
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 01 March 2021
Sec. Thoracic Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.631949
This article is part of the Research TopicTreatment for Non Small Cell Lung Cancer in Distinct Patient PopulationsView all 32 articles
 Donghui Wang1
Donghui Wang1 Cen Chen2
Cen Chen2 Yanli Gu1
Yanli Gu1 Wanjun Lu3
Wanjun Lu3 Ping Zhan3
Ping Zhan3 Hongbing Liu3
Hongbing Liu3 Tangfeng Lv3
Tangfeng Lv3 Yong Song3*
Yong Song3* Fang Zhang1,3*
Fang Zhang1,3*Background: Immune-related adverse events (irAEs) have been reported to be associated with the efficacy of immunotherapy. Herein, we conducted a meta-analysis to demonstrate that irAEs could predict the efficacy of immune checkpoint inhibitors (ICIs) in lung cancer patients.
Methods: Literature on the correlation between irAEs and the efficacy of immunotherapy in lung cancer patients were searched to collect the data on objective response rate (ORR), overall survival (OS), or progression-free survival (PFS) of the patients. These data were incorporated into the meta-analysis.
Results: A total of 34 records encompassing 8,115 patients were examined in this study. The irAEs occurrence was significantly associated with higher ORR {risk ratio (RR): 2.43, 95% confidence interval (CI) [2.06–2.88], p < 0.00001} and improved OS {hazard ratio (HR): 0.51, 95% CI [0.43–0.61], p < 0.00001}, and PFS (HR: 0.50, 95% CI [0.44–0.57], p < 0.00001) in lung cancer patients undergoing ICIs. Subgroup analysis revealed that OS was significantly longer in patients who developed dermatological (OS: HR: 0.53, 95%CI [0.42–0.65], p < 0.00001), endocrine (OS: HR: 0.55, 95%CI [0.45–0.67], p < 0.00001), and gastrointestinal irAEs (OS: HR: 0.58, 95%CI [0.42–0.80], p = 0.0009) than in those who did not. However, hepatobiliary, pulmonary, and high-grade (≥3) irAEs were not correlated with increased OS and PFS.
Conclusion: The occurrence of irAEs in lung cancer patients, particularly dermatological, endocrine, and gastrointestinal irAEs, is a predictor of enhanced ICIs efficacy.
Immunotherapy has led to unprecedented improvements in the life expectancy of cancer patients, particularly those with lung cancer, by priming the immune system to fight against the tumor cells. ICIs target cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1), which are critical molecules that negatively regulate the T-cell activity and aid the tumor cells in evading immune surveillance. However, owing to the heightened immune response, the irAEs caused by ICIs are frequent and ineluctable. A meta-analysis showed that the probability of irAE incidence ranges from 54% to 76% and varies according to the types of ICIs (1). Any organ system could be involved, including the skin, endocrine glands, gastrointestinal tract, liver, pulmonary, and, less commonly, the cardiovascular and central nervous systems. Although the underlying mechanism has not been completely elucidated, increased T-cell activity, B-cell-mediated autoantibody production, and cross-reactive tumoral antigenicity have been suggested to be involved in the occurrence of irAEs (2).
The irAEs inevitably affect the treatment and prognosis of the patients. Once these events occur, clinicians adopt different management strategies according to the types and grades of irAEs. The National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology (ASCO), and European Society of Medical Oncology (ESMO) have issued guidelines for the management of irAEs and have provided comprehensive general treatment algorithms for the clinicians (3, 4). In terms of prognosis, discontinuing ICIs may affect the life expectancy of the patients. However, the clinicians have noticed that patients who developed irAEs were more likely to benefit from ICIs. Meanwhile, theoretically, both antitumor and anti-self-adverse effects could result when the immunity is enhanced. Thus, there seems to exist a correlation between the occurrence of irAEs and the efficacy of immunotherapy.
A systemic review of melanoma has been conducted, which revealed that irAEs could predict survival and response in patients treated with ICIs (5), however, comprehensive meta-analyses focusing on lung cancer have not yet been performed. Herein, our work aimed to elucidate whether the occurrence of irAEs could predict the efficacy of ICIs in patients with lung cancer. Furthermore, we performed a subgroup analysis to decipher the association of organ-specific and grade-specific irAEs with the clinical outcomes of the patients.
PubMed, Embase, and Cochrane Library databases from inception to October 15, 2020 were searched to locate eligible studies reporting the association between the efficacy of ICIs and irAEs in lung cancer patients undergoing immunotherapy. The search strategy was “[immune-related (Title/Abstract)] AND [adverse events (Title/Abstract)] AND {[lung cancer (Title)] OR [SCLC (Title)]}.” The citations of relevant articles were also reviewed in case of omission. The language was restricted to English, and conference abstracts were also included.
The studies that met the following criteria were included in the review:
1. Involving patients who were diagnosed with lung cancer.
2. Involving patients who were treated with CTLA-4, PD-1, or PD-L1 inhibitors.
3. Studies reporting the association between irAEs and ICIs efficacy.
4. Studies that provided data on ORR, OS, or PFS of the patients.
5. Studies that provided the HR of OS or PFS in patients who developed irAEs versus patients without irAEs and 95% CI.
6. Studies published in the English language.
The exclusion criteria of the studies to be included in the review were as follows:
1. Studies that provided OS and PFS, but not HR, or provided HR and P-value, but not 95% CI.
2. Duplicated data or overlapping study populations (the most recent report was included).
3. The adverse events were not caused by the use of ICIs.
DW and CC independently extracted the data from the included studies. The following data were extracted: author, year of publication, trial design, landmark analysis, sample size, irAE type and grade, ORR of patients with and without irAEs, HRs, and 95% CIs for OS or PFS of patients with global, organ-specific, and grade-specific irAEs. Several studies provided both multivariate and univariate HRs, and we selected the former. If studies provided HRs with and without landmark analysis, we chose the former. If studies provided the HRs of global as well as organ-specific irAEs, we opted for the former. If studies provided the HRs of both grade-specific and all-grade irAEs, we selected the latter. The Newcastle-Ottawa scale (NOS) criteria were applied to assess the quality of the included studies. Discrepancies were resolved by discussions among DW, CC, and YG.
