
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 26 March 2021
Sec. Pharmacology of Anti-Cancer Drugs
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.593337
This article is part of the Research Topic Molecular Mechanisms of Drug Resistance And Strategies of Sensitization in Breast Cancer View all 16 articles
Breast cancer is a highly complicated disease. Advancement in the treatment and prevention of breast cancer lies in elucidation of the mechanism of carcinogenesis and progression. Rodent models of breast cancer have developed into premier tools for investigating the mechanisms and genetic pathways in breast cancer progression and metastasis and for developing and evaluating clinical therapeutics. Every rodent model has advantages and disadvantages, and the selection of appropriate rodent models with which to investigate breast cancer is a key decision in research. Design of a suitable rodent model for a specific research purpose is based on the integration of the advantages and disadvantages of different models. Our purpose in writing this review is to elaborate on various rodent models for breast cancer formation, progression, and therapeutic testing.
Breast cancer is the most commonly diagnosed cancer and one of the most common cause of cancer death in women worldwide. Advancement in treatment and prevention of breast cancer lies in elucidation of the mechanism of carcinogenesis and progression. Rodent models of breast cancer have developed into premier tools for breast cancer research, and they have generated important insights into the mechanisms that underpin development of the disease and interventional therapies. This review summarizes various rodent models for breast cancer formation, progression, and therapeutic testing.
In the past century, rodent models have proved to be powerful tools in improving knowledge of the underlying mechanisms and genetic pathways of breast cancer and have also created approaches for modeling clinical tumor subtypes and developing innovative cancer therapies. Certain mouse lines can naturally develop breast cancer, or they can be transplanted with breast cancer. Tumors can also be induced by manipulating the mouse genome or by delivery of a viral infection or carcinogen. The relatively low cost of mice and their high reproductive cycle of only 9 weeks make them excellent models for cancer research. In 1911, the first transplantable mouse mammary tumor line and the epithelial origin of a spontaneous mammary tumor were described by Haaland (1). Jacksons Laboratories showed that a retrovirus caused a high incidence of mammary tumors in mice in 1936 (2). The first xenograft breast cancer model was reported in 1962 via the heterotransplantation of human breast cancer into an immune-deficient mouse (3). The development of genetically engineered animal models offered a great leap in understanding the genetic basis of breast cancer. The first report of a transgenic mouse model of breast cancer, Oncomouse. In 1984, The Philip Leder research group generated transgenic mice using mouse mammary tumor virus (MMTV)/c-myc fusion gene expression. The mice developed mammary adenocarcinomas spontaneously (4), and 3 years later, they produced transgenic mice with coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes, which resulted in a dramatic and synergistic acceleration of tumor formation (5). These milestones established an entirely new research tool with which to explore the genetic processes of breast cancer. The first transgenic mouse model (GEMM) of HER2-positive breast cancer, reported in 1988, represented another milestone in breast cancer research (6). In 1999, Chuxia Deng and colleagues succeeded in developing a mouse model that ablated BRCA1 specifically in mammary epithelial cells, resulting in mammary tumors (7). This mouse model offered a notably large amount of information that greatly facilitated our understanding of the gender- and tissue-specific tumor suppressor functions of BRCA1 and enriched insights into applying these preclinical models of disease to breast cancer research. However, the GEMM requires time-consuming and expensive work, and another main drawback is that it is highly difficult to control the spatial and temporal expression of a gene of interest. Actually, most human breast cancers are not due to hereditary mutations, rather they arise from normal cells that later suffered spontaneous mutations. The technique of virus-mediated gene transfer into selected mammary cells (such as stem cells and specific progenitor cells) at selected times can overcome many of the shortcomings of the existing mouse models and more closely mimics human breast cancer formation in which only one or a few cells mutate to initiate cancer development (8).
Human cancer cells can be grown as transplants in mice. These transplantable models are simple but have been proven to be highly useful for assessment of breast cancer features, biological behaviors, metastatic potential, and response to therapy.
Cell-derived xenograft (CDX) of human breast cancer is performed from aggressive cancer cell lines. The CDX model from different tumor cell lines has unique characteristics, including relatively homogenous histological features, molecular subtype, and metastatic potential, among other features. The CDX model makes it possible for different mammary cancer cell lines to be transferred to the mouse in a short time, allowing validation of the target genes of interest and the possibility of research on metastasis and therapy response. It represents a relatively homogenous mass but cannot mimic the heterogeneity or the tumor microenvironment of human breast cancer (9). This technique is usually performed in nude mice (deficient in T-cell function) or other immunocompromised mice but cannot mimic the immune system reaction. If the cancer cells are derived from mouse, they can also be grafted into mice with an intact immune system (10, 11). And the long-term growth in vitro can alter some features from its origin cell. Triple-negative breast cancer cell lines such as MDA-MB-231, MDA-MB-435, and SUM1315 can be used to generate stable orthotopic or spontaneous metastasis models of breast cancer via orthotopic injection in the mammary fat pad (12). The metastatic MDA-MB-231 and SUM149 CDX models can also be generated by injection into the mouse tail vein (13). Not all breast cancer cell lines from human can be used to successfully establish a CDX mouse model (14). ER-positive luminal A cell lines such as T47D or MCF-7 can only form a tumor mass in immunodeficient mice supplemented with subcutaneous estradiol pellets (15), which produces 18–40 times the physiological levels of estrogen in mice (16, 17). HER2 subtype cell lines such as SKBR3 and MDA-MB-453 cells have poor tumorigenic potential (18).
Due to the limitation of CDX models, the patient-derived xenograft (PDX) is generated by xenografting fresh human tumor biopsies that recapitulate the diversity of breast cancer into host mice. This model reflects the tumor original behavior, histopathology, drug response, and metastatic potential of the original tumor (19). In brief, human tumor fragments or tumor cell suspensions are implanted into the immunocompromised mice and subsequently passaged through several generations in mice. The more heavily immunocompromised mice are usually used to generate PDX, such as NOD-SCID mice (deficient in T-cell and B-cell functions) and NSG mice (deficient in T-cells, B-cells and NK cells).
The PDX modes are relatively stable after the third passage in mice (20) and have relatively stable biological behaviors, such as gene expression profiles, intrinsic phenotypes, and genomic alteration, that are similar to the source of human breast cancer (21–24). PDX also has selected structural variation or mutation differences with the original primary tumor, perhaps due to the adaption to transplantation into the new microenvironment (25). PDX models appear to retain the heterogeneity of the parental tumor of origin and experience a “bottlenecking” clonal selection during transplantation and serial passaging (26). Ding et al. reported comprehensive genomic sequence analysis of DNA samples from an African-American patient with basal-like breast cancer for peripheral blood, the primary tumor, a brain metastasis, and a xenograft derived from the primary tumor (25). That group found that the PDX derived from the primary tumor contained all of the primary tumor mutations and displayed a mutation enrichment pattern that resembled the metastasis. The metastatic subclone was present within the primary tumor, the aggressive subclone with clonal selection in PDX. The PDX drug screening test can mimic and predict drug efficacy, especially in triple negative breast cancer (27), and is a pivotal preclinical tool for evaluating drug response and study of the clonal evolution of tumors and new biomarkers (15). TNBC tumors and to a lesser degree the HER2+ tumors, readily adapt to growth in mice, whereas only 2.5% of ER+ tumors successfully formed stable patient-derived breast cancer xenografts (28). PDX models can’t mimic the immune system and tumor-host interaction because it must also be grown in immunocompromised mice.
The breast ductal system is a complex series of branching tubules extending from intralobular ductules to the major lactiferous ducts that terminate in the nipple. The mouse intraductal model is based on the special structure of the mammary mouse gland. Human cancer cells can be introduced directly into the mouse mammary ducts in immunodeficient mice to mimic the natural progression of cancer cells in the mammary microenvironment. Behbod et al. established the ductal carcinoma in situ (DCIS) model by injecting the human DCIS cell lines (MCF-10 and SUM-255) into mouse mammary ducts via up-the-teat injection (29). This approach mimicked breast tumor carcinogenesis and progression from in situ to invasive disease and spontaneous metastasis to the relevant sites. In contrast to fat-pad-grafted ER+ tumor cell lines that require estrogen supplement, the MIND of MCF-7 achieved a high engraftment rate without hormone supplements and recapitulated the histopathology and kinetics of human ER-positive tumors (16, 17). These MIND models also often developed bone, lung, and brain metastases, whereas fat pad injection xenografts developed few brain and no bone metastases. The Ki67 indices of MIND MCF-7 tumors were 23%, highly similar to MCF-7 cell lines, and the gene expression signatures are highly similar to the luminal B subtype of clinical samples, as shown by Affymetric microarray and PAM50 (30). For the triple-negative breast cancer mouse model, a fully immunocompetent 4T1-based intraductal model can mimic breast cancer advancement and metastasis to the lungs via lymphatic or hematogenous dissemination within 4 weeks (31–33), and it can also disseminate to the liver, brain and kidney (34). 4T1 is a mouse breast cancer line derived from a spontaneously arising mammary tumor in BALB/cfC3H mice (35). The 4T1 MIND models overcome immunosuppression and allow effective immunotherapy research for TNBC (33, 36). This model is predictive, retransplantable, and genomically stable and is an economical and practical mouse model for translational research and study of physiologically relevant hormone action in breast carcinogenesis.
