- Department of Thoracic Surgery, The Second Affiliated Hospital of Harbin Medical University, Harbin, China
Background: Esophageal cancer is one of the most common cancer types, with its most common distant metastatic site being the lung. Currently, population-based data regarding the proportion and prognosis of patients with esophageal cancer with lung metastases (ECLM) at the time of diagnosis is insufficient. Therefore, we aimed to determine the proportion of patients with ECLM at diagnosis, as well as to investigate the prognostic parameters of ECLM.
Methods: This population-based observational study obtained data from the Surveillance, Epidemiology, and End Results (SEER) database registered between 2010 and 2016. Multivariable logistic regression was performed to identify predictors of the presence of ECLM at diagnosis. Multivariable Cox regression and competing risk analysis were used to assess prognostic factors in patients with ECLM. Median survival was estimated using Kaplan–Meier curves.
Results: Of 10,965 patients diagnosed with esophageal cancer between 2010 and 2016, 713 (6.50%) presented with initial lung metastasis at diagnosis. Lung metastasis represented 27.15% of all cases with metastatic disease to any distant site. Considering all patients with esophageal cancer, multivariable logistic regression indicated that pathology grade, pathology type, T staging, N staging, race, and number of extrapulmonary metastatic sites were predictive factors for the occurrence of lung metastases at diagnosis. The median survival time of patients with ECLM was 4.0 months. Patients receiving chemotherapy or chemoradiotherapy had the longest median overall survival, 7.0 months. Multivariable Cox regression indicated that age, histology type, T2 staging, number of extrapulmonary metastatic sites, and treatment (chemotherapy, radiotherapy, or chemoradiotherapy) were independent predictors for overall survival (OS). Multivariable competing risk analysis determined that age, number of extrapulmonary metastatic sites, and treatment were independent predictors for esophageal cancer-specific survival (CSS).
Conclusion: The findings of this study may provide important information for the early diagnosis of ECLM, as well as aid physicians in choosing appropriate treatment regimens for these patients.
Introduction
Esophageal cancer is the seventh most prevalent malignant tumor and has the sixth highest mortality rate worldwide (1). In 2019, 18,440 new esophageal cancer cases and 16,171 esophageal cancer-related deaths were registered in the United States (2). In Western countries, the incidence of esophageal cancer—especially esophageal adenocarcinoma—has increased over the recent decades (3–5). At the time of diagnosis, approximately 50% of patients with esophageal cancer have metastases to distant lymph nodes or other organs (6, 7). In general, the most common distant metastasis organs for esophageal cancer are, in descending order, the liver, lung, bone, and brain (8–10). The prognosis of esophageal cancer patients with distant metastases is very poor, with a 5-year survival rate <5% (11, 12). In recent years, the overall 5-year survival rate for patients with metastatic esophageal cancer may increase to approximately 20% in many countries owing to the development of new treatment methods and use of targeted drugs (13). Nonetheless, the establishment of an optimal treatment for esophageal cancer with distant metastasis (M1) requires further studies and clinical trials.
Wu et al. (13) suggested that surgery combined with radiotherapy could improve survival in patients with metastatic esophageal cancer; however, other studies had different perspectives (14, 15). Tanaka et al. (11) proposed chemoradiotherapy as an effective treatment for esophageal squamous cell carcinoma (ESCC) patients with distant metastasis. Additionally, the National Comprehensive Cancer Network (NCCN) guidelines recommend solely supportive and palliative care for these patients (16). Thus, treatment strategies for patients with M1 esophageal cancer remain controversial. To the best of our knowledge, a few population-based studies on esophageal cancer with distant metastasis have been published (17, 18). However, these studies did not provide population-level estimates of prognosis parameters for patients newly diagnosed with esophageal cancer with lung metastases (ECLM). Therefore, a population-based study providing detailed information about ECLM remains necessary to clarify the epidemiologic characteristics and prognosis associated with this disease.
The NCCN guidelines recommend using computed tomography (CT) and positron emission tomography (PET)/CT to estimate the clinical stage of esophageal cancer and determine whether distant metastases are present (16). For lung metastasis diagnosis, CT may be combined with fiberoptic bronchoscopy, endoscopic ultrasonography, and chest X-ray (19). Although CT is a routine imaging technique to monitor potential lung metastasis, its detection at the earliest stage remains a great challenge because of its small size and low density (20). With the advent of multidetector computed tomography (MDCT), especially 64-slice systems, the detection of small pulmonary nodules has improved (21). PET/CT has great advantages for excluding metastatic lesions; moreover, its positive and negative predictive values for distant metastatic diseases reached 68 and 99%, respectively (22). However, PET/CT is expensive, making it not cost-effective to use in diagnosis and evaluation. Therefore, identifying esophageal cancer patients who are at high-risk of lung metastases and determining predictive factors for ECLM occurrence are important. We believe that combining predictive factors with 64-slice MDCT may improve early and accurate diagnosis of ECLM.
In this population-based study, we used data of esophageal cancer patients with or without lung metastases from the Surveillance, Epidemiology, and End Results (SEER) database between 2010 and 2016 to determine the incidence of lung metastasis at diagnosis and to investigate predictive factors of lung metastasis detection at diagnosis on a population level. Additionally, we analyzed prognostic factors affecting the survival of ECLM patients and compared the effect of different treatments in disease prognosis.
Methods
Cohort Selection and Data Collection
This retrospective study analyzed publicly available data from the SEER database. We used SEER*stat software version 8.3.4 (with additional treatment from 1975 to 2016) to extract data from the SEER-18 database, which includes information on cancer incidence, treatment, and survival for approximately 28% of the US population (23). The most recent datapoints available in the SEER database date from 2016 based on submissions up until November 2018; these became available in April 2019. Moreover, the SEER database did not include any information regarding metastases location until 2010. Therefore, we analyzed data from patients diagnosed with esophageal cancer between January 1, 2010 and December 31, 2016, including as many medical records as possible.
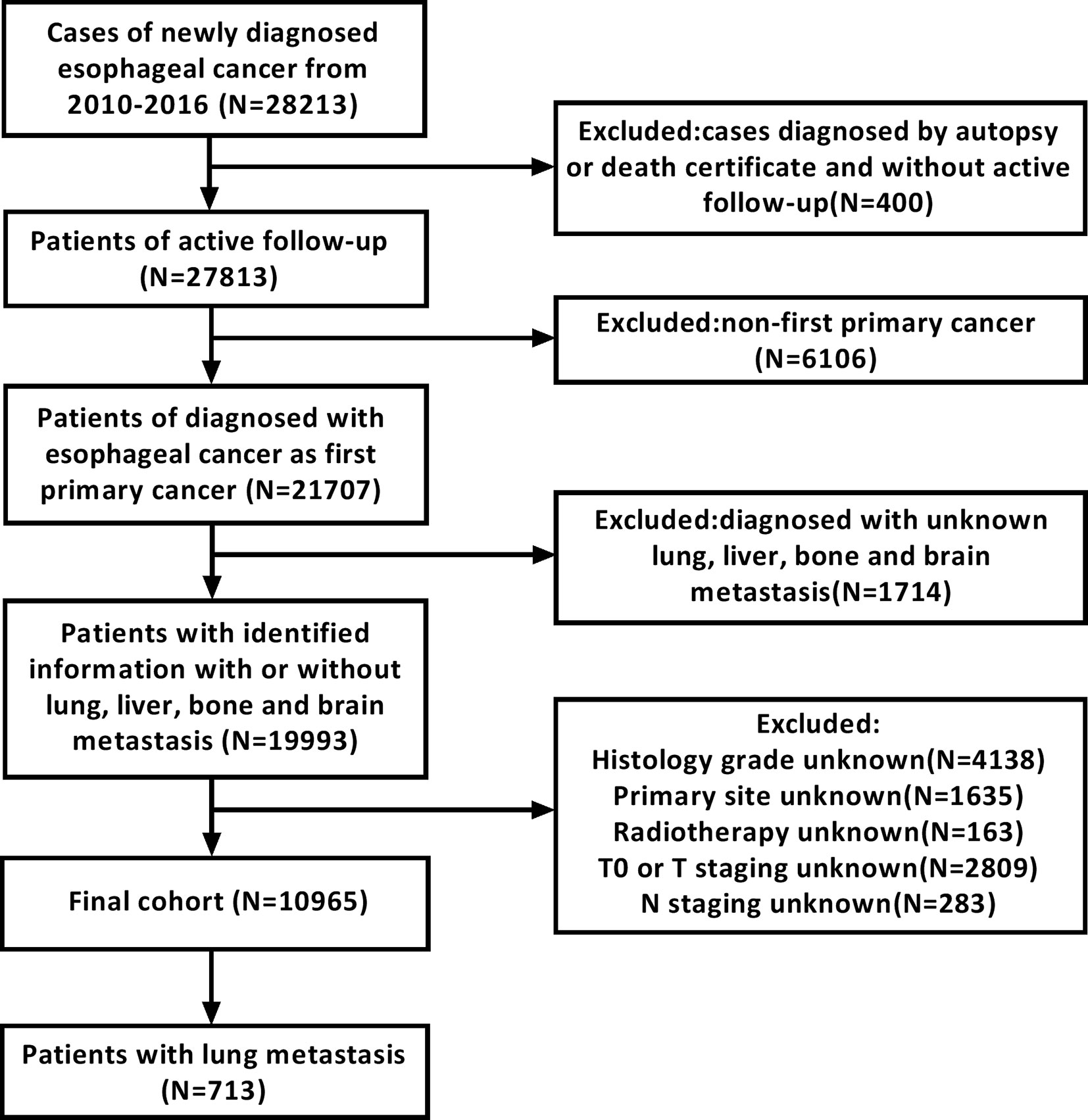
We screened the records of 28,213 patients who were initially diagnosed with esophageal cancer between 2010 and 2016. All patients were 18 years old or older. Exclusion criteria were as follows: 1) patients diagnosed based on death certificate or autopsy; 2) patients without active follow-up; 3) patients whose esophageal cancer was not the primary cancer; 4) lack of information about lung, liver, bone, and brain metastases at diagnosis; 5) patients whose esophageal cancer was stage T0; and 6) lack of information regarding histology grade, primary site, radiotherapy regimen, T staging, and N staging. A detailed data extraction flowchart is shown in Figure 1. Finally, 10,965 patients were included in the final study cohort. Of those, 2,626 patients were diagnosed with metastases to any distant site and 713 patients were diagnosed with lung metastases. Subsequently, we collected clinical and sociodemographic variables to conduct a descriptive statistical analysis and summarize the demographic and tumor characteristics of patients. Clinical variables included sex, age at diagnosis, tumor location, pathology grade, pathology type, tumor staging, T staging, N staging, treatment, race, and extra lung metastases number. Sociodemographic variables included insurance status, marital status, high school education, and median family income.
