- 1School of Biomedical Engineering & Imaging Sciences, King’s College London, London, United Kingdom
- 2Department of Neuroradiology, King’s College Hospital NHS Foundation Trust, London, United Kingdom
- 3Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, United Kingdom
- 4Brainstrust, Cowes, United Kingdom
- 5Leeds Institute of Medical Research at St James’s, St James’s University Hospital, Leeds, United Kingdom
- 6Faculty of Medicine and Health, Leeds Institute of Health Sciences, University of Leeds, Leeds, United Kingdom
- 7Siemens Healthineers, Frimley, United Kingdom
- 8The Brain Tumour Charity, Fleet, United Kingdom
- 9Department of Neurosurgery, King’s College Hospital NHS Foundation Trust, London, United Kingdom
- 10ACT for Cancer, London, United Kingdom
- 11The Tessa Jowell Brain Cancer Mission, London, United Kingdom
- 12Lysholm Department of Neuroradiology, National Hospital for Neurology and Neurosurgery, London, United Kingdom
- 13Department of Oncology, Christie Hospital NHS Foundation Trust, Manchester, United Kingdom
- 14Department of Neuroradiology, The Walton Centre NHS Foundation Trust, Liverpool, United Kingdom
- 15Department of Radiology, Wales Research and Diagnostic PET Imaging Centre, Cardiff University School of Medicine, Cardiff, United Kingdom
- 16King’s College Trials Unit, King’s College London, London, United Kingdom
- 17King’s Health Economics, King’s College London, London, United Kingdom
- 18Department of Oncology, Velindre Cancer Centre, Cardiff, United Kingdom
- 19Department of Neuroradiology, Western General Hospital, Edinburgh, United Kingdom
- 20Birmingham Brain Cancer Program, University of Birmingham, Birmingham, United Kingdom
- 21University Hospitals Birmingham NHS Foundation Trust, Birmingham, United Kingdom
- 22Department of Neuro-oncology, Imperial College Healthcare NHS Trust, London, United Kingdom
- 23Institute of Translational Medicine, University of Liverpool, Liverpool, United Kingdom
- 24Department of Neurosurgery, The Walton Centre NHS Foundation Trust, Liverpool, United Kingdom
Objectiv e: To summarise current evidence for the utility of interval imaging in monitoring disease in adult brain tumours, and to develop a position for future evidence gathering while incorporating the application of data science and health economics.
Methods: Experts in ‘interval imaging’ (imaging at pre-planned time-points to assess tumour status); data science; health economics, trial management of adult brain tumours, and patient representatives convened in London, UK. The current evidence on the use of interval imaging for monitoring brain tumours was reviewed. To improve the evidence that interval imaging has a role in disease management, we discussed specific themes of data science, health economics, statistical considerations, patient and carer perspectives, and multi-centre study design. Suggestions for future studies aimed at filling knowledge gaps were discussed.
Results: Meningioma and glioma were identified as priorities for interval imaging utility analysis. The “monitoring biomarkers” most commonly used in adult brain tumour patients were standard structural MRI features. Interval imaging was commonly scheduled to provide reported imaging prior to planned, regular clinic visits. There is limited evidence relating interval imaging in the absence of clinical deterioration to management change that alters morbidity, mortality, quality of life, or resource use. Progression-free survival is confounded as an outcome measure when using structural MRI in glioma. Uncertainty from imaging causes distress for some patients and their caregivers, while for others it provides an important indicator of disease activity. Any study design that changes imaging regimens should consider the potential for influencing current or planned therapeutic trials, ensure that opportunity costs are measured, and capture indirect benefits and added value.
Conclusion: Evidence for the value, and therefore utility, of regular interval imaging is currently lacking. Ongoing collaborative efforts will improve trial design and generate the evidence to optimise monitoring imaging biomarkers in standard of care brain tumour management.
Introduction
Over the last decade the treatment landscape for adult brain tumours has changed incrementally for some tumour types, such as metastases, where there have been improvements in systemic therapy and brain radiotherapy (1). For other tumour types there has been little change. The management of glioblastoma remains largely based on maximum safe resection and radiotherapy with concomitant and adjuvant temozolomide chemotherapy (2). Evidence from randomised controlled trials [level 1 (3)] underpins clinical treatments of adult brain tumours. In contrast, there is little evidence (< level 3) to support the current imaging practices used to monitor disease progression or response to treatment (4, 5). Therefore, the clinical utility [the relevance and usefulness of an intervention in patient care using all sources of evidence (6)] of interval imaging (imaging at pre-planned time-points to assess tumour status, as compared with scanning for reasons of clinical deterioration) is largely unknown.
“Interval imaging” was first introduced into neuro-oncology in 1977 by Victor Levin, shortly after the introduction of computed tomography (CT) (7). In 1981 the World Health Organisation (WHO) convened two expert meetings on the “Standardization of Reporting Results of Cancer Treatment”. The recommendations were widely adopted to ensure consistency of timing between centres and became the basis of subsequent iterations of high-grade glioma treatment monitoring and result reporting (8). These evolved with the development of MRI. In 1990, Macdonald also recommended assessing factors affecting imaging appearance such as corticosteroid use (9) which subsequently formed the basis of the AVAglio trial response criteria (10) and the 2010 response assessment in neuro-oncology (RANO) trial guidelines (11). There is a demonstrable historical lineage following the advent of CT for how enhancing tumour size, following exogenous contrast administration, has been incorporated into current clinical and trial practice. The expert committees were informed by observational studies, supported by a biologically plausible assumption that the images from each time-point, or the rate of change in a series of imaging investigations, are reliable “monitoring biomarkers” (12) reflecting tumour behaviour. The assumption is that changes in tumour size identify progression of disease, potentially before it becomes clinically apparent, resulting in a lead time improvement for therapeutic intervention. Indeed, there may be benefits in changing management before the development of irreversible disability or before the extent of tumour precludes intervention. Some justification for enhancement as a disease proxy has been inferred from data showing that enhancing tumour size and extent of resection are “prognostic biomarkers” (12) at both first presentation and recurrence (13–15). However, there is no evidence that earlier diagnosis influences prognosis pre- or post-operatively, and individual enhancing tumour growth trajectories vary between individuals with the same histological tumour type.
Deriving an evidence base surrounding current imaging practices is important for several reasons. There is a lack of biological specificity for contrast enhancement, particularly in the context of treatment effects and pseudophenomena, which can confound imaging assessment. There is also variability in clinical adoption of interval imaging practice across UK and European neuro-oncology centres which is unlikely to be in the best interests of patients or healthcare systems (16, 17). It is also noteworthy that the widely reported observation that increasing enhancement tracks growth of most brain tumour types, has extended the use of contrast-enhancing tumour size as a biomarker beyond high-grade glioma. The impetus to derive an evidence-base is driven by researchers (4, 5, 16), but more importantly patients and carers (Box 1) (18). This in part relates to factors such as understanding the anxiety surrounding the imaging event and awaiting the results – so-called “scanxiety” (19). Furthermore, determining the health economics related to interval imaging is equally important. These include understanding the direct costs of subsequent investigations (for example, ‘advanced imaging’ requiring additional sequences and processing time, or earlier interval imaging than usual), additional hospital appointments that may follow uncertain tumour changes, continuation of futile therapies, as well as indirect and opportunity costs.
The purpose of this position statement is to summarise the current evidence base for the utility of interval imaging in brain tumours and to propose potential studies for future evidence gathering incorporating the disciplines of data science and health economics.
Box 1. A National Institute for Health Research (NIHR) James Lind Alliance (JLA) Priority.
The National Institute for Health Research (NIHR) James Lind Alliance (JLA) brings patients, carers and clinicians together in Priority Setting Partnerships stating that “addressing uncertainties about the effects of a treatment should become accepted as a routine part of clinical practice” and that “patients, carers and clinicians should work together to agree which, among those uncertainties, matter most and deserve priority attention”.
