
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 05 March 2021
Sec. Thoracic Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.601185
A correction has been applied to this article in:
Corrigendum: Clinical Characteristics and Prognosis of Patients With Pulmonary Mucoepidermoid Carcinoma: A SEER-Based Analysis
 Lingxiao Qiu1,2†‡
Lingxiao Qiu1,2†‡ Pan Song3†‡
Pan Song3†‡ Pingmei Chen4†
Pingmei Chen4† Huaqi Wang1,5,6
Huaqi Wang1,5,6 Fangfang Li1
Fangfang Li1 Mengxuan Shu7
Mengxuan Shu7 Gen-cheng Gong8
Gen-cheng Gong8 Xiangjin Song1
Xiangjin Song1 Chun Huang1
Chun Huang1 Hongxia Jia1
Hongxia Jia1 Nana Li1
Nana Li1 Guojun Zhang1,5,6*†
Guojun Zhang1,5,6*†Background: Primary pulmonary mucoepidermoid carcinoma (PMEC) is an extremely rare malignancy. Its clinical characteristics and prognosis are not fully understood. This study evaluated clinical characteristics and prognostic factors of PMEC and established a nomogram to predict its 1-, 3-, 5- and 10-year cancer-specific survival (CSS) rates.
Methods: In the Surveillance, Epidemiology, and End Results database from January 1, 1975 to December 31, 2016, patients pathologically diagnosed with PMEC were identified. Kaplan–Meier analysis and Cox regression were performed to evaluate the CSS stratified by different covariates. A predictive nomogram model was built and validated by the concordance index (C-index) and calibration curves.
Results: A total of 585 PMEC patients were identified. A total of 408 (70%) of patients were placed into the training cohort, and 177 (30%) patients were placed into the validation cohort. The 5- and 10-year CSS rates of stage I–II PMEC patients were 91.4 and 88.9, respectively. The 1-, 3- and 5-year CSS rates of stage III–IV PMEC were 56.5, 39.45, and 32.1%, respectively. Survival curves showed that older age, large tumor size, poor differentiation, and high TNM stage were associated with a significantly worse prognosis. CSS outcomes were significantly better in patients who received surgical treatments (surgical alone, surgery plus radiation and/or chemotherapy). Patients who received radiation and/or chemotherapy had the worst prognosis. Multivariate Cox results revealed that covariates, including age, tumor laterality, tumor sizes, pathological differentiation, lymph node metastasis, distant metastasis, TNM stage and therapy, were independent prognostic factors for PMEC. These factors were used to construct a nomogram. The C-index of the nomogram was 0.921. The calibration curve presented favorable consistency between the predicted CSS and actual observations. This nomogram was validated by the validation cohort. The C-index of the validation cohort was 0.968.
Conclusion: Age, bilateral tumors, tumor size, pathological differentiation grade, lymph node metastasis, distant metastasis, TNM stage and therapy were independent prognostic factors of PMEC patients. The first nomogram for predicting the CSS of PMEC was built and validated, showing its potential value in practice.
Lung cancer is the most commonly diagnosed cancer in both males and females globally (1–3). It has become the leading cause of cancer-associated death among males in both developed and developing countries, as well as the leading cause of cancer death among females in developed countries (1–3). Mucoepidermoid carcinoma (MEC) is a subtype of salivary gland tumors (SGTs), which represent a group of fairly rare lung tumors (4). It is estimated that MEC accounts for only less than 1% of primary malignant lung neoplasms (5, 6). MEC often occurs in the parotid gland, submandibular gland, sublingual gland and lacrimal gland, and is infrequently seen in the lungs (7–9). Due to its low frequency of pulmonary origination, little is known about the demographics, treatment, survival, or prognostic factors associated with primary pulmonary mucoepidermoid carcinoma (PMEC). To date, the current characterization and prognosis of PMEC have been informed mostly by case reports and small case series (6, 10–12). Even the largest SGT series to date—with 699 MEC patients from the National Cancer Database (NCDB)—did not analyze PMEC patients separately from MEC alone (13). Thus, there is an opportunity to profile the understanding of PMEC by assessing it in a more targeted series.
The Surveillance, Epidemiology, and End Results (SEER) database consists of 18 cancer registries across the United States, which covers approximately 30% of the US population with cancer diagnosis, treatment, and survival data. The extensive tumor patient information in the SEER database has a large advantage for analyzing the features and prognosis of rare cancers. In our study, we aimed to examine the characteristics, survival, and prognostic factors of PMEC patients with the SEER database.
The data in this study were derived from the SEER database with the software SEER*Stat version 8.3.5. Patients diagnosed with primary PMEC were retrospectively identified from January 1, 1975 to December 31, 2016.
Patients were eligible if they met the following criteria: (1) patients were diagnosed with lung cancer; and (2) the diagnosis of PMEC was confirmed by histology or exfoliative cytology.
The following criteria were used for data exclusion: (1) multiple primary tumors; (2) unclear or incomplete follow-up time; and (3) the survival state at the end of follow-up was unclear.
The following basic characteristics were collected and sorted: age at diagnosis (≤35, 36–60, 61–74, and ≥75), sex (male or, female), race (white, black, and other races, including American Indian and Asian/Pacific Islander), primary tumor site (main bronchus, upper lobe, middle lobe, lower lobe, overlapping lesion of the lung, and lung NOS), tumor laterality (left, right, one side but side unspecified, and bilateral), tumour sizes, pathological differentiation grade (well differentiated, moderate differentiation, poor differentiation, and undifferentiated), T stage (T1, T2, T3, T4, and Tx), N stage (N0, N1, N2, N3, and Nx), M stage (M0, M1, and Mx), therapy (surgery, radiation or chemotherapy, and combined therapy, including surgery + radiation, surgery + chemotherapy, and surgery + radiation +chemotherapy), survival months and survival status (alive, dead from PMEC, and dead from other reasons). Cancer-specific survival (CSS) was the main outcome.
The included patients were randomly divided into a training cohort (70%) and validation cohort (30%) by the random number methods. Baseline characteristics including age, sex, race, primary tumor site, laterality, tumour sizes, pathological differentiation grade, TNM stage, T stage, N stage, M stage, and therapy, were described. Kaplan–Meier analysis was utilized for the CSS of PMEC patients according to the above factors. The 1-, 5-, 10-, and 20-year CSS rates of PMEC patients with AJCC TNM stages I–II and III–I III–IV were calculated with survival tables. Survival curves were constructed, and hazard ratios (HRs) with 95% confidence intervals (95% CIs) were calculated.
Univariate Cox regression analysis was used to evaluate each variable’s value for predicting CSS. A multivariate Cox regression model was used to analyze variables with P < 0.05 in univariate analyses. A nomogram model was built with the coefficients of each factor in multivariate Cox analysis. The concordance index (C-index) and calibration curves were performed to evaluate the predictive accuracy of the nomogram. Validation of the nomogram model was conducted with the validation cohort.