Chemical compounds can induce breast cancer. For example, the carcinogen 7,12-dimethylbenzathracene (DMBA), delivered intragastrically by gavage, can induce mammary adenocarcinomas with several morphological types in mice (37). The induced tumors were luminal-like and mostly ER/PR+ (38, 39). Previous research indicated that estrogen exposure was closely related to elevated breast cancer risk in women (40, 41). The 17β-estradiol-induced mammary cancers highly express ER, PR, and GATA binding protein 3 (42–45), and others such as N-nitroso-N-methylurea (NMU) can induce mouse breast cancer similar to that of low-grade estrogen-receptor positive human breast cancer (46–48). Spontaneous chemically induced mouse models are helpful for investigation of the pathogenesis and therapeutics of breast cancer (49).
Genetically engineered mouse models (GEMM) of breast cancer have supplied a wealth of knowledge for both basic cancer research and translational oncology by introducing DNA into the mouse genome. GEMMs reflect some of the diversity of genetic lesions in human breast cancer. These models include three broad groups: transgenic mouse model, knockout mouse model, and genetic models of breast cancer based on intraductal injection of virus to modify the genes of the mammary cells.
Transgenic mouse models refer to those which have exogenous DNA integrated in their germline as a consequence of experimental DNA transfer application. The integrated DNA may or may not be derived from the same species as the host genome, it may or may not be targeted to an intended site of incorporation in the genome, and it may or may not encode for a functional gene.
The MMTV-PyMT transgenic mouse is a model that carries the polyoma virus middle T-antigen under the control of the mouse mammary tumor virus (MMTV) promoter. The PyMT is involved in multiple oncogenic pathways that lead to an aggressive tumor phenotype such as Src, Ras, and PI3K (50–54). MMTV-PyMT females develop multifocal, poorly differentiated, highly invasive ductal carcinoma as early as 4 weeks of age, reaching the maximum tumor burden at 12 weeks of age, and they also exhibit lung metastasis near 10 weeks of age (55–59). This model is used in breast cancer research to analyze the mechanism of carcinogenesis and alter the tumor microenvironment. Maglione also reported that atypical lesions had levels of detectable ER expression, and the mammary intraepithelial neoplasia and tumor cells had variable sporadic ER-positive nuclei staining (58). Previous research indicated that the PyVT mammary tumors were shown to be ER+, PR+, P53+, and HER-2+ via immunohistochemistry at the early stage of tumor formation, progressing to the triple-negative subtype (57, 58). This model has drug resistance to cisplatin and paclitaxel, but tamoxifen is effective in the prestage and early stage of tumor formation (57).
The Wnt-1 (int-1) proto-oncogene was originally cloned following activation by MMTV insertion in mouse mammary tumors (60, 61). The MMTV-wnt-1 mouse model was established with the MMTV-LTR upstream of wnt1 insertion in the opposite transcription orientation (62, 63). This model is characterized by grossly ductal hyperplasia with extensive fibrosis, and these mice can develop breast cancer at an onset of 24 weeks (64). Females cannot deliver milk to their young because of extensive ductal hyperplasia (64). The tumors in MMTV-wnt-1 transgenic mice are composed of myoepithelial (basal-like) and luminal epithelial cells. β-catenin is an integral player in the Wnt signal transduction pathway, and β-catenin transgenic (MMTV‐βcatΔN) mice exhibit mammary gland hyperplasia and mammary adenocarcinoma, which are highly similar to the corresponding lesions in MMTV-wnt-1 mice (65). Wnt10b is a ligand that activates the canonical Wnt/β-catenin pathway, and MMTV-Wnt-10b transgenic mice showed hyperplastic mammary development involving highly branched mammary ducts and gynecomastia (66). LRP6 is a Wnt signaling coreceptor, and MMTV‐LRP6 mice exhibit significant hyperplasia with upregulated expression of Axin2, Cyclin D1, and c-Myc (67). MMTV-c-Myc and MMTV-int2 mice also develop pronounced mammary hyperplasia and adenocarcinoma in proportion (65, 68). Indeed, the wnt-associated mouse model has made a great contribution to elaboration of the wnt pathway in breast carcinogenesis.
The first MMTV-ErbB2 transgenic mouse model expressed an activated Erbb2 under promoter of MMTV-LTR, and these mice are viable and fertile (6). There is no phenotypic effect in males. This transgene is expressed at low levels in the normal mammary epithelium, salivary gland, and lung (69, 70), and higher expression is detected in tumor tissue. This model produces multifocal and stochastic mammary tumor formation near 15 weeks of age (69, 71) and lung metastasis with long latency (approximately 32 weeks or longer) (72) and had positive cyclin D1 and CDK4 expression and a high Ki-67 proliferative index. In contrast to the MMTV-ErbB2 mouse line, Muller et al. later established transgenic mice carrying unactivated neu under the MMTV promoter (73). The mice began to develop focal mammary adenocarcinoma surrounded with hyperplastic mammary epithelium at 16 weeks of age, with decreased neu intrinsic tyrosine kinase activity. Many of these tumor-bearing transgenic mice with unactivated neu also developed metastatic tumors in the lung (73). Li et al. found that 37% of tumors in the MMTV-ErbB2 mouse had mis-sense mutations in p53 (74), and thus, they established bitransgenic mice carrying MMTV-neu and a 172Arg-to-His p53 mutant (p53-172H). The bitransgenic mice developed anaplastic and aneuploidy tumors that exhibited increased apoptosis, distinct from the tumors with diminished apoptosis arising in p53-null mice (74).
The C3(1)/SV 40/t-antigen (C3(1)/Tag) mouse model contained a recombinant gene expressing the simian virus 40 early-region transforming sequence under rat prostatic steroid binding protein [C3(1)]. Female hemizygous mice generally developed mammary hyperplasia at 9 weeks of age and mammary intraepithelial neoplasia with similarities to DCIS at 12 weeks, with subsequent development of mammary adenocarcinoma at an onset of 24 weeks in 100% of the animals and 15% incidence of lung metastasis (75–81). This model develops invasive carcinoma independently of hormones or pregnancy (72). All mammary adenocarcinomas were diffuse immunopositive for CK14, CK18, and p53 and negative for αSMA, ERα, PR, and C-erbB-2 (81). Previous study indicated that human basal-like breast cancer exhibits a high frequency of p53 mutation of deletion. It is a suitable mouse model for research on basal-like breast cancer because of the gene expression and DNA somatic alteration levels.
Cyclin D1 is essential in breast carcinogenesis induced by c-neu and v-Ha-ras and not induced by c-myc or Wnt-1 (82). The MMTV-cyclin D1 mouse can develop mammary adenocarcinomas quite late stochastically (83, 84). Cyclin E is a cancer marker that is the limiting factor for the transition from G1 to the S-phase of the cell cycle, which determines ignition of DNA duplication. Previous research indicated that the 27% low-molecular-weight isoform of cyclin E transgenic mice can induce metastatic mammary carcinoma (85).
Knockout animals are mice with targeted disruption of selected endogenous gene sequences. These models are used to reveal valuable clues related to the biological and molecular function of a gene in the setting of a developing or developed tumor. The constitutive knockout model refers to the whole-body knockout model, i.e., the target gene is knocked out in all tissues at all times. Many tumor suppressors often result in lethality during embryonic development or at developmental stages prior to tumor formation. This obstacle has been effectively overcome by applying the conditional knockout model (86) in which the gene knockout can be spatially and even temporally regulated. With a conditional KO, gene inactivation can occur in a certain tissue type, made possible by Cre-LoxP and Flp-Frt recombinase system. Today, the development of the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 technique has made conditional knockouts even more popular and widely used. This new technology is more efficient and easier than the Cre-LoxP or Flp-Frt recombinase technology. Therefore, we summarize the tumor phenotype of the popular conditional knockout strains reported in the literature.
