- 1Department of Breast Surgery, First Affiliated Hospital, Xi’an Jiaotong University, Xi’an, China
- 2School of Medicine, Xi’an Jiaotong University, Xi’an, China
Background: Contralateral prophylactic mastectomy (CPM) in female breast cancer (FBC) is supported by multiple clinical studies and consensus guidelines, but knowledge of preventive contralateral mastectomy in male breast cancer (MaBC) is very limited and its benefits are still controversial.
Methods: A retrospective cohort study was enrolled with 4,405 MaBC patients who underwent unilateral mastectomy (UM) or CPM from the Surveillance, Epidemiology, and End Results (SEER) database from 1998 to 2015. A nomogram was built based on the corresponding parameters by competing risks regression to predict the 3-year, 5-year, and 8-year probabilities of BCSD (breast cancer-specific death). C-index and calibration curves were chosen for validation. Net reclassification index (NRI) and integrated discrimination improvement (IDI) were used to estimate the nomogram’s clinical utility.
Results: A total of 4,197 patients received UM and 208 patients received CPM, with 63-months median follow-up. In the competing risks regression, six variables (surgery, marital status, T-stage, N-stage, histology, tumor grade) were significantly associated with BCSD. Based on these independent prognosis factors, a nomogram model was constructed. The C-index 0.75 (95%CI: 0.73-0.77) in the training cohort and 0.73 (95%CI: 0.71-0.74) in the internal validation group suggested robustness of the model. In addition, the calibration curves exhibited favorably. The NRI values (training cohort: 0.54 for 3-year, 0.55 for 5-year, and 0.49 for 8-year BCSD prediction; validation cohort: 0.51 for 3-year, 0.45 for 5-year, and 0.33 for 8-year BCSD prediction) and IDI values (training cohort: 0.02 for 3-year, 0.03 for 5-year, and 0.04 for 8-year BCSD prediction; validation cohort: 0.02 for 3-year, 0.04 for 5-year, and 0.04 for 8-year BCSD prediction) indicated that the model performed better than the AJCC criteria-based tumor staging alone.
Conclusions: The administration of CPM was associated with the decrease in risk of BCSD in patients with MaBC. The nomogram could provide a precise and personalized prediction of the cumulative risk in patients with MaBC after CPM.
Introduction
Contralateral prophylactic mastectomy (CPM) is a controversial but hot topic in the world. The application of CPM could reduce risk of contralateral breast cancer (CBC) for female patients with unilateral breast cancer (1–5). However, almost all prospective clinical trials concerning CPM are conducted in female breast cancer (FBC) patients. Consequently, the benefit of CPM on male breast cancer (MaBC) patients remains unknown due to its rarity (6).
As a rare primary breast malignancy, MaBC accounts for less than 1% of all breast cancers (7–10). Compared with FBC, previous studies suggested that patients with MaBC had different biological characteristics such as advanced age, a higher percentage of lymph node metastases, and were estrogen receptor-positive (ER+) (9, 11, 12). In contrast to FBC, MaBC tends to present BRCA2 mutation rather than BRCA1 mutation (13). Therefore, more clinical evidence for surgical strategies and subsequent treatment methods are needed for MaBC patients since current guidelines are based on female clinic data.
To further explore and identify the curative effects of CPM in patients with resectable MaBC, we followed a large cohort of males with MaBC from 1998 to 2015 from the population-based database Surveillance, Epidemiology, and End Results (SEER) cancer registry program. In the study, we established a competing risks nomogram to predict and identify those patients who could benefit from CPM.
Materials and Methods
Data Resource
The recent version of the SEER 18 registries’ custom data (with additional treatment fields) was used as the data source for the present population-based investigation. This database consists of 18 population-based cancer registries and covers approximately 26% of the US population across several geographic regions (14). SEER*-Stat Software version 8.3.6 (https://seer.cancer.gov/seerstat/) (Information Management Service, Inc. Calverton, MD, USA) was used to generate the case listing. All procedures were performed in accordance with approved guidelines. This study was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University. Informed patient consent was not required to access and use SEER data.
