
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 18 March 2021
Sec. Hematologic Malignancies
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.614215
This article is part of the Research TopicAcute Promyelocytic Leukemia - Towards a Chemotherapy-Free Approach to Cure in All PatientsView all 11 articles
The indication of hematopoietic stem cell transplantation (HSCT) in acute promyelocytic leukemia (APL) has evolved historically from a widespread use in front-line therapy during the pre-ATRA era to a virtual rejection of this indication for patients treated with modern treatments. HSCT in first complete remission could only be considered for an extremely small fraction of patients with persistent MRD at the end of consolidation or for those who relapse. In the pre-ATO era, relapsed patients were usually treated with readministration of ATRA and chemotherapy as salvage therapy, generally containing high-dose cytarabine and an anthracycline, followed by further post-remission chemotherapy and/or HSCT. ATO-based regimens are presently regarded as the first option for relapsed APL. The selection of the most appropriate post-remission treatment option for patients in second CR (CR2), as well as the modality of HSCT when indicated, depends on several variables, such as pre-transplant molecular status, duration of first remission, age, and donor availability. Although with a moderate level of evidence, based on recent retrospective studies, autologous HSCT would be at present the preferred option for consolidation for patients in molecular CR2. Allogeneic HSCT could be considered in patients with a very early relapse or those beyond CR2. Nevertheless, the superiority of HSCT as consolidation over other alternatives without transplantation has recently been questioned in some studies, which justify a prospective controlled study to resolve this still controversial issue.
Modern treatment approaches for patients with newly diagnosed APL, using the combination of all-trans retinoic acid (ATRA) with either arsenic trioxide (ATO), chemotherapy or both, result in 90% to 95% complete remission (CR) rates with virtual absence of primary resistance, and 85% to 90% rates of long-term survival (1). Different to most subtypes of acute myeloid leukemia, these outstanding results in APL have been obtained without consolidating patients in first CR (CR1) with hematopoietic stem cell transplantation (HSCT). In this context, HSCT has virtually ceased to play any role in CR1 and relegated to consolidate patients in CR2 or beyond after salvage therapy for relapsed APL (1–3).
In this review article, we will discuss the clinical outcomes of HSCT in patients with APL and discuss the issues related to this indication, including the preferred choice of donor, source of hematopoietic stem cells, and conditioning regimen, among other controversial matter. We will also address the main prognostic factors for transplant outcomes that have been identified. We aim to provide insight into decision making regarding the optimal use of HSCT in patients with APL.
The indication of HSCT in patients with APL has evolved historically from a widespread use of this procedure in front-line therapy during the pre-ATRA era to a virtual rejection of this indication when patients are treated with modern treatments containing ATRA. Except for the beginning of the ATRA era, in which many groups still continued to indicate an HSCT in CR1 (4, 5), this has gradually been abandoned and explicitly rejected by the European LeukemiaNet (ELN) recommendations (1, 2) and the National Comprehensive Cancer Network (NCCN) guidelines (3).
Currently, the general consensus is that HSCT has no role patients in CR1, even in high-risk patients.
Only a few small retrospective and uncontrolled studies have compared the therapeutic efficacy of HSCT versus consolidation without transplantation in relapsed patients with APL (Table 1). Most of these studies showed that transplanted patients had a higher event-free survival (EFS), ranging from 61% to 83% for auto-HSCT (6, 7, 9) and from 41% to 71% for allo-HSCT (6, 9, 10), compared to those not transplanted, ranging from 30% to 45% (6, 7, 10). It should be noted, however, that these differences were statistically significant in only two of these studies (6, 7).
