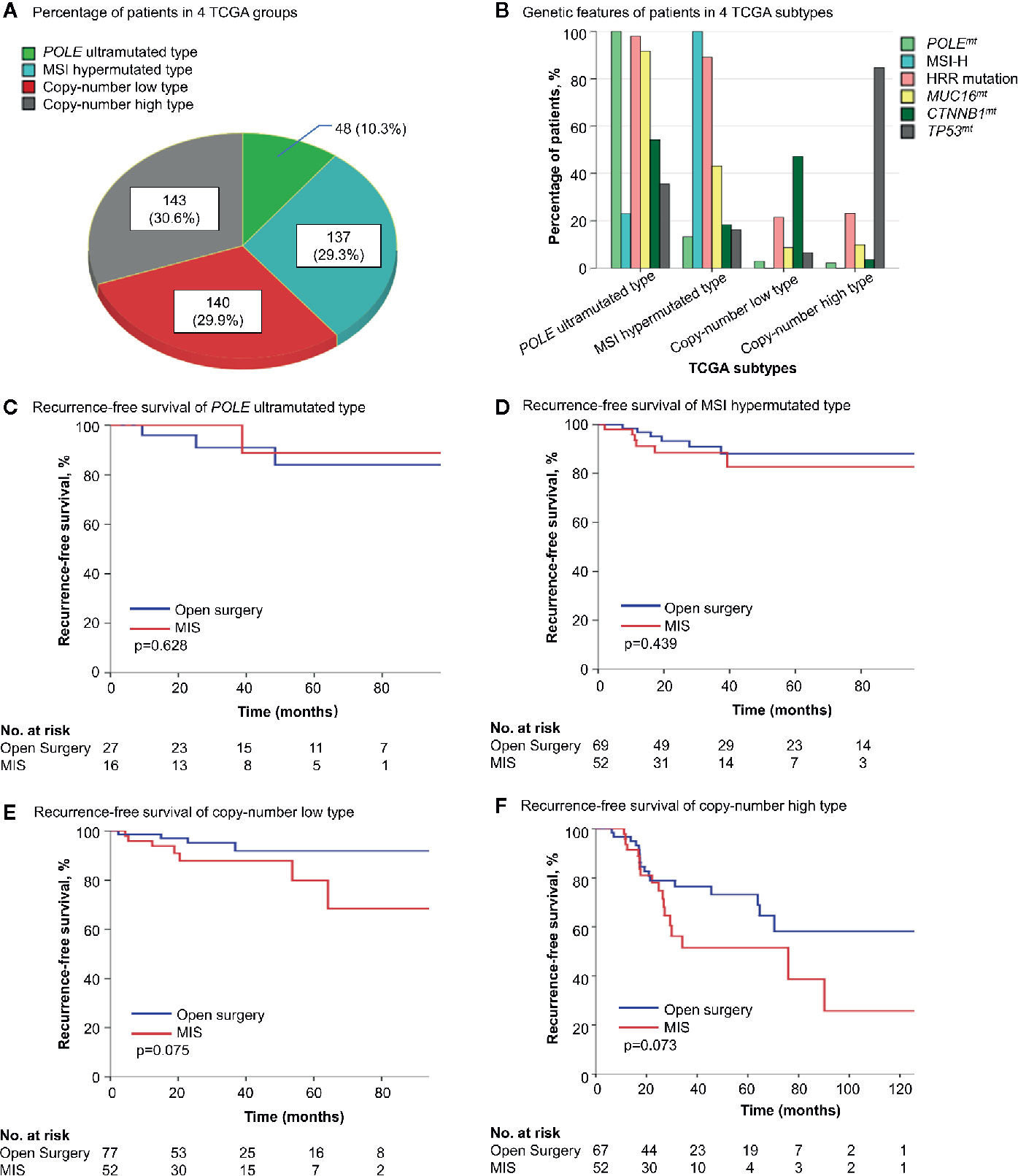
Figure 2 The survival influence of surgical approach in different TCGA molecular subgroups. (A) The distribution of 4 TCGA molecular subtypes in the cohort. (B) Genetic alterations in each molecular subtype. (C–F) Kaplan-Meier survival curves of patients with different molecular features by surgical approach. TCGA, the Cancer Genome Atlas; POLEmt, POLE mutation; MSI-H, microsatellite-instability high; HRR, homologous recombination repair; MUC16mt, MUC16 mutation; CTNNB1mt, CTNNB1 mutation; CTNNB1wt, CTNNB1 wild type; TP53mt, TP53 mutation; TP53wt, TP53 wild type; MIS, minimally invasive surgery.
