- 1Radiation Oncology, Rocky Mountain Cancer Centers, Denver, CO, United States
- 2Statistics, Linasmar Consulting, Houston, TX, United States
- 3Radiology, Invision Sally Jobe, Greenwood Village, CO, United States
- 4Surgery, SurgOne, Greenwood Village, CO, United States
Purpose: To report a primary objective clinical outcome of ipsilateral breast recurrence following accelerated partial breast irradiation (APBI) in women with triple negative and other high risk breast cancer (as described in 2017 ASTRO guidelines) (i.e., age 40–49, size 2.1–3.0 cm, estrogen receptor negative and invasive lobular breast cancer). Secondary objectives of axillary and regional failure as well as overall survival are also reported.
Methods and Material: Patients from two clinical trials (NCT01185145, NCT01185132) were treated with 38.5 Gy IMRT or 3D-CRT APBI w/3.85 Gy fraction/BID fractionation for 10 fractions. Triple negative and other high risk patients (n=269) were compared to a total of 478 low risk patients which ASTRO defined as “suitable” for APBI. High risk patients, for the purpose of this study, were defined as those who possess one or more high risk criteria: triple negative (n=30), tumor size >2 cm <3 cm (n=50), HER 2+ (n=54), age range 40–50 years (n=120), ER- (n=43), and ILC histology (n=52).
Results: Median follow up was 4.0 years for all patients. No significant difference was found for this high-risk cohort at 5 years for ipsilateral breast, or regional recurrences. Axillary recurrence was significantly adversely impacted by triple negative and ER- statuses (p=0.01, p=0.04). There were significant correlations between triple negative type and axillary recurrence on multivariate analysis (p=0.03). Overall survival for all patients was unaffected by any of the high-risk categories.
Conclusion: The data from this study suggests that women possessing high risk features are at no more meaningful risk for recurrence than other patients considered to be acceptable for APBI treatment. However, the finding of axillary recurrence in patients with triple negative breast cancer does warrant a degree of caution in proceeding with accelerated partial breast irradiation technique in this patient group.
Introduction
Accelerated partial breast radiotherapy (APBI) recently has been widely accepted as an alternative breast radiotherapy option for the post-lumpectomy adjuvant management of breast cancer. APBI has the benefit of shortened treatment time and reduced radiation exposure to surrounding tissues when compared to whole breast irradiation (WBI).
Contemporary external beam and brachytherapy APBI reports, including those of the authors, have reported that local control rates in certain early-stage invasive breast cancer patients may be comparable to those treated with standard whole breast (1–4). Optimal treatment outcomes of APBI are contingent upon proper patient selection.
The American Society of Radiation Oncology (ASTRO) has previously issued guidelines for patient categorization into “suitable”, “cautionary”, and “unsuitable” groups (2). Currently, these guidelines were revised to expand the suitable category to include characteristics previously felt to be cautionary (3). The GEC-ESTRO Brachytherapy Committee have also published recommended APBI clinical guidelines. These guidelines state that APBI could be offered as standard therapy to eligible patients >50 years of age who have T1 invasive ductal carcinoma with a minimum of 2 mm margins (4). The National Comprehensive Cancer Network (NCCN) panel accepts the updated 2016 version of the ASTRO APBI guideline, which now defines patients “suitable” for APBI to be the following: 1) 50 years or older with invasive ductal carcinoma (IDCA) measuring ≤2 cm (T1 disease) with negative margin widths of ≥2 mm, no lymphvascular invasion, estrogen receptor (ER) positive, and BRCA 1/2 negative or 2) screening-detected ductal carcinoma in situ (DCIS) with low/intermediate nuclear grade, and tumor size measuring ≤2.5 cm with negative margin widths of ≥3 mm (1).
The cautionary group of patient characteristics now includes: age 40–49, size of 2.1–3 cm, estrogen receptor negative, and invasive lobular histology (according to ASTRO). There have been only a few reports which document the APBI experience with this cautionary subgroup of patients and these pertain nearly exclusively to brachytherapy techniques (5–13).
This is a retrospective analysis of a total of 269 patients with high risk characteristics, including triple negative, who have been enrolled into two separate accelerated partial breast trials, prospective phase II (NCT01185145) and phase III (NCT01185132) clinical trials. Historically, reports have been divided in the outcomes of these patients. There are whole breast radiotherapy reports which state that the local/ipsilateral breast control in patients with triple negative breast cancer are significantly lower than patients without triple negative or basal type tumors (14–19). There were similar conclusions in young patients (20–31) and in patients with infiltrating lobular histologies (32–34) and HER2/neu positive cancers (15–17, 25, 35) which show higher recurrence rates than in older patients with non- lobular or HER2/neu positive tumors. In contrast, other reports utilizing external beam/brachytherapy irradiation have not observed any worse loco-regional recurrence outcomes in patients with triple negative/basal type of breast cancer (36–41), young age (42–44) or infiltrating lobular histologies (45–51) when compared to older patients with non- lobular or HER2/neu positive tumors.