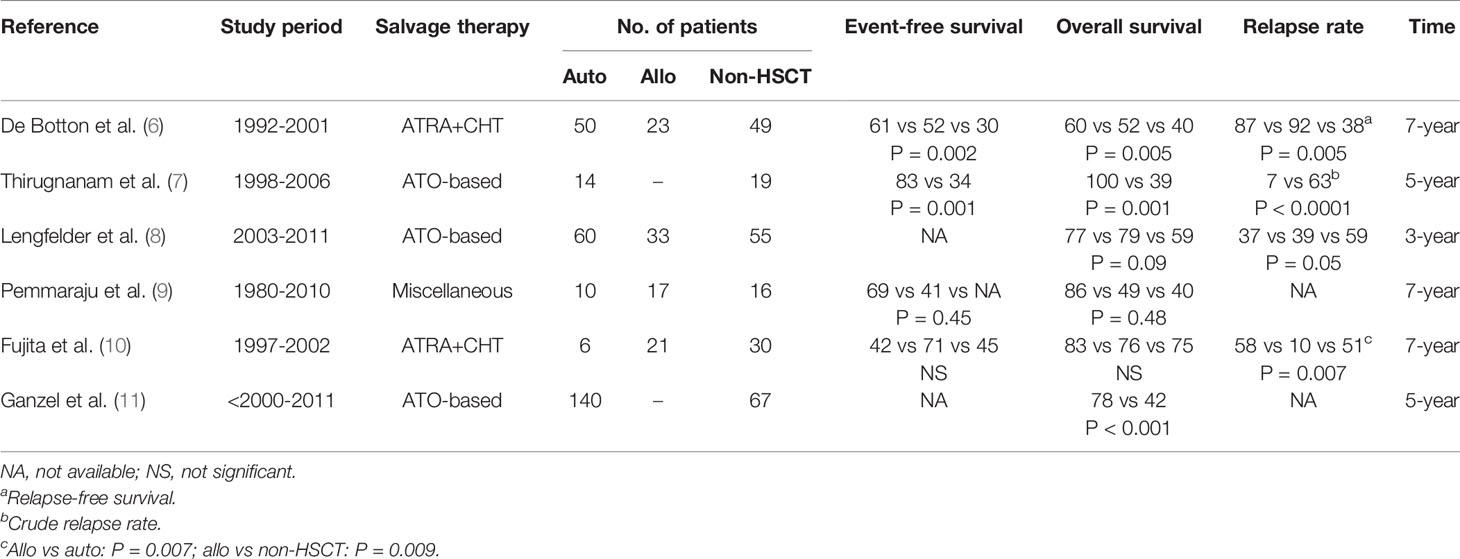
Table 1 Hematopoietic stem cell transplantation (autologous and/or allogeneic) compared with non-transplantation in relapsed APL.
The superiority of transplantation versus non-transplantation was even more apparent for overall survival (OS), which ranged from 60% to 100% for auto-HSCT (6–11) and 49% to 79% for allo-HSCT (6, 8–10), while it was 39% to 75% for those not transplanted (6–11). Except from two small studies (9, 10), the remaining ones showed a significantly higher OS (6–8, 11). Not without reason, it can be argued that differences in survival outcomes could be explained, at least in part, by a selection bias, since transplantation is ruled out in a sizable proportion of patients because they are considered clinically unfit. However, this explanation would not be enough, since practically all the studies have shown a higher relapse rate in patients undergoing consolidation treatment without transplantation (6, 7, 10), including a retrospective ELN study in relapsed APL treated with ATO-based regimens (8). It should be noted, however, that some groups have recently reported prolonged remissions in series of patients relapsing after ATRA plus chemotherapy treated with ATO plus ATRA without transplant.
Based on the previously mentioned studies, the ELN recommendations (1) and the NCCN guidelines (3) consider transplantation as the best option to consolidate patients in CR2 or beyond after salvage therapy for relapsed APL. This is, however, an unresolved issue, since some recent reports question the need for transplantation, at least in patients who achieve molecular remission with ATO and ATRA (1) or ATO, ATRA, mitoxantrone and bortezomib (12). Therefore, a prospective controlled study is warranted to address this still controversial issue.
Once it has been shown that consolidation with HSCT, either auto-HSCT or allo-HSCT, results in better survival outcomes than non-transplant strategies for patients in CR2 or beyond, the next question that arises is the appropriate type of transplant to be done. The choice of autologous or allogeneic transplantation should be based on the modality that has shown higher efficacy in this setting. Again a few small, retrospective and uncontrolled studies have compared the therapeutic efficacy of auto-HSCT and allo-HSCT in relapsed patients with APL (Table 2). None of these studies was able to demonstrate superiority of one modality of HSCT over the other in EFS, except from an analysis of the Acute Leukemia Working Party (ALWP) of the European Blood and Marrow Transplantation (EBMT) that has been recently published (16). In this large study that included 341 and 228 APL patients in CR2 who underwent auto-HSCT and allo-HSCT, respectively, EFS was significantly higher in the former group (75% vs 55%). Auto-HSCT was also superior to allo-HSCT in terms of OS, not only in this study, but also in other large studies (6, 15). The most likely explanations for finding a clearer benefit in OS than in EFS could be, on the one hand, because the potential benefit of a graft-versus-leukemia (GVL) effect in patients undergoing allo-HSCT would be widely counterbalanced by a higher transplant related mortality (TRM) systematically reported in that setting compared with patients undergoing auto-HSCT (5, 6, 9, 10, 13–15). In fact, no study was able to demonstrate a significant reduction in the relapse rate, while the two largest studies reported a significantly higher rate of TRM in the allo-HSCT (15). On the other hand, a second relapse after auto-HSCT is probably a clinical situation with a higher chance of subsequent salvage as compared to a second relapse after allo-HSCT. This would also explain that, in most series, the increase in the OS rate with respect to the EFS is significantly greater in patients undergoing auto-HSCT compared to those undergoing allo-HSCT (9, 10, 15).
