- 1Unità Operativa Complessa Radioterapia Oncologica, Dipartimento di Diagnostica per Immagini, Radioterapia Oncologica ed Ematologia, Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Roma, Italy
- 2Istituto di Radiologia, Università Cattolica del Sacro Cuore, Roma, Italy
- 3Department of Radiation Oncology, University Medical Center Utrecht, Utrecht, Netherlands
- 4Department of Human Oncology, University of Wisconsin-Madison, Madison, WI, United States
- 5Department of Radiation Oncology, Eberhard Karls Universität Tübingen, Tübingen, Germany
- 6German Cancer Consortium (DKTK), Partner Site, Tübingen, Germany
Online MRI-guided radiotherapy (MRgRT) is one of the most recent technological advances in radiotherapy. MRgRT permits the visualization of tumorous and healthy tissue while the patient is on the treatment table and online daily plan adaptations following the observed anatomical changes. In the context of rectal cancer, online MRgRT is a very promising modality due to the pronounced geographical variability of tumor tissues and the surrounding healthy tissues. This current paper will discuss the possible applications of online MRgRT, in particular, in terms of radiotherapy dose escalation and response prediction in organ preservation approaches for rectal cancer.
Introduction
Neoadjuvant (chemo)radiotherapy (NCRT) represents the reference standard in the treatment of locally advanced rectal cancer (LARC), primarily aiming to reduce local recurrence rates after surgery (1). MRI with its superior soft-tissue contrast has gained a crucial role in the initial staging and response assessment of rectal cancer and can stratify patients into different prognostic groups with risk-adjusted personalized therapeutic approaches (2, 3).
A promising driver of precision RT in rectal cancer is the recent introduction of linear accelerators with an onboard MR scanner, the MR-Linac. This new treatment machine enables online MRI-guided RT (MRgRT) which opens a new era for an image-guided and online adaptive RT (4). At the time of writing, two commercial 35 solutions are available for clinical use: the MRIdian system by ViewRay (ViewRay Inc, USA), which was first released in 2014 coupling a low tesla scanner (0.35 T) with a triplet of 60Co heads and was later replaced by a 6 MV linac, and the Unity system by Elekta (Elekta AB, Sweden), which uses a 1.5 T scanner and a 7 MV linac, released in 2017 (5–7). Despite the low number of active hybrid units, there is a growing interest on the role of this advanced irradiation technique (4, 8).
One of the areas of interest and current research on rectal cancer is the organ preservation approach (9). With the current therapeutic approaches, pathological complete response (pCR) after NCRT for LARC is in the range of 10–20% in most trials. There has been a great interest in developing strategies with tolerable toxicity to increase the number of patients who achieve a complete clinical response and, therefore, could be managed in a non-operative manner in the framework of a “watch and wait” approach (10, 11). These strategies include the intensification of systemic treatment, the prolongation of the interval between neoadjuvant therapies and response assessment or surgery, total neoadjuvant therapy, hyperthermia, and radiotherapy dose escalation (12–16). The latter strategy has been hampered so far by the very limited resolution of cone-beam CT (CBCT)-based images, resulting in large safety margins. Furthermore, response prediction is a field with a need for tailored treatment approaches.
This review aims to present and discuss opportunities with online MRgRT in the treatment of rectal cancer.