Review Manager (RevMan) Version 5.4 (Nordic Cochrane Center, Copenhagen, Denmark) was used to perform the statistical analyses. The log HRs of irAEs versus non-irAEs were obtained by calculating RevMan. If the HR of non-irAEs versus irAEs was provided instead of the opposite comparison, the HR and 95% CI of irAEs versus non-irAEs were calculated by determining the reciprocal of the original HR and 95% CI (6). Heterogeneity was assessed by applying the chi-square test and I2 statistic. A chi-square p<0.05 or an I2>50% was considered to indicate significant heterogeneity (7). The random-effects model was used in case of significant heterogeneity (8); if not, the fixed-effects model was used. Publication bias was evaluated using Begg’s test and Egger’s test. If there is a significant publication bias, we will further use the trim and fill method to evaluate.
A total of 848 records were retrieved from PubMed, Embase, and Cochrane Library databases, and two were acquired from additional resources. The flowchart of the records selection is given in Figure 1. After removing the duplicate records and excluding the irrelevant studies, 81 records were assessed for their eligibility. Finally, 34 records encompassing 8115 patients were chosen for this meta-analysis (9–42). Among the included studies, 31 had employed a retrospective cohort design, and 12 had adopted a landmark analysis. Twenty-one studies had reported the ORR of patients with or without irAEs, 23 had provided the HR of OS and 25 had provided the HR of PFS. Seventeen studies had reported organ-specific irAEs, and four had reported grade-specific irAEs. The characteristics of the included studies were listed in Table 1 and Supplementary Material 1 (Table S1).
A total of 21 studies involving 5,256 patients had reported the ORR of patients with or without irAEs. The pooled RR for ORR was 2.43 (95% CI [2.06–2.88], p < 0.00001), which means that patients experiencing irAEs respond better to ICIs than those who do not (Figure 2). A random-effect model was utilized because of significant heterogeneity (I2 = 55%, p = 0.001). The occurrence of irAEs also predicted improved OS and PFS in lung cancer patients undergoing immunotherapy. The pooled HR OS was 0.51 for OS (95% CI [0.43–0.61], p < 0.00001) and 0.50 for PFS (95% CI [0.44–0.57], p < 0.00001). However, significant heterogeneities were observed for OS (I2 = 69%, p < 0.00001) and PFS (I2 = 49%, p = 0.003) (Figures 3, 4).
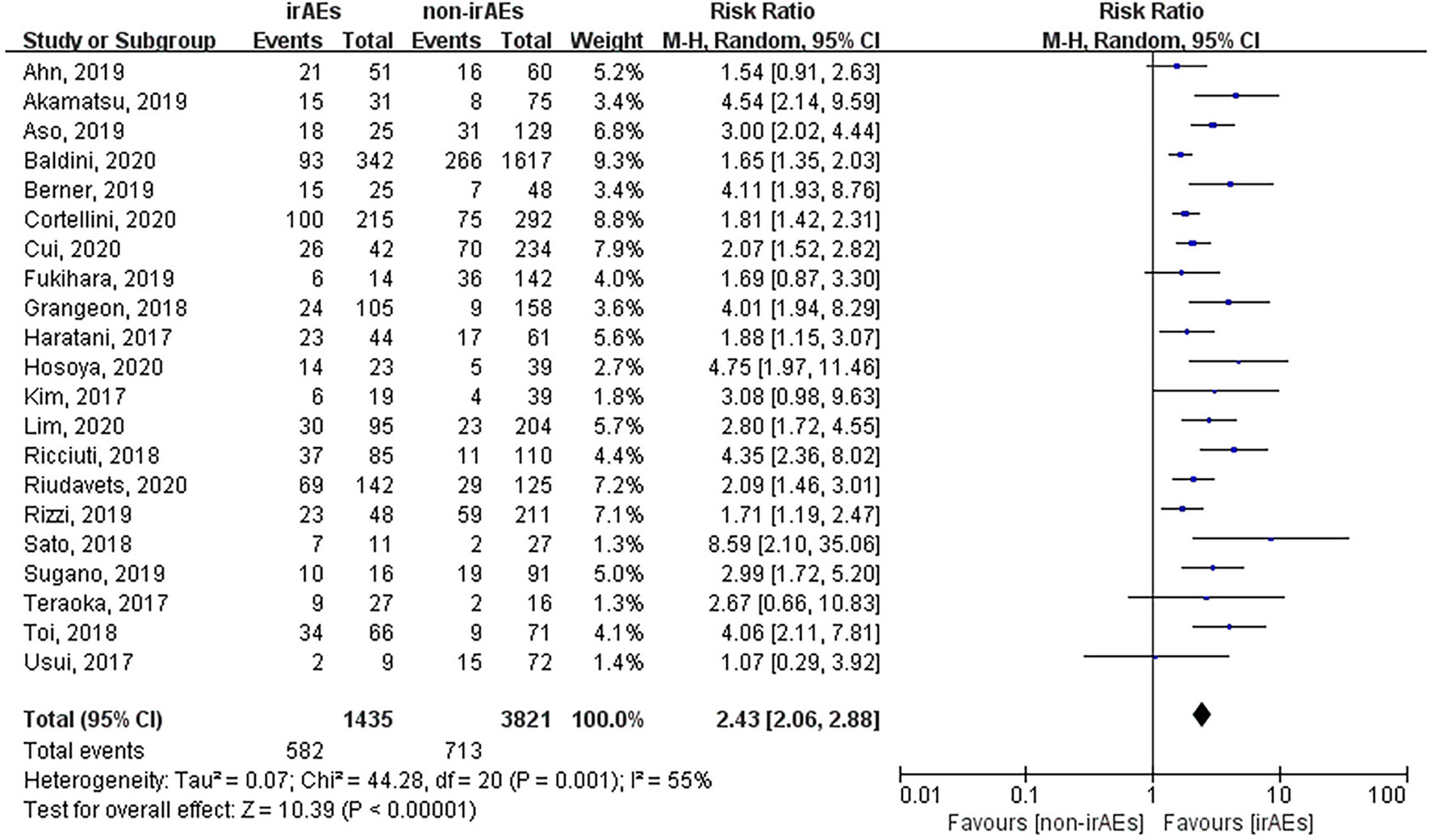
Figure 2 Forest plot of the association between the occurrence of irAEs and ORR. ORR, objective response rate; irAEs, immune-related adverse events; non-irAEs, non-immune-related adverse events.