All statistical analyses were performed with SPSS version 25, R software version 3.2.3 and GraphPad Prism 7.0. P < 0.05 was considered statistically significant.
The sociodemographics, tumor attributes, and treatment of PMEC are outlined in Table 1. A total of 585 eligible PMEC patients with a median age of 54 (35–68) years were identified. PMEC was more common in younger populations (59.4% of patients were aged less than 60 years versus 40.5% of patients were aged more than 60 years). Surgical resection was performed in 83.7% of PMEC tumor patients. Of the patients who were treated without surgery, the majority were treated with chemotherapy and/or radiation (9.4% of all patients). A total of 408 (70%) patients were randomly placed into a training cohort, and 177 (30%) patients were placed into a validation cohort. The median follow-up time of the included patients was 37 (8–124) months.
The OS and CSS rates of PMEC patients varied greatly with TNM stage (Table 2). For the patients with stage I–II PMEC, the 3-, 5- and 10-year OS rates were 88.3, 85.5, and 79.6, respectively. The 3-, 5-, and 10- year CSS rates were 94.7, 94.2, and 91.4%, respectively. For the patients with stage III–IV PMEC, the 1-, 3- and 5- year OS rates were 47.7, 28.2, and 23.8%, respectively. The 1-, 3- and 5- year of CSS rates were 56.5, 39.45, and 32.1%, respectively.
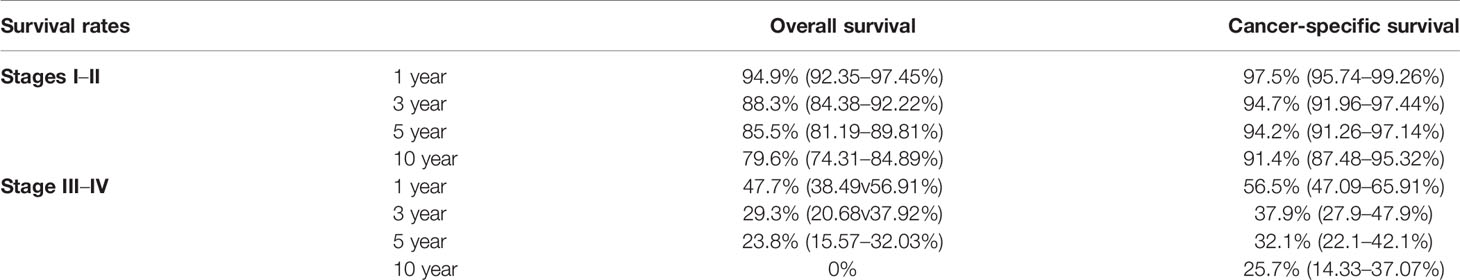
Table 2 1-, 3-, 5- and 10-year overall survival and cancer-specific survival rates of patients with PMEC stages I–II and stages III–IV.
As shown in Figure 1A, the prognosis for PMEC patients decreased significantly with age (P < 0.001). There was no association between prognosis and sex (Figure 1B) or race (Figure 1C). PMEC patients with tumors originating in the upper lobe had a worse prognosis than those with primary tumors in the middle or lower lobes (Figure 1D). The CSS of PMEC patients with bilateral tumors was significantly shorter than that of PMEC patients with unilateral tumors (P < 0.001) (Figure 1E). Differences were observed in the survival curve between the periods of 2010–2016 and 1975–2009 (Figure 1F).
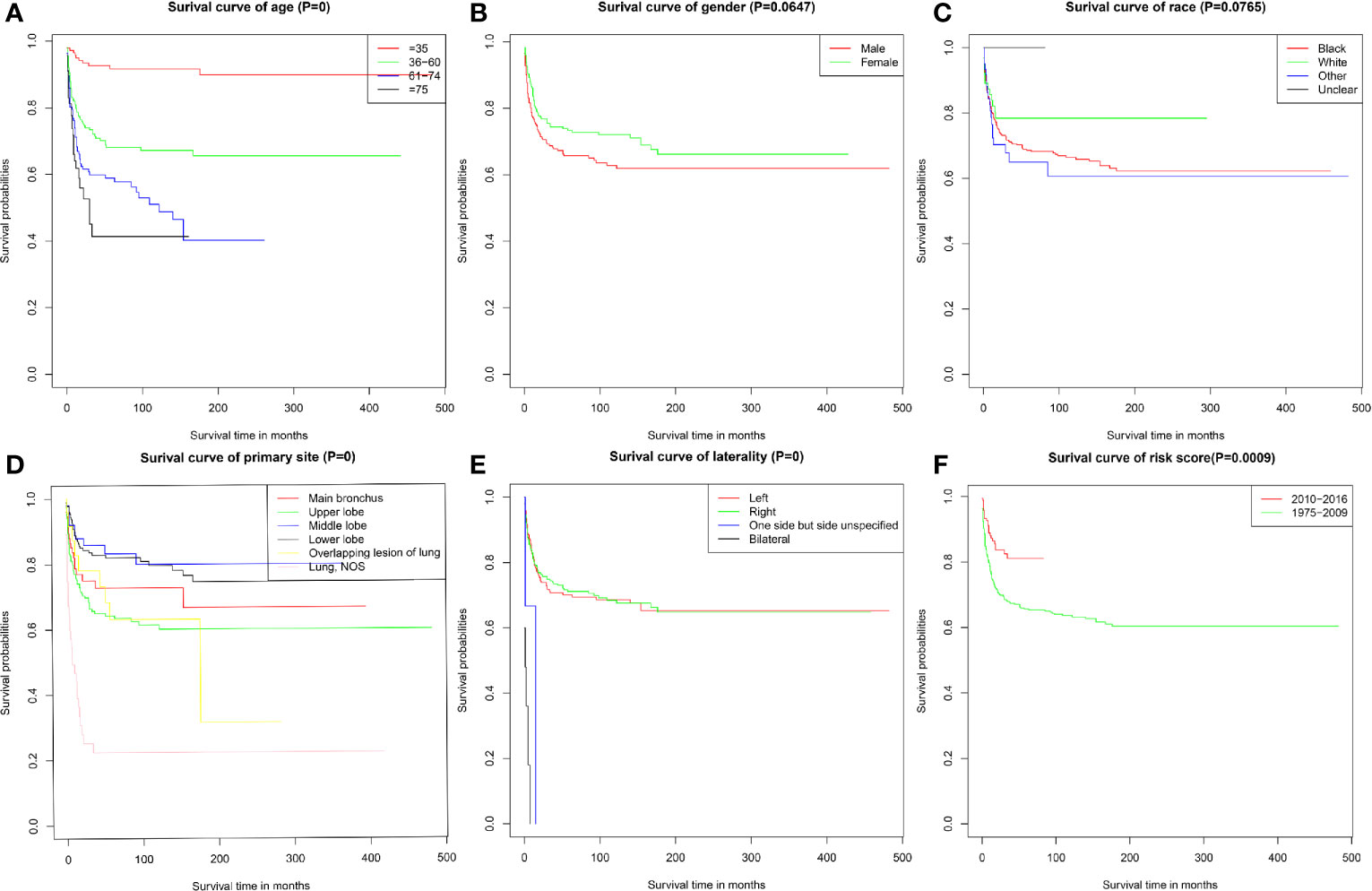
Figure 1 Cancer-specific survival of PMEC patients stratified by (A) age; (B) gender; (C) race; (D) primary site; (E) laterality; (F) time.