Methods
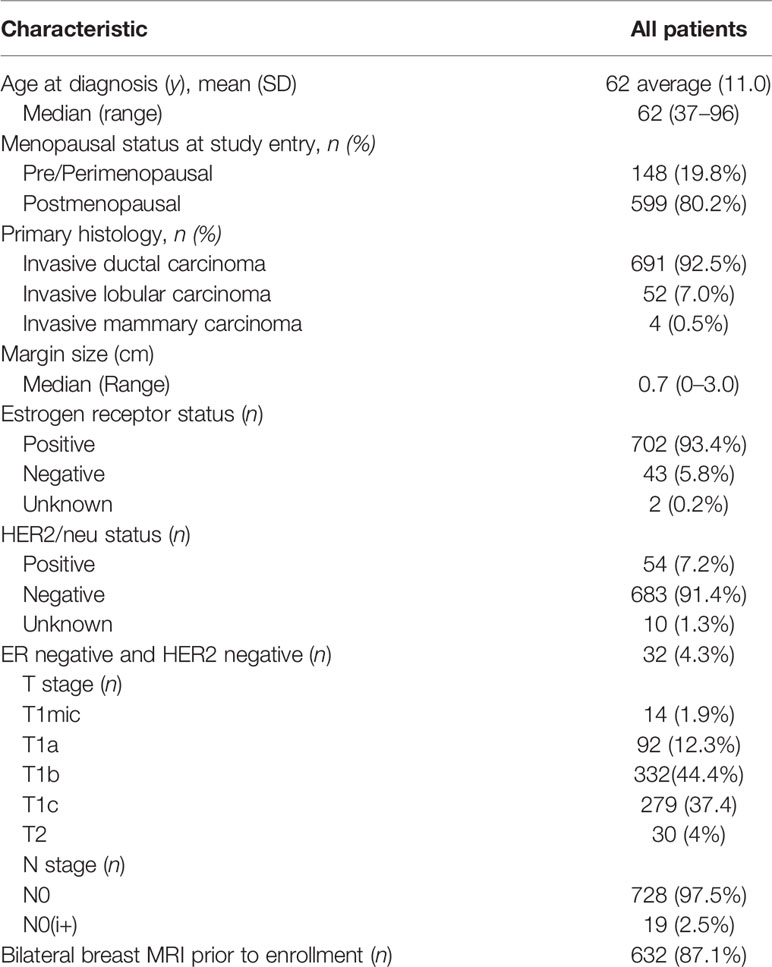
A total of 747 patients enrolled in two accelerated partial breast protocols were used in this analysis. Eligibility for both trials were very similar and included patients with clinically unifocal invasive breast cancer which measured up to 3 cm in size. Patient characteristics are in Table 1 and protocol eligibility requirements including a minimum of ≥2 mm margins and treatment guidelines have been previously reported (52, 53). High risk patients, for the purpose of this study, were defined as those who possess one or more high risk criteria: triple negative (n=30), tumor size ≥2 cm ≤3 cm (n=50), HER2 + (n=54), age range 40-50 years (n=120), ER- (n=43), and ILC histology (n=52). Data collection did not include variables such as limited/focal lymph vascular invasion (LVI) or extensive intraductal component (EIC). Table 2 also delineates an analysis of shared high risk characteristics. Clinical outcomes of ipsilateral breast, axillary, and combined regional recurrences (ipsilateral or axillary) (RR), and overall survival (OS) were analyzed and compared in each high-risk cohort.
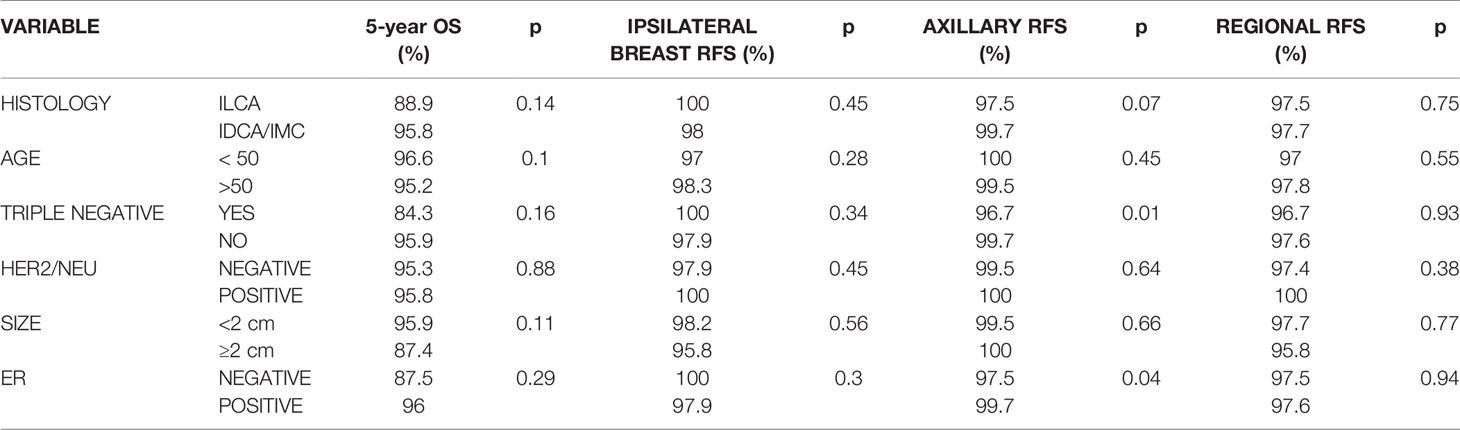
Table 2 Actuarial 5-year overall survival, ipsilateral breast recurrence-free survival (RFS), axillary RFS, and regional (breast and axillary) RFS.
Statistical Analyses
Continuous variables were presented as mean with standard deviation and median with ranges. Categorical variables were expressed as counts with percentages. Kaplan-Meier method with log-rank test was used to estimate the overall survival and the recurrence-free survivals. Univariate and multivariable Cox regression models, which including variables of age, histology, tumor size, and hormone receptor status, were performed to evaluate risk factors associated with death and recurrences.
