- 1Division of Otolaryngology and Head and Neck Surgery, IEO, European Institute of Oncology IRCCS, Milan, Italy
- 2Department of Experimental Oncology, IEO, European Institute of Oncology IRCCS, Milan, Italy
- 3Department of Mathematics, DMAT, Politecnico di Milano, Milan, Italy
- 4Center for Analysis Decisions and Society, CADS, Human Technopole, Milan, Italy
- 5Division of Pathology, IEO, European Institute of Oncology IRCCS, Milan, Italy
- 6Department of Otorhinolaryngology, Policlinico San Matteo, IRCCS, Pavia, Italy
- 7Department of Otorhinolaryngology, ASST Ovest Milanese, Legnano, Italy
- 8Division of Data Manager, IEO, European Institute of Oncology IRCCS, Milan, Italy
- 9Division of Radiotherapy, IEO, European Institute of Oncology IRCCS, Milan, Italy
- 10Department of Medical Oncology, Urogenital and Head and Neck Tumors Medical Treatment, IEO, European Institute of Oncology IRCCS, Milan, Italy
- 11Department of Biomedical Sciences, University of Sassari, Sassari, Italy
Objective: The prognostic role of age among patients affected by Oral Tongue Squamous Cell Carcinoma (OTSCC) is a topic of debate. Recent cohort studies have found that patients diagnosed at 40 years of age or younger have a better prognosis. The aim of this cohort study was to clarify whether age is an independent prognostic factor and discuss heterogeneity of outcomes by stage and treatments in different age groups.
Methods: We performed a study on 577 consecutive patients affected by primary tongue cancer and treated with surgery and adjuvant therapy according to stage, at European Institute of Oncology, IRCCS. Patients with age at diagnosis below 40 years totaled 109 (19%). Overall survival (OS), disease-free survival (DFS), tongue specific free survival (TSFS) and cause-specific survival (CSS) were compared by age groups. Multivariate Cox proportional hazards models were used to assess the independent role of age.
Results: The median follow-up time was 5.01 years (range 0–18.68) years with follow-up recorded up to February 2020. After adjustment for all the significant confounding and prognostic factors, age remained independently associated with OS and DSF (respectively, p = 0.002 and p = 0.02). In CSS and TSFS curves, the role of age seems less evident (respectively, p = 0.14 and p = 0.0.37). In the advanced stage sub-group (stages III–IV), age was significantly associated with OS and CSS with almost double increased risk of dying (OS) and dying from tongue cancer (CSS) in elderly compared to younger groups (OS: HR = 2.16 95%, CI: 1.33–3.51, p= 0.001; CSS: HR = 1.76 95%, CI: 1.03–3.01, p = 0.02, respectively). In our study, young patients were more likely to be treated with intensified therapies (glossectomies types III–V and adjuvant radio-chemotherapy). Age was found as a prognostic factor, independently of other significant factors and treatment. Also the T–N tract involved by disease and neutrophil-to-lymphocyte ratio ≥3 were independent prognostic factors.
Conclusions: Young age at diagnosis is associated with a better overall survival. Fewer younger people than older people died from tongue cancer in advanced stages.
Introduction
One of the hot topics in the field of head and neck tumors concerns the increased incidence of oral tongue squamous cell carcinoma (OTSCC) in young people (1, 2). The reason for these new epidemiological data is still undefined. Above all, etiopathogenesis and prognosis remain unclear when compared to the traditional population affected by oral tongue cancer, which is generally composed of people of over fifty years old with known risk factors such as heavy smoking and alcohol habits (3, 4).
The incidence of mobile tongue cancer in young patients is reported as increasing worldwide, especially in the last three decades (5–8). In particular, the analysis carried out by the National Cancer Institute Surveillance, Epidemiology and End Results Program (SEER) in 2011 on North American data shows that the overall incidence of OTSCC remained stable from 1975 to 2007 but increased in women and more specifically in the sub-population of young white women (9). Many conflicting groups have been published on the etiology, natural history, and prognosis of OTSCC in young adults. As early as 1970, tongue cancer in young people was supposed to be a distinct clinical entity that needed to be treated more aggressively than that in older adults (10). In 2011, Patel et al. also hypothesized a possible hormonal influence as the cause of these tumors (9). In this scenario, also chronic mucosal trauma is considered a possible cause for OTSS in young patients (11).
It is well known that the onset of OTSCC is related to smoking and alcohol abuse and in some countries with the habit of chewing betel leaves (12, 13). In addition, some authors have reported that gender distribution is different depending on the age of onset of oral cancer (12, 14, 15). While in the elderly, males account for over 70% of cases, this percentage drops to 50–65% under 45 years of age. This difference is consolidated if we consider that most of the young, non-smoking and non-drinking patients are reported to be women (12, 14, 15).
Currently, the primary treatment strategy for OTSCC is upfront surgery followed, in advanced stages, by radiotherapy or radio-chemotherapy based on final histopathological findings, according to international guidelines (16, 17).
Locoregional control of OTSCC has improved in recent decades; the reason could be more aggressive surgical resections supported by modern reconstructive methods with free flaps and advances in adjuvant treatment (18–21).
However, despite improved locoregional control, survival rates have remained stable or slightly improved over the past two decades with 5-year overall survival (OS) of approximately 60% for all stages and 33–54% in patients with locally advanced disease (16, 22).
Other treatment strategies such as neoadjuvant chemotherapy followed by surgery or upfront radio-chemotherapy have been employed for OTSCC without any significant improvement in survival (22–24).
In young patients, the prognosis of OTSCC is still controversial. Several early reports on squamous cell carcinoma (SCC) concluded that the disease was more aggressive and the prognosis lower in young adults compared to older patients (10, 25–27). However, recent studies have not found any significant differences in OS between different age groups (2, 28–32). At the same time, several other investigators claim that younger OTSCC patients have better survival compared to elderly patients (6, 8, 29, 33, 34). Conversely, the Memorial Sloan-Kettering Cancer Center reported that younger OTSCC patients had a higher rate of locoregional recurrence, with no significant difference in survival between young and old groups (35).
Many studies reported no difference in biological behavior among young and elderly patients with OTSCC (36–41). In particular, the latest next-generation sequencing techniques indicated that genomic profiles and mutations in the guide genes were very similar among young and elderly OTSCC patients, with similar mechanisms of tumorigenesis (36–41). Moreover, gene-specific mutation and copy number alteration frequencies were the same between young and old OTSCC patients in two independent cohorts (40).
Different results on prognosis and etiology may be attributed both to the small size of the patient cohorts and the different cut-off to define “young” age. Furthermore, there was a considerable heterogeneity both between and also within samples (i.e., matched/unmatched, early/advanced tumor stage). Finally, they did not specify whether or not studies were adjusted for factors such as treatment modality, stage of the disease, presence and absence of metastatic lymph nodes, and percentage of patients between the two age groups. With these premises, the fundamental question about the role of age in cancer outcome of oral tongue cancer remains unanswered.
The aim of the study was to investigate the prognostic role of age and its influence on OTSCC cancer relapse and survival. We collected information on clinical and demographic characteristics of a retrospective cohort of OTSCC patients treated with homogenous modality in our institution, to define which factors could mostly influence survival outcomes.
Material and Methods
Between January 2000 and December 2018, a total of 891 patients with oral cavity cancer underwent surgery at the Division of Otolaryngology and Head and Neck surgery of the European Institute of Oncology, IRCCS (IEO). The inclusion criteria were: patients with a histologically confirmed primary diagnosis of OTSCC and primary surgical treatment received at IEO. Among these, 173/891 patients were excluded because of previous surgery and/or excisional biopsy which were considered as a complete surgical therapy and not as a mere diagnostic procedure; 105/891 patients were excluded due to histology other than SCC, and 13/891 were excluded because the tumor origin was attributable to the base of the tongue. At the end of the selection, 577 patients with primary diagnosis of OTSCC and primary surgical treatment received at IEO were included in the study cohort.
The current study was approved by the IEO Ethics Committee (cod. IEO 225).
Staging referred to the TNM classification in accordance with the 7th edition American Joint Committee on Cancer system, and all cases were re-classified according to the 8th edition (42, 43). We reported the clinical stages according the 7th TNM editions and the pathological stages in both 7th and 8th TNM editions for completeness of results.
For the study of the paper outcomes, we used the new staging system (8th TNM edition) because it is the prognostic TNM currently used.
Preoperative diagnoses were assessed by a simple biopsy of the lesion, while magnetic resonance imaging or a computed tomography scan was performed for local disease study. Positron emission tomography/computedtomography or total body computed tomography was used for the pre-operative patients’ systemic evaluation.
The retrospective information extracted from the electronic medical records included:
- anthropometric and demographic patient characteristics: height, weight, age, gender, pre-operative neutrophil-to-lymphocyte ratio (NLR);
- epidemiologic data: smoking history, family history for tongue cancer and other cancers, alcoholic habits;
- histopathologic features and staging: surgical margins, clinical and pathological TNM 7th and 8th editions, tumor grading, the status of T–N tract (44, 45);
- performed treatment: type of surgery, glossectomies I to V according to the Ansarin et al. classification (from transoral to total glossectomy) (46), adjuvant treatment strategies (radiotherapy and/or chemotherapy)
- follow-up information: type of recurrence (local/locoregional) and/or distant metastases, secondary primary and the patients’ status at the last follow-up.
The definition of pack–years of cigarette smoking was used for the evaluation of tobacco consumption (47). Smoking and alcohol status at diagnosis were collected to classify patients as current, former, and never-smoking/drinking.
