- 1Radiotherapy Department I, Yantaishan Hospital, Yantai, China
- 2Department of Oncology, Shengli Oilfield Central Hospital, Dongying, China
Background: Several recent studies have investigated the prognostic and clinicopathological significance of epithelial cadherin (E-cadherin) in pancreatic cancer; however, conclusions from these studies remain inconsistent. Therefore, we performed a meta-analysis to evaluate the effects of E-cadherin expression on the prognosis and clinicopathological characteristics of pancreatic cancer.
Methods: Embase, PubMed, and Web of Science were searched to identify articles associated with E-cadherin and pancreatic cancer. Hazard ratios (HRs) and odds ratios (ORs) with corresponding confidence intervals (CIs) were calculated and summarized. All eligible studies were searched until May 20, 2020. Heterogeneity among studies was assessed using the Chi-square test and I2 statistic.
Results: Overall, 25 studies were identified, of which 12 reports with 1,032 cases concerned the prognosis of pancreatic cancer, and 22 involved the risk and clinical characteristics of pancreatic cancer. The overall results revealed that E-cadherin expression was significantly related to overall survival, gender, tumor grade, lymph node metastasis, tumor differentiation, and risk of pancreatic cancer. In the subgroup analysis, no significant heterogeneity or publication bias was observed.
Conclusions: E-cadherin expression is strongly associated with the risk, clinical features, and prognosis of pancreatic cancer, suggesting that E-cadherin may be an effective biomarker for the clinical assessments and predicting prognosis of pancreatic cancer.
Introduction
Pancreatic cancer is one of the deadliest human malignancies, with a reported 5-year relative survival rate of less than 5% (1). According to published studies, approximately 88% of individuals diagnosed with pancreatic cancer are elderly patients (2). Meanwhile, 90% of newly diagnosed patients with pancreatic cancer are at an advanced stage, with 60% presenting lymph node metastasis, 69% involving nerve invasion, and free viable tumor cells detected in the peritoneal fluid of approximately 33% of patients (3, 4). Early detection could significantly improve the survival rate of pancreatic carcinoma, but early detection is hampered by several challenges. Prior to diagnosis, some commonly encountered symptoms associated with pancreatic cancer include loss of weight, nausea, indigestion or pain, abdominal or back pain, fatigue, or diabetes. However, these symptoms are often associated with several other conditions; thus, patients hardly ever visit a hospital or consider that they could be presenting signs of cancer. Frequently, only extreme pain or symptoms such as jaundice motivate patients to visit a physician, with tumor cells having metastasized at this stage. Although radiation therapy and chemotherapy are common therapeutic strategies during pancreatic cancer, surgery is the only potential cure for pancreatic cancer. However, patients who undergo surgery are known to ultimately present local or metastatic recurrence (5). Considering the poor prognosis and difficulties in early detection, novel detection and prognostic markers need to be identified. In recent years, investigations have focused on the effects of epithelial-mesenchymal transition (EMT) on pancreatic cancer. EMT is a process in which epithelial cells acquire motile mesenchymal features and promote cell migration and invasion (6). Accumulating evidence has revealed that EMT is closely related to the invasion and metastasis of pancreatic cancer (6, 7).
Epithelial cadherin (E-cadherin) is a typical type I classical cadherin, which is expressed mainly in epithelial cells and can mediate cell-cell interactions (8). Published studies have revealed that loss of E-cadherin can promote tumor cell invasiveness and correlates with poor prognosis of cancers (9, 10). Somatic mutations, silencing of the E-cadherin gene promoter, chromosomal deletions, or proteolytic cleavage of E-cadherin could lead to the suppression of E-cadherin expression, which is often detected in human tumors (11, 12). A recent study has reported that E-cadherin expression was downregulated in pancreatic tumor cells and correlated with tumor metastasis (13). Reportedly, the level of cadherin expression affects adhesion among cells. In contrast, E-cadherin inactivation may be involved in the differentiation of pancreatic tumor cells. However, several factors, including sample size, gender, and cancer stage, limit the credibility of observed results. To accurately elucidate the role of E-cadherin in the clinical progress and prognosis of pancreatic cancer, we collected published data to perform a meta-analysis.
Methods
Publication Search
Eligible reports regarding the association of E-cadherin expression with clinicopathological features and prognosis of pancreatic cancer were systematically searched in electronic databases, including PubMed, Embase, and Web of Science, until May 20, 2020. We searched these electronic databases using the following keywords: “pancreatic cancer,” “pancreatic carcinoma,” “E-cadherin,” “cadherin,” “CDH1” and “prognosis.” Two independent investigators collected the included studies based on the inclusion and exclusion criteria. Furthermore, the references cited in included studies were screened to identify other eligible studies.
Inclusion and Exclusion Criteria
Eligible studies were included according to predefined inclusion and exclusion criteria. All included studies had to meet the following inclusion criteria: (1) studies published in English; (2) case-control studies, retrospective studies, or cohort studies; (3) patients had to present detailed pathological diagnostic criteria; (4) studies had to detail sufficient data to calculate hazard ratios (HRs), odds ratios (ORs), and 95% CIs. Conversely, studies were excluded based on the following exclusion criteria: (1) reviews, comments, letters, conferences, case reports, editorials, and meta-analyses; (2) studies with duplicated data; (3) no available data.
Data Extraction and Quality Assessment
Data regarding the relationship between E-cadherin expression and risk, prognosis, and clinicopathological parameters of pancreatic cancer were extracted by two researchers. The following information was collected from each study: first author’s name, publication year, country, ethnicity, protein expression detection method, cancer subtype, sample type, HRs and 95% confidence intervals (CIs), survival curve, and the number of cases and controls in different groups. The Newcastle–Ottawa Scale (NOS) table (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp, accessed on June 1, 2019) was applied to comprehensively evaluate the quality of included studies that involved the relationship between the risk of pancreatic cancer and E-cadherin expression. The included studies were categorized as low (<4), medium (4–6), or high (>6) according to their quality scores (14).