More efforts were made to establish a simplified model for deciding surgical approach based on molecular markers. According to the survival data, in patients with one or more of the four features (POLEmt, MSI-H, HRR mutation and MUC16mt), open surgery and MIS group showed similar survival outcomes, regardless of CTNNB1 and TP53 status. While for the rest, MIS was associated with shortened RFS (p = 0.001 in Kaplan-Meier survival analysis and Cox regression). Further stratification was made among the latter based on CTNNB1 and TP53 status, and three subgroups were generated: CTNNB1 mutant subgroup, TP53 mutant subgroup, no specific molecular feature subgroup (Table S5). Since only in TP53 mutant subgroup was the prognostic effect of MIS significant (p < 0.001 in Kaplan-Meier survival analysis, p = 0.001 in Cox regression for RFS, Table S5), we combined the other two subgroups together, and got three independent molecular subtypes. Patients with ≥1 of the four features (POLEmt, MSI-H, HRR mutation and MUC16mt) were named subtype 1. Among the remaining, patients without specific molecular features were named subtype 2, and those with TP53 mutations were named subtype 3.
The three molecular subtypes varied greatly in multiple clinicopathological characteristics (Table 2). We then compared the baseline features of open surgery and MIS cohort within each molecular subtype (Table S6). Since postoperative chemotherapy rate was significantly higher in the MIS group in subtype 3 (p = 0.043), PSM was used to adjust for it (matching variable: postoperative chemotherapy). And survival analyses based on the cohorts after PSM (Table S7) further confirmed the result that open surgery was associated with longer RFS in this subtype (Figure S3).
