- 1Vessel-Organ Interaction Research Center, College of Pharmacy, Kyungpook National University, Daegu, South Korea
- 2Research Institute of Pharmaceutical Sciences, Kyungpook National University, Daegu, South Korea
- 3Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
- 4Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
- 5Department of Surgery, School of Medicine, Kyungpook National University, Kyungpook National University Chilgok Hospital, Daegu, South Korea
- 6Department of Oncology/Hematology, School of Medicine, Kyungpook National University, Kyungpook National University Chilgok Hospital, Daegu, South Korea
- 7Department of Pathology, School of Medicine, Kyungpook National University, Kyungpook National University Chilgok Hospital, Daegu, South Korea
- 8Department of Digital Health, Samsung Advanced Institute for Health Sciences & Technology, Sungkyunkwan University, Seoul, South Korea
- 9Statistics and Data Center, Research Institute for Future Medicine, Samsung Medical Center, Seoul, South Korea
- 10R&D Center, Gencurix Inc., Seoul, South Korea
- 11Laboratory of Molecular Pathology and Cancer Genomics, Research Institute of Pharmaceutical Sciences and College of Pharmacy, Seoul National University, Seoul, South Korea
- 12Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, South Korea
Background: The prognostic or predictive value of commonly used multigene assays in young patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) early breast cancer is unclear. In this study, we assessed the prognostic value of the GenesWell BCT assay according to age group.
Methods: We identified patients with pN0-1, HR+/HER2− breast cancer in a prospective cohort of women who underwent surgery between 2005 and 2017. The GenesWell BCT assay was performed on tissue samples from selected patients. Distant metastasis-free survival (DMFS) and disease-free survival (DFS) were compared between the risk groups assigned by the BCT score.
Results: A total of 712 patients were eligible for analysis. The median follow-up time was 7.47 years. The BCT score was prognostic in patients aged ≤50 years (n = 404) and those aged >50 years (n = 308). In both age groups, the 10-year DMFS and DFS rates for patients classified as high risk by the BCT score were significantly lower than those for patients classified as low risk. A multivariate analysis revealed that the BCT score was an independent prognostic factor for DFS in patients aged ≤50 years (hazard ratio, 1.28; 95% CI, 1.05–1.56; P = 0.015), as well as those aged >50 years.
Conclusion: The BCT score could be used to identify low-risk patients who will not benefit from adjuvant chemotherapy to treat HR+/HER2− early breast cancer regardless of age. A further prospective study to assess the prognostic and predictive value of the BCT score is required.
Introduction
Young age at diagnosis is a negative prognostic factor for patients with early breast cancer, particularly those with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) breast cancer (1–3). Accordingly, young age is often used as an indication for adjuvant chemotherapy, and some studies have reported that most (>80%) young patients with early breast cancer receive adjuvant chemotherapy (4, 5). However, HR+/HER2− breast cancer patients benefit less from chemotherapy than HER2+ or triple-negative breast cancer patients (6), and young age alone should not be a reason to expand chemotherapy indications in early breast cancer (7, 8) Therefore, it is important to identify patients who will not benefit from chemotherapy to avoid unnecessary chemotherapy in young patients with HR+/HER2− breast cancer.
Several multigene assays, such as MammaPrint (9) and Oncotype DX (10), have been developed to predict the risk of recurrence or response to adjuvant chemotherapy in early breast cancer. However, most of those assays were developed using data mainly from postmenopausal women in Western countries (11, 12), and recent prospective clinical trials (e.g., TAILORx for Oncotype DX and MINDACT for MammaPrint) also included only a small number of young breast cancer patients (13, 14). Few data are available regarding the prognostic or predictive value of the commonly used multigene assays in young breast cancer patients. Moreover, recent TAILORx results show that the Oncotype DX recurrence score (RS) has different treatment implications for patients aged ≤50 and those aged >50 years (14), and they further revealed that the clinical risk classification in combination with RS provided prognostic information for identifying young patients who could benefit from chemotherapy (15). Those findings raised concerns about the value of existing multigene assays for deciding whether to use adjuvant chemotherapy in young breast cancer patients.
Another concern is that the median and peak age of breast cancer patients in Asian populations, including South Korea, Japan, Singapore, and Taiwan, is younger than in Western countries (16, 17). Breast cancer in young Asian women has distinctive disease characteristics compared with that in Western countries (18, 19). Therefore, it is particularly important to elucidate the reliability of multigene assays in young Asian women with breast cancer.
The GenesWell Breast Cancer Test (BCT) is a prognostic assay that predicts the risk of recurrence in patients with HR+/HER2− early breast cancer (20). This assay was developed using data from Asian patients, including a higher percentage of young patients than was used for previous assays. A recent study comparing the BCT score and the Oncotype DX RS for risk classification found that the concordance between the two risk scores was low in women aged ≤50 years, suggesting the need to find more adequate tests in that population (21). In this study, we analyzed the distribution of BCT scores by age group and assessed its prognostic value in patients aged ≤50 years and >50 years.
Materials and Methods
Patients and Tumor Samples
A total of 3,289 patients with T1–3, N0–1, HR+/HER2− early breast cancer who underwent surgery at SMC between July 2005 and December 2017 or at KNUH between January 2009 and December 2017 were screened in a prospectively collected patient cohort, and their clinical information and survival data were collected. Clinical information included their age at operation, tumor size, pathologic nodal (pN) status, pathologic stage according to the 7th edition of the American Joint Committee on Cancer classification, histologic grade, nuclear grade, lymphovascular invasion (LVI), multiplicity, Ki-67 (%), estrogen receptor (ER)/progesterone receptor (PR)/HER2 status, and use of hormone therapy or chemotherapy. ER/PR/HER2 status was obtained from the pathological report. ER and PR results determined by immunohistochemistry (IHC) were considered positive when at least 1% of tumor cells showed nuclear staining, according to American Society of Clinical Oncology/College of American Pathologists guidelines (22). HER2 was considered positive if ≥10% of tumor cells showed 3+ staining by IHC or 2+ staining by IHC with amplification using fluorescent or silver in situ hybridization (23).
Patients lacking clinical information or survival data, and those with short follow-up duration (≤12 months) were excluded from the sample. We stratified the included patients by age group and nodal status (pN0 or pN1) and then selected patients from each age group (31–40, 41–50, 51–60, >60 years) so that the ratio of patients with pN0 and pN1 tumors in each age group was 2:1. All patients in their 20s or 70s were included. The quantity and quality of formalin-fixed, paraffin-embedded (FFPE) tumor samples from the selected patients were evaluated, and only patients with sufficient FFPE tumor samples were then used to test the GenesWell BCT assay. In case of multiplicity, paraffin block from the largest mass was used to assess the BCT score.
GenesWell BCT Assay
Total RNA was isolated from the FFPE samples, and the GenesWell BCT assay was performed as previously described (20). The BCT score was calculated from the relative expression values of six prognostic genes (UBE2C, TOP2A, RRM2, FOXM1, MKI67, and BTN3A2), normalized by three reference genes (CTBP1, CUL1, and UBQLN1), and two clinical variables (tumor size and pN status). Patients were categorized as high risk for recurrence or distant metastasis if the BCT score was ≥4, whereas patients with a BCT score <4 were categorized as low risk.