Adaptations for the Changing Anatomy
Variations in the target position during radiotherapy for rectal cancer are largely due to daily changes in bladder and bowel filling. For the elective target volumes used to treat patients in the neoadjuvant setting, the position variations are most prominent in the mesorectum, specifically in the anterior part of the upper mesorectum, where the position of the mesorectum is dependent on both rectal and bladder filling, and with deformations of up to 7 mm (17–19). Besides interfraction variations due to changes in the filling of the organs in the pelvis, there is also a possibility of tumor regression during the treatment course, which changes the anatomy. On average, rectal tumors can reduce almost 50% in volume during the treatment course (20, 21). These variations are, in most cases, not relevant in the neoadjuvant setting as the gross target volume (GTV) is inside the mesorectum in most cases but becomes very relevant in the setting of dose escalation. Taking into account these uncertainties at the target position, generally large clinical target volume (CTV) to planning target volume (PTV) margins are used around the target volumes of up to even 2.3 cm, which leads to a considerable burden on the healthy tissues (18, 19). Different adaptive strategies have been proposed to reduce the need for large margins in the neoadjuvant treatment setting. One of the most promising techniques is the library of plans (LOP) strategy (22). In this strategy, the CTV from a single planning CT is contracted and expanded based on population variation statistics, and multiple radiation treatment plans are generated based on different CTVs. For each fraction, the plan is chosen with the CTV that best matches the actual volume as visualized on the localization CBCT. Applying the LOP strategy allows reductions of, on average, 15% in the PTV compared to conventional treatment, but the daily selection of the appropriate plan can be challenging due to poor CBCT image quality (20, 21). Furthermore, while this approach is useful for an adequate coverage of the mesorectum, it is no longer a reliable tool for dose escalation of the tumor itself. With MRgRT, it is possible to adapt daily treatment plans based on MRI-visualized anatomy. The superior soft-tissue contrast of MRI compared to CBCT gives the opportunity to not only see the mesorectum and organs at risk but also to visualize the primary tumor and pathological lymph nodes during each fraction. Figure 1 shows representative scans from the 0.35 T and 1.5 T MR-Linac. Based on this daily visualized anatomy, different adaptive treatment strategies can be chosen from a simple translation of the treatment fields to full online replanning (7). The radiotherapy dose escalation strategy takes around 50 min for each treatment fraction and allows the use of smaller CTV to PTV margins of 4–6 mm (23). Reduced margins and daily adaptation of treatment fields lead to a reduced spread of the dose in the surrounding healthy structures, such as the surrounding uninvolved rectal wall, the small bowel, the bladder, and the anal sphincter, potentially resulting in less radiotherapy-related short- and long-time side effects. This is particularly important for the expanding group of patients who are treated with watchful waiting strategies, as, for these patients, a treatment with limited toxicity and a satisfactory anorectal function after (chemo)radiotherapy is of utmost importance (24).
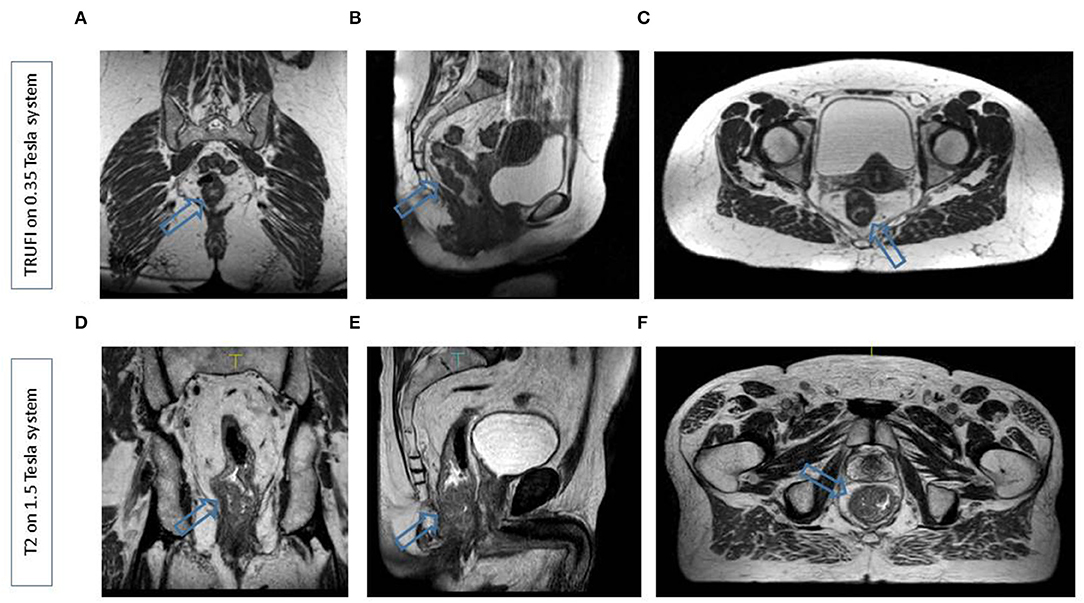
Figure 1. Representative scans from the 0.35 T MR LINAC (A–C) in the coronal, sagittal, and transverse planes and the 1.5 T MR LINAC (D–F) in the coronal, sagittal, and transverse planes. In both cases, a patient with a distally located rectal tumur was scanned. Arrows indicate the tumur.