In this study, the optimal age cut-off points were determined using the X-tile program (http://www.tissuearray.org/rimmlab/), which can divide the cohort into three subsets and determine two optimal cut-off values using the minimum p-value from the log-rank χ2 statistics for patients’ age based on survival rates (24). Therefore, we used X-tile to identify the optimal age cut-off based on the esophageal cancer-specific mortality rate of ECLM patients. As shown in Figure 2, the optimal age cut-off points were 58 and 74 years; thus, we stratified the cohort into three age groups: 18–58 years old, 59–74 years old, and ≥75 years old. Tumor locations included lesions in the upper, middle, and lower esophagus, as well as overlapping lesions. Cancer pathology grade was classified into three categories: well-differentiated (Grade I), moderately differentiated (Grade II), and poorly differentiated or undifferentiated (Grade III/IV). Histological types were determined using the International Classification of Diseases for Oncology, third edition (ICD-O-3) (adenocarcinoma: 8140, 8144, 8145, 8210, 8211, 8244, 8255, 8260–8263, and 8323; squamous carcinoma: 8051, 8052, 8070–8075, and 8083; others: 8000, 8010, 8013, 8020, 8033, 8041, 8046, 8094, 8480, 8490, 8560, 8574, and 8980).
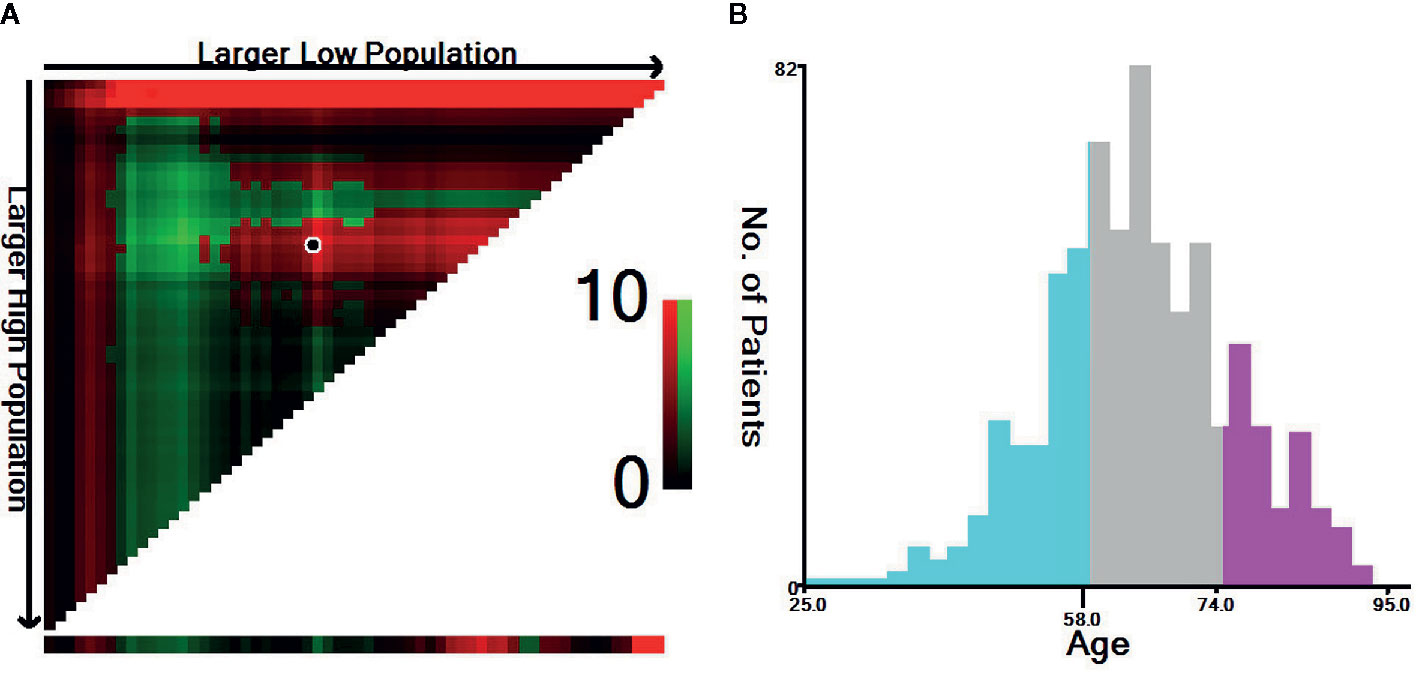
Figure 2 Identification of the optimal age cut-off points for esophageal cancer patients with lung metastasis. (A) X-tile plots based on age according to esophageal cancer-specific mortality. The plots show χ2 log-rank values; the brightest pixel represents the maximum χ2 log-rank value. (B) Distribution of patients according to age ranging from 25 to 95 years old. The optimal age cut-off of ECLM patients age is shown as 58 and 74 years old (χ2 = 9.65, P < 0.001).
TNM staging was based on the seventh edition of the Cancer Staging Manual of the American Joint Committee on Cancer (AJCC) (25). Because the eighth edition is the most commonly used classification at present, we converted the codes from the previous edition to their corresponding eighth-edition codes. T staging included T1, T2, T3, and T4; N staging included N0, N1, N2, and N3. Patients were also stratified according to treatment regimens: those who received radiotherapy (Ra), those who received chemotherapy (Che), those who received chemoradiotherapy (Che+Ra), and those who received none of these (No). In this study, the number and proportion of patients who received chemoradiotherapy were determined based on with/without chemotherapy patients number and with/without radiotherapy patients number.
Race contained white, black, and others. Insurance status was classified into insured, uninsured, and unknown. Marital status was divided into married, unmarried, and unknown. Educational levels were stipulated based on 10% increments with high school education as the base level. Median household income levels were defined using $20,000 increments. High school education and median family income were derived from the US Census American Community Survey obtained from the SEER*Stat software (26). Additionally, we investigated if the number of extrapulmonary metastases (in the liver, bone, brain, and other sites) at diagnosis was associated with the presence of metastasis in the lung and prognosis of ECLM patients.
Statistical Analysis
We used descriptive statistics to calculate the absolute number, percentage, and median survival (in months) of patients with ECLM at diagnosis. ECLM incidence was determined based on the proportion of ECLM patients among the entire cohort, as well as among those diagnosed with metastatic esophageal cancer to any distant site. All data were stratified by sex, age, tumor location, and other variables. Multivariable logistic regression was used to determine predictive factors for the presence of lung metastases at diagnosis. The variables included in the multivariable logistic regression analysis were selected based on an univariable logistic regression analysis to identify statistically significant variables (P < 0.05).