Patient Cohort
Male patients diagnosed with unilateral breast cancer from 1998 to 2015 were enrolled in the study. Patients were included by following criteria: 1) primary breast cancer; 2) TNM (Breast-Adjusted American Joint Committee on Cancer, AJCC 6th) stages 0, I, II, or III; and 3) unilateral mastectomy (UM) or CPM. The demographic and clinicopathological variables were shown as follows: sex (male), age, race, site, behavior years of diagnosis, tumor grade, tumor T stage, tumor N stage, type of surgery, radiotherapy, chemotherapy, ER status, PR status, survival months, vital status, reasons of death, marital status, and breast-adjusted AJCC 6th TNM stage.
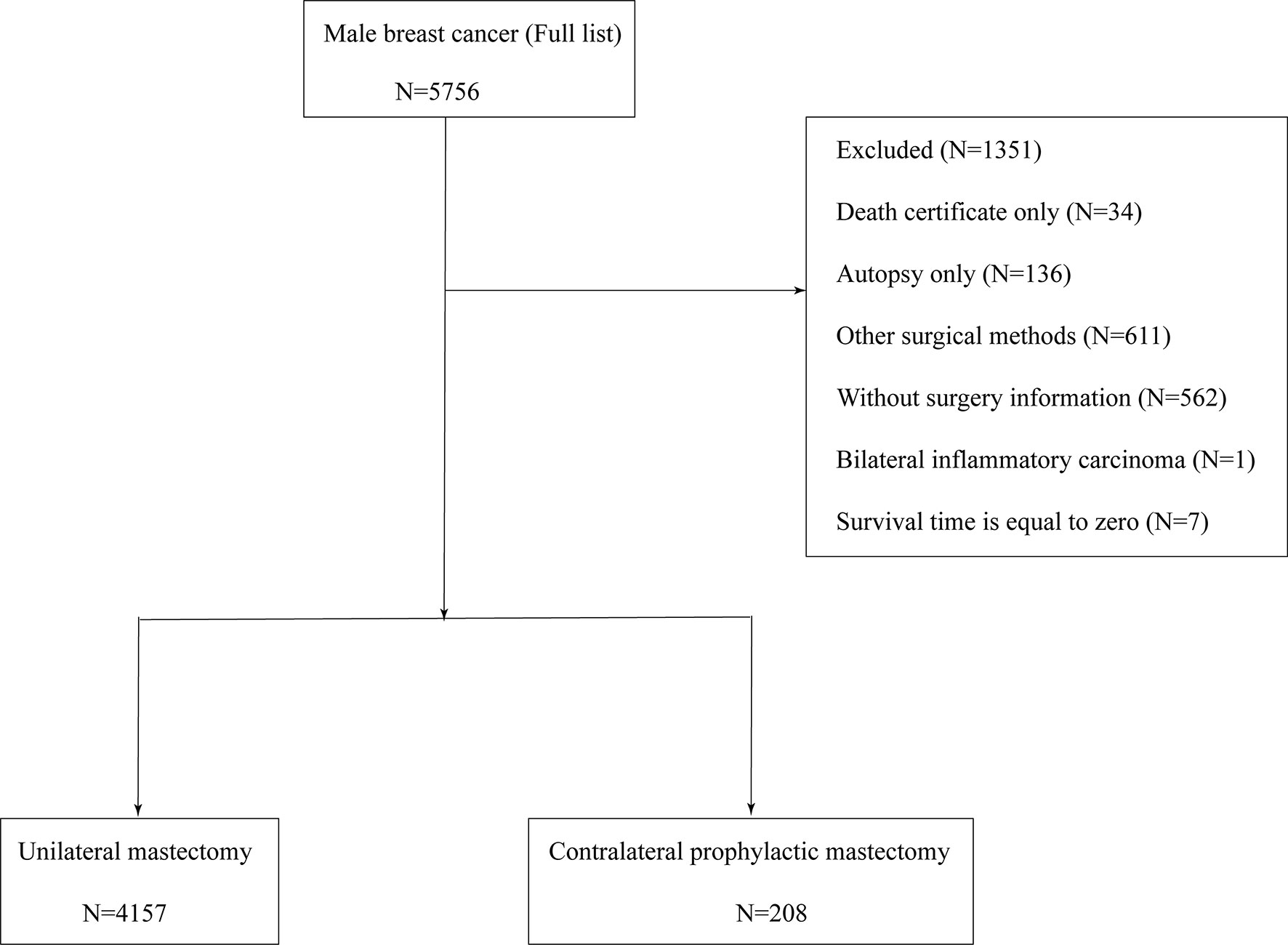
After the preliminary selection, patients were excluded by following criteria: (1) unknown AJCC stage; (2) the follow-up type of autopsy or death certificate; (3) distant metastasis (M1); (4) aged below 20 years; (5) missing surgical records; and (6) survival months is zero. Figure 1 shows the entire screening process.