In addition to comparative studies providing evidence that auto-HSCT is a better treatment strategy than allo-HSCT in relapsed APL (Table 2), other large case series have also demonstrated the outstanding efficacy and feasibility of that procedure after salvage therapy with ATO-based treatment (17), improving the outcomes of those with ATRA plus chemotherapy-based approaches (18).
As far as we know, there are no prospective and controlled studies comparing results between peripheral blood and bone marrow as a source of hematopoietic progenitors in the specific context of APL. However, it is a fact that peripheral blood has been by far the preferred stem cell source used in most studies analyzing auto-HSCT in this disease (Table 3). Except for the previous EBMT study conducted between 1993 and 2003 (5), in which the proportion of patients transplanted with peripheral blood was 53%, all the remaining comparative studies reported percentages in the narrow range between 86% and 100%, including the last EBMT study in which the proportion increased to 92% (16). It should be noted, however, that the few retrospective studies that compared bone marrow with peripheral blood showed that the stem cell source did not affect either of the outcomes (5, 21). In contrast, the most common stem cell source in the allo-HSCT setting was bone marrow (range, 64% to 87%), except for a couple of series that reported a lower proportion of 47% and 18% (9). Interestingly, most studies in patients with APL undergoing allo-HSCT include a very high proportion of those from matched sibling donor (MSD), sometimes up to 100%, which is significantly higher than would be due to the average availability of this type of donor (roughly 30%). In some studies, this reflects the eligibility criteria established for the study, which exclude transplants from alternative sources and donors, but in other studies it may reflect the attitude of many reluctant physicians to choose alternative donors when a MSD is not available. Probably also, the retrospective studies currently available in relapsed APL do not reflect the great advances and growing use of alternative donors that are taking place in the field of allo-HSCT. It is a fact that significant differences in survival in the past between MSD, matched unrelated mismatched related and unrelated donors are gradually being curtailed.
Regarding conditioning intensity, myeloablative conditioning regimens (MAC) were almost universally used for auto-HSCT, except a few patients who received reduced intensity regimen (RIC) (15). Although MAC was also the most common preparative regimen in allo-HSCT, RIC is increasingly used for older and medically unfit patients up to 32% (16). Unfortunately, data following RIC in APL are currently lacking.
As shown in Table 4, TBI-based regimens were preferred for auto-HSCT in the CIBMTR registry data (76%) (15) and in a single center study (10). In contrast, non-TBI-based regimens were preferred in most recent report of the EBMT registry (85%) (16), the Japan Adult Leukemia Study Group (100%) (17), and the Japanese Society for Hematopoietic Cell Transplantation (JSHCT) (96%) (21), as well as other single center studies (7, 13, 19). None of these studies with an appropriate sample size have addressed the comparison of results according to the conditioning regimen to draw significant conclusions.
In allo-HSCT, TBI and non-TBI-based regimens were equally distributed in the CIBMTR registry data (15) and the previous EBMT study (5). However, TBI-based regimens were preferred in in some studies (6, 10, 13) and non-TBI in others (9), including the most recent of the EBMT registry (16).