The survival curve also revealed that PMEC patients with poorly differentiated or undifferentiated tumors had much worse survival outcomes than those with highly or moderately differentiated tumors (P < 0.001) (Figure 2A). Patients with more advanced TNM stages had obviously worse survival outcomes (Figure 2B). With TNM stage I as the reference, the HRs and 95%CIs of stages II, III, and IV were 6.68 (2.72–16.41), 9.70 (5.0–18.83) and 34.33 (18.99–62.06), respectively. Patients with stage T3 and T4 disease had significantly worse survival outcomes than those with stage T1 and T2 disease (P < 0.001) (Figure 2C). The prognosis for PMEC patients was much worse for patients with the invasion of lymph nodes (P < 0.001) (Figure 2D). The CSS of PMEC patients with distant metastases was significantly shorter than that of PMEC patients without distant metastases (P < 0.001) (Figure 2E).
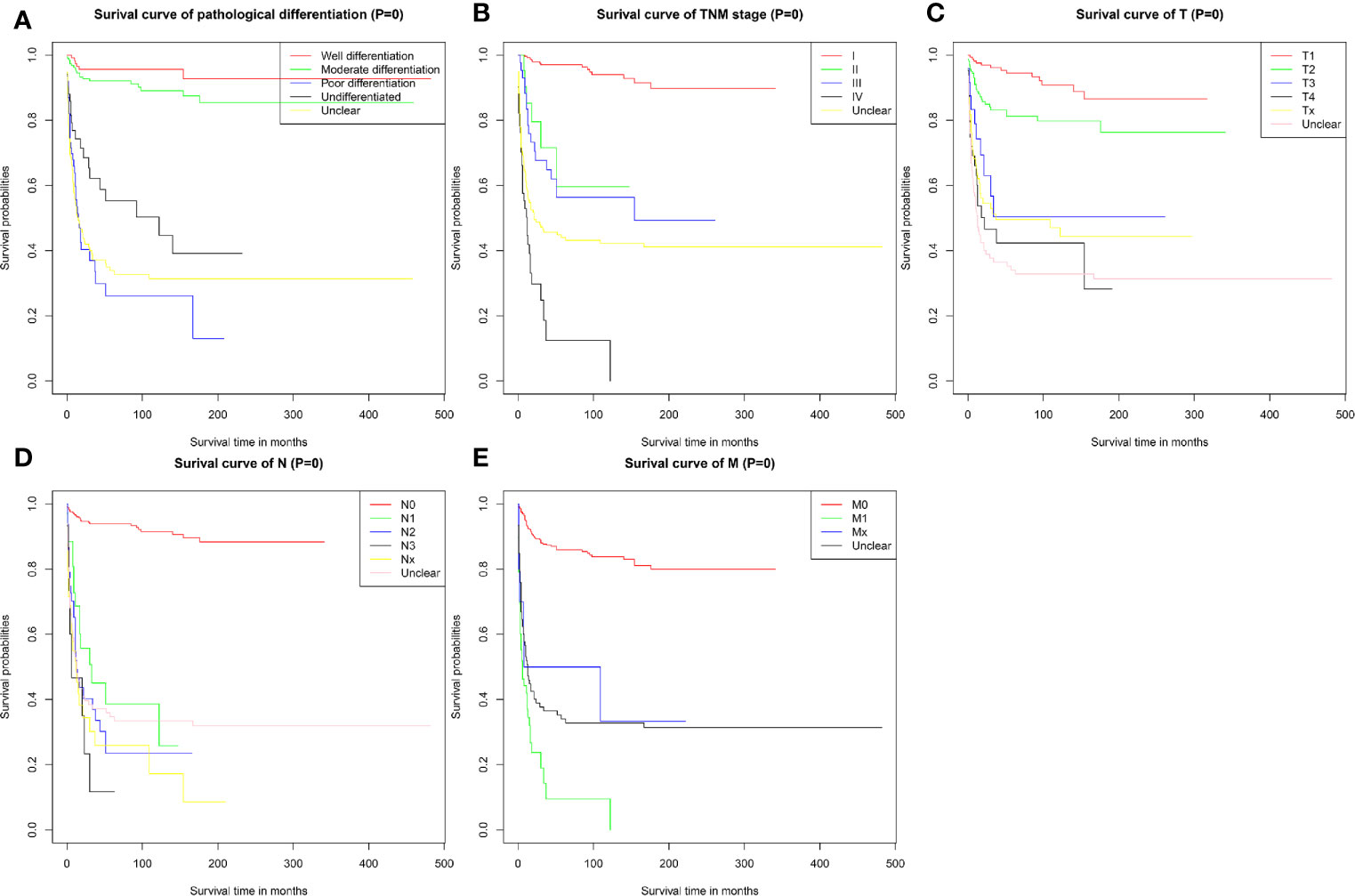
Figure 2 CSS for patients with PMEC stratified by (A) pathological differentiation grade; (B) TNM stage; (C) T stage; (D) N stage; (E) M stage.
For PMEC patients with TNM stage I–II, the results of survival curves showed that patients who underwent cancer-directed surgery had obviously better survival outcomes than those who underwent other treatments (radiation and/or chemotherapy, surgery plus radiation and/or chemotherapy) [CSS HR: 13.07, 95% CI (2.25–76.03), Figure 3A1; OS HR: 5.77, 95% CI (1.89–17.62), Figure 3A2].