Significant differences in OS and RFS were observed among different subtypes (p < 0.001 for OS and RFS in Kaplan-Meier survival analysis, Figure 3). And the prognostic difference was influenced by surgical approaches. In the open surgery cohort, OS and RFS were similar (p = 0.052 and 0.603, respectively), while in the MIS cohort, three subtypes varied significantly in OS and RFS (p < 0.001 for both), with subtype 1 prognostically the best and subtype 3 the worst (Figure 3). In univariate Cox regressions, the influence of molecular subtype was significant for both OS and RFS in the MIS cohort, but not in the open surgery cohort (Table 3). And in multivariate stepwise regressions, molecular subtype was independently associated with disease recurrence in the MIS cohort (p < 0.001) (Table 4).
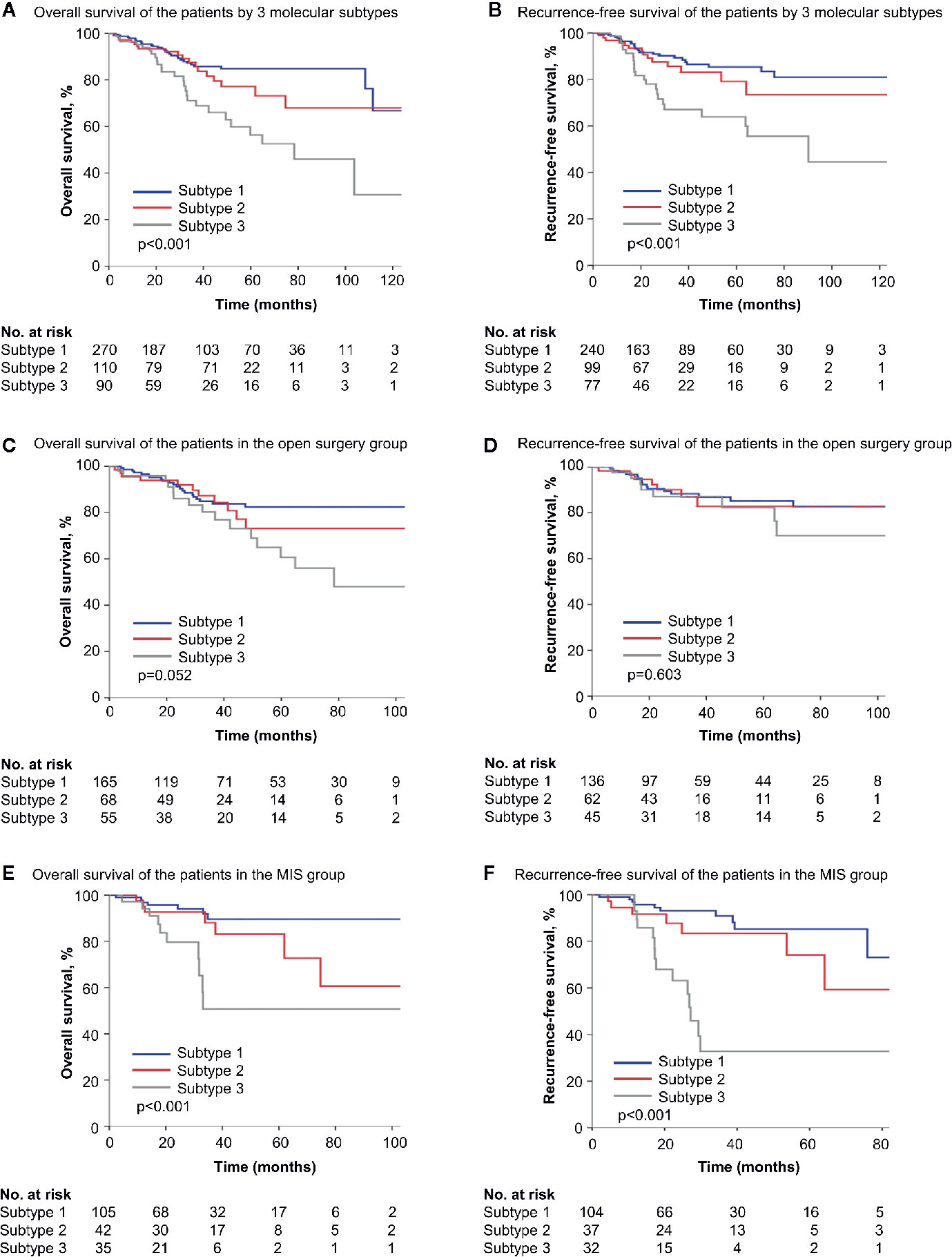
Figure 3 Survival of patients by simplified molecular subtypes. (A, B) Kaplan-Meier survival curves of all patients by three molecular subtypes. (C, D) Kaplan-Meier survival curves of the open surgery cohort by three molecular subtypes. (E, F) Kaplan-Meier survival curves of the MIS cohort by three molecular subtypes. MIS, minimally invasive surgery.
Since the TCGA molecular classification of endometrial cancer was proposed (12), some genomic studies have been conducted to establish an integrated molecular risk stratification system (21, 25, 26). And further efforts were made to deliver therapies tailored to certain molecular alterations (27). In this study, we analyzed the influence of surgical approaches on patients in different molecular subgroups. Certain molecular features were proved to influence endometrial cancer patients’ survival after open surgeries or MIS. A TCGA-based model for choosing surgical approaches and a simplified model based on three molecular subtypes were established accordingly (Figure 4).