Propensity Score Matching (PSM)
We used PSM with a 1:1 ratio to match control and treatment groups and enable us to evaluate causal treatment effects by excluding the effects of confounding factors (24). This analysis was performed using a nearest-neighbor matching algorithm in the “MatchIt” package for R software. The propensity score of the cohort was calculated using clinicopathological factors that significantly affect survival (P < 0.05 in Cox proportional hazard analysis), and the caliper was set to 0.04.
Statistical Analysis
Patient characteristics between the groups were compared using independent t-tests for continuous variables and the chi-square or Fisher’s exact test for categorical variables. Disease-free survival (DFS) was defined as the time from the date of surgery to the date of any recurrence, including locoregional recurrence and distant metastasis of breast cancer, and distant metastasis-free survival (DMFS) was defined as the time between the date of surgery and the date of distant metastasis. The probability of DFS and DMFS were estimated using the Kaplan-Meier method, and the log-rank test was used to assess statistical differences in survival rates between groups. Univariate and multivariate analyses were performed using Cox regression and proportional hazard models to evaluate the association between the clinical variables or BCT score and patient outcomes. All hazard ratios are reported with 95% confidence intervals (CIs). All statistical tests were two-sided, and a P < 0.05 was regarded as statistically significant. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA) and R 3.6.2 (http://www.R-project.org).
Results
Patient Characteristics
Of the 1,043 FFPE samples on which the GenesWell BCT assay was performed, 325 samples that returned invalid GenesWell BCT results and six samples from patients receiving hormone therapy <2 years were excluded. We thus used 712 patients with valid BCT scores in our analyses (Figure 1).
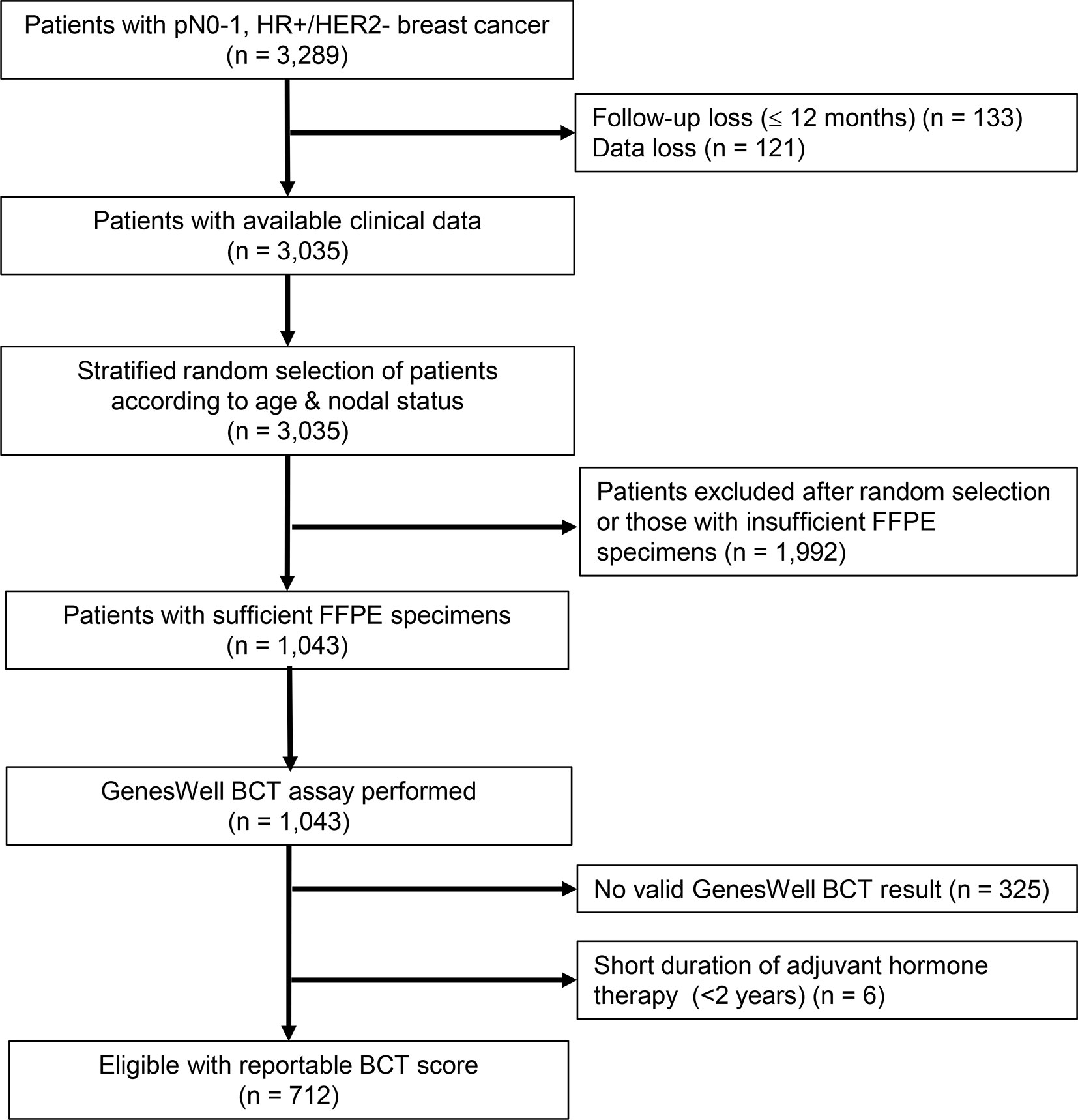
Figure 1 Study flow diagram. SMC, Samsung Medical Center; KNUH, Kyungpook National University Hospital; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; FFPE, formalin-fixed, paraffin-embedded.