Survival estimates were obtained using the Kaplan–Meier method and compared using the log-rank test. We conducted an univariable Cox regression model to identify statistically significant variables (P < 0.05); subsequently, these variables were included in the multivariable Cox regression model to determine the covariates associated with all-cause mortality. To analyze the variables that affected esophageal cancer-specific mortality, we conducted Fine and Gray’s competing risk regression for univariable and multivariable analysis (27).
Statistical analyses were performed using SPSS (version 25.0; IBM Corp., Armonk, NY, USA) and R (version 3.3.2; R Foundation for Statistical Computing, Vienna, Austria) software. Logistic and Cox regression analyses were performed using SPSS. Prism 7.0 (GraphPad Software, San Diego, CA, USA) was used to plot Kaplan–Meier survival curves. R was used for competing risk analysis using the package “cmprsk” (version 2.2-72014). Two-sided P-values <0.05 were considered statistically significant.
Results
Patient Characteristics
Overall, 10,965 patients were diagnosed with esophageal cancer between 2010 and 2016 in the USA. Of these, 8,867 (80.87%) were men and 2,098 (19.13%) were women, with a median age of 66 years (range, 18–102 years). The proportion of patients diagnosed with esophageal adenocarcinoma was more than two-fold of patients diagnosed with ESCC (64.30 vs. 28.15%). Patients’ median survival was 11 months. Among all patients diagnosed with esophageal cancer, 2,626 presented with metastatic disease to any distant site and 713 presented with metastases in the lung. Regarding those with lung metastases, 594 (83.31%) were men and 119 (16.69%) were women. Moreover, those with lung metastases represented 6.50% of the entire cohort and 27.15% of patients with metastatic disease to any distant site. Patients’ clinical and demographic characteristics are shown in Table 1.
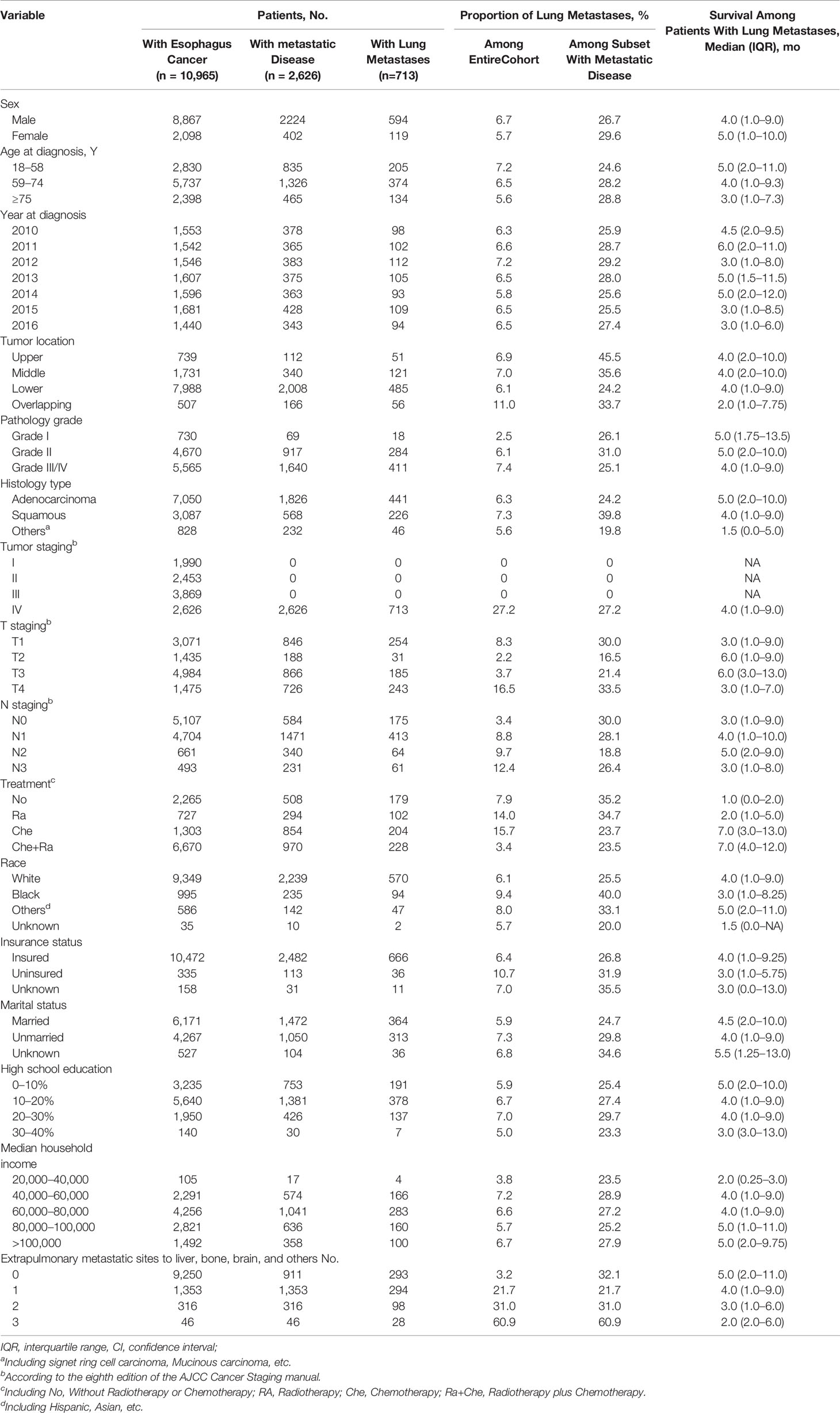
Table 1 Clinical Characteristics of Patients With Esophagus Cancer With Identified Lung Metastases at Diagnosis.
Predictors for the Presence of Lung Metastases
Univariable logistic regression analysis (Supplementary Table 1) identified 10 statistically significant variables (P < 0.05) among the entire cohort: age, tumor location, pathology grade, pathology type, T staging, N staging, race, insurance status, marital status, and number of extrapulmonary metastatic sites. These variables were included in the multivariable logistic regression analysis. As shown in Table 2, pathology grade, pathology type, T staging, N staging, race, and number of extrapulmonary metastatic sites were identified as statistically significant among the entire cohort.
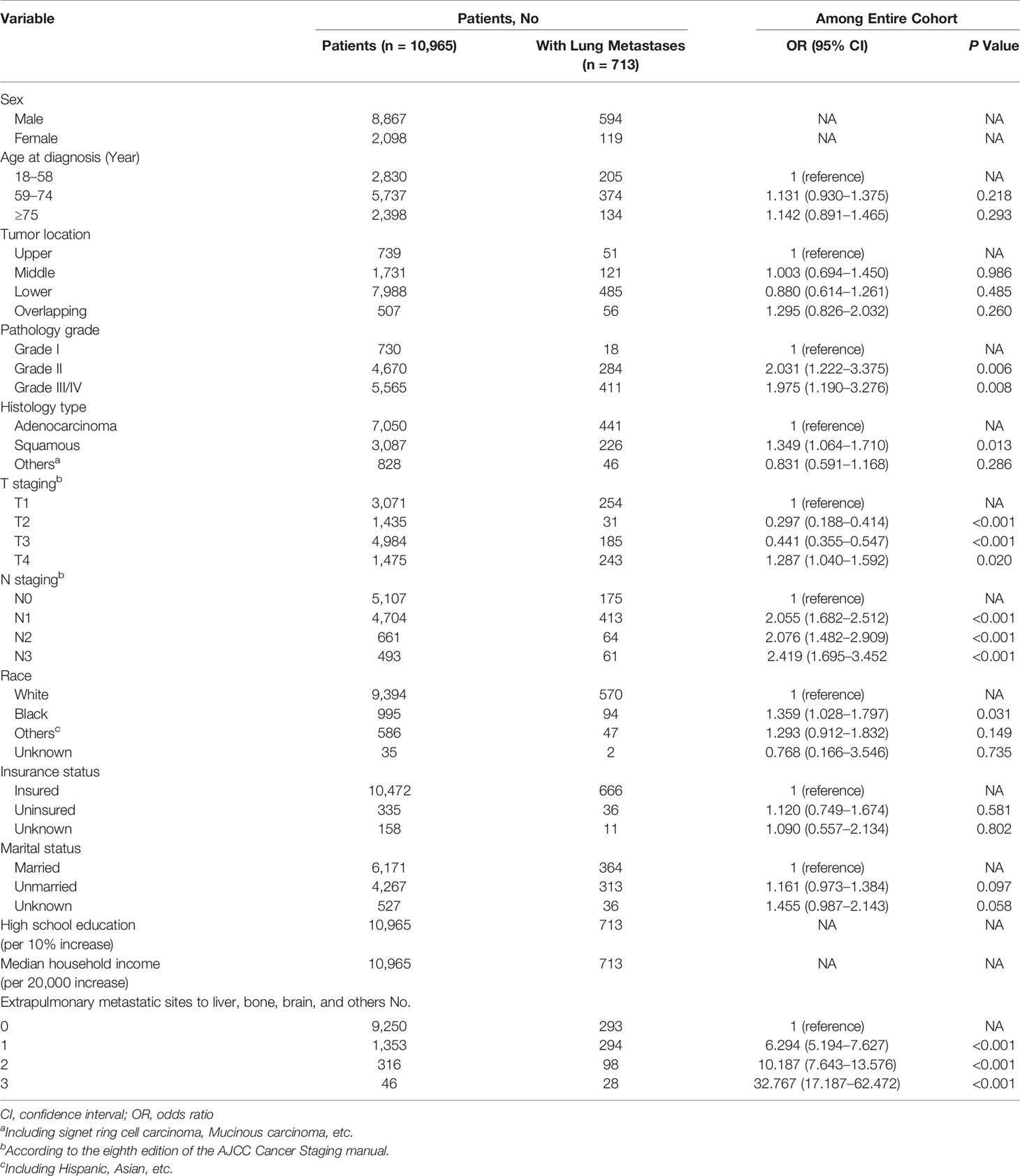
Table 2 Multivariable Logistic Regression for the Presence of Lung Metastases at Diagnosis of Esophagus Cancer.