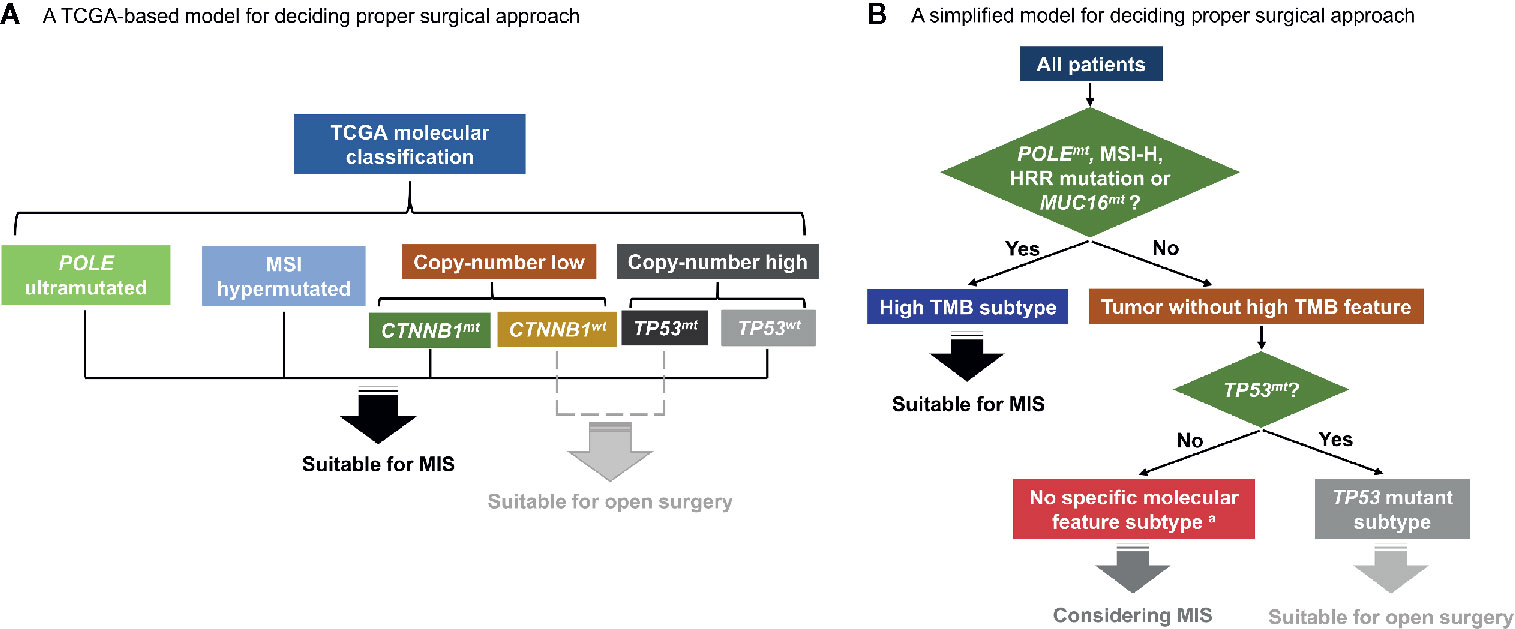
Figure 4 Hypothetical schemas for choosing surgical approaches based on patients’ molecular features. (A) A TCGA-based model for deciding proper surgical approach. (B) A simplified model for deciding proper surgical approach. TCGA, the Cancer Genome Atlas; MSI, microsatellite-instability; CTNNB1mt, CTNNB1 mutation; CTNNB1wt, CTNNB1 wild type; TP53mt, TP53 mutation; TP53wt, TP53 wild type; MIS, minimally invasive surgery; POLEmt, POLE mutation; MSI-H, microsatellite-instability high; HRR, homologous recombination repair; MUC16mt, MUC16 mutation; TMB, tumor mutation burden. a Patients with concurrent TP53mt and CTNNB1mt are excluded and should be separately discussed.
Our previous work has demonstrated that MIS was associated with shorter RFS in microsatellite-stable (MSS) endometrioid endometrial cancer patients compared with the open counterpart, while in other patients, long-term survival in the two surgery groups was similar (14). Surgical manipulations and tumor biological properties, especially elevated mutation load, were thought to account for the difference (14). Recently, compromised DNA damage repair, including mutations in POLE exonuclease domain, mismatch repair pathways and HRR pathways, were found to be associated with higher tumor mutation burden (TMB), key clinicopathological features and patients’ prognosis, and could be used for further stratification in endometrial cancer (12, 20, 28–32). In a pan-cancer study by Wang et al. (20), mutations in base-excision repair, mismatch repair, and HRR pathways were demonstrated to be highly correlated with tumor mutation load. And in another recent study, tumors with elevated global mutation load typically showed overall higher mutation rates in multiple DNA damage repair pathways (33). Furthermore, another biomarker, MUC16mt was proved to be also related with tumor mutation burden and better survival in multiple tumor types, including endometrial cancer (18, 34–36). In our study, the four molecular markers were all included, and their prognostic relevance was examined in detail.