Statistical Analysis
Statistical analyses were performed using STATA Statistical Software (Version 14.2; StataCorp LP, College Station, TX, USA). Initially, HRs, ORs, 95% CIs, and the weight of each included study were calculated with a fixed-effect model and random-effects model to estimate the role of E-cadherin expression in risk, prognosis, and clinicopathological features of pancreatic cancer (15). Statistical significance was set at P < 0.05. The Chi-square test and I2 statistic were used to evaluate the significance of heterogeneity among the studies (16). If significant heterogeneity was detected, the random-effects model (DerSimonian–Laird method) was applied; otherwise, the fixed-effect model (the Mantel-Haenszel method) was used (15). Begg’s rank test and Egger’s regression test were used for evaluating the publication bias of each study, which could be visually measured by the asymmetry of Begg’s funnel plot (17). In addition, the leave-one-out sensitivity analysis was performed to further assess the effects of individual studies on overall results (18). Finally, a meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Results
Search Strategy and Selection
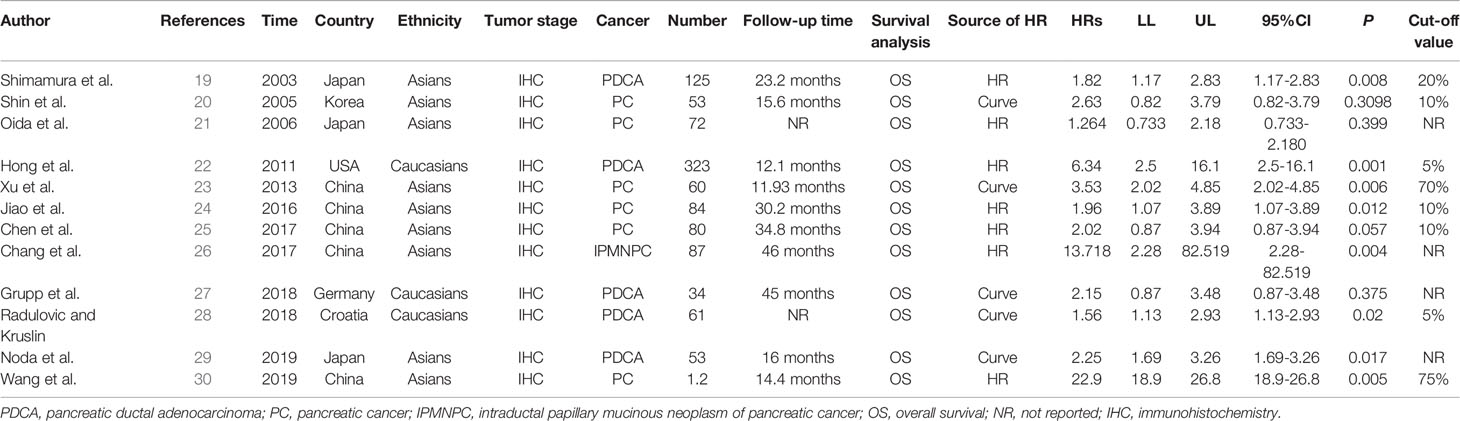
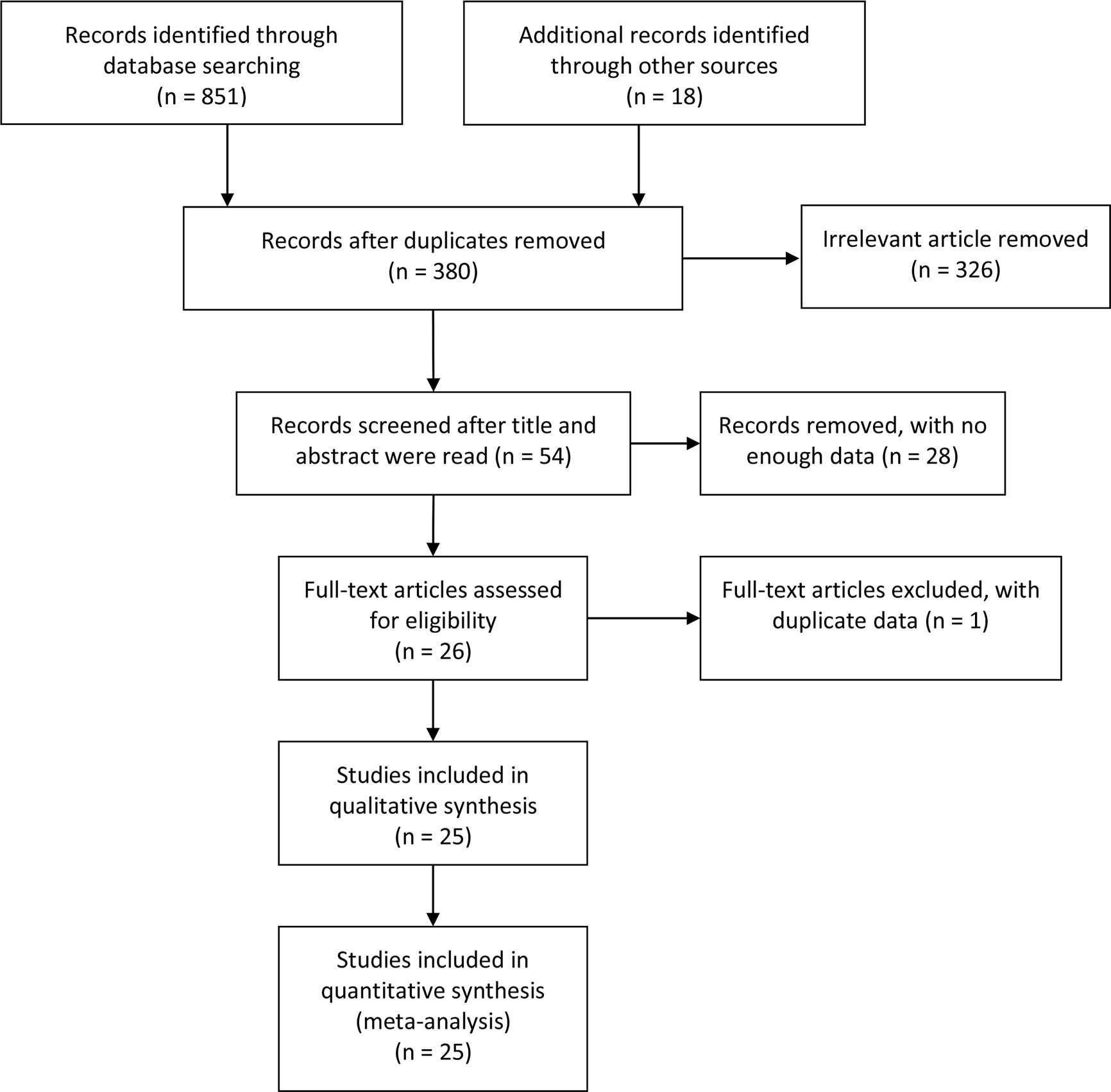
Of the 381 studies initially identified, 12 studies involving 1,032 pancreatic cancer patients analyzed the influence of E-cadherin on the prognosis of pancreatic cancer, whereas 22 studies assessed the risk and clinicopathological features of pancreatic cancer (19–43). However, the study by Wang et al. significantly affected the stability of pooled HRs and was excluded according to the sensitivity analysis (30). Therefore, in the final 11 studies on prognosis, three studies were reported in Caucasian populations (22, 27, 28), with eight concentrating on Asian populations (19–21, 23–26, 29). Furthermore, four reports were collected to assess the risk of pancreatic cancer (24, 25, 28, 31), while 18 studies were included in the analysis of clinicopathological features of pancreatic cancer. In all eligible studies, E-cadherin expression was detected using immunohistochemistry (IHC). Seven studies directly provided HR estimates and 95% CIs, while other studies reported survival curves in which Engauge Digitizer 4.1 software was applied to extracted HRs and 95% CIs (Tables 1 and 2) (Figure 1).