In addition to the main analysis, we performed a sub-analysis matching the recurrent patients with non-recurrent ones. Variables used for matching were age, histology, tumor size and hormone receptor status. For each sub-analysis, we matched variables for patients with and without recurrences and analyzed one risk factor which was not matched. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
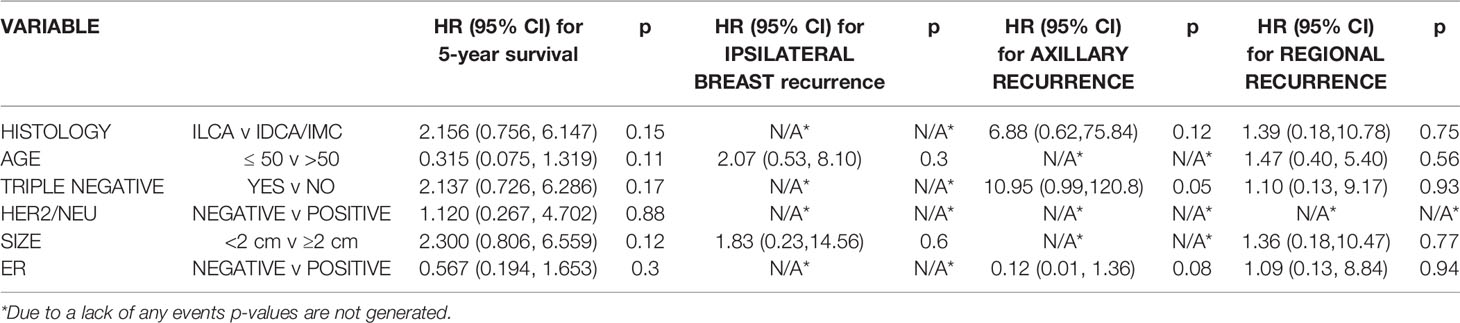
There were 269 patients in the high-risk study group which also includes 30 patients with triple negative subtype breast cancer. High-risk/triple negative patients were compared against a total of 478 patients. Median follow up was 4.0 years for all patients. Of all high- risk patients/triple negative, 70 patients had two or more high-risk characteristics. Table 3 shows that no significant overall survival, ipsilateral breast or regional relapse-free survival differences were found for this high-risk cohort at 5 years as compared to low risk patients. There were also no significant differences for ipsilateral breast, axillary or regional (ipsilateral breast or axillary) recurrences in the infiltrating lobular, age ≤50, HER2/neu positive or tumor size ≥2 cm between cohorts. However, the triple negative subtype was found to significantly adversely impact axillary recurrence. Other “high risk” variables such as the ER negative subtypes were also found to significantly adversely impact axillary recurrence.
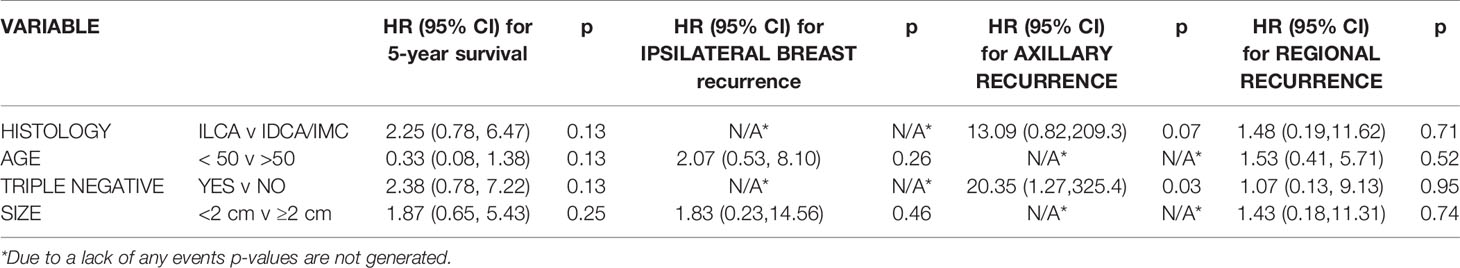
On univariate analysis, triple negative status was also associated with decreased axillary recurrence-free survival (p=0.051) (Table 3). The multivariate analysis in Table 4 depicts the only significant correlations which were between triple negative type and decreased axillary recurrence-free survival (p=0.03).
Matched Pair Analysis
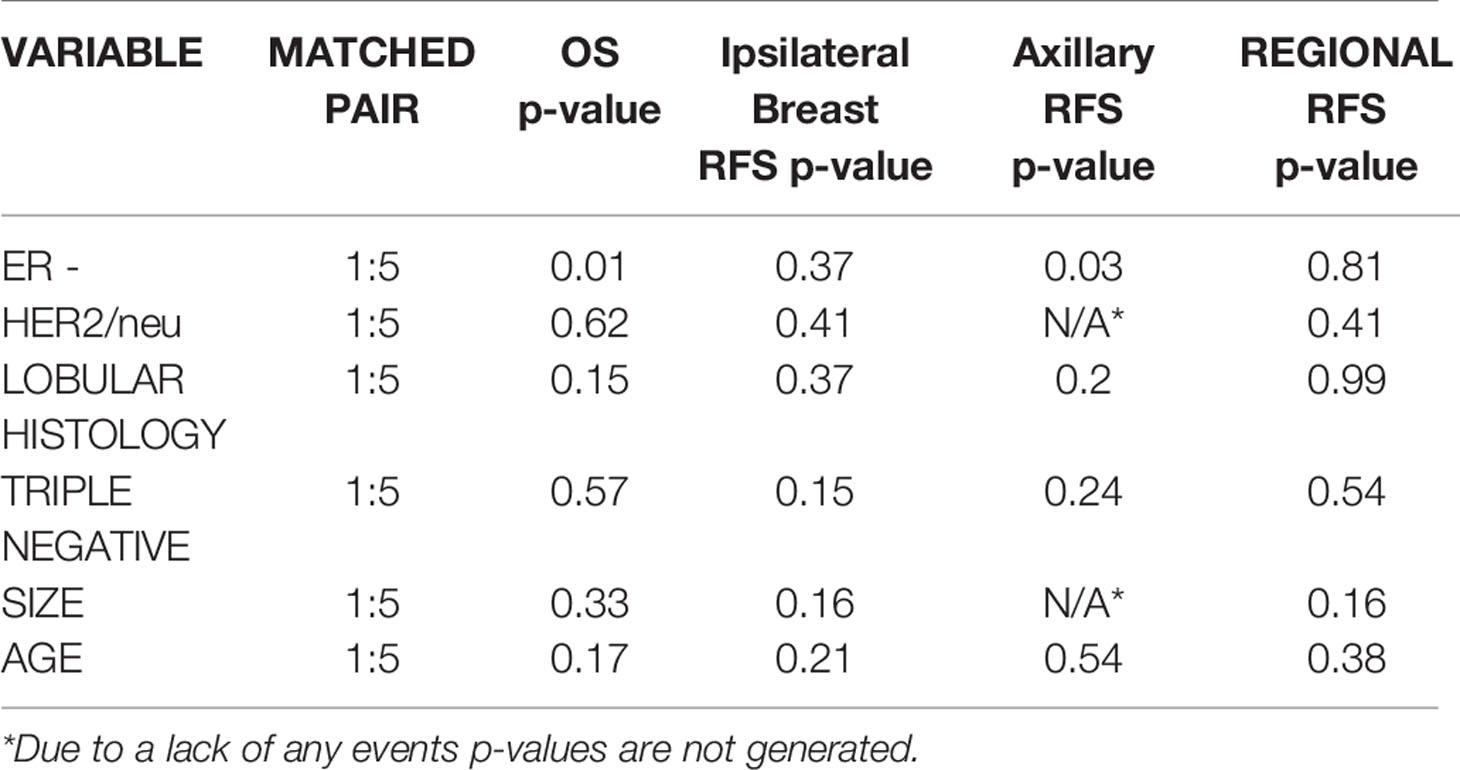
The matched pair analysis is shown in Table 5. The only significant difference between the high and low risk APBI cohorts was for axillary recurrence free survival and overall survival for ER – patients (p=0.03, p=0.013). There were no significant differences in the remaining high risk cohorts for overall survival, ipsilateral breast recurrence-free, axillary recurrence- free, or regional recurrence-free survival outcomes.
Discussion
The current guidelines from various organizations are not firmly based on APBI data which document that ipsilateral breast tumor recurrences (IBTR) are higher among certain subsets of patients including those with triple negative tumors. Rather, these groupings represent a conservative approach to patient APBI eligibility due to available contradicting data. The authors do recognize that these guidelines are for the use of APBI outside of clinical trial and are updated to reflect new research findings to provide continuing direction for the use of APBI. One can even find a lack of consistency between the ASTRO and GESTRO consensus guideline statements, including tumor size and estrogen receptor status (2, 4).