In 2015, the group met to establish the ten highest clinical priority uncertainties in neuro-oncology in the UK. Number two is: “what is the effect on prognosis of interval scanning to detect tumour recurrence compared with scanning on symptomatic recurrence in people with a brain tumour?” Patients expect imaging will give an accurate account of the effect of treatment and either reassure or initiate a change in the treatment plan. If there is uncertainty regarding progression, that leads to anxiety until the next scan or specialist MR imaging. In addition, if there is minor imaging progression only, there may be the clinical dilemma as to whether to change management if there are further options, even though the patient has had no clinical deterioration and it is not known whether earlier pre-symptomatic intervention improves survival.
This might be interpreted as:
“For me, a patient, does earlier scanning to detect asymptomatic progression improve my quality of life and survival, or does it not make any difference, or make it worse?”
And:
“Would I, as a doctor, improve the quality of life and survival of my patients if I monitored them more proactively and detected progression before it became symptomatic or does it not make any difference or make it worse?”
Materials And Methods
Clinicians, scientists, and patient advocates and representatives with expertise in interval imaging, data science, health economics or trial management of adult brain tumours, convened in London, UK, in April 2019 in conjunction with a National Cancer Research Institute (NCRI) Brain Tumour group workshop. Available evidence for interval imaging pathways for different tumour types was discussed in the context of research recommendations of previous publications, including the UK’s National Institute for Health and Care Excellence (NICE) brain tumour guidelines NG99 (Supplementary Table 1) and a systematic review of glioma imaging (4, 5, 16). Specifically, we sought to assess value in the context of morbidity, mortality, quality of life and resource use (together these outcomes give the additional outcome measure of cost effectiveness). Clinical utility incorporates all these outcome measures as well as considering the interests and goals of stakeholders (6). In addition, the results of a UK national clinical practice survey on the use of internal imaging in glioblastoma management were reviewed. Opportunities to generate evidence were explored in the context of specific study designs, with a focus on the utility and limitations of applying each design to a specific scenario. We outlined the advantages and disadvantages of each design based on current evidence and expert opinion. The discussion was compiled into a manuscript and circulated to NCRI Brain Tumour Group members and invited experts in attendance as well as those unable to attend. Edits and feedback were incorporated until all authors were in agreement with the content, and a position statement was produced around potential approaches to studying interval imaging in glioma and meningioma.
Results
Targeting of Interval Imaging Studies
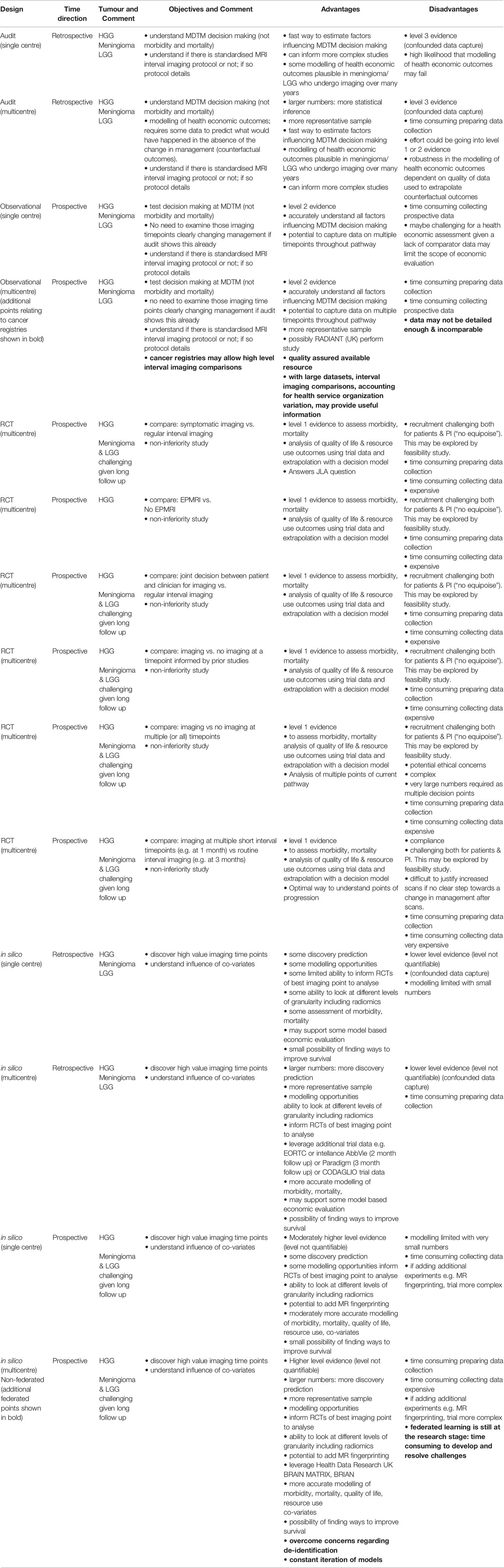
Following explicit agreement that there was an evidence gap, and that the JLA priority (Box 1) should be addressed, an initial question was to determine which brain tumour types should be included in the position statement. Whilst central nervous system (CNS) tumours comprise a range of diverse histological and molecular entities (20), meningioma and gliomas are the two commonest accounting for 36% and 28% of tumours, respectively. These were selected as the focus for interval imaging studies. Although brain metastases are more common overall, because of the rapidly evolving treatment paradigms according to primary cancer site (stereotactic radiosurgery, chemotherapy, immunotherapy) (1), the reliance on disease response assessment in the body to systemic treatments, and the fact that this can even vary between different intracranial lesions in the same individual (1), it was agreed that metastases were beyond the scope of the workshop (21–23). It was acknowledged that high-grade (WHO III-IV) and lower-grade (WHO II) infiltrating gliomas should be treated separately. Individual imaging biomarker techniques beyond standard structural clinical MRI have been reviewed extensively elsewhere (24, 25) and the uptake of these “advanced” MRI and positron emission tomography (PET) techniques is limited and highly heterogeneous, even in specialist neuro-oncology centres across Europe (17). The focus at the workshop was therefore on determining the value of the structural MRI interval imaging pathway and methods to interrogate this, although the potential role of additional imaging techniques remains relevant. Improving the diagnostic performance of structural or “advanced” imaging biomarkers is a further means to rationalise imaging timepoints by reducing repeat imaging while there is ongoing uncertainty. Key points regarding study design types are summarised in Table 1.
Interval Imaging Overview
Neuro-oncology multi-disciplinary team meetings (MDTs, referred to as “Tumor Boards” in North America) consider longitudinal patient management, with serial imaging follow-up having a central role. Meningioma and glioma imaging follow up schedules from UK’s National Institute for Health and Care Excellence (NICE) brain tumour guidelines NG99 are shown in Supplementary Tables 2 and 3 (4). In the broadest sense, interval imaging is typically performed in order to determine whether a tumour is growing, which may initiate or change treatment. A planned imaging schedule provides clinicians with a framework to track an aspect of tumour biology just before clinical review and allows easier administrative timetabling for the imaging department, MDT planning, and the patient diary planning, but cannot determine what the symptoms will be at the point of imaging. The radiologist only has information of symptoms at the time of request, which may limit interpretation. This allows decisions to be made on commencing, continuing, or discontinuing treatment and provides insight into whether treatment has caused a meaningful alteration in tumour biology. Standard structural MRI is routinely used for this purpose. In some centres, “advanced” MRI techniques (e.g. dynamic-susceptibility contrast enhanced, DSC, imaging or 1H-magnetic resonance spectroscopy MRS) or PET (targeting glucose or amino acid uptake) helps problem solving in instances when structural imaging is indeterminate (16, 17). As an alternative to a planned imaging schedule, imaging can also be triggered by a change in symptomatology or clinician concern, regardless of any scheduled follow-up interval. While triggered imaging is more difficult to organise at short notice, the strategy benefits from addressing patient concerns regarding the cause of new symptoms and providing the radiologist with contemporaneous clinical information at the time of imaging. In most centres the strategy for interval imaging is a combination of both a planned schedule and triggered imaging (16). Clinical and other non-imaging biomarkers of disease progression, whether as triggers for imaging or additional treatment response biomarkers, have the potential to be incorporated into the patient pathway and would benefit from further research (Supplementary Information 1).