In total, 4,405 patients with MaBC were included in our cohort. To estimate the impact of CPM on prognosis, the study cohort was classified into two groups by different operation selections: UM group and CPM group. “No radiation and/or cancer-directed surgery” were regarded as no radiotherapy. “No/Unknown” chemotherapy records were regarded as no chemotherapy.
End Points
Patients were followed up until November 2015, and the median follow-up was 63 months (ranging from 1 month to 227 months). The primary indexes, breast cancer-specific death (BCSD) and breast cancer-specific survival (BCSS), were defined as the time interval between the date of diagnosis and death due to breast cancer. The secondary outcome measurement was overall survival (OS) which was deemed as the interval from the date of diagnosis to the date of death for any reason.
Statistical Analysis
All analyses were performed by using R statistical software version 3.6.3 (https://www.r-project.org). We used descriptive statistics to summarize demographic and clinical variables, continuous variables with normal distribution were described as means and standard deviations, categorical variables were compared using Chi-squared test or Fisher’s exact test as appropriate. Firstly, Kaplan-Meier curves and log-rank test were performed to determine the statistical differences among groups of overall survival (OS) and breast cancer-specific survival (BCSS). Secondly, a Cox proportional hazards model was constructed to find prognostic factors of MaBC by the R package of rms. Thirdly, the competing risk analysis model was used to estimate the hazard of the cumulative incidence function while controlling for the competing risks of death, which predicted BCSD by the R package of cmprsk and competing risks regression (15, 16). Fourthly, in order to predict the prognosis of MaBC after three, five, and eight years, based on the coefficients from the competing risks regression models, a nomogram was built by the R packages mstate and regplot (17). Lastly, during the validation process, concordance indexes (C-index) and calibration curves were used to determine predictive accuracy and discriminability. Net reclassification index (NRI) and integrated discrimination improvement (IDI) were performed to estimate the nomogram’s clinical utility compared with the AJCC-TNM stage system. All P-values were bilateral and P< 0.05 was considered to be statistically significant.