It is generally accepted that patients undergoing transplantation with minimal residual disease (MRD) positive have a worse prognosis than in MRD-negative status. The clinical situation in which APL patients do not achieve MRD-negative status can occur in two different scenarios, one in which after first-line consolidation treatment the patient does not achieve molecular remission (primary molecular resistance or molecular persistence) and another in which the patient does not achieve molecular remission after salvage consolidation therapy (secondary molecular resistance). For the extremely low fraction of patients in the former scenario, given their poor prognosis, unless they are promptly managed aggressively prior to the occurrence of a hematologic relapse (22), allo-HSCT has traditionally been considered as the first option when patients are suitable for transplantation. It should be noted that molecular persistence at this point was already uncommon in early studies (3%-4%) (23), but has almost disappeared (<1%) in patients receiving state-of-the art treatments with either ATRA plus ATO (24) or ATRA plus chemotherapy-based approaches (25). As far as we know, the only study whose objective was to analyze the outcomes in patients with molecular persistence at the end of first-line consolidations was reported in 2004 and with a very small sample size (22). Four patients undergoing allo-HSCT were alive in hematologic and molecular remission at 64, 92, 98 and 118 months, while three patients treated with chemotherapy followed by auto-HSCT were alive in hematologic and molecular remission at 64, 96 and 98 months. These three patients were in molecular remission at the time of auto-HSCT.
Regarding the second scenario, that is, in patients MRD-positive who underwent transplantation during CR2, data are even scarcer. Some studies in the past showed data that today could provide clues for the interpretation of more current, apparently paradoxical data. While Meloni et al. reported 7 patients with relapsed APL undergoing auto-HSCT being MRD-positive and all patients relapsed within 9 months of transplantation (26), two single case reports showed long-term molecular remission in patients transplanted in CR2 after receiving MRD-positive autografts (27, 28). It was subsequently described in four patients who had molecular evidence of disease in at least one of the harvested samples and remained MRD negative after auto-HSCT (29). This could be explained, among other hypothesis, by the non-clonogenic nature of the PML/RARA-positive cells present in the graft. In fact, the persistence of differentiating PML/RARA-positive cells and spontaneously cleared during follow-up is a common event in patients receiving ATRA. In addition, long-term hematopoiesis after autologous HSCT would be sustained by the subset of CD34+/CD38− progenitor cells administered, and these immature progenitors have been shown to lack the PML/RAR rearrangement in APL patients.
Some recent studies have analyzed transplant outcomes according to MRD status before transplantation. Despite the bias inherent in the generally recommended strategy of limiting autologous transplantation for MRD-negative patients, relegating allogeneic transplantation for MRD-positive patients, these studies have provided some interesting data. Surprisingly, as in the CIBMTR study (15), the most recent EBMT study also found that transplant outcomes in MRD-positive recipients were not statistically different between autologous and allogeneic HSCT (16). Although these data should be interpreted with caution for a number of reasons, such as sample size and selection bias, among others, both studies have confirmed that a proportion of MRD-positive recipients can achieve long-term disease control not only undergoing allo-HSCT, but surprisingly also after auto-HSCT. This interesting finding apparently contradicts the notion that performing auto-HSCT in patients MRD positive is hopelessly doomed to failure. A recent study of the JSHCT, in a large series of APL patients undergoing auto-HSCT, with 35 being MRD-positive and 293 MRD-negative pre-transplantation, reported no association between MRD status and TRM, relapse, and OS rates (21). The cumulative incidence of relapse at 5 years was 10.3% (95% CI 7.1–14.3%) for patients with negative PML-RARA, and 8.9% (95% CI 2.3–21.3%) for patients with positive PML-RARA at transplant. In contrast to the study by Meloni et al. (26), in which bone marrow was the stem cell source, the vast majority of the patients included in the IBMTR, EBMT and JSHCT studies used peripheral blood stem cells (15, 21). These findings raise the question of the feasibility of performing auto-HSCT in patients MRD-positive when using peripheral blood as a of stem cell source and deserve to be confirmed in prospective studies.
A few registry-based studies have addressed a reliable analysis of prognostic factors associated to transplant outcomes in APL. Although originally introduced for allo-HSCT in chronic myeloid leukemia (30), and later demonstrated its applicability to acute myeloid leukemia and other malignant and non-malignant diseases (22), the classical EBMT risk score has never been tested for patients with APL. However, some of the few available studies on prognostic factors in APL have demonstrated the predictive value of some of the factors considered in the EBMT risk score. Thus, age (> 40 years) and a shorter duration of CR1 adversely influenced overall mortality in the CIBMTR study (15, 21), while in the EBMT study (16), age assessed as a continuous variable (per 10 years) and also a shorter interval from diagnosis to transplant showed an adverse impact not only on TRM (only age), but also in relapse risk, leukemia-free and overall survival. In another retrospective pan-European study in patients receiving ATO as salvage therapy (8), in addition to molecular persistence, a shorter duration of CR1 (<18 months) had an adverse impact on transplant outcomes (LFS and OS) in multivariable analysis.
