- 1Division of Thyroid Surgery, China-Japan Union Hospital of Jilin University, Jilin Provincial Key Laboratory of Surgical Translational Medicine, Jilin Provincial Engineering Laboratory of Thyroid Disease Prevention and Control, Changchun, China
- 2Division for Endocrine and Minimally Invasive Surgery, Department of Human Pathology in Adulthood and Childhood “G. Barresi”, University Hospital G. Martino, University of Messina, Messina, Italy
Objective: To investigate the association between the presence of female-specific tumors and aggressive clinicopathological features in papillary thyroid cancer (PTC).
Methods: This study retrospectively analyzed 9,822 female cases between June 2008 and December 2017. Odds ratios and corresponding 95% confidence intervals were calculated. Findings were stratified by age and body mass index (BMI) in different models.
Results: 1443/9822 (14.7%) patients with PTC had a female-specific tumor. Presence of a benign breast mass was an independent risk factor for a primary PTC lesion > 1 cm in diameter (adjusted OR = 1.446, 95% CI 1.136–1.840, P = 0.003), but a protective factor against extrathyroidal extension of PTC (adjusted OR = 0.650, 95%CI 0.500–0.845, P = 0.001). Presence of a benign uterine mass was an independent risk factor for multifocal PTC (adjusted OR = 1.305, 95%CI 1.113–1.531, P = 0.001). Analyses stratified by age and BMI revealed the presence of a benign breast mass was an independent risk factor for a primary PTC lesion > 1 cm in diameter in patients aged <36 years (adjusted OR = 1.711, 95% CI 1.063–2.754, P = 0.027), and a protective factor against extrathyroidal extension of PTC in patients aged ≥36 - <42 years (OR adjusted = 0.533, 95% CI 0.302–0.941, P = 0.030) or with a BMI ≥ 23.4 kg/m2 (BMI ≥ 23.4 to < 25.7 kg/m2, adjusted OR = 0.441, 95% CI 0.246–0.792, P = 0.006; BMI ≥25.7 kg/m2, adjusted OR = 0.558, 95% CI 0.315–0.998, P2 = 0.045). Presence of a benign uterine mass was an independent risk factor for multifocal PTC in patients aged ≥49 years (adjusted OR = 1.397, 95% CI 1.088–1.793, P = 0.009) or with a BMI <21.5 kg/m2 (OR adjusted = 1.745, 95% CI 1.214–2.509, P = 0.003).
Conclusion: The presence of a benign breast mass was an independent risk factor for a primary PTC lesion > 1 cm in diameter and a protective factor against extrathyroidal extension of PTC, while the presence of a benign uterine mass was an independent risk factor for multifocal PTC. Data from this study may help surgeons propose more personalized treatment plans when encountering patients with PTC and female-specific benign tumors.
Introduction
Evidence suggests that less women than men are affected by cancer. The National Cancer Institute estimates that one in three women and one in two men will be diagnosed with cancer during their lifetime, and women are more likely to survive cancer than men (1).
Despite this, the prevalence of thyroid cancer is higher in women than men (2–6), possibly due to the effects of endogenous female sex hormones, mood, stress, and genetic factors. Specifically, in vitro experiments show that estrogens promote the proliferation and invasion of thyroid cancer cell lines. Clinical data confirm a role for estrogen in the incidence of thyroid cancer (7–12), suggesting breast cancer and thyroid cancer share a common etiology (13). In one study, women with a history of breast cancer were 1.55 times more likely to develop thyroid cancer as a secondary malignancy compared to women with no history of breast cancer, while women with a history of thyroid cancer were 1.18 time more likely to develop breast cancer as a secondary malignancy compared to women with no history of thyroid cancer (14). These data imply that women with a history of breast or thyroid cancer should adhere to the appropriate guidelines for prevention of and screening for secondary malignancies during follow-up of their primary cancer.
The presence of female-specific tumors has also been associated with the incidence of thyroid lesions (15, 16). Benign breast (HR = 1.47, 95% CI 1.09–1.99) (17) and uterine (HR = 1.72, 95% CI 1.18–2.50) (13) masses may increase the risk of thyroid cancer. However, few reports have described the role of female-specific tumors in the progression of thyroid cancer.
The objective of this study was to investigate the association between the presence of female-specific tumors and aggressive clinicopathological features in papillary thyroid cancer (PTC). Understanding the molecular mechanisms responsible for sex-bias differences in thyroid cancer progression may improve the management of patients with thyroid cancer and inform the development of personalized therapeutic strategies.
Methods
Study Design and Data Sources
This retrospective analysis used data collected from an institutional prospective web-based registry platform. The study was conducted in compliance with the Declaration of Helsinki. The Health Care Ethics Committee of the China-Japan Union Hospital of Jilin University, Changchun, China, approved the protocol (NO. 2019040806). All patients had signed informed consent.
Study Patients
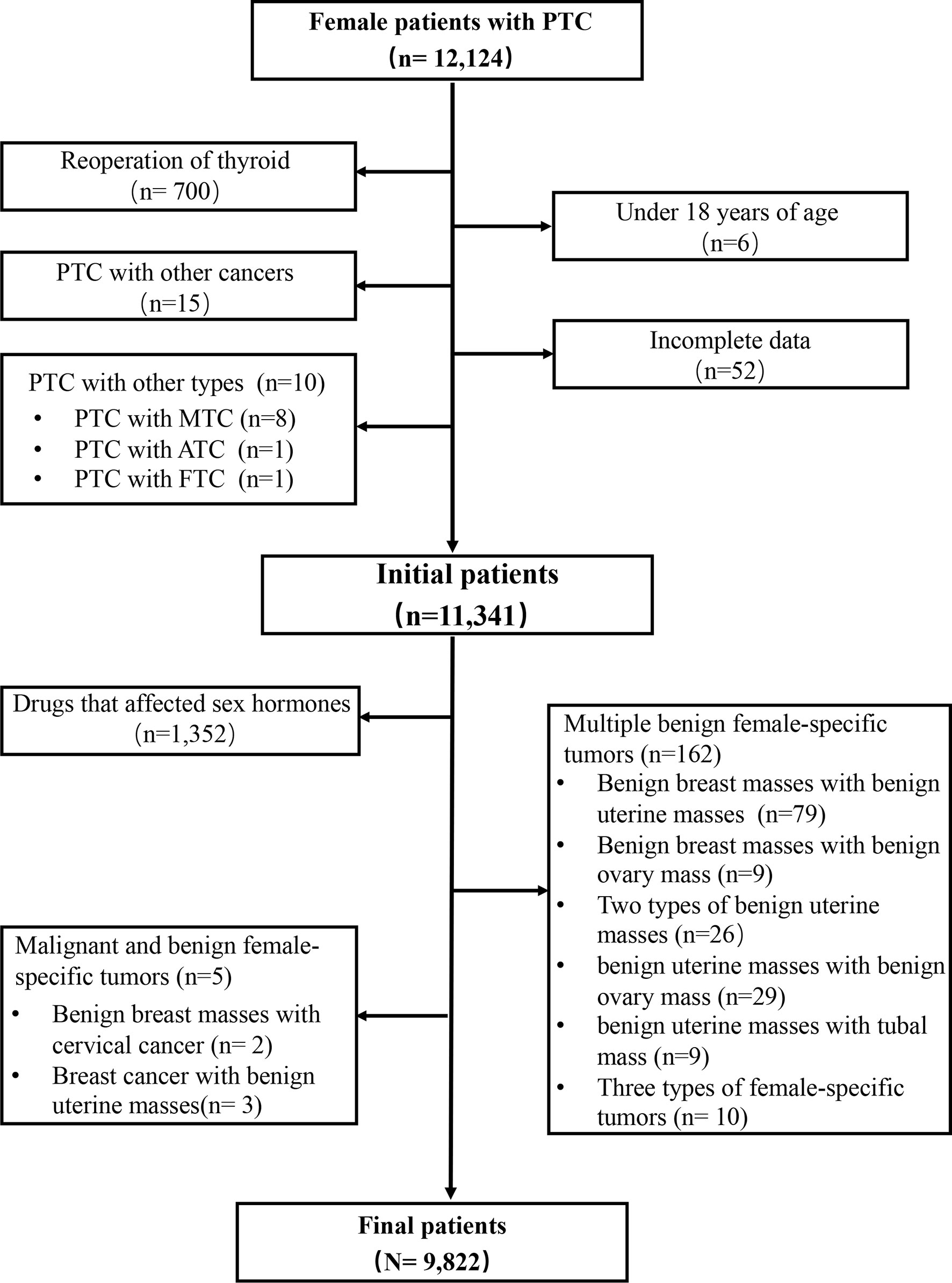
Patients with PTC (age ≥ 18 years) who were treated at the Division of Thyroid Surgery of the China-Japan Union Hospital of Jilin University (Changchun, China), a high-volume university-affiliated teaching hospital, between June 2008 and December 2017 were eligible for this study. Exclusion criteria were: 1) age < 18 years; 2) underwent reoperation; 3) multiple female-specific tumors; 4) history of other cancer or subtype of thyroid cancer; 5) long-term use of drugs that might affect the levels of sex hormones; 6) missing data (Figure 1).