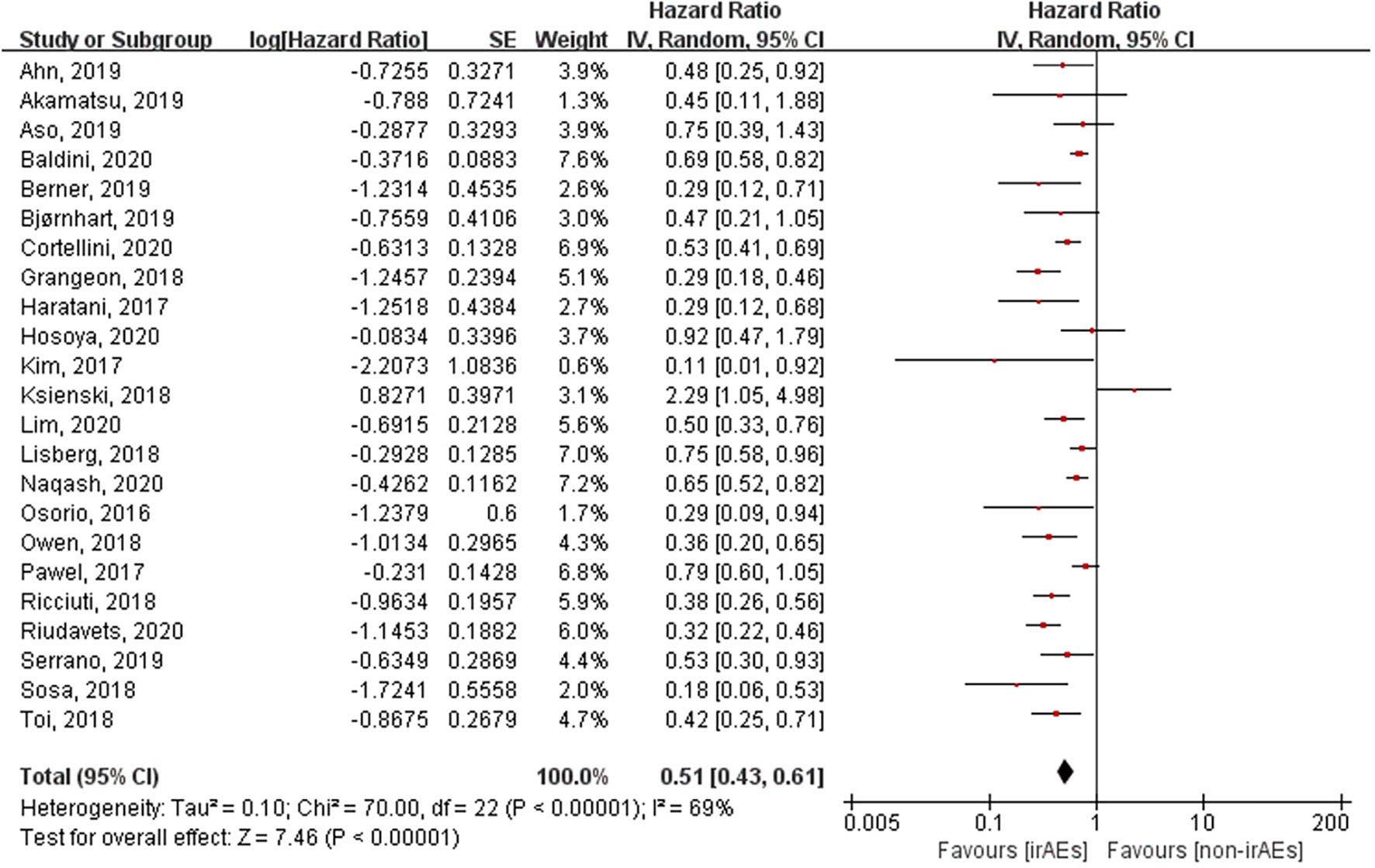
Figure 3 Forest plot of the association between the occurrence of irAEs and OS. OS, overall survival; irAEs, immune-related adverse events; non-irAEs, non-immune-related adverse events.
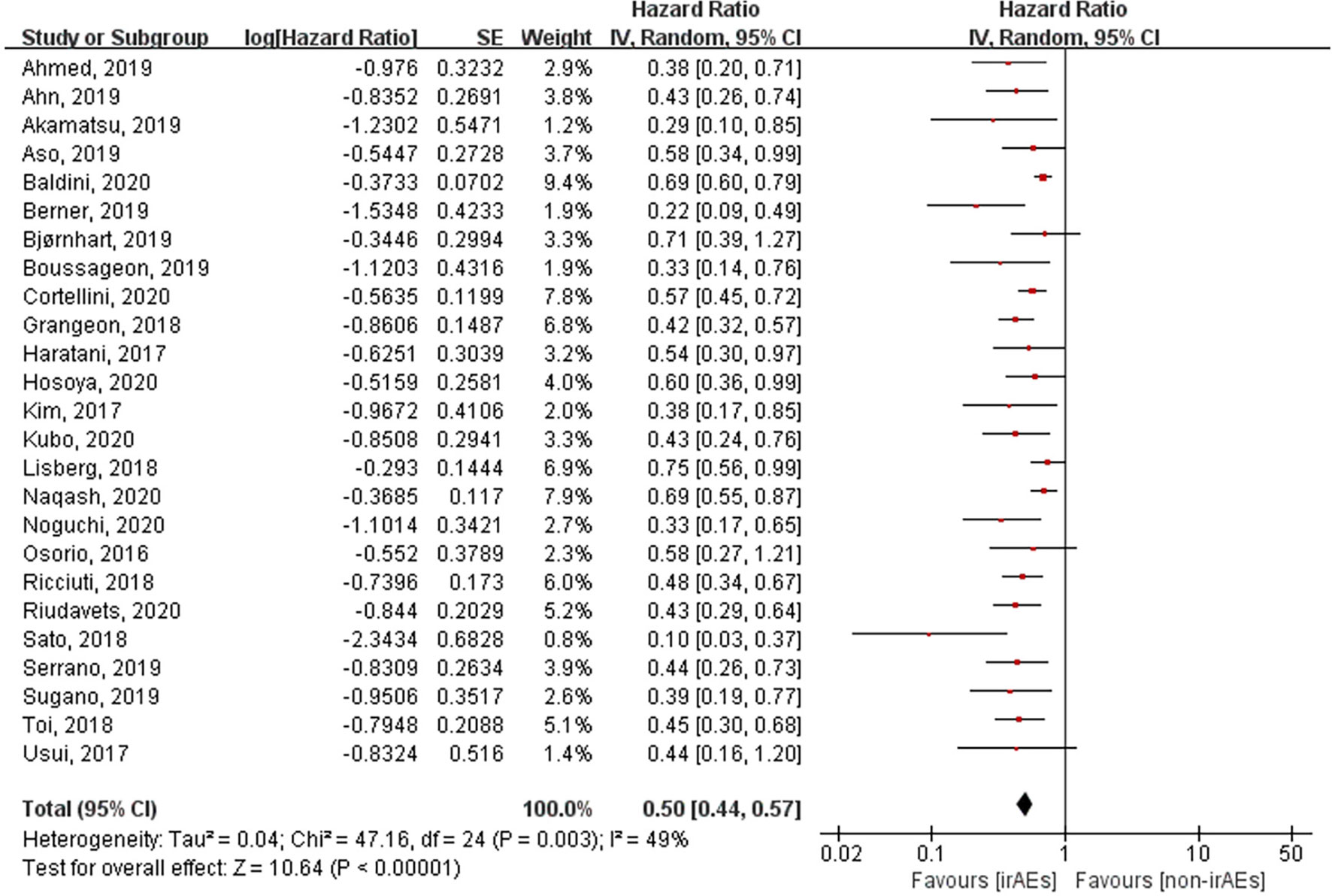
Figure 4 Forest plot of the association between the occurrence of irAEs and PFS. PFS, progression-free survival; irAEs, immune-related adverse events; non-irAEs, non-immune-related adverse events.