BRCA1 inherited mutations predispose carriers to female breast and ovarian cancers. Constitutive knockout of mouse BRCA1 causes recessive mouse embryonic lethality (87), and therefore, the BRCA1 conditional mutant mouse model was used to overcome this obstacle (88). Exon 11 is a large central exon of 3426 bp that represents 60% of the coding sequence in BRCA1 (89). In 1999, Xu established a BRCA1flox11 mutant mouse, which was achieved by deleting only exon 11 of the full-length BRCA1 gene and leaving expression of the short BRCA1 transcript with loxP sites (BRCA1flox11) (7). The 25% BRCA1flox11 mutant mouse develops mammary tumors after a long latency (7). The 94% BRCA1flox11 mouse develops mammary tumors with a long latency (T50 = 17 months), and the tumors exhibit an atypical medullary phenotype strongly reminiscent of basal-like breast tumors (90). Xu et al. found that the BRCA1flox11 mutation mouse often had had spontaneous p53 mutation, and thus they introduced heterozygous deletion of p53 in the BRCA1flox11 mouse, which accelerated tumor formation (91). Weaver et al. also revealed that certain of the tumors had structural abnormalities on the map location of c-myc gene, Rb1, and p53, similar to BRCA1-associated breast cancer in patients (92).
Other conditional BRCA1 alleles are reported to cause functionally null BRCA1 alleles by flanking exon 2 (BRCAf2) (90), exons 5–6 (BRCA1f5–6) (93), exons 5–13 (BRCA1f5–13) (94), or exons 22–24 (BRCA1f22–24) (95). The BRCA1f5–13 mouse had intermediate to high grade tumors with high mitotic count, expansive growth, moderate to high nuclear grade also displayed ER-negative immunohistochemistry staining with pushing borders, and increased expression of basal epithelial markers, similar to human basal-like breast cancer (94). The 64% mouse with BRCA1f22–24 mutation combined with heterozygosity for a p53 mutation developed tumors with basal-like markers in all cases before 22 months of age. This model had high histological grade, central necrotic areas, and presence of homologous metaplastic elements and is a suitable model for metaplastic basal-like breast cancers (95).
Germline mutations of BRCA2 are associated with one-third of hereditary breast cancer. Jonker et al. generated a mouse model with conditional mutants of BRCA2f11 (flanking exon 11 of the gene with loxP sites) and found that no BRCA2f11 mice developed tumors. The mammary glands and skin frequently developed tumors in females carrying conditional BRCA2f11 and p53 knockout alleles (96). The vast majority of the mammary tumors were carcinomas with myoepithelial or basal cell types. Most tumors arising in the conditional BRCA2f11 and p53 knockout mice had high-grade invasive ductal carcinoma, with a solid growth pattern, a large CK8-positive and ER-negative cell type with high mitotic count, high-grade nuclei and with pushing borders (96). The tumors often harbor the undifferentiated basal cell type. Based on the results from cross-species comparison by unsupervised clustering, these tumors are closely similar to human BRCA1-mutated breast cancers with basal-like phenotypes. Ludwig generated mice with BRCA2f3–4 (flanking exons 3 and 4 of the gene with loxP sites) mutation, which had a high incidence (77%) of breast tumors that developed in one or more glands after a long latency (time for median tumor-free survival of approximately 1.4 years; total of 40 tumors in 20 animals) (97). In addition, Cheung generated a mouse model with BRCA2f9–11 (flanking exon 9–10 of the gene with loxP sites), which had mammary adenocarcinomas after a long latency (average, 1.6 years). A subset of these tumors also showed downregulated p53 expression (98).
As mentioned previously, the p53 mutation is linked tightly with breast cancer. The conditional knockout p53Δ2–10 (deletion of exon 2–10 of the gene with Cre recombinase) model generated by Jonker et al. develops lymphomas and sarcoma rather than epithelial tumors (96), and therefore, those researchers crossed p53Δ2–10 mice with K14-cre transgenic mice (Cre recombinase expression is restricted to skin and mammary gland epithelium and other epithelial tissues). The resulting K14-cre p53Δ2–10 mice developed mammary tumors with a median latency (T50) of 288 days. Interestingly, 38% of the mammary tumors were pure epithelial tumors (intermediate to high-grade), 48% were poorly differentiated biphasic carcinoma, and 14% were well differentiated biphasic carcinoma. The molecular signatures of these tumors showed a significant association with human sporadic ER-negative tumors (94). These tumors closely mimic human sporadic basal-like breast cancer. Lin et al. generated a mouse breast cancer model with inactivated p53 (deletion of exon 2–10 of the gene with Cre/loxp) in mammary epithelial cells (99). The tumors are heterogeneous, including adenocarcinoma, myoepithelial adenocarcinoma, spindle cell tumor, and adenosquamous carcinoma, and most were poorly differentiated invasive adenocarcinomas, which share the most histopathological similarity with human breast cancer. A total of 35% had c-myc amplification, and 66% had erbB2 overexpression. The tumors were initially ERα-positive but progressed to ERα-positive and -negative tumors (99), similar to human breast cancer. Multistep histopathological changes and alterations in the ERα expression pattern are observed during progression of mammary carcinogenesis in these models.
PTEN is a tumor suppressor that is frequently mutated in breast cancers. Germline PTEN mutations cause inherited syndromes that lead to an increased risk of breast cancer. Wu Hong and colleague generated PTENΔ5 allele (flanking exon 5 of PTEN with loxP sites) and established mammary-specific PTEN deletion mice (100, 101). PTEN null mammary epithelial cells were hyperproliferative and showed decreased apoptosis. Mutant females developed mammary tumors with upregulated populations of cells expressing cytokeratin 5 and 6 within 400 days (101). When a PTEN conditional allele was mated with MMTV-NIC mice, which coupled expression of Cre and activated ErbB2 from the bicistronic transgenic transcript, all female mice developed multifocal mammary tumors and high lung metastases, which displayed histopathological and molecular characteristics of the luminal subtype of primary human breast cancer (102).
Based on molecular biology, breast cancer is highly complicated. Most human cancers are not due to hereditary mutations, and instead, they arise from normal cells that later suffer spontaneous mutations. It is notably difficult to manipulate the spatial and temporal expression of genes in mouse. Genetic models of breast cancer based on intraductal injection of a virus can circumvent selected disadvantages of the typical transgenic or knockout mouse models. Currently, in clinical and basic research, compound techniques of mouse models have more practical applications. The avian leukosis-sarcoma virus (ALSV) and its specific receptor tumor virus A (TVA) play a vital role in this model. Mammalian cells lack the TVA gene sequence, and the transfer of the TVA gene to specific cells in mouse renders them uniquely susceptible to infection by ALSV-based RCAS virus (103). RCAS viruses can be delivered into mice by injection of virus-producing cells or by injection of concentrated virus (103, 104). Harold Varmus and colleagues constructed the RCAS-TVA avian retroviral system, which can carry oncogenes (e.g., K-ras, c-myc), marker genes (e.g., green fluorescent protein, alkaline phosphatase), dominant negative tumor suppressors (e.g., mutant p53), or recombinases (e.g., Cre) (105). This method offers a precise way to manipulate the temporal and spatial expression of genes in the mammary epithelium. A single TVA mouse stain can be used to evaluate the effects of multiple genes, individually or in combination, instead of generating a mouse line for each gene of interest. Yi Li modeled breast cancer in a mouse with the RCAS-TVA system by mammary gland intraductal injection (106) (Figure 1). Precancerous lesions can be detected by 7 days following RCAS-PyMT injection (107). The PyMT oncogene delivered by RCAS-TVA caused multifocal mammary tumors after a notably short median latency of only 12.5 days. The tumors were composed of myoepithelial (basal-like) and luminal epithelial cells and were relatively well differentiated, consisting of many acini and heterogeneous cell types with ER positive expression (8, 108). In mice injected with RCAS-erbB2, precancerous lesions can be detected 14 days after injection (109). The mice developed high grade, poorly differentiated, stroma-rich, and ER-negative mammary tumors (109–111).