Dose Escalation
In order to increase the number of patients eligible for organ preservation strategies, innovative and novel treatment protocols to maximize complete response rates are needed. This can be achieved by increasing the radiotherapy dose to the primary tumor as shown in the dose-response curve presented by Appelt et al. (25). This dose-response curve was constructed based on studies that delivered a brachytherapy boost after external beam radiotherapy for locally advanced rectal cancer. Moreover, a systemic review by Burbach et al. showed a potential effect of external beam radiotherapy dose escalation. At the same time, two recent prospective randomized trials did show an increased tumor response with external beam dose escalation, but not in terms of pathological complete response or sustained clinical complete response (26, 27). An explanation for these negative results can at least partly be seen in the limitations of CBCT-based dose escalation, in particular, limited target coverage. Large safety margins had to be used because of the aforementioned poor target, and organ-at-risk visibility with CBCT imaging and organ-at-risk constraints resulted in reduced coverage of the tumor in many cases (28). Another aspect in both the clinical trials was the high complete rate observed in the standard arm underlining the critical need for parameters that identify patients who are unlikely to benefit from dose escalation since they already have a very favorable phenotype. More precise delivery of the external beam irradiation with online MR guidance dose can probably solve the issue of target volume coverage as tumors can be visualized with MRI immediately before and during dose delivery. This solution has a clear advantage of online adaptive MRgRT over “offline” adaptive strategies with pre-defined time points for adaptation (29). Besides, by daily online replanning, the margins needed can be minimized and treatment volumes for dose escalation will be smaller, potentially facilitating dose escalation beyond the biologically effective dose of ~65–70 Gy used in the recently published dose escalation studies (26, 27). This is supported by a recent radiotherapy planning study by Bonomo et al. based on sequential MRI scans, showing that an online adaptive boost strategy results in lower doses to the rectum and the anal canal (30). MR-guided dose escalation strategies are currently under development, and the organ preservation potential of these new schemes will be tested in innovative trials. While the safety of extreme dose escalation under MRI guidance needs clinical proving, the experience in prostate cancer suggests that the rectum can tolerate a high dose localized to a small volume (31). MR-guided dose escalation may also help facilitate an R0 resection in challenging surgical cases. Rectal tumors with threatened mesorectal margins, pelvic sidewall invasion, or iliac lymph node involvement are at high risk for incurable local recurrences. Radiation boost can be used to decrease the risk of positive surgical margin in these cases or to eradicate tumor cells in lymph nodes that are not routinely resected.
Future Perspectives: Precision RT With the Inclusion of Prediction Models and Functional Imaging
Besides the intuitive approach of using anatomical MR sequences for the adaption of treatment plans following the anatomy of the day, there has been a great interest in using functional imaging data and advanced image analysis for precision radiotherapy of rectal cancer (32, 33). Data from the literature supports various hypotheses on how diffusion-weighted imaging in particular might be a very useful tool. First, it has been shown that early changes in the apparent diffusion coefficient (ADC) can predict response to radiochemotherapy more accurately than early changes in tumor volume (34). Interestingly, the predictive value of early changes in ADC, for instance from baseline to week two of treatment, was superior to baseline ADC values, likely reflecting biological properties of the tumor. As described earlier, adequate patient selection is a key component of dose escalation strategies. A considerable number of patients can achieve a complete response without dose escalation and will have no benefit from dose escalation. Therefore, selecting patients for dose escalation based on changes in functional imaging data is a very interesting approach. With MR-linac hybrid devices, it is possible to acquire these data “in one go,” while the patient is on the table and being treated. Moving one step beyond, one could also envision defining tumor subvolumes that have a high likelihood of harboring residual tumor cells and use these volumes for dose-escalated treatment as supported by a study by Shaverdian et al. (35). Furthermore, the large amount of imaging data that is acquired during the course of the treatment can be used to generate, optimize, and validate prediction models in the context of quantitative imaging data and radiomic analysis (36, 37).