The emergence of novel therapies, such as immunotherapy have created challenges for follow-up imaging of glioma in clinical trials, with pseudophenomena occurring in up to 5% of patients leading to the development of modified response assessment approaches such as iRANO (26). The central modification is that the moratorium on progressive disease is extended to cover the first 6 months of treatment. Stopping routine interval imaging for 6 months is, however, not recommended given the potential for the side effects of these therapies. Immunotherapy is not currently recommended as a second-line treatment option in most countries outside of research trials.
Interval Imaging and Confounds
Although MRI is a safe and effective technique, structural imaging can lead to false positive, false negative, and indeterminate results, particularly relating to post-treatment related pseudophenomena in glioma. In glioblastoma, pseudoprogression is an early post-treatment related effect typically occurring within 6 month of finishing concomitant temozolomide and radiotherapy whereas pseudoresponse typically occurs after anti-angiogenic agents such as bevacuzimab have been administered. False positive progression and false negative treatment response are manifest as an increase or decrease in MRI contrast enhancing volume respectively. Confounding of treatment response commonly occurs with the use of current standard interval imaging conventions in therapeutic and novel imaging glioma trials due to the impact of such pseudophenomena (27). Delayed treatment effects such as increased enhancement due to radiation necrosis can similarly cause false positive progression. Other examples of non-specificity include post-operative peritumoral parenchymal enhancement following operative “tissue handling”; or following operative infarction. Confounding is particularly relevant if progression-free survival is used as an outcome measure which is fundamentally based on, and therefore affected by, the timing of routine interval imaging. In part to mitigate this, objective criteria such as RANO require a threshold of enhancing size change (a 25% increase or 50% decrease in the product of perpendicular dimensions) and an indication of clinical status, corticosteroid dose, and other possible confounds of deterioration such as unrelated health issues; and for true positive progression there is a requirement for sustained size change beyond one time point. It is noteworthy that overall survival is also confounded by differences in treatment at progression. Management typically consists of second-line chemotherapy including the combination of procarbazine, lomustine and vincristine (PCV) (28–30), TMZ re-challenge (31, 32) or supportive care. Not only is management heterogenous, but pseudophenomona will confound this management choice. Furthermore, such detail to understand these co-variates are rarely included in studies (33).
The Patient and Carer Experience of Interval Imaging
Patients undergoing MRI often experience anxiety prior to and during scanning (34). Up to 37% of patients undergoing MRI experience moderate to high levels of anxiety related to the procedure itself (35–37). When the patient is aware they have a brain tumour that is being assessed for response or progression, anxiety is likely to be more frequent and magnified. Such “scanxiety” is a recognised consequence of interval imaging in cancer (19). Incorporating phenomena such as “scanxiety” into neuro-oncological studies requires patient-reported outcome (PRO) measures which are well defined and reliable and therefore can generate high-quality evidence (38).
Uncertainty is defined as an individual’s “lack of ability to determine the meaning of illness‐related events” (39). In patients with primary brain tumours this has a direct impact on all negative mood states (tension, depression, anger, fatigue, and confusion) measured using the Profile of Mood States‐Short Form (POMS‐SF)) (40). These negative mood states impact on symptom severity, with higher levels of uncertainty associated with worse negative mood states and symptom severity. Due of the high likelihood of disease progression or recurrence in glioma, negative mood states may be exacerbated when patients who have symptoms are awaiting MRI results. Interventions designed to reduce uncertainty may help lessen patients’ perception of symptom severity, which may subsequently result in better treatment outcomes and quality of life. One solution might be “one-stop” clinics in neuro-oncology, but this can be logistically challenging due to managing scanner capacity and radiologist availability for providing direct access reporting. Another approach to reduce uncertainty might be to provide re-assurance that the disease is better or to give a clear management plan for treatment at the point when the imaging results are conveyed to them. Conversely, any new uncertainty or uncertainty that persists following imaging, such as the consideration of pseudophenomena, might reinforce or perpetuate negative mood states. In the typically slower progressing lower grade glioma and meningioma, where there is less impact of pseudophenomena and delayed treatment effects, it is unclear how the relatively long imaging intervals influence uncertainty.
There are several sources of low-level evidence (level 4) indicating that patient and carer anxiety related to perceived unnecessary MRI scans or inaccurate or indeterminate imaging findings in primary brain tumours is a concern. This was a motivating factor behind the James Lind Alliance Priority Setting Partnership priority to establish the value and benefit of neuro-oncological interval scanning (18). Study design into interval imaging would benefit from including patient-reported outcomes (PRO) so that “uncertainty reduction” can be measured.
Interval Imaging Practices With a Focus on Glioblastoma
There is no robust evidence (< level 3) to support the value or lack of value for the imaging practices currently used to monitor disease or to determine the response of any treatment given in adult brain tumours (4). There is also a lack of evidence around the utility of early post-operative MRI (EPMRI; within 72 h) on adult brain tumour patients after surgical resection of glioblastoma (16, 41–43). Interval imaging conventions are based predominantly on expert opinion and have been primarily motivated by efforts to standardise outcomes for comparing therapeutic trials (9, 11). For EPMRI, it is also noted that indirect contributors to value, such as improving surgical practice (5, 44), are challenging to measure, particularly at the start of a complex treatment pathway. Any study design into interval imaging value must consider current or planned therapeutic trials where outcomes are based on interval imaging regimens. Similarly, there should be awareness that current imaging conventions and the reliance of regulatory approval pathways on them [e.g. FDA endorsing RANO-based treatment outcomes (45)] might impede the development of innovative imaging solutions and other biomarkers designed to rationalise or optimise the imaging pathway.
An understanding of current practice is critical to subsequent study design. A recent UK-wide national clinical practice survey on the use of interval imaging in glioblastoma management (GIN CUP study) showed considerable variation between centres (16). Similarly, the timing and interval length between MRI examinations in the period following completion of adjuvant chemotherapy, shows considerable inter-institutional variation. It is also noted that current UK, European, and international guidelines (4, 46–49) show variation and lack of consensus on the frequency and timing of neuroimaging during the post treatment follow-up period, likely as a result of the lack of objective evidence base and different resourcing between jurisdictions.
In summary, there is considerable variation in interval imaging practice between centres during the glioblastoma post treatment follow-up period which should be considered in the study design of interval imaging value. Neuroimaging is believed to be crucial in making subsequent plausible management decisions once treatment is initiated, however there is a paucity of evidence for this assertion at all timepoints. Additional evidence, therefore, needs to be obtained to determine whether imaging protocols used in current routine clinical practice, and the type of neuroimaging performed at each component of the pathway, result in a measurable and impactful change in management (as opposed to the perception of a change or impact). Determining whether there is a change in outcome and value (morbidity, mortality, quality of life or resource use) is key.
Health Economics of Interval Imaging
Economic evaluation addresses issues of efficiency and cost effectiveness: are the resources required to provide the intervention, in this case MRI scans, justified by the health benefits? If the MRI scan offers no health benefit, then the intervention is not considered cost effective, with robust economic evaluation required to determine under which circumstances cost-effectiveness is achieved. Whilst retrospective analyses may determine to some extent whether there has been a change in management, prospective studies are needed to quantify the benefit in the context of confounds.