Interestingly, in our study, the above four features (POLEmt, MSI-H, HRR mutation, and MUC16mt) were all related with non-inferior survival outcomes after MIS. Based on previous research, weakened tumor cell viability, more neoantigens and stronger antitumor immune response were found to be common features shared by high TMB tumors (20, 28, 29, 37, 38). And peritoneal disseminated tumor cells caused by MIS procedures, which was thought to be one of the sources of disease recurrence (39–41), may be thereby more vulnerable in these circumstances. According to our previous assumption based on tumor microsatellite status (14), which can be generalized to high TMB endometrial cancer, the unique features of such tumors may counterbalance the negative effect of MIS, and results in similar prognosis compared with open surgeries.
In 2015, Stelloo et al. (25) proposed a simplified endometrial cancer classification system using surrogate molecular markers, and identified four prognostic groups including POLE mutant, MSI-H, TP53 mutant, and no specific molecular profile (NSMP) group. In accordance with the TCGA classification, POLE mutant and MSI-H group showed relatively better survival outcome and TP53 mutant group was prognostically inferior (25). Further attempts were made to incorporate genetic, immunohistochemical, and clinicopathological features into the classification system (21). In the risk stratification system described by Stelloo et al. (21), TP53mt was shown to be one of the unfavorable features, while another molecular marker associated with endometrial cancer recurrence, CTBBN1mt, was an intermediate risk factor (19, 21).
In our study, CTNNB1 status also influenced patients’ survival after different surgical approaches in TCGA copy-number low cohort. But in the simplified model, after considering other concurrent molecular alterations, the value of CTNNB1 in determining suitable surgical approaches was not significant enough. Besides, the survival data of three molecular subtypes in our model was basically consistent with the established models, as mentioned above (21, 25, 26). But a novel finding of our study is that the difference in survival status among three subtypes was greatly influenced by surgical approaches, especially for TP53 mutant tumors. As shown in our data, patients with TP53mt (subtype 3) typically showed worse survival than the other two subtypes in the entire cohort. But in the open surgery cohort, similar prognosis was seen, especially as to patients’ RFS, indicating that for TP53 mutant tumors, the advantage of open surgery was much more prominent. Previous studies demonstrated that TP53mt in endometrial cancer was associated with more adverse pathological factors, advanced stage and worse survival (21, 25, 26, 42), which was in accordance with our data based on TCGA cohort. And for advanced stage endometrial cancer, the completeness of surgical resection is essential for better prognosis (43, 44). In this regard, for endometrial cancer with TP53mt, open surgery may be more suitable due to its better intraoperative detection, larger surgical extent and greater number of lymph nodes resected (though not significant enough in our data, see Table S2).
In this study, a simplified model based on TCGA data was established, and statistical methods, including PSM and multivariate analyses were conducted for internal validation. Similar to TCGA molecular classification, our model also showed strong associations with clinicopathological parameters, and exhibited good prognostic value. But instead of using multi-omics data, we established the model based on surrogate molecular markers, which enhanced its feasibility in practical use. Besides, the model further helped elucidate the association between tumor molecular features and surgical decisions, providing some novel insights in endometrial cancer treatment.
But some limitations still exist. Firstly, the method of patients’ classification in our model was based on next-generation sequencing (NGS) data, which can hardly be adopted in most medical centers for clinical application, especially in less developed areas. Clinically feasible methods, such as immunohistochemistry, should be studied, and more data are needed to prove the efficacy of surrogate methods. Secondly, in this study, only endometrioid and serous type endometrial cancer were included, further verification of the model in other histological subtypes, like clear cell carcinoma, etc., is essential. Thirdly, as we have pointed out before (14), TCGA as a public database, is limited in inter-case consistency, especially as to surgical manipulations, which may differ among different regions, hospitals and surgeons. Besides, in TCGA cohort, the follow-up time was relatively short, as median OS and RFS were not seen in most subgroups. Further studies based on different cohorts with longer follow-up time are needed as external verification of the results. Finally, though statistical adjustments were conducted, the study was still limited in controlling confounders due to the retrospective nature.