Specifically, the following factors were associated with greater odds of lung metastasis presence at diagnosis: pathology grade II (vs. grade I; odds ratio [OR], 2.031; 95% confidence interval [CI], 1.222–3.375; P = 0.006), pathology grade III/IV (vs. grade I; OR, 1.975; 95% CI, 1.190–3.276; P = 0.008), squamous cell carcinoma (vs. adenocarcinoma; OR, 1.349; 95% CI, 1.064–1.710; P = 0.013), stage T4 (vs. T1; OR, 1.287; 95% CI, 1.040–1.592; P = 0.020), stage N1 (vs. N0; OR, 2.055; 95% CI, 1.682–2.512; P < 0.001), stage N2 (vs. N0; OR, 2.076; 95% CI, 1.482–2.909; P < 0.001), stage N3 (vs. N0; OR, 2.419; 95% CI, 1.695–3.452; P < 0.001), black race (vs. white; OR, 1.359; 95% CI, 1.028–1.797; P = 0.031), 1 extrapulmonary metastatic site (vs. 0 extrapulmonary metastatic site; OR, 6.294; 95% CI, 5.194–7.627; P < 0.001), 2 extrapulmonary metastatic sites (vs. 0 extrapulmonary metastatic site; OR, 10.187; 95% CI, 7.643–13.576; P < 0.001), and 3 extrapulmonary metastatic sites (vs. 0 extrapulmonary metastatic site; OR, 32.767; 95% CI, 17.187–62.472; P < 0.001). Conversely, the multivariable model indicated that sex, age, tumor location, insurance status, and marital status were not associated with the risk of lung metastasis presence at diagnosis. Additionally, stage T2 (vs. T1; OR, 0.297; 95% CI, 0.188–0.414; P < 0.001) and stage T3 (vs. T1; OR, 0.441; 95% CI, 0.355–0.547; P < 0.001) were associated with marginally lower odds of lung metastasis presence at diagnosis.
Considering these results, patients with esophageal cancer who presented with poor tumor grade, squamous cell carcinoma, T4 staging, late N staging, and presence of more metastatic sites, as well as black patients, had a higher risk of presenting with lung metastases at diagnosis, whereas T2 and T3 staging were considered protective factors for lung metastasis at diagnosis.
Survival
For all-cause mortality among patients with metastatic disease to any distant site, univariable Cox regression analysis identified twelve variables that were significantly associated with overall survival (P < 0.05): sex, age, pathology type, T staging, N staging, number of extrapulmonary metastatic sites, race, insurance situation, marital status, high school education, median household income, and treatment (Supplementary Table 2). These variables were included in the multivariable Cox regression analyses. The statistics showed that eight variables were significantly associated with all-cause mortality for esophageal cancer patients with distant metastases (P < 0.05), including sex, age, pathology type, T staging, number of extrapulmonary metastatic sites, marital status, median household income, and treatment. Detailed statistical results are shown in Supplementary Table 3.
Univariable Cox regression analysis for all-cause mortality among patients with ECLM identified seven variables that were significantly associated with overall survival (P < 0.05): age, tumor location, pathology type, T staging, number of extrapulmonary metastatic sites, median household income, and treatment type. Regarding esophageal cancer-specific mortality, univariable competing risk analysis identified four variables that were significantly associated with cancer-specific survival (P < 0.05): age, pathology type, number of extrapulmonary metastatic sites, and treatment type. Details of the univariable analysis of mortality among patients with ECLM are displayed in Supplementary Table 4.
The variables identified using univariable analyses were included in multivariable analyses (Table 3). Multivariable Cox regression analysis indicated that the following factors were associated with an increase in all-cause mortality among patients with ECLM: age ≥75 years (vs. 18–58 years; hazard ratio [HR], 1.481; 95% CI, 1.168–1.877; P = 0.001), other pathology types (vs. adenocarcinoma; HR, 1.769; 95% CI, 1.275–2.454; P = 0.001), 1 extrapulmonary metastatic site (vs. 0 extrapulmonary metastatic site; HR, 1.190; 95% CI, 1.333–1.107; P=0.002), and ≥2 extrapulmonary metastatic sites (vs. 0 extrapulmonary metastatic site; HR, 1.822; 95% CI, 1.419–2.339; P < 0.001). Conversely, stage T2 (vs. T1; HR, 0.633; 95% CI, 0.404–0.992; P = 0.046), Ra treatment (vs. No treatment; HR, 0.648; 95% CI, 0.500–0.839; P = 0.001), Che treatment (vs. No treatment; HR, 0.260; 95% CI, 0.209–0.325; P < 0.001), and Che+Ra treatment (vs. No treatment; HR, 0.259; 95% CI, 0.206–0.325; P < 0.001) were significantly associated with a decrease in all-cause mortality. Tumor location and median household income were not significantly associated with all-cause mortality (P > 0.05).
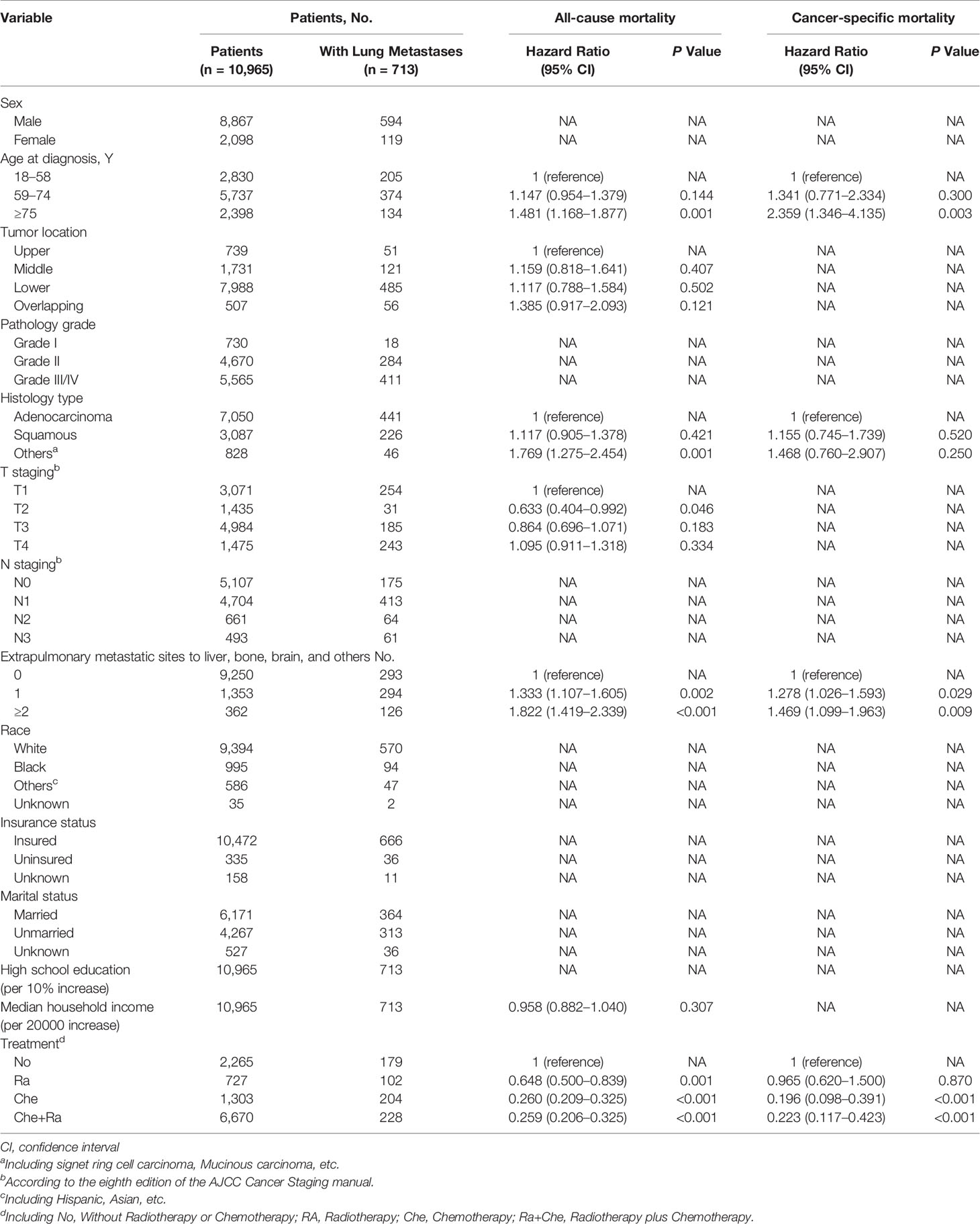
Table 3 Multivariable Cox Regression for All-Cause Mortality and Esophageal Cancer Specific Mortality Among Patients With Lung Metastases.