To determine cost effectiveness, an estimate of the impact of imaging on overall resource use is required as well as an estimate of the impact of imaging on survival and quality of life. Consideration of resource data collection is important in the design of future studies. Prospective collection of quality of life data can support a within-trial analysis of quality adjusted life-years (QALYs: a measure of the impact of the intervention on health-related quality of life and survival). To inform QALYs, quality of life is typically measured using generic quality of life instruments, of which the most commonly used are the EuroQol EQ-5D (50), the SF-6D (51) (based on the Rand SF-36 questionnaire) and the health utilities index (52). The EQ-5D is the most commonly used measure, and reports health status as the level of functioning in five domains. The five level (5L) instrument differentiates 3,125 response combinations each of which has an associated tariff ranging from 1 for full health, through 0 for dead, to negative scores for a small number of health states considered worse than dead. Measurement might be performed at baseline and 3 monthly intervals during the imaging period, in a similar fashion to interval imaging. More extensive questionnaires such as the European Co-operative Oncology Group Quality of Life Questionnaire Core 30 (EORTC QLQ C30) (53) may provide a more targeted capture of quality of life and may prove to be suitable instruments. Further work is required to determine more detailed evidence surrounding patients’ views on quality of life in relation to interval imaging cost effectiveness. Other sources of information to capture outcomes such as mortality or resource use can come from case report forms (CRF) within a trial or from clinical registers, e.g. the Surveillance, Epidemiology and End Results database in the US (SEER) (54) and the National Cancer Registration and Analysis Service (NCRAS) in the UK (55). Administrative databases may also provide useful data on diagnosis, treatments and survival (56). The literature also provides estimates of the cost of care for relevant events that may not be observed during a trial such as the cost of end-of-life-care (EOLC) (57).
Trial follow-up is frequently insufficient to capture the full implications of monitoring and treatment on patient costs and outcomes. A decision model is commonly used to extrapolate costs and outcomes beyond trial follow-up, often over the remaining lifetime of the patient cohort. The most commonly used is the Markov model, which captures patient trajectories as a sequence of health states representing progression of the disease (58). Estimation of lifetime costs and outcomes of different monitoring and treatment strategies allows quantification of the difference in costs and outcomes across strategies. The ratio of incremental costs to incremental outcomes, known as the incremental cost-effectiveness ratio (ICER), reports the efficiency of more effective strategies in terms of the cost per unit improvement in outcome. These data typically influence recommendations from national health technology agencies on the use of new technology and care pathways, although the application of an explicit upper limit or threshold with regard to cost-effectiveness is limited to the UK, Australia and Canada (59).
Deriving evidence to determine the cost effectiveness of interval imaging requires consideration of the impact of imaging on downstream costs and outcomes. Downstream costs for surgical treatments such as craniotomy or licensed chemotherapy drugs can greatly outweigh the costs of the imaging. Hence quantifying small changes in treatments arising from imaging is important. Therefore, any design or modelling to optimise interval imaging cost effectiveness in routine clinical practice should incorporate changes in the costs of any subsequent alteration in treatment i.e. related and opportunity costs. For example, the model should incorporate changes in the costs of continuing expensive and ineffective therapies which themselves may be associated with adverse effects; changes to surgical procedures which themselves may be associated with reduced or prolonged hospital stays; and changes to the costs of rehabilitation if the clinical impacts of progression of underlying disease are altered.
The conclusions of any health economic design framework described above are most applicable to integrated healthcare systems such as the UK. In these healthcare systems, imaging was historically considered relatively costly, and most agencies endorse rationing which can limit use. However, other reimbursement models in other healthcare systems can incentivise additional investigation, as reflected by the wide discrepancy in MRI use between countries (60).
Beyond providers, there are individual financial implications for imaging. For example, 54% of carers of US patients with high-grade gliomas out of active treatment had costs of $271 per month with transportation to hospitals amongst the greatest out of pocket costs (61). These personal costs may be lower in healthcare systems such as the UK where hospitals and charities provide additional support, but evidence suggests they remain substantial (62). Health economic modelling would benefit from incorporating such individual costs and regional/international variations.
More evidence to determine the cost effectiveness of interval imaging incorporating the patient, carer, and healthcare system is required. Careful study design using standard tools should achieve this. Evidence on cost-effectiveness will improve care pathways in all systems, and is central to the efficient use of resources in centrally funded healthcare systems.
Data Science In Silico Interval Imaging Studies
In silico studies are those performed on a computer or via computer simulation. Sophisticated algorithms or simulations can advance scientific understanding, although the inferences drawn must recognise the limitations introduced by the simplified or reductive framework. The results of these simulations can be tested in existing trials or serve as a guide for future trials. Machine learning applications may move beyond inferential statistical approaches to attempt to extract more accurate predictions from complex datasets. Such approaches for imaging monitoring biomarkers in neuro-oncology are at an early stage of development in terms of clinical validation and applied techniques are not yet ready to be incorporated into the clinic (63, 64). A recent systematic review using PRISMA-DTA and QUADAS-2 methodology, showed that the small numbers of patients included in machine learning studies, the high risk of bias and concerns of applicability in the study designs, and the low level of evidence given that the monitoring biomarker studies are retrospective, suggest that limited conclusions can be drawn from the data (33).
Studies may take advantage of enhanced computational approaches to build data-rich neuro-oncology monitoring biomarker models, although more involved or computationally expensive approaches such as those used in deep learning, may not de facto outperform more traditional machine learning techniques, for example multivariate logistic regression (63). It is also notable that studies applying machine learning to build neuro-oncology monitoring biomarker models have yet to show overall advantage over those using traditional statistical methods in terms of analytical validation and diagnostic performance (63, 65, 66). Such statistical methodology is wide ranging and includes generalised estimating equations and mixed models (67) but for clarity, we note that there is a continuum between the two fields, a pertinent example being non-parametric orthogonal transformations for dimensionality reduction.
We note several barriers in translating machine learning which the neuro-oncology community must appreciate for in silico study design: (1) the clinical context may not be represented with a decreased ability to perform holistic evaluations of patients, with loss of valuable and irreducible aspects of the human experience such as psychological, relational, social, and organizational issues (68); (2) accuracy-driven performance metrics have led to more opaque models (69) although advances in interpretability and explainability may mitigate this somewhat (70); (3) binding the empirical data to categorical interpretation misses an intrinsic ambiguity in the observed phenomena (71) which might negatively affect performance (68); (4) overreliance on the capabilities of automation can lead to the related phenomenon of deskilling (72). Furthermore, there are several technical limitations that make many algorithms unreliable: domain adaptation is still in its infancy and further solutions are required to help algorithms extrapolate well to new hospitals. Uncertainty estimation is still underdeveloped, and necessary to know when algorithms are out-of-distribution or when the accuracy might be poor. Robustness to data issues, such as artefacts, is very much needed but also at its infancy. Lastly, the presence of multiple pathologies (for example, tumours and stroke) can also confound algorithms as these cases are rare and often unlabelled.
Nonetheless, we emphasise that machine learning models have key advantages: whilst three decades ago it was noted that they require less formal statistical training given developments in software (73, 74), more recently there has also been a transformative reduction in the requisite programming expertise for researchers which has been enabled by open source software standardised implementations (75–77); have the ability to detect implicitly any complex non-linear relationship between independent and dependent variables (73, 78); and have the ability to detect all possible interactions between predictor variables (69). Indeed, new approaches have proven to bring new perspectives and insights to the diagnosis of neuro-oncology pathologies, such as glioma (79, 80). In particular, some of these models are currently used as diagnostic biomarkers (12) for prediction of tumour grading and genomics from imaging as well as automating diagnosis from histopathology; furthermore prognostic biomarkers can provide insights into survival (80).