Patients with PTC were classified into two groups according to the presence or absence of female-specific tumors, including benign breast masses, benign uterine masses, benign ovarian masses, breast cancer, or gynecological cancers. Benign breast masses included breast fibroadenoma, breast cysts, breast intraductal papilloma, and breast lipoma assigned a Breast Imaging Reporting and Data System (BI-RADS) 1–3 assessment on ultrasound and confirmed by long-term follow-up or biopsy (18). Benign uterine masses included uterine fibroids, uterine polyps, cervical cysts and uterine adenomyoma confirmed by ultrasound or pathology. Benign ovarian masses included ovarian cysts and mature teratoma confirmed by ultrasound or pathology. Breast cancers and gynecological cancers, including endometrial cancer, cervical cancer, and ovarian cancer were verified by postoperative paraffin pathology (Table S1).
PTC was diagnosed and characterized on postoperative paraffin pathology. Aggressive clinicopathological characteristics in PTC included a tumor diameter >1 cm, multifocal PTC, extrathyroidal extension of PTC, lymph node metastasis, T3-T4, clinical stage III or IV, and distant metastasis found on pathology or imaging in lung, bone, lung and bone combined, or other sites (19).
Data Collection
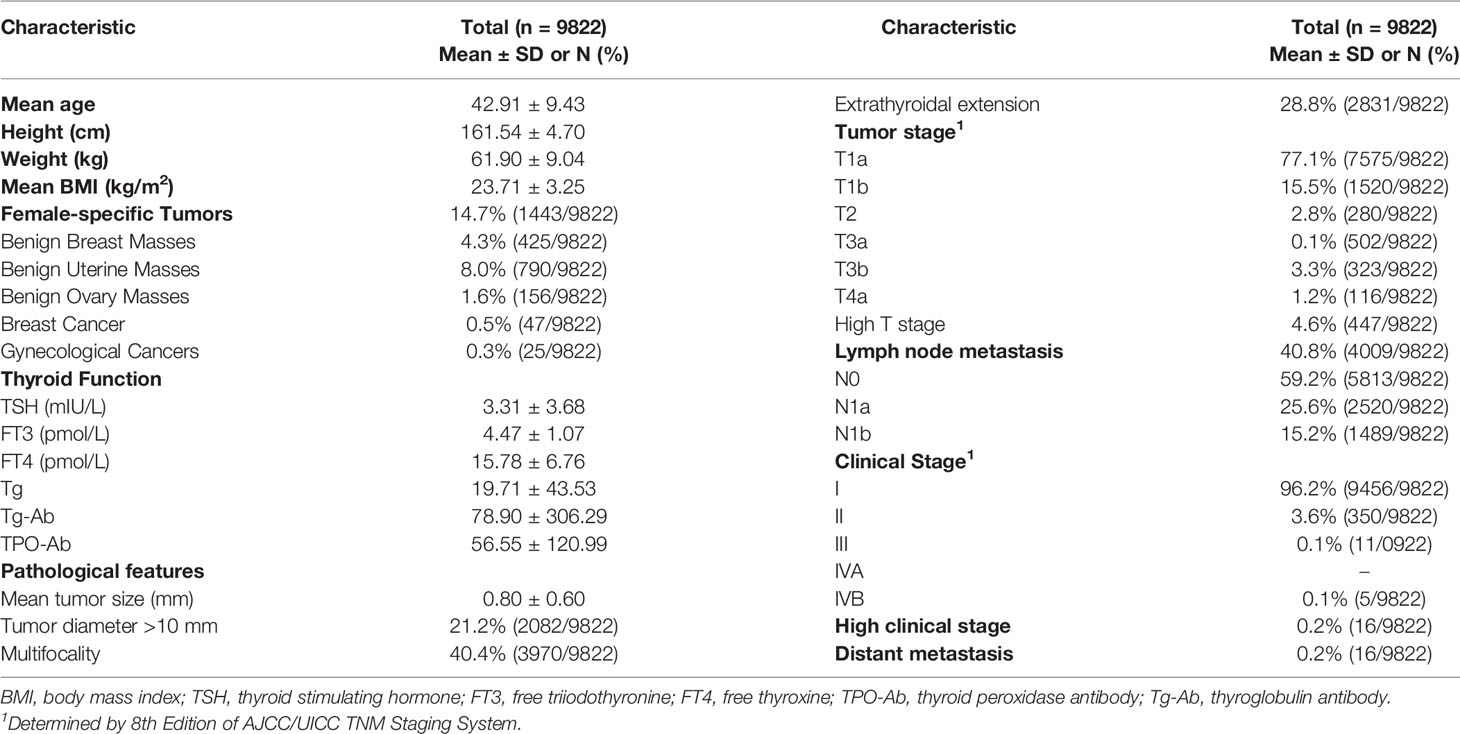
Patients demographic and clinical characteristics were recorded, including height, weight, gender, age, previous medical history, thyroid function (thyroid stimulating hormone [TSH], tri-iodothyronine [FT3], thyroxine [FT4], thyroid peroxidase antibody [TPO-Ab], thyroglobulin [Tg] and thyroglobulin antibodies [Tg-Ab]), and clinicopathological characteristics of PTC (size of the primary lesion, number of lesions, extrathyroidal extension, number of lymph node metastases [including the central area and lateral neck area], distant metastasis, tumor, node and metastasis [TNM] and clinical staging). TNM and clinical staging were conducted according to the AJCC guidelines 8th edition (20).