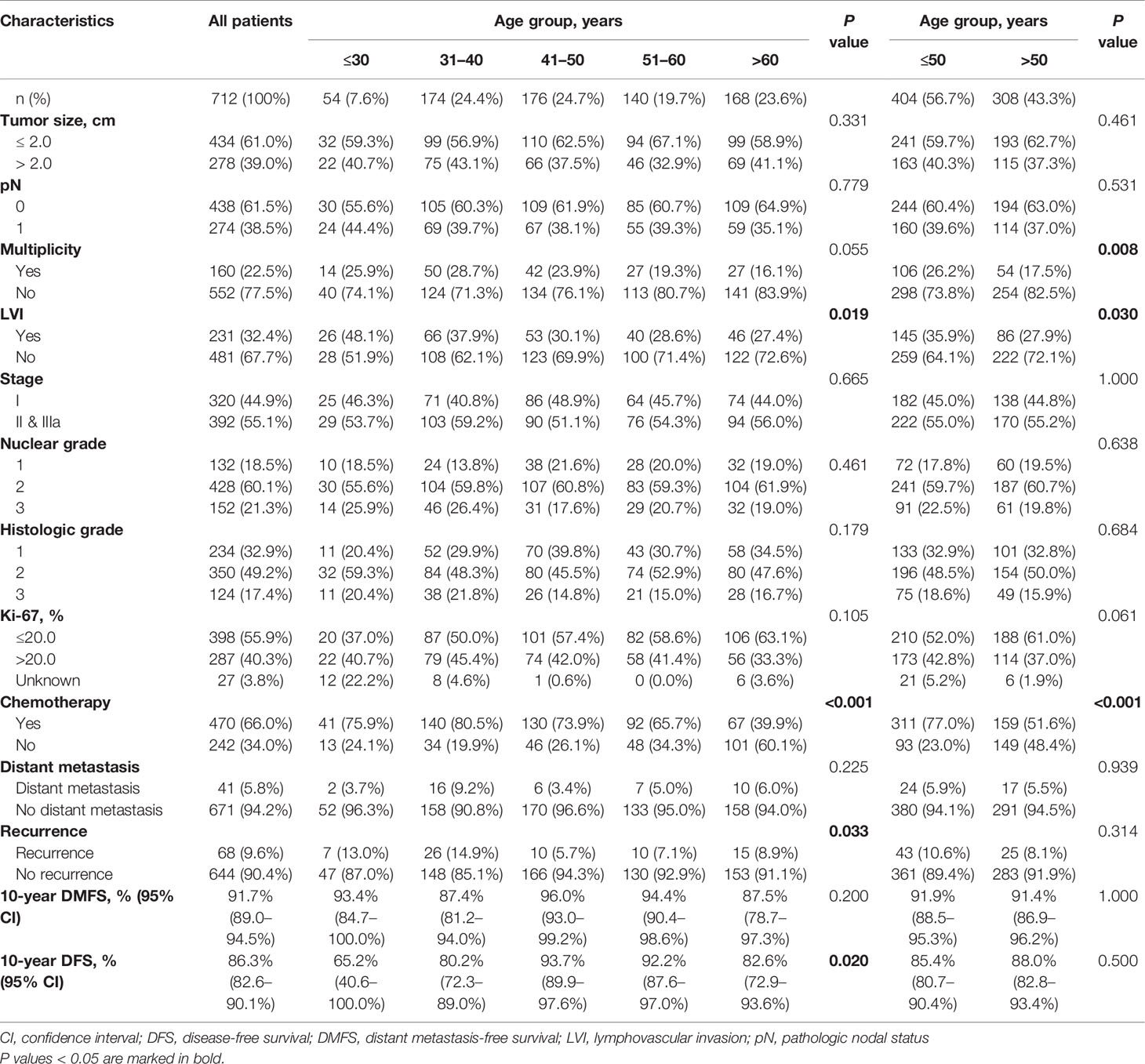
The patient characteristics are presented in Table 1. The median age of the 712 patients was 48.5 years (range 21–80), and 56.7% of patients (n = 404) were aged ≤50 years. Four hundred thirty-eight (61.5%) patients had pN0 tumors, and 434 (61.0%) patients had small tumors ≤2.0 cm. The median follow-up duration was 7.47 years (range 1.12–13.05). Sixty-six percent of patients were treated with hormone therapy plus chemotherapy, whereas 34.0% of patients received hormone therapy alone. When we compared the clinical characteristics of patients by age group, we found no significant differences in tumor size, pN, nuclear grade, histologic grade, or Ki-67 between age groups (Table 1). However, young women with breast cancer were more likely to have a higher rate of LVI (P = 0.019) and receive chemotherapy (P < 0.001) than older patients (Table 1). The incidence of distant metastasis and recurrence in all patients was 5.8 and 9.6%, respectively. Younger patients showed significantly lower 10-year DFS rates than older patients (P = 0.020). In contrast, 10-year DMFS did not differ significantly between the age groups (P = 0.200). The 10-year DFS rate of patients aged ≤30 years was 65.2%, whereas patents aged >30 years had a >80% of 10-year DFS rate.
Prognostic Validation of the BCT Score
The median BCT score of all patients was 3.89 (range 0–10.00), with 52.4% of patients categorized into the BCT low-risk group and 47.6% of patients classified as high risk (Figure 2A). The BCT high-risk group was significantly associated with unfavorable clinicopathological factors, including larger tumor size and advanced pN status (Supplementary Table 1).
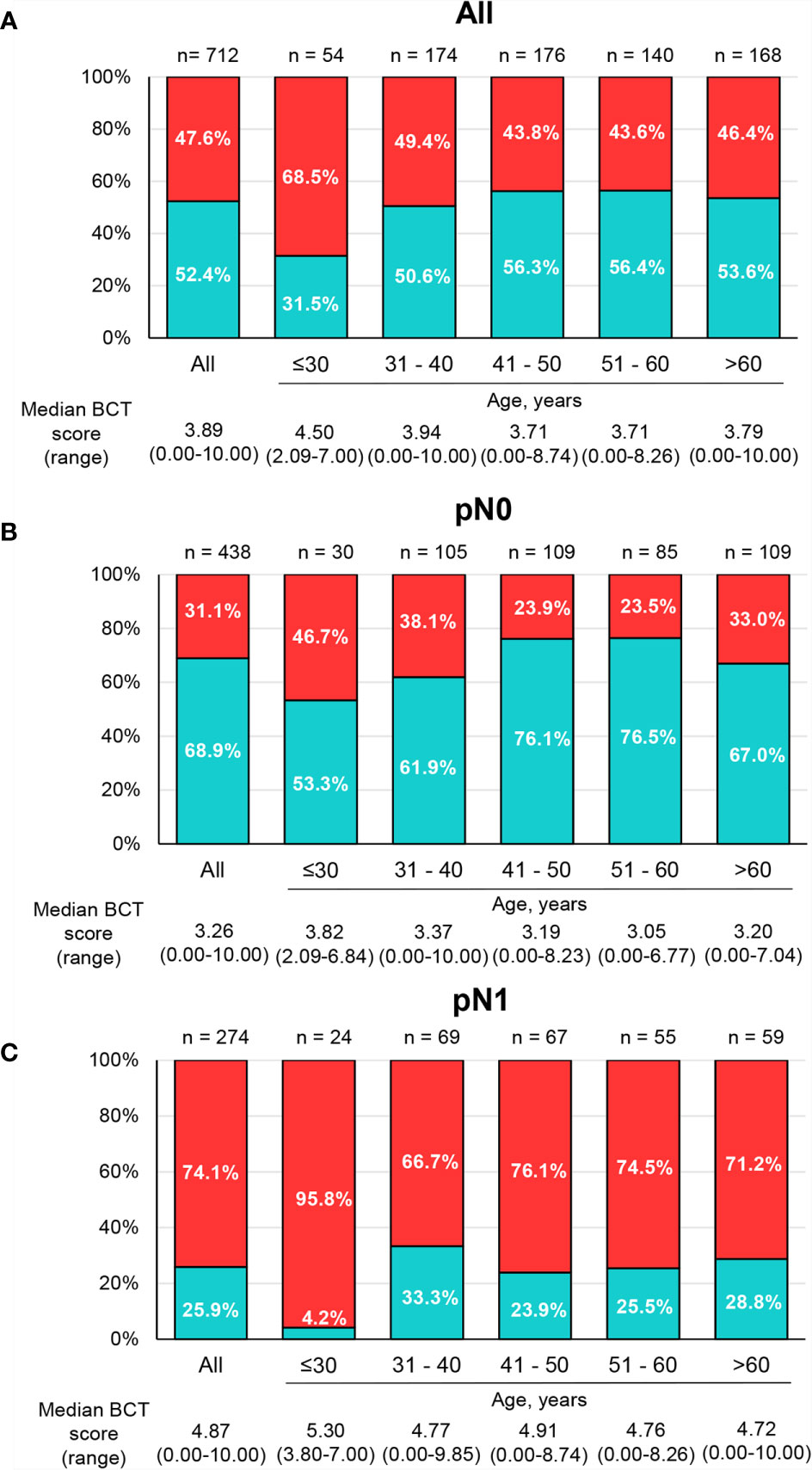
Figure 2 Distribution of BCT scores and risk groups by age group. The percentage of patients within each BCT risk group (blue for the BCT low-risk group and red for the BCT high-risk group) among (A) all patients (n = 712), (B) pN0 patients (n = 438), and (C) pN1 patients (n = 274) by age group. The median BCT score of each age group is also depicted.