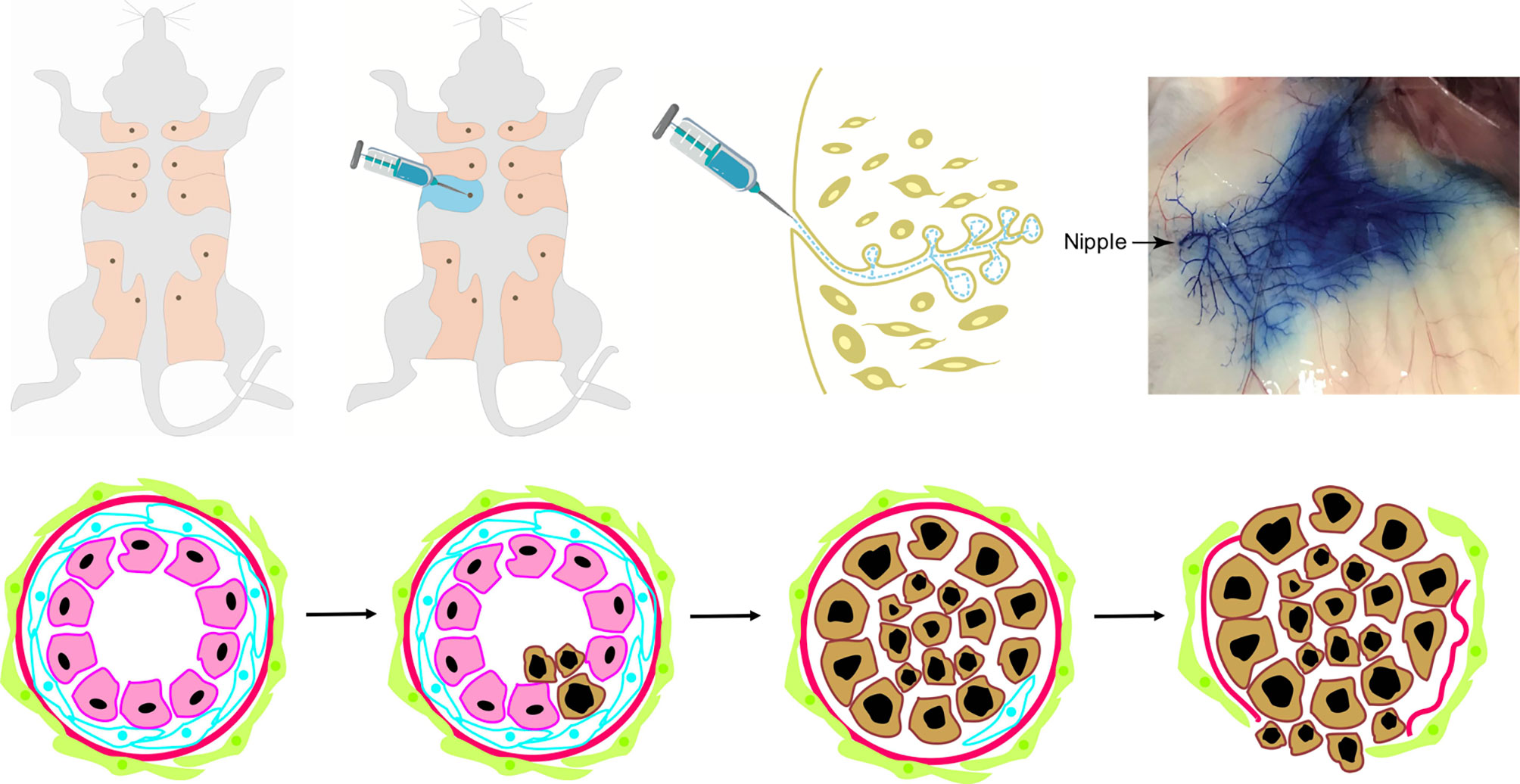
Figure 1 The intraductal injection of retrovirus into mammary glands in vivo with virus vector initiates and promotes carcinogenesis in mouse models. It is a novel mouse mammary gland cancer model which mimics human breast cancer non-invasive-to-invasive progression with virus vector. Most human breast cancers are not due to hereditary mutations, rather they arise from normal cells that later suffered spontaneous mutations. This mouse model was established by intraductal injection of retrovirus carrying the oncogenes with blue dye into one of the fifth mouse mammary glands. The technique of virus-mediated gene transfers into selected mammary cells (such as stem cells and specific progenitor cells) at selected times can overcome many of the shortcomings of the existing mouse models and more closely mimics human breast cancer formation in which only one or a few cells mutate to initiate cancer development. It allows temporal analysis of many processes involved in early breast cancer invasive progression including intraductal cancer cell growth, the cell interactions with the surrounding normal epithelial and myoepithelial cells, and their escape into the surrounding stroma. Photo of nipple injection -- courtesy of Wen Bu (Baylor College of Medicine).
Majority GEMMs are ER negative and most xenograft mouse models are based on few ER+ cancer cell lines (112). And there are no reliable mouse models of ER+ breast cancer that are also estrogen-dependent (113, 114). For example, STAT1−/− mice express abundant amounts of ER and PR (115), but tumor development is not hormone-dependent (116). A K-Ras mutant has been reported to induce ER+ tumors in mice (114), but the resulting tumors have not been thoroughly tested for estrogen dependency. As we previous indicated that the RACS-TVA approach can especially introduce genetic alterations into only a small number of the mammary cells (103). Lentiviral PyMT produces both luminal and basal-like tumors (55). TVA-PyMT mice and TVA-erbB2 mice had ER expression in greater than 10% of mammary tumor cells (117). And PyMT-induced tumors exhibited a two-fold increased ER-positivity versus erbB2-induced tumors. Compare with mice mammary glands, rats are more similar to the human breast, rats mammary glands had a ductal tree terminates in TDLUs with connective tissues and organized fibroblasts as sheath around and shows extensive alveolar development (118). Oral DMBA or intravenous or subcutaneous of NMU induced ER+ and PR+ tumors in rats (119), and many of these tumors harbor Ras mutations (49, 120). Ras signaling is frequently activated in human breast cancer, usually not by mutations in a Ras gene per se, but by mutations and overexpression of upstream signaling components such as receptor tyrosine kinases and NF1 mutations (121). Wang et al. found that intraductal injection of retrovirus expressing activated versions of Ras or erbB2 into Sprague/Dawley rats led to ER+ tumors (122). This intraductal model has a defined genetic mutation and is more relevant to human breast cancer etiology than DMBA models. NF1 mutations are enriched in ER+ breast cancers of patients. Crispr-mediated germline knockout of NF1 has been reported to induce ER+ tumors that are estrogen dependent (123). The CRISPRs technology is already widely used to edit somatic cells, and CAS9 rats are already commercially available. Therefore, intraductal injection of a virus carrying gRNA could be used to mutate NF1 and other genes associated with human ER+ cancer to generate somatic models of ER+ cancer in rats. Wen and Yi also described the intraductal injection of lentiviral vector FUCGW carrying the mutated oncogene HrasQ61L to Sprague/Dawley rats led to mammary tumors with high positive expression of both ER and PR (124). This technique is an efficient tool for modeling formation, prevention, and treatment of human breast cancer, especially ER+ breast cancer.
Actually, to mimic human breast cancer accurately is very difficult, especially in breast cancer therapy. CDX or PDX models are widely used because of its easy application, large and rapid tumor cohort generation, and simple preclinical data assessment. They can’t recapitulate tumorigenicity and treatment response in immunocompromized or immune-competent host system. In clinic, cyclin dependent kinase 4/6 (CDK4/6) inhibitors PD0332991 (palbociclib) has shown great efficacy in the treatment of hormone receptor-positive breast cancer, has received conditional approval from the FDA for metastatic breast cancer. Roberts et al. indicated that palbociclib is effective in a HER2-positive mouse model of breast cancer (MMTV-c-neu) but had no effect in the basal-like breast model C3-TAg (125, 126). The combination of carboplatin plus PD0332991 decreased antitumor activity compared with carboplatin alone in MMTV-c-neu with hematopoietic stem cell dormancy (125). It mimicked the therapy response of palbociclib in different subtype breast cancer. Usary et al. examined a range of therapeutics focused on MEK, mTOR, and PIK3CA/mTOR inhibitors in basal-like (C3-TAg), luminal B (MMTV-c-neu), and claudin-low (T11/TP53−/− OST) GEMM (127). They found variable responses in different GEMM. The MMTV-c-neu and basal-like breast model C3-Tag was the most responsive in general and claudin-low T11/TP53−/− model was the most resistant with only small responses. GEMMs recapitulated characteristics of human breast cancer have become a promising tool in cancer research to predict clinical outcome. A successful GEMM is very slow and laborious so that it has not been widely used. And there are still a lot of deficiencies with GEMM in preclinical research. The “co-clinical” trials which are validated in vivo models to pursue high-throughput drug screening and rapid translation of effective anticancer drugs into the clinical setting (128, 129). The co-clinical trials are underway in breast cancer, and we are looking forward to better rodent models for therapeutic testing of breast cancer.