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
All authors were involved in the writing of this perspective paper. CG and LB provided the figure.
Funding
The MRgRT program in Tübingen received funding from the German Research Council (ZI 736/2-1, 438106095).
Conflict of Interest
LB has active research agreements with ViewRay Inc and has received speaker honoraria for scientific presentations. CG: University Hospital Tübingen receives financial and technical support, including costs for travels (CG) and symposia from Elekta AB (Stockholm, Sweden), under a research agreement.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. (2012) 30:1926–33. doi: 10.1200/JCO.2011.40.1836
2. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rodel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv22–40. doi: 10.1093/annonc/mdx224
3. Curvo-Semedo L. Rectal cancer: staging. Magn Reson Imaging Clin N Am. (2020) 28:105–15. doi: 10.1016/j.mric.2019.09.003
4. Chiloiro G, Boldrini L, Meldolesi E, Re A, Cellini F, Davide C, et al. MR-guided radiotherapy in rectal cancer: first clinical experience of an innovative technology. Clin Transl Radiat Oncol. (2019) 18:80–6. doi: 10.1016/j.ctro.2019.04.006
5. Klüter S. Technical design and concept of a 0.35 T MR-Linac. Clin Transl Radiat Oncol. (2019) 18:98–101. doi: 10.1016/j.ctro.2019.04.007
6. Slotman B, Gani C. Online MR-guided radiotherapy – a new era in radiotherapy. Clin Transl Radiat Oncol. (2019) 18:102–3. doi: 10.1016/j.ctro.2019.04.011
7. Winkel D, Bol GH, Kroon PS, van Asselen B, Hackett SS, Werensteijn-Honingh AM, et al. Adaptive radiotherapy: the Elekta Unity MR-linac concept. Clin Transl Radiat Oncol.. (2019) 18:102–3.
8. Gani C, Boldrini L, Valentini V. Online MR guided radiotherapy for rectal cancer. New opportunities. Clin Transl Radiat Oncol. (2019) 18:66–7. doi: 10.1016/j.ctro.2019.04.005
9. Gani C, Bonomo P, Zwirner K, Schroeder C, Menegakis A, Rodel C, et al. Organ preservation in rectal cancer - challenges and future strategies. Clin Transl Radiat Oncol. (2017) 3:9–15. doi: 10.1016/j.ctro.2017.02.002
10. Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. (2011) 29:4633–40. doi: 10.1200/JCO.2011.37.7176
11. Gani C, Kirschniak A, Zips D. Watchful Waiting after Radiochemotherapy in Rectal Cancer: When Is It Feasible? Visc Med. (2019) 35:119–23. doi: 10.1159/000499167
12. Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C, et al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6). J Clin Oncol. (2016) 34:3773–80. doi: 10.1200/JCO.2016.67.6049
13. Gani C, Schroeder C, Lamprecht U, Bamberg M, Berger B. Rates of pathologic complete response and sphincter-sparing surgery after neoadjuvant radiochemotherapy with or without regional pelvic hyperthermia for locally advanced rectal cancer. J Clin Oncol. (2012) 30:622. doi: 10.1200/jco.2012.30.4_suppl.622
14. Burbach JP, den Harder AM, Intven M, van Vulpen M, Verkooijen HM, Reerink O. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: a systematic review and meta-analysis. Radiother Oncol. (2014) 113:1–9. doi: 10.1016/j.radonc.2014.08.035
15. Rödel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. (2012) 13:679–87. doi: 10.1016/S1470-2045(12)70187-0
16. Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo Pa, Varma MG, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. (2015) 2045:1–10. doi: 10.1016/S1470-2045(15)00004-2