Statistical Analysis
Statistical analysis was performed using SPSS 22.0 for windows (SPSS, Chicago, IL, USA). Continuous data are reported as mean ± standard deviation and were compared using the t-test or analysis of variance. Categorical data are reported as percentages. and were compared with the chi-square test or Fisher’s exact test. Binary logistic regression was used to calculate odds ratios (OR) and 95% confidence intervals (CI). The independent variable was the coexistence of PTC and a female-specific tumor, and the dependent variables were the aggressive clinicopathological characteristics in PTC. Further binary logistic regression considered age or BMI as continuous variables in different models. ORs and corresponding 95% CIs were calculated to investigate the association between the presence of a benign breast mass and a primary PTC lesion > 1 cm in diameter, or extrathyroidal extension of PTC or the presence of a benign uterine mass and multifocal PTC. The subjects were divided into four categories based on quartiles of age and BMI in Table 6 and Table 7. P<0.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 12,124 female patients with PTC were identified from the institutional prospective web-based registry platform, of which 9,822 met the inclusion criteria (Figure 1). Included patients were all female, with a mean (SD) age of 42.91 ± 9.43 years, and a mean (SD) BMI of 23.71 ± 3.25 kg/m2. 85.3% (8379/9822) of patients with PTC did not have a female-specific tumor. 14.7% (1443/9822) of patients with PTC had a female-specific tumor. Of these, 4.3% (425/9822) of patients had a benign breast mass, 0.5% (47/9822) of patients had a breast cancer, 8.0% (790/9822) of patients had a benign uterine mass, 1.6% (156/9822) of patients had a benign ovarian mass, and 0.3% (25/9822) of patients had a gynecological cancer. Considering aggressive clinicopathological characteristics in PTC, 21.2% (2082/9822) of patients had a primary PTC lesion > 1 cm in diameter, 40.4% (3970/9822) of patients had multifocal PTC, 28.8% (2831/9822) of patients had extrathyroidal extension of PTC, 40.8% (4009/9822) of patients had lymph node metastasis, 4.6% (447/9822) were T3 or T4, and 0.2% (16/9822) of patients were clinical stage III or IV or had distant metastasis (Table 1).
Association Between Female-Specific Tumors and Aggressive Clinicopathological Characteristics in PTC
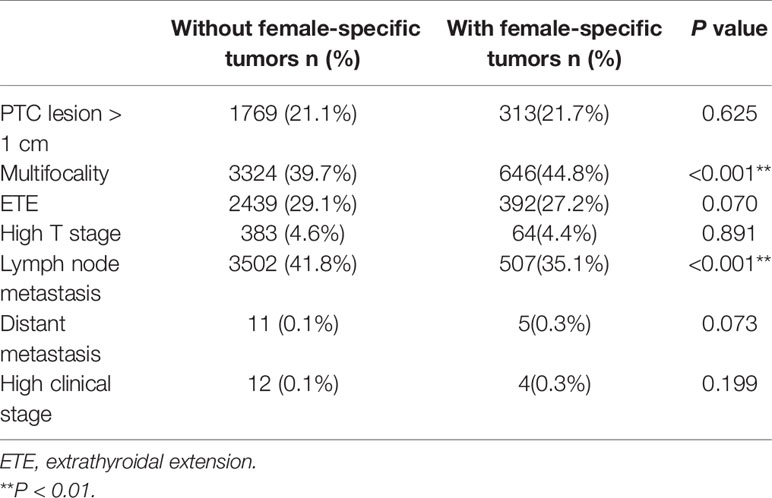
Compared to patients with PTC without a female-specific tumor, more patients with PTC and a female-specific tumor had multifocal PTC (44.8%, vs. 39.7%, P<0.001) and less in lymph node metastasis (35.1% vs. 41.8%, P<0.01) (Table 2). There was correlation between PTC lesion > 1 cm and high BMI, younger age, high TSH and high Tg; between multifocality and high BMI, older age, high TPO-Ab, Tg-Ab; between ETE and high BMI, older age, high FT3, low TPO-Ab, high Tg, high Tg-Ab, low Tg/TSH; between lymph node metastasis and high BMI, younger age, high Tg, high Tg-Ab. When analyzing corresponding parameters, we would use related variables as covariates to adjust the OR value of aggressive clinicopathological features (Table 3).

Table 3 The association between different variables and aggressive clinicopathological features of PTC.
On univariate analysis, compared to patients with PTC without a female-specific tumor, more patients with PTC and a benign breast mass had a primary PTC lesion > 1 cm in diameter (25.4% vs. 21.1%, P<0.05) but fewer patients with PTC and a benign breast mass had extrathyroidal extension of PTC (20.2% vs. 29.1%, P<0.001). Compared to patients with PTC without a female-specific tumor, more patients with PTC and a benign uterine mass had multifocal PTC (47.0% vs. 39.7%, P<0.001) or distant metastases (0.6% vs. 0, P<0.01), but fewer patients with PTC and a benign uterine mass had lymph node metastases (33.7% vs. 41.8%, P<0.001). The presence of a benign ovarian mass, breast cancer, or gynecological cancer was not associated with aggressive clinicopathological characteristics in PTC (Table 4). Binary logistic regression showed that the presence of a benign breast mass was an independent risk factor for a primary PTC lesion > 1 cm in diameter (adjusted OR = 1.446, 95% CI 1.136–1.840, P = 0.003), but a protective factor against extrathyroidal extension of PTC (adjusted OR = 0.650, 95%CI 0.500–0.845, P = 0.001). The presence of a benign uterine mass was an independent risk factor for multifocal PTC (adjusted OR = 1.305, 95%CI 1.113–1.531, P = 0.001) (Table 5).
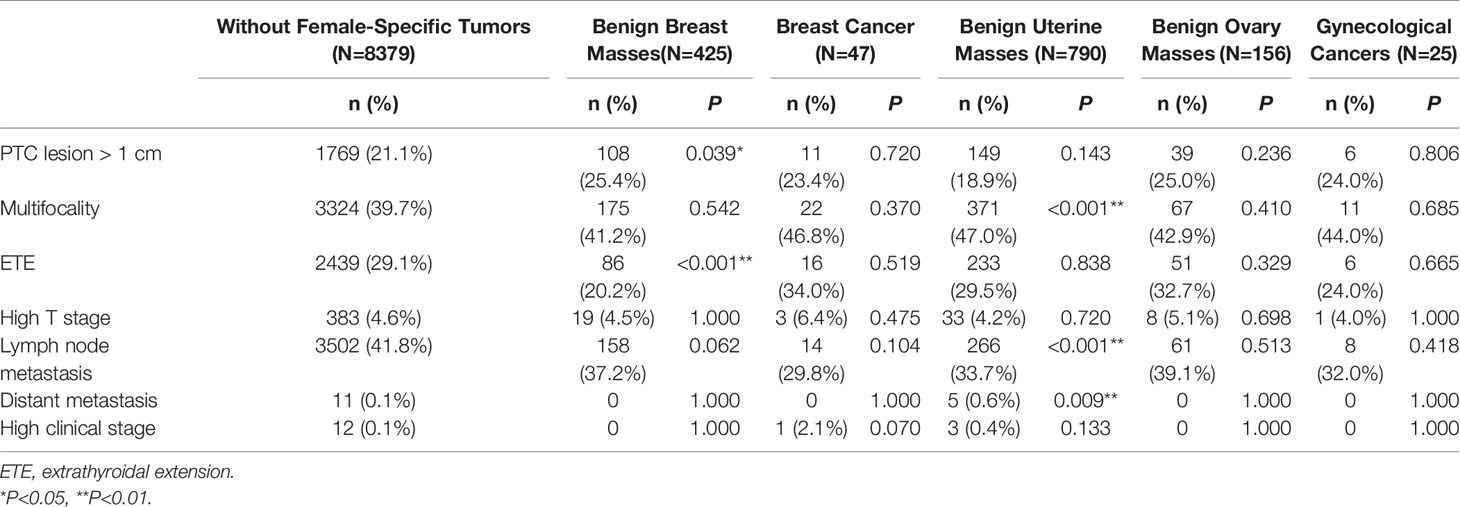
Table 4 Univariate analysis of the association between female-specific tumors and aggressive clinicopathological features in PTC.