As well, the publication of other reports would suggest that the standard use of APBI might extend beyond the scope of these recommended patient groups.
Current reports have been relatively inconsistent in identifying particular variables which may impact ipsilateral breast tumor recurrence and have had inconsistent findings in other aspects of regional/distant control. As discussed previously, accelerated partial breast radiotherapy can be administered with brachytherapy as well as external beam radiotherapy (3-dimensional, intensity modulated and proton techniques). Several reports document the APBI brachytherapy experience with patients who are categorized in the “cautionary” and/or “unsuitable” poor prognostic variables (5–13). A combined Mammosite Registry and William Beaumont experience with partial breast brachytherapy reported that there were no significant differences in ipsilateral breast failures in the unsuitable cohort versus the “suitable” or “cautionary” cohorts (4.6% versus 2.5% and 3.3% respectively; p=0.2). However, age (<50 vs ≥50) as well as estrogen receptor status (negative versus positive) were significant factors for ipsilateral breast failures (7).
The University of Wisconsin published findings in patients with “high” risk/cautionary features (17, 18). On univariate analysis, both ER negative receptor status and lobular histology were significantly associated with ipsilateral breast failure (p= 0.002 and 0.0004, respectively). Multivariate analysis, however, failed to identify any cautionary feature associated with breast failure. William Beaumont Hospital did not find any significant differences in local breast failure across “suitable”, “cautionary”, or “unsuitable” subgroups in 199 APBI patients when compared to a matched cohort of 199 whole breast patients after a median follow-up of 9 and 13 years for the two groups respectively (10). Univariate analysis of APBI patients did not result in any variable which was significantly associated with ipsilateral breast recurrence. However, as noted in our study, regional nodal failure was significantly associated with ER negative receptor status and positive nodal status in the APBI cohort.
Several other accelerated partial breast irradiation reports found that negative estrogen receptor status could result in a higher ipsilateral breast recurrence and/or distant failure (6, 12, 13).
Studies examining the efficacy of WBI on high risk patients have reported similarly inconsistent results as APBI studies in identifying suitable characteristics for treatment (14, 15, 26, 28, 34, 36, 43, 44). Just as in the case of APBI data, these WBI studies have had equivocal conclusions and, as a whole, have not consistently agreed on all exclusion/inclusion criteria for APBI patients.
While continued, supporting data is needed, the comparability in study outcomes of APBI vs WBI treatment suggests that high risk patients are at no more meaningful risk for recurrence when treated with APBI than WBI. The data reported here as well the other studies cited above suggest that APBI might also be used as a standard of care treatment for the cautionary group analyzed in this study.