Result
Baseline Characteristics
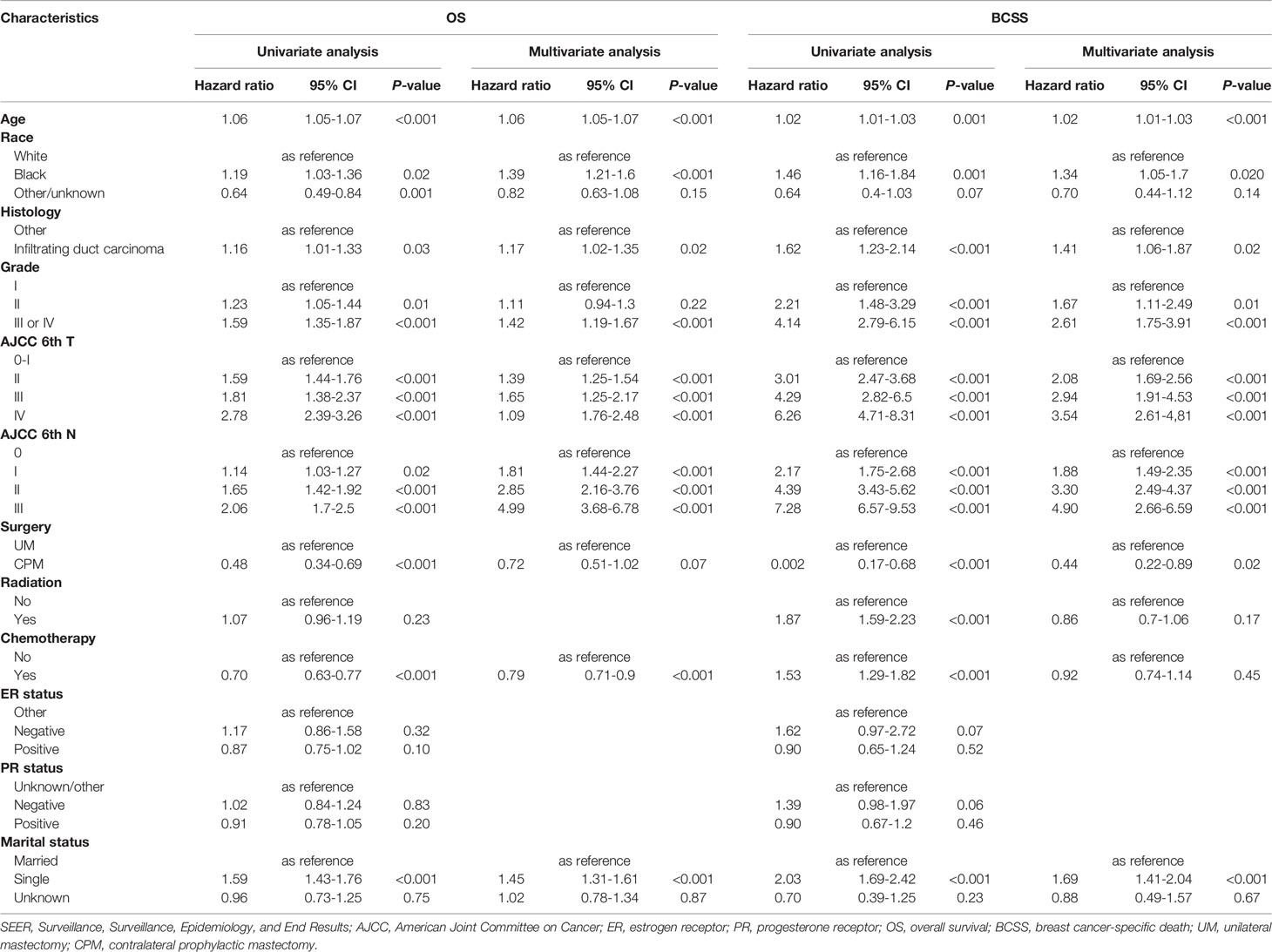
Among the 4,405 patients from our study cohort, 95.3% (4,197/4,405) of patients received UM, while 4.7% (208/4,405) had CPM. Among these men, 82.5% of patients were white, 52.7% of patients had moderate differentiated tumors, 85% of patients had infiltrating duct carcinoma, 49.5% of patients were in the early T-stage (T0 and T1), 56.2% of patients were in the N0 stage, 23.7% of patients received chemotherapy and 38% of patients received radiation, 90.8% of patients were ER-positive (ER+), 81.2% of patients were PR-positive (PR+), and 69.7% of patients were married. Compared with patients who received UM, patients who received CPM were younger in age (59 ± 12 years versus 67 ± 12 years), and more likely to receive chemotherapy (49% versus 37.4%), while the ratio of T2 stage (38.5% versus 41.2%) and grade II (44.7% versus 53.1%) were lower. There were no statistical significance in race, histology, N stage, received radiation, ER status, PR status, and marital status. Detailed information is shown in Table 1.