Advances in brain tumour database curation will facilitate integration of imaging data with demographic, clinical, and molecular marker data into large databases [in the UK, for example, these include Health Data Research UK, the Tessa Jowell BRAIN MATRIX (81) or BRIAN – the Brain tumouR Information and Analysis Network (82)]. The capture of large volumes of data and the inclusion of a wider spectrum of imaging phenotypes, typically results in improved diagnostic performance during machine learning or statistical tasks; the relative improvement of deep learning model performance is particularly marked (83–85). Note that for deep learning, the dependency on very large datasets can be reduced by data augmentation and transfer learning; the latter, where an already developed model for a task is reused as the starting point or a model on a second task, is especially advantageous for medical tasks since these pre-trained models not only obviate the need for very large datasets but are less computationally expensive (70, 79, 80). Once established, incoming data from each of these larger scale live repositories will facilitate ongoing refinement and assessment of impacts. Examples of machine learning tools that have been used with large datasets in neuro-oncology, as well as generic approaches to multi-centre machine learning which might overcome privacy issues, are contained in the Supplementary Information 2.
Initiatives and consensus statements have provided recommended frameworks (86–89) for standardising imaging biomarker discovery, analytical validation, and clinical validation (12), which can help to improve the robustness of study design of machine learning applied to neuro-oncology. It is clear that for such an approach large, well-annotated datasets, and therefore, multi-disciplinary and multi-centre collaborations are mandated (63), and this will require a collaborative approach to reach meaningful dataset size and quality.
Interval Imaging Study Design and Statistical Considerations
The overarching purpose of any study design would be to determine the value of interval imaging and to maximise this value where possible. Ideally, studies would provide robust evidence (≤ level 3) for morbidity, mortality, quality of life and resource use (together these outcomes give the additional outcome measure of cost effectiveness) of three tumour groups (meningiomas, lower grade gliomas, and high-grade gliomas) undergoing interval imaging. Further Patient and Public Involvement (PPI) work is underway to refine measurable metrics although a primary outcome of mortality and secondary outcomes of quality of life e.g. the EUROQOL EQ 5D–5L or EORTC QLQ C30 score, may be sensible. Outcomes are confounded by treatment type and motivate thorough co-variate collection. Progression-free survival is especially confounded as described above and must be considered carefully as an outcome measure in glioma study design. This is a major driver for the consideration of adopting “advanced imaging” in more robustly defining a progression event through imaging.
Given these, it is likely that different approaches are required to construct an evidence framework surrounding interval imaging (Table 1); building the framework is likely to be stepwise (90), using less robust evidence (< level 3) initially as well as determining baseline quality of life and resource use outcomes. Whilst the trial giving the highest level of evidence would be a randomised controlled trial (RCT), and likely a non-inferiority design, knowing which aspects of the pathway to randomise will require additional supportive intermediate evidence from preliminary studies. For example, data can be acquired using audit or observational studies to determine whether there is a change in management or not. If management is changed, an RCT may be able to address whether there is additional value from the change in management in terms of morbidity, mortality, quality of life and resource use. However, there may be challenges for recruitment of patients into an RCT, predominantly influenced by tumour type. For example, in a high-grade glioma RCT with reduced imaging in one arm, some participants and recruiters may oppose reduced imaging in a tumour where changes in disease can be rapid. It is plausible that there would be less concern for lower grade gliomas or meningioma interval imaging studies.
In silico studies using statistical or machine learning approaches might provide an alternative to inform which aspects of the pathway should be randomised in an RCT (91, 92). Alternatively, such techniques might be used to approximate outcomes themselves, however, as with an RCT, a large number of centres would be required to provide sufficient data, particularly if PROs and health economic measures are also incorporated. It is noteworthy that within existing provision and clinical trials, there will be natural jitter and missed time points in the follow-up of patients. With large datasets this might provide an opportunity using appropriate modelling techniques to assess the impacts of these natural timing differences and missing data points. Despite the potential of in silico studies, a disadvantage is that they do not produce level 1 evidence nor is it clear how the most complex modelling studies equate with traditional levels of evidence (3).
Whilst the focus of study design relates to the structural MRI interval imaging pathway and by default the “when” of imaging, the “how” and “what else” remain important avenues for research (5). It is conceivable that the interrogation of biomarkers such as MRI radiomic features, advanced MRI or PET studies can be added as secondary objectives. It is acknowledged that these are not routinely used nor widely available modalities, and in the case of PET in particular, have a distinct risk and cost effectiveness profile compared to structural MRI. We note other expert consortia are looking specifically into advanced imaging and processing techniques to develop international recommendations and guidelines on their application as monitoring biomarkers (93, 94).
Regardless of the approach to achieve accurate, complete, and transparent reporting of studies contributing to the evidence of interval imaging in standard of care brain tumour management, we strongly recommend following reporting guidelines from the EQUATOR Network (95), available for example in prospective biomarker studies (96, 97), RCTs (98) or economic evaluations (99).
Discussion
Determining the value, and therefore the utility, of interval imaging in brain tumour management remains a key priority in neuro-oncology. Meningioma and glioma were identified as priorities for interval imaging utility analysis. Any study design that changes imaging regimens should consider the potential for influencing current or planned therapeutic trials; ensure opportunity costs are measured; and that indirect contributions to value are identified and assessed.
Whilst it was agreed that an RCT would provide level 1 evidence, no consensus was reached on specific trial design, reflecting the immense challenge faced in addressing this evidence gap. While development of level 1 evidence is the desired goal, given that current practice is predominantly based on expert opinion (level 5) there is a role for establishing “intermediate level” evidence that might support a future RCT. The outcomes of any study must include overall survival, quality of life and resource use. The panel agreed that this “intermediate level” evidence was unlikely to be obtained solely through descriptive and inferential statistics of existing datasets and would benefit from modelling and advanced statistical and machine learning approaches, and that larger, aggregate datasets would be required involving multicentre collaborations. Overall, no consensus was reached as to the specific studies which should be undertaken, but types of study have been described here for consideration along with their strengths and limitations.
This position statement aims to provide a framework for developing the evidence base for the value of interval imaging in primary brain tumours and, thereafter, practice recommendations. The panel welcomes any collaborative approach from groups interested in aggregating data and contributing to study design. Ongoing collaborative efforts will improve trial design and generate the evidence to optimise monitoring imaging biomarkers in standard of care brain tumour management.
Author Contributions
FB, TB HB, CB, JC, LD, RG, MJ, CMc, SM, NM, CMu, MP, JP, DS, GT andMWcontributed conception and design of the study. TB wrote the first draft of the manuscript. FB, TB, HB, JC, RG, MJ, SM, NM, MP, JP, DS, GT, AW and MW wrote sections of the manuscript. DJ, KA, JK, NH, AL, SO, AW and CW: Not in attendance at incubator day; provided subsequent input. All authors contributed to the article and approved the submitted version.
Funding
This Interval Imaging Incubator Day meeting was facilitated and funded by brainstrust – the brain cancer people. No participants were remunerated for their contribution. National Cancer Research Institute (NCRI) separately provided travel and accommodation for those panel members who had travelled for either the main brain tumour group meeting or the glioma subgroup. This work was supported by the Wellcome/EPSRC Centre for Medical Engineering [WT 203148/Z/16/Z].
Conflict of Interest
TB, speaker’s bureau for AbbVie and Siemens Healthineers. Craig Buckley, Head of Research and Innovation – Siemens Healthineers GB&I. JC, BrainMiner Founder. Involved in machine learning enterprise and business. JK, involved in enterprise and business. MM, BrainMiner Founder. Involved in machine learning enterprise and business. SO, BrainMiner Founder. Involved in machine learning enterprise and business. MP, received payment for consultancy work from Merck not related to cancer. AW, unrestricted educational grant, Bayer Schering, consultancy work and honoraria not related to cancer.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Jonathon Buwanabala and Stephen J. Price also attended the incubator day.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.620070/full#supplementary-material
References
1. Anvold ND, Lee EQ, Mehta MP, Margolin K, Alexander BM, Lin NU, et al. Updates in the management of brain metastases. Neuro Oncol (2016) 18:1043–65. doi: 10.1093/neuonc/now127
2. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med (2005) 352:987–96. doi: 10.1056/NEJMoa043330
3. Howick J, Chalmer I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, et al. Oxford Centre for Evidence-Based Medicine The Oxford 2011 levels of evidence. Oxford (2016). Available at: http://www.cebm.net/index.aspx?o1/45653 (Accessed 1 August, 2018).