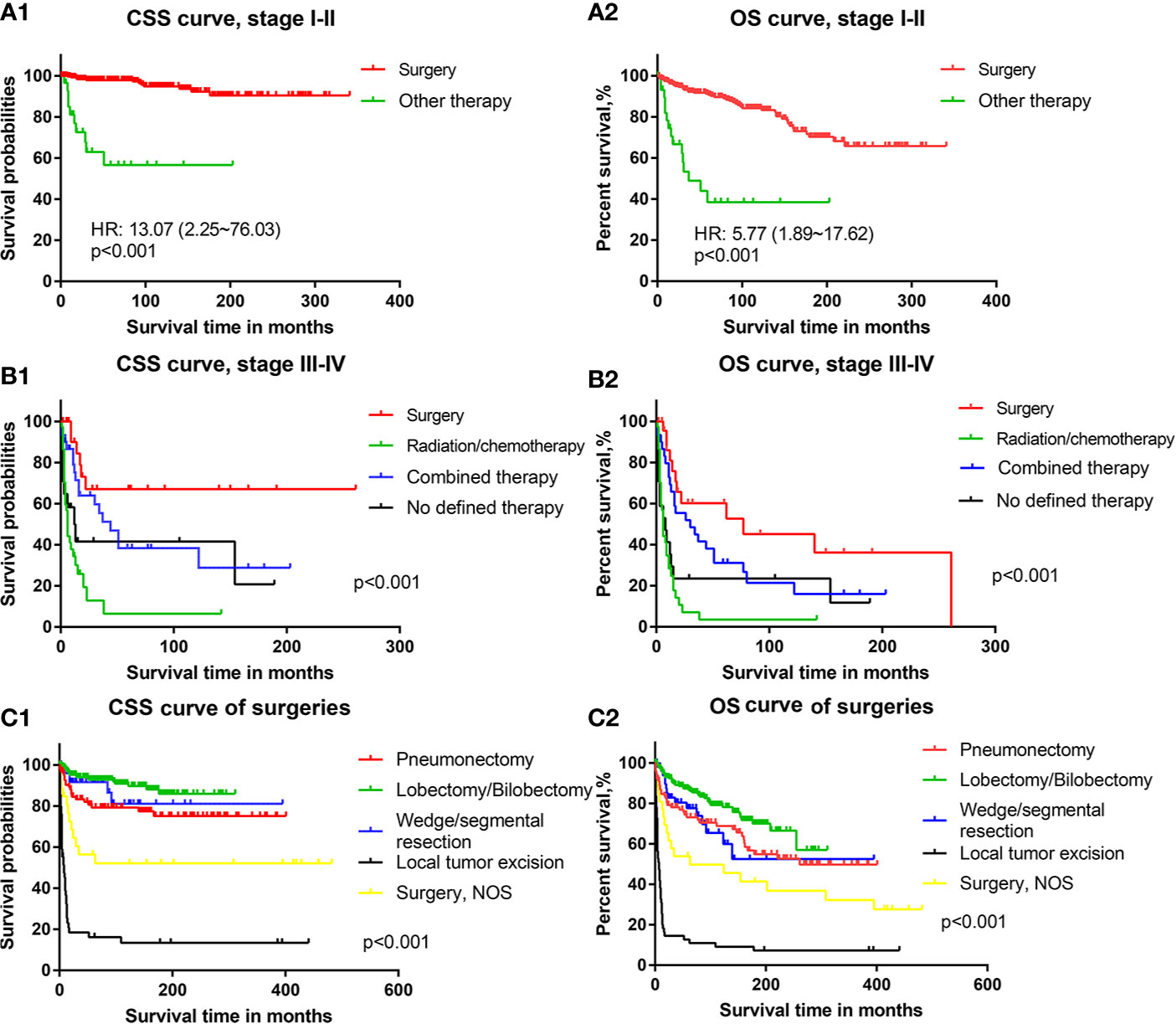
Figure 3 The cancer-specific survival and overall survival of patients with different treatments: (A1) Cancer-specific survival of PMEC patients in TNM stages I–II with surgery or other therapy. (A2) Overall survival of PMEC patients in TNM stages I–II with surgery or other therapy. (B1) Cancer-specific survival of PMEC patients in TNM stages III–IV with surgery, radiation/chemotherapy, combined therapy and no defined therapy. (B2) Overall survival of PMEC patients in TNM stages III–IV with surgery, radiation/chemotherapy, combined therapy and no defined therapy. (C1) Cancer-specific survival of PMEC patients with pneumonectomy, lobectomy/bilobectomy, wedge/segmental resection and local tumor excision. (C2) Overall survival of PMEC patients with pneumonectomy, lobectomy/bilobectomy, wedge/segmental resection and local tumor excision.
For stage III–IV PMEC patients, the survival results showed that surgical therapy was associated with the highest CSS rate, followed by combined surgery plus radiation and/or chemotherapy. Radiation and/or chemotherapy alone was the worst among all selective therapies (Figures 3B1, B2). With surgical therapy as the reference, the CSS HRs (95% CIs)of combined therapy and radiation/chemotherapy were 2.29 (1.01–5.20) and 5.79 (2.95–11.39), respectively. For the OS of PMEC patients with stage III–IV, there was no significant difference between surgery and combined therapy. Survival outcomes of radiation and/or chemotherapy were still the worst among all therapies. With surgical therapy as the reference, the HR and 95% CI of combined therapy and radiation and/or chemotherapy were 1.74 (0.90–3.37) and 3.95 (2.16–7.22), respectively.
Among patients undergoing surgical treatments, those who received pneumonectomy, lobectomy/bilobectomy and wedge/segmental resection had an excellent long-term CSS (Figure 3C1). Patients receiving lobectomy/bilobectomy were associated with better prognoses than those receiving pneumonectomy. Prognoses of patients who underwent local tumor excision were the worst among all methods of surgery. With lobectomy/bilobectomy therapy as the reference, the CSS HR and 95% CI of pneumonectomy, wedge/segmental resection and local tumor excision were 2.37 (1.20–4.67), 1.61 (0.58–4.62) and 17.87 (8.13–39.31), respectively. Similar results were revealed by the OS curve of patients with PMEC (Figure 3C2). Patients with local tumor excision had a poor prognosis in terms of both CSS outcomes (Figure 3C1) and OS outcomes (Figure 3C2). With lobectomy/bilobectomy therapy as the reference, the OS HR and 95% CI of pneumonectomy, wedge/segmental resection and local tumor excision were 1.68 (1.06–2.65), 1.80 (0.90–3.61) and 9.92 (5.13–19.18), respectively.
Univariate and multivariate Cox results revealing potential prognostic factors were presented in Table 3. Multivariate Cox analysis revealed that elderly age (>60 years), bilateral tumors, large tumor sizes (>30 mm), poorly differentiated and undifferentiated tumors, lymph node metastases and distant metastases were independent prognostic factors of worse survival in PMEC patients (Table 3). Conversely, cancer-directed surgery was an independent protective prognostic factor for PMEC patients (Table 3).