The Kaplan-Meier survival curves show statistically significant differences in DMFS (P < 0.001) and DFS (P < 0.001) between the BCT low-risk and high-risk groups (Figure 3). The probability of 10-year DMFS for patients in the low-risk and high-risk groups was 96.9 and 86.2%, respectively. Recurrence rates at 10 years in patients categorized by the BCT as low risk and high risk were 8.8 and 19.1%, respectively.
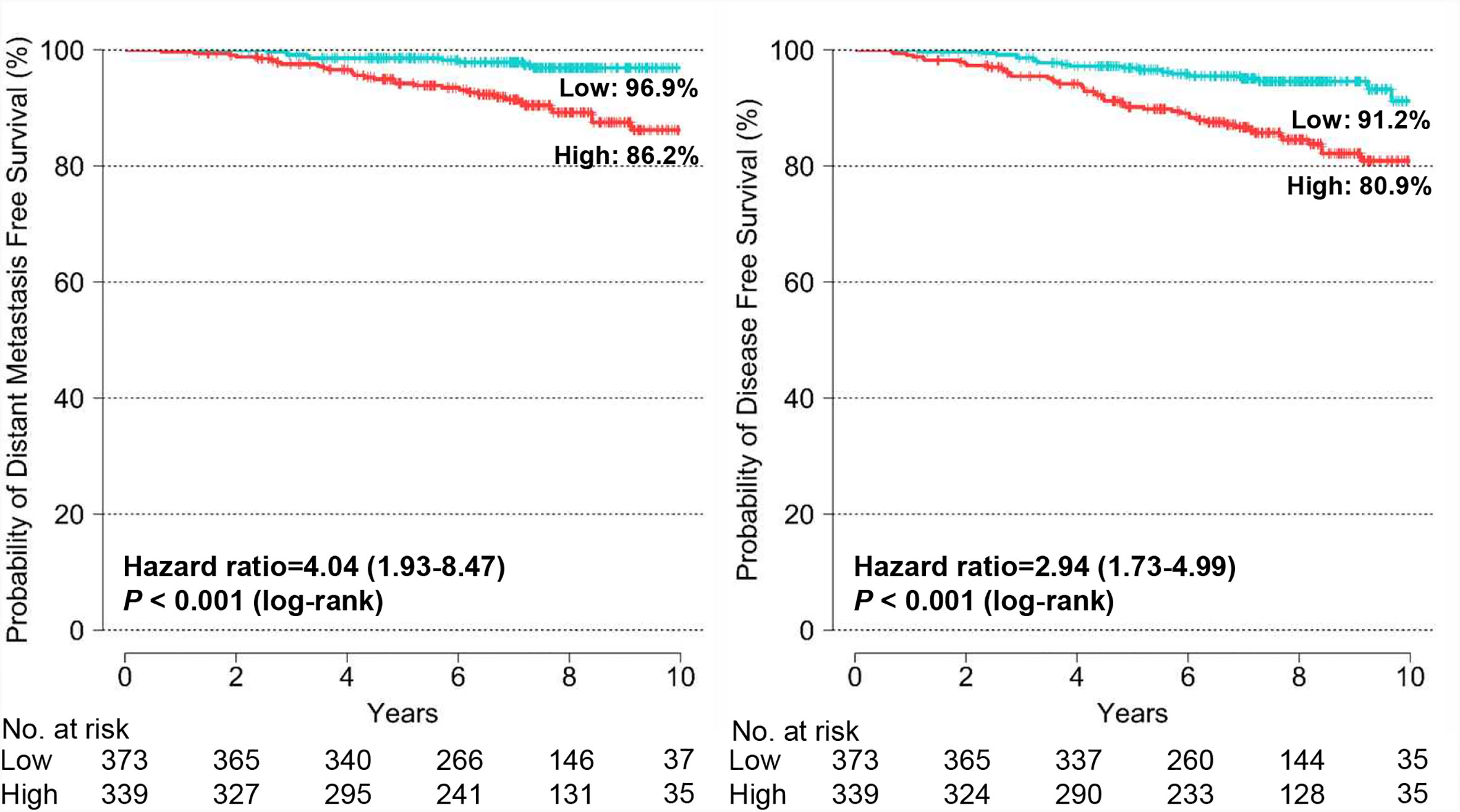
Figure 3 Kaplan-Meier estimates of 10-year distant metastasis-free survival and disease-free survival by BCT risk group in all patients. Patients were classified into the low-risk (blue) or high-risk (red) group according to their BCT scores.
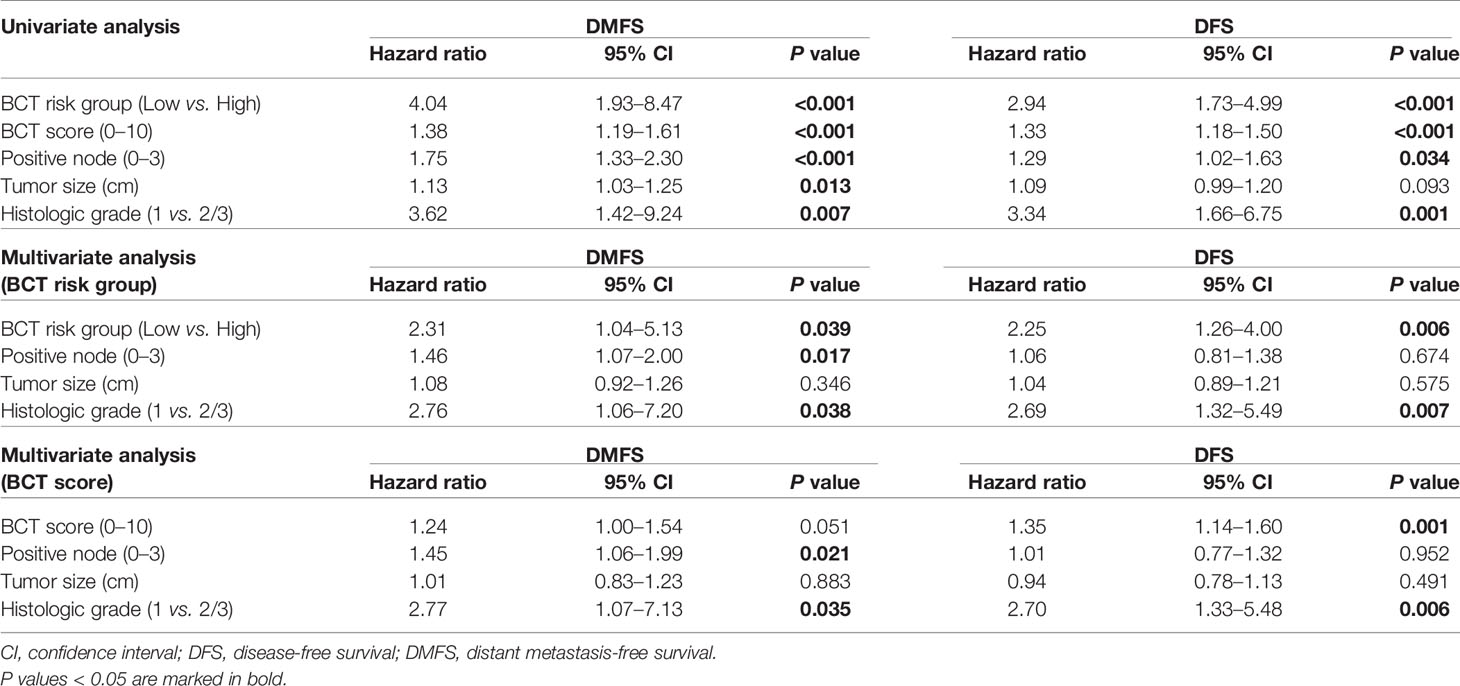
We examined the association between the BCT score and patient survival using Cox’s proportional hazard model. In the univariate analysis for DMFS and DFS, the BCT high-risk group was significantly associated with an increased risk of distant metastasis and recurrence (P < 0.001) (Table 2). A high BCT score also correlated with an increased risk of recurrence (P < 0.001). The prognostic significance of the BCT risk group and BCT score was retained in the multivariate analysis. Being in the BCT high-risk group was an independent negative prognostic factor for DMFS (hazard ratio, 2.31; 95% CI, 1.04–5.13; P = 0.039) and DFS (hazard ratio, 2.25; 95% CI, 1.26–4.00; P = 0.006) (Table 2). The BCT score taken as a continuous variable was also independently associated with the risk of recurrence.