The larger phase III trials which randomized APBI versus WBI have had varying but similar eligibility criteria (54–59). Generally, these trials have included patients age ≥40 except RTOG 0413 which included patients ≥18 and IMPORT-LOW which only allowed patients ≥50. None of these specifically excluded ER negative patients, HER2/neu positive patients and the Import Low and RAPID trials disallowed invasive lobular. However, infiltrating ductal comprised greater than 85% of the patient populations of these studies with RTOG 0413 stating 4% of their APBI cohort was infiltrating lobular. None of these studies disallowed ER negative patients but this population only was approximately 5%–8% (RTOG 0413 had 19% ER/PR negative patients) of their APBI cohort. Tumor size for the RAPID, IMPORT-LOW, and RTOG 0413 was ≤3 cm and was 2.5 cm and 2 cm for the Florence and Hungarian trials respectively. Although HER2/neu positivity was not considered to an exclusion criterium in these trials, it has only been reported in the Florence (2.8%) and IMPORT-LOW trials (4%). At this time, however, there have been no data from these phase III studies which have driven any consensus toward definitive data- driven conclusions.
Limitations for this study include the sample size and the length of follow-up. Of all 747 patients that were enrolled in our clinical trials, 269 patients were defined as high risk for the purpose of this study. To our knowledge this is the largest study analyzing the use of APBI in high risk women. Further studies with increased sample sizes are needed for corroboration of the results presented. The median follow-up for this study is 4.0 years. Prior reports have shown that median times to ipsilateral breast relapse in patients with ASTRO defined cautionary characteristics such as triple negative, estrogen receptor negative and HER2/neu positive range from 3–4 years (32, 34, 60). Other studies have also reported median disease-free intervals of 2–3 years in this category (19, 61–63).
Conclusion
The data presented in this study shows that there should be continued reconsideration for inclusion of at least several high-risk variables such as estrogen receptor negative, triple negative, HER2/neu positive,/2–3 cm primary tumors, age 40–50 patients, and patients with infiltrating lobular tumors. Age, histology, and tumor size do not appear to affect favorable outcomes. However, although there is evidence to suggest that there should be continued caution for APBI patient selection of triple negative and estrogen receptor negative tumors, these differences may not be of any meaningful clinical differences whether WBI or APBI is utilized. Further studies and/or follow-up must be done to further corroborate whether these patients, especially those with triple negative disease, should be included or not as eligible for APBI.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Western IRB. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LA and YW provided statistical analysis and manuscript writing. AG, ST, and CL contributed to the data analysis and manuscript preparation. LB and JW contributed to the manuscript preparation. All authors contributed to the article and approved the submitted version.
Conflict of Interest
LA and ST were employed by Linasmar Consulting.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. National Comprehensive Cancer Network. Breast Cancer (Version 3.2020). Available at: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (Accessed May 7, 2020).
2. Smith BD, Arthur DW, Buchholz TA, Haffty BG, Hahn CA, Hardenbergh PH, et al. Accelerated Partial Breast Irradiation Consensus Statement for the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys (2009) 74:987–1001. doi: 10.1016/j.ijrobp.2009.02.031
3. Correa C, Harris EE, Leonardi MC, Smith BD, Taghian AG, Thompson AM, et al. Accelerated Partial Breast Irradiation: Executive summary for the update of an ASTRO Evidence-Based Consensus Statement. Pract Radiat Oncol (2017) 7:73–9. doi: 10.1016/j.prro.2016.09.007
4. Polgár C, Limbergen EV, Pötter R, Kovács G, Polo A, Lyczek J, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: Recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiotherapy and Oncology (2010) 94(3):264–73. doi: 10.1016/j.ijrobp.2010.07.581
5. Mchaffie DR, Patel RR, Adkison JB, Das RK, Geye HM, Cannon GM. Outcomes after accelerated partial breast irradiation in patients with ASTRO consensus statement cautionary features. Int J Radiat Oncol Biol Phys (2011) 81(1):46–51. doi: 10.1016/j.ijrobp.2010.05.011
6. Christoudias MK, Collett AE, Stull TS, Gracely EJ, Frazier TG, Barrio AV. Are the American Society for Radiation Oncology Guidelines accurate predictors of recurrence in early stage breast cancer patients treated with balloon-based brachytherapy? Int J Surg Oncol (2013) 8:2013. doi: 10.1155/2013/829050
7. Wilkinson JB, Beitsch PD, Shah C, Arthur D, Haffty BG, Wazer DE, et al. Evaluation of current consensus statement recommendations for accelerated partial breast irradiation: a pooled analysis of William Beaumont Hospital and American Society of Breast SurgeonMammoSite Registry Trial Data. Int J Radiat Oncol Biol Phys (2012) 85:1179–85. doi: 10.1016/j.ijrobp.2012.10.010
8. Patel RR, Christensen ME, Hodge CW, Adkison JB, Das RK. Clinical outcomeanalysis in ‘‘high-risk’’ vs. ‘‘low-risk’’ patients eligible for national surgical adjuvant breast and bowel B- 39/radiation therapy oncology group 0413 trial: Five-year results. Int J Radiat Oncol Biol Phys (2008) 70:970–3. doi: 10.1016/j.ijrobp.2007.12.005