Nowadays, genetic features of endometrial cancer are getting more and more attention from the gynecologic oncology community not only for its significance in identifying patients with higher recurrence risk, but also for selecting patients potentially benefiting from cancer immune and molecular targeted therapies. On one hand, accumulating evidences support the usage of NGS-guided targeted treatment in endometrial cancer patients for its definite survival benefits (27). And immunotherapies, as an example, is getting increasingly wider clinical application, even beyond MSI-H or mismatch repair deficient tumors (45). On the other hand, molecular-based risk stratifications of endometrial cancer are getting closer association with clinical decision-making. In this study, we linked patients’ genetic features to surgical treatment, and, for the first time, established surgical selection models for precise treatment strategy design in endometrial cancer patients harboring certain molecular alterations. Our results, once again, highlighted the vital role of gene testing in clinical cancer treatment and suggested that surgeries, in combination with adjuvant therapy and targeted drugs, could be delivered in an individualized manner. In the future, further refinement of the model is essential, and further attempts of incorporating clinicopathological factors to establish integrated stratification system may be necessary. Besides, as mentioned above, more studies based on prospective cohorts are needed to validate the models in different countries and larger populations.
Publicly available datasets were analyzed in this study. This data can be found here: https://portal.gdc.cancer.gov/.
YD and ZW planned the study. YD, JYW and LZ contributed to methodology, data extraction and formal analysis. YD wrote the original draft. ZW and JLW supervised the whole study, reviewed, and revised the manuscript. ZW and JLW contributed to funding acquisition. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (grant number 81972426, 81874108), Special Projects for Strengthening Basic Research of Peking University (grant number BMU2018JC005), and National Key Technology R&D Program of China (grant number 2019YFC1005200, 2019YFC1005201).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We appreciate the great efforts of the TCGA Research Network since all the results of this study are based upon data from the TCGA project: https://www.cancer.gov/tcga.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.634857/full#supplementary-material
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
2. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer (2019) 144(8):1941–53. doi: 10.1002/ijc.31937
3. Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol (2009) 27(32):5331–6. doi: 10.1200/JCO.2009.22.3248
4. Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol (2012) 30(7):695–700. doi: 10.1200/JCO.2011.38.8645
5. Matei D, Filiaci V, Randall ME, Mutch D, Steinhoff MM, Disilvestro PA, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med (2019) 380(24):2317–26. doi: 10.1056/NEJMoa1813181
6. Creutzberg CL, Van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. Lancet (2000) 355(9213):1404–11. doi: 10.1016/S0140-6736(00)02139-5
7. Nout R, Smit V, Putter H, Jürgenliemk-Schulz I, Jobsen J, Lutgens L, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet (2010) 375(9717):816–23. doi: 10.1016/S0140-6736(09)62163-2
8. De Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol (2018) 19(3):295–309. doi: 10.1016/s1470-2045(18)30079-2
9. Janda M, Gebski V, Brand A, Hogg R, Jobling TW, Land R, et al. Quality of life after total laparoscopic hysterectomy versus total abdominal hysterectomy for stage I endometrial cancer (LACE): a randomised trial. Lancet Oncol (2010) 11(8):772–80. doi: 10.1016/S1470-2045(10)70145-5
10. Janda M, Gebski V, Davies LC, Forder P, Brand A, Hogg R, et al. Effect of total laparoscopic hysterectomy vs total abdominal hysterectomy on disease-free survival among women with stage I endometrial cancer: A randomized clinical trial. JAMA (2017) 317(12):1224–33. doi: 10.1001/jama.2017.2068