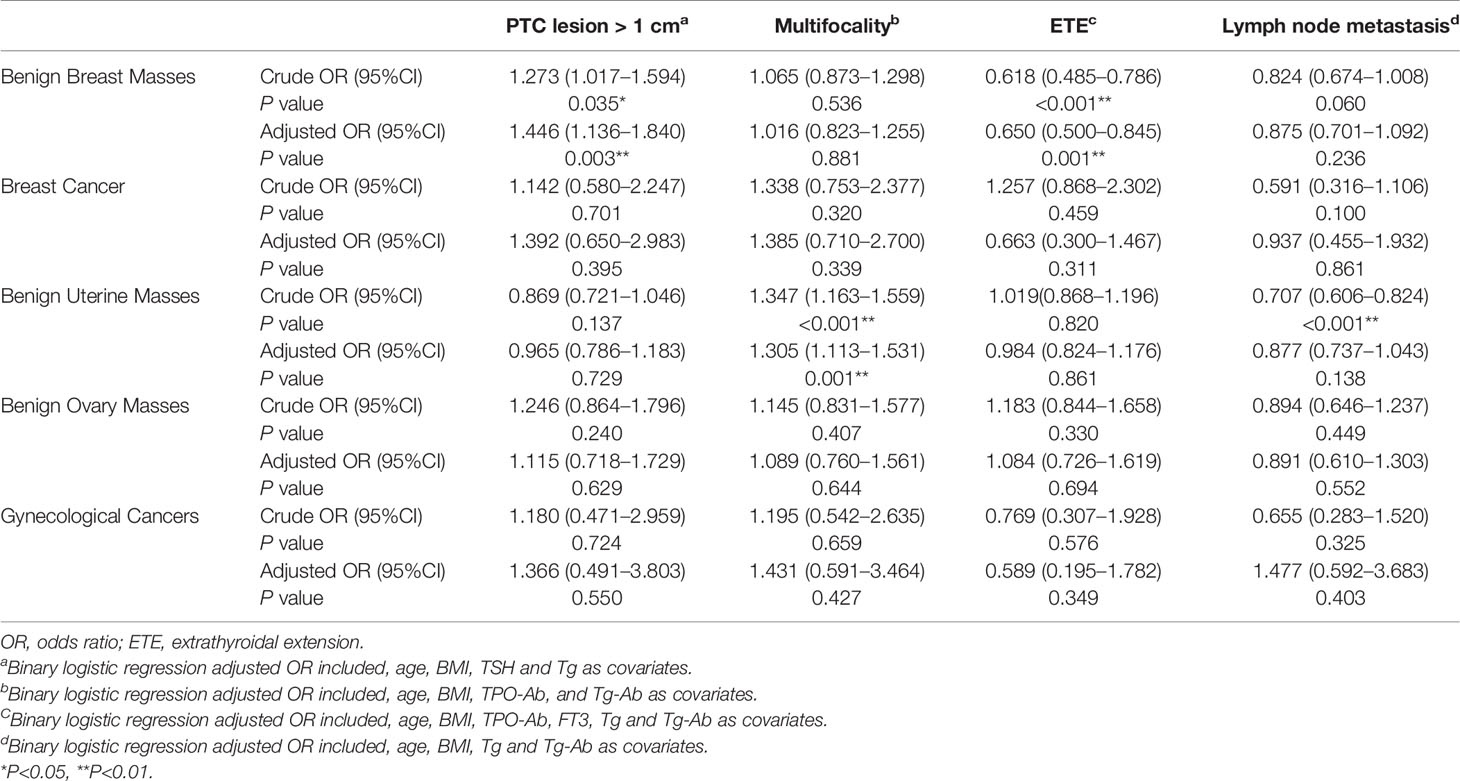
Table 5 Binary logistic regression analysis of the association between female-specific tumors and aggressive clinicopathological features in PTC.
Association Between Benign Breast Mass and Aggressive Clinicopathological Characteristics in PTC Stratified by Age and BMI
The presence of a benign breast mass was an independent risk factor for a primary PTC lesion > 1 cm in diameter in patients aged <36 years (adjusted OR = 1.711, 95% CI 1.063–2.754, P = 0.027). The presence of a benign breast mass was a protective factor against extrathyroidal extension of PTC in patients aged ≥36 - <42 years (OR adjusted = 0.533, 95% CI 0.302–0.941, P = 0.030).
The presence of a benign breast mass had no effect on the risk of developing a primary PTC lesion > 1 cm in diameter in patients with a BMI <21.5 - >25.7 kg/m2. The presence of a benign breast mass was a protective factor against extrathyroidal extension of PTC in patients with a BMI ≥ 23.4 kg/m2 (BMI ≥ 23.4 - < 25.7 kg/m2, adjusted OR = 0.441, 95% CI 0.246–0.792, P = 0.006; BMI ≥25.7 kg/m2, adjusted OR = 0.558, 95% CI 0.315–0.998, P2 = 0.045) (Table 6).
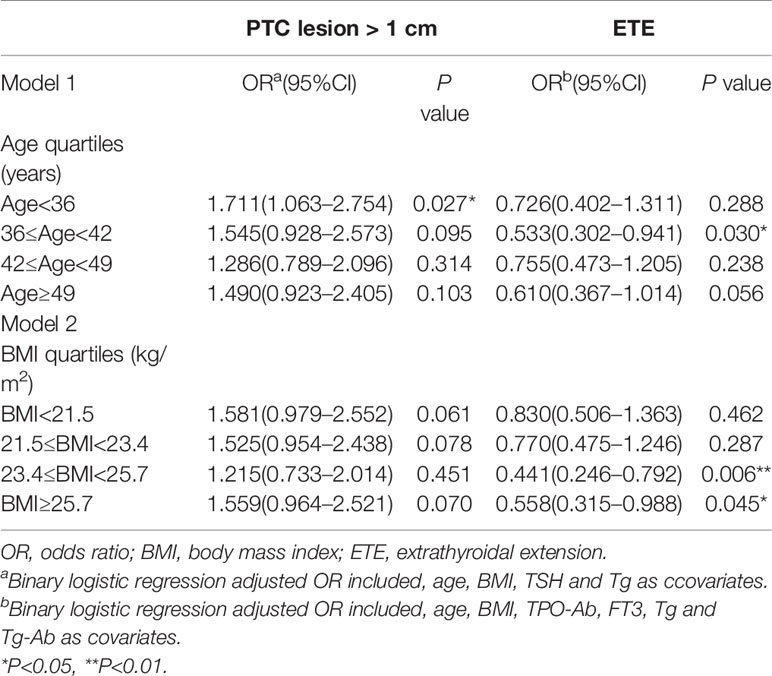
Table 6 Association between benign breast masses and aggressive clinicopathological features in PTC stratified by age and BMI.