4. The National Institute for Health and Care Excellence. Guideline NG99. Available at: https://www.nice.org.uk/guidance/ng99 (Accessed 1 March 2019).
5. Thompson G, Lawrie TA, Kernohan A, Jenkinson MD. Interval brain imaging for adults with cerebral glioma. Cochrane Database System Rev (2019) 10. doi: 10.1002/14651858.CD013137
6. Lesko LJ, Zineh I, Huang S-M. What is clinical utility and why should we care. Clin Pharm Ther (2010) 88:729–33. doi: 10.1038/clpt.2010.229
7. Levin VA Crafts DC, Norman DM, Hoffer PB, Spire JP, Wilson CB. Criteria for evaluating patients undergoing chemotherapy for malignant brain tumors. J Neurosurg (1977) 47:329–35. doi: 10.3171/jns.1977.47.3.0329
8. Miller AB, Hoogstraten B, Staquest M, Winkler A. Reporting results of cancer treatment. Cancer (1981) 47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::AID-CNCR2820470134>3.0.CO;2-6
9. Macdonald DR, Cascino TL, Schold SC, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol (1990) 8:1277–80. doi: 10.1200/JCO.1990.8.7.1277
10. Chinot OL, de La Motte R, Moore N. AVAglio: Phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv Ther (2011) 28:334–40. doi: 10.1007/s12325-011-0007-3
11. Wen PY, MacDonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J Clin Oncol (2010) 28:1963–72. doi: 10.1200/JCO.2009.26.3541
12. FDA-NIH Biomarker Working Group. In: BEST (biomarkers, EndpointS, and other tools) resource. 1st edn. Silver Spring, MD: Food and Drug Admin- istration (US), co-published by Bethesda, MD: National Institutes of Health (US) (2016). Available at: https://pubmed.ncbi.nlm.nih.gov/27010052/; https://www.ncbi.nlm.nih.gov/books/NBK326791
13. Ellingson BM, Harris RJ, Woodworth DC, Leu K, Zaw O, Mason WP, et al. Baseline pretreatment contrast enhancing tumor volume including central necrosis is a prognostic factor in recurrent glioblastoma: evidence from single- and multicenter trials. Neuro Oncol (2017) 19:89–98. doi: 10.1093/neuonc/now187
14. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg (2001) 95:190–8. doi: 10.3171/jns.2001.95.2.0190
15. Sanai N, Polley M-Y, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg (2011) 115:3–8. doi: 10.3171/2011.2.JNS10998
16. Booth TC, Luis A, Brazil L, Thompson G, Daniel RA Shuaib H, et al. Glioblastoma post-operative Imaging in Neuro-oncology: Current UK practice (GIN CUP study). Eur Radiol (2020). doi: 10.1007/s00330-020-07387-3
17. Thust S, Heiland S, Falini AR, Jäger H, Waldman AC, Sundgren P, et al. Glioma imaging in Europe: a survey of 220 centres and recommendations for best clinical practice. Eur Radiol (2018) 28:3306–17. doi: 10.1007/s00330-018-5314-5
18. The James Lind Alliance Priority Setting Partnerships. Available at: http://www.jla.nihr.ac.uk/priority-setting-partnerships/neuro-oncology/ (Accessed 1 August 2018).
19. Baumi JM, Troxel A, Epperson CN, Cohen RB, Schmitz K, Stricker C, et al. Scan-associated distress in lung cancer: Quantifying the impact of “scanxiety”. Lung Cancer (2016) 100:110–3. doi: 10.1016/j.lungcan.2016.08.002
20. Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol (2014) 32:3810–6. doi: 10.1200/JCO.2014.57.2909
21. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol (2016) 131:803–20. doi: 10.1007/s00401-016-1545-1
22. Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol (2014) 15:387–95. doi: 10.1016/S1470-2045(14)70221-9
23. Liu Y, Alexander BM, Chen Y-H, Horvath MC, Aizer AA, Claus EB, et al. Salvage whole brain radiotherapy or stereotactic radiosurgery after initial stereotactic radiosurgery for 1-4 brain metastases. J Neurooncol (2015) 124:429–37. doi: 10.1007/s11060-015-1855-5
24. Waldman AD, Jackson A, Price SJ, Clark CA, Booth TC, Auer DP, et al. Quantitative imaging biomarkers in neuro-oncology. Nat Rev Clin Oncol (2009) 6:445–54. doi: 10.1038/nrclinonc.2009.92
25. Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol (2010) 9:906–20. doi: 10.1016/S1474-4422(10)70181-2
26. Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol (2015) 16(15):e534–42. doi: 10.1016/s1470-2045(15)00088-1
27. Buwanabala J, Mirchandani A, Booth TC. (2019). The (mis)use of imaging criteria in the assessment of glioblastoma treatment response. In: Proceedings of the 57th American Society of Neuroradiology, Boston, USA. Oak Brook, 2019 May 18-23. p. 1.
28. Weller M, van den Bent M, Tonn JC, Stupp R, Preusser M, Cohen-Jonathan-Moyal E, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol (2017) 18(6):e315–29. doi: 10.1016/S1470-2045(17)30194-8
29. Weller M, Cloughesy T, Perry JR. Wick W Standards of care for treatment of recurrent glioblastoma–are we there yet? Neuro Oncol (2013) 15(1):4–27. doi: 10.1093/neuonc/nos273
30. Parasramka S, Talari G, Rosenfeld M, Guo J, Villano JL. Procarbazine, lomustine and vincristine for recurrent high-grade glioma. Cochrane Database Syst Rev (2017) 7:CD011773. doi: 10.1002/14651858.CD011773.pub2
31. Perry JR, Bélanger K, Mason WP, Fulton D, Kavan P, Easaw J, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol (2010) 28(12):2051–7. doi: 10.1200/JCO.2009.26.5520
32. Weller M, Tabatabai G, Kästner B, Felsberg J, Steinbach JP, Wick A, et al. MGMT promoter methylation is a strong prognostic biomarker for benefit from dose- intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin Cancer Res (2015) 21(9):2057–64. doi: 10.1158/1078-0432.CCR-14-2737
33. Booth TC, Akpinar B, Roman A, Shuaib H, Luis A, Chelliah A, et al. Machine learning and glioblastoma: treatment response monitoring biomarkers in 2021. In: Crimi A, editor. MLCN/RNO-AI 2020, LNCS. Switzerland AG: Springer Nature (2020). p. 12449.