About the “neck lymph node” status we considered clinical (c)/pathological (p) Nx, N0, N+ as distinct variables. All patients’ follow-up were collected and updated to assess their status at the last clinical evaluation and the last contact. Patient deaths and their possible causes were assessed using the Italian national death register.
The NLR cutoff of < or ≥3 was determined based on prior publications (48–51).
All the data were collected in a well-designed database according to good clinical practice guidelines.
In previous studies of OTSCC the most frequent used age cutoff to define young adulthood was 40; moreover, it is reported that the role of risk factors, as smoke and alcohol, start to be significant after the age of 40 (1, 52–54). Thus, the main analysis was carried out considering 40 years of age as the cutoff of interest.
Treatment Modality
All patients received the standard surgical treatment according to the IEO protocol for tongue cancer (46). The clinical early stages (clinical stags I and II) underwent trans-oral glossectomies (type I or II) followed by delayed (within 30 days) neck dissection (I–IV levels) in cases of deep of infiltration (DOI)>3 mm in the tongue. The “wait and see” policy was chosen where DOI was less than 3 mm.
The variable “neck dissection” (Table 1) refers to neck dissections performed en bloc with the tongue cancer (glossectomies types III to V) or as prophylactic neck dissection after 4 weeks from trans-oral glossectomies (types I or II) for tumor DOI.
Tumor clinically staged as intermediate and advanced (III and IV) were treated with glossectomies types III–to V en bloc neck dissection removing the T–N tract in pull-through or with trans-mandibular approaches (compartmental tongue surgery). Adjuvant treatment, such as radiotherapy or radio-chemotherapy, was recommended based on definitive histopathological findings.
Definition of Endpoints
Local recurrence was defined as recurrence in the original tumor bed with the same histopathologic features of the primary tumor in the first three years after treatment. Regional recurrence was described as a metastatic disease in the head and neck region. Distant recurrence was defined as the presence of metastatic disease in all other locations. Any recurrence was reported as any local, regional, or distant metastasis, whichever occurred first. Disease-free survival (DFS) was defined as the time from surgery until any kind of tumor recurrence, including the occurrence of a second primary tumor or death from any cause, or the last contact date if alive with no recurrence. The last contact date was considered the last follow-up visit performed with the patient or the last telephone call ascertaining the patient’s state of health.
We considered the second tumor as an event of interest if it appeared after three years of treatment in the oral cavity or in other districts at any time after treatment (55).
Overall survival (OS) was defined as the time from surgery until the date of death from any cause, or the last contact date if alive (56). Patients’ deaths were assessed using the Italian national death registers.
The following tumor-specific clinical outcomes were also evaluated: Cause-specific survival (CSS) defined as the time from surgery until the date of death for tongue cancer. In case of no death for tongue cancer the observation was censored at the last follow-up visit or the date of death for other causes. Tongue specific free survival (TSFS) included the period after a successful treatment during which there were no signs and symptoms of the disease that was treated (tongue cancer) (56–59). The events considered for the TSFS were: local, locoregional recurrence and metastases only for tongue cancer. In case of no events or death for tongue cancer, the observation was censored at the last follow-up visit or the date of death for other causes.
Statistical Analysis
Patient clinical-pathological and tumor characteristics were expressed as relative frequencies and percentages according to age. We choose 40 years old as a cutoff point for age in compliance with previous published studies that evaluated age in head and neck cancer (1, 10, 60–62). We conducted also a sensitivity analysis looking for the best cutoff point in our group for each survival outcome, and we investigated the role of age also as a continuous variable.
Univariate models were performed to evaluate the association of age and other prognostic factors (e.g., smoking pack/year, T–N tract, surgery and stage of 8th TNM edition) with clinical outcomes (DFS, OS, CSS and TSFS). Differences between survival curves were investigated with Log-rank tests and estimated using the Kaplan–Meier method. We assessed the independent prognostic role of age for each outcome with multivariate Cox Proportional Hazard models adjusted for all significant prognostic factors. Hazard ratio (HR) with 95% confidence intervals (CIs) from multivariate Cox proportional hazard models were reported. Sub-group analyses were conducted to investigate whether stage (8th TNM edition) and surgery were associated with any cancer event as local recurrence, secondary primary (DFS) or death of any cause (OS) and recurrence related only to tongue cancer (TSFS) or death of tongue cancer (CSS) depending on age (56–59). We used a Chi-squared test to assess the association of age with frequencies of patients diagnosed recently (between 2010 and 2020), to investigate the influence of time of diagnosis and whether recent diagnoses were associated with sex. Finally, we evaluated whether the proportion of young patients (≤40 years old) was significantly associated with the type of surgery and stage (8th TNM edition). All analyses were carried out with R 4.0 software (http://cran.r-project.org/), and p-values <0.05 were considered statistically significant.
Results
Patients’ Characteristics
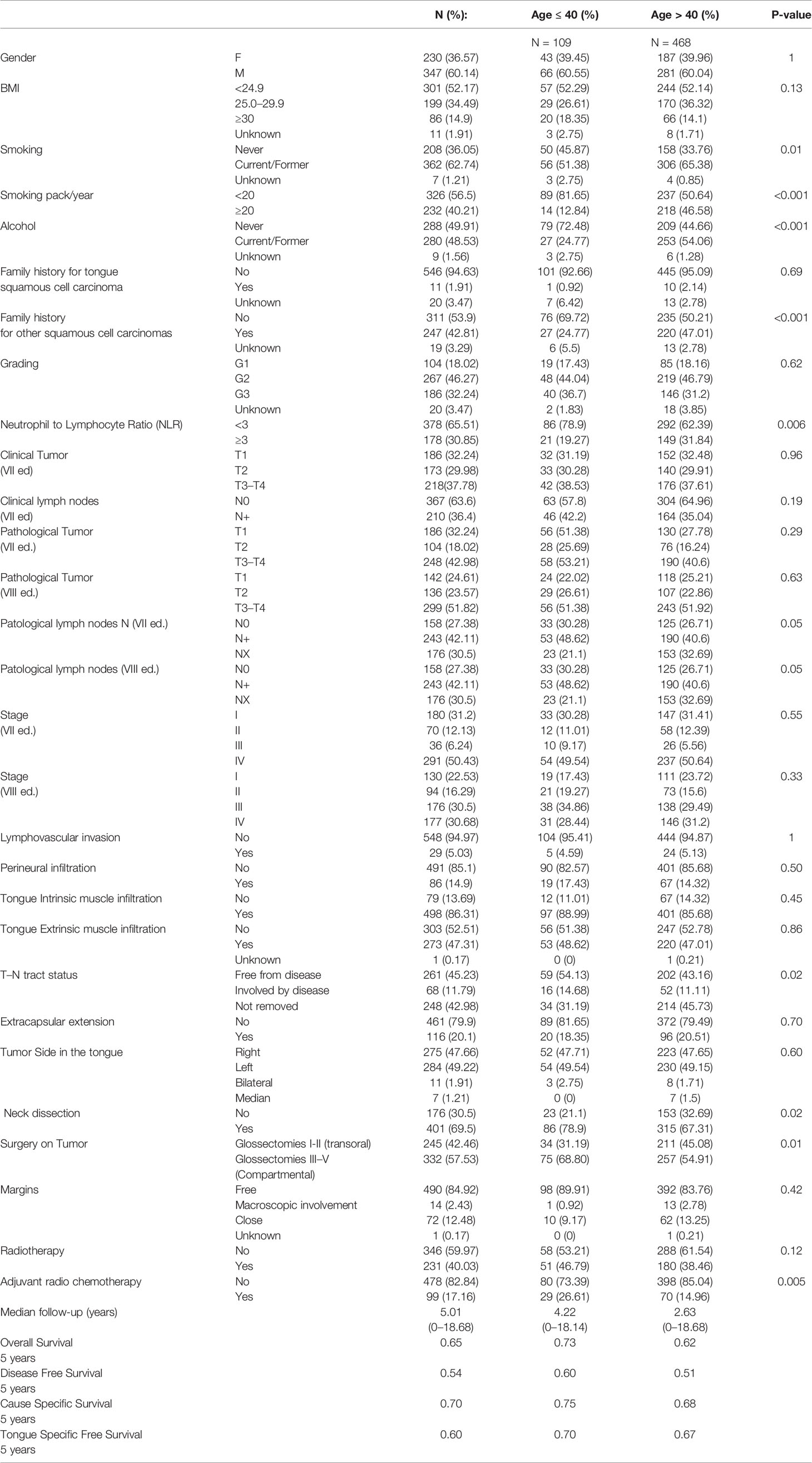
Clinical-pathological and tumor characteristics of the study population are reported in Table 1. Among the young group (n = 109), the median age was 32 (range 27–37), while the median age was 61 (range 50–71) in the elderly group (n = 468).
We did not find any significant difference between the two groups (young and elderly) in terms of sex, BMI, family history for oral tongue tumor, tumor stage (I–IV, 7th and 8th TNM editions), pT (7th and 8th TNM editions), cT and cN according the 7th TNM edition, the status of post-surgery margins, and radiotherapy (RT) as adjuvant treatment.
Among the elderly patients, we found a significantly higher number of current/former smokers (65.38 vs 51.38% for older vs younger respectively, p = 0.01) as well as patients with family history for other family members with SCC (47.01 vs 24.77% for older vs younger respectively, p = 0.001). Moreover, comparing the two groups, the youngest population was significantly more treated with type III–V glossectomies (54.9 vs 68.8% for older vs younger respectively; p =0.01) and with neck dissections (67.31 vs 78.9% for older vs younger, respectively; p = 0.02).
In the two age groups considered, the preoperative NLR ratio was highly significant for the population over >40 years old with a value equal or greater than 3 (p = 0.006).