The presence of a benign breast mass had no effect on the risk of developing a primary PTC lesion > 1 cm in diameter in patients aged <36 years with a BMI<21.5 kg/m2 (adjusted OR = 1.957, 95% CI 0.937–4.087, P = 0.074). The presence of a benign breast mass was a protective factor against extrathyroidal extension of PTC in patients aged ≥36 - <42 years with a BMI ≥23.4 kg/m2 (adjusted OR = 0.289, 95% CI 0.101–0.828, P = 0.021) (Figure 2).
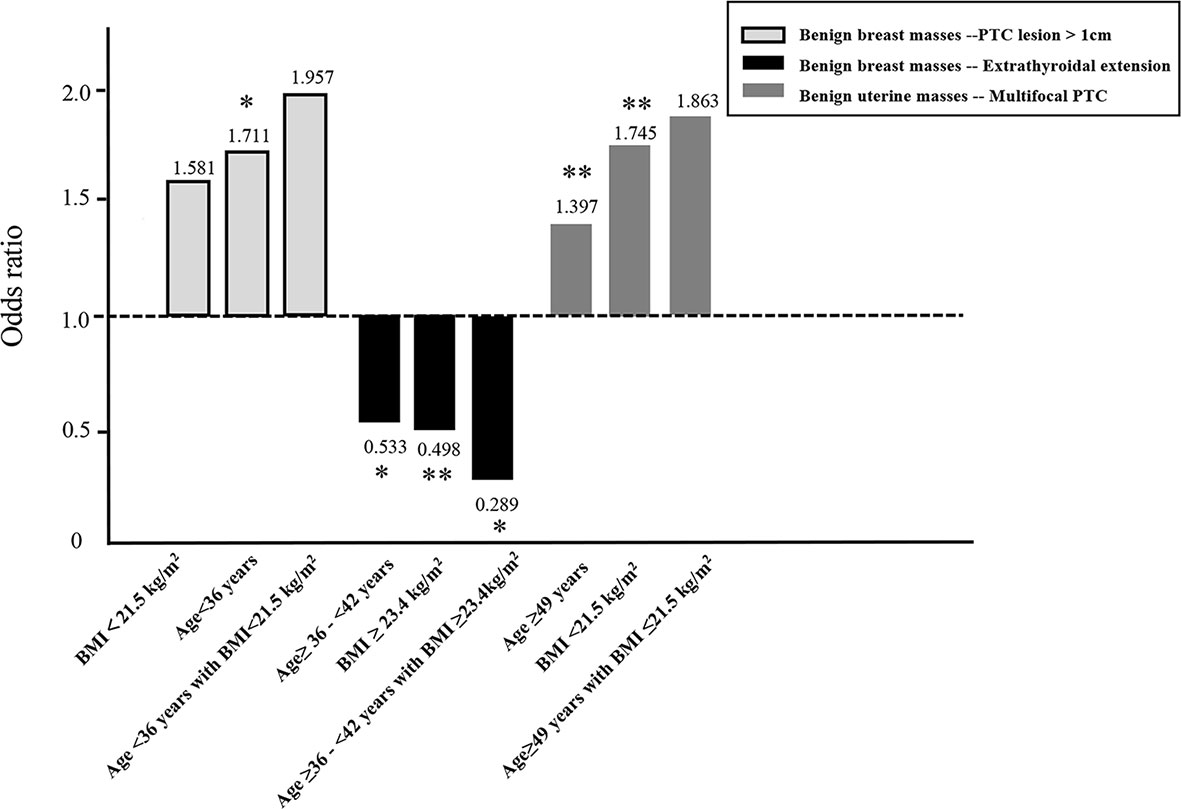
Figure 2 Influence of age and/or BMI on the association between the presence of a benign breast mass and a primary PTC lesion > 1cm in diameter or extrathyroidal extension of PTC, and the presence of a benign uterine mass and multifocal PTC. *P<0.05, **P<0.01.
In addition, age was a protective factor of PTC lesion >1 cm (adjusted OR 36≤age<42 = 0.564, adjusted OR 42≤age<49 = 0.513, adjusted OR age≥49 = 0.649). With age, the risk of extrathyroidal extension gradually increased (adjusted OR 36≤age<42 = 1.189, adjusted OR 42≤age<49 = 1.233, adjusted OR age≥49 = 1.420) (Table S2).
Association Between Benign Uterine Mass and Aggressive Clinicopathological Characteristics in PTC Stratified by Age and BMI
The presence of a benign uterine mass was an independent risk factor for multifocal PTC in patients aged ≥49 years (adjusted OR = 1.397, 95% CI 1.088–1.793, P = 0.009) or a BMI <21.5 kg/m2 (OR adjusted = 1.745, 95% CI 1.214–2.509, P = 0.003) (Table 7).
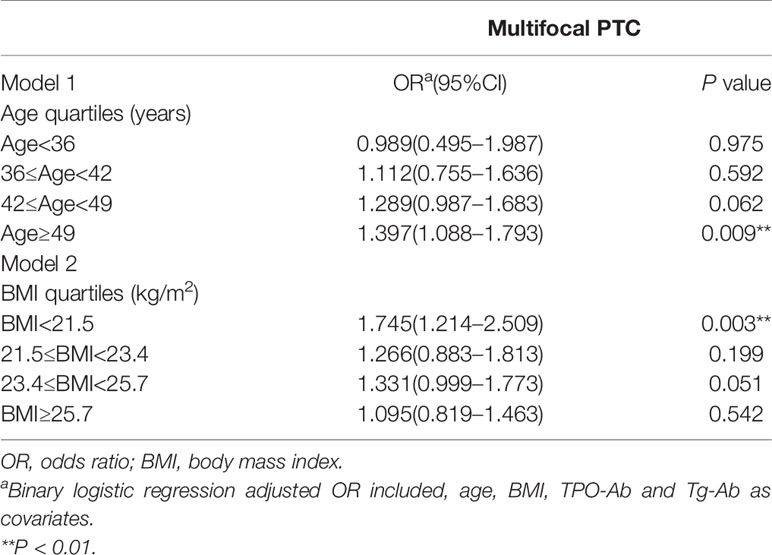
Table 7 Association between benign uterine masses and aggressive clinicopathological features in PTC stratified by age and BMI.
The presence of a benign uterine mass had no effect on the risk of developing multifocal PTC in patients aged ≥49 years with a BMI ≤21.5 kg/m2 (adjusted OR = 1.863, 95% CI = 0.977–3.554, P = 0.059) (Figure 2).
Discussion
This retrospective analysis of 9,822 female patients investigated the association between the presence of female-specific tumors and aggressive clinicopathological features in PTC. A benign breast mass was an independent risk factor for a primary PTC lesion > 1 cm in diameter and a protective factor against extrathyroidal extension of PTC, while the presence of a benign uterine mass was an independent risk factor for multifocal PTC. Findings revealed that the presence of benign breast or uterine masses could influence invasive growth of PTC. Patients were stratified by age and BMI. In young female (age <36 years) patients, the presence of a benign breast mass was an independent risk factor for a primary PTC lesion > 1 cm in diameter. Among young and middle-aged overweight/obese women (age 36–42 years; BMI ≥ 23.4 kg/m2), the presence of a benign breast mass was a protective factor against extrathyroidal extension of PTC. In middle-aged (>49 years) or lean (BMI<21.5 kg/m2) patients, the presence of a benign uterine mass was an independent risk factor for multifocal PTC. This study demonstrated that the presence of female-specific benign tumors influences aggressive clinicopathological features in PTC. These data have implications for PTC surveillance and treatment.