Various organ systems could be affected by irAEs. Zhou et al. had found that the association between the different irAE types and patient outcomes was inconsistent by conducting a meta-analysis that covered all cancer types (43). However, this issue remains unclear in case of lung cancer. Hence, we performed subgroup analysis according to irAE types and grades exclusively in lung cancer patients. After pooling the HRs of OS according to the irAE types, the occurrence of dermatological (HR: 0.53, 95% CI [0.42–0.65], p < 0.00001), endocrine (HR: 0.55, 95% CI [0.45–0.67], p < 0.00001), and gastrointestinal (HR: 0.58, 95% CI [0.42–0.80], p = 0.0009) irAEs was found to be significantly correlated with longer OS of lung cancer patients treated with ICIs (Figure 5). Nevertheless, no significant association was seen between OS and the occurrence of hepatobiliary (HR: 1.06, 95% CI [0.76–1.48], p = 0.73) and pulmonary (HR: 1.28, 95% CI [0.58–2.85], p = 0.54) irAEs (Figure 6). The association between irAE type and the PFS of patients was identical to that of OS (Supplementary Material 1: Figures S1, S2). Furthermore, we conducted a subgroup analysis based on the irAE grades. It was inferred that high irAE grades (≥ 3) were not significantly associated with OS and PFS (OS: HR:0.83, 95% CI [0.49–1.43], p = 0.51; PFS: HR: 0.88, 95% CI [0.67–1.16], p = 0.37) (Supplementary Material 1: Figure S3). Owing to data limitations, the association between low irAE grades (≤ 2) and patient outcomes could not be analyzed. In addition, we performed subgroup analysis based on the drug types. The pooled HR of OS was 0.57 (95%CI [0.45–0.72], p < 0.00001) for nivolumab monotherapy and 0.56 (95%CI [0.24-1.31], p = 0.18) for pembrolizumab monotherapy (Supplementary Material 1: Figure S4).
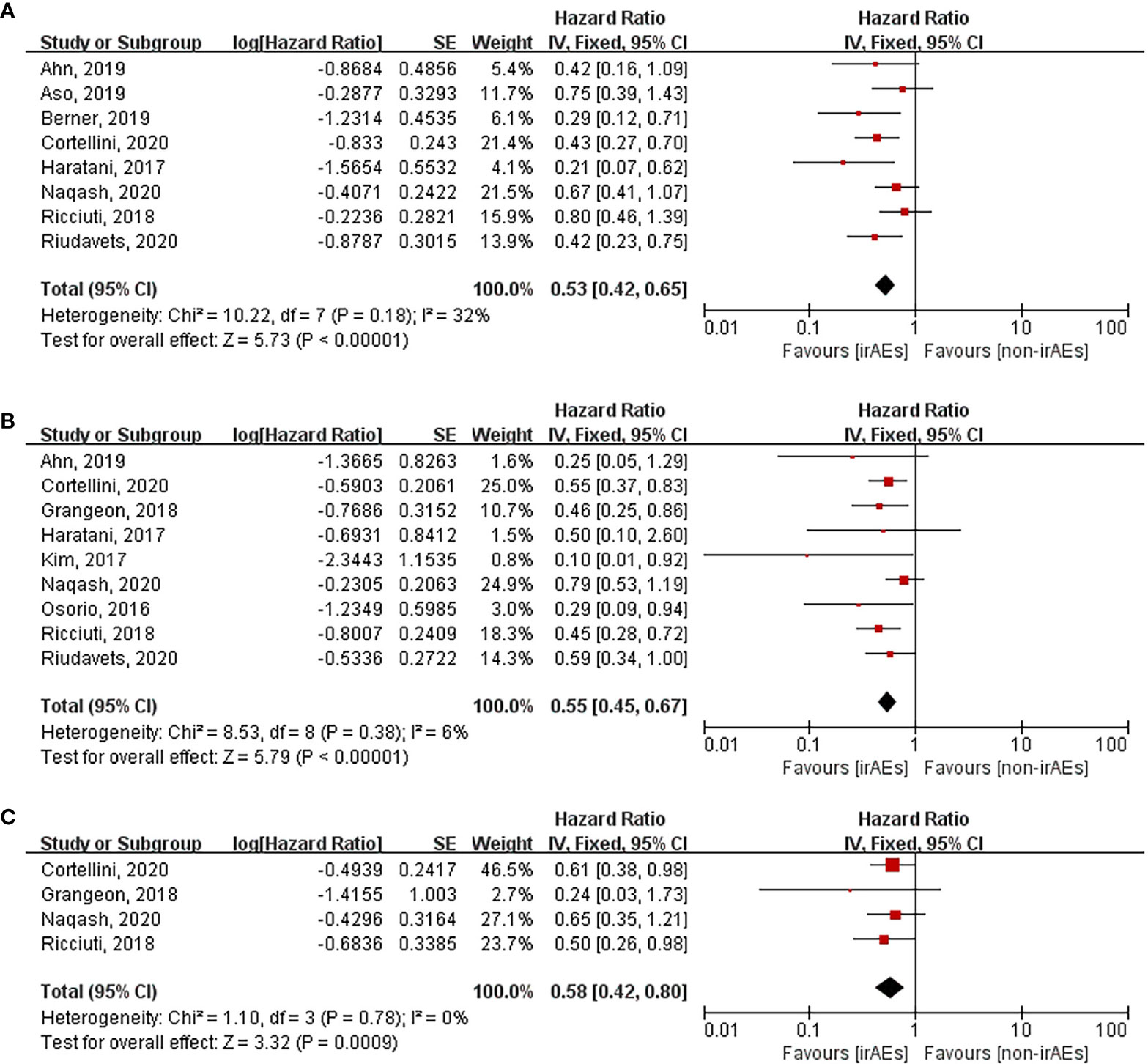
Figure 5 Forest plot of the association between the occurrences of different irAEs types and OS. (A) dermatological irAEs; (B) endocrine irAEs; (C) gastrointestinal irAEs. OS, overall survival; irAEs, immune-related adverse events; non-irAEs, non-immune-related adverse events.