Also, for the pathological state of the lymph nodes (pN 7th and 8th editions) and of the T–N tract status (free from disease, involved by disease, not removed), we find a difference between the two populations under examination (p = 0.05 and p = 0.02, respectively). Moreover, adjuvant radio-chemotherapy was administered more in young patients than in elderly (14.96 vs 26.61% for older vs younger respectively; p = 0.005).
In our sample, the proportion of young diagnoses (≤40 years old) does not seem to be significantly increased over the years: 55% were ≤40 years old between 2000 and 2010, while they were 45% between 2010 and 2020 (p = 0.63).
Furthermore, we also found that the proportion of female cancers was not different from the past: 53% female patients were found between 2000 and 2010, while they totaled 47% between 2010 and 2018 (p = 1).
Events During Follow-Up
The median follow-up time was 5.01 years (range 0–18.68 years) with follow-up recorded up to February 2020.
Overall, we observed 149 (25.8%) locoregional recurrences, 38 (6.5%) distant metastases, and 15 (2.6%) locoregional with synchronous distant metastases as the first site of relapse.
Two hundred and forty-six patients (42.6%) died: 66% died from primary tumor (locoregional-distant recurrence), 10% died from a second different tumor, 12% from no cancer related causes, and 12% died from unknown causes.
Forty-six patients had a second primary tumor, of whom two were ≤40 years old.
Regarding OS, elderly 5-year survival was 62% compared to 73% among younger patients, while elderly 10-year survival was 51%, and 70% in younger patients (log rank test p = 0.0006, Figure 1).
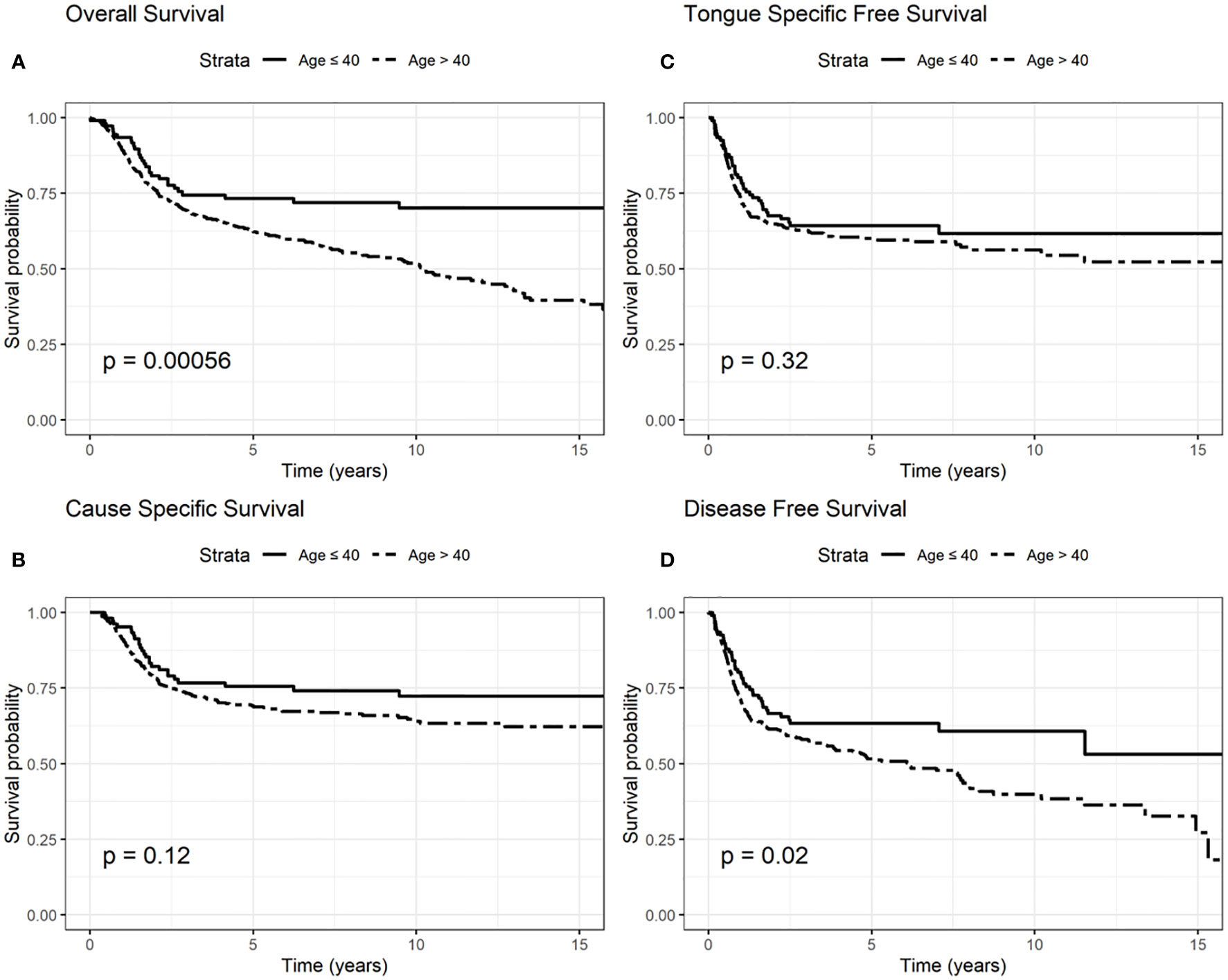
Figure 1 Survival probability: overall survival (OS) (A), cause-specific survival (CSS) (B), tongue specific free survival (TSFS) (C), disease-free survival (DFS) (D) according to age.
CSS 5-year survival was 68 and 75% in elderly and younger patients, respectively; CSS 10-year survival was 63 and 72% among elderly and younger patients (log rank test p = 0.12, Figure 1).
TSFS at 5-year was 67% for elderly patients and 70% for younger patients; 10-year TSFS was 40% in case of the elderly compared to 50% in younger patients (log-rank test p = 0.32, Figure 1).
DFS at 5-year was 51% in elderly than 60% in younger patients; DFS at 10-year was 38 and 53% in elderly and younger patients, respectively (log-rank test p = 0.02, Figure 1).
We presented OS, CSS, TSFS, and DFS curves by age, T–N tract involvement and stage 8th edition (Figures 1–3). Data on OS, CSS, TSFS, and DFS on smoking pack/year (p/y) with 20 p/y as cutoff point, type of intervention (III–V vs I, II glossectomies) and stage 8th edition by univariate analysis in association with age (≤/>40) were not significant (data not shown).
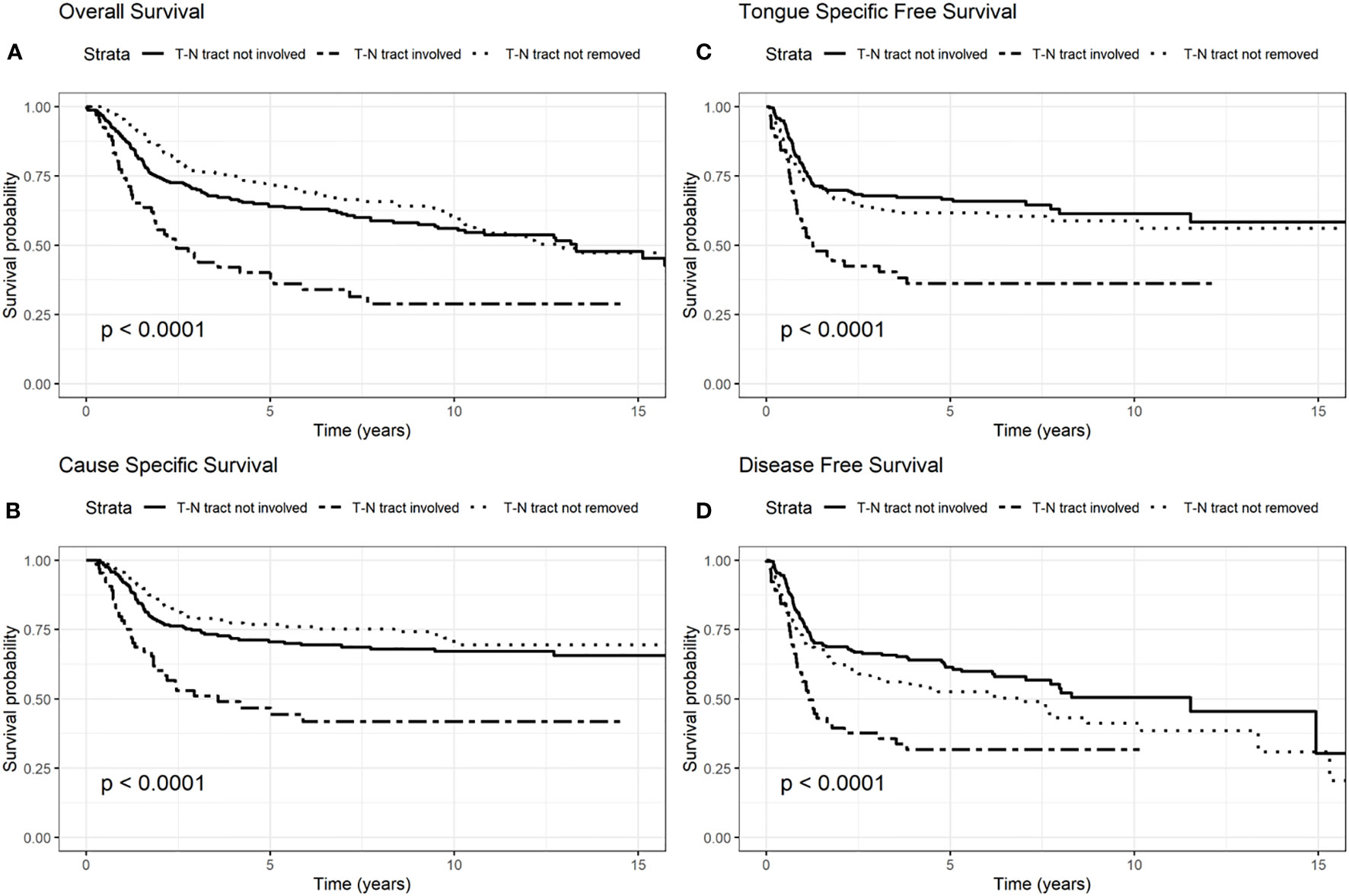
Figure 2 Survival probability: overall survival (OS) (A), cause-specific survival (CSS) (B), tongue specific free survival (TSFS) (C), disease-free survival (DFS) (D) according to T–N tract status.