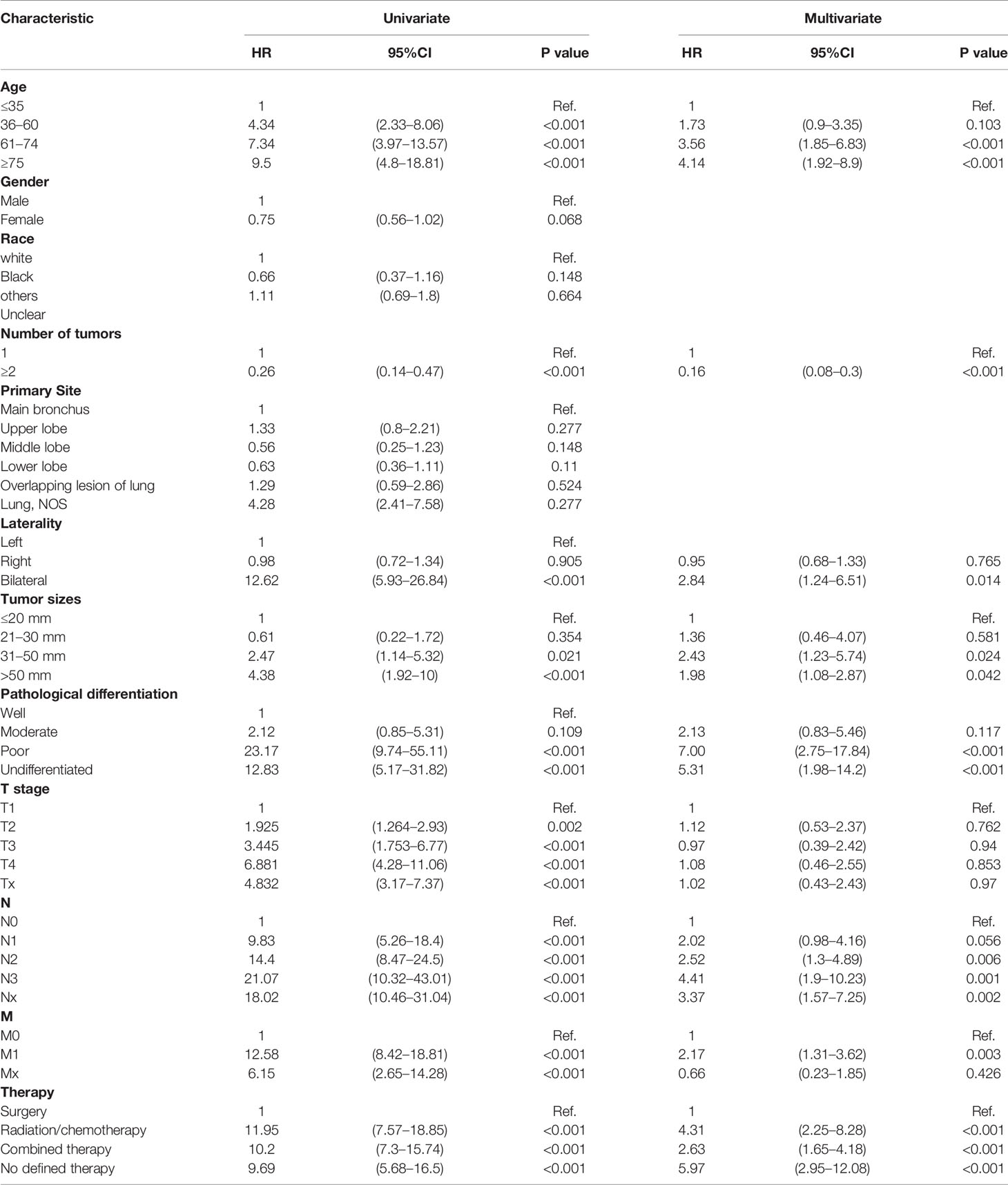
Table 3 Multivariate Cox regression analysis of prognostic factors for patients with pulmonary mucoepidermoid carcinoma.
A nomogram was established based on results of multivariate Cox analysis (Figure 4). The C-index of this nomogram was 0.921, indicating that the model was reliable (Figure 4). In addition, the calibration curve showed that the predicted curve and the actual observation curve were very close, which indicated that the result was reliable (Figure 5). The validation cohort was utilized to verify the nomogram and its C-index was 0.968 (Figure 5). The calibration curve showed high favorable consistency, indicating that the nomogram could be trusted.
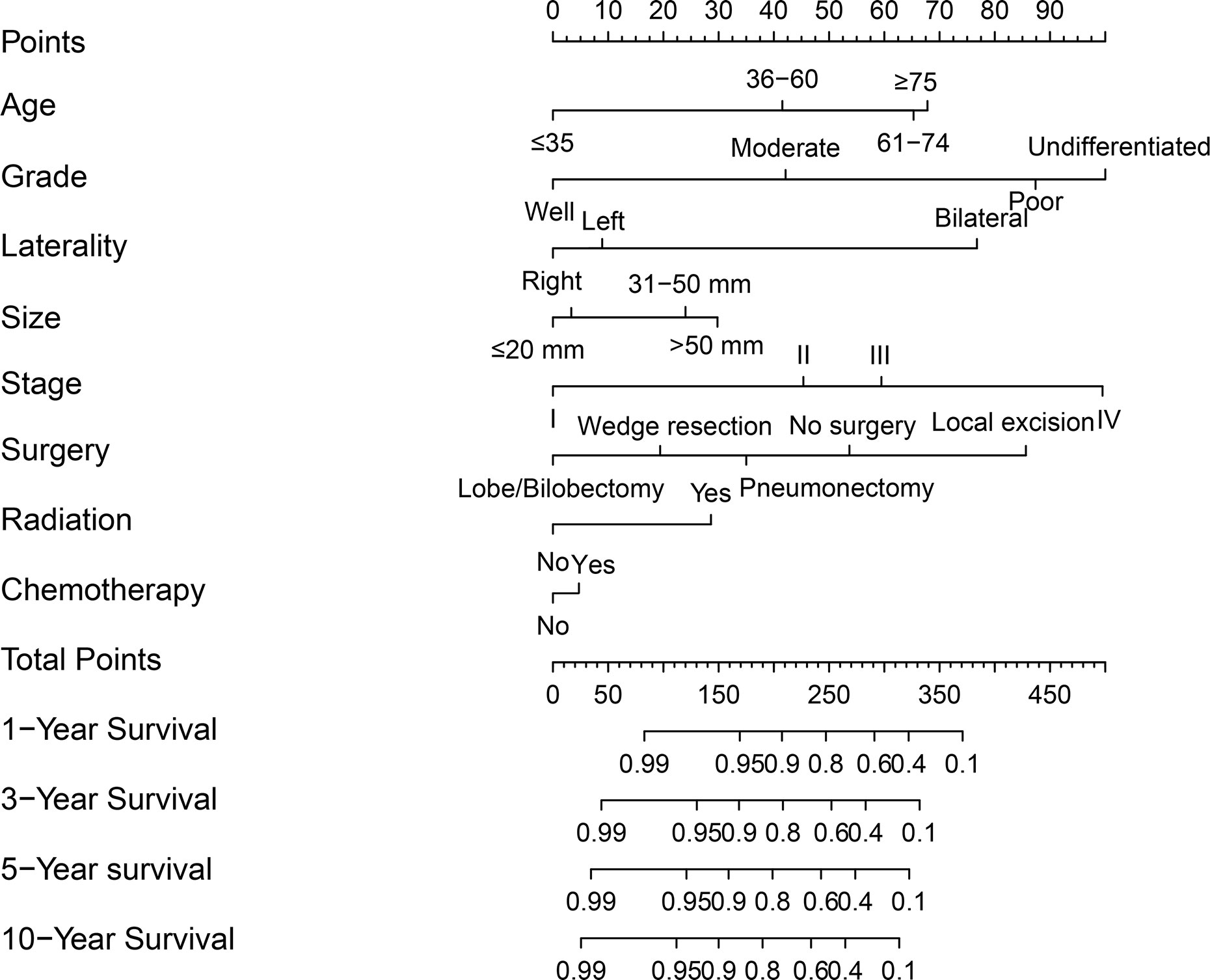
Figure 4 A nomogram of predicting the 1-year, 3-year, 5-year, and 10-year cancer-specific survival rates for PMEC patients.