Benign breast masses are commonly found in women. The prevalence of benign breast masses in China is estimated at 18.6% (16). In this study, 4.3% of patients had PTC and a benign breast mass. Previous studies have shown that postmenopausal women with a benign breast mass have a higher risk of developing thyroid cancer than women without a benign breast mass (HR = 1.38, 95% CI 1.10–1.73) (21), or the presence of a benign breast mass increased the risk of thyroid cancer by 47% (HR = 1.47, 95%CI 1.09–1.99) (17, 20). However, data describing the influence of a benign breast mass on the progression of thyroid cancer are scarce.
The present study revealed that the presence of a benign breast mass was a risk factor for a primary PTC lesion > 1 cm in diameter in women aged < 36 years(adjusted OR = 1.711, P = 0.027), but not in 36≤Age<42 (adjusted OR = 1.545, P = 0.095), 42≤Age<49 (adjusted OR = 1.286, P = 0.314), Age≥49 (adjusted OR = 1.490, P = 0.103).We suppose that the mechanism underlying the co-occurrence of a benign breast mass and thyroid cancer may involve estrogen. Estrogen plays an important role in the incidence and progression of benign breast masses (19, 22), and estrogen receptor expression is a common occurrence in thyroid tumor tissues. Vannucchi et al. proved that ERα and PR expression was found in 66.5% and 75.8% of patients respectively and was significantly correlated with larger tumor size and with a non-incidental diagnosis. A trend toward a higher prevalence of local metastases was observed in ER- and PR-expressing tumors (23). Similar to our study, the author concluded that although no impact on outcome was found, the evaluation of ERα and PR receptor expression could add insights into the biological behavior of tumors and could modify the follow-up, particularly in fertile women affected with persistent disease. And in vitro studies show that estrogens promote the proliferation of thyroid cancer cells (24–27). Ovarian reserve begins to decline at 35 years of age, the number of ovarian follicles and the quality of oocytes decreases, and estrogen secretion is reduced (28–30).
Besides, one study hypothesized that differences between young and older patients with PTC had a biological/genetic basis (31). However, they didn’t find a difference in the frequency or type of somatic mutations between young and older patients, six genes (extracellular matrix protein 1 [ECM1], v‐erb‐2 erythroblastic leukemia viral oncogene homolog 2 [ERBB2], urinary plasminogen activator [UPA], 6‐phosphofructo‐2‐kinase/fructose‐2,6‐biphosphatase 2 [PFKFB2], meis homeobox 2 [MEIS2], and carbonic anhydrase II [CA2]) had significant expression between the young and older patients. In another study, age was a protective factor of the primary PTC lesion > 1 cm (32). In present study, the presence of female-specific tumors was the risk factor for PTC lesion > 1 cm. With increasing age, this effect might be offset.
Interestingly, the presence of a benign breast mass was a protective factor against extrathyroidal extension of PTC. According to the data, age was a risk factor for extrathyroidal extension. With age, the risk of extrathyroidal extension gradually increased (adjusted OR 36≤age<42 = 1.189, adjusted OR 42≤age<49 = 1.233, adjusted OR age≥49 = 1.420). The protective effect of benign breast tumors might counteract the risk of extrathyroidal extension conducted related to age. It is clear that this could only be a hypothesis, it must be supported by clinical and experiemntal studies. And the effect that is unlikely to be explained by a mechanism involving estrogen (12, 33–35). but might be mediated by the tumor microenvironment. We speculate that in benign breast conditions, the immune system is challenged, and inflammation in the mammary gland may influence the thyroid gland to initiate a ‘self-protection’ mechanism, which prevents thyroid cancer invading through the capsule. Consequently, women with PTC and benign breast tumors may have larger diameter primary lesions but no extrathyroidal extension.
Stratifying patients by age and BMI demonstrated that the protective effect of benign breast masses on extrathyroidal extension of PTC was significant in overweight/obese women aged 36 to 42 years. Evidence suggests anti-Müllerian hormone is obviously decreased in women in their upper reproductive years, which is indicative of ovarian dysfunction. We speculate that fluctuations in anti-Müllerian hormone may mediate extrathyroidal extension of PTC (36).
Benign uterine masses are commonly found in women, with an estimated prevalence of 4.5% to 68.6% (37). The prevalence of benign uterine masses is age dependent, reaching > 70% in women aged > 50 years (38–40). One previous study showed that the presence of a benign uterine mass was associated with an increased risk of thyroid cancer (HR = 1.72, 95% CI 1.18–2.50) (13); however, data describing the influence of a benign uterine mass on the progression of thyroid cancer is scarce.
The present study revealed that the presence of a benign breast mass was a risk factor for a primary PTC lesion > 1 cm in diameter in women aged < 36 years. The multifocality of PTC has been explained at the genetic level in different ways, being two hypothesis the more relevant to date: a) different foci composed by different clones with different genetic pattern; b) intrathyroidal metastatization. Some studies suggest discordant heterogeneous BRAF mutation patterns found in approximately 40% of the multifocal PTCs in adults (41). Multifocal PTCs had higher expression of mRNAs in Wnt- and pluripotency‐related pathways when BRAF mutation was present (42). It is still primitive to give conclusions about the mechanisms, but these appear to be the most credible to explain the difference between multifocal and unifocal PTCs.
In addition, it was proved that programmed death-ligand 1 (PD-L1) expression was elevated in multifocal PTC tumors (43). The expression of programmed death-1 (PD-1) and its ligand PD-L1 is upregulated in uterine disease and promoted by 17beta-estradiol (44). Therefore, we speculated the presence of a benign uterine mass was an independent risk factor for multifocal PTC.
We found that there was correlation between PTC lesion > 1 cm and high BMI, younger age, high TSH and high Tg; between multifocality and high BMI, older age, high TPO-Ab, Tg-Ab; between ETE and high BMI, older age, high FT3, low TPO-Ab, high Tg, high Tg-Ab, low Tg/TSH; between lymph node metastasis and high BMI, younger age, high Tg, high Tg-Ab. When analyzing corresponding parameters, we would use related variables as covariates to adjust the OR value of aggressive clinicopathological features. Besides, Preoperative Tg levels may have an extremely limited value. They could be influenced by several factors not related to thyroid cancer, for example gland size (45). Thus, we analyzed Tg/TSH levels and found that lower Tg/TSH was a risk factor of ETE. These findings were similar to other studies (32, 45–49).
This research had several strengths. First, the study revealed the association between the presence of female-specific tumors and aggressive clinicopathological features in PTC. Second, it was a relatively large single-center retrospective analysis of the relationship between age, BMI, female-specific tumors and PTC. Last, all cases of thyroid cancer were confirmed by medical records, avoiding potential differential selection and recall bias.
This study was assoictaed with several limitations. First, it addresses an evidence gap — is there an etiologic link between benign tumors and aggressive clinicopathological features in PTC. The study used a large sample size to fill this evidnce gap, but the precise mechanism underlying the co-occurrence of a benign breast/uterine mass and thyroid cancer has yet to be clearly defined. Second, as the study had a retrospective design without follow-up data, the impact on prognosis is unknown. Third, age of diagnosis of female-specific tumors and PTC were not confirmed; therefore, cause and effect cannot be established. Last, women with small benign tumors that were undetected may have been included in the analysis. Large-scale randomized controlled studies are warranted to provide further clarity to the influence of female-specific tumors on aggressive clinicopathological features in PTC.