Multivariable competing risk analysis for esophageal cancer-specific mortality among patients with ECLM identified the following factors associated with an increase in esophageal cancer-specific mortality: age ≥75 years (vs. 18–58 years; HR, 2.359; 95% CI, 1.346–4.135; P = 0.003), 1 extrapulmonary metastatic site (vs. 0 extra-pulmonary metastatic site; HR, 1.278; 95% CI, 1.026–1.593; P=0.029), ≥2 extrapulmonary metastatic sites (vs. 0 extrapulmonary metastatic site; HR, 1.469; 95% CI, 1.099–1.963; P = 0.009). Conversely, Che treatment (vs. No treatment; HR, 0.169; 95% CI, 0.098–0.391; P < 0.001) and Che+Ra treatment (vs. No treatment; HR, 0.223; 95% CI, 0.117–0.423; P < 0.001) were significantly associated with a decrease in esophageal cancer-specific mortality. Pathology type (including squamous cell carcinoma, adenocarcinoma, and others) and Ra treatment were not associated with esophageal cancer-specific mortality.
Multivariable Cox regression analysis indicated that other histology types were associated with poor overall survival, whereas T2 staging was associated with improved overall survival. Furthermore, considering the results of both multivariable models, patients with ECLM who were 75 years old or older had a worse prognosis, which was consistent with the optimal age cut-off points for patients with ECLM (Figure 2). Moreover, the multivariable analyses indicated that those with an extensive systemic disease at diagnosis had poor survival, while those who received positive treatment (radiotherapy, chemotherapy, or chemoradiotherapy) had improved survival.
Survival estimates obtained using the Kaplan–Meier method indicated that the median survival of patients with ECLM was 4.0 months (interquartile range [IQR], 1.0–9.0 months) (Figure 3A). The median survival of patients with ECLM aged 18–58 years was 5.0 months (IQR, 2.0–11.0 months); 59–74 years, 4.0 months (IQR, 1.0–9.3 months); and ≥75 years, 3.0 months (IQR, 1.0–7.3 months) (Figure 3B). The median survival of those with no extrapulmonary metastatic site was 5.0 months (IQR, 2.0–11.0 months); 1 extrapulmonary metastatic site, 4.0 months (IQR, 1.0–9.0 months); with ≥2 extrapulmonary metastatic sites, 3.0 months (IQR, 1.0–6.0 months) (Figure 3C). Finally, the median survival of patients with ECLM who received No treatment was 1.0 month (IQR, 0.0–2.0 months); Ra treatment, 2.0 months (IQR, 1.0–5.0 months); Che treatment, 7.0 months (IQR, 3.0–13.0 months); and Che+Ra treatment, 7.0 months (IQR, 4.0–12.0 months) (Figure 3D). In summary, those treated with chemotherapy or chemoradiotherapy had the best prognosis, while those who received no treatment had the worst prognosis.
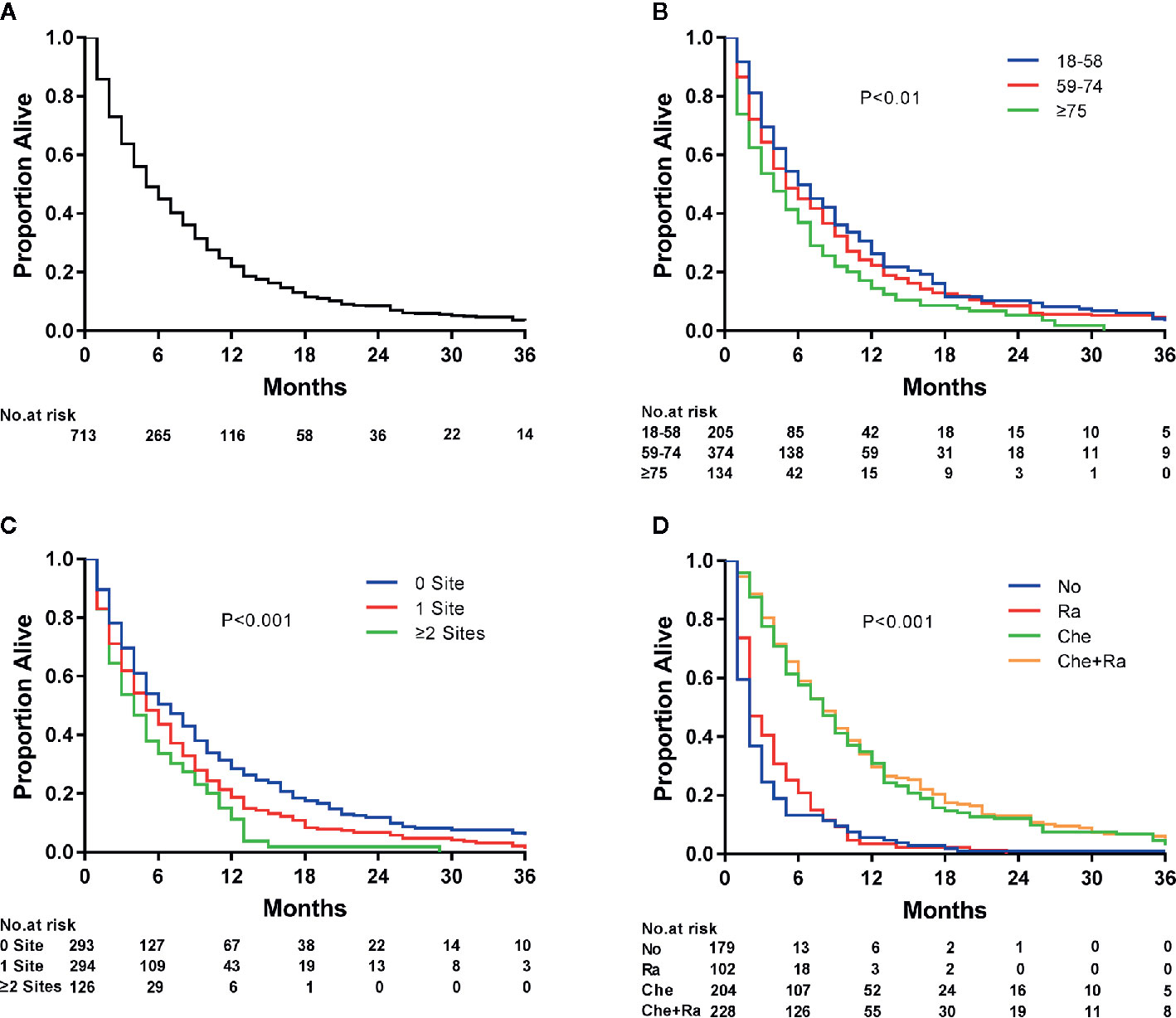
Figure 3 Kaplan–Meier analysis of overall survival among esophageal cancer patients with lung metastasis at diagnosis. (A) overall, (B) stratified by age, (C) stratified by the extent of extrapulmonary metastatic disease, and (D) stratified by type of treatment.