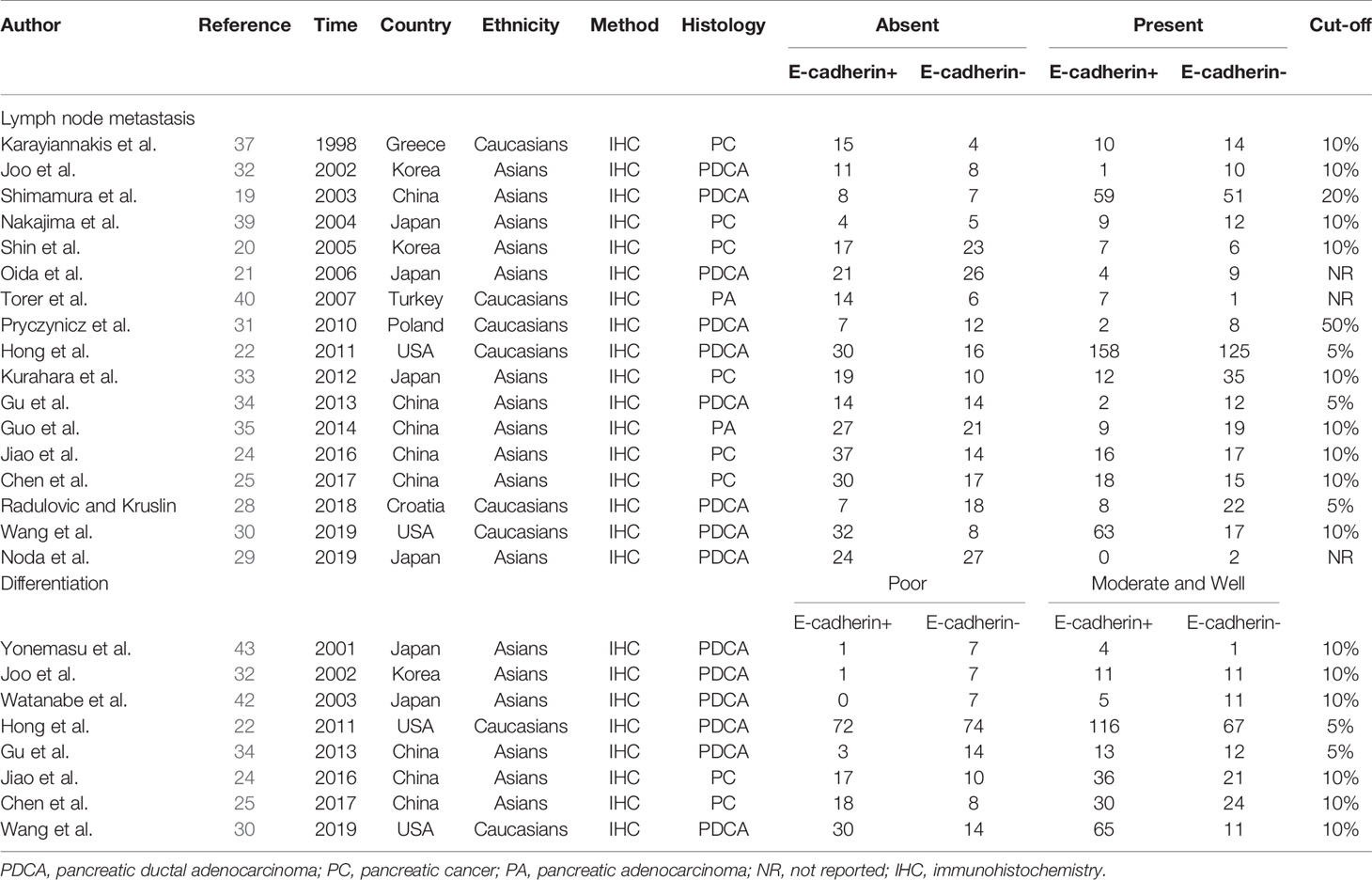
Table 2 Characteristics of eligible studies in the meta-analysis for the clinical features of pancreatic cancer.
Correlation Between E-Cadherin Expression and Prognosis of Pancreatic Cancer
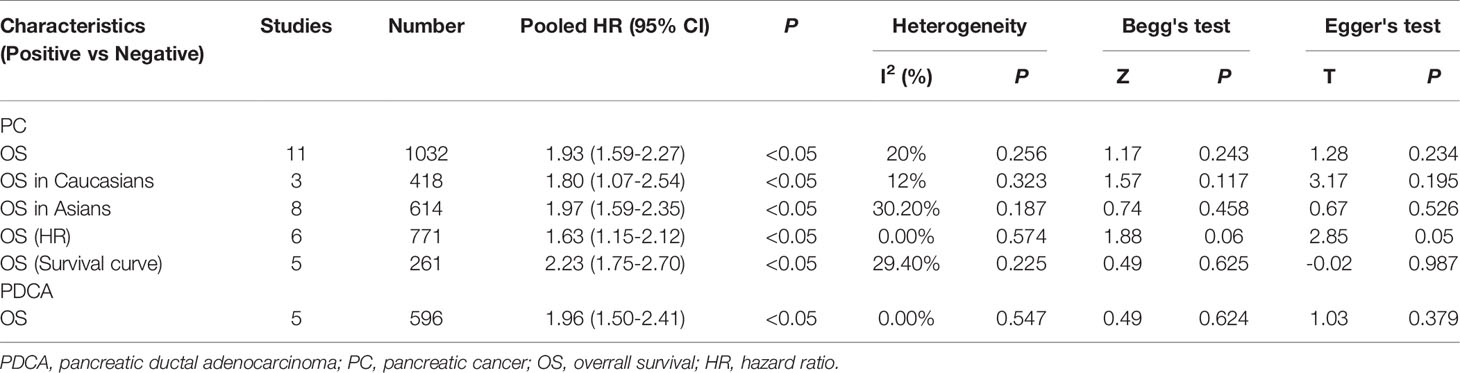
The overall results indicated a significant relationship between E-cadherin expression and overall survival (OS) of pancreatic cancer (HR = 1.93, 95% CI = 1.59-2.27, P < 0.05). No significant heterogeneity was detected; therefore, a fixed-effects model was used to calculate the pooled HRs. The pooled HR was 1.93, indicating that decreased expression levels of E-cadherin were significantly associated with the poor OS of pancreatic cancer. To further assess the association between aberrant E-cadherin expression and OS in pancreatic cancer, subgroup analyses based on race, source of HRs, and cancer subtype were conducted. The subgroup analysis showed similar results both in Caucasian (HR = 1.80, 95% CI = 1.07-2.54, P < 0.05) and Asian populations (HR = 1.97, 95% CI = 1.59-2.35, P < 0.05). In addition, the subgroup analysis for pancreatic ductal adenocarcinoma (PDCA) revealed that low E-cadherin expression led to a poor OS in pancreatic cancer (HR = 1.96, 95% CI = 1.50-2.41, P < 0.05). None of the subgroup analyses detected significant heterogeneity across studies (Table 3) (Figure 2).