11. Galaal K, Donkers H, Bryant A, Lopes AD. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst Rev (2018) 10:Cd006655. doi: 10.1002/14651858.CD006655.pub3
12. The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature (2013) 497:67–73. doi: 10.1038/nature12113
13. Wortman BG, Bosse T, Nout RA, Lutgens LCHW, van der Steen-Banasik EM, Westerveld H, et al. Molecular-integrated risk profile to determine adjuvant radiotherapy in endometrial cancer: Evaluation of the pilot phase of the PORTEC-4a trial. Gynecol Oncol (2018) 151(1):69–75. doi: 10.1016/j.ygyno.2018.07.020
14. Dai Y, Wang Z, Wang J. Survival of microsatellite-stable endometrioid endometrial cancer patients after minimally invasive surgery: An analysis of the Cancer Genome Atlas data. Gynecol Oncol (2020) 158(1):92–8. doi: 10.1016/j.ygyno.2020.04.684
15. Goldman M, Craft B, Hastie M, Repečka K, McDade F, Kamath A, et al. The UCSC Xena platform for public and private cancer genomics data visualization and interpretation. bioRxiv (2019) 326470. doi: 10.1101/326470
16. Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio Cancer Genomics Portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discovery (2012) 2(5):401–4. doi: 10.1158/2159-8290.CD-12-0095
17. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal (2013) 6(269):pl1. doi: 10.1126/scisignal.2004088
18. Hu J, Sun J. MUC16 mutations improve patients’ prognosis by enhancing the infiltration and antitumor immunity of cytotoxic T lymphocytes in the endometrial cancer microenvironment. Oncoimmunology (2018) 7(10):e1487914. doi: 10.1080/2162402X.2018.1487914
19. Kurnit KC, Kim GN, Fellman BM, Urbauer DL, Mills GB, Zhang W, et al. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod Pathol (2017) 30:1032–41. doi: 10.1038/modpathol.2017.15
20. Wang Z, Zhao J, Wang G, Zhang F, Zhang Z, Zhang F, et al. Comutations in DNA damage response pathways serve as potential biomarkers for immune checkpoint blockade. Cancer Res (2018) 78(22):6486. doi: 10.1158/0008-5472.CAN-18-1814
21. Stelloo E, Nout RA, Osse EM, Jürgenliemk-Schulz IJ, Jobsen JJ, Lutgens LC, et al. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin Cancer Res (2016) 22(16):4215–24. doi: 10.1158/1078-0432.CCR-15-2878
22. Riaz N, Blecua P, Lim RS, Shen R, Higginson DS, Weinhold N, et al. Pan-cancer analysis of bi-allelic alterations in homologous recombination DNA repair genes. Nat Commun (2017) 8(1):857. doi: 10.1038/s41467-017-00921-w
23. Creasman W, Odicino F, Maisonneuve P, Quinn M, Beller U, Benedet J, et al. Carcinoma of the corpus uteri. Int J Gynaecol Obstet (2006) 95:S105–43. doi: 10.1016/S0020-7292(06)60031-3
24. Bardou P, Mariette J, Escudié F, Djemiel C, Klopp C. jvenn: an interactive Venn diagram viewer. BMC Bioinf (2014) 15(1):293. doi: 10.1186/1471-2105-15-293
25. Stelloo E, Bosse T, Nout RA, MacKay HJ, Church DN, Nijman HW, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol (2015) 28(6):836–44. doi: 10.1038/modpathol.2015.43
26. Talhouk A, McConechy MK, Leung S, Li-Chang HH, Kwon JS, Melnyk N, et al. A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer (2015) 113(2):299–310. doi: 10.1038/bjc.2015.190
27. Urick ME, Bell DW. Clinical actionability of molecular targets in endometrial cancer. Nat Rev Cancer (2019) 19(9):510–21. doi: 10.1038/s41568-019-0177-x
28. Temko D, Van Gool IC, Rayner E, Glaire M, Makino S, Brown M, et al. Somatic POLE exonuclease domain mutations are early events in sporadic endometrial and colorectal carcinogenesis, determining driver mutational landscape, clonal neoantigen burden and immune response. J Pathol (2018) 245(3):283–96. doi: 10.1002/path.5081
29. Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res (2016) 22(4):813–20. doi: 10.1158/1078-0432.CCR-15-1678
30. Zhao H, Thienpont B, Yesilyurt BT, Moisse M, Reumers J, Coenegrachts L, et al. Mismatch repair deficiency endows tumors with a unique mutation signature and sensitivity to DNA double-strand breaks. eLife (2014) 3:e02725. doi: 10.7554/eLife.02725