9. McHaffie DR, Patel RR, Adkison JB, Das RK, Geye HM, Cannon GM. Outcomes after accelerated partial breast irradiation in patients with ASTRO Consensus Statement cautionary features. Int J Radiat Oncol Biol Phys (2011) 81:46–51. doi: 10.1016/j.ijrobp.2010.05.011
10. Vicini F, Arthur D, Wazer D, Chen P, Mitchell C, Wallace M, et al. Limitations of the American Society of Therapeutic Radiology and Oncology Consensus Panel guidelines on the use of accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys (2011) 79:977–84. doi: 10.1016/j.ijrobp.2009.12.047
11. Zauls AJ, Watkins JM, Wahlquist AE, Brackett C III, Aguero EG, Baker MK, et al. Outcomes in women treated with MammoSite brachytherapy or whole breast irradiation stratified by ASTRO accelerated partial breast irradiation consensus statement groups. Int J Radiat Oncol Biol Phys (2012) 82:21–9. doi: 10.1016/j.ijrobp.2010.08.034
12. Shaitelman S, Vicini F, Beitsch P, Haffty B, Keisch M, Lyden M. Five-year Outcome of Patients Classified Using the American Society for Radiation Oncology Consensus Statement Guidelines for the Application of Accelerated Partial Breast Irradiation. Cancer (2010) 116:4677–85. doi: 10.1002/cncr.25383
13. Stull TS, Goodwin MC, Gracely EJ, Chernick MR, Carella RJ, Frazier TG, et al. A single-institution review of accelerated partial breast irradiation in patients considered “cautionary” by the American Society for Radiation Oncology. Ann Surg Oncol (2012) 19:553–9. doi: 10.1245/s10434-011-1941-7
14. Solin LJ, Hwang WT, Vapiwala N. Outcome after breast conservation treatment with radiation for women with triple negative early stage invasive breast carcinoma. Clin Breast Cancer (2009) 9:96–100. doi: 10.3816/CBC.2009.n.018
15. Hattangadi-Gluth JA, Wo JY, Nguyen PL, Raad RF, Sreedhara M, Niemierko A, et al. Basal subtype of invasive breast canceris associated with a higher risk of true recurrence after conventional breast-conserving therapy. Int J Radiat Oncol Biol Phys (2012) 82(3):1185–91. doi: 10.1016/j.ijrobp.2011.02.061
16. Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol (2008) 26(14):2373–8. doi: 10.1200/JCO.2007.14.4287
17. Chen J, Jiang P, Wang HJ, Zhang JY, Xu Y, Guo MH, et al. The efficacy of molecular subtyping in predicting postoperative recurrence in breast- conserving therapy: a 15-study meta-analysis. World J Surg Oncol (2014) 12(1):212. doi: 10.1186/1477-7819-12-212
18. Zaky SS, Lund M, May KA, Godette KD, Beitler JJ, Holmes LR, et al. The negative effect of triple-negative breast cancer on outcome after breast-conserving therapy. Ann Surg Oncol (2011) 18(10):2858–65. doi: 10.1245/s10434-011-1669-4
19. Lowery AJ, Kell MR, Glynn RW, Kerin MJ, Sweeney KJ. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat (2012) 133(3):831–41. doi: 10.1007/s10549-011-1891-6
20. Cèfaro GA, Genovesi D, Marchese R, Ursini LA, Cianchetti E, Ballone E, et al. Predictors of local recurrence after conservative surgery and whole-breast irradiation. Breast Cancer Res Treat (2006) 98(3):329–35. doi: 10.1007/s10549-006-9169-0
21. Voogd AC, Nielsen M, Peterse JL, Blichert-Toft M, Bartelink H, Overgaard M, et al. Danish Breast Cancer Cooperative Group and the Breast Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol (2001) 19(6):1688–97. doi: 10.1200/JCO.2001.19.6.1688
22. Fourquet A, Campana F, Zafrani B, Mosseri V, Vielh P, Durand JC, et al. Prognostic factors of breast recurrence in the conservative management of early breast cancer: a 25-year follow-up. Int J Radiat Oncol Biol Phys (1989) 17(4):719–25. doi: 10.1016/0360-3016(89)90057-6
23. Oh JL, Bonnen M, Outlaw ED, Schechter NR, Perkins GH, Strom EA, et al. The impact of young age on locoregional recurrence after doxorubicin-based breast conservation therapy in patients 40 years old or younger: How young is “young”? Int J Radiat Oncol Biol Phys (2006) 65(5):1345–52. doi: 10.1016/j.ijrobp.2006.03.028
24. Komoike Y, Akiyama F, Iino Y, Ikeda T, Akashi-Tanaka S, Ohsumi S, et al. Ipsilateral breast tumor recurrence (IBTR) after breast- conserving treatment for early breast cancer: risk factors and impact on distant metastases. Cancer (2006) 106(1):35–41. doi: 10.1002/cncr.21551
25. Livi L, Meattini I, Saieva C, Borghesi S, Scotti V, Petrucci A, et al. The impact of young age on breast cancer outcome. Eur J Surg Oncol (EJSO) (2010) 36(7):639–45. doi: 10.1016/j.ejso.2010.05.016
26. Jobsen JJ, Van der Palen J, Meerwaldt JH. The impact of age on local control in women with pT1 breast cancer treated with conservative surgery and radiation therapy. Eur J Cancer (2001) 37(15):1820–7. doi: 10.1016/S0959-8049(01)00173-3
27. Recht A, Connolly JL, Schnitt SJ, Silver B, Rose MA, Love S, et al. The effect of young age on tumor recurrence in the treated breast after conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys (1988) 14(1):3–10. doi: 10.1016/0360-3016(88)90043-0
28. Vrieling C, Collette L, Fourquet A, Hoogenraad WJ, Horiot JC, Jager JJ, et al. Can patient-, treatment-and pathology-related characteristics explain the high local recurrence rate following breast-conserving therapy in young patients? Eur J Cancer (2003) 39(7):932–44. doi: 10.1016/S0959-8049(03)00123-0
29. Boyages J, Recht A, Connolly JL, Schnitt SJ, Gelman R, Kooy H, et al. Early breast cancer: predictors of breast recurrence for patients treated with conservative surgery and radiation therapy. Radiother Oncol (1990) 19(1):29–41. doi: 10.1016/0167-8140(90)90163-Q
30. Harrold EV, Turner BC, Matloff ET, Pathare P, Beinfield M, McKhann C, et al. Local recurrence in the conservatively treated breast cancer patient: a correlation with age and family history. Cancer J Sci Am (1998) 4(5):302–7.