The selection of appropriate rodent models for investigation of breast cancer is an important experimental decision. Every mouse model has advantages and disadvantages, and thus it is highly important to design a suitable mouse model for each research purpose based on integration of the advantages and disadvantages of different models, and compound techniques of mouse models have more practical application. The rodent models may help to improve the knowledge of breast carcinogenesis mechanism and genetic pathways, as well as creating therapy for modeling clinical breast cancer subtypes and develop innovative cancer therapy.
All authors made substantial contributions to articles reviewed in this manuscript, were involved in the drafting and revision, and approved the final version of this manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (No. 81972791). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Wen Bu (Baylor College of Medicine) for kindly providing the nipple injection photo (Figure 1).
1. Cardiff RD, Kenney N. A compendium of the mouse mammary tumor biologist: from the initial observations in the house mouse to the development of genetically engineered mice. Cold Spring Harb Perspect Biol (2011) 3(6):a003111. doi: 10.1101/cshperspect.a003111
2. Bittner JJ. Some Possible Effects of Nursing on the Mammary Gland Tumor Incidence In Mice. Science (New York NY) (1936) 84(2172):162. doi: 10.1126/science.84.2172.162
3. Kim JB, O’Hare MJ, Stein R. Models of breast cancer: is merging human and animal models the future? Breast Cancer Res (2004) 6(1):22–30. doi: 10.1186/bcr645
4. Stewart TA, Pattengale PK, Leder P. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell (1984) 38(3):627–37. doi: 10.1016/0092-8674(84)90257-5
5. Sinn E, Muller W, Pattengale P, Tepler I, Wallace R, Leder P. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell (1987) 49(4):465–75. doi: 10.1016/0092-8674(87)90449-1
6. Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell (1988) 54(1):105–15. doi: 10.1016/0092-8674(88)90184-5
7. Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, et al. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet (1999) 22(1):37–43. doi: 10.1038/8743
8. Du Z, Podsypanina K, Huang S, McGrath A, Toneff MJ, Bogoslovskaia E, et al. Introduction of oncogenes into mammary glands in vivo with an avian retroviral vector initiates and promotes carcinogenesis in mouse models. Proc Natl Acad Sci U S A (2006) 103(46):17396–401. doi: 10.1073/pnas.0608607103
9. Silva JG, Corrales-Medina FF, Maher OM, Tannir N, Huh WW, Rytting ME, et al. Clinical next generation sequencing of pediatric-type malignancies in adult patients identifies novel somatic aberrations. Oncoscience (2015) 2(2):187–92. doi: 10.18632/oncoscience.131
10. Tao K, Fang M, Alroy J, Sahagian GG. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer (2008) 8:228. doi: 10.1186/1471-2407-8-228
11. Paschall AV, Liu K. An Orthotopic Mouse Model of Spontaneous Breast Cancer Metastasis. J Vis Exp (2016) 114:54040. doi: 10.3791/54040
12. Zhang C, Yan Z, Arango ME, Painter CL, Anderes K. Advancing bioluminescence imaging technology for the evaluation of anticancer agents in the MDA-MB-435-HAL-Luc mammary fat pad and subrenal capsule tumor models. Clin Cancer Res (2009) 15(1):238–46. doi: 10.1158/1078-0432.Ccr-08-0897
13. Kuperwasser C, Dessain S, Bierbaum BE, Garnet D, Sperandio K, Gauvin GP, et al. A mouse model of human breast cancer metastasis to human bone. Cancer Res (2005) 65(14):6130–8. doi: 10.1158/0008-5472.Can-04-1408
14. Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res (2011) 13(4):215. doi: 10.1186/bcr2889
15. Whittle JR, Lewis MT, Lindeman GJ, Visvader JE. Patient-derived xenograft models of breast cancer and their predictive power. Breast Cancer Res (2015) 17(1):17. doi: 10.1186/s13058-015-0523-1
16. Sikora MJ, Cooper KL, Bahreini A, Luthra S, Wang G, Chandran UR, et al. Invasive lobular carcinoma cell lines are characterized by unique estrogen-mediated gene expression patterns and altered tamoxifen response. Cancer Res (2014) 74(5):1463–74. doi: 10.1158/0008-5472.Can-13-2779
17. Sflomos G, Dormoy V, Metsalu T, Jeitziner R, Battista L, Scabia V, et al. A Preclinical Model for ERα-Positive Breast Cancer Points to the Epithelial Microenvironment as Determinant of Luminal Phenotype and Hormone Response. Cancer Cell (2016) 29(3):407–22. doi: 10.1016/j.ccell.2016.02.002
18. Holen I, Speirs V, Morrissey B, Blyth K. In vivo models in breast cancer research: progress, challenges and future directions. Dis Models Mech (2017) 10(4):359–71. doi: 10.1242/dmm.028274
19. Murayama T, Gotoh N. Patient-Derived Xenograft Models of Breast Cancer and Their Application. Cells (2019) 8(6):621. doi: 10.3390/cells8060621
20. Kawaguchi T, Foster BA, Young J, Takabe K. Current Update of Patient-Derived Xenograft Model for Translational Breast Cancer Research. J Mammary Gland Biol Neoplasia (2017) 22(2):131–9. doi: 10.1007/s10911-017-9378-7
21. Li S, Shen D, Shao J, Crowder R, Liu W, Prat A, et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep (2013) 4(6):1116–30. doi: 10.1016/j.celrep.2013.08.022
22. DeRose YS, Wang G, Lin YC, Bernard PS, Buys SS, Ebbert MT, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med (2011) 17(11):1514–20. doi: 10.1038/nm.2454
23. Reyal F, Guyader C, Decraene C, Lucchesi C, Auger N, Assayag F, et al. Molecular profiling of patient-derived breast cancer xenografts. Breast Cancer Res (2012) 14(1):R11. doi: 10.1186/bcr3095
24. Zhang X, Claerhout S, Prat A, Dobrolecki LE, Petrovic I, Lai Q, et al. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res (2013) 73(15):4885–97. doi: 10.1158/0008-5472.Can-12-4081
25. Ding L, Ellis MJ, Li S, Larson DE, Chen K, Wallis JW, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature (2010) 464(7291):999–1005. doi: 10.1038/nature08989
26. Eirew P, Steif A, Khattra J, Ha G, Yap D, Farahani H, et al. Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature (2015) 518(7539):422–6. doi: 10.1038/nature13952
27. Engebraaten O, Vollan HKM, Børresen-Dale AL. Triple-negative breast cancer and the need for new therapeutic targets. Am J Pathol (2013) 183(4):1064–74. doi: 10.1016/j.ajpath.2013.05.033
28. Kabos P, Finlay-Schultz J, Li C, Kline E, Finlayson C, Wisell J, et al. Patient-derived luminal breast cancer xenografts retain hormone receptor heterogeneity and help define unique estrogen-dependent gene signatures. Breast Cancer Res Treat (2012) 135(2):415–32. doi: 10.1007/s10549-012-2164-8
29. Behbod F, Kittrell FS, LaMarca H, Edwards D, Kerbawy S, Heestand JC, et al. An intraductal human-in-mouse transplantation model mimics the subtypes of ductal carcinoma in situ. Breast Cancer Res (2009) 11(5):R66. doi: 10.1186/bcr2358
30. Haricharan S, Lei J, Ellis M. Mammary Ductal Environment Is Necessary for Faithful Maintenance of Estrogen Signaling in ER+ Breast Cancer. Cancer Cell (2016) 29(3):249–50. doi: 10.1016/j.ccell.2016.02.017
31. Ghosh A, Sarkar S, Banerjee S, Behbod F, Tawfik O, McGregor D, et al. MIND model for triple-negative breast cancer in syngeneic mice for quick and sequential progression analysis of lung metastasis. PloS One (2018) 13(5):e0198143. doi: 10.1371/journal.pone.0198143
32. Atiya HI, Dvorkin-Gheva A, Hassell J, Patel S, Parker RL, Hartstone-Rose A, et al. Intraductal Adaptation of the 4T1 Mouse Model of Breast Cancer Reveals Effects of the Epithelial Microenvironment on Tumor Progression and Metastasis. Anticancer Res (2019) 39(5):2277–87. doi: 10.21873/anticanres.13344
33. Steenbrugge J, Breyne K, Demeyere K, De Wever O, Sanders NN, Van Den Broeck W, et al. Anti-inflammatory signaling by mammary tumor cells mediates prometastatic macrophage polarization in an innovative intraductal mouse model for triple-negative breast cancer. J Exp Clin Cancer Res (2018) 37(1):191. doi: 10.1186/s13046-018-0860-x
34. Luo XL, Lin L, Hu H, Hu FL, Lin Y, Luo ML, et al. Development and characterization of mammary intraductal (MIND) spontaneous metastasis models for triple-negative breast cancer in syngeneic mice. Sci Rep (2020) 10(1):4681. doi: 10.1038/s41598-020-61679-8
35. Dexter DL, Kowalski HM, Blazar BA, Fligiel Z, Vogel R, Heppner GH. Heterogeneity of tumor cells from a single mouse mammary tumor. Cancer Res (1978) 38(10):3174–81.