31. Auguste A, Genestie C, De Bruyn M, Adam J, Le Formal A, Drusch F, et al. Refinement of high-risk endometrial cancer classification using DNA damage response biomarkers: a TransPORTEC initiative. Mod Pathol (2018) 31(12):1851–61. doi: 10.1038/s41379-018-0055-1
32. Ashley CW, Da Cruz Paula A, Kumar R, Mandelker D, Pei X, Riaz N, et al. Analysis of mutational signatures in primary and metastatic endometrial cancer reveals distinct patterns of DNA repair defects and shifts during tumor progression. Gynecol Oncol (2019) 152(1):11–9. doi: 10.1016/j.ygyno.2018.10.032
33. Knijnenburg TA, Wang L, Zimmermann MT, Chambwe N, Gao GF, Cherniack AD, et al. Genomic and molecular landscape of DNA damage repair deficiency across the Cancer Genome Atlas. Cell Rep (2018) 23(1):239–54. doi: 10.1016/j.celrep.2018.03.076
34. Li X, Pasche B, Zhang W, Chen K. Association of MUC16 mutation with tumor mutation load and outcomes in patients with gastric cancer. JAMA Oncol (2018) 4(12):1691–8. doi: 10.1001/jamaoncol.2018.2805
35. Wang X, Yu X, Krauthammer M, Hugo W, Duan C, Kanetsky PA, et al. The association of MUC16 mutation with tumor mutation burden and its prognostic implications in cutaneous melanoma. Cancer Epidemiol Biomarkers Prev (2020) 29(9):1792–9. doi: 10.1158/1055-9965.EPI-20-0307
36. Yu Y, Lin D, Li A, Chen Y, Ou Q, Hu H, et al. Association of immune checkpoint inhibitor therapy with survival in patients with cancers with MUC16 variants. JAMA Netw Open (2020) 3(6):e205837. doi: 10.1001/jamanetworkopen.2020.5837
37. Bellone S, Centritto F, Black J, Schwab C, English D, Cocco E, et al. Polymerase ϵ (POLE) ultra-mutated tumors induce robust tumor-specific CD4+ T cell responses in endometrial cancer patients. Gynecol Oncol (2015) 138(1):11–7. doi: 10.1016/j.ygyno.2015.04.027
38. Fan Y, Ying H, Wu X, Chen H, Hu Y, Zhang H, et al. The mutational pattern of homologous recombination (HR)-associated genes and its relevance to the immunotherapeutic response in gastric cancer. Cancer Biol Med (2020) 17(4):1002–13. doi: 10.20892/j.issn.2095-3941.2020.0089
39. Melamed A, Margul DJ, Chen L, Keating NL, Del Carmen MG, Yang J, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med (2018) 379(20):1905–14. doi: 10.1056/NEJMoa1804923
40. Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med (2018) 379(20):1895–904. doi: 10.1056/NEJMoa1806395
41. Song J, Le T, Hopkins L, Fung-Kee-Fung M, Lupe K, Gaudet M, et al. A comparison of disease recurrence between robotic versus laparotomy approach in patients with intermediate-risk endometrial cancer. Int J Gynecol Cancer (2020) 30(2):160–6. doi: 10.1136/ijgc-2019-000838
42. Raffone A, Travaglino A, Mascolo M, Carbone L, Guida M, Insabato L, et al. TCGA molecular groups of endometrial cancer: Pooled data about prognosis. Gynecol Oncol (2019) 155(2):374–83. doi: 10.1016/j.ygyno.2019.08.019
43. Rajkumar S, Nath R, Lane G, Mehra G, Begum S, Sayasneh A. Advanced stage (IIIC/IV) endometrial cancer: Role of cytoreduction and determinants of survival. Eur J Obstet Gynecol Reprod Biol (2019) 234:26–31. doi: 10.1016/j.ejogrb.2018.11.029
44. Rauh L, Staples JN, Duska LR. Chemotherapy alone may have equivalent survival as compared to suboptimal surgery in advanced endometrial cancer patients. Gynecol Oncol Rep (2020) 32:100535. doi: 10.1016/j.gore.2020.100535
Keywords: endometrial neoplasms, minimally invasive surgical procedures, molecular features, survival, recurrence
Citation: Dai Y, Wang J, Zhao L, Wang Z and Wang J (2021) Tumor Molecular Features Predict Endometrial Cancer Patients’ Survival After Open or Minimally Invasive Surgeries. Front. Oncol. 11:634857. doi: 10.3389/fonc.2021.634857
Received: 29 November 2020; Accepted: 20 January 2021;
Published: 26 February 2021.
Edited by:
Shannon Neville Westin, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Sara Yvonne Brucker, University of Tübingen, GermanyCopyright © 2021 Dai, Wang, Zhao, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiqi Wang, d2FuZ3pxbmV0QHNpbmEuY29t; Jianliu Wang, d2FuZ2ppYW5saXUxMjAzQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.