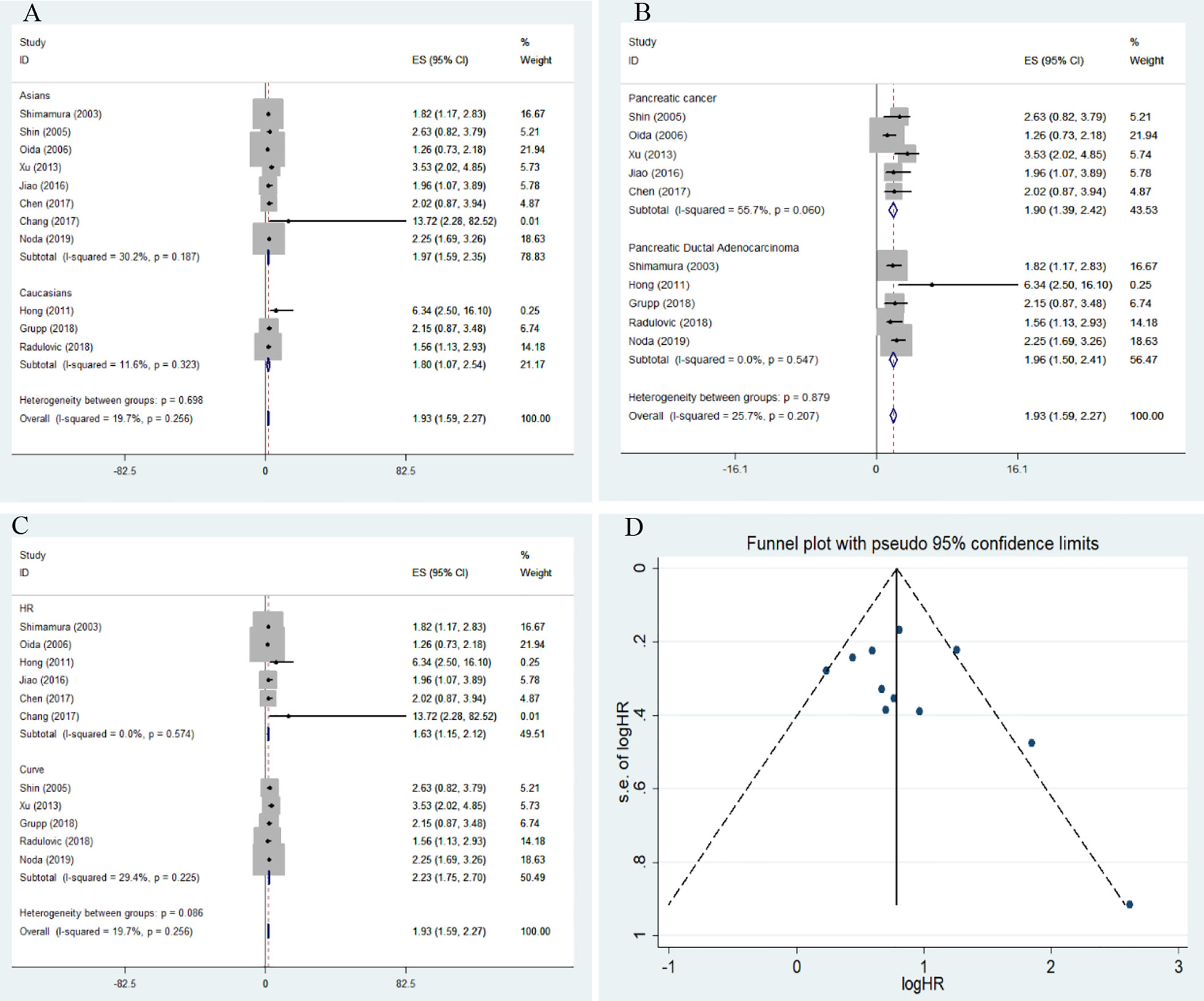
Figure 2 Forest plot and funnel plot for the association between E-cadherin expression and overall survival of pancreatic cancer. (A) subgroup analysis for ethnicity; (B) subgroup analysis for cancer subtype; (C) subgroup analysis for the source of hazard ratios (HRs); (D) funnel plot for the association between E-cadherin expression and overall survival of pancreatic cancer.
Correlation Between E-Cadherin Expression and Clinicopathological Features of Pancreatic Cancer
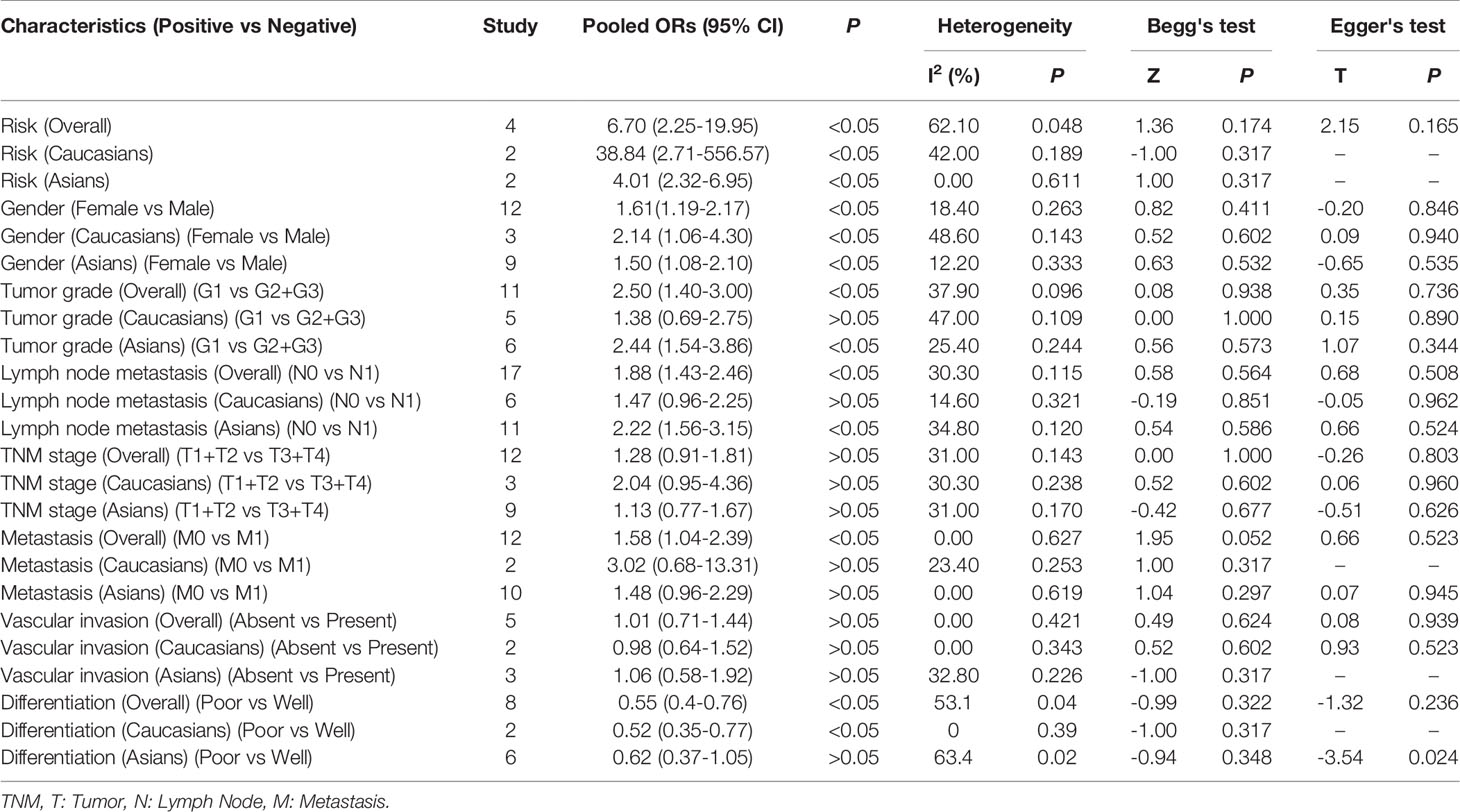
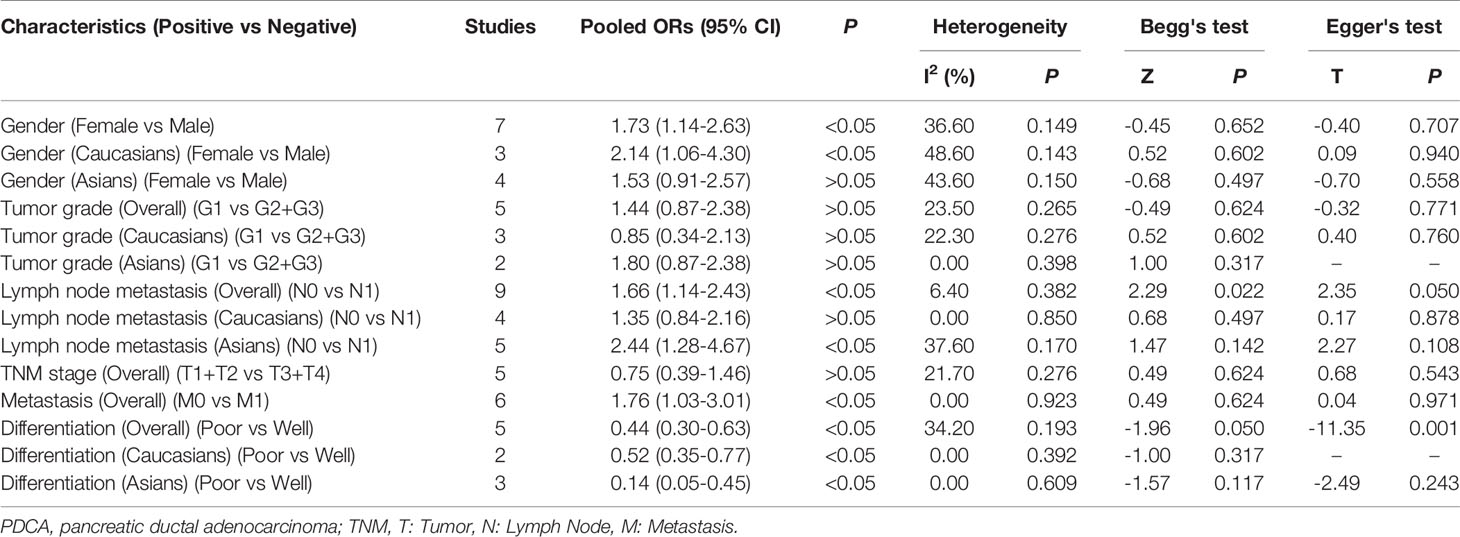
Among the included studies, four investigated the association between E-cadherin expression and the risk of pancreatic cancer, and a significant association was observed among Caucasian and Asian populations (overall results, OR = 6.70, 95% CI = 2.25-19.95, P < 0.05; Caucasian, ORs = 38.84, 95% CI = 2.71-556.57, P < 0.05; Asian, ORs = 4.01, 95% CI = 2.32-6.95, P < 0.05). Therefore, decreased E-cadherin expression may lead to an increased risk of pancreatic cancer. Next, we assessed the correlation between decreased E-cadherin expression and the clinicopathological features of patients with pancreatic cancer. The results are summarized in Table 4. As expected, decreased E-cadherin expression was significantly correlated with poor differentiation of pancreatic cancer (overall results, ORs = 0.55, 95% CI = 0.40-0.76, P < 0.05), which was observed in Caucasian (OR = 0.52, 95% CI = 0.35-0.77, P < 0.05) but not in Asian populations (Asian, OR = 0.62, 95% CI = 0.37-1.05, P > 0.05). In addition, we noted that decreased E-cadherin expression was significantly associated with tumor grade (overall results, ORs = 2.50, 95% CI = 1.40-3.00, P < 0.05; Caucasian, OR = 1.38, 95% CI = 0.69-2.75, P > 0.05; Asian, ORs = 2.44, 95% CI = 1.54-3.86, P < 0.05) and lymph node metastasis (overall results, OR = 1.88, 95% CI = 1.43-2.46, P < 0.05; Caucasian, OR = 1.47, 95% CI = 0.96-2.25, P > 0.05; Asian, ORs = 2.22, 95% CI = 1.56-3.15, P < 0.05), which indicated that decreased E-cadherin