17. Ippolito E, Mertens I, Haustermans K, Gambacorta MA, Pasini D, Valentini V. IGRT in rectal cancer. Acta Oncol. (2008) 47:1317–24. doi: 10.1080/02841860802256459
18. Nijkamp J, de Jong R, Sonke JJ, Remeijer P, van Vliet C, Marijnen C. Target volume shape variation during hypo-fractionated preoperative irradiation of rectal cancer patients. Radiother Oncol. (2009) 92:202–9. doi: 10.1016/j.radonc.2009.04.022
19. Brierley JD, Dawson LA, Sampson E, Bayley A, Scott S, Moseley JL, et al. Rectal motion in patients receiving preoperative radiotherapy for carcinoma of the rectum. Int J Radiat Oncol Biol Phys. (2011) 80:97–102. doi: 10.1016/j.ijrobp.2010.01.042
20. Roels S, Slagmolen P, Nuyts J, Lee JA, Loeckx D, Maes F, et al. Biological image-guided radiotherapy in rectal cancer: challenges and pitfalls. Int J Radiat Oncol Biol Phys. (2009) 75:782–90. doi: 10.1016/j.ijrobp.2008.11.031
21. Van den Begin R, Kleijnen JP, Engels B, Philippens M, van Asselen B, Raaymakers B, et al. Tumor volume regression during preoperative chemoradiotherapy for rectal cancer: a prospective observational study with weekly MRI. Acta Oncol. (2018) 57:723–7. doi: 10.1080/0284186X.2017.1400689
22. Chris B, Baukelien vT, Suzanne vB, Jan-Jakob S, Peter R. Margin and PTV volume reduction using a population based library of plans strategy for rectal cancer radiotherapy. Medical Physics. (2018)
23. Intven MPW, de Mol van Otterloo SR, Mook S, Doornaert PAH, de Groot-van Breugel EN, Sikkes GG, et al. Online adaptive MR-guided radiotherapy for rectal cancer; feasibility of the workflow on a 1.5T MR-linac; clinical implementation and initial experience. Radiother Oncol. (2020) 154:172–8. doi: 10.1016/j.radonc.2020.09.024
24. Gani C, Gani N, Zschaeck S, Eberle F, Schaeffeler N, Hehr T, et al. Organ preservation in rectal cancer: the patients' perspective. Front Oncol. (2019) 9:318. doi: 10.3389/fonc.2019.00318
25. Appelt AL, Ploen J, Vogelius IR, Bentzen SM, Jakobsen A. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys. (2013) 85:74–80. doi: 10.1016/j.ijrobp.2012.05.017
26. Couwenberg AM, Burbach JPM, Berbee M, Lacle MM, Arensman R, Raicu MG, et al. Efficacy of dose-escalated chemoradiation on complete tumor response in patients with locally advanced rectal cancer (RECTAL-BOOST): a phase 2 randomized controlled trial. Int J Radiat Oncol Biol Phys. (2020) 108:1008–18. doi: 10.1016/j.ijrobp.2020.06.013
27. Valentini V, Gambacorta MA, Cellini F, Aristei C, Coco C, Barbaro B, et al. The INTERACT Trial: Long-term results of a randomised trial on preoperative capecitabine-based radiochemotherapy intensified by concomitant boost or oxaliplatin, for cT2 (distal)-cT3 rectal cancer. Radiother Oncol. (2019) 134:110–8. doi: 10.1016/j.radonc.2018.11.023
28. Burbach JP, Verkooijen HM, Intven M, Kleijnen JP, Bosman ME, Raaymakers BW, et al. RandomizEd controlled trial for pre-operAtive dose-escaLation BOOST in locally advanced rectal cancer (RECTAL BOOST study): study protocol for a randomized controlled trial. Trials. (2015) 16:58. doi: 10.1186/s13063-015-0586-4
29. Passoni P, Fiorino C, Slim N, Ronzoni M, Ricci V, Di Palo S, et al. Feasibility of an adaptive strategy in preoperative radiochemotherapy for rectal cancer with image-guided tomotherapy: boosting the dose to the shrinking tumor. Int J Radiat Oncol Biol Phys. (2013) 87:67–72. doi: 10.1016/j.ijrobp.2013.05.004