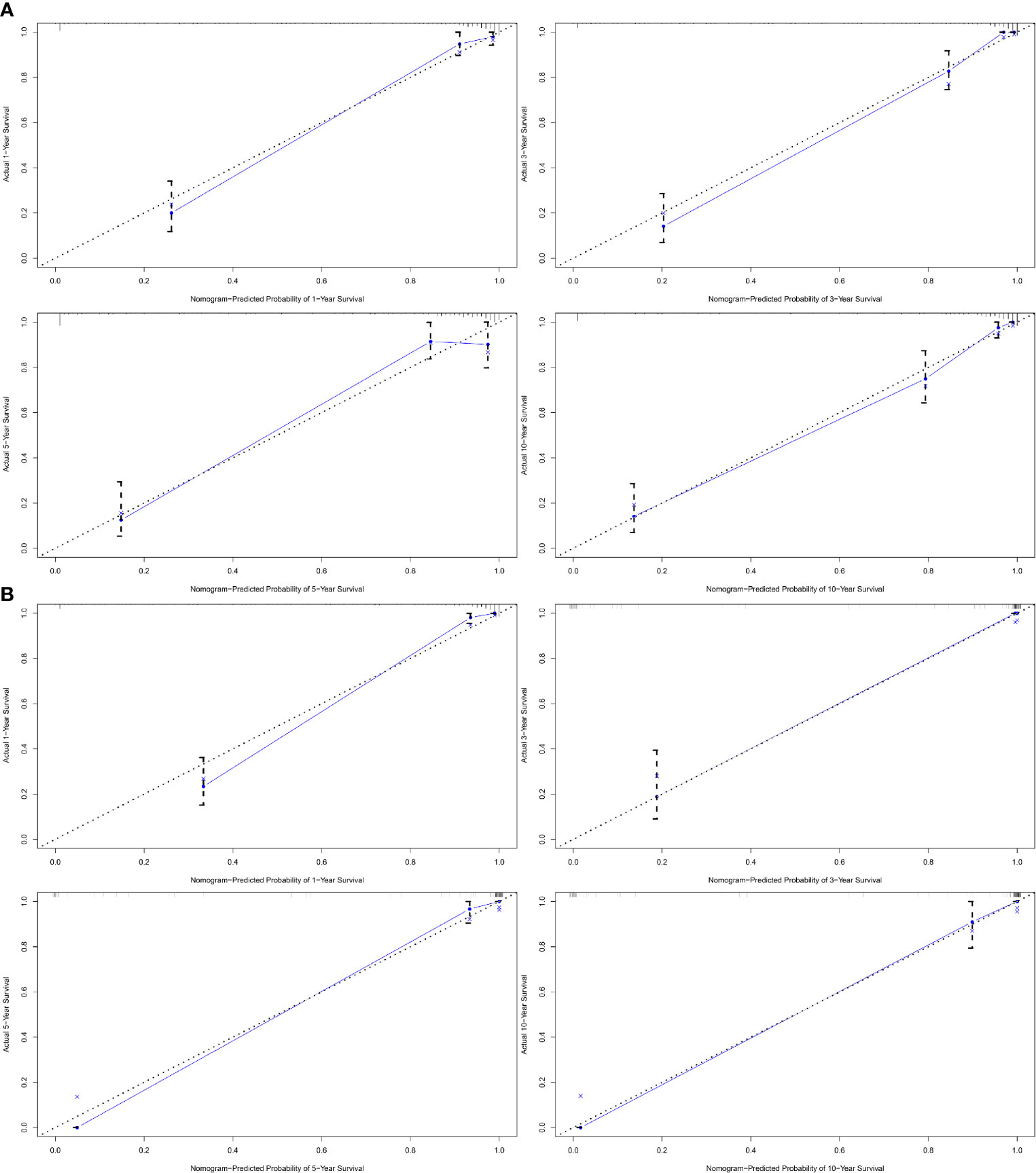
Figure 5 The calibration curve of nomogram-predicted probability. (A1) The calibration curve of 1-year cancer-specific survival outcomes. (A2) The calibration curve of 3-year cancer-specific survival outcomes. (A3) The calibration curve of 5-year cancer-specific survival outcomes. (A4) The calibration curve of 10-year cancer-specific survival outcomes. (B1) The validation curve of 1-year cancer-specific survival outcomes. (B2) The validation curve of 3-year cancer-specific survival outcomes. (B3) The validation curve of 5-year cancer-specific survival outcomes. (B4) The validation curve of 10-year cancer-specific survival outcomes.
PMECs are rare pulmonary malignancies, and most PMEC studies are small series or single case reports due to their scarcity. Therefore, characteristics and outcomes of this disease remain unclear. The SEER database is one of the largest cancer registries worldwide. It covers a wide range of data on patients, tumors, treatments, and survival outcomes. In the present study, we examined the characteristics and prognostic factors of PMECs and identified variables affecting survival, utilizing data from the SEER database between 1975 and 2016. We constructed a predictive nomogram of PMEC for survival rates in this study.
Consistent with previous reports, our study showed that there was a similar distribution of PMEC incidence between males and females (12, 14). It was previously observed that the onset age of MEC ranged from 4 years old to 86 years old, and nearly 50% of patients were below 40 years old (12, 14, 15). Similar to other types of MEC, PMEC still showed a similar trend of younger-onset (59.5% of patients were under 60, and 40.5% of patients were over 60) in our study. The vast majority of SGTs are endobronchial lesions, predominantly involving the stem bronchi, carina, and trachea (6, 12, 16). More than 66% of patients with SGTs have endobronchial masses (12). However, we observed that PMEC most frequently occurred in the upper lobes (35.2%) and lower lobes (33.1%), instead of in the bronchus. This result aligned with previous database-based PMEC studies (13) and revealed the diversity of its characteristics.