The stage of the disease as such, another factor considered in the EBMT risk score, has been little studied in APL. In fact, as far as we know, only a retrospective study carried out in five Italian transplant centers has addressed this issue in a relatively small series (20). The study reported the outcome of 31 APL patients who underwent allo-HSCT in CR2 (n=15) or beyond (n=16), with OS being worse in patients with more advanced disease, but at the limit of statistical significance (p = 0.05) in the univariate analysis, while relapse rate was not statistically significant. Although it is expected that other factors considered in the EBMT risk score, such as donor type and donor recipient sex combinations, could also be predictive of transplant outcomes in APL, we are not aware of any study that has analyzed these factors in this disease.
Regarding prognostic factors in auto-HSCT, in addition to the duration of CR1, it has recently been suggested that salvage therapy with ATO is associated with a delayed hematopoietic recovery after transplantation. This association was initially suggested in two cases (31), but later confirmed in a retrospective review of 58 APL patients undergoing auto-HSCT at 21 institutions in the United States and Japan (32). This study found that ATO exposure prior to hematopoietic progenitor cell collection has negative impact on hematopoietic recovery after auto-HSCT. Fortunately, this delay in hematopoietic recovery does not appear to have a significant impact on TRM and other transplant outcomes.
Patients with CNS or other extramedullary relapse have classically been associated with a poorer outcome than those with isolated bone marrow relapse (33). However, recent data have not been able to demonstrate a negative impact of extramedullary disease on transplant outcomes after salvage therapy (8, 11).
The high cure rate currently obtained in patients with APL using modern treatments with ATRA plus chemotherapy or ATRA plus ATO point out that there is no role for HSCT in front-line therapy. The indication of HSCT has been relegated as consolidation of relapsed patients who achieve second CR after salvage therapy whenever possible. Although with a moderate level of evidence, based on recent retrospective studies, autologous HSCT would be the preferred option for consolidation for patients in molecular CR2. Allogeneic HSCT could be considered in patients with a very early relapse or those beyond CR2. Nevertheless, the superiority of HSCT as consolidation over other alternatives without transplantation has recently been questioned in some studies, which justify a prospective controlled study to resolve this still controversial issue.
All authors have contributed equally. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Sanz MA, Fenaux P, Tallman MS, Estey EH, Löwenberg B, Naoe T, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood (2019) 133(15):1630–43. doi: 10.1182/blood-2019-01-894980
2. Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood (2009) 113(9):1875–91. doi: 10.1182/blood-2008-04-150250
4. Mandelli F, Labopin M, Granena A, Iriondo A, Prentice G, Bacigalupo A, et al. European survey of bone marrow transplantation in acute promyelocytic leukemia (M3). Working Party on Acute Leukemia of the European Cooperative Group for Bone Marrow Transplantation (EMBT). Bone Marrow Transpl (1994) 14(2):293–8.
5. Sanz MA, Labopin M, Gorin N-C, la Rubia de J, Arcese W, Meloni G, et al. Hematopoietic stem cell transplantation for adults with acute promyelocytic leukemia in the ATRA era: a survey of the European Cooperative Group for Blood and Marrow Transplantation. Bone Marrow Transpl (2007) 39(8):461–9. doi: 10.1038/sj.bmt.1705620
6. De Botton S, Fawaz A, Chevret S, Dombret H, Thomas X, Sanz M, et al. Autologous and allogeneic stem-cell transplantation as salvage treatment of acute promyelocytic leukemia initially treated with all-trans-retinoic acid: a retrospective analysis of the European acute promyelocytic leukemia group. J Clin Oncol (2005) 23(1):120–6. doi: 10.1200/JCO.2005.03.127
7. Thirugnanam R, George B, Chendamarai E, Lakshmi KM, Balasubramanian P, Viswabandya A, et al. Comparison of clinical outcomes of patients with relapsed acute promyelocytic leukemia induced with arsenic trioxide and consolidated with either an autologous stem cell transplant or an arsenic trioxide-based regimen. Biol Blood Marrow Transpl (2009) 15(11):1479–84. doi: 10.1016/j.bbmt.2009.07.010