Among all patients with ECLM, 102 (14.31%) received radiotherapy, 204 (28.61%) received chemotherapy, and 228 (31.98%) received chemoradiotherapy. Among the entire cohort, 727 (6.63%) patients received radiotherapy, 1,303 (11.88%) received chemotherapy, and 6,670 (60.83%) received chemoradiotherapy. Therefore, those who received chemoradiotherapy represented the majority in the entire cohort and among patients with ECLM.
Discussion
To the best of our knowledge, this is the first population-based study to describe the incidence proportion of ECLM and analyze the predictive and prognostic factors associated with the outcomes of patients with ECLM at diagnosis. We used the most recent data on esophageal cancer available in the SEER database, identifying and including 713 patients with ECLM in our study—possibly the largest number of patients with ECLM included in a study. We used descriptive statistical and logistic regression analyses to investigate the factors associated with ECLM at diagnosis. Moreover, we used three statistical methods (Kaplan–Meier curves, Cox regression, and competing risk analyses) to obtain survival estimates. The results presented in this study may aid physicians in timely detecting lung metastases and choosing appropriate treatment methods.
We demonstrated that 6.50% of patients with esophageal cancer had lung metastases at diagnosis, and 27.15% of esophageal cancer patients with any distant metastases at diagnosis had lung metastases. These results were similar to those of a previous study that also used data from the SEER database (17); conversely, our results were higher than those reported by another study based on the SEER database (28). This discrepancy may be caused by the use of different exclusion criteria. Nonetheless, our study included a larger cohort than the abovementioned studies, indicating that our analysis had greater statistical power. A previous study reported that lung metastases represented 20% of all metastatic esophageal cancer cases (14), a proportion slightly lower than that found in our study. This difference may be explained by an increase in the proportion of metastatic diseases over the past 20 years, as well as the development of diagnostic technologies that can detect the presence of lung metastases more efficiently. An autopsy-based study reported that 52% of patients had lung metastases, the largest proportion ever reported (29). The authors of this study hypothesized that differences in race and sex between patients included in their cohort and those included in other studies were the main reason for this discrepancy, as well as the use of data with very few postoperative deaths.
Our and other studies have shown that patients with squamous cell carcinoma were more likely to develop lung metastasis compared with those with other esophageal cancer histological types (17, 28). The aforementioned autopsy-based study (29) was based solely on autopsy findings of patients with ESCC, representing an additional reason for the high proportion of lung metastases found in their study. Another autopsy-based study reported that 31% of patients with metastatic esophageal cancer had lung metastases (30), a proportion slightly higher than that found in our study. Their autopsy series included patients with recurred or post-treatment lung metastases, whereas we exclusively included those with lung metastases at diagnosis. In addition, metastases are more easily detected during autopsy than during physical and imaging examinations performed at the time of esophageal cancer diagnosis. Accordingly, the proportion of patients with ECLM found in our study and in other SEER-based studies are consistently lower than those found in autopsy-based studies (17, 28–30).
In our study, we used multivariable logistic regression analysis to determine predictive factors for the presence of lung metastasis at diagnosis and identify those at increased risk of having lung metastases. We showed that patients with poor tumor grade, squamous cell carcinoma histological type, T4 staging, late N staging, and more extrapulmonary metastatic sites, as well as black patients, had an increased risk of having lung metastases at diagnosis compared with the entire cohort. Regarding pathology grades, among the entire cohort, 6.66, 42.59, and 50.75% of patients with lung metastases had diseases grade I, II, and III/IV, respectively. Among those with metastatic esophageal cancer, 2.63, 34.92, and 62.45% of patients with lung metastases had diseases grade I, II, and III/IV, respectively. Those with disease grade II and III/IV had a significantly greater likelihood of presenting with lung metastases at diagnosis than those with disease grade I. These results are in accordance with previous studies (17, 31, 32). Higher pathology grades are considered more malignant; accordingly, several studies have demonstrated that pathology grade is a strong survival predictor, with higher grades indicating a poor prognosis (33, 34). Nonetheless, the mechanism behind the association between higher pathology grades and lung metastasis occurrence requires further elucidation.
Regarding tumor pathology types, patients diagnosed with squamous cell carcinoma had a higher proportion of lung metastases than those diagnosed with adenocarcinoma. This finding is in accordance with previously mentioned studies (17, 28), as well as with other Japanese studies that reported the lung as the most common distant metastasis site in ESCC (35, 36). We hypothesized that the higher proportion of lung metastasis in ESCC may be related with tumor location because most esophageal adenocarcinomas were located in the lower esophagus, whereas ESCC was more evenly distributed throughout the upper, middle, and lower esophagus, with the middle esophagus representing the largest proportion (8, 31). However, the underlying mechanisms of distant metastasis responsible for the differences observed between these two pathology subtypes remain unclear.
Regarding T staging, tumors in T4 stage had a significantly higher percentage of lung metastasis compared with those of tumors in T2 and T3 stages. Furthermore, tumors in late N staging had a higher proportion of lung metastasis than that of tumors in early N staging. Accordingly, a previous study reported that T and N staging were the greatest contributors in metastasis prediction (32). Sakanaka et al. (37) demonstrated that patients with larger metastatic lymph nodes were at a higher risk of developing diseases with distant metastases. Therefore, many studies have recognized late T staging and N staging as important factors for the occurrence of distant metastasis in esophageal cancer. In our study, T2 and T3 staging were associated with a lower risk of lung metastasis at diagnosis. Most T and N staging included in the SEER database were based on clinical staging using CT and other imaging examinations; consequently, the staging may be less accurate than pathological and autopsy-based staging used in other studies (38).
Black patients had a significantly greater likelihood of presenting with lung metastases at diagnosis than white patients. The reason for this difference remains unknown and requires further elucidation (38). In our study, esophageal cancer patients with more extrapulmonary metastatic sites had a significantly higher risk of lung metastasis. This finding is similar to those of studies on other metastatic malignant tumors, such as breast and gastric cancers (38, 39). We hypothesized that hematogenous and lymphatic dissemination caused by metastatic sites may increase the occurrence of distant metastases. Further studies focusing on this topic are warranted.
In clinical practice, CT scanning is often used to detect lung metastases; however, the detection of early metastatic lesions in the lung using this imaging technique is challenging (20). Conversely, 64-slice MDCT has great advantages for detecting small lung nodules (21). Patients poor tumor grade, squamous cell carcinoma histological type, T4 staging, late N staging, and more extrapulmonary metastatic sites, as well as black patients, should receive increased attention during clinical examination. Specifically, we suggest that these patients should undergo 64-slice MDCT scanning for lung nodules screening and, if necessary, pulmonary puncture pathology to ensure the early diagnosis of lung metastases. Timely diagnosis is important to assure that patients will receive appropriate treatment as soon as possible, significantly prolonging their survival and improving their quality of life (11, 17, 31).
In our study, we used competing risks analysis to identify variables affecting esophageal cancer-specific mortality. This analysis is based on events that occurred prior to the primary event of interest. Therefore, when predicting disease-specific outcomes, competing risk analysis provides a better estimation for the clinical prognosis of patients, helping clinicians to apply appropriate therapy strategies (40). Multivariable Cox regression analysis among patients with lung metastases indicated that patients with other histology types (e.g., signet ring cell carcinoma) had poorer OS than those with adenocarcinoma owing to their more aggressive biological behaviors (41). Interestingly, patients with T2-stage tumors had a better prognosis than those with T1-stage tumors. This finding may have been influenced by T staging inaccuracies in the SEER database.
Our study showed that patients who were 75 years old or older and with more extrapulmonary metastatic sites had a significantly lower overall survival and cancer-specific survival, while patients who received positive treatment (radiotherapy, chemotherapy, or chemoradiotherapy) had better prognoses. Patients aged ≥75 years had a shorter survival time than those aged 18–58 years. This discrepancy may be a result of poorer physical fitness and reduced natural life expectancy of older adults. Furthermore, from our findings, we observed that more extrapulmonary metastatic sites were consistently associated with poorer survival, which is in accordance with the results of other studies (17, 18). This trend is similar to that observed in other malignant tumors (38, 39). Therefore, a higher number of metastatic sites may indicate a poor prognosis in malignant diseases in general.