31. Dewar JA, Arriagada R, Benhamou S, Benhamou E, Bretel JJ, Pellae-Cosset B, et al. Local relapse and contralateral tumor rates in patients with breast cancer treated with conservative surgery and radiotherapy (Institut Gustave Roussy 1970–1982). Cancer (1995) 76(11):2260–5. doi: 10.1002/1097-0142(19951201)76:11<2260::AID-CNCR2820761113>3.0.CO;2-D
32. Mate TP, Carter D, Fischer DB, Hartman PV, McKhann C, Merino M, et al. A clinical and histopathologic analysis of the results of conservation surgery and radiation therapy in stage I and II breast carcinoma. Cancer (1986) 58(9):1995–2002. doi: 10.1002/1097-0142(19861101)58:9<1995::AID-CNCR2820580907>3.0.CO;2-1
33. Du Toit RS, Locker AP, Elis IO, Elston CW, Blarney RW. Evaluation of the prognostic value of triple node biopsy in early breast cancer. Br J Surg (1990) 77(2):163–7. doi: 10.1002/bjs.1800770216
34. Livi L, Paiar F, Saieva C, Scoccianti S, Dicosmo D, Borghesi S, et al. Survival and breast relapse breast cancer after conserving in 3834 patients with T1-T2 surgery and adjuvant treatment. Radiother Oncol (2007) 82:287–93. doi: 10.1016/j.radonc.2006.11.009
35. Braunstein LZ, Taghian AG, Niemierko A, Salama L, Capuco A, Bellon JR, et al. Breast-cancer subtype, age, and lymph node status as predictors of local recurrence following breast-conserving therapy. Breast Cancer Res Treat (2017) Jan 1161(1):173–9. doi: 10.1007/s10549-016-4031-5
36. Freedman GM, Anderson PR, Li T, Nicolaou N. Locoregional recurrence of triple-negative breast cancer after breast-conserving surgery and radiation. Cancer (2009) 115(5):946–51. doi: 10.1002/cncr.24094
37. Zumsteg ZS, Morrow M, Arnold B, Zheng J, Zhang Z, Robson M, et al. Breast-conserving therapy achieves locoregional outcomes comparable to mastectomy in women with T1-2N0 triple-negative breast cancer. Ann Surg Oncol (2013) 20(11):3469–76. doi: 10.1245/s10434-013-3011-9
38. Adkins FC, Gonzalez-Angulo AM, Lei Xrnandez-Aya LF, Mittendorf EA, Litton JK, Wagner J, et al. Triple-negative breast cancer is not a contraindication for breast conservation. Ann Surg Oncol (2011) 18(11):3164. doi: 10.1245/s10434-011-1920-z
39. Gangi A, Chung A, Mirocha J, Liou DZ, Leong T, Giuliano AE. Breast-conserving therapy for triple-negative breast cancer. JAMA Surg (2014) 149(3):252–8. doi: 10.1001/jamasurg.2013.3037
40. Barbieri V, Sanpaolo P, Genovesi D. Prognostic impact of triple negative phenotype in conservatively treated breast cancer. Breast J (2011) 17(4):377–82. doi: 10.1111/j.1524-4741.2011.01100.x
41. Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol (2010) 28(10):1684–91. doi: 10.1200/JCO.2009.24.9284
42. Solin LJ, Fowble B, Schultz DJ, Goodman RL. Age as a prognostic factor for patients treated with definitive irradiation for early stage breast cancer. Int J Radiat Oncol Biol Phys (1989) 16(2):373–81. doi: 10.1016/0360-3016(89)90333-7
43. Fowble BL, Schultz DJ, Overmoyer B, Solin LJ, Fox K, Jardines L, et al. The influence of young age on outcome in early stage breast cancer. Int J Radiat Oncol Biol Phys (1994) 30(1):23–33. doi: 10.1016/0360-3016(94)90515-0
44. Elkhuizen PH, van de Vijver MJ, Hermans JO, Zonderland HM, van de Velde CJ, Leer JW. Local recurrence after breast-conserving therapy for invasive breast cancer: high incidence in young patients and association with poor survival. Int J Radiat Oncol Biol Phys (1998) 40(4):859–67. doi: 10.1016/S0360-3016(97)00917-6
45. Peiro G, Bornstein BA, Connolly JL, Gelman R, Hetelekidis S, Nixon AJ, et al. The influence of infiltrating lobular carcinoma on the outcome of patients treated with breast-conserving surgery and radiation therapy. Breast Cancer Res Treat (2000) 59(1):49–54. doi: 10.1023/A:1006384407690
46. Kurtz JM, Jacquemier J, Torhorst J, Spitalier JM, Amalric R, Hünig R, et al. Conservation therapy for breast cancers other than infiltrating ductal carcinoma. Cancer (1989) 63(8):1630–5. doi: 10.1002/1097-0142(19890415)63:8<1630::AID-CNCR2820630833>3.0.CO;2-U
47. Poen JC, Tran L, Juillard G, Selch MT, Giuliano A, Silverstein M, et al. Conservation therapy for invasive lobular carcinoma of the breast. Cancer (1992) 69(11):2789–95. doi: 10.1002/1097-0142(19920601)69:11<2789::AID-CNCR2820691126>3.0.CO;2-J
48. White JR, Gustafson GS, Wimbish K, Ingold JA, Lucas RJ, Levine AJ, et al. Conservative surgery and radiation therapy for infiltrating lobular carcinoma of the breast: The role of preoperative mammograms in guiding treatment. Cancer (1994) 74(2):640–7. doi: 10.1002/1097-0142(19940715)74:2<640::AID-CNCR2820740216>3.0.CO;2-V