PMECs are a pathological subtype of p-SGTs, representing a rare category of lung carcinomas (13). Pulmonary salivary gland tumors (p-SGTs) were first identified as early as the 1950s and were initially classified as benign bronchial adenomas (17). In the 1960s, researchers observed that this broad group of tumors were factually capable of invasive growth and metastasis but were more indolent than the prevalent neoplasms of bronchial origin (18). The prognosis of MEC patients is generally considered optimistic. A previous large series suggested that the 5-year and 10-year OS rates of p-SGTs were 80.0 and 62.7%, respectively. Our results found that the 5-year CSS and OS rates of patients with PMEC stage I–II were 94.2 and 85.5%, respectively. The 5-year CSS and OS rates of patients with PMEC stage III–IV disease were 32.1 and 23.8%, respectively. The survival outcomes of patients with PMEC seemed to be better than those of patients with non-small cell lung cancer (NSCLC) and small-cell lung cancer (SCLC). It was reported that the 5-year survival rate of all NSCLC patients was 52.6% (19). The 5-year overall survival of NSCLC in different stages was as follows: IA, 66%; IB, 53%; IIA, 42%; IIB, 36%; IIIA, 10%; IIIB, 12%; and IV, 4% (20). For small cell lung cancer, it was reported that the 5-year survival rate of patients was 22% for local disease and 1% for extensive disease [13]. PMECs belong to low-grade lung malignancies and are considered to have a more favorable survival outcome than NSCLC and SCLC. However, the 3- and 5-CSS rates of patients with stage III–IV PMEC were only 39.45 and 32.1%, respectively, in our study. The results of this study revealed that the prognosis is not good for advanced patients with PMEC. Considering that our data span a large number of years, we analyzed data from the past ten years and ten years ago. A difference was observed in the survival curves of the two periods of time. The results may be related to living conditions, pharmaceutical treatments and medical conditions in the past decade.
The prognostic factors of MEC are controversial. Important prognostic factors of MEC include age, TNM stage and pathological differentiation grade (21–23). Poor OS was associated with older age, higher clinical stages, larger tumour size and non-surgical treatments (24–26). Our study found that the survival of PMEC patients was not only significantly affected by factors of age, pathological differentiation, TNM stage, tumor size, lymph node metastasis, distant metastasis and treatment approaches. The primary tumor site was also identified as a survival-associated factor. Moreover, age, bilateral tumors, tumor size, pathological differentiation grade, TNM stage, lymph node metastasis and treatment approaches were independent prognostic factors for PMEC in the current study. In addition, our study revealed that age >60, poor differentiation, tumor sizes >30 mm, lymph node metastases and distant metastases were especially associated with worse survival outcomes.
Previously, surgical resection was considered the most effective treatment for MEC (6, 26). However, the usefulness of chemotherapy and radiotherapy for advanced diseases remains controversial (23). In our study, considering the significant difference in survival between stage I–II and stage III–IV patients, we separated stage I–II and stage III–IV patients and examined the effect of treatment options on their survival curve alone. Our results revealed that cancer-directed surgical resection was the main treatment for stage I–II PMEC. It had excellent long-term survival outcomes in both CSS and OS. In patients with stage III–IV PMEC, surgical treatments (surgery alone and surgery plus radiation and/or chemotherapy) were still associated with improved survival outcomes compared with radiation and/or chemotherapy alone. Surgical resection treatment was the optimal treatment option for PMEC patients. This finding is in agreement with the previous research results (26–28). Furthermore, we further analyzed the impact of surgical options on survival. We found that patients who chose local surgical resection had the worst survival, while patients who chose pneumonectomy, lobectomy, or wedge procedures obtained good survival benefits. Therefore, we believe that for PMEC patients, such as MEC patients, surgery is still the best treatment option. However, local surgical resection should be avoided. Surgical treatment is associated with obviously improved survival outcomes. Pneumonectomy, lobectomy, and wedge procedures should be the top three recommended surgical options, but which surgical option should be chosen is based on the actual situation.
In the past, survival predictions for PMEC were based only on the predictive nomogram of SGTs or MEC (13, 25). No separate PMEC nomogram from a large series of model studies had been constructed. Since CSS may be more representative of the nature of the tumor itself than OS, we constructed a nomogram of PMEC CSS to forecast the 1-, 3-, 5- and 10-year CSS rates of PMEC patients. This is the first time that PMEC-specific nomogram was constructed. The C-index of this nomogram in the training and validation cohorts reached 0.921 and 0.968, respectively. The C-index and calibration curves showed the strength of this model. Validations of this model also supported the reliability and accuracy of the nomogram. Therefore, we believe that our model can provide clinicians with good model predictions for individual PMEC patients.
Even though a total of 585 clinical cases of PMEC were included and analyzed in our study, there were still some limitations. First, this study was retrospective, thus there were some inevitable confounding factors that affected the accuracy of our results. Second, our results were conducted with the data from the SEER database. These results might only represent American patients and might not be applicable to all PMEC patients worldwide. Third, with the limited data on PMEC patients, our study did not conduct validations for the main outcomes. Consequently, the reliability and accuracy of our results might have been affected and need to be reevaluated by future studies. Finally, adverse effects of treatments, quality of life, etc. were not analyzed in the current study. This may have caused our analysis to be incomplete. Therefore, high-quality studies are still needed in the future to evaluate clinical and survival outcomes of PMEC patients.
Publicly available datasets were analyzed in this study. This data can be found here: Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/).