34. Tugwell JR, Goulden N, Mullins P. Alleviating anxiety in patients prior to MRI: A pilot single-centre single-blinded randomised controlled trial to compare video demonstration or telephone conversation with a radiographer versus routine intervention. Radiography (2018) 24:122–1329. doi: 10.1016/j.radi.2017.10.001
35. van Minde D, Klaming L, Weda H. Pinpointing moments of high anxiety during an MRI examination. Int J Behav Med (2014) 21:487–95. doi: 10.1007/s12529-013-9339-5
36. Törnqvist E, Månsson Å, Larsson E-M, Hallström I. Impact of extended written information on patient anxiety and image motion artifacts during magnetic resonance imaging. Acta Radiologica (2006) 47:474–80. doi: 10.1080/02841850600690355
37. Eshed I, Althoff CE, Hamm B, Hermann KA. Claustrophobia and premature termination of magnetic resonance imaging examinations. J Magn Reson Imaging (2007) 26:401–4. doi: 10.1002/jmri.21012
38. Dirven L, Armstrong TS, Blakeley JO, Brown PD, Grant R, Jalali R, et al. Working Plan for the Use of Patient-Reported Outcome Measures in Adults With Brain Tumours: A Response Assessment in Neuro-Oncology (RANO) Initiative. Lancet Oncol (2018) 19:173–80. doi: 10.1016/S1470-2045(18)30004-4
39. Mishel MH. Uncertainty in illness. Image J Nurs Sch (1988) 20:225–32. doi: 10.1111/j.1547-5069.1988.tb00082.x
40. Lin L, Chiang H-S, Acquaye AA, Vera-Bolanos E, Gilbert MR, Armstrong TS. Uncertainty, mood states, and symptom distress in patients with primary brain tumors. Cancer (2013) 119:2796–806. doi: 10.1002/cncr.28121
41. Bette S, Gempt K, Huber T, Boeckh-Behrens T, Ringel F, Meyer B, et al. Patterns and Time Dependence of Unspecific Enhancement in Postoperative Magnetic Resonance Imaging After Glioblastoma Resection. World Neurosurg (2016) 90:440–7. doi: 10.1016/j.wneu.2016.03.031
42. Lescher S, Schniewindt S, Jurcoane CA, Senft C, Hattingen E. Time window for postoperative reactive enhancement after resection of brain tumors: less than 72 hours. Neurosurg Focus (2014) 37:E3. doi: 10.3171/2014.9.FOCUS14479
43. Kläsner B, Buchmann N, Gempt J, Ringel F, Lapa C, Krause BJ. Early 18F]FET-PET in Gliomas after Surgical Resection: Comparison with MRI and Histopathology. PLoS One (2015) 10:e0141153. doi: 10.1371/journal.pone.0141153
44. Mrowczynski OD, Zammar S, Bourcier AJ, Langan ST, Liao J, Specht C, et al. Utility of Early Post-operative MRI after Glioblastoma Resection: Implications on Patient Survival. World Neurosurg (2018) 120:e1171–4. doi: 10.1016/j.wneu.2018.09.027
45. Wen PY, Cloughesy TF, Ellingson BM, Reardon DA, Fine HA, Abrey L, et al. Report of the Jumpstarting Brain Tumor Drug Development Coalition and FDA clinical trials neuroimaging endpoint workshop (January 30, 2014, Bethesda MD). Neuro-Oncology (2014) 16:vii36–47. doi: 10.1093/neuonc/nou226
46. Sanghera P, Rampling R, Haylock B, Jefferies S, McBain C, Rees JH, et al. The concepts, diagnosis and management of early imaging changes after therapy for glioblastomas. Clin Oncol (R Coll Radiol) (2012) 24:216–27. doi: 10.1016/j.clon.2011.06.004
47. Stupp R, Brada M, van den Bent MJ, Tonn J-C, Pentheroudakis G. High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2014) 25:iii93–iii101. doi: 10.1093/annonc/mdu050
48. Weller M, van den Bent MJ, Tonn J-C, Stupp R, Preusser M, Cohen-Jonathan-Moyal E, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol (2017) 18:e315–29. doi: 10.1016/S1470-2045(17)30194-8
49. National Cancer Comprehensive Network Foundation. NCCN guidelines for treatment of cancer by site. Central Nervous System Cancers (2019). Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#cns (Accessed 1 September 2019).
50. EuroQol Research Foundation. EQ-5D (2017). Available at: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/ (Accessed 1 February 2017).
51. Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health economics (2002) 21(2):271–92. doi: 10.1016/S0167-6296(01)00130-8
52. Feeny D, Furlong W, Torrance GW, Goldsmith CH, Zhu Z, DePauw S, et al. Multiattribute and single-attribute utility functions for the health utilities index mark 3 system. Med Care (2002) 40(2):113–28. doi: 10.1097/00005650-200202000-00006
53. EORTC Quality of Life Group. (2019). Available at: https://qol.eortc.org (Accessed 4 November 2019).
54. Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist (2007) 12(1):20–37. doi: 10.1634/theoncologist.12-1-20
55. The National Cancer Registration and Analysis Service. (2019). Available at: http://www.ncin.org.uk/publications/data_briefings/astrocytic_brain_tumours_survival_rates_in_england (Accessed 4 November 2019).
56. Mazzali C, Duca P. Use of administrative data in healthcare research. Internal Emergency medicine (2015) 10(4):517–24. doi: 10.1007/s11739-015-1213-9
57. Engelhardt JB, McClive-Reed KP, Toseland RW, Smith TL, Larson DG, Tobin DR. Effects of a program for coordinated care of advanced illness on patients, surrogates, and healthcare costs: a randomized trial. Am J Manag Care (2006) 12:93–100.
58. York Health Economics Consortium. (2016). Available at: https://yhec.co.uk/glossary/markov-model/ (Accessed 4 November 2019).
59. Thokala P, Ochalek J, Leech AA, Tong T. Cost-effectiveness thresholds: the past, the present and the future. Pharmacoeconomics (2018) 36(5):509–22. doi: 10.1007/s40273-017-0606-1
60. Papanicolas I, Woskie LR, Jha AK. Health Care Spending in the United States and Other High-Income Countries. JAMA (2018) 319:1024–39. doi: 10.1001/jama.2018.1150
61. Raizer JJ, Fitzner KA, Jacobs DI, Bennet CL, Liebling DB, Luu TH. Economics of Malignant Gliomas: A Critical Review. J Oncol Pract (2015) 11:e59–65. doi: 10.1200/JOP.2012.000560
62. Finney A, Davies S, Hayes D, Collard S. Cancer's hidden price tag: revealing the costs behind the illness (2013). Available at: https://www.macmillan.org.uk/_images/Cancers-Hidden-Price-Tag-report-England_tcm9-270862.pdf (Accessed 4 October 2020).
63. Booth TC, Williams M, Luis A, Cardoso J, Keyoumars A, Shuaib H. Machine learning and glioma imaging biomarkers. Clin Radiol (2019) 75:20–32. doi: 10.1016/j.crad.2019.07.001
64. Booth TC. An Update on Machine Learning in Neuro-oncology Diagnostics. In: Crimi A, editor. LNCS, vol. 11383 . BrainLes: Springer Nature Switzerland AG (2019). p. 1–8.
65. Hansen MR, Pan E, Wilson A, McCreary M, Wang Y, Stanley T, et al. Post-gadolinium 3-dimensional spatial, surface, and structural characteristics of glioblastomas differentiate pseudoprogression from true tumor progression. J Neurooncol (2018) 139:731–8. doi: 10.1007/s11060-018-2920-7
66. Ceschin R, Kurlan BF, Abberbock SR, Ellingson BM, Okada H, Jakacki RI, et al. Parametric response mapping of apparent diffusion coefficient (ADC) as an imaging biomarker to distinguish pseudoprogression from true tumor progression in peptide-based vaccine therapy for pediatric diffuse instrinsic pontine glioma. AJNR Am J Neuroradiol (2015) 36:2170–6. doi: 10.3174/ajnr.A4428
67. Williamson JM, Datta S, Satten GA. Marginal analyses of clustered data when cluster size is informative. Biometrics (2003) 59(1):36–42. doi: 10.1111/1541-0420.00005
68. Cabitza F, Rasoini R, Genisi GF. Unintended consequences of machine learning in medicine. JAMA (2017) 318(6):517–8. doi: 10.1001/jama.2017.7797
69. Tu J. Advantages and disadvantages of using artificial neural networks versus logistic regression for predicting medical outcomes. J Clin Epidemiol (1996) 49:1225–31. doi: 10.1016/S0895-4356(96)00002-9
70. Reyes M, Meier R, Pereira S, Silva CA, Dahlweid F-M, von Tengg-Kobligk, et al. On the Interpretability of Artificial Intelligence in Radiology: Challenges and Opportunities. Radiol Artif Intell (2020) 2(3):e190043. doi: 10.1148/ryai.2020190043
71. Dharmarajan K, Strait KM, Tinetti ME, Lagu T, Lindenauer PK, Lynn J, et al. Treatment for multiple acute cardiopulmonary conditions in older adults hospitalized with pneumonia, chronic obstructive pulmonary disease, or heart failure. J Am Geriatr Soc (2016) 64(8):1574– 1582. doi: 10.1111/jgs.14303
72. Hoff T. Deskilling and adaptation among primary care physicians using two work innovations. Health Care Manage Rev (2011) 36(4):338–48. doi: 10.1097/HMR.0b013e31821826a1
73. White H. Learning in artificial neural networks: A statistical perspective. Neural Comput (1989) 1:425–64. doi: 10.1162/neco.1989.1.4.425
74. Astion ML, Wilding P. The application of back-propagation neural networks to problems in pathology and laboratory medicine. Arch Pathol Lab Med (1992) 116:995–1001.