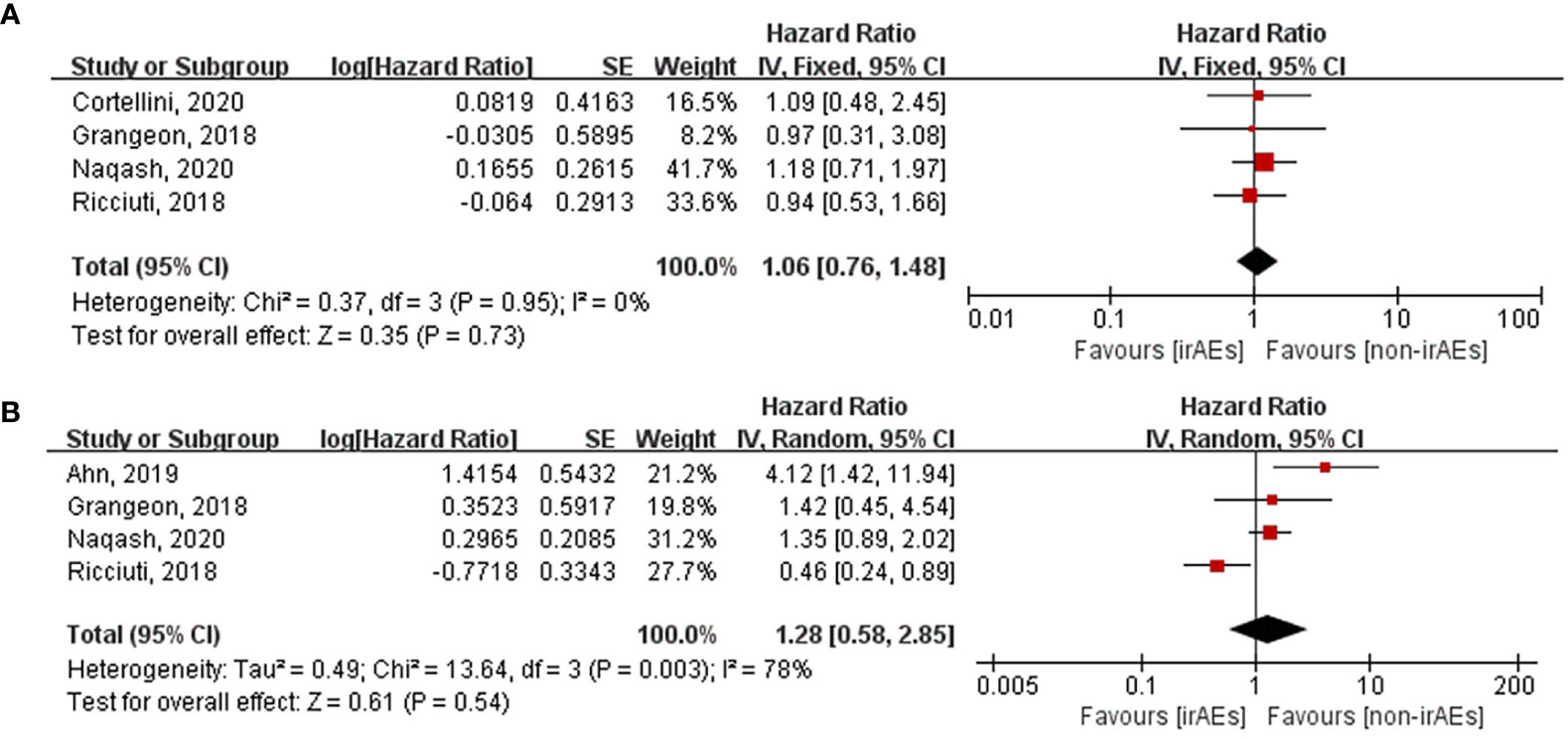
Figure 6 Forest plot of the association between the occurrences of different irAEs types and OS. (A) hepatobiliary irAEs; (B) pulmonary irAEs. OS, overall survival; irAEs, immune-related adverse events; non-irAEs, non-immune-related adverse events.
Sensitivity analyses were conducted to assess the stability of our results. The results of OS and PFS remained consistent even upon deleting any included study or changing the random-effect model into the fixed-effect model (Supplementary Material 1: Figures S5, S6). The funnel plots assessing the publication bias of OS and PFS are presented in Supplementary Material 1 (Figures S7, S8). Regarding OS analysis, Begg’s test (p = 0.369) revealed no publication bias, however, Egger’s test (p = 0.043) presented with the opposite result. We further adopted the trim and fill method to evaluate the publication bias. The result revealed that no study was trimmed or filled and that the pooled result of OS was unchanged, which indicated that our results were stable. Regarding PFS analysis, Begg’s test (p = 0.016) and Egger’s test (p < 0.001) suggested the existence of significant publication bias. The result of the trim and fill method was similar to that of OS.
Although the underlying pathophysiology has not been clearly elucidated until date, accumulating evidence suggest that the occurrence of irAEs can predict the outcomes of patients receiving immunotherapy. In our meta-analysis focusing on lung cancer, the results demonstrated that the occurrence of irAEs was significantly associated with ORR, OS, and PFS of patients treated with ICIs. In addition, patients who experienced dermatological, endocrine, and gastrointestinal irAEs had longer OS and PFS than those who did not. Nevertheless, pulmonary and hepatobiliary irAEs and high-grade (≥3) irAEs were not correlated with OS and PFS.