49. Santiago RJ, Harris EE, Qin L, Hwang WT, Solin LJ. Similar long-term results of breast- conservation treatment for Stage I and II invasive lobular carcinoma compared with invasive ductal carcinoma of the breast: The University of Pennsylvania experience. Cancer (2005) 103(12):2447–54. doi: 10.1002/cncr.21071
50. Molland JG, Donnellan M, Janu NC, Carmalt HL, Kennedy CW, Gillett DJ. Infiltrating lobular carcinoma—a comparison of diagnosis, management and outcome with infiltrating duct carcinoma. Breast (2004) 13(5):389–96. doi: 10.1016/j.breast.2004.03.004
51. Salvadori B, Biganzoli E, Veronesi P, Saccozzi R, Rilkes F. Conservative surgery for infiltrating lobular breast carcinoma. Br J Surg (1997) 84(1):106–9. doi: 10.1046/j.1365-2168.1997.02540.x
52. Lei RY, Leonard CE, Howell KT, Henkenberns PL, Johnson TK, Hobart TL, et al. Four-year clinical update from a prospective trial of accelerated partial breast intensity-modulated radiotherapy (APBIMRT). Breast Cancer Res Treat (2013) 140(1):119–33. doi: 10.1007/s10549-013-2623-x
53. Leonard C, Carter D, Kercher J, Howell K, Henkenberns P, Tallhamer M, et al. Prospective trial of accelerated partial breast intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys (2007) 67(5):1291–8. doi: 10.1016/j.ijrobp.2006.11.016
54. Polgár C, Fodor J, Major T, Sulyok Z, Kásler M. Breast-conserving therapy with partial or whole breast irradiation: ten-year results of the Budapest randomized trial. Radiother Oncol (2013) 108(2):197–202. doi: 10.1016/j.radonc.2013.05.008
55. Vicini FA, Cecchini RS, White JR, Arthur DW, Julian TB, Rabinovitch RA, et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet (2019) 394(10215):2155–64. doi: 10.1016/S0140-6736(19)32514-0
56. Whelan TJ, Julian JA, Berrang TS, Kim DH, Germain I, Nichol AM, et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): a randomised controlled trial. Lancet (2019) 394(10215):2165–72. doi; 10.1016/S0140-6736(19)32515-2
57. Meattini I, Marrazzo L, Saieva C, Desideri I, Scotti V, Simontacchi G, et al. Accelerated partial-breast irradiation compared with whole-breast irradiation for early breast cancer: Long-term results of the randomized phase III APBI-IMRT-Florence trial. J Clin Oncol (2020) 38(35):4175–83. doi: 10.1200/JCO.20.00650
58. Coles CE, Griffin CL, Kirby AM, Titley J, Agrawal RK, Alhasso A, et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet (2017) 390(10099):1048–60. doi: 10.1016/S0140-6736(17)31145-5
59. Strnad V, Ott OJ, Hildebrandt G, Kauer-Dorner D, Knauerhase H, Major T, et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet (2016) 387(10015):229–38. doi: 10.1016/S0140-6736(15)00471-7
60. Parikh RR, Housman D, Yang Q, Toppmeyer D, Wilson LD, Haffty BG. Prognostic value of triple-negative phenotype at the time of locally recurrent, conservatively treated breast 19 cancer. Int J Radiat Oncol Biol Phys (2008) 72(4):1056–63. doi: 10.1016/j.ijrobp.2008.02.066
61. Gosset M, Hamy AS, Mallon P, Delomenie M, Mouttet D, Pierga JY, et al. Prognostic impact of time to ipsilateral breast tumor recurrence after breast conserving surgery. PloS One (2016) 11(8). doi: 10.1371/journal.pone.0159888
62. Wangchinda P, Ithimakin S. Factors that predict recurrence later than 5 years after initial treatment in operable breast cancer. World J Surg Oncol (2016) 14(1):223. doi: 10.1186/s12957-016-0988-0
63. Chumsri S, Li Z, Serie DJ, Mashadi-Hossein A, Colon-Otero G, Song N, et al. Incidence of Late Relapses in Patients with HER2-Positive Breast Cancer Receiving Adjuvant Trastuzumab: Combined Analysis of NCCTG N9831 (Alliance) and NRG Oncology/NSABP B-31. J Clin Oncol (2019) 37(35):3425–35. doi: 10.1200/JCO.19.00443
Keywords: young age group, infiltrating lobular breast cancer, HER2 breast cancer +, estrogen receptor negative breast cancer, triple negative breast cancer, partial breast external beam radiotherapy
Citation: Goulding A, Asmar L, Wang Y, Tole S, Barke L, Widner J and Leonard C (2021) Outcomes After Accelerated Partial Breast Irradiation in Women With Triple Negative Subtype and Other “High Risk” Variables Categorized as Cautionary in The ASTRO Guidelines. Front. Oncol. 11:617439. doi: 10.3389/fonc.2021.617439
Received: 14 October 2020; Accepted: 01 February 2021;
Published: 11 March 2021.
Edited by:
Jaroslaw T. Hepel, Rhode Island Hospital, United StatesReviewed by:
Vivek Verma, Allegheny General Hospital, United StatesDiane Ling, University of Southern California, United States
Copyright © 2021 Goulding, Asmar, Wang, Tole, Barke, Widner and Leonard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charles Leonard, Y2hhcmxlcy5sZW9uYXJkQHVzb25jb2xvZ3kuY29t
 Anabel Goulding
Anabel Goulding Lina Asmar
Lina Asmar Yunfei Wang2
Yunfei Wang2 Charles Leonard
Charles Leonard