In conclusion, this study retrospectively analyzed data collected from patients with PTC and a female-specific tumor in a single institutional database to reveal the association between the presence of a female-specific tumor and PTC proliferation and invasion. Findings showed the presence of a benign breast mass was an independent risk factor for a primary PTC lesion > 1 cm in diameter and a protective factor against extrathyroidal extension of PTC, while the presence of a benign uterine mass was an independent risk factor for multifocal PTC. The underlying mechanisms may be mediated by estrogen. Data from this study may help surgeons propose more personalized treatment plans when encountering patients with PTC and female-specific tumors.
Data Availability Statement
The data sets generated and/or analyzed during the current study are not publicly available to protect patient privacy but are available from the corresponding author on reasonable request. Requests to access the data sets should be directed to HS,c19oQGpsdS5lZHUuY24=.
Ethics Statement
This study was approved by the Health Care Ethics Committee of the China-Japan Union Hospital of Jilin University (no. 2019040806). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conception/design: HS and JZ. Collection and assembly of data: JZ, DZ, LNZ, NL, and GX. Data analysis and interpretation: JZ and LZ. Manuscript writing: JZ and GD. Final approval of manuscript: JZ, LZ, GD, DZ, LNZ, NL, GX, and HS. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.611471/full#supplementary-material
References
1. Olson E, Wintheiser G, Wolfe KM, Droessler J, Silberstein PT. Epidemiology of Thyroid Cancer: A Review of the National Cancer Database, 2000-2013. Cureus (2019) 11(2):e4127. doi: 10.7759/cureus.4127
2. Suh S, Kim YH, Goh TS, Lee J, Jeong DC, Oh S-O, et al. Outcome prediction with the revised American joint committee on cancer staging system and American thyroid association guidelines for thyroid cancer. Endocrine (2017) 58(3):495–502. doi: 10.1007/s12020-017-1449-4
3. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet (2016) 388(10061):2783–95. doi: 10.1016/S0140-6736(16)30172-6
4. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res (2014) 74(11):2913–21. doi: 10.1158/0008-5472.CAN-14-0155
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin (2015) 65(1):5–29. doi: 10.3322/caac.21254
6. Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”–screening and overdiagnosis. N Engl J Med (2014) 371(19):1765–7. doi: 10.1056/NEJMp1409841
7. Luo J, Hendryx M, Manson JE, Liang X, Margolis KL. Hysterectomy, Oophorectomy, and Risk of Thyroid Cancer. J Clin Endocrinol Metab (2016) 101(10):3812–9. doi: 10.1210/jc.2016-2011
8. Zahid M, Goldner W, Beseler CL, Rogan EG, Cavalieri EL. Unbalanced estrogen metabolism in thyroid cancer. Int J Cancer (2013) 133(11):2642–9. doi: 10.1002/ijc.28275
9. Derwahl M, Nicula D. Estrogen and its role in thyroid cancer. Endocr Relat Cancer (2014) 21(5):T273–T83. doi: 10.1530/ERC-14-0053
10. Kim YA, Kim YA, Cho SW, Song YS, Min HS, Park IA, et al. Increased expression of thyroid hormone receptor alpha and estrogen receptor alpha in breast cancer associated with thyroid cancer. Eur J Surg Oncol (2021) 22:S0748-7983(21):00042–1. doi: 10.1016/j.ejso.2021.01.015
11. Chen GG, Vlantis AC, Zeng Q, van Hasselt CA. Regulation of cell growth by estrogen signaling and potential targets in thyroid cancer. Curr Cancer Drug Targets (2008) 8(5):367–77. doi: 10.2174/156800908785133150
12. Rajoria S, Suriano R, Shanmugam A, Wilson YL, Schantz SP, Geliebter J, et al. Metastatic phenotype is regulated by estrogen in thyroid cells. Thyroid (2010) 20(1):33–41. doi: 10.1089/thy.2009.0296
13. Braganza MZ, de González AB, Schonfeld SJ, Wentzensen N, Brenner AV, Kitahara CM. Benign breast and gynecologic conditions, reproductive and hormonal factors, and risk of thyroid cancer. Cancer Prev Res (Phila) (2014) 7(4):418–25. doi: 10.1158/1940-6207.CAPR-13-0367
14. Bolf EL, Sprague BL, Carr FE. A Linkage Between Thyroid and Breast Cancer: A Common Etiology? Cancer Epidemiol Biomarkers Prev (2019) 28(4):643–9. doi: 10.1158/1055-9965.EPI-18-0877
15. Spinos N, Terzis G, Crysanthopoulou A, Adonakis G, Markou KB, Vervita V, et al. Increased frequency of thyroid nodules and breast fibroadenomas in women with uterine fibroids. Thyroid (2007) 17(12):1257–9. doi: 10.1089/thy.2006.0330
16. Li H, Wang Z, Liu J-S, Zou B-S, Chen H-R, Xu Z, et al. Association Between Breast and Thyroid Lesions: A Cross-Sectional Study Based on Ultrasonography Screening in China. Thyroid (2020) 30(8):1150–8. doi: 10.1089/thy.2019.0184
17. Schonfeld SJ, Ron E, Kitahara CM, Brenner A, Park Y, Sigurdson AJ, et al. Hormonal and reproductive factors and risk of postmenopausal thyroid cancer in the NIH-AARP Diet and Health Study. Cancer Epidemiol (2011) 35(6):e85–90. doi: 10.1016/j.canep.2011.05.009
18. Elezaby M, Li G, Bhargavan-Chatfield M, Burnside ES, DeMartini WB. Acr bi-rads assessment category 4 subdivisions in diagnostic mammography: Utilization and outcomes in the national mammography database. Radiology (2018) 287:416–22. doi: 10.1148/radiol.2017170770
19. Rohan TE, Negassa A, Chlebowski RT, Lasser NL, McTiernan A, Schenken RS, et al. Estrogen plus progestin and risk of benign proliferative breast disease. Cancer Epidemiol Biomarkers Prev (2008) 17(9):2337–43. doi: 10.1158/1055-9965.EPI-08-0380
20. Nam SH, Bae MR, Roh J-L, Gong G, Cho K-J, Choi S-H, et al. A comparison of the 7th and 8th editions of the AJCC staging system in terms of predicting recurrence and survival in patients with papillary thyroid carcinoma. Oral Oncol (2018) 87:158–64. doi: 10.1016/j.oraloncology.2018.11.003
21. Bach AG, Abbas J, Jasaabuu C, Schramm D, Wienke A, Surov A. Comparison between incidental malignant and benign breast lesions detected by computed tomography: a systematic review. J Med Imaging Radiat Oncol (2013) 57(5):529–33. doi: 10.1111/1754-9485.12046
22. Rohan TE, Negassa A, Chlebowski RT, Habel L, McTiernan A, Ginsberg M, et al. Conjugated equine estrogen and risk of benign proliferative breast disease: a randomized controlled trial. J Natl Cancer Inst (2008) 100(8):563–71. doi: 10.1093/jnci/djn075