Several biomarkers have been investigated to predict the efficacy of ICIs prior to the treatment. Clinical practice suggests that not all predictors are effective. PD-L1 is the commonly adopted defective biomarker in clinical practice. Its expression in tumor cells, especially when ≥50%, was found to be significantly associated with the outcomes of lung cancer patients (44). Nevertheless, patients with negative PD-L1 expression also benefit from immunotherapy (45). Another acknowledged biomarker is the tumor mutational burden (TMB), whose predictive value is comparable to that of PD-L1 (46). Both tumor tissue and blood TMB have been shown to be associated with superior survival in lung cancer patients (47–50). However, technical limitations and the absence of standardization hampered its clinical application. Our results indicate that irAEs could be predictors of clinical outcomes in lung cancer patients after the initiation of treatment. These events are routinely witnessed within a few weeks of the initial administration, which helps clinicians in the early prediction of ICI efficacy. Most irAEs related to ipilimumab develop within 8–12 weeks of initiating the medication, with skin toxicity occurring at around 2–3 weeks, gastrointestinal and hepatic toxicity at 6–7 weeks, and endocrine toxicity at 9 weeks (51). The onset time of irAEs differs between anti-CTLA-4 and anti-PD-1 inhibitors. In patients treated with nivolumab, skin irAEs occur at about 5 weeks, followed by gastrointestinal irAEs at 7 weeks, hepatic irAEs at 8 weeks, pulmonary irAEs at 9 weeks, and endocrine irAEs at 10 weeks (51).
As mentioned previously, not all irAE occurrences are related to the efficacy of immunotherapy. The overall incidence of immune-related pneumonitis (IRP) was 4% (52, 53), with grade ≥3 accounting for 16.7%–42.9% of the cases (24, 25). A meta-analysis found that IRP was the major cause of death due to irAEs (54). Immune-related hepatitis (IRH) is usually asymptomatic, with elevated serum transaminase or bilirubin levels. Most patients developing IRH tended to receive corticosteroid treatment (55). For patients with grade ≥3 irAEs, which are usually life-threatening, ICIs should be permanently discontinued and treatment with immunosuppressive drugs such as glucocorticoids should be commenced. An increased risk of mortality and treatment with corticosteroids may together counteract the efficacy of ICIs.
While Zhou et al. had conducted a meta-analysis which mainly covered melanoma and non-small cell lung cancer to investigate the association between irAEs and the efficacy of ICIs (43), there are some key differences between our work and theirs. First, irAE profiles tend to vary according to the cancer type (56). For example, gastrointestinal irAEs occur more frequently in melanoma than in lung cancer, while the opposite is true for pneumonia. Therefore, the results obtained for other cancers may not be applicable for lung cancer. Although the cancer types studied by us are not as extensive as those investigated by Zhou et al., our results are more representative of the actual scenario in the specific field of lung cancer. Second, we pooled the RRs of ORR, which is lacking in Zhou et al.’s work. ORR is an important indicator of the response to immunotherapy, and our results demonstrated that patients experiencing irAEs respond better to ICIs than those who do not.
However, there are some limitations in our research. First, most of the included studies were retrospective, which might affect the quality of the evidences analyzed. Second, heterogeneities were significant in the analysis of ORR, OS, and PFS. After conducting a subgroup analysis based on the types of irAEs, the heterogeneities were significantly reduced. Third, Begg’s test and Egger’s test suggested the existence of significant publication bias. We further adopted the trim and fill method, which indicated that our results were stable. The last, but not the least, owing to the availability of only limited data, we were unable to analyze the association between low grade irAEs and patient outcomes. Hence, more cohort studies need to be performed in the future.
In conclusion, our meta-analysis has demonstrated that the occurrence of irAEs, especially dermatological, endocrine, and gastrointestinal irAEs, could predict the enhanced efficacy of immunotherapy in lung cancer patients treated with ICIs.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conceptualization, supervision: FZ and YS. Data extraction: DW and CC. Writing – original draft preparation: DW. Methodology, software: YG and WL. Writing – reviewing and editing: PZ, HL, and TL. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (grant numbers 81970034 and 81570078).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.631949/full#supplementary-material
1. Xu C, Chen YP, Du XJ, Liu JQ, Huang CL, Chen L, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ (Clinical Res ed) (2018) 363:k4226. doi: 10.1136/bmj.k4226
2. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chavez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers (2020) 6:38. doi: 10.1038/s41572-020-0160-6
3. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36:1714–68. doi: 10.1200/JCO.2017.77.6385
4. Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2017) 28:iv119–42. doi: 10.1093/annonc/mdx225
5. Ouwerkerk W, van den Berg M, van der Niet S, Limpens J, Luiten RM, et al. Biomarkers, measured during therapy, for response of melanoma patients to immune checkpoint inhibitors: a systematic review. Melanoma Res (2019) 29(5):453–64. doi: 10.1097/CMR.0000000000000589
6. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
7. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Res ed) (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
8. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin trials (2007) 28:105–14. doi: 10.1016/j.cct.2006.04.004
9. Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol Off J Eur Soc Med Oncol (2017) 28:583–9. doi: 10.1093/annonc/mdw640
10. Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, et al. Early Immune-Related Adverse Events and Association with Outcome in Advanced Non–Small Cell Lung Cancer Patients Treated with Nivolumab: A Prospective Cohort Study. J Thoracic Oncol (2017) 12:1798–805. doi: 10.1016/j.jtho.2017.08.022
11. Kim HI, Kim M, Lee SH, Park SY, Kim YN, Kim H, et al. Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. Oncoimmunology (2018) 7:e1375642. doi: 10.1080/2162402X.2017.1375642
12. Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung cancer (Amsterdam. Netherlands) (2018) 115:71–4. doi: 10.1016/j.lungcan.2017.11.019
13. Lisberg A, Tucker DA, Goldman JW, Wolf B, Carroll J, Hardy A, et al. Treatment-Related Adverse Events Predict Improved Clinical Outcome in NSCLC Patients on KEYNOTE-001 at a Single Center. Cancer Immunol Res (2018) 6:288–94. doi: 10.1158/2326-6066.CIR-17-0063
14. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-small cell lung cancer. JAMA Oncol (2018) 4:374–8. doi: 10.1001/jamaoncol.2017.2925
15. Owen DH, Wei L, Bertino EM, Edd T, Villalona-Calero MA, He K, et al. Incidence, Risk Factors, and Effect on Survival of Immune-related Adverse Events in Patients With Non-Small-cell Lung Cancer. Clin Lung Cancer (2018) 19:e893–900. doi: 10.1016/j.cllc.2018.08.008
16. Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet-Bressand M, Khobta N, et al. Association Between Immune-related Adverse Events and Efficacy of Immune Checkpoint Inhibitors in Non–small-cell Lung Cancer. Clin Lung Cancer (2019) 20:201–7. doi: 10.1016/j.cllc.2018.10.002
17. Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol (2019) 145:479–85. doi: 10.1007/s00432-018-2805-3
18. Cortellini A, Friedlaender A, Banna GL, Porzio G, Bersanelli M, Cappuzzo F, et al. Immune-related Adverse Events of Pembrolizumab in a Large Real-world Cohort of Patients With NSCLC With a PD-L1 Expression ≥ 50% and Their Relationship With Clinical Outcomes. Clin Lung Cancer (2020) 21(6):498–508. doi: 10.1016/j.cllc.2020.06.010
19. Toi Y, Sugawara S, Sugisaka J, Ono H, Kawashima Y, Aiba T, et al. Profiling Preexisting Antibodies in Patients Treated with Anti-PD-1 Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol (2019) 5:376–83. doi: 10.1001/jamaoncol.2018.5860
20. Ahn BC, Pyo KH, Xin CF, Jung D, Shim HS, Lee CY, et al. Comprehensive analysis of the characteristics and treatment outcomes of patients with non-small cell lung cancer treated with anti-PD-1 therapy in real-world practice. J Cancer Res Clin Oncol (2019) 145:1613–23. doi: 10.1007/s00432-019-02899-y
21. Berner F, Bomze D, Diem S, Ali OH, Fässler M, Ring S, et al. Association of Checkpoint Inhibitor-Induced Toxic Effects With Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol (2019) 5:1043–7. doi: 10.1001/jamaoncol.2019.0402
22. Fukihara J, Sakamoto K, Koyama J, Ito T, Iwano S, Morise M, et al. Prognostic Impact and Risk Factors of Immune-Related Pneumonitis in Patients With Non-Small-Cell Lung Cancer Who Received Programmed Death 1 Inhibitors. Clin Lung Cancer (2019) 20:442–450.e4. doi: 10.1016/j.cllc.2019.07.006
23. Baldini E, Lunghi A, Cortesi E, Turci D, Signorelli D, Stati V, et al. Immune-related adverse events correlate with clinical outcomes in NSCLC patients treated with nivolumab: The Italian NSCLC expanded access program. Lung Cancer (2020) 140:59–64. doi: 10.1016/j.lungcan.2019.12.014
24. Cui P, Huang D, Wu Z, Tao H, Zhang S, Ma J, et al. Association of immune-related pneumonitis with the efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer. Ther Adv Med Oncol (2020) 12:1–10. doi: 10.1177/1758835920922033
25. Naqash AR, Ricciuti B, Owen DH, Florou V, Toi Y, Cherry C, et al. Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with nivolumab: a pooled exploratory analysis from a global cohort. Cancer Immunol Immunother CII (2020) 69:1177–87. doi: 10.1007/s00262-020-02536-5
26. Saavedra Serrano C, Barquín García A, Corral de la Fuente E, Serrano Domingo JJ, Albarrán Artahona V, Martin Huertas R, et al. Association of immune-related adverse events (irAEs) and immunotherapy (IT) benefit in advanced non-small cell lung cancer (NSCLC). Ann Oncol (2019) 30:ii57–8. doi: 10.1093/annonc/mdz063.053
27. Akamatsu H, Murakami E, Oyanagi J, Shibaki R, Kaki T, Takase E, et al. Immune-Related Adverse Events by Immune Checkpoint Inhibitors Significantly Predict Durable Efficacy Even in Responders with Advanced Non-Small Cell Lung Cancer. Oncologist (2020) 25:e679–83. doi: 10.1634/theoncologist.2019-0299
28. Von Pawel J, Syrigos K, Mazieres J, Cortinovis D, Dziadziuszko R, Gandara DR, et al. Association between immune-related adverse events (irAEs) and atezolizumab efficacy in advanced NSCLC: Analyses from the phase III study OAK. Ann Oncol (2017) 28:v469. doi: 10.1093/annonc/mdx380.017
29. Hosoya K, Fujimoto D, Morimoto T, Kumagai T, Tamiya A, Taniguchi Y, et al. Association Between Early Immune-related Adverse Events and Clinical Outcomes in Patients With Non–Small Cell Lung Cancer Treated With Immune Checkpoint Inhibitors. Clin Lung Cancer (2020) 21:e315–28. doi: 10.1016/j.cllc.2020.01.003
30. Boussageon M, Ortiz-Cuaran S, Chabaud S, Pérol D, Avrillon V, Mastroianni B, et al. P1.01-116 Early Immune-Related Adverse Events Under PD-1/PD-L1 Inhibitors Predict Better Progression-Free Survival in NSCLC. J Thoracic Oncol (2019) 14:S407. doi: 10.1016/j.jtho.2019.08.831
31. Melián Sosa M, Puchades C, Mendez JA, Torres A, Rodrigo C, Gimeno G, et al. Immune-related adverse events (irAEs) and survival (OS) in metastatic non-small cell lung cancer (mNSCLC) patients (pts) treated with immunotherapy. Ann Oncol (2018) 29:viii501. doi: 10.1093/annonc/mdy292.014
32. Rizzi M, Rizzo M, Minatta N, Recondo G, Naveira M, Lupinacci L, et al. P1.04-82 Toxicity as a Clinical Marker for Efficacy of Immunotherapy in NSCLC: A Multicentric Experience from Argentina. J Thoracic Oncol (2019) 14:S474–5. doi: 10.1016/j.jtho.2019.08.985
33. Aso M, Toi Y, Sugisaka J, Aiba T, Kawana S, Saito R, et al. Association Between Skin Reaction and Clinical Benefit in Patients Treated with Anti-Programmed Cell Death 1 Monotherapy for Advanced Non-Small Cell Lung Cancer. Oncologist (2020) 25:e536–44. doi: 10.1634/theoncologist.2019-0550