36. Steenbrugge J, Vander Elst N, Demeyere K, De Wever O, Sanders NN, Van Den Broeck W, et al. Comparative Profiling of Metastatic 4T1- vs. Non-metastatic Py230-Based Mammary Tumors in an Intraductal Model for Triple-Negative Breast Cancer. Front Immunol (2019) 10:2928:2928. doi: 10.3389/fimmu.2019.02928
37. Barros AC, Muranaka EN, Mori LJ, Pelizon CH, Iriya K, Giocondo G, et al. Induction of experimental mammary carcinogenesis in rats with 7,12-dimethylbenz(a)anthracene. Rev Hosp Clin (2004) 59(5):257–61. doi: 10.1590/s0041-87812004000500006
38. Danforth DN Jr, Tamarkin L, Lippman ME. Melatonin increases oestrogen receptor binding activity of human breast cancer cells. Nature (1983) 305(5932):323–5. doi: 10.1038/305323a0
39. Sutherland RL, Murphy LC, San Foo M, Green MD, Whybourne AM, Krozowski ZS. High-affinity anti-oestrogen binding site distinct from the oestrogen receptor. Nature (1980) 288(5788):273–5. doi: 10.1038/288273a0
40. Travis RC, Key TJ. Oestrogen exposure and breast cancer risk. Breast Cancer Res (2003) 5(5):239–47. doi: 10.1186/bcr628
41. Seymour L, Bezwoda WR, Meyer K, Behr C. Detection of P24 protein in human breast cancer: influence of receptor status and oestrogen exposure. Br J Cancer (1990) 61(6):886–90. doi: 10.1038/bjc.1990.198
42. Mense SM, Remotti F, Bhan A, Singh B, El-Tamer M, Hei TK, et al. Estrogen-induced breast cancer: alterations in breast morphology and oxidative stress as a function of estrogen exposure. Toxicol Appl Pharmacol (2008) 232(1):78–85. doi: 10.1016/j.taap.2008.06.007
43. Harvell DM, Strecker TE, Tochacek M, Xie B, Pennington KL, McComb RD, et al. Rat strain-specific actions of 17beta-estradiol in the mammary gland: correlation between estrogen-induced lobuloalveolar hyperplasia and susceptibility to estrogen-induced mammary cancers. Proc Natl Acad Sci U S A (2000) 97(6):2779–84. doi: 10.1073/pnas.050569097
44. Jiang S, Katayama H, Wang J, Li SA, Hong Y, Radvanyi L, et al. Estrogen-induced aurora kinase-A (AURKA) gene expression is activated by GATA-3 in estrogen receptor-positive breast cancer cells. Horm Cancer (2010) 1(1):11–20. doi: 10.1007/s12672-010-0006-x
45. Ruhlen RL, Willbrand DM, Besch-Williford CL, Ma L, Shull JD, Sauter ER. Tamoxifen induces regression of estradiol-induced mammary cancer in the ACI.COP-Ept2 rat model. Breast Cancer Res Treat (2009) 117(3):517–24. doi: 10.1007/s10549-008-0169-0
46. Kassayová M, Bobrov N, Strojný L, Orendáš P, Demečková V, Jendželovský R, et al. Anticancer and Immunomodulatory Effects of Lactobacillus plantarum LS/07, Inulin and Melatonin in NMU-induced Rat Model of Breast Cancer. Anticancer Res (2016) 36(6):2719–28.
47. Chan MM, Lu X, Merchant FM, Iglehart JD, Miron PL. Serial transplantation of NMU-induced rat mammary tumors: a model of human breast cancer progression. Int J Cancer (2007) 121(3):474–85. doi: 10.1002/ijc.22684
48. Martinez VG, Crown J, Porter RK, O’Driscoll L. Neuromedin U alters bioenergetics and expands the cancer stem cell phenotype in HER2-positive breast cancer. Int J Cancer (2017) 140(12):2771–84. doi: 10.1002/ijc.30705
49. Zeng L, Li W, Chen CS. Breast cancer animal models and applications. Zool Res (2020) 41(5):477–94. doi: 10.24272/j.issn.2095-8137.2020.095
50. Raptis L, Brownell HL, Corbley MJ, Wood KW, Wang D, Haliotis T. Cellular ras gene activity is required for full neoplastic transformation by the large tumor antigen of SV40. Cell Growth Differ (1997) 8(8):891–901.
51. Raptis L, Marcellus R, Corbley MJ, Krook A, Whitfield J, Anderson SK, et al. Cellular ras gene activity is required for full neoplastic transformation by polyomavirus. J Virol (1991) 65(10):5203–10. doi: 10.1128/JVI.65.10.5203-5210.1991
52. Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, et al. The genomic landscapes of human breast and colorectal cancers. Science (New York NY) (2007) 318(5853):1108–13. doi: 10.1126/science.1145720
53. Fromowitz FB, Viola MV, Chao S, Oravez S, Mishriki Y, Finkel G, et al. ras p21 expression in the progression of breast cancer. Hum Pathol (1987) 18(12):1268–75. doi: 10.1016/s0046-8177(87)80412-4
54. Garcia R, Yu CL, Hudnall A, Catlett R, Nelson KL, Smithgall T, et al. Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ (1997) 8(12):1267–76.
55. Smith BA, Shelton DN, Kieffer C, Milash B, Usary J, Perou CM, et al. Targeting the PyMT Oncogene to Diverse Mammary Cell Populations Enhances Tumor Heterogeneity and Generates Rare Breast Cancer Subtypes. Genes Cancer (2012) 3(9-10):550–63. doi: 10.1177/1947601913475359
56. Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol (1992) 12(3):954–61. doi: 10.1128/mcb.12.3.954
57. Shishido SN, Delahaye A, Beck A, Nguyen TA. The anticancer effect of PQ1 in the MMTV-PyVT mouse model. Int J Cancer (2014) 134(6):1474–83. doi: 10.1002/ijc.28461
58. Maglione JE, Moghanaki D, Young LJ, Manner CK, Ellies LG, Joseph SO, et al. Transgenic Polyoma middle-T mice model premalignant mammary disease. Cancer Res (2001) 61(22):8298–305.
59. Maglione JE, McGoldrick ET, Young LJ, Namba R, Gregg JP, Liu L, et al. Polyomavirus middle T-induced mammary intraepithelial neoplasia outgrowths: single origin, divergent evolution, and multiple outcomes. Mol Cancer Ther (2004) 3(8):941–53.
60. Nusse R, Brown A, Papkoff J, Scambler P, Shackleford G, McMahon A, et al. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell (1991) 64(2):231. doi: 10.1016/0092-8674(91)90633-a
61. McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell (1990) 62(6):1073–85. doi: 10.1016/0092-8674(90)90385-r
62. Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell (1988) 55(4):619–25. doi: 10.1016/0092-8674(88)90220-6
63. Foo AL, Sly PD. Pulmonary function in a hospital population of asthmatic children. J Asthma (1991) 28(4):273–80. doi: 10.3109/02770909109073384
64. Li Y, Hively WP, Varmus HE. Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene (2000) 19(8):1002–9. doi: 10.1038/sj.onc.1203273
65. Michaelson JS, Leder P. beta-catenin is a downstream effector of Wnt-mediated tumorigenesis in the mammary gland. Oncogene (2001) 20(37):5093–9. doi: 10.1038/sj.onc.1204586
66. Lane TF, Leder P. Wnt-10b directs hypermorphic development and transformation in mammary glands of male and female mice. Oncogene (1997) 15(18):2133–44. doi: 10.1038/sj.onc.1201593
67. Zhang J, Li Y, Liu Q, Lu W, Bu G. Wnt signaling activation and mammary gland hyperplasia in MMTV-LRP6 transgenic mice: implication for breast cancer tumorigenesis. Oncogene (2010) 29(4):539–49. doi: 10.1038/onc.2009.339
68. Muller WJ, Lee FS, Dickson C, Peters G, Pattengale P, Leder P. The int-2 gene product acts as an epithelial growth factor in transgenic mice. EMBO J (1990) 9(3):907–13. doi: 10.1002/j.1460-2075.1990.tb08188.x
69. Cardiff RD, Sinn E, Muller W, Leder P. Transgenic oncogene mice. Tumor phenotype predicts genotype. Am J Pathol (1991) 139(3):495–501.