expression predicted poor clinical outcomes of pancreatic cancer. However, no statistical correlations were observed between E-cadherin expression and TNM stage (OR = 1.28, 95% CI = 0.91-1.81, P > 0.05) or vascular invasion of pancreatic cancer (OR = 1.01, 95% CI = 0.71-1.44, P > 0.05), both in Caucasian and Asian populations. Although the overall results revealed that E-cadherin expression was significantly associated with metastasis of pancreatic cancer, no significant association was observed in the subgroup analysis based on ethnicity (overall results, OR = 1.58, 95% CI = 1.04-2.39, P < 0.05; Caucasian, OR = 3.02, 95% CI = 0.68-13.31, P > 0.05; Asian, OR = 1.48, 95% CI = 0.96-2.29, P > 0.05). . Heterogeneity was detected in the analysis for the differentiation of pancreatic cancer, and subgroup analysis found no significant heterogeneity in the subgroup analysis of Caucasian populations. Heterogeneity in Asian populations revealed that other factors might affect the accuracy of results for differentiation. Moreover, the incidence of decreased E-cadherin expression in males (51.94%) was higher than that in females (43.24%) (overall, OR = 1.61, 95% CI = 1.19-2.17, P < 0.05), which was also observed in the stratified analysis based on ethnicity.
We further performed a subgroup analysis to evaluate clinical feature data for PDCA, and these results revealed that downregulation of E-cadherin was associated with gender (overall results, OR = 1.73, 95% CI = 1.14-2.63, P < 0.05; Caucasian, ORs = 2.14, 95% CI = 1.06-4.30, P < 0.05), lymph node metastasis (Overall results, ORs = 1.66, 95% CI = 1.14-2.43, P < 0.05; Asian, ORs = 2.44, 95% CI = 1.28-4.67, P < 0.05) and metastasis (overall results, OR = 1.76, 95% CI = 1.03-3.01, P < 0.05), and differentiation (Overall results, ORs = 0.44, 95% CI = 0.30-0.63, P < 0.05; Caucasian, ORs = 0.52, 95% CI = 0.35-0.77, P < 0.05; Asian, ORs = 0.14, 95% CI = 0.05-0.45, P < 0.05) in pancreatic cancer. Based on Cochran’s Q and I2 statistics, heterogeneity was not detected among included studies (Table 5) (Figure 3).