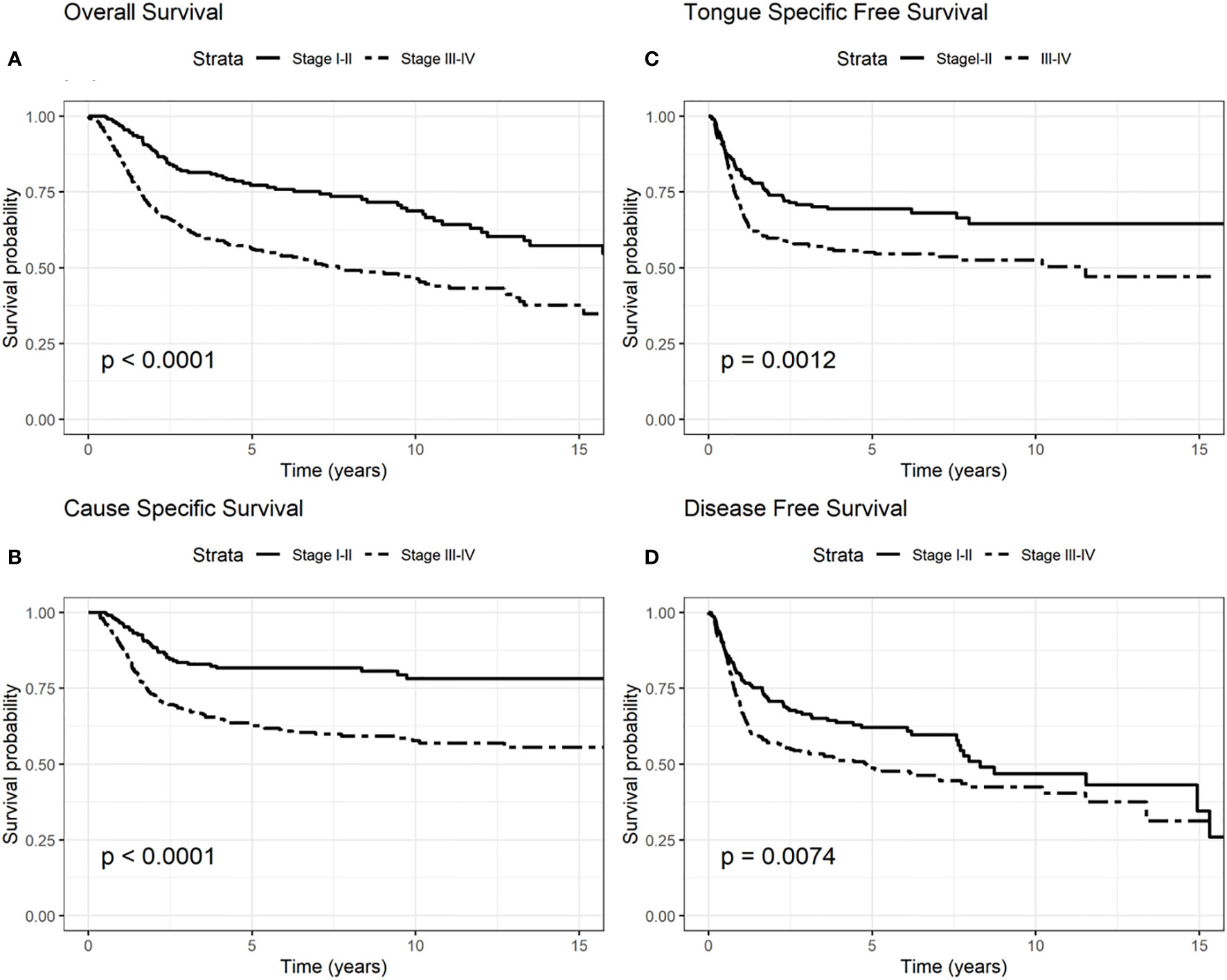
Figure 3 Survival probability: overall survival (OS) (A), cause-specific survival (CSS) (B), tongue specific free survival (TSFS) (C), disease-free survival (DFS) (D) according to stages I–II and III–IV according to the 8th edition.
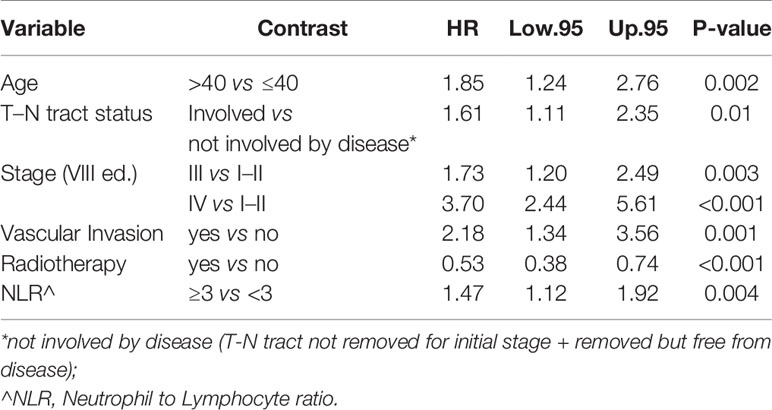
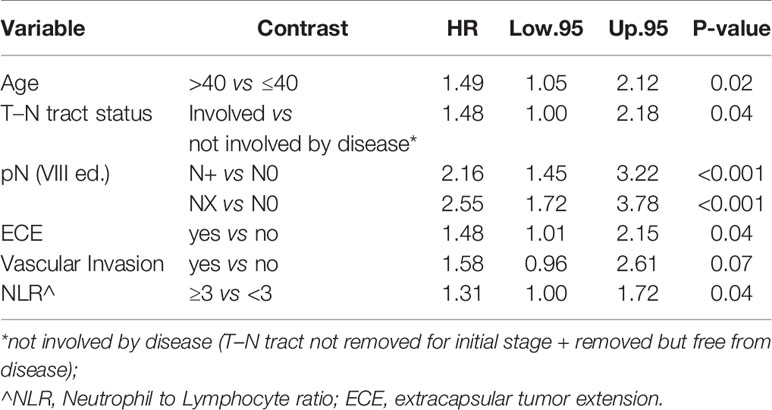
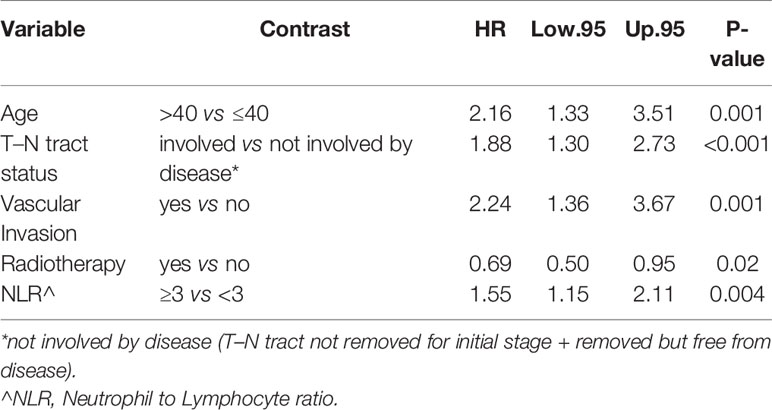
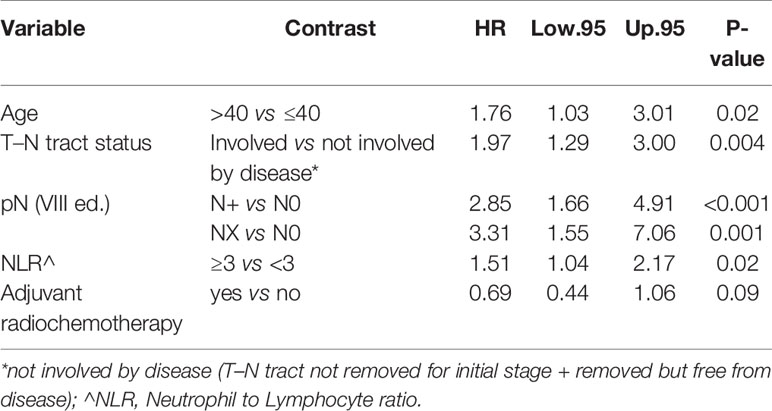
In particular, we found that the elderly group was associated with worse overall survival (log-rank test p < 0.001) (Figure 1). These results were confirmed by the multivariate analysis, after adjusting for all significant prognostic factors, revealing an almost double risk of death in elderly patients (HR = 1.85 95%, CI: 1.24–2.76; p = 0.002) (Table 2). Age was still significantly associated with DFS, as a categorical variable, both in univariate (log-rank test p = 0.02) and multivariate Cox models with a 49% increased risk of relapse in elderly patients (HR = 1.49 95%, CI: 1.05–2.12; p = 0.02) (Figure 1, Table 3).