8. Lengfelder E, Lo-Coco F, Adès L, Montesinos P, Grimwade D, Kishore B, et al. Arsenic trioxide-based therapy of relapsed acute promyelocytic leukemia: registry results from the European LeukemiaNet. Leukemia (2015) 29(5):1084–91. doi: 10.1038/leu.2015.12
9. Pemmaraju N, Tanaka MF, Ravandi F, Lin H, Baladandayuthapani V, Rondon G, et al. Outcomes in patients with relapsed or refractory acute promyelocytic leukemia treated with or without autologous or allogeneic hematopoietic stem cell transplantation. Clin Lymphoma Myeloma Leuk (2013) 13(4):485–92. doi: 10.1016/j.clml.2013.02.023
10. Fujita H, Asou N, Iwanaga M, Hyo R, Nomura S, Kiyoi H, et al. Role of hematopoietic stem cell transplantation for relapsed acute promyelocytic leukemia: a retrospective analysis of JALSG-APL97. Cancer Sci (2013) 104(10):1339–45. doi: 10.1111/cas.12230
11. Ganzel C, Mathews V, Alimoghaddam K, Ghavamzadeh A, Kuk D, Devlin S, et al. Autologous transplant remains the preferred therapy for relapsed APL in CR2. Bone Marrow Transpl (2016) 51(9):1180–3. doi: 10.1038/bmt.2016.96
12. Kulkarni U, Ganesan S, Alex AA, Palani H, David S, Balasundaram N, et al. A phase II study evaluating the role of bortezomib in the management of relapsed acute promyelocytic leukemia treated upfront with arsenic trioxide. Cancer Med (2020) 9(8):2603–10. doi: 10.1002/cam4.2883
13. Kohno A, Morishita Y, Iida H, Yanada M, Uchida T, Hamaguchi M, et al. Hematopoietic stem cell transplantation for acute promyelocytic leukemia in second or third complete remission: a retrospective analysis in the Nagoya Blood and Marrow Transplantation Group. Int J Hematol (2008) 87(2):210–6. doi: 10.1007/s12185-008-0020-8
14. Alimoghaddam K, Ghavamzadeh A, Jahani M, Jalali A, Jorjani H, Iravani M, et al. Hematopoietic stem cell transplantation in acute promyelocytic leukemia, experience in Iran. Arch Iran Med (2011) 14(5):332–4.
15. Holter Chakrabarty JL, Rubinger M, Le-Rademacher J, Wang HL, Grigg A, Selby GB, et al. Autologous is superior to allogeneic hematopoietic cell transplantation for acute promyelocytic leukemia in second complete remission. Biol Blood Marrow Transpl (2014) 20(7):1021–5. doi: 10.1016/j.bbmt.2014.03.025
16. Sanz J, Labopin M, Sanz MA, Aljurf M, Botelho Sousa A, Craddock C, et al. Hematopoietic stem cell transplantation for adults with relapsed acute promyelocytic leukemia in second complete remission. Bone Marrow Transpl (2020). doi: 10.1038/s41409-020-01162-0
17. Yanada M, Tsuzuki M, Fujita H, Fujimaki K, Fujisawa S, Sunami K, et al. Phase 2 study of arsenic trioxide followed by autologous hematopoietic cell transplantation for relapsed acute promyelocytic leukemia. Blood (2013) 121(16):3095–102. doi: 10.1182/blood-2012-11-466862
18. Yanada M, Yano S, Kanamori H, Gotoh M, Emi N, Watakabe K, et al. Autologous hematopoietic cell transplantation for acute promyelocytic leukemia in second complete remission: outcomes before and after the introduction of arsenic trioxide. Leuk Lymphoma (2017) 58(5):1061–7. doi: 10.1080/10428194.2016.1231406
19. Ferrara F, Finizio O, Izzo T, Riccardi C, Criscuolo C, Carbone A, et al. Autologous stem cell transplantation for patients with acute promyelocytic leukemia in second molecular remission. Anticancer Res (2010) 30(9):3845–9.
20. Ramadan SM, Di Veroli A, Camboni A, Breccia M, Iori AP, Aversa F, et al. Allogeneic stem cell transplantation for advanced acute promyelocytic leukemia in the ATRA and ATO era. Haematologica (2012) 97(11):1731–5. doi: 10.3324/haematol.2012.065714
21. Yanada M, Takami A, Mizuno S, Mori J, Chou T, Usuki K, et al. Autologous hematopoietic cell transplantation for acute myeloid leukemia in adults: 25 years of experience in Japan. Int J Hematol (2020) 111(1):93–102. doi: 10.1007/s12185-019-02759-y
22. Breccia M, Diverio D, Noguera NI, Visani G, Santoro A, Locatelli F, et al. Clinico-biological features and outcome of acute promyelocytic leukemia patients with persistent polymerase chain reaction-detectable disease after the AIDA front-line induction and consolidation therapy. Haematologica (2004) 89(1):29–33.