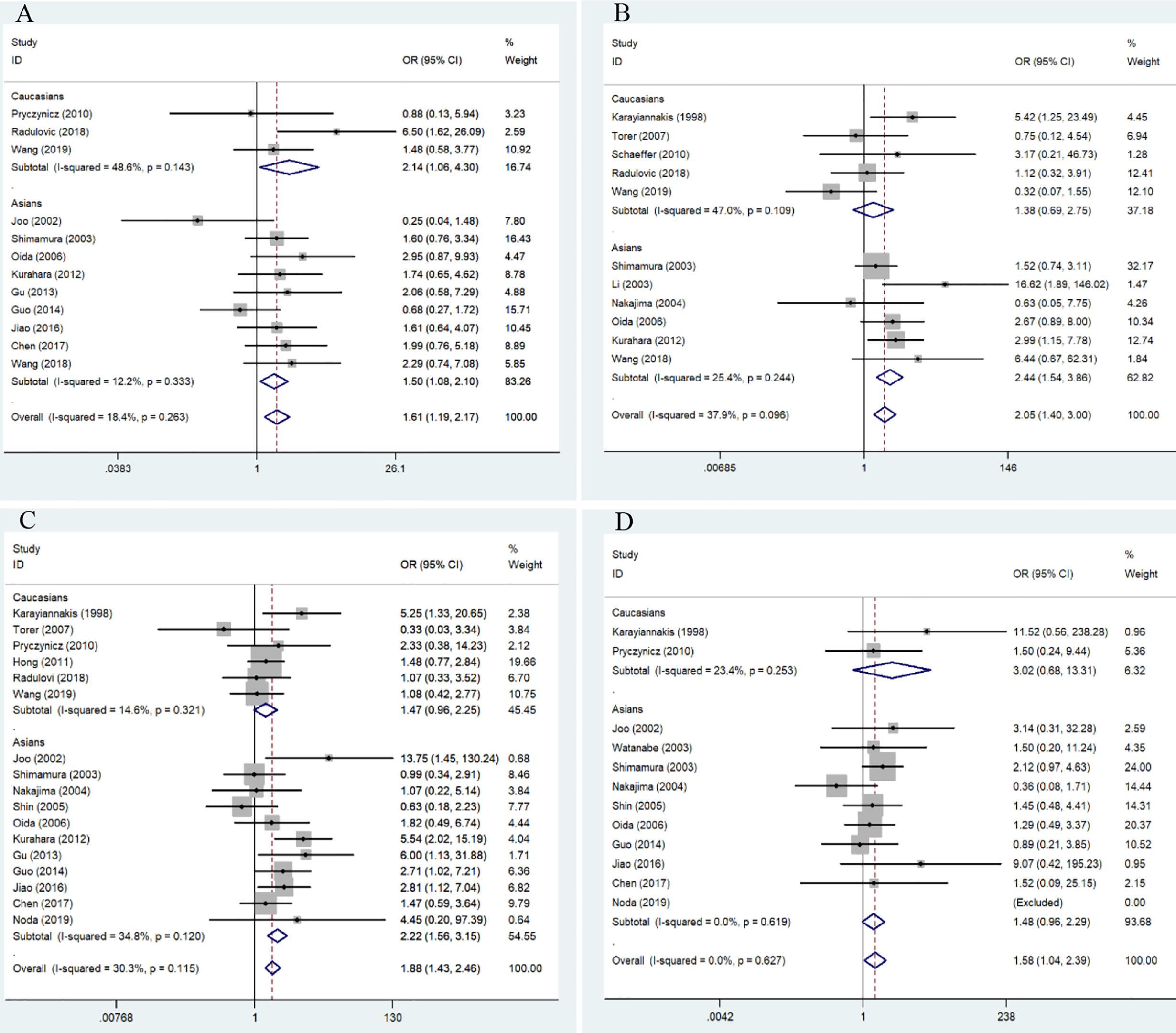
Figure 3 Forest plot for the association of E-cadherin expression with gender, grade, lymph node metastasis, and differentiation of patients with pancreatic cancer. (A) gender; (B) tumor grade; (C) lymph node metastasis; (D) differentiation.
Publication Bias and Sensitivity Analysis
Sensitivity analysis was performed to examine the effects of each included study on pooled HRs and ORs. For OS in pancreatic cancer, the results of Wang et al. (30) significantly affected the pooled HRs; accordingly, we removed this study from all meta-analyses for the OS of pancreatic cancer. In the other subgroup analysis for risk and clinical features, no individual study significantly altered the pooled ORs; however, heterogeneity was still observed in the analysis for differentiation in Asian populations, which indicated that other factors might have led to the main source of heterogeneity. Egger’s test and Begg’s funnel plot revealed the absence of publication bias in the analysis.
Discussion
During EMT, the cobblestone epithelial appearance of cultured epithelial cells gradually changed to a spindle-shaped, mesenchymal morphology. This transformation is reversible, in which mesenchymal cells can convert back into an epithelial state, known as a mesenchymal–epithelial transition (MET) (44). EMT plays a crucial role in embryogenesis and tissue morphogenesis; therefore, several studies focused on the relationship between EMT, as well as development and wound healing in adults (45). Normally, epithelial cells in tissues are connected through tight junctions and adherens junctions, and the adherens junctions are linked to cell surface E-cadherin molecules. Increasing evidence indicates that epigenetic changes regulate EMT, which is not dependent on DNA sequence alterations in normal and cancer cells (46). Furthermore, the identified somatic mutations and hypermethylation of the E-cadherin promoter did not result in its downregulation (47). Reportedly, recruitment of the histone deacetylases, HDAC1 and HDAC2, by the transcriptional repressor ZEB1 might be responsible for changes in E-cadherin expression (47). Following the activation of EMT, the expression of E-cadherin is repressed, which weakens the connection between cells and leads to the loss of typical polygonal, cobblestone morphology of epithelial cells (48). The activation of the EMT program often works in association with other signaling pathways to promote the conversion of epithelial cells into a fully mesenchymal state in normal cells, which is also observed in cancer cells (49). As one of the key regulators of EMT, E-cadherin may play an important role in primary tumor development and progression. Decreased expression of E-cadherin might reduce the adhesive strength and suppress the function of downstream transcription factors such as snail, LEF-1, and ZEB1.
In the present meta-analysis, the pooled results suggested that cancer tissues present a lower E-cadherin expression than normal tissues or adjacent tissues (24, 25, 28, 31), which indicated that EMT occurred during pancreatic cancer carcinogenesis. E-cadherin expression was significantly correlated with worse histopathological grade (19, 21, 28, 30, 33, 36–41), lymphatic invasion-positive tumor (19–22, 24, 25, 28–35, 37, 39, 40), and poor differentiation of pancreatic cancer (22, 24, 25, 30, 32, 34, 42, 43), which revealed that E-cadherin expression was significantly linked to malignant biological behaviors and tumor progression. Moreover, the pooled HR was 1.80 in Caucasian and 1.97 in Asian populations for OS, revealing that decreased E-cadherin expression predicted poorer prognosis and might be a potential biomarker for estimating the survival rate in pancreatic cancer patients. Furthermore, the number of male patients with pancreatic cancer presenting decreased E-cadherin expression was higher than female patients, and different lifestyle factors such as smoking and drinking in men could be responsible for these findings.
Tissue samples from different subtypes of pancreatic tumors were included in the current meta-analysis. Therefore, subgroup analysis based on cancer type was performed to investigate the association between E-cadherin expression and PDCA, which is the most frequent type of pancreatic cancer. A significant correlation was detected between E-cadherin downregulation and the prognosis and clinical progress of PDCA. Furthermore, we observed a more obvious association between E-cadherin downregulation and poor PDCA differentiation. In addition, according to the pooled ORs, E-cadherin downregulation promoted the metastasis of PDCA cells. These observations were in line with the perspective that E-cadherin is a tumor suppressor molecule in cancer development (50), which further highlighted that the EMT process is pivotal in cancer invasion and metastasis (3). Accordingly, E-cadherin may be a useful therapeutic target for PDCA to inhibit cancer progression. Wang et al. have observed that the level of E-cadherin expression gradually decreases over time with microenvironmental changes, indicating that the microenvironment might reduce E-cadherin expression (36).
There is growing evidence that the tumor microenvironment (TME) plays an important role in the progression and prognosis of pancreatic cancer. The TME consists of the extracellular matrix (ECM), aberrant immune cells, pancreatic stellate cells, and abnormal tumor vascularization (51). It has been reported that E-cadherin mutations and aberrant downregulation of E-cadherin can be observed in breast cancer, which might be associated with an abnormal TME (52). In the present study, E-cadherin expression was significantly associated with grade, metastasis, and differentiation of pancreatic cancer. In a published study, E-cadherin was significantly associated with lymph node metastasis in gastric cancer (53). Cancer-associated fibroblasts could lead to the deposition of the ECM and mediate the process of EMT, which includes regulation of E-cadherin expression level (54). Therefore, it might be crucial to clarify the level of E-cadherin expression in different cells in future studies.