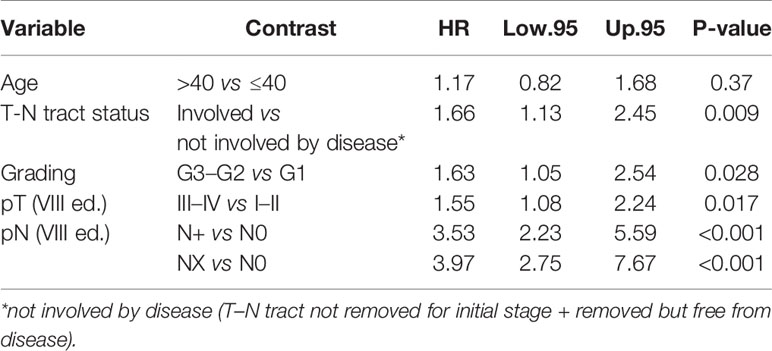
Conversely, age was not significantly associated with CSS and TSFS (log-rank test p = 0.14 and p = 0.37 respectively) (Tables 4, 5).
The difference between heavy (<20 p/y) and non-heavy smokers (≥20 p/y) was found to be significantly associated with OS but only in the univariate analysis (log-rank p = 0.05).
The involvement of the T–N tract was found to be significantly associated with all the evaluated clinical outcomes (OS, CSS, DFS, TSFS with p < 0.001) (Figure 2, Tables 2–5). Similarly, patients with advanced stage IV appeared to have a worse prognosis (p < 0.001 for OS, CSS, and DFS) (Figure 3).
The multivariable Cox model for OS showed that age remained independently associated with death (p = 0.002), adjusting for T–N tract, stage, vascular invasion together with adjuvant RT and NLR (Table 2).
Age was independently associated with DFS (p = 0.02), adjusting for other significant prognostic factors such as the T–N tract involvement, pN, extra capsular tumor spread (ECE), vascular invasion, and NLR (Table 3).
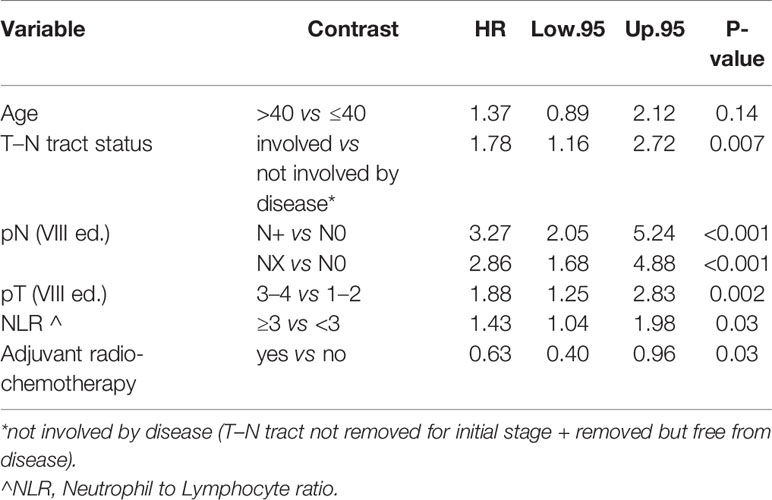
On the other hand age was not found to be associated with CSS (p = 0.14) adjusting for T–N tract status, pN, pT NLR, and adjuvant radio-chemotherapy. The latter factors were found to be significantly associated with dying of tongue cancer (Table 4).
Finally, only T–N tract status, grading, pT and pN were independent prognostic factors associated with TSFS (Table 5).
In all the multivariate models in which the variable pN was found to be significant, we see how patients with pN+ and Nx always had a poorer prognosis compared to pN0 patients.
We also conducted stratified analyses to evaluate whether the association of age with recurrence (DFS and TSFS) and survival (OS and CSS) was different by stage (8th edition) and treatment.
In the early stages (I–II), age was not found to be significantly associated with death (OS: HR = 1.56 95%, CI: 0.77–3.16, p = 0.21; CSS HR = 0.80 95% CI: 0.38–1.70, p = 0.57).
On the other hand, in multivariate analysis, for the advanced stages (III–IV, 8th edition), age was found to be significantly associated with OS and CSS models, revealing a worse outcome for patients diagnosed at age >40 years (OS: HR = 2.16 95%, CI: 1.33–3.51, p= 0.001; CSS HR = 1.76 95%, CI: 1.03–3.01, p = 0.02) (Tables 6, 7).
Furthermore, we investigated the relationship between age, stage, and performed treatment (glossectomy types I–II vs IV–V): we found that for advanced stages (III–IV 8th edition) there were no statistically significant different distributions of types of glossectomies between the two age groups considered (p = 0.07); instead in the initial stages (I–II 8th edition), glossectomies III–V were significantly more frequent at age ≤40 (30%) than at age >40 (14%), (p = 0.02) (Table S1).
Then, we analyzed the role of age in multivariate analysis for patients treated with the same surgery stratified by stage.
In the initial stages I–II according to TNM 8th edition for OS and CSS, in glossectomies types I–II we did not find any significant prognostic factor. In contrast, for type III–V glossectomies and early stages low number of events did not allow to estimate independent prognostic factors (data not shown).
For TSFS in type I–II glossectomies and stages I–II, the lymph nodal status (pN+ and pNx) remained significant (p < 0.001) (Table S2). Low number of events in early stage treated with type III–V glossectomies did not allow to identify independent prognostic factors associated with TSFS (data not shown).
Focusing on the DFS for early stages, in glossectomies types I–II we found that lymph node status was significantly associated with relapse: worse DFS was found in patients with laterocervical disease (p = 0.001) (Table S3). In early stage type III–V glossectomies, the NLR ratio remained significant (p = 0.03) (Table S4).
Concerning the advanced stages, for OS we had significance for the NLR ratio (p = 0.006) in type I–II glossectomies (data not shown). In type III–V glossectomies, age remained significant with the T–N tract, vascular invasion, post-operative radiotherapy treatment and NLR ratio (p = 0.001, p = 0.003, p = 0.006, p = 0.007and p = 0.04 respectively) (Table S5).
We did not find significant elements in CSS for type I–II glossectomies in advanced stages. In type III–V glossectomies for advanced stages CSS, we confirmed the variables: age, T–N tract, lymph node status and adjuvant radio-chemotherapy as independent risks factors for worse prognosis (p = 0.01, p = 0.01, p < 0.001, and p = 0.06, respectively) (Table S6).
In the TSFS model for advanced stages, in type I–II glossectomies, only the presence of pNx remained associated with prognosis (p = 0.05), while in type III–V glossectomies we found that the T–N tract and pN+ status were significantly associated with worse TSFS (p = 0.005 and < 0.001 respectively) (data not shown).
In DFS of advanced stages, NLR ratio was found to be significantly associated with relapse for type I–II glossectomies (p = 0.05) (data not shown); while for type III–V glossectomies age, T–N tract status, lymph node status, ECE and vascular invasion remained significantly associated with relapse (p = 0.04, p = 0.07, p = 0.001, p = 0.07 and p = 0.04 respectively) (Table S7).
Regarding the female sub-group, age appears to be significantly directly associated with prognosis also in multivariate analysis: patients older than 40 years have almost double increased risk of dying (OS) compared to younger groups (OS: HR = 2.02 95%, CI: 1.12–3.84, p = 0.01) (Table S8). In the male sub-group results were similar.
We also assessed several sensitivity analyses considering age as continuous variable and considering as cut-off point of age 45 and conclusion did not change (data not shown).
Discussion
Our analyses confirmed better survival outcomes in young patients than in elderly patients.
We choose 40 as the cutoff age to distinguish young from older people in line with the study by Oliver et al. and others (1, 52, 53); moreover it is reported that the role of risk factors, such as smoke and alcohol, seems to be significant after the age of 40 (54).
In the multivariate Cox analysis for OS and CSS, the variable “age” remained highly significant for the OS model: young people were characterized by a better OS regardless of tumor stage, while CSS did not seem to be significantly correlated with age. However, CSS was related to pT, pN, T–N tract status, NLR, and adjuvant radio-chemotherapy.
Analyzing the advanced stage sub-group (stages III–IV), age seemed to be significant in OS and in CSS models with a double risk of dying and dying for tongue cancer in elderly compared to young people.
Focusing on the role of age in multivariate analysis for patients treated with the same surgery stratified by stage, “age” did not seem to play a role for glossectomies types I–II and stages I–II.
In advanced-stage and glossectomies type III–IV patients at age ≥40 showed to have approximately double risk of death compared to younger patients (CSS) and 50% increased risk of relapse.
Many published studies have shown no significant difference in prognosis between young and elderly patients (31, 34, 35, 63–65). However, Goldenberg D et al. affirmed that a better prognosis characterized young patients than older ones (29).
In a matched-pair analysis, Farquhar et al. described a greater recurrence incidence in young people <45 years old, but no differences in overall mortality in the two groups (30).
In 2019 Oliver at al. published a study with a high number of cases (under 40 years old) and revealed that young patients did not have a worse survival than elderly, as previously found in smaller cohorts: controlling for all confounding factors, patients under 40 had a significantly 9% higher 5‐year survival (77.1 vs 68.2%). In this work, Oliver et al. underline that age alone could not be a factor for treatment intensification beyond the standard of care (1).
Over the years, other authors have demonstrated that young patients have a worse prognosis and suggested that more aggressive approaches could improve locoregional control and OS (8, 10, 66, 67).
Conversely, Oliver et al. reported in a considerable sample that: “the intensification of treatment could be a source of significant increases in morbidity and cost of treatment, without any proven benefits at this time” (1).