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
LQ, PS, and GZ conceived the idea and edited the manuscript. LQ, PS, PC, and MS wrote the manuscript. XS, CH, and NL contributed to literature search. LQ, PS, and G-cG were involved in data collection and classification. LQ, PS, FL, and HJ prepared the tables and figures. HW and GZ supervised the final version of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by grants from National Natural Science Foundation of China (Grant No. 81874042 and Grant No. 81972182).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: Cancer J Clin (2015) 65(2):87–108. doi: 10.3322/caac.21262
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA: Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
4. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumours: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thoracic Oncol (2015) 10(9):1243–60. doi: 10.1097/JTO.0000000000000630
5. Roden AC, García JJ, Wehrs RN, Colby TV, Khoor A, Leslie KO, et al. Histopathologic, immunophenotypic and cytogenetic features of pulmonary mucoepidermoid carcinoma. Modern Pathol: An Off J U States Can Acad Pathol Inc (2014) 27(11):1479–88. doi: 10.1038/modpathol.2014.72
6. Zhu F, Liu Z, Hou Y, He D, Ge X, Bai C, et al. Primary salivary gland-type lung cancer: clinicopathological analysis of 88 cases from China. J Thoracic Oncol Off Publ Int Assoc Study Lung Cancer (2013) 8(12):1578–84. doi: 10.1097/JTO.0b013e3182a7d272
7. Li LJ, Li Y, Wen YM, Liu H, Zhao HW. Clinical analysis of salivary gland tumour cases in West China in past 50 years. Oral Oncol (2008) 44(2):187–92. doi: 10.1016/j.oraloncology.2007.01.016
8. Liao WC, Chih-Chao C, Ma H, Hsu CY. Salivary Gland Tumours: A Clinicopathologic Analysis From Taipei Veterans General Hospital. Ann Plast Surg (2020) 84(1S Suppl 1):S26–s33. doi: 10.1097/SAP.0000000000002178
9. Eveson JW, Cawson RA. Salivary gland tumours. A review of 2410 cases with particular reference to histological types, site, age and sex distribution. J Pathol (1985) 146(1):51–8. doi: 10.1002/path.1711460106
10. Nguyen CV, Suster S, Moran CA. Pulmonary epithelial-myoepithelial carcinoma: a clinicopathologic and immunohistochemical study of 5 cases. Hum Pathol (2009) 40(3):366–73. doi: 10.1016/j.humpath.2008.08.009
11. Nowak K, Menzies-Gow A, Hull J, Jordan S. Bronchial sleeve resection for a mucoepidermoid carcinoma, mimicking asthma in a 27-year-old female. Am J Respir Crit Care Med (2012) 186(9):e14–5. doi: 10.1164/rccm.201201-0103IM
12. Molina JR, Aubry MC, Lewis JE, Wampfler JA, Williams BA, Midthun DE, et al. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer (2007) 110(10):2253–9. doi: 10.1002/cncr.23048
13. Resio BJ, Chiu AS, Hoag J, Dhanasopon AP, Blasberg JD, Boffa DJ. Primary Salivary Type Lung Cancers in the National Cancer Database. Ann Thoracic Surg (2018) 105(6):1633–9. doi: 10.1016/j.athoracsur.2018.01.055
14. Yousem SA, Hochholzer L. Mucoepidermoid tumours of the lung. Cancer (1987) 60(6):1346–52. doi: 10.1002/1097-0142(19870915)60:6<1346::AID-CNCR2820600631>3.0.CO;2-0
15. Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol (2001) 25(7):835–45. doi: 10.1097/00000478-200107000-00001
16. Elnayal A, Moran CA, Fox PS, Mawlawi O, Swisher SG, Marom EM. Primary salivary gland-type lung cancer: imaging and clinical predictors of outcome. AJR Am J Roentgenol (2013) 201(1):W57–63. doi: 10.2214/AJR.12.9579
17. Falk N, Weissferdt A, Kalhor N, Moran CA. Primary Pulmonary Salivary Gland-type Tumors: A Review and Update. Adv Anat Pathol (2016) 23(1):13–23.
18. Burcharth F, Axelsson C. Bronchial adenomas. Thorax (1972) 27(4):442–9. doi: 10.1136/thx.27.4.442
19. Goya T, Asamura H, Yoshimura H, Kato H, Shimokata K, Tsuchiya R, et al. Prognosis of 6644 resected non-small cell lung cancers in Japan: a Japanese lung cancer registry study. Lung Cancer (Amsterdam Netherlands) (2005) 50(2):227–34. doi: 10.1016/j.lungcan.2005.05.021
20. Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, Edell ES, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest (2005) 128(1):452–62. doi: 10.1378/chest.128.1.452
21. Li X, Guo Z, Liu J, Wei S, Ren D, Chen G, et al. Clinicopathological characteristics and molecular analysis of primary pulmonary mucoepidermoid carcinoma: Case report and literature review. Thoracic Cancer (2018) 9(2):316–23. doi: 10.1111/1759-7714.12565
22. Hsieh CC, Sun YH, Lin SW, Yeh YC, Chan ML. Surgical outcomes of pulmonary mucoepidermoid carcinoma: A review of 41 cases. PloS One (2017) 12(5):e0176918. doi: 10.1371/journal.pone.0176918
23. Xi JJ, Jiang W, Lu SH, Zhang CY, Fan H, Wang Q. Primary pulmonary mucoepidermoid carcinoma: an analysis of 21 cases. World J Surg Oncol (2012) 10:232. doi: 10.1186/1477-7819-10-232
24. Kim BG, Lee K, Um SW, Han J, Cho JH, Kim J, et al. Clinical outcomes and the role of bronchoscopic intervention in patients with primary pulmonary salivary gland-type tumours. Lung Cancer (Amsterdam Netherlands) (2020) 146:58–65. doi: 10.1016/j.lungcan.2020.05.016
25. Qin BD, Jiao XD, Liu K, Wu Y, He X, Liu J, et al. Clinical, pathological and treatment factors associated with the survival of patients with primary pulmonary salivary gland-type tumours. Lung Cancer (Amsterdam Netherlands) (2018) 126:174–81. doi: 10.1016/j.lungcan.2018.11.010
26. Heitmiller RF, Mathisen DJ, Ferry JA, Mark EJ, Grillo HC. Mucoepidermoid lung tumours. Ann Thoracic Surg (1989) 47(3):394–9. doi: 10.1016/0003-4975(89)90380-9
27. Kang DY, Yoon YS, Kim HK, Choi YS, Kim K, Shim YM, et al. Primary salivary gland-type lung cancer: surgical outcomes. Lung Cancer (Amsterdam Netherlands) (2011) 72(2):250–4. doi: 10.1016/j.lungcan.2010.08.021
Keywords: pulmonary mucinous epidermoid carcinoma, clinical characteristics, prognosis, Surveillance, Epidemiology, and End Results (SEER), nomogram
Citation: Qiu L, Song P, Chen P, Wang H, Li F, Shu M, Gong G-c, Song X, Huang C, Jia H, Li N and Zhang G (2021) Clinical Characteristics and Prognosis of Patients With Pulmonary Mucoepidermoid Carcinoma: A SEER-Based Analysis. Front. Oncol. 11:601185. doi: 10.3389/fonc.2021.601185
Received: 31 August 2020; Accepted: 07 January 2021;
Published: 05 March 2021.
Edited by:
Sara Pilotto, University of Verona, ItalyReviewed by:
Alberto Bongiovanni, Romagnolo Scientific Institute for the Study and Treatment of Tumors (IRCCS), ItalyCopyright © 2021 Qiu, Song, Chen, Wang, Li, Shu, Gong, Song, Huang, Jia, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guojun Zhang, Z2p6aGFuZ3p6dUAxMjYuY29t
†ORCID: Lingxiao Qiu, orcid.org/0000-0002-1703-0797
Pan Song, orcid.org/0000-0001-8509-2351
Pingmei Chen, orcid.org/0000-0002-8555-2386
Guojun Zhang, orcid.org/0000-0003-1756-0082
‡These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.