We also assessed the prognostic value of the BCT score in subgroups of patients divided by treatment and pN status. The BCT score was prognostic for DMFS and DFS among patients treated with hormone therapy plus chemotherapy (Supplementary Figure 1). The BCT high-risk group had significantly shorter DMFS (P = 0.005) and DFS (P = 0.005) than the BCT low-risk group. The BCT score was also prognostic for DFS in patients treated with hormone therapy alone (P = 0.002), but it was not prognostic for DMFS in that group. The subgroup analysis by pN status revealed that the BCT score was more prognostic in patients with pN0 tumors than in those with pN1 tumors (Supplementary Figure 2). There was a significant difference in DMFS (P = 0.040) and DFS (P = 0.004) between the BCT low-risk and high-risk groups in patients with pN0 tumors. For patients with pN1 tumors, the BCT score was prognostic for DFS (P = 0.030) but not DMFS.
Prognostic Value of the BCT Score by Age Group
The distribution of BCT scores and risk classification by age group are shown in Figure 2. The percentage of young patients categorized into the BCT high-risk group was significantly higher than the percentage of older patients (P = 0.019). The median BCT score in younger patients was also significantly higher than that in older patients (P = 0.009). In particular, very young patients (aged ≤30 years) had a higher median BCT score (4.50) and a higher likelihood of being in the BCT high-risk group (68.5%) than those in other age groups (Figure 2A). Similar results were observed in patients with pN0 tumors and those with pN1 tumors (Figures 2B, C). However, the BCT risk classifications and median BCT score did not differ significantly between patients aged ≤50 years and >50 years (Supplementary Figure 3).
We assessed the prognostic value of the BCT score in patients aged ≤50 years and >50 years and found that the BCT score was prognostic in both age groups. Kaplan-Meier survival curves showed that the BCT high-risk group had a significantly shorter DMFS and DFS than the BCT low-risk group in both age groups (P < 0.001 for DMFS and P < 0.001 for DFS in patients aged >50 years; P = 0.020 for DMFS and P = 0.030 for DFS in patients aged ≤50 years) (Figure 4). A high BCT score was significantly associated with an increased risk of recurrence or distant metastasis in patients aged ≤50 years and >50 years (Table 3). Moreover, in both age groups, a high BCT score was an independent negative prognostic factor for recurrence (hazard ratio, 1.64; 95% CI, 1.10–2.46; P = 0.016 in patients aged >50 years and hazard ratio, 1.28; 95% CI, 1.05–1.56; P = 0.015 in patients aged ≤50 years) (Table 3).
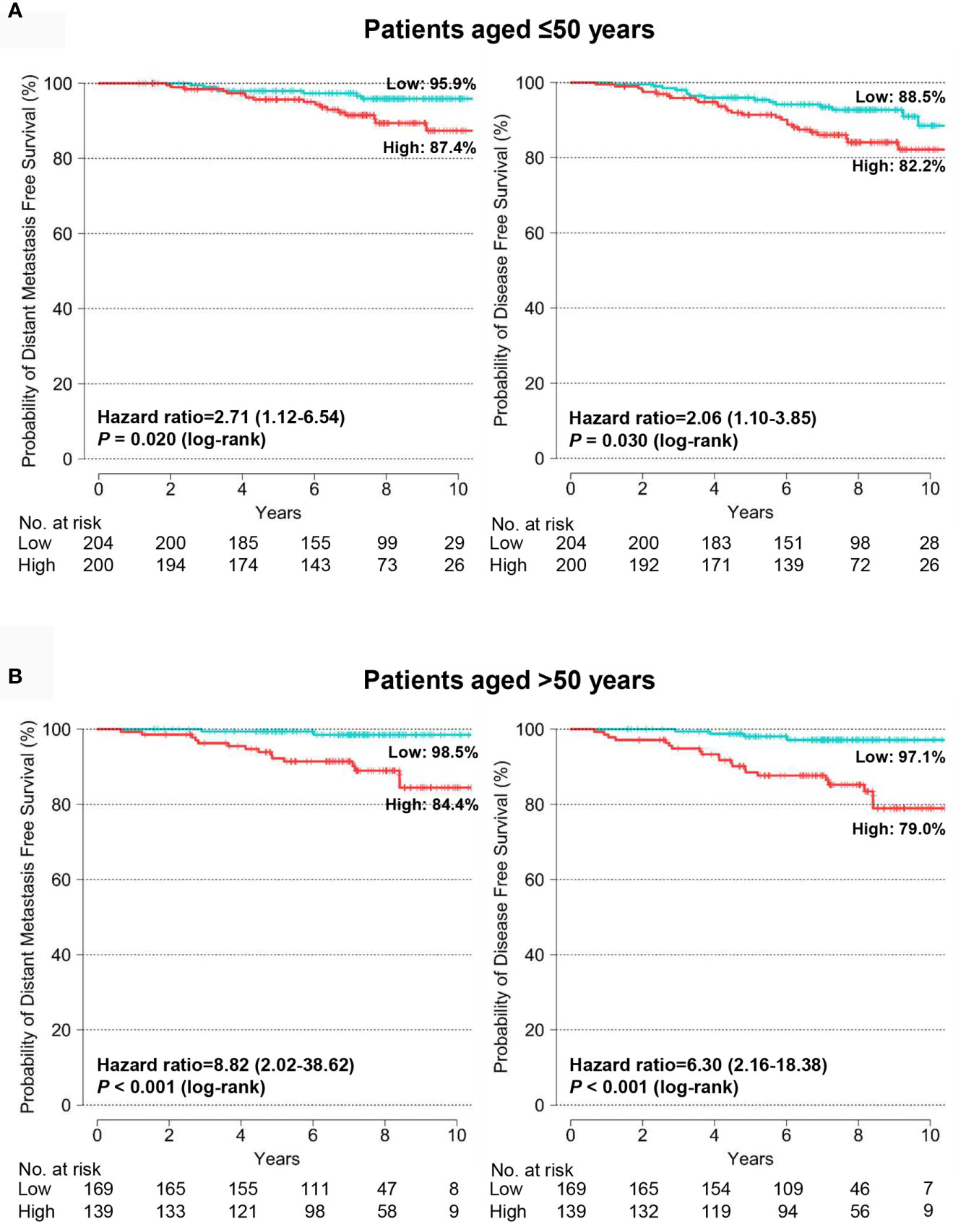
Figure 4 Kaplan-Meier estimates of 10-year distant metastasis-free survival and disease-free survival by BCT risk group in (A) patients aged ≤50 years (n = 404) and (B) patients aged >50 years (n = 308). Patients were classified into the low-risk (blue) or high-risk (red) group according to their BCT scores.