30. Bonomo P, Lo Russo M, Nachbar M, Boeke S, Gatidis S, Zips D, et al. 1.5 T MR-linac planning study to compare two different strategies of rectal boost irradiation. Clin Transl Radiat Oncol. (2020) 26:86–91. doi: 10.1016/j.ctro.2020.11.016
31. Olsson CE, Jackson A, Deasy JO, Thor M. A systematic post-QUANTEC review of tolerance doses for late toxicity after prostate cancer radiation therapy. Int J Radiat Oncol Biol Phys. (2018) 102:1514–32. doi: 10.1016/j.ijrobp.2018.08.015
32. Joye I, Deroose CM, Vandecaveye V, Haustermans K. The role of diffusion-weighted MRI and 18 F-FDG PET/CT in the prediction of pathologic complete response after radiochemotherapy for rectal cancer: a systematic review. Radiotherapy Oncol. (2014) 113:158–65. doi: 10.1016/j.radonc.2014.11.026
33. Thorwarth D, Ege M, Nachbar M, Mönnich D, Gani C, Zips D, et al. Quantitative magnetic resonance imaging on hybrid magnetic resonance linear accelerators: Perspective on technical and clinical validation. Phys Imaging Radiation Oncol. (2020) 16:69–73. doi: 10.1016/j.phro.2020.09.007
34. Lambrecht M, Vandecaveye V, De Keyzer F, Roels S, Penninckx F, Van Cutsem E, et al. Value of diffusion-weighted magnetic resonance imaging for prediction and early assessment of response to neoadjuvant radiochemotherapy in rectal cancer: preliminary results. Int J Radiat Oncol Biol Phys. (2012) 82:863–70. doi: 10.1016/j.ijrobp.2010.12.063
35. Shaverdian N, Yang Y, Hu P, Hart S, Sheng K, Lamb J, et al. Feasibility evaluation of diffusion-weighted imaging using an integrated MRI-radiotherapy system for response assessment to neoadjuvant therapy in rectal cancer. Br J Radiol. (2017) 90:20160739. doi: 10.1259/bjr.20160739
36. Boldrini L, Cusumano D, Chiloiro G, Casa C, Masciocchi C, Lenkowicz J, et al. Delta radiomics for rectal cancer response prediction with hybrid 0.35 T magnetic resonance-guided radiotherapy (MRgRT): a hypothesis-generating study for an innovative personalized medicine approach. Radiol Med. (2019) 124:145–53. doi: 10.1007/s11547-018-0951-y
37. Cusumano D, Boldrini L, Yadav P, Yu G, Musurunu B, Chiloiro G, et al. External validation of early regression index (ERITCP) as predictor of pathologic complete response in rectal cancer using magnetic resonance-guided radiation therapy. Int J Radiat Oncol Biol Phys. (2020) doi: 10.1016/j.ijrobp.2020.07.2323
Keywords: radiotherapy, rectal cancer, MRI, MR-linac, dose escalation
Citation: Boldrini L, Intven M, Bassetti M, Valentini V and Gani C (2021) MR-Guided Radiotherapy for Rectal Cancer: Current Perspective on Organ Preservation. Front. Oncol. 11:619852. doi: 10.3389/fonc.2021.619852
Received: 21 October 2020; Accepted: 08 February 2021;
Published: 30 March 2021.
Edited by:
Christopher Schultz, Medical College of Wisconsin, United StatesReviewed by:
Joshua Pohyun Kim, Henry Ford Health System, United StatesJ. Joshua Smith, Memorial Sloan Kettering Cancer Center, United States
Copyright © 2021 Boldrini, Intven, Bassetti, Valentini and Gani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cihan Gani, Y2loYW4uZ2FuaUBtZWQudW5pLXR1ZWJpbmdlbi5kZQ==
 Luca Boldrini1,2
Luca Boldrini1,2 Martijn Intven
Martijn Intven Michael Bassetti
Michael Bassetti Vincenzo Valentini
Vincenzo Valentini Cihan Gani
Cihan Gani