Based on findings of the current meta-analysis, E-cadherin expression was significantly decreased in pancreatic cancer tissue samples and presented a close relationship with poor prognosis and malignant progression of the tumor patients. In the analysis of pancreatic cancer differentiation, we detected heterogeneity among studies of Asian populations; accordingly, subgroup analysis based on cancer type was further performed in PDCA. No heterogeneity was observed in the analysis of PDCA. Additionally, the sensitivity analysis results revealed that no individual study significantly affected the stability of the pooled HRs and ORs.
Although heterogeneity and publication bias were rarely observed, some limitations persist in the present study. First, we extracted HRs from the survival curve when eligible studies did not directly provide HRs, and the extracted HRs might minimally differ from the real data. Second, the sample number in some subgroup analyses was small. Third, although we included all eligible literature in English, several studies in other languages were not included, which might have excluded some potential data.
In conclusion, this meta-analysis demonstrated that decreased E-cadherin expression was positively correlated with advanced clinical features and poor OS in patients with pancreatic cancer, suggesting that it might be a potential biomarker for clinical assessments and predicting the prognosis of pancreatic cancer.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.627116/full#supplementary-material
Abbreviations
HRs, Hazard ratios; ORs, Odds ratios; CIs, confidence intervals; PDCA, pancreatic ductal adenocarcinoma; PC, pancreatic cancer; IPMNPC, intraductal papillary mucinous neoplasm of pancreatic cancer; OS, overall survival; NR, not reported; PA, pancreatic adenocarcinoma; IHC, immunohistochemistry.
References
1. Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology (2013) 144(6):1316–26. doi: 10.1053/j.gastro.2013.01.078
2. Surveillance, Epidemiology, and End Results (SEER) Program. Percent of new cases by age group: pancreatic cancer, SEER 2010–2014, all races, both sexes. Available at: https://seer.cancer.gov (Accessed October 9, 2017).
3. Kalluri R, Weinberg RA. The basics of epithelialemesenchymal transition. J Clin Invest (2009) 119(6):1420e8. doi: 10.1172/JCI39104
4. Beuran M, Negoi I, Paun S, Ion AD, Bleotu C, Negoi IR, et al. The epithelial to mesenchymal transition in pancreatic cancer: A systematic review. Pancreatology (2015) 15(3):217–25. doi: 10.1016/j.pan.2015.02.011
5. Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet (2004) 363(9414):1049e57. doi: 10.1016/S0140-6736(04)15841-8
6. Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol (2012) 22(5-6):396–403. doi: 10.1016/j.semcancer.2012.04.001
7. Jiang JH, Liu C, Cheng H, Lu Y, Qin Y, Xu YH, et al. Epithelial-mesenchymal transition in pancreatic cancer: Is it a clinically significant factor? Biochim Biophys Acta (2015) 1855(1):43–9. doi: 10.1016/j.bbcan.2014.11.004
8. Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science (1991) 251(5000):1451–5. doi: 10.1126/science.2006419
9. Christofori G, Semb H. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem Sci (1999) 24(2):73–6. doi: 10.1016/S0968-0004(98)01343-7
10. Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer (2004) 4(2):118–32. doi: 10.1038/nrc1276
11. Strathdee G. Epigenetic versus genetic alterations in the inactivation of E-cadherin. Semin Cancer Biol (2002) 12(5):373–9. doi: 10.1016/S1044-579X(02)00057-3
12. Christofori G. New signals from the invasive front. Nature (2006) 441(7092):444–50. doi: 10.1038/nature04872
13. von Burstin J, Eser S, Paul MC, Seidler B, Brandl M, Messer M, et al. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology (2009) 137(1):361–371, 371. doi: 10.1053/j.gastro.2009.04.004
14. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
15. Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Internal Med (1997) 127(9):820–6. doi: 10.7326/0003-4819-127-9-199711010-00008
16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
17. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
18. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
19. Takamura M, Sakamoto M, Ino Y, Shimamura T, Ichida T, Asakura H, et al. Expression of liver-intestine cadherin and its possible interaction with galectin-3 in ductal adenocarcinoma of the pancreas. Cancer Sci (2003) 94(5):425–30. doi: 10.1111/j.1349-7006.2003.tb01459.x
20. Shin SJ, Kim KO, Kim MK, Lee KH, Hyun MS, Kim KJ, et al. Expression of E-cadherin and uPA and their association with the prognosis of pancreatic cancer. Jpn J Clin Oncol (2005) 35(6):342–8. doi: 10.1093/jjco/hyi094
21. Oida Y, Yamazaki H, Tobita K, Mukai M, Ohtani Y, Miyazaki M, et al. Increased S100A4 expression combined with decreased E-cadherin expression predicts a poor outcome of patients with pancreatic cancer. Oncol Rep (2006) 16(3):457–63. doi: 10.3892/or.16.3.457
22. Hong SM, Li A, Olino K, Wolfgang CL, Herman JM, Schulick RD, et al. Loss of E-cadherin expression and outcome among patients with resectable pancreatic adenocarcinomas. Mod Pathol (2011) 24(9):1237–47. doi: 10.1038/modpathol.2011.74
23. Xu B, Jin DY, Lou WH, Wang DS. Lipocalin-2 is associated with a good prognosis and reversing epithelial-to-mesenchymal transition in pancreatic cancer. World J Surg (2013) 37(8):1892–900. doi: 10.1007/s00268-013-2009-6
24. Jiao F, Hu H, Han T, Zhuo M, Yuan CC, Yang HY, et al. Aberrant expression of nuclear HDAC3 and cytoplasmic CDH1 predict a poor prognosis for patients with pancreatic cancer. Oncotarget (2016) 7(13):16505–16. doi: 10.18632/oncotarget.7663
25. Chen L, Ma C, Bian Y, Shao CW, Wang TG, Li J, et al. Aberrant expression of STYK1 and E-cadherin confer a poor prognosis for pancreatic cancer patients. Oncotarget (2017) 8(67):111333–45. doi: 10.18632/oncotarget.22794
26. Chang YR, Park T, Park SH, Kim YK, Lee KB, Kim SW, et al. Prognostic significance of E-cadherin and ZEB1 expression in intraductal papillary mucinous neoplasm. Oncotarget (2018) 9(1):306–20. doi: 10.18632/oncotarget.23012
27. Grupp K, Melling N, Bogoevska V, Reeh M, Uzunoglu FG, Gammal ATE, et al. Expression of ICAM-1, E-cadherin, periostin and midkine in metastases of pancreatic ductal adenocarcinomas. Exp Mol Pathol (2018) 104(2):109–13. doi: 10.1016/j.yexmp.2018.01.005