23. Sanz MA, Martín G, González M, León A, Rayón C, Rivas C, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood (2004) 103(4):1237–43. doi: 10.1182/blood-2003-07-2462
24. Platzbecker U, Avvisati G, Cicconi L, Thiede C, Paoloni F, Vignetti M, et al. Improved Outcomes With Retinoic Acid and Arsenic Trioxide Compared With Retinoic Acid and Chemotherapy in Non-High-Risk Acute Promyelocytic Leukemia: Final Results of the Randomized Italian-German APL0406 Trial. J Clin Oncol (2017) 35(6):605–12. doi: 10.1200/JCO.2016.67.1982
25. Sanz MA, Montesinos P, Rayón C, Holowiecka A, la Serna de J, Milone G, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood (2010) 115(25):5137–46. doi: 10.1182/blood-2010-01-266007
26. Meloni G, Diverio D, Vignetti M, Avvisati G, Capria S, Petti MC, et al. Autologous bone marrow transplantation for acute promyelocytic leukemia in second remission: prognostic relevance of pretransplant minimal residual disease assessment by reverse-transcription polymerase chain reaction of the PML/RAR alpha fusion gene. Blood (1997) 90(3):1321–5. doi: 10.1182/blood.V90.3.1321.1321_1321_1325
27. Sanz MA, la Rubia de J, Bonanad S, Barragán E, Sempere A, Martín G, et al. Prolonged molecular remission after PML/RAR alpha-positive autologous peripheral blood stem cell transplantation in acute promyelocytic leukemia: is relevant pretransplant minimal residual disease in the graft? Leukemia (1998) 12(6):992–5. doi: 10.1038/sj.leu.2401024
28. Ruiz-Argüelles GJ, Garcés-Eisele J, Ruiz-Argüelles A. Continued complete remission after a PML/RAR-alpha+ autograft in acute promyelocytic leukaemia. Eur J Haematol (1996) 56(3):186–7. doi: 10.1111/j.1600-0609.1996.tb01342.x
29. Thomas X, Dombret H, Cordonnier C, Pigneux A, Gardin C, Guerci A, et al. Treatment of relapsing acute promyelocytic leukemia by all-trans retinoic acid therapy followed by timed sequential chemotherapy and stem cell transplantation. APL Study Group. Acute promyelocytic leukemia. Leukemia (2000) 14(6):1006–13. doi: 10.1038/sj.leu.2401800
30. Gratwohl A, Hermans J, Goldman JM, Arcese W, Carreras E, Devergie A, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet (1998) 352(9134):1087–92. doi: 10.1016/S0140-6736(98)03030-X
31. Ueki T, Ohashi K, Jinta M, Okuyama Y, Hiruma K, Akiyama H, et al. Delayed hematological recovery following autologous transplantation utilizing peripheral blood stem cells harvested after treatment with arsenic trioxide. Pathol Oncol Res (2008) 14(4):387–90. doi: 10.1007/s12253-008-9049-5
32. Mannis GN, Logan AC, Leavitt AD, Yanada M, Hwang J, Olin RL, et al. Delayed hematopoietic recovery after auto-SCT in patients receiving arsenic trioxide-based therapy for acute promyelocytic leukemia: a multi-center analysis. Bone Marrow Transpl (2015) 50(1):40–4. doi: 10.1038/bmt.2014.201
Keywords: acute promyelocytic leukaemia, hematopoietic (stem) cell transplantation (HCT), all-trans retinoic acid (ATRA), arsenic trioxide, relapse
Citation: Sanz J, Montesinos P and Sanz MA (2021) Role of Hematopoietic Stem Cell Transplantation in Acute Promyelocytic Leukemia. Front. Oncol. 11:614215. doi: 10.3389/fonc.2021.614215
Received: 05 October 2020; Accepted: 26 February 2021;
Published: 18 March 2021.
Edited by:
Yok Lam Kwong, The University of Hong Kong, Hong KongReviewed by:
Richard Champlin, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2021 Sanz, Montesinos and Sanz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miguel A. Sanz, bXNhbnpAdXYuZXM=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.