70. Ray D, Terao Y, Nimbalkar D, Hirai H, Osmundson EC, Zou X, et al. Hemizygous disruption of Cdc25A inhibits cellular transformation and mammary tumorigenesis in mice. Cancer Res (2007) 67(14):6605–11. doi: 10.1158/0008-5472.Can-06-4815
71. Hodgson MC, Vanostran G, Alghamdi S, Poppiti RJ, Agoulnik AI, Agoulnik IU. Reduced androgen receptor expression accelerates the onset of ERBB2 induced breast tumors in female mice. PloS One (2013) 8(4):e60455. doi: 10.1371/journal.pone.0060455
72. Fantozzi A, Christofori G. Mouse models of breast cancer metastasis. Breast Cancer Res (2006) 8(4):212. doi: 10.1186/bcr1530
73. Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA (1992) 89(22):10578–82. doi: 10.1073/pnas.89.22.10578
74. Li B, Rosen JM, McMenamin-Balano J, Muller WJ, Perkins AS. neu/ERBB2 cooperates with p53-172H during mammary tumorigenesis in transgenic mice. Mol Cell Biol (1997) 17(6):3155–63. doi: 10.1128/mcb.17.6.3155
75. Maroulakou IG, Anver M, Garrett L, Green JE. Prostate and mammary adenocarcinoma in transgenic mice carrying a rat C3(1) simian virus 40 large tumor antigen fusion gene. Proc Natl Acad Sci U S A (1994) 91(23):11236–40. doi: 10.1073/pnas.91.23.11236
76. Pathania R, Ramachandran S, Elangovan S, Padia R, Yang P, Cinghu S, et al. DNMT1 is essential for mammary and cancer stem cell maintenance and tumorigenesis. Nat Commun (2015) 6:6910. doi: 10.1038/ncomms7910
77. Shibata MA, Jorcyk CL, Liu ML, Yoshidome K, Gold LG, Green JE. The C3(1)/SV40 T antigen transgenic mouse model of prostate and mammary cancer. Toxicol Pathol (1998) 26(1):177–82. doi: 10.1177/019262339802600121
78. Wild R, Yokoyama Y, Dings RP, Ramakrishnan S. VEGF-DT385 toxin conjugate inhibits mammary adenocarcinoma development in a transgenic mouse model of spontaneous tumorigenesis. Breast Cancer Res Treat (2004) 85(2):161–71. doi: 10.1023/B:BREA.0000025407.02896.ec
79. Song W, Hwang Y, Youngblood VM, Cook RS, Balko JM, Chen J, et al. Targeting EphA2 impairs cell cycle progression and growth of basal-like/triple-negative breast cancers. Oncogene (2017) 36(40):5620–30. doi: 10.1038/onc.2017.170
80. Liu R, Varghese S, Rabkin SD. Oncolytic herpes simplex virus vector therapy of breast cancer in C3(1)/SV40 T-antigen transgenic mice. Cancer Res (2005) 65(4):1532–40. doi: 10.1158/0008-5472.Can-04-3353
81. Erisman JW, Domburg N, de Vries W, Kros H, de Haan B, Sanders K. The Dutch N-cascade in the European perspective. Sci China C Life Sci (2005) 48(Suppl 2):827–42. doi: 10.1007/bf03187122
82. Sutherland RL, Musgrove EA. Cyclin D1 and mammary carcinoma: new insights from transgenic mouse models. Breast Cancer Res (2002) 4(1):14–7. doi: 10.1186/bcr411
83. Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature (1994) 369(6482):669–71. doi: 10.1038/369669a0
84. van Rossum AG, van Bragt MP, Schuuring-Scholtes E, van der Ploeg JC, van Krieken JH, Kluin PM, et al. Transgenic mice with mammary gland targeted expression of human cortactin do not develop (pre-malignant) breast tumors: studies in MMTV-cortactin and MMTV-cortactin/-cyclin D1 bitransgenic mice. BMC Cancer (2006) 6:58. doi: 10.1186/1471-2407-6-58
85. Garcia Y, Kahn O, Rabardel L, Chansou B, Salmon L, Tuchagues JP. Two-Step Spin Conversion for the Three-Dimensional Compound Tris(4,4’-bis-1,2,4-triazole)iron(II) Diperchlorate. Inorg Chem (1999) 38(21):4663–70. doi: 10.1021/ic990511q
86. Deng CX. Conditional knockout mouse models of cancer. Cold Spring Harb Protoc (2014) 2014(12):1217–33. doi: 10.1101/pdb.top074393
87. Hakem R, de la Pompa JL, Sirard C, Mo R, Woo M, Hakem A, et al. The tumor suppressor gene Brca1 is required for embryonic cellular proliferation in the mouse. Cell (1996) 85(7):1009–23. doi: 10.1016/s0092-8674(00)81302-1
88. Deng CX. Tumor formation in Brca1 conditional mutant mice. Environ Mol Mutagen (2002) 39(2-3):171–7. doi: 10.1002/em.10069
89. Raval C, Saeed K. Anaesthetic management of a patient of Brugada syndrome for an emergency appendicectomy. Anesth Essays Res (2012) 6(1):101–4. doi: 10.4103/0259-1162.103390
90. Shakya R, Szabolcs M, McCarthy E, Ospina E, Basso K, Nandula S, et al. The basal-like mammary carcinomas induced by Brca1 or Bard1 inactivation implicate the BRCA1/BARD1 heterodimer in tumor suppression. Proc Natl Acad Sci U S A (2008) 105(19):7040–5. doi: 10.1073/pnas.0711032105
91. Xu X, Qiao W, Linke SP, Cao L, Li WM, Furth PA, et al. Genetic interactions between tumor suppressors Brca1 and p53 in apoptosis, cell cycle and tumorigenesis. Nat Genet (2001) 28(3):266–71. doi: 10.1038/90108
92. Weaver Z, Montagna C, Xu X, Howard T, Gadina M, Brodie SG, et al. Mammary tumors in mice conditionally mutant for Brca1 exhibit gross genomic instability and centrosome amplification yet display a recurring distribution of genomic imbalances that is similar to human breast cancer. Oncogene (2002) 21(33):5097–107. doi: 10.1038/sj.onc.1205636
93. McPherson JP, Lemmers B, Hirao A, Hakem A, Abraham J, Migon E, et al. Collaboration of Brca1 and Chk2 in tumorigenesis. Genes Dev (2004) 18(10):1144–53. doi: 10.1101/gad.1192704
94. Liu X, Holstege H, van der Gulden H, Treur-Mulder M, Zevenhoven J, Velds A, et al. Somatic loss of BRCA1 and p53 in mice induces mammary tumors with features of human BRCA1-mutated basal-like breast cancer. Proc Natl Acad Sci U S A (2007) 104(29):12111–6. doi: 10.1073/pnas.0702969104
95. McCarthy A, Savage K, Gabriel A, Naceur C, Reis-Filho JS, Ashworth A. A mouse model of basal-like breast carcinoma with metaplastic elements. J Pathol (2007) 211(4):389–98. doi: 10.1002/path.2124
96. Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet (2001) 29(4):418–25. doi: 10.1038/ng747
97. Ludwig T, Fisher P, Murty V, Efstratiadis A. Development of mammary adenocarcinomas by tissue-specific knockout of Brca2 in mice. Oncogene (2001) 20(30):3937–48. doi: 10.1038/sj.onc.1204512
98. Cheung AM, Elia A, Tsao MS, Done S, Wagner KU, Hennighausen L, et al. Brca2 deficiency does not impair mammary epithelium development but promotes mammary adenocarcinoma formation in p53(+/-) mutant mice. Cancer Res (2004) 64(6):1959–65. doi: 10.1158/0008-5472.can-03-2270
99. Lin SC, Lee KF, Nikitin AY, Hilsenbeck SG, Cardiff RD, Li A, et al. Somatic mutation of p53 leads to estrogen receptor alpha-positive and -negative mouse mammary tumors with high frequency of metastasis. Cancer Res (2004) 64(10):3525–32. doi: 10.1158/0008-5472.Can-03-3524
100. Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis (New York NY 2000) (2002) 32(2):148–9. doi: 10.1002/gene.10036
101. Li G, Robinson GW, Lesche R, Martinez-Diaz H, Jiang Z, Rozengurt N, et al. Conditional loss of PTEN leads to precocious development and neoplasia in the mammary gland. Development (Cambridge England) (2002) 129(17):4159–70.