As evidenced by the Kaplan–Meier curves, radiotherapy, chemotherapy, and chemoradiotherapy treatment increased the median survival of patients with ECLM by 1, 6, and 6 months, respectively, compared with no treatment. The median survival of patients who received chemotherapy or chemoradiotherapy did not differ significantly (P = 0.366). Furthermore, patients who received radiotherapy had a better prognosis than patients who received no therapy (P < 0.01). However, similarly to the results of another SEER-based study (42), radiotherapy did not significantly affect cancer-specific mortality. In contrast, other studies have reached different conclusions (12, 43). Differently from these studies, we focused on patients with ECLM and their cancer-specific mortality, which may explain the result discrepancy. A prospective study suggested that chemoradiotherapy was superior to radiotherapy alone for treating patients with esophageal cancer (44). Another retrospective study showed that patients with stage IV B ESCC who underwent multimodality therapy, especially chemoradiotherapy, had significantly better survival than those who underwent single-modality therapy and supportive care (11). Similarly, we also found that chemoradiotherapy was superior to radiotherapy alone (P < 0.01). Furthermore, Wang et al. (45) reported that local therapy with radiation (median dose, 5,040 cGy) after initial palliative chemotherapy could achieve better local control and long-term survival in patients with stage IV B esophageal cancer. Guttmann et al. (46) demonstrated that chemotherapy combined with definitive dose radiotherapy (≥5,040 cGy) to the primary tumor could improve survival in patients with metastatic esophageal cancer compared with chemotherapy alone, whereas chemotherapy combined with palliative dose radiotherapy (<5,040 cGy) had a slightly worse prognosis than that of chemotherapy alone. Thus, radiation dose has a significant impact on the prognosis of patients with metastatic esophageal cancer. Because the SEER database did not provide radiation dose information, we were unable to evaluate this aspect. This lack of radiotherapy dose data may also explain why we observed no differences in prognosis between chemotherapy and chemoradiotherapy.
The NCCN guidelines recommend solely palliative and supportive care for patients with metastatic esophageal cancer (16). Previous studies also indicated that patients with stage IV esophageal cancer should not undergo esophagectomy (14, 15). In our study, among 713 patients with ECLM, only 22 underwent surgery for primary tumor treatment. Thus, the number patients in the surgery sub-cohort was insufficient to perform a survival analysis and assess the effect of surgery on patients’ outcomes. Other studies have encountered this same limitation (18, 47). Therefore, randomized controlled and multicenter trials are required to determine whether surgery is an effective treatment option for patients with M1 esophageal cancer.
Presently, treatment strategies for esophageal cancer with distant metastasis remain controversial. Several studies, as well as the NCCN guidelines, demonstrated that surgery was inappropriate for M1 esophageal cancer because of patients’ short life expectancy and attendant risks (14–16). Moreover, no large-scale prospective studies have demonstrated whether surgery has a beneficial effect on the prognosis of patients with M1 esophageal cancer. Conversely, many studies showed that multimodality therapy (chemoradiotherapy) provided better treatment results for these patients (11, 43–45). In our study, most patients received chemoradiotherapy, achieving a median survival of 7 months. Over the past 10 years, targeted therapy (e.g., trastuzumab) for treating HER2-positive advanced and metastatic esophageal cancer has received increased attention and achieved positive results (13, 48). Thus, non-surgical multimodality treatments may represent the most appropriate choices for treating M1 esophageal cancer, including ECLM.
Limitation
This study has some limitations. First, we utilized information from the SEER database, which is derived from a retrospective study and may carry inherent biases. Second, we only considered patients who had lung metastasis at initial diagnosis because data regarding patients who developed lung metastases during their disease course was not included in the SEER database. Third, all statistical analyses were based on the population of the United States and may not represent the population of other countries or regions. Fourth, education level and median family income were determined at a county level instead of a patient level, which may have affected the results of the uni- and multivariate analysis conducted in this study. Fifth, the SEER database did not include information regarding recurrence rate and mortality after treatment, which may have affected the evaluation of treatment effects. Finally, the SEER database did not provide details regarding chemotherapy drugs or radiotherapy dose and target. The incomplete information may have affected grouping accuracy, as well as the results derived from these groupings.
Conclusion
To the best of our knowledge, this population-based study was the first to analyze patients with ECLM at initial diagnosis. Our study provided information regarding the epidemiology of lung metastases in these patients. Considering the factors that may predict the occurrence of lung metastasis at diagnosis, high-risk patients should undergo a 64-slice MDCT examination for small lung nodules screening. According to our findings, chemotherapy or chemoradiotherapy may represent the most advantageous treatments for patients with ECLM. Therefore, our study may provide useful information to help physicians in early diagnosis and selection of appropriate treatment for patients with ECLM, ultimately improving the outcomes of these patients.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/.
Author Contributions
JG, SZ, HL, and LZ contributed to the conception and design of this research. JG, SZ, HL, and LZ performed the data acquisition and analysis. JG and SZ wrote the first draft of the manuscript. MH, TL, and JZ wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the staff members of the National Cancer Institute and the researchers who have been involved with the Surveillance, Epidemiology, and End Results (SEER) Program.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.603953/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.1002/ijc.31937
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
3. Coleman HG, Xie SH, Lagergren J. The Epidemiology of Esophageal Adenocarcinoma. Gastroenterology (2018) 154:390–405. doi: 10.1053/j.gastro.2017.07.046
4. Rubenstein JH, Shaheen NJ. Epidemiology, diagnosis, and management of esophageal adenocarcinoma. Gastroenterology (2015) 149(2):302–17. doi: 10.1053/j.gastro.2015.04.053
5. Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut (2015) 64(3):381–7. doi: 10.1136/gutjnl-2014-308124
6. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med (2003) 349(23):2241–52. doi: 10.1056/NEJMra035010
7. Horner MJ, Ries LAG, Krapcho M, Aminou R, Howlader N, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2006. Bethesda, MD: National Cancer Institute (2009). Available at: http://seer.cancer.gov/csr/1975_2006/.
8. Mariette C, Balon JM, Piessen G, Fabre S, Van Seuningen I, Triboulet JP. Pattern of recurrence follow-ing complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer (2003) 97(7):1616–23. doi: 10.1002/cncr.11228
9. D’Journo XB. Clinical implication of the innovations of the 8 edition of the TNM classification for esophageal and esophago-gastric cancer. J Thorac Dis (2018) 10:S2671–81. doi: 10.21037/jtd.2018.03.182
10. Chen MQ, Xu BH, Zhang YY. Analysis of prognostic factors for esophageal squamous cell carcinoma with distant organ metastasis at initial diagnosis. J Chin Med Assoc (2014) 77(11):562–6. doi: 10.1016/j.jcma.2014.05.014
11. Tanaka T, Fujita H, Matono S, Nagano T, Nishimura K, Murata K, et al. Outcomes of multimodalitytherapy for stage IVB esophageal cancer with distant organ metastasis (M1-Org). Dis Esophagus (2010) 23(8):646–51. doi: 10.1111/j.1442-2050.2010.01069.x
12. Wu SG, Xie WH, Zhang ZQ, Sun JY, Li FY, Lin HX, et al. Surgery combined with radiotherapy improved survival in metastatic esophageal cancer in a surveillance epidemiology and end results population-based study. Sci Rep (2016) 6:28280. doi: 10.1038/srep28280
13. Forde PM, Kelly RJ. Chemotherapeutic and targeted strategies for locally advanced and metastatic eso-phageal cancer. J Thorac Oncol (2013) 8(6):73–84. doi: 10.1097/JTO.0b013e31828b5172
14. Quint LE, Hepburn LM, Francis IR, Whyte RI, Orringer MB. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer (1995) 76(7):1120–5. doi: 10.1002/1097-0142(19951001)76:7<1120::aid-cncr2820760704>3.0.co;2-w
15. Saddoughi SA, Reinersman JM, Zhukov YO, Taswell J, Mara K, Harmsen SW, et al. Survival After Surgical Resection of Stage IV Esophageal Cancer. Ann Thorac Surg (2017) 103(1):261–6. doi: 10.1016/j.athoracsur.2016.06.070
16. Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and EsophagogastricJunction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2019) 17(7):855–83. doi: 10.6004/jnccn.2019.0033
17. Ai D, Zhu H, Ren W, Chen Y, Liu Q, Deng J, et al. Patterns of distant organ metastases in esophageal cancer: a population-based study. J Thorac Dis (2017) 9(9):3023–30. doi: 10.21037/jtd.2017.08.72
18. Wu SG, Zhang WW, He ZY, Sun JY, Chen YX, Guo L. Sites of metastasis and overall survival in esophageal cancer: a population-based study. Cancer Manag Res (2017) 9:781–8. doi: 10.2147/CMAR.S150350
19. van Vliet EP, Steyerberg EW, Eijkemans MJ, Kuipers EJ, Siersema PD. Detection of distant metastases inpatients with oesophageal or gastric cardia cancer: a diagnostic decision analysis. Br J Cancer (2007) 97(7):868–76. doi: 10.1038/sj.bjc.6603960
20. Chen H, Huang S, Zeng Q, Zhang M, Ni Z, Li X, et al. A retrospective study analyzing missed diagnosis of lung metastases at their early stages on computed tomography. J Thorac Dis (2019) 11(8):3360–8. doi: 10.21037/jtd.2019.08.19
21. Xie X, Willemink Martin J, de Jong Pim A, van Ooijen PM, Oudkerk M, Vliegenthart R, et al. Small irregular pulmonary nodules in low-dose CT: observer detection sensitivity and volumetry acc-uracy. AJR Am J Roentgenol (2014) 202(3):202–9. doi: 10.2214/AJR.13.10830
22. Noble F, Bailey D, SWCIS Upper Gastrointestinal Tumour Panel, Tung K, Byrne JP. Impact of integrated PET/CT in the staging of oesophageal cancer: a UK population-based cohort study. Clin Radiol (2009) 64(7):699–705. doi: 10.1016/j.crad.2009.03.003
23. Surveillance Epidemiology and End Results (SEER) Program. (2019). Available at: http://www.seer.cancer.gov (Accessed October 27, 2019).