Actually, also in our sample, young patients underwent more aggressive treatments as shown by a significant number of glossectomies types II–V (compartmental surgery), neck dissection, and adjuvant therapies.
Studying our data by type of surgery, the difference in surgical treatment seemed to be statistically evident in the initial stages (I–II) where young patients were treated more with aggressive surgery, but these differences did not remain significant in multivariate analysis. In this regard, as reported by Oliver et al., it will be interesting to further investigate whether the intensification of therapy, generally reserved for young patients, really leads to better disease control in young people than in the elderly (1).
Focusing on adjuvant therapy, regardless of age, multivariate analysis showed that radiotherapy remained significantly associated with OS and radio-chemotherapy with CSS; among surgical treatments were not significant, taking into account other factors as stage, pT, pN, and LNR.
Another aspect highlighted in our results was the role of the T–N tract. The T–N tract is the soft tissue between the primary tumor (T) and the neck lymph nodes (N) and it is composed by the sublingual and submandibular glands, mylohyoid muscle, lingual nerve, artery, and vein and all the stromal tissue, and lingual and sublingual lymph nodes of the compartment. This study confirmed that, independently of age, and other factors analyzed in multivariate analysis, the involvement of T–N tract was significant in all studied survival models (OS, DFS, TSFS, and CSS). Consequently, the status of the T–N tract played an important role in prognosis for patients with OTSCC regardless of age. In fact, patients with the T–N tract involved by disease had a 60% increased risk of dying (OS) and a 78% higher risk of dying from tongue cancer (CSS). These data got worse in advanced stages III–IV where OS and CSS worsen almost twice as much as in those who do not have the disease in the T-N tract.
The presence of cervical metastases at diagnosis and the T–N tract status were confirmed as important prognostic factors in all the survival models stratified by stage.
Focusing on lymph nodal status, patients with pN+ and pNx always showed a worse oncological outcome in multivariate analysis. The presence of neck metastases is directly related to the oncological stage and, consequently, to the prognosis.
Special consideration should be made for patients with pNx. In case of pNx, many published works showed that these patients have a worse OS and a higher frequency of local recurrence, especially compared to pN0 patients. In fact, pNx patients generally did not undergo the neck dissection because of clinical condition or because the “wait and see” protocol was applied. In this way, as reported by the literature, about 30% of these patients remained with undiagnosed neck micro-metastases and then a worse prognosis for local relapses (68–71). Our data confirm this evidence.
Regardless of treatment modality, the role of smoking is still debated among risk factors for the young. The prognosis of young patients with OTSCC is still undefined, and there exists a lack of clear definition of young and old patients in the published literature. Analyzing the OTSCC literature, as already reported, the concept of “young age” has been considered in varying ways: from below 30 going up to 45 years old. However, the majority of studies considered 40 years old as the major age below which patients were defined young (21, 22, 35, 52, 60, 61, 72–76). There is an agreement that in patients below 40 years old, there is too short smoking exposure to develop carcinogenic activity. Thus, chronic mucosal trauma, genetic and/or hormonal features could cause oral tongue cancer, but to date, no certain data have been proven (11, 40, 54, 77).
In our data smoking and alcohol did not influence the prognosis of the two groups in multivariate analysis.
In our sample the proportion of female cancers was not different from the past. On the contrary, in 2011 Patel et al. reported that OTSCC was increasing among young white individuals with age 18 to 44 years, particularly among white women (9). A recent study on Asiatic patients with tongue cancer described an increasing incidence particularly in young females. Younger females with tongue SCC had no significant history of smoking (78, 79). Regarding the prognosis of our study cohort, females older than 40 years have almost double increased risk of dying (OS) compared to younger.
Moreover, our study highlighted the important role of the NLR as independent prognostic factor. As well reported not only in head and neck cancers, a tumor-induced change of the immune system toward a pre-tumoral pattern and worse prognosis are related with high values of the NLR ratio (80). In our sample, an NLR value greater than three was associated with a worse prognosis in the curves for OS, CSS, and DFS, and the pejorative role was confirmed in the OS and CSS for advanced stage in patients treated with both type I–II and III–V glossectomies. These data also showed how the role of the immune system, in addition to the age and stage of the disease, could be a determining factor in cancer aggressiveness. However, further studies are needed to better understand it.
In this study, we presented survival data specifically related to tongue cancer highlighting how young patients died less specifically of tongue cancer (CSS and TSFS).
In the advanced stages, young age and NLR smaller than three were correlated with a better prognosis in terms of OS and CSS.
As reported by the literature, the elderly group shows worse outcomes, and this fact could be related to the associated comorbidities of older people regarding OS (6, 33). Instead, for CSS we may hypothesize a role of the immune system. Some studies attested an increase of LNR in the elderly: these data could favor tumor aggression with a worsening of the specific cancer outcomes (81).
Moreover, the independent and pejorative role of the pNx was well defined in multivariate analysis for CSS, DFS and TSFS. In our sample the pNx was mostly referred to the oldest group which generally had the less invasive surgery and to the application of the “wait and see” protocol with “personalized” surgical approaches also based on their health status.
Nevertheless, this work has several limitations, such as the limited number of young patients, the monocentric and retrospective nature of the data.
Despite this, to the best of our knowledge, this is one of the largest monocentric cohort studies, the first work to describe patients who underwent standard and replicable surgical treatments over a period of years, reporting comprehensive data of known risk factors, with a long and complete period of follow-up and in which the prognostic role of age, T–N tract, and NLR is clearly demonstrated.
Conclusion
The peer-reviewed biomedical literature has shown that the role played by age in OTSCC prognosis is a matter of controversy. Our study revealed that young patients had a better prognosis and survived longer than elderly patients. Moreover, young people showed a slightly better recurrence-free survival, and they died less from tongue cancer than older patients, even in advanced tumor stages. In our sample, young patients seem more likely to be treated with intensified mode. Future studies, prospective and multicentric, will be needed to investigate the role of treatment intensification in young patients with OTSCC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the European Institute of Oncology cometee (cod. IEO 225). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
MA and MT conceptualized and drafted the study. RBe carried out literature review and revised the manuscript. SZ and LB revised the manuscript. RBr, GG, MC, FM, SC, and DA critically reviewed the manuscript for important intellectual content. DS, FS, and CP collected patients’ data. SG and FC realized statistical analysis. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by the Italian Ministry of Health with Ricerca Corrente and 5x1000 funds.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank William Russell-Edu for assistance with the English text.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.616653/full#supplementary-material
References
1. Oliver JR, Wu SP, Chang CM, Roden DF, Wang B, Hu KS, et al. Survival of oral tongue squamous cell carcinoma in young adults. Head Neck (2019) 41(9):2960–8. doi: 10.1002/hed.25772
2. Paderno A, Morello R, Piazza C. Tongue carcinoma in young adults: a review of the literature. Acta Otorhinolaryngol Ital (2018) 38(3):175–80. doi: 10.14639/0392-100X-1932
3. Choi G, Song JS, Choi SH, Nam SY, Kim SY, Roh JL, et al. Comparison of Squamous Cell Carcinoma of the Tongue between Young and Old Patients. J Pathol Transl Med (2019) 53(6):369–77. doi: 10.4132/jptm.2019.09.16
4. Oliveira LL, Bergmann A, Melo AC, Thuler LC. Prognostic factors associated with overall survival in patients with oral cavity squamous cell carcinoma. Med Oral Patol Oral Cir Bucal (2020) 25(4):e523–31. doi: 10.4317/medoral.23558
5. Tota JE, Anderson WF, Coffey C, Califano J, Cozen W, Ferris RL, et al. Rising incidence of oral tongue cancer among white men and women in the United States, 1973-2012. Oral Oncol (2017) 67:146–52. doi: 10.1016/j.oraloncology.2017.02.019
6. Xu Q, Wang C, Li B, Kim K, Li J, Mao M, et al. The impact of age on oral squamous cell carcinoma: A longitudinal cohort study of 2,782 patients. Oral Dis (2019) Apr25(3):730–41. doi: 10.1111/odi.13015
7. Ng JH, Iyer NG, Tan MH, Edgren G. Changing epidemiology of oral squamous cell carcinoma of the tongue: A global study. Head Neck (2017) 39(2):297–304. doi: 10.1002/hed.24589
8. Annertz K, Anderson H, Biörklund A, Möller T, Kantola S, Mork J, et al. Incidence and survival of squamous cell carcinoma of the tongue in Scandinavia, with special reference to young adults. Int J Cancer (2002) 101(1):95–9. doi: 10.1002/ijc.10577
9. Patel SC, Carpenter WR, Tyree S, Couch ME, Weissler M, Hackman T, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol (2011) 29(11):1488–94. doi: 10.1200/JCO.2010.31.7883
10. Sarkaria JN, Harari PM. Oral tongue cancer in young adults less than 40 years of age: rationale for aggressive therapy. Head Neck (1994) 16(2):107–11. doi: 10.1002/hed.2880160202
11. Singhvi HR, Malik A, Chaturvedi P. The Role of Chronic Mucosal Trauma in Oral Cancer: A Review of Literature. Indian J Med Paediatr Oncol (2017) 38(1):44–50. doi: 10.4103/0971-5851.203510
12. Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer (2007) 110(7):1429–35. doi: 10.1002/cncr.22963
13. Sankaranarayanan R, Masuyer E, Swaminathan R, Ferlay J, Whelan S. Head and neck cancer: a global perspective on epidemiology and prognosis. Anticancer Res (1998) 18(6B):4779–86.