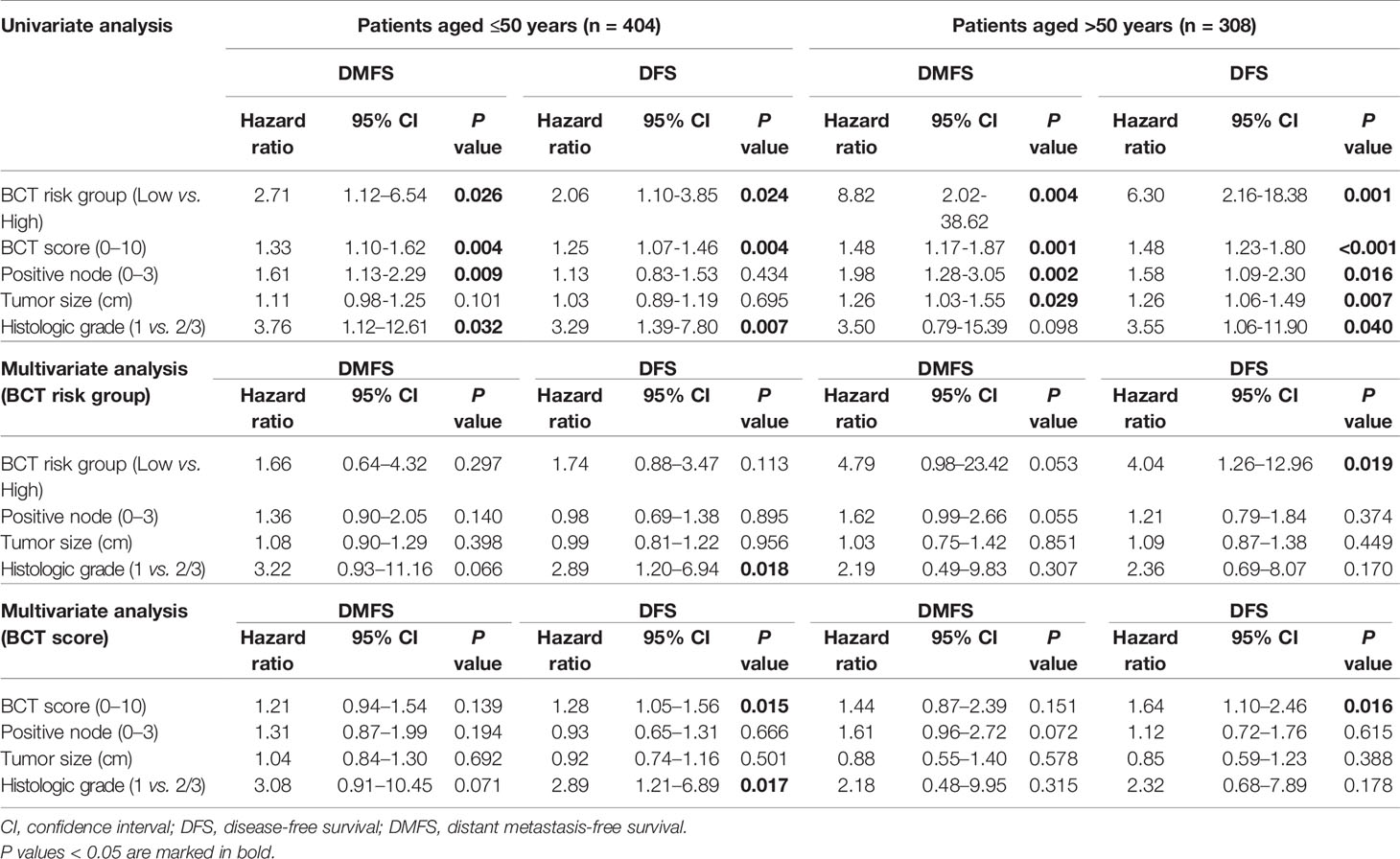
Table 3 Univariate and multivariate analyses for DMFS and DFS in patients aged ≤50 years and >50 years.
Predictive Value of the BCT Score for Chemotherapy Benefit in the PSM Cohort
In the original cohort, clinicopathological characteristics differed significantly between treatment groups (hormone therapy alone vs. hormone therapy plus chemotherapy) within the BCT risk groups. Patients treated with hormone therapy plus chemotherapy had unfavorable clinicopathological status, including larger tumor size, higher histologic grade, and advanced pN status, compared with those treated with hormone therapy alone in both the BCT low-risk and high-risk groups (Supplementary Table 2). Therefore, it was not feasible to assess the predictive value of the BCT score for chemotherapy benefit in the original cohort (Supplementary Figure 4). Using the PSM method, we generated a matched cohort in which the clinical characteristics did not differ significantly between treatment groups (Supplementary Table 2). In the PSM cohort, the 10-year DFS for the BCT high-risk group (n = 90) improved significantly, from 77.8 to 95.5%, after the addition of chemotherapy to hormone therapy (hazard ratio, 0.20; 95% CI, 0.04–0.94; P = 0.020) (Figure 5). In contrast, 10-year DFS did not differ significantly between the two treatment groups in the BCT low-risk group (n = 180) (Figure 5).

Figure 5 Kaplan-Meier estimates of 10-year distant metastasis-free survival and disease-free survival according to treatment group in the PSM cohort. (A) BCT low-risk (n = 180) and (B) BCT high-risk (n = 90) group. Patients were treated with either hormone therapy alone (HT) or hormone therapy plus chemotherapy (HT+CT). PSM, Propensity score matching.