75. Medical Open Network for AI (MONAI). Available at: https://monai.io/ (Accessed 30 Dec 2020).
76. Ratib O, Rosset A, Heuberger J. Open source software and social networks: disruptive alternatives for medical imaging. Eur J Radiol (2011) 78(2):259–65. doi: 10.1016/j.ejrad.2010.05.004
77. Caban JJ, Joshi A, Nagy P. Rapid development of medical imaging tools with open-source libraries. J Digit Imaging (2007) 20(Suppl 1):83–93. doi: 10.1007/s10278-007-9062-3
78. Hinton GE. How neural networks learn from experience. Sci Am (1992) 267:145–51. doi: 10.1038/scientificamerican0992-144
79. Rudie JD, Rauschecker AM, Bryan RN, Davatzikos C, Mohan S. Emerging Applications of Artificial Intelligence in Neuro-Oncology. Radiology (2019) 290(3):607–18. doi: 10.1148/radiol.2018181928
80. Sotoudeh H, Shafaat O, Bernstock JD, Brooks MD, Elsayed GA, Chen JA, et al. Artificial Intelligence in the Management of Glioma: Era of Personalized Medicine. Front Oncol (2019) 9:768. doi: 10.3389/fonc.2019.00768
81. Jowell T. BRAIN MATRIX (2020). Available at: https://www.birmingham.ac.uk/research/activity/mds/trials/crctu/trials/brain-matrix/index.aspx (Accessed 2 May 2020).
82. BRIAN The Brain Tumour Charity. (2020). Available at: https://www.askbrian.org.uk (Accessed 2 May 2020).
83. Hestness J, Narang S, Ardalani N, Diamos G, Jun H, Kianinejad H, et al. Deep Learning Scaling is Predictable, Empirically (2017). (Accessed 4 December, 2019).
84. Sun C, Shrivastava A, Singh S, Gupta A. Revisiting unreasonable effectiveness of data in deep learning era (2017). (Accessed 4 December, 2019).
85. Lei S, Zhang H, Wang K, Su Z. How training data affect the accuracy & robustness of neural networks for image classification. In: Proceedings of the 2019 International Conference on Learning Representations (ICLR-2019). New Orleans, USA (2019).
86. Zwanenburg A, Leger S, Vallières M. Image biomarker standardisation initiative (2019). Available at: https://arXiv.preprint.arXiv:1612.07003 (Accessed 1 November, 2019).
87. O’Connor JPB, Aboagye EO, Adams JE, Aerts HJW, Barrington SF, Beer AJ, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol (2017) 14:169–86. doi: 10.1038/nrclinonc.2016.162
88. Sullivan DC, Obuchowski NA, Kessler LG, Raunig DL, Gatsonis C Huang EP, et al. Metrology standards for quantitative imaging biomarkers. Radiology (2015) 277:813–25. doi: 10.1148/radiol.2015142202
89. Collins GS, Reitsma JB, Altman DG, Moons KGTransparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. Br J Cancer (2020) 112:251–9. doi: 10.1038/bjc.2014.639
90. McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet (2009) 374(9695):1105–12. doi: 10.1016/S0140-6736(09)61116-8
91. Kim A, Thompson E, Governale L, Santa C, Cahill K, Kieran M, et al. Recurrence after gross-total resection of low-grade pediatric brain tumors: the frequency and timing of postoperative imaging. J Neurosurg Pediatr (2014) 14:356–64. doi: 10.3171/2014.6.PEDS1321
92. Zaazoue MA, Manley PE, Mehdar MA, Ullrich NJ, Dasenbrock HH, Chordas CA, et al. Optimizing Postoperative Surveillance of Pediatric Low-Grade Glioma Using Tumor Behavior Patterns. Neurosurgery (2020) 86:288–97. doi: 10.1093/neuros/nyz072
93. Clement P, Booth T, Borovečki F, Emblem KE, Figueiredo P, Hirschler L, et al. GliMR: Cross-Border Collaborations to Promote Advanced MRI Biomarkers for Glioma. J Med Biol Eng (2020). doi: 10.1007/s40846-020-00582-z
94. Society of Neuro-Oncology. (2020). https://soc-neuro-onc.org/WEB/About_Content/News_Pages/RANO_at_SNO.aspx.
95. Equator Network. (2020). https://www.equator-network.org/.
96. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD Group. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. Radiology (2015) 277(3):826–32. doi: 10.1148/radiol.2015151516
97. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. Eur J Clin Invest (2015) 45(2):204–14. doi: 10.1111/eci.12376
98. Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med (2010) 152(11):726–32. doi: 10.7326/0003-4819-152-11-201006010-00232
Keywords: glioblastoma, high grade glioma, glioma, meningioma, interval imaging, magnetic resonance imaging, utility, monitoring biomarker
Citation: Booth TC, Thompson G, Bulbeck H, Boele F, Buckley C, Cardoso J, Dos Santos Canas L, Jenkinson D, Ashkan K, Kreindler J, Huskens N, Luis A, McBain C, Mills SJ, Modat M, Morley N, Murphy C, Ourselin S, Pennington M, Powell J, Summers D, Waldman AD, Watts C, Williams M, Grant R and Jenkinson MD (2021) A Position Statement on the Utility of Interval Imaging in Standard of Care Brain Tumour Management: Defining the Evidence Gap and Opportunities for Future Research. Front. Oncol. 11:620070. doi: 10.3389/fonc.2021.620070
Received: 21 October 2020; Accepted: 06 January 2021;
Published: 09 February 2021.
Edited by:
Jose R. Pineda, University of the Basque Country, SpainReviewed by:
Margarida Julià-Sapé, Centre for Biomedical Network Research (CIBER), SpainAlfredo Vellido, Universitat Politecnica de Catalunya, Spain
Andrea Salmaggi, ASST Lecco, Italy
Copyright © 2021 Booth, Thompson, Bulbeck, Boele, Buckley, Cardoso, Dos Santos Canas, Jenkinson, Ashkan, Kreindler, Huskens, Luis, McBain, Mills, Modat, Morley, Murphy, Ourselin, Pennington, Powell, Summers, Waldman, Watts, Williams, Grant and Jenkinson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas C. Booth, tombooth@doctors.org.uk
†These authors share first authorship
‡These authors share last authorship
 Thomas C. Booth
Thomas C. Booth Gerard Thompson3†
Gerard Thompson3† Helen Bulbeck
Helen Bulbeck Florien Boele
Florien Boele Liane Dos Santos Canas
Liane Dos Santos Canas David Jenkinson
David Jenkinson Keyoumars Ashkan
Keyoumars Ashkan Jack Kreindler
Jack Kreindler Nick Morley
Nick Morley Sebastian Ourselin
Sebastian Ourselin James Powell
James Powell Adam D. Waldman
Adam D. Waldman Michael D. Jenkinson
Michael D. Jenkinson