24. Camp RL, Dolled-Filhart M, Rimm DL. X-tile:a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res (2004) 10(21):7252–9. doi: 10.1158/1078-0432.CCR-04-0713
25. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol (2010) 17(6):1471–4. doi: 10.1245/s10434-010-0985-4
26. US Census Bureau. US Census 2000 Gateway (2019). Available at: http://www.census.gov/main/www/cen2000.html (Accessed October 27, 2019).
27. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc (1999) 94:496–509. doi: 10.1080/01621459.1999.10474144
28. Wu SG, Zhang WW, Sun JY, Li FY, Lin Q, He ZY, et al. Patterns of Distant Metastasis Between Histological Types in Esophageal Cancer. Front Oncol (2018) 8:302:302. doi: 10.3389/fonc.2018.00302
29. Anderson LL, Lad TE. Autopsy findings in squamous-cell carcinoma of the esophagus. Cancer (1982) 50(8):1587–90. doi: 10.1002/1097-0142(19821015)50:8<1587::aid-cncr2820500820>3.0.co;2-s
30. Mandard AM, Chasle J, Marnay J, Villedieu B, Bianco C, Roussel A, et al. Autopsy findings in 111cases of esophageal cancer. Cancer (1981) 48(2):329–35. doi: 10.1002/1097-0142(19810715)48:2<329::aid-cncr2820480219>3.0.co;2-v
31. Ai D, Chen Y, Liu Q, Deng J, Zhao K. The effect of tumor locations of esophageal cancer on themetastasis to liver or lung. J Thorac Dis (2019) 11(10):4205–10. doi: 10.21037/jtd.2019.09.67
32. Zhu C, You Y, Liu S, Ji Y, Yu J. A Nomogram to Predict Distant Metastasis for Patients with Esophageal Cancer. Oncol Res Treat (2020) 43:2–9. doi: 10.1159/000503613
33. Rice TW, Rusch VW, Apperson-Hansen C, Allen MS, Chen LQ, Hunter JG, et al. Worldwide esophageal cancer collaboration. Dis Esophagus (2009) 22(1):1–8. doi: 10.1111/j.1442-2050.2008.00901.x
34. Dickson GH, Singh KK, Escofet X, Kelley K. Validation of a modified GTNM classification in peri-junctional oesophago-gastric carcinoma and its use as a prognostic indicator. Eur J Surg Oncol (2001) 27(7):641–4. doi: 10.1053/ejso.2001.1200
35. Natsugoe S, Okumura H, Matsumoto M, Uchikado Y, Setoyama T, Uenosono Y, et al. The role of salvagesurgery for recurrence of esophageal squamous cell cancer. Eur J Surg Oncol (2006) 32(5):544–7. doi: 10.1016/j.ejso.2006.02.014
36. Sugiyama M, Morita M, Yoshida R, Ando K, Egashira A, Takefumi O, et al. Patterns and time of re-currence after complete resection of esophageal cancer. Surg Today (2012) 42(8):752–8. doi: 10.1007/s00595-012-0133-9
37. Sakanaka K, Ishida Y, Itasaka S, Ezoe Y, Aoyama I, Miyamoto S, et al. Identification of a predictive factor for distant metastasis in esophageal squamous cell carcinoma after definitive chemoradiotherapy. Int J Clin Oncol (2016) 21(5):899–908. doi: 10.1007/s10147-016-0967-z
38. Sun Z, Zheng H, Yu J, Huang W, Li T, Chen H, et al. Liver Metastases in Newly Diagnosed Gastric Cancer:A Population-Based Study from SEER. J Cancer (2019) 10(13):2991–3005. doi: 10.7150/jca.30821
39. Martin AM, Cagney DN, Catalano PJ, Warren LE, Bellon JR, Punglia RS, et al. Brain Metastases in Newly Diagnosed Breast Cancer: A Population-Based Study. JAMA Oncol (2017) 3(8):1069–77. doi: 10.1001/jamaoncol.2017.0001
40. Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation (2016) 133(6):601–9. doi: 10.1161/CIRCULATIONAHA.115.017719
41. Nafteux PR, Lerut TE, Villeneuve PJ, Dhaenens JM, De Hertogh G, Moons J, et al. Signet ring cells in esophageal and gastroesophageal junction carcinomas have a more aggressive biological behavior. Ann Surg (2014) 260(6):1023–9. doi: 10.1097/SLA.0000000000000689
42. Wu XX, Chen RP, Chen RC, Gong HP, Wang BF, Li YL, et al. Nomogram predicting cancer-specific mortality in patients with esophageal adenocarcinoma: a competing risk analysis. J Thorac Dis (2019) 11(7):2990–3003. doi: 10.21037/jtd.2019.07.56
43. Wojcieszynski AP, Berman AT, Wan F, Plastaras JP, Metz JM, Mitra N, et al. The impact of radiation therapy sequencing on survival and cardiopulmonary mortality in the combined modality treatment of patients with esophageal cancer. Cancer (2013) 119(11):1976–84. doi: 10.1002/cncr.27970
44. Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med (1992) 326(24):1593–8. doi: 10.1056/NEJM199206113262403
45. Wang J, Suri JS, Allen PK, Liao Z, Komaki R, Ho L, et al. Factors Predictive of Improved Outcomes With Multimodality Local Therapy After Palliative Chemotherapy for Stage IV Esophageal Cancer. Am J Clin Oncol (2016) 39(3):228–35. doi: 10.1097/COC.0000000000000066
46. Guttmann DM, Mitra N, Bekelman J, Metz JM, Plastaras J, Feng W, et al. Improved Overall Survival with Aggressive Primary Tumor Radiotherapy for Patients with Metastatic Esophageal Cancer. J Thorac Oncol (2017) 12(7):1131–42. doi: 10.1016/j.jtho.2017.03.026
47. Liu M, Wang C, Gao L, Lv C, Cai X. A nomogram to predict long-time survival for patients with M1 diseases of esophageal cancer. J Cancer (2018) 9(21):3986–90. doi: 10.7150/jca.27579
48. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet (2010) 376(9742):687–97. doi: 10.1016/S0140-6736(10)61121-X
Keywords: esophageal cancer, lung metastases, survival, treatment, SEER program
Citation: Guo J, Zhang S, Li H, Hassan MOO, Lu T, Zhao J and Zhang L (2021) Lung Metastases in Newly Diagnosed Esophageal Cancer: A Population-Based Study. Front. Oncol. 11:603953. doi: 10.3389/fonc.2021.603953
Received: 04 December 2020; Accepted: 11 January 2021;
Published: 25 February 2021.
Edited by:
Zhe-Sheng Chen, St. John’s University, United StatesReviewed by:
Jun Lyu, First Affiliated Hospital of Jinan University, ChinaXibao Yu, Sun Yat-sen University Cancer Center (SYSUCC), China
Zhongbing Wu, Hebei Medical University, China
Copyright © 2021 Guo, Zhang, Li, Hassan, Lu, Zhao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linyou Zhang, aG11bHl6aGFuZ0BvdXRsb29rLmNvbQ==
†These authors have contributed equally to this work
 Jida Guo
Jida Guo Shengqiang Zhang
Shengqiang Zhang Huawei Li
Huawei Li