Discussion
Following a previous study (20), this study further validated the prognostic ability of the BCT score to predict recurrences in patients with HR+/HER2− early breast cancer according to age group in an independent cohort. DMFS and DFS differed significantly between the BCT low-risk and high-risk groups, and being in the BCT high-risk group was an independent negative prognostic factor for both DMFS and DFS. Importantly, our subgroup analysis by age group demonstrated that the BCT score was prognostic in patients aged ≤50 years, whereas it had more prognostic value in patients aged >50 years. These results suggest that the BCT score is prognostic irrespective of age. However, although using the BCT score as a continuous variable or a risk group indicator retained its significance for DFS in the multivariate analysis, the BCT score was not independently associated with DMFS in patients aged ≤50 years or >50 years. This finding might be attributable to the very low rate of distant metastasis in this study (5.8%) compared with that found in a previous study (13.1%) (20) due to our short follow-up period or to the treatment effect of chemotherapy, particularly in the BCT high-risk group. Most patients (83.8%, 284/339) classified into the BCT high-risk group received adjuvant chemotherapy, which could have affected the patient outcomes. To validate the prognostic value of the BCT score for DMFS in each age group, a longer follow-up to this study or an additional study with more patients will be needed.
Multigene assays, such as MammaPrint and Oncotype DX, are commonly used to predict the prognosis of early breast cancer patients of all ages, but their prognostic or predictive value in young patients is unclear. Moreover, some assays, such as Prosigna and EndoPredict, are indicated or validated only for use in postmenopausal patients (11, 25–28); only a few studies have tested their prognostic significance in premenopausal patients. EndoPredict was shown to be prognostic in premenopausal patients who are node-positive and have received chemotherapy (29). A recent study also showed that continuous Prosigna ROR scores were prognostic in high-risk premenopausal patients, most of whom were lymph node–positive and received cyclophosphamide-based adjuvant chemotherapy (30). Given that young patients are more likely to receive chemotherapy than older patients, as confirmed in this study, it is notable that the BCT score can be used to avoid unnecessary chemotherapy in young patients who are likely to receive aggressive therapy by accurately identifying low-risk patients who will not benefit from chemotherapy.
When we compared the BCT score distribution and clinicopathological parameters according to age group, we found that younger patients had tumors with higher LVI and that a higher percentage of young patients was classified into the BCT high-risk group than older patients. In particular, very young patients (aged ≤30 years) composed the highest percentage of the BCT high-risk group and had shorter DFS than patients of other ages, indicating that very young patients with HR+/HER2− early breast cancer have a poorer prognosis than older patients. These results are in line with previous studies, which showed that the prognosis of patients aged <35 years with HR+ breast cancer is worse than that of patients aged 35–50 years (3) and that patients in their 20s who had HR+ breast cancer had significantly worse outcomes than those in 30s and 40s (2). A larger difference between DMFS and DFS in patients aged ≤30 years compared with older age groups was observed in this study. This difference might be due to a shorter follow-up period of the youngest subgroup than that of other age groups (median follow-up duration, 4.95 years vs. 6.82 to 7.97 years). Similar to a previous study (21), we found that the distribution of BCT scores between patients aged ≤50 years and those >50 years was similar, whereas patients with pN1 tumors formed a higher percentage of the BCT high-risk group than patients with pN0 tumors.
In the cohort matched using the PSM method, patients classified as high risk by their BCT scores showed a significant improvement in survival after adding chemotherapy to hormone therapy. In contrast, those at low risk according to their BCT scores received no significant survival benefit from adding chemotherapy. In line with a previous study (31), our results also suggest that the BCT score can predict whether patients with HR+/HER2− early breast cancer will benefit from adding chemotherapy to hormone therapy.
Despite its strengths, this study has some limitations. Many (~30%) FFPE tissue samples could not be evaluated by the GenesWell BCT assay because of significant degradation in the mRNA extracted from FFPE samples stored for longer than 10 years. RNA degradation increases with the storage time of FFPE tissues (32). Moreover, due to a very low rate of distant metastasis in patients included in this study, the BCT score was an independent prognostic factor for DFS, but not for DMFS. This study is a retrospective study; a prospective study to assess the prognostic and predictive of the BCT score is required. For this reason, a randomized prospective trial is being conducted to evaluate 10-year DMFS according to adjuvant chemotherapy in patients classified as clinical high and BCT low risk (ClinicalTrials.gov number NCT04278469).
Conclusions
This study demonstrated that the BCT score is prognostic in patients aged ≤50 years and those aged >50 years. The BCT score can be used to identify low-risk patients who will not benefit from adjuvant chemotherapy to treat early breast cancer, irrespective of age. A further prospective study to assess the prognostic and predictive value of the BCT score is required.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (SMC) (Seoul) and Kyungpook National University Hospital (KNUH) (Daegu) (IRB No.: 2017-01-054 and 2017-01-029) in the Republic of Korea and performed in accordance with the Declaration of Helsinki. Because the study is retrospective and patient information was anonymized and de-identified prior to analysis, the requirement for informed consent was waived. A general consent to use the samples for research purpose was obtained from patients at the time of surgery.
Author Contributions
JEL and YKS conceived the study and participated in its design. JMR, SYC, JL, SJL and J-YP were involved in data acquisition. MJK and JMR drafted the manuscript. MJK, JMR, JH, KK and JEL analyzed and interpreted the data. JH and SH performed statistical analyses. SJN, SWK, and YM provided administrative, technical, or material support. KK supervised the statistical analysis. YKS participated in critical revisions of the manuscript with respect to important intellectual content. HYP and JEL supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI17C1142) (JEL) and by the National Research Foundation of Korea (NRF) grant, funded by the Korea government (MSIT) (NRF-2020R1A5A2017323) (MJK). The funders had no role in study design, collection, analysis and interpretation of data, preparation of the manuscript or decision to publish.