102. Schade B, Rao T, Dourdin N, Lesurf R, Hallett M, Cardiff RD, et al. PTEN deficiency in a luminal ErbB-2 mouse model results in dramatic acceleration of mammary tumorigenesis and metastasis. J Biol Chem (2009) 284(28):19018–26. doi: 10.1074/jbc.M109.018937
103. Ahronian LG, Lewis BC. Using the RCAS-TVA system to model human cancer in mice. Cold Spring Harb Protoc (2014) 2014(11):1128–35. doi: 10.1101/pdb.top069831
104. Ahronian LG, Lewis BC. In vivo delivery of RCAS virus to mice. Cold Spring Harb Protoc (2014) 2014(11):1167–9. doi: 10.1101/pdb.prot077982
105. Fisher GH, Orsulic S, Holland E, Hively WP, Li Y, Lewis BC, et al. Development of a flexible and specific gene delivery system for production of murine tumor models. Oncogene (1999) 18(38):5253–60. doi: 10.1038/sj.onc.1203087
106. Reddy JP, Li Y. The RCAS-TVA system for introduction of oncogenes into selected somatic mammary epithelial cells in vivo. J Mammary Gland Biol Neoplasia (2009) 14(4):405–9. doi: 10.1007/s10911-009-9157-1
107. Sun H, Zhu Y, He QF, Shu LY, Zhang W, Chai YM. Reinforcement strategy for lateral rafting plate fixation in posterolateral column fractures of the tibial plateau: The magic screw technique. Injury (2017) 48(12):2814–26. doi: 10.1016/j.injury.2017.10.033
108. Siwko SK, Bu W, Gutierrez C, Lewis B, Jechlinger M, Schaffhausen B, et al. Lentivirus-mediated oncogene introduction into mammary cells in vivo induces tumors. Neoplasia (New York NY) (2008) 10(7):653–662, 651 p following 662. doi: 10.1593/neo.08266
109. Haricharan S, Hein SM, Dong J, Toneff MJ, Aina OH, Rao PH, et al. Contribution of an alveolar cell of origin to the high-grade malignant phenotype of pregnancy-associated breast cancer. Oncogene (2014) 33(50):5729–39. doi: 10.1038/onc.2013.521
110. Haricharan S, Dong J, Hein S, Reddy JP, Du Z, Toneff M, et al. Mechanism and preclinical prevention of increased breast cancer risk caused by pregnancy. eLife (2013) 2:e00996. doi: 10.7554/eLife.00996
111. Sinha VC, Qin L, Li Y. A p53/ARF-dependent anticancer barrier activates senescence and blocks tumorigenesis without impacting apoptosis. Mol Cancer Res (2015) 13(2):231–8. doi: 10.1158/1541-7786.Mcr-14-0481-t
112. Hakamy A, Bolton CE, Gibson JE, McKeever TM. Risk of fall in patients with COPD. Thorax (2018) 73(11):1079–80. doi: 10.1136/thoraxjnl-2017-211008
113. Champagne E, Scarpellino L, Lane P, Acha-Orbea H. CD28/CTLA4-B7 interaction is dispensable for T cell stimulation by mouse mammary tumor virus superantigen but not for B cell differentiation and virus dissemination. Eur J Immunol (1996) 26(7):1595–602. doi: 10.1002/eji.1830260728
114. Andò S, Malivindi R, Catalano S, Rizza P, Barone I, Panza S, et al. Conditional expression of Ki-Ras(G12V) in the mammary epithelium of transgenic mice induces estrogen receptor alpha (ERα)-positive adenocarcinoma. Oncogene (2017) 36(46):6420–31. doi: 10.1038/onc.2017.252
115. Chan SR, Vermi W, Luo J, Lucini L, Rickert C, Fowler AM, et al. STAT1-deficient mice spontaneously develop estrogen receptor α-positive luminal mammary carcinomas. Breast Cancer Res (2012) 14(1):R16. doi: 10.1186/bcr3100
116. Chan SR, Rickert CG, Vermi W, Sheehan KC, Arthur C, Allen JA, et al. Dysregulated STAT1-SOCS1 control of JAK2 promotes mammary luminal progenitor cell survival and drives ERα(+) tumorigenesis. Cell Death Differ (2014) 21(2):234–46. doi: 10.1038/cdd.2013.116
117. Holloway KR, Sinha VC, Bu W, Toneff M, Dong J, Peng Y, et al. Targeting Oncogenes into a Defined Subset of Mammary Cells Demonstrates That the Initiating Oncogenic Mutation Defines the Resulting Tumor Phenotype. Int J Biol Sci (2016) 12(4):381–8. doi: 10.7150/ijbs.12947
118. Masso-Welch PA, Darcy KM, Stangle-Castor NC, Ip MM. A developmental atlas of rat mammary gland histology. J Mammary Gland Biol Neoplasia (2000) 5(2):165–85. doi: 10.1023/a:1026491221687
119. Russo J, Russo IH. Atlas and histologic classification of tumors of the rat mammary gland. J Mammary Gland Biol Neoplasia (2000) 5(2):187–200. doi: 10.1023/a:1026443305758
120. Shirai K, Uemura Y, Fukumoto M, Tsukamoto T, Pascual R, Nandi S, et al. Synergistic effect of MNU and DMBA in mammary carcinogenesis and H-ras activation in female Sprague-Dawley rats. Cancer Lett (1997) 120(1):87–93. doi: 10.1016/s0304-3835(97)00293-0
121. Griffith OL, Spies NC, Anurag M, Griffith M, Luo J, Tu D, et al. The prognostic effects of somatic mutations in ER-positive breast cancer. Nat Commun (2018) 9(1):3476. doi: 10.1038/s41467-018-05914-x
122. Wang B, Kennan WS, Yasukawa-Barnes J, Lindstrom MJ, Gould MN. Difference in the response of neu and ras oncogene-induced rat mammary carcinomas to early and late ovariectomy. Cancer Res (1992) 52(15):4102–5.
123. Dischinger PS, Tovar EA, Essenburg CJ, Madaj ZB, Gardner EE, Callaghan ME, et al. NF1 deficiency correlates with estrogen receptor signaling and diminished survival in breast cancer. NPJ Breast Cancer (2018) 4:29. doi: 10.1038/s41523-018-0080-8
124. Bu W, Li Y. Intraductal Injection of Lentivirus Vectors for Stably Introducing Genes into Rat Mammary Epithelial Cells in Vivo. J Mammary Gland Biol Neoplasia (2020). doi: 10.1007/s10911-020-09469-w
125. Roberts PJ, Bisi JE, Strum JC, Combest AJ, Darr DB, Usary JE, et al. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst (2012) 104(6):476–87. doi: 10.1093/jnci/djs002
126. Roberts PJ, Usary JE, Darr DB, Dillon PM, Pfefferle AD, Whittle MC, et al. Combined PI3K/mTOR and MEK inhibition provides broad antitumor activity in faithful murine cancer models. Clin Cancer Res (2012) 18(19):5290–303. doi: 10.1158/1078-0432.Ccr-12-0563
127. Usary J, Zhao W, Darr D, Roberts PJ, Liu M, Balletta L, et al. Predicting drug responsiveness in human cancers using genetically engineered mice. Clin Cancer Res (2013) 19(17):4889–99. doi: 10.1158/1078-0432.Ccr-13-0522
128. Lunardi A, Ala U, Epping MT, Salmena L, Clohessy JG, Webster KA, et al. A co-clinical approach identifies mechanisms and potential therapies for androgen deprivation resistance in prostate cancer. Nat Genet (2013) 45(7):747–55. doi: 10.1038/ng.2650
Keywords: mouse model, mouse intraductal model, transgenic mouse model, breast cancer, rodent model
Citation: Liu C, Wu P, Zhang A and Mao X (2021) Advances in Rodent Models for Breast Cancer Formation, Progression, and Therapeutic Testing. Front. Oncol. 11:593337. doi: 10.3389/fonc.2021.593337
Received: 26 October 2020; Accepted: 27 January 2021;
Published: 26 March 2021.
Edited by:
Ceshi Chen, Kunming Institute of Zoology, ChinaReviewed by:
Yongliang Huo, Guangzhou Medical University, ChinaCopyright © 2021 Liu, Wu, Zhang and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyun Mao, eHltYW9AY211LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.