14. Harris SL, Kimple RJ, Hayes DN, Couch ME, Rosenman JG. Never-smokers, never-drinkers: unique clinical subgroup of young patients with head and neck squamous cell cancers. Head Neck (2010) 32(4):499–503. doi: 10.1002/hed.21220
15. Hussein AA, Helder MN, de Visscher JG, Leemans CR, Braakhuis BJ, de Vet HCW, et al. Global incidence of oral and oropharynx cancer in patients younger than 45 years versus older patients: A systematic review. Eur J Cancer (2017) 82:115–27. doi: 10.1016/j.ejca.2017.05.026
16. Chinn SB, Myers JN. Oral Cavity Carcinoma: Current Management, Controversies, and Future Directions. J Clin Oncol (2015) 33(29):3269–76. doi: 10.1200/JCO.2015.61.2929
17. National Comprehensive Cancer Network guidelines. Available at: http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (Accessed October, 2020).
18. Calabrese L, Bruschini R, Giugliano G, Ostuni A, Maffini F, Massaro MA, et al. Compartmental tongue surgery: Long term oncologic results in the treatment of tongue cancer. Oral Oncol (2011) 47(3):174–9. doi: 10.1016/j.oraloncology.2010.12.006
19. Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med (2004) 350(19):1937–44. doi: 10.1056/NEJMoa032646
20. Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med (2004) 350(19):1945–52. doi: 10.1056/NEJMoa032641
21. Piazza C, Grammatica A, Montalto N, Paderno A, Del Bon F, Nicolai P. Compartmental surgery for oral tongue and floor of the mouth cancer: Oncologic outcomes. Head Neck (2019) 41(1):110–5. doi: 10.1002/hed.25480
22. Kies MS, Boatright DH, Li G, Blumenschein G, El-Naggar AK, Brandon Gunn G, et al. Phase II trial of induction chemotherapy followed by surgery for squamous cell carcinoma of the oral tongue in young adults. Head Neck (2012) 34(9):1255–62. doi: 10.1002/hed.21906
23. Gore SM, Crombie AK, Batstone MD, Clark JR. Concurrent chemoradiotherapy compared with surgery and adjuvant radiotherapy for oral cavity squamous cell carcinoma. Head Neck (2015) 37(4):518–23. doi: 10.1002/hed.23626
24. Zhong LP, Zhang CP, Ren GX, Guo W, William WN Jr, Sun J, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol (2013) 31(6):744–51. doi: 10.1200/JCO.2012.43.8820
25. Byers RM. Squamous cell carcinoma of the oral tongue in patients less than thirty years of age. Am J Surg (1975) 130(4):475–8. doi: 10.1016/0002-9610(75)90487-0
26. Brägelmann J, Dagogo-Jack I, El Dinali M, Stricker T, Brown CD, Zuo Z, et al. Oral cavity tumors in younger patients show a poor prognosis and do not contain viral RNA. Oral Oncol (2013) 49(6):525–33. doi: 10.1016/j.oraloncology.2013.02.003
27. Son YH, Kapp DS. Oral cavity and oropharyngeal cancer in a younger population. Review of literature and experience at Yale. Cancer (1985) 55(2):441–4. doi: 10.1002/1097-0142(19850115)55:2<441::AID-CNCR2820550225>3.0.CO;2-5
28. Goepfert RP, Kezirian EJ, Wang SJ. Oral tongue squamous cell carcinoma in young women: a matched comparison-do outcomes justify treatment intensity? ISRN Otolaryngol (2014) 2014:529395. doi: 10.1155/2014/529395
29. Goldenberg D, Brooksby C, Hollenbeak CS. Age as a determinant of outcomes for patients with oral cancer. Oral Oncol (2009) 45(8):e57–61. doi: 10.1016/j.oraloncology.2009.01.011
30. Farquhar DR, Tanner AM, Masood MM, Patel SR, Hackman TG, Olshan AF, et al. Oral tongue carcinoma among young patients: An analysis of risk factors and survival. Oral Oncol (2018) 84:7–11. doi: 10.1016/j.oraloncology.2018.06.014
31. Atula S, Grénman R, Laippala P, Syrjänen S. Cancer of the tongue in patients younger than 40 years. A distinct entity? Arch Otolaryngol Head Neck Surg (1996) 122(12):1313–9. doi: 10.1001/archotol.1996.01890240021006
32. Myers JN, Elkins T, Roberts D, Byers RM. Squamous cell carcinoma of the tongue in young adults: increasing incidence and factors that predict treatment outcomes. Otolaryngol Head Neck Surg (2000) 122(1):44–51. doi: 10.1016/S0194-5998(00)70142-2
33. Mukdad L, Heineman TE, Alonso J, Badran KW, Kuan EC, St John MA. Oral tongue squamous cell carcinoma survival as stratified by age and sex: A surveillance, epidemiology, and end results analysis. Laryngoscope (2019) 129(9):2076–81. doi: 10.1002/lary.27720
34. Verschuur HP, Irish JC, O’Sullivan B, Goh C, Gullane PJ, Pintilie M. A matched control study of treatment outcome in young patients with squamous cell carcinoma of the head and neck. Laryngoscope (1999) 109(2 Pt 1):249–58. doi: 10.1097/00005537-199902000-00015
35. Friedlander PL, Schantz SP, Shaha AR, Yu G, Shah JP. Squamous cell carcinoma of the tongue in young patients: a matched-pair analysis. Head Neck (1998) 20(5):363–8. doi: 10.1002/(SICI)1097-0347(199808)20:5<363::AID-HED1>3.0.CO;2-W
36. Barnabé L, Batista AC, Mendonça EF, Nonaka CFW, Alves PM. Cell cycle markers and apoptotic proteins in oral tongue squamous cell carcinoma in young and elderly patients. Braz Oral Res (2019) 36:1–7. doi: 10.1590/1807-3107bor-2019.vol33.0103
37. Dos Santos Costa SF, Brennan PA, Gomez RS, Fregnani ER, Santos-Silva AR, Martins MD, et al. Molecular basis of oral squamous cell carcinoma in young patients: Is it any different from older patients? J Oral Pathol Med (2018) 47(6):541–6. doi: 10.1111/jop.12642
38. O’Regan EM, Toner ME, Smyth PC, Finn SP, Timon C, Cahill S, et al. Distinct array comparative genomic hybridization profiles in oral squamous cell carcinoma occurring in young patients. Head Neck (2006) 28(4):330–8. doi: 10.1002/hed.20354
39. Benevenuto TG, Nonaka CF, Pinto LP, de Souza LB. Immunohistochemical comparative analysis of cell proliferation and angiogenic index in squamous cell carcinomas of the tongue between young and older patients. Appl Immunohistochem Mol Morphol (2012) 20(3):291–7. doi: 10.1097/PAI.0b013e31823277f6
40. Pickering CR, Zhang J, Neskey DM, Zhao M, Jasser SA, Wang J, et al. Squamous cell carcinoma of the oral tongue in young non-smokers is genomically similar to tumors in older smokers. Clin Cancer Res (2014) 20(14):3842–8. doi: 10.1158/1078-0432.CCR-14-0565
41. Santos-Silva AR, Ribeiro AC, Soubhia AM, Miyahara GI, Carlos R, Speight PM, et al. High incidences of DNA ploidy abnormalities in tongue squamous cell carcinoma of young patients: an international collaborative study. Histopathology (2011) 58(7):1127–35. doi: 10.1111/j.1365-2559.2011.03863.x
42. Amin MB, Edge S, Greene FL, Byrd DR, Brookland RK, Washington MK. AJCC Cancer Staging Manual. American Joint Commitee on Cancer (AJCC): Springer International Publishing (2017).
43. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed Vol. 2010. . New York, NY: Springer (2010).
44. Tagliabue M, Gandini S, Maffini F, Navach V, Bruschini R, Giugliano G, et al. The role of the T-N tract in advanced stage tongue cancer. Head Neck (2019) 41(8):2756–67. doi: 10.1002/hed.25761
45. Calabrese L, Giugliano G, Bruschini R, Ansarin M, Navach V, Grosso E, et al. Compartmental surgery in tongue tumours: description of a new surgical technique. Acta Otorhinolaryngol Ital (2009) 29(5):259–64.
46. Ansarin M, Bruschini R, Navach V, Giugliano G, Calabrese L, Chiesa F, et al. Classification of GLOSSECTOMIES: Proposal for tongue cancer resections. Head Neck (2019) 41(3):821–7. doi: 10.1002/hed.25466
47. Cammarata LM, Zagà V, Pistone G. The pack-year as expression of tobacco use in smokers’ lifetime. Tabaccologia (2014) 1(2):31–4.