34. Riudavets M, Mosquera J, Garcia-Campelo R, Serra J, Anguera G, Gallardo P, et al. Immune-Related Adverse Events and Corticosteroid Use for Cancer-Related Symptoms Are Associated With Efficacy in Patients With Non-small Cell Lung Cancer Receiving Anti-PD-(L)1 Blockade Agents. Front Oncol (2020) 10:1677. doi: 10.3389/fonc.2020.01677
35. Kubo S, Kobayashi N, Somekawa K, Hirata M, Kamimaki C, Aiko H, et al. Identification of biomarkers for non-small-cell lung cancer patients treated with an immune checkpoint inhibitor. Anticancer Res (2020) 40:3889–96. doi: 10.21873/anticanres.14379
36. Lim SM, Kim SW, Cho BC, Kang JH, Ahn MJ, Kim DW, et al. Real World Experience of Nivolumab in Non-Small Cell Lung Cancer in Korea. Cancer Res Treat Off J Korean Cancer Assoc (2020) 52(4):1112–9. doi: 10.4143/crt.2020.245
37. Noguchi S, Suminaga K, Kaki T, Kawachi H, Fukao A, Terashita S, et al. Correlation of immune-related adverse events and effects of pembrolizumab monotherapy in patients with non-small cell lung cancer. Lung Cancer: Targets Ther (2020) 11:53–7. doi: 10.2147/LCTT.S254146
38. Sugano T, Seike M, Saito Y, Kashiwada T, Terasaki Y, Takano N, et al. Immune checkpoint inhibitor-associated interstitial lung diseases correlate with better prognosis in patients with advanced non-small-cell lung cancer. Thoracic Cancer (2020) 11:1052–60. doi: 10.1111/1759-7714.13364
39. Ahmed Y, Lee J, Calvert P. EP1.01-47 Thyroid Related Adverse Events Predict Survival in NSCLC Patients Receiving Anti-PD-1/ PD-L1 Therapy. J Thoracic Oncol (2019) 14:S930. doi: 10.1016/j.jtho.2019.08.2020
40. Usui Y, Udagawa H, Kirita K, Umemura S, Matsumoto S, Yoh K, et al. Association between immune-related adverse events and efficacy of nivolumab in advanced non-small cell lung cancer. Ann Oncol (2017) 28:ix86. doi: 10.1093/annonc/mdx697.053
41. Bjørnhart B, Hansen KH, Jørgensen TL, Herrstedt J, Schytte T. Efficacy and safety of immune checkpoint inhibitors in a Danish real life non-small cell lung cancer population: a retrospective cohort study. Acta Oncol (Stockholm Sweden) (2019) 58:953–61. doi: 10.1080/0284186X.2019.1615636
42. Ksienski D, Wai ES, Croteau N, Fiorino L, Brooks E, Poonja Z, et al. Efficacy of Nivolumab and Pembrolizumab in Patients With Advanced Non–Small-Cell Lung Cancer Needing Treatment Interruption Because of Adverse Events: A Retrospective Multicenter Analysis. Clin Lung Cancer (2019) 20:e97–e106. doi: 10.1016/j.cllc.2018.09.005
43. Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med (2020) 18:87. doi: 10.1186/s12916-020-01549-2
44. Xu Y, Wan B, Chen X, Zhan P, Zhao Y, Zhang T, et al. The association of PD-L1 expression with the efficacy of anti-PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: a meta-analysis of randomized controlled trials. Transl Lung Cancer Res (2019) 8:413–28. doi: 10.21037/tlcr.2019.08.09
45. Liu X, Guo CY, Tou FF, Wen XM, Kuang YK, Zhu Q, et al. Association of PD-L1 expression status with the efficacy of PD-1/PD-L1 inhibitors and overall survival in solid tumours: A systematic review and meta-analysis. Int J Cancer (2020) 147:116–27. doi: 10.1002/ijc.32744
46. Lu S, Stein JE, Rimm DL, Wang DW, Bell JM, Johnson DB, et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade: A Systematic Review and Meta-analysis. JAMA Oncol (2019) 5:1195–204. doi: 10.1001/jamaoncol.2019.1549
47. Wang Z, Duan J, Cai S, Han M, Dong H, Zhao J, et al. Assessment of Blood Tumor Mutational Burden as a Potential Biomarker for Immunotherapy in Patients With Non-Small Cell Lung Cancer With Use of a Next-Generation Sequencing Cancer Gene Panel. JAMA Oncol (2019) 5:696–702. doi: 10.1001/jamaoncol.2018.7098
48. Hellmann MD, Callahan MK, Awad MM, Calvo E, Ascierto PA, Atmaca A, et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell (2018) 33:853–861.e4. doi: 10.1016/j.ccell.2018.04.001
49. Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med (2018) 24:1441–8. doi: 10.1038/s41591-018-0134-3
50. Velcheti V, Kim ES, Mekhail T, Dakhil C, Stella PJ, Shen X, et al. Prospective clinical evaluation of blood-based tumor mutational burden (bTMB) as a predictive biomarker for atezolizumab (atezo) in 1L non-small cell lung cancer (NSCLC): Interim B-F1RST results. J Clin Oncol (2018) 36:12001–1. doi: 10.1200/JCO.2018.36.15_suppl.12001
51. Sosa A, Lopez Cadena E, Simon Olive C, Karachaliou N, Rosell R. Clinical assessment of immune-related adverse events. Ther Adv Med Oncol (2018) 10:1758835918764628. doi: 10.1177/1758835918764628
52. Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J, et al. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest (2017) 152:271–81. doi: 10.1016/j.chest.2017.04.177
53. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol (2016) 2:1607–16. doi: 10.1001/jamaoncol.2016.2453
54. Sun X, Roudi R, Dai T, Chen S, Fan B, Li H, et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: a PRISMA systematic review and meta-analysis. BMC Cancer (2019) 19:558. doi: 10.1186/s12885-019-5701-6
55. Romanski NA, Holmstroem RB, Ellebaek E, Svane IM. Characterization of risk factors and efficacy of medical management of immune-related hepatotoxicity in real-world patients with metastatic melanoma treated with immune checkpoint inhibitors. Eur J Cancer (Oxford Engl 1990) (2020) 130:211–8. doi: 10.1016/j.ejca.2020.02.041
Keywords: immune-related adverse events (irAEs), efficacy, immune checkpoint inhibitors (ICIS), lung cancer, meta-analysis
Citation: Wang D, Chen C, Gu Y, Lu W, Zhan P, Liu H, Lv T, Song Y and Zhang F (2021) Immune-Related Adverse Events Predict the Efficacy of Immune Checkpoint Inhibitors in Lung Cancer Patients: A Meta-Analysis. Front. Oncol. 11:631949. doi: 10.3389/fonc.2021.631949
Received: 21 November 2020; Accepted: 22 January 2021;
Published: 01 March 2021.
Edited by:
Junji Uchino, Kyoto Prefectural University of Medicine, JapanReviewed by:
Yunfang Yu, Sun Yat-Sen Memorial Hospital, ChinaCopyright © 2021 Wang, Chen, Gu, Lu, Zhan, Liu, Lv, Song and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Zhang, emhhbmdmYW5nbGFiQDE2My5jb20=; Yong Song, eW9uZy5zb25nQG5qdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.