Conflict of Interest
JH and YM are employees of Gencurix. Gencurix provided a support in the form of salaries for the authors JH and YM, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.588728/full#supplementary-material
References
1. Partridge AH, Hughes ME, Warner ET, Ottesen RA, Wong YN, Edge SB, et al. Subtype-Dependent Relationship Between Young Age at Diagnosis and Breast Cancer Survival. J Clin Oncol (2016) 34(27):3308–14. doi: 10.1200/JCO.2015.65.8013
2. Ryu JM, Yu J, Kim SI, Kim KS, Moon HG, Choi JE, et al. Different prognosis of young breast cancer patients in their 20s and 30s depending on subtype: a nationwide study from the Korean Breast Cancer Society. Breast Cancer Res Treat (2017) 166(3):833–42. doi: 10.1007/s10549-017-4472-5
3. Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea–a report from the Korean Breast Cancer Society. J Clin Oncol (2007) 25(17):2360–8. doi: 10.1200/JCO.2006.10.3754
4. Han W, Kang SY. Relationship between age at diagnosis and outcome of premenopausal breast cancer: age less than 35 years is a reasonable cut-off for defining young age-onset breast cancer. Breast Cancer Res Treat (2010) 119(1):193–200. doi: 10.1007/s10549-009-0388-z
5. Copson E, Eccles B, Maishman T, Gerty S, Stanton L, Cutress RI, et al. Prospective observational study of breast cancer treatment outcomes for UK women aged 18-40 years at diagnosis: the POSH study. J Natl Cancer Inst (2013) 105(13):978–88. doi: 10.1093/jnci/djt134
6. Early Breast Cancer Trialists’ Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet (2005) 365(9472):1687–717. doi: 10.1016/S0140-6736(05)66544-0
7. Paluch-Shimon S, Pagani O, Partridge AH, Abulkhair O, Cardoso MJ, Dent RA, et al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast (2017) 35:203–17. doi: 10.1016/j.breast.2017.07.017
8. Suter MB, Pagani O. Should age impact breast cancer management in young women? Fine tuning of treatment guidelines. Ther Adv Med Oncol (2018) 10:1758835918776923. doi: 10.1177/1758835918776923
9. van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med (2002) 347(25):1999–2009. doi: 10.1056/NEJMoa021967
10. Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med (2004) 351(27):2817–26. doi: 10.1056/NEJMoa041588
11. Varga Z, Sinn P, Seidman AD. Summary of head-to-head comparisons of patient risk classifications by the 21-gene Recurrence Score(R) (RS) assay and other genomic assays for early breast cancer. Int J Cancer (2019) 145(4):882–93. doi: 10.1002/ijc.32139
12. Kwon MJ. Emerging immune gene signatures as prognostic or predictive biomarkers in breast cancer. Arch Pharm Res (2019) 42(11):947–61. doi: 10.1007/s12272-019-01189-y
13. Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med (2016) 375(8):717–29. doi: 10.1056/NEJMoa1602253
14. Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N Engl J Med (2018) 379(2):111–21. doi: 10.1056/NEJMoa1804710
15. Sparano JA, Gray RJ, Ravdin PM, Makower DF, Pritchard KI, Albain KS, et al. Clinical and Genomic Risk to Guide the Use of Adjuvant Therapy for Breast Cancer. N Engl J Med (2019) 380(25):2395–405. doi: 10.1056/NEJMoa1904819
16. Lin CH, Yap YS, Lee KH, Im SA, Naito Y, Yeo W, et al. Contrasting Epidemiology and Clinicopathology of Female Breast Cancer in Asians vs the US Population. J Natl Cancer Inst (2019) 111(12):1298–306. doi: 10.1093/jnci/djz090
17. Park EH, Min SY, Kim Z, Yoon CS, Jung KW, Nam SJ, et al. Basic Facts of Breast Cancer in Korea in 2014: The 10-Year Overall Survival Progress. J Breast Cancer (2017) 20(1):1–11. doi: 10.4048/jbc.2017.20.1.1
18. Kan Z, Ding Y, Kim J, Jung HH, Chung W, Lal S, et al. Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun (2018) 9(1):1725. doi: 10.1038/s41467-018-04129-4
19. Yap YS, Lu YS, Tamura K, Lee JE, Ko EY, Park YH, et al. Insights Into Breast Cancer in the East vs the West: A Review. JAMA Oncol (2019) 5(10):1489–96. doi: 10.1001/jamaoncol.2019.0620
20. Gong G, Kwon MJ, Han J, Lee HJ, Lee SK, Lee JE, et al. A new molecular prognostic score for predicting the risk of distant metastasis in patients with HR+/HER2- early breast cancer. Sci Rep (2017) 7:45554. doi: 10.1038/srep45554
21. Kwon MJ, Lee JE, Jeong J, Woo SU, Han J, Kang BI, et al. Comparison of GenesWell BCT Score With Oncotype DX Recurrence Score for Risk Classification in Asian Women With Hormone Receptor-Positive, HER2-Negative Early Breast Cancer. Front Oncol (2019) 9:667. doi: 10.3389/fonc.2019.00667
22. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med (2010) 134(6):907–22. doi: 10.1200/JOP.777003
23. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med (2014) 138(2):241–56. doi: 10.5858/arpa.2013-0953-SA
24. Ho DE, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Political Anal (2007) 15(3):199–236. doi: 10.1093/pan/mpl013
25. Gnant M, Filipits M, Greil R, Stoeger H, Rudas M, Bago-Horvath Z, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol (2014) 25(2):339–45. doi: 10.1093/annonc/mdt494
26. Sestak I, Cuzick J, Dowsett M, Lopez-Knowles E, Filipits M, Dubsky P, et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol (2015) 33(8):916–22. doi: 10.1200/JCO.2014.55.6894
27. Filipits M, Rudas M, Jakesz R, Dubsky P, Fitzal F, Singer CF, et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res (2011) 17(18):6012–20. doi: 10.1158/1078-0432.CCR-11-0926
28. Fitzal F, Filipits M, Rudas M, Greil R, Dietze O, Samonigg H, et al. The genomic expression test EndoPredict is a prognostic tool for identifying risk of local recurrence in postmenopausal endocrine receptor-positive, her2neu-negative breast cancer patients randomised within the prospective ABCSG 8 trial. Br J Cancer (2015) 112(8):1405–10. doi: 10.1038/bjc.2015.98
29. Martin M, Brase JC, Calvo L, Krappmann K, Ruiz-Borrego M, Fisch K, et al. Clinical validation of the EndoPredict test in node-positive, chemotherapy-treated ER+/HER2- breast cancer patients: results from the GEICAM 9906 trial. Breast Cancer Res (2014) 16(2):R38. doi: 10.1186/bcr3642
30. Jensen MB, Laenkholm AV, Nielsen TO, Eriksen JO, Wehn P, Hood T, et al. The Prosigna gene expression assay and responsiveness to adjuvant cyclophosphamide-based chemotherapy in premenopausal high-risk patients with breast cancer. Breast Cancer Res (2018) 20(1):79. doi: 10.1186/s13058-018-1012-0
31. Kwon MJ, Lee SB, Han J, Lee JE, Lee JW, Gong G, et al. BCT score predicts chemotherapy benefit in Asian patients with hormone receptor-positive, HER2-negative, lymph node-negative breast cancer. PloS One (2018) 13(11):e0207155. doi: 10.1371/journal.pone.0207155
32. Cronin M, Pho M, Dutta D, Stephans JC, Shak S, Kiefer MC, et al. Measurement of gene expression in archival paraffin-embedded tissues: development and performance of a 92-gene reverse transcriptase-polymerase chain reaction assay. Am J Pathol (2004) 164(1):35–42. doi: 10.1016/S0002-9440(10)63093-3
Keywords: GenesWell BCT assay, BCT score, prognostic value, predictive value, young breast cancer patients, HR+/HER2− early breast cancer
Citation: Kwon MJ, Ryu JM, Cho SY, Nam SJ, Kim SW, Lee J, Lee SJ, Park J-Y, Park HY, Hong S, Kim K, Han J, Moon Y, Shin YK and Lee JE (2021) Validation of the GenesWell BCT Score in Young Asian Women With HR+/HER2− Early Breast Cancer. Front. Oncol. 11:588728. doi: 10.3389/fonc.2021.588728
Received: 29 July 2020; Accepted: 13 January 2021;
Published: 23 February 2021.
Edited by:
Alberto Farolfi, Romagnolo Scientific Institute for the Study and Treatment of Tumors (IRCCS), ItalyReviewed by:
Julio de la Torre-Montero, Comillas Pontifical University, SpainGaia Griguolo, Università di Padova, Italy
Copyright © 2021 Kwon, Ryu, Cho, Nam, Kim, Lee, Lee, Park, Park, Hong, Kim, Han, Moon, Shin and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeong Eon Lee, cGFvamx1c0BoYW5tYWlsLm5ldA==
†These authors have contributed equally to this work
 Mi Jeong Kwon
Mi Jeong Kwon Jai Min Ryu3†
Jai Min Ryu3† Soo Youn Cho
Soo Youn Cho Jeeyeon Lee
Jeeyeon Lee Sungjun Hong
Sungjun Hong Jinil Han
Jinil Han Young Kee Shin
Young Kee Shin Jeong Eon Lee
Jeong Eon Lee