48. Wu CN, Chuang HC, Lin YT, Fang FM, Li SH, Chien CY. Prognosis of neutrophil-to-lymphocyte ratio in clinical early-stage tongue (cT1/T2N0. cancer Onco Targets Ther (2017) 10:3917–24. doi: 10.2147/OTT.S140800
49. Zhang B, Du W, Gan K, Fang Q, Zhang X. Significance of the neutrophil-to-lymphocyte ratio in young patients with oral squamous cell carcinoma. Cancer Manag Res (2019) 11:7597–603. doi: 10.2147/CMAR.S211847
50. Abbate V, Dell’Aversana Orabona G, Salzano G, Bonavolontà P, Maglitto F, Romano A, et al. Pre-treatment Neutrophil-to-Lymphocyte Ratio as a predictor for occult cervical metastasis in early stage (T1-T2 cN0) squamous cell carcinoma of the oral tongue. Surg Oncol (2018) 27(3):503–7. doi: 10.1016/j.suronc.2018.06.002
51. Hasegawa T, Iga T, Takeda D, Amano R, Saito I, Kakei Y, et al. Neutrophil-lymphocyte ratio associated with poor prognosis in oral cancer: a retrospective study. BMC Cancer (2020) 20(1):568. doi: 10.1186/s12885-020-07063-1
52. Garavello W, Spreafico R, Gaini RM. Oral tongue cancer in young patients: a matched analysis. Oral Oncol (2007) 43(9):894–7. doi: 10.1016/j.oraloncology.2006.10.013
53. de Morais EF, Mafra RP, Gonzaga AKG, de Souza DLB, Pinto LP, da Silveira ÉD. Prognostic Factors of Oral Squamous Cell Carcinoma in Young Patients: A Systematic Review. J Oral Maxillofac Surg (2017) 75(7):1555–66. doi: 10.1016/j.joms.2016.12.017
54. Mohideen K, Krithika C, Jeddy N, Bharathi R, Thayumanavan B, Sankari SL. Meta-analysis on risk factors of squamous cell carcinoma of the tongue in young adults. J Oral Maxillofac Pathol (2019) 23(3):450–7. doi: 10.4103/jomfp.JOMFP_118_19
55. Priante AV, Castilho EC, Kowalski LP. Second primary tumors in patients with head and neck cancer. Curr Oncol Rep (2011) 13(2):132–7. doi: 10.1007/s11912-010-0147-7
56. Birgisson H, Wallin U, Holmberg L, Glimelius B. Survival endpoints in colorectal cancer and the effect of second primary other cancer on disease free survival. BMC Cancer (2011) 11:438–2407-11-438. doi: 10.1186/1471-2407-11-438
58. Schatzkin A, Freedman LS, Schiffman MH, Dawsey SM. Validation of intermediate end points in cancer research. J Natl Cancer Inst (1990) 82(22):1746–52. doi: 10.1093/jnci/82.22.1746
59. Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol (2007) 25(15):2127–32. doi: 10.1200/JCO.2006.10.3523
60. Veness MJ, Morgan GJ, Sathiyaseelan Y, Gebski V. Anterior tongue cancer: age is not a predictor of outcome and should not alter treatment. ANZ J Surg (2003) 73(11):899–904. doi: 10.1046/j.1445-2197.2003.02818.x
61. Hyam DM, Conway RC, Sathiyaseelan Y, Gebski V, Morgan GJ, Walker DM, et al. Tongue cancer: do patients younger than 40 do worse? Aust Dent J (2003) 48(1):50–4. doi: 10.1111/j.1834-7819.2003.tb00009.x
62. Fang QG, Shi S, Liu FY, Sun CF. Tongue squamous cell carcinoma as a possible distinct entity in patients under 40 years old. Oncol Lett (2014) 7(6):2099–102. doi: 10.3892/ol.2014.2054
63. Randall CJ, Shaw HJ. Malignant tumours of the tongue in young adults. Experience of a secondary referral centre. J Laryngol Otol (1986) 100(11):1295–8. doi: 10.1017/S0022215100101008
64. Lund VJ, Howard DJ. Head and neck cancer in the young: a prognostic conundrum? J Laryngol Otol (1990) 104(7):544–8. doi: 10.1017/S002221510011312X
65. Siegelmann-Danieli N, Hanlon A, Ridge JA, Padmore R, Fein DA, Langer CJ. Oral tongue cancer in patients less than 45 years old: institutional experience and comparison with older patients. J Clin Oncol (1998) 16(2):745–53. doi: 10.1200/JCO.1998.16.2.745
66. Depue RH. Rising mortality from cancer of the tongue in young white males. N Engl J Med (1986) 315(10):647. doi: 10.1056/NEJM198609043151013
67. Jeon JH, Kim MG, Park JY, Lee JH, Kim MJ, Myoung H, et al. Analysis of the outcome of young age tongue squamous cell carcinoma. Maxillofac Plast Reconstr Surg (2017) 39(1):41. doi: 10.1186/s40902-017-0139-8
68. Jiang Q, Tang A, Long S, Qi Q, Song C, Xin Y, et al. Development and validation of a nomogram to predict the risk of occult cervical lymph node metastases in cN0 squamous cell carcinoma of the tongue. Br J Oral Maxillofac Surg (2019) 57(10):1092–7. doi: 10.1016/j.bjoms.2019.09.024
69. D’Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, et al. Head and Neck Disease Management Group.Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. N Engl J Med (2015) 373(6):521–9. doi: 10.1056/NEJMoa1506007
70. Abu-Ghanem S, Yehuda M, Carmel NN, Leshno M, Abergel A, Gutfeld O, et al. Elective Neck Dissection vs Observation in Early-Stage Squamous Cell Carcinoma of the Oral Tongue With No Clinically Apparent Lymph Node Metastasis in the Neck: A Systematic Review and Meta-analysis. JAMA Otolaryngol Head Neck Surg (2016) 142(9):857–65. doi: 10.1001/jamaoto.2016.1281
71. Tsushima N, Sakashita T, Homma A, Hatakeyama H, Kano S, Mizumachi T, et al. The role of prophylactic neck dissection and tumor thickness evaluation for patients with cN0 tongue squamous cell carcinoma. Eur Arch Otorhinolaryngol (2016) 273(11):3987–92. doi: 10.1007/s00405-016-4077-3
72. Pitman KT, Johnson JT, Wagner RL, Myers EN. Cancer of the tongue in patients less than forty. Head Neck (2000) 22(3):297–302. doi: 10.1002/(SICI)1097-0347(200005)22:3<297::AID-HED14>3.0.CO;2-3
73. Vargas H, Pitman KT, Johnson JT, Galati LT. More aggressive behavior of squamous cell carcinoma of the anterior tongue in young women. Laryngoscope (2000) 110(10 Pt 1):1623–6. doi: 10.1097/00005537-200010000-00009
74. Liao CT, Wang HM, Hsieh LL, Chang JT, Ng SH, Hsueh C, et al. Higher distant failure in young age tongue cancer patients. Oral Oncol (2006) 42(7):718–25. doi: 10.1016/j.oraloncology.2005.11.012
75. Kabeya M, Furuta R, Kawabata K, Takahashi S, Ishikawa Y. Prevalence of human papillomavirus in mobile tongue cancer with particular reference to young patients. Cancer Sci (2012) 103(2):161–8. doi: 10.1111/j.1349-7006.2011.02149.x
76. Yang AK, Liu TR, Chen FJ, Ma XF, Guo ZM, Song M, et al. Survival analysis of 229 patients with advanced squamous cell carcinoma of the oral tongue. Ai Zheng (2008) 27(12):1315–20.
77. Gamez ME, Kraus R, Hinni ML, Moore EJ, Ma DJ, Ko SJ, et al. Treatment outcomes of squamous cell carcinoma of the oral cavity in young adults. Oral Oncol (2018) 87:43–8. doi: 10.1016/j.oraloncology.2018.10.014
78. Satgunaseelan L, Allanson BM, Asher R, Reddy R, Low HTH, Veness M, et al. The incidence of squamous cell carcinoma of the oral tongue is rising in young non-smoking women: An international multi-institutional analysis. Oral Oncol (2020) 110:104875. doi: 10.1016/j.oraloncology.2020.104875
79. Lin NC, Hsu JT, Tsai KY. Difference between Female and Male Patients with Oral Squamous Cell Carcinoma: A Single-Center Retrospective Study in Taiwan. Int J Environ Res Public Health (2020) 17(11):3978. doi: 10.3390/ijerph17113978
80. Mattavelli D, Lombardi D, Missale F, Calza S, Battocchio S, Paderno A, et al. Prognostic Nomograms in Oral Squamous Cell Carcinoma: The Negative Impact of Low Neutrophil to Lymphocyte Ratio. Front Oncol (2019) 9:339. doi: 10.3389/fonc.2019.00339
Keywords: tongue cancer, age, prognosis, head neck cancer, T-N tract, overall survival
Citation: Ansarin M, De Berardinis R, Corso F, Giugliano G, Bruschini R, De Benedetto L, Zorzi S, Maffini F, Sovardi F, Pigni C, Scaglione D, Alterio D, Cossu Rocca M, Chiocca S, Gandini S and Tagliabue M (2021) Survival Outcomes in Oral Tongue Cancer: A Mono-Institutional Experience Focusing on Age. Front. Oncol. 11:616653. doi: 10.3389/fonc.2021.616653
Received: 12 October 2020; Accepted: 11 January 2021;
Published: 12 April 2021.
Edited by:
Alberto Paderno, University of Brescia, ItalyReviewed by:
Giuseppe Mercante, Humanitas University, ItalyMarco Ferrari, University of Brescia, Italy
Copyright © 2021 Ansarin, De Berardinis, Corso, Giugliano, Bruschini, De Benedetto, Zorzi, Maffini, Sovardi, Pigni, Scaglione, Alterio, Cossu Rocca, Chiocca, Gandini and Tagliabue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marta Tagliabue, marta.tagliabue@ieo.it; Rita De Berardinis, rita.deberardinis@ieo.it
†ORCID: Marta Tagliabue, orcid.org/0000-0002-7879-4846
Rita De Berardinis, orcid.org/0000-0003-4959-587X
‡These authors share last authorship
 Mohssen Ansarin
Mohssen Ansarin Rita De Berardinis
Rita De Berardinis Federica Corso2,3,4
Federica Corso2,3,4 Gioacchino Giugliano
Gioacchino Giugliano Fausto Maffini
Fausto Maffini Daniela Alterio
Daniela Alterio Maria Cossu Rocca
Maria Cossu Rocca Susanna Chiocca
Susanna Chiocca Sara Gandini
Sara Gandini Marta Tagliabue
Marta Tagliabue