28. Radulovic P, Kruslin B. Immunohistochemical expression of NEDD9, E-cadherin and gamma-catenin and their prognostic significance in pancreatic ductal adenocarcinoma (PDAC). Bosn J Basic Med Sci (2018) 18(3):246–51. doi: 10.17305/bjbms.2018.2378
29. Noda Y, Goshima S, Tsuji Y, Tomita H, Hara A, Kawaguchi M, et al. Prognostic evaluation of pancreatic ductal adenocarcinoma: Associations between molecular biomarkers and CT imaging findings. Pancreatology (2019) 19(2):331–9. doi: 10.1016/j.pan.2019.01.016
30. Wang M, Estrella JS, Katz MH, Kim M, Rashid A, Lee JE, et al. Expression of Epithelial-Mesenchymal Transition Markers in Treated Pancreatic Ductal Adenocarcinoma. Pancreas (2019) 48(10):1367–72. doi: 10.1097/MPA.0000000000001432
31. Pryczynicz A, Guzinska-Ustymowicz K, Kemona A, Czyzewska J. Expression of the E-cadherin-catenin complex in patients with pancreatic ductal adenocarcinoma. Folia Histochem Cytobiol (2010) 48(1):128–33. doi: 10.2478/v10042-008-0089-1
32. Joo YE, Rew JS, Park CS, Kim SJ. Expression of E-cadherin, alpha- and beta-catenins in patients with pancreatic adenocarcinoma. Pancreatology (2002) 2(2):129–37. doi: 10.1159/000055903
33. Kurahara H, Takao S, Maemura K, Mataki Y, Kuwahata T, Maeda K. Epithelial-mesenchymal transition and mesenchymal-epithelial transition via regulation of ZEB-1 and ZEB-2 expression in pancreatic cancer. J Surg Oncol (2012) 105(7):655–61. doi: 10.1002/jso.23020
34. Gu W, Liu W, Shen X, Shi Y, Wang L, Liu HL. Emergence of heterogeneous nuclear ribonucleoprotein A2/B1 vs loss of E-cadherin: their reciprocal immunoexpression profiles in human pancreatic cancer. Ann Diagn Pathol (2013) 17(1):14–7. doi: 10.1016/j.anndiagpath.2012.04.004
35. Guo S, Xu J, Xue R, Liu YQ, Yu HB. Overexpression of AIB1 correlates inversely with E-cadherin expression in pancreatic adenocarcinoma and may promote lymph node metastasis. Int J Clin Oncol (2014) 19(2):319–24. doi: 10.1007/s10147-013-0549-2
36. Wang W, Dong L, Zhao B, Lu J, Zhao YP. Ecadherin is downregulated by microenvironmental changes in pancreatic cancer and induces EMT. Oncol Rep (2018) 40(3):1641–9. doi: 10.3892/or.2018.6528
37. Karayiannakis AJ, Syrigos KN, Chatzigianni E, Papanikolaou S, Alexiou D, Kalahanis N, et al. Aberrant E-cadherin expression associated with loss of differentiation and advanced stage in human pancreatic cancer. Anticancer Res (1998) 18(6A):4177–80. doi: 10.1016/j.biomaterials.2021.120680
38. Li YJ, Ji XR. Relationship between expression of E-cadherin-catenin complex and clinicopathologic characteristics of pancreatic cancer. World J Gastroenterol (2003) 9(2):368–72. doi: 10.3748/wjg.v9.i2.368
39. Nakajima S, Doi R, Toyoda E, Tsuji S, Wada M, Koizumi M, et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin Cancer Res (2004) 10(12 Pt 1):4125–33. doi: 10.1158/1078-0432.CCR-0578-03
40. Torer N, Kayaselcuk F, Nursal TZ, Yildirim S, Tarim A, Nòyan T, et al. Adhesion molecules as prognostic markers in pancreatic adenocarcinoma. J Surg Oncol (2007) 96(5):419–23. doi: 10.1002/jso.20654
41. Schaeffer DF, Assi K, Chan K, Buczkowski AK, Chung SW, Scudamore CH, et al. Tumor expression of integrin-linked kinase (ILK) correlates with the expression of the E-cadherin repressor snail: an immunohistochemical study in ductal pancreatic adenocarcinoma. Virchows Arch (2010) 456(3):261–8. doi: 10.1007/s00428-009-0866-z
42. Watanabe I, Hasebe T, Sasaki S, Konishi M, Inoue K, Nakagohri T, et al. Advanced pancreatic ductal cancer: fibrotic focus and beta-catenin expression correlate with outcome. Pancreas (2003) 26(4):326–33. doi: 10.1097/00006676-200305000-00003
43. Yonemasu H, Takashima M, Nishiyama KI, Ueki T, Yao T, Tanaka M, et al. Phenotypical characteristics of undifferentiated carcinoma of the pancreas: a comparison with pancreatic ductal adenocarcinoma and relevance of E-cadherin, alpha catenin and beta catenin expression. Oncol Rep (2001) 8(4):745–52. doi: 10.3892/or.8.4.745
44. Nieto MA. Epithelial-Mesenchymal Transitions in development and disease: old views and new perspectives. Int J Dev Biol (2009) 53(8-10):1541–7. doi: 10.1387/ijdb.072410mn
45. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol (2019) 20(2):69–84. doi: 10.1038/s41580-018-0080-4
46. Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med (2013) 19(11):1438–49. doi: 10.1038/nm.3336
47. Aghdassi A, Sendler M, Guenther A, Mayerle J, Behn CO, Heidecke CD, et al. Recruitment of histone deacetylases HDAC1 and HDAC2 by the transcriptional repressor ZEB1 downregulates E-cadherin expression in pancreatic cancer. Gut (2012) 61(3):439–48. doi: 10.1136/gutjnl-2011-300060
48. Thiery JP, Acloque H, Huang RY, Nieto MA, et al. Epithelial-mesenchymal transitions in development and disease. Cell (2009) 139(5):871–90. doi: 10.1016/j.cell.2009.11.007
49. Bierie B, Pierce SE, Kroeger C, Stover DG, Pattabiraman DR, Thiru P, et al. Integrin-beta4 identifies cancer stem cell-enriched populations of partially mesenchymal carcinoma cells. Proc Natl Acad Sci USA (2017) 114(12):E2337–46. doi: 10.1073/pnas.1618298114
50. Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene (2008) 27(55):6920–9. doi: 10.1038/onc.2008.343
51. Huang X, Ding L, Liu X, Tong R, Ding J, Qian Z, et al. Regulation of tumor microenvironment for pancreatic cancer therapy. Biomater Int J Mol Sci (2020) 21(19):7307.
52. Berx G, Van Roy F. The E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res (2001) 3(5):289–93. doi: 10.1186/bcr309
53. Eom BW, Ryu KW, Yoon HM, Kook MC. Predictive value of E-cadherin and EpCAM for detection of metastatic lymph node in early gastric cancer. Chin J Cancer Res (2020) 32(5):614–20. doi: 10.21147/j.issn.1000-9604.2020.05.06
Keywords: E-cadherin, pancreatic cancer, susceptibility, prognosis, meta-analysis
Citation: Wang P and Zhu Z (2021) Prognostic and Clinicopathological Significance of E-Cadherin in Pancreatic Cancer Patients: A Meta-Analysis. Front. Oncol. 11:627116. doi: 10.3389/fonc.2021.627116
Received: 08 November 2020; Accepted: 08 March 2021;
Published: 12 April 2021.
Edited by:
Manish A. Shah, Cornell University, United StatesReviewed by:
Zhifang Zhang, Beckman Research Institute, City of Hope, United StatesAmira Kamal El-hawary, Mansoura University, Egypt
Copyright © 2021 Wang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zengkuan Zhu, emVuZ2t6dTEwQGFsaXl1bi5jb20=
 Pengbo Wang1
Pengbo Wang1 Zengkuan Zhu
Zengkuan Zhu