23. Vannucchi G, De Leo S, Perrino M, Rossi S, Tosi D, Cirello V, et al. Impact of estrogen and progesterone receptor expression on the clinical and molecular features of papillary thyroid cancer. Eur J Endocrinol (2015) 173(1):29–36. doi: 10.1530/EJE-15-0054
24. Xue L, Yan H, Chen Y, Zhang Q, Xie X, Ding X, et al. EZH2 upregulation by ERα induces proliferation and migration of papillary thyroid carcinoma. BMC Cancer (2019) 19(1):1094. doi: 10.1186/s12885-019-6306-9
25. Faria CC, Peixoto MS, Carvalho DP, Fortunato RS. The Emerging Role of Estrogens in Thyroid Redox Homeostasis and Carcinogenesis. Oxid Med Cell Longev (2019) 2019:2514312. doi: 10.1155/2019/2514312
26. Li M, Chai H-F, Peng F, Meng Y-T, Zhang L-Z, Zhang L, et al. Estrogen receptor β upregulated by lncRNA-H19 to promote cancer stem-like properties in papillary thyroid carcinoma. Cell Death Dis (2018) 9(11):1120. doi: 10.1038/s41419-018-1077-9
27. Meng D, Wu W, Li Z, Qin G. IQGAP1 modulates the proliferation and invasion of thyroid cancer cells in response to estrogen. Int J Mol Med (2015) 36(2):588–94. doi: 10.3892/ijmm.2015.2232
28. Liu C-M, Ding L-J, Li J-Y, Dai J-W, Sun H-X. Advances in the study of ovarian dysfunction with aging. Yi Chuan (2019) 41(9):816–26. doi: 10.16288/j.yczz.19-134
29. Birch Petersen K, Hvidman HW, Forman JL, Pinborg A, Larsen EC, Macklon KT, et al. Ovarian reserve assessment in users of oral contraception seeking fertility advice on their reproductive lifespan. Hum Reprod (2015) 30(10):2364–75. doi: 10.1093/humrep/dev197
30. Sills ES, Alper MM, Walsh APH. Ovarian reserve screening in infertility: practical applications and theoretical directions for research. Eur J Obstet Gynecol Reprod Biol (2009) 146(1):30–6. doi: 10.1016/j.ejogrb.2009.05.008
31. Goldfarb M, Freyer DR. Comparison of secondary and primary thyroid cancer in adolescents and young adults. Cancer (2014) 120(8):1155–61. doi: 10.1002/cncr.28463
32. Li CL, Dionigi G, Zhao YS, Liang N, Sun H. Influence of body mass index on the clinicopathological features of 13,995 papillary thyroid tumors. J Endocrinol Invest (2020) 43(9):1283–99. doi: 10.1007/s40618-020-01216-6
33. Wang Z, He L, Sun W, Qin Y, Dong W, Zhang T, et al. miRNA-299-5p regulates estrogen receptor alpha and inhibits migration and invasion of papillary thyroid cancer cell. Cancer Manag Res (2018) 10:6181–93. doi: 10.2147/CMAR.S182625
34. Zhu P, Liao L-Y, Zhao T-T, Mo X-M, Chen GG, Liu Z-M. GPER/ERK&AKT/NF-κB pathway is involved in cadmium-induced proliferation, invasion and migration of GPER-positive thyroid cancer cells. Mol Cell Endocrinol (2017) 442:68–80. doi: 10.1016/j.mce.2016.12.007
35. Magri F, Capelli V, Rotondi M, Leporati P, La Manna L, Ruggiero R, et al. Expression of estrogen and androgen receptors in differentiated thyroid cancer: an additional criterion to assess the patient’s risk. Endocr Relat Cancer (2012) 19(4):463–71. doi: 10.1530/ERC-11-0389
36. Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, et al. Association Between Biomarkers of Ovarian Reserve and Infertility Among Older Women of Reproductive Age. JAMA (2017) 318(14):1367–76. doi: 10.1001/jama.2017.14588
37. Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG (2017) 124(10):1501–12. doi: 10.1111/1471-0528.14640
39. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol (2003) 188(1):100–7. doi: 10.1067/mob.2003.99
40. Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nat Rev Dis Primers (2016) 2:16043. doi: 10.1038/nrdp.2016.43
41. Giannini R, Ugolini C, Lupi C, Proietti A, Elisei R, Salvatore G, et al. The heterogeneous distribution of BRAF mutation supports the independent clonal origin of distinct tumor foci in multifocal papillary thyroid carcinoma. J Clin Endocrinol Metab (2007) 92(9):3511–6. doi: 10.1210/jc.2007-0594
42. Pak K, Suh S, Goh TS, Kim S-J, Oh S-O, Seok JW, et al. BRAF-positive multifocal and unifocal papillary thyroid cancer show different messenger RNA expressions. Clin Endocrinol (Oxf) (2019) 90(4):601–7. doi: 10.1111/cen.13928
43. Shi R-L, Qu N, Luo T-X, Xiang J, Liao T, Sun G-H, et al. Programmed Death-Ligand 1 Expression in Papillary Thyroid Cancer and Its Correlation with Clinicopathologic Factors and Recurrence. Thyroid (2017) 27(4):537–45. doi: 10.1089/thy.2016.0228
44. Wu L, Lv C, Su Y, Li C, Zhang H, Zhao X, et al. Expression of programmed death-1 (PD-1) and its ligand PD-L1 is upregulated in endometriosis and promoted by 17beta-estradiol. Gynecol Endocrinol (2019) 35(3):251–6. doi: 10.1080/09513590.2018.1519787
45. Lin Y, Li T, Liang J, Li X, Qiu L, Wang S, et al. Predictive value of preablation stimulated thyroglobulin and thyroglobulin/thyroid-stimulating hormone ratio in differentiated thyroid cancer. Clin Nucl Med (2011) 36(12):1102–5. doi: 10.1097/RLU.0b013e3182291c65
46. Tam AA, Ozdemir D, Aydın C, Bestepe N, Ulusoy S, Sungu N, et al. Association between preoperative thyrotrophin and clinicopathological and aggressive features of papillary thyroid cancer. Endocrine (2018) 59(3):565–72. doi: 10.1007/s12020-018-1523-6
47. Jung SP, Kim M, Choe J-H, Kim JS, Nam SJ, Kim J-H. Clinical implication of cancer adhesion in papillary thyroid carcinoma: clinicopathologic characteristics and prognosis analyzed with degree of extrathyroidal extension. World J Surg (2013) 37(7):1606–13. doi: 10.1007/s00268-013-2034-5
48. Morand GB, da Silva SD, Mlynarek AM, Black MJ, Payne RJ, Hier MP. Clinicopathological relevance of antithyroglobulin antibodies in low-risk papillary thyroid cancer. Clin Otolaryngol (2017) 42(6):1130–4. doi: 10.1111/coa.12835
Keywords: female, thyroid, benign tumors, papillary thyroid cancer, pathology, surgery
Citation: Zhang J, Zhou L, Dionigi G, Zhang D, Zhao L, Liang N, Xue G and Sun H (2021) Association Between the Presence of Female-Specific Tumors and Aggressive Clinicopathological Features in Papillary Thyroid Cancer: A Retrospective Analysis of 9,822 Cases. Front. Oncol. 11:611471. doi: 10.3389/fonc.2021.611471
Received: 29 September 2020; Accepted: 25 January 2021;
Published: 11 March 2021.
Edited by:
Cesare Piazza, University of Brescia, ItalyCopyright © 2021 Zhang, Zhou, Dionigi, Zhang, Zhao, Liang, Xue and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Sun, c19oQGpsdS5lZHUuY24=
 Jiao Zhang
Jiao Zhang Le Zhou
Le Zhou Gianlorenzo Dionigi
Gianlorenzo Dionigi Daqi Zhang1
Daqi Zhang1 Nan Liang
Nan Liang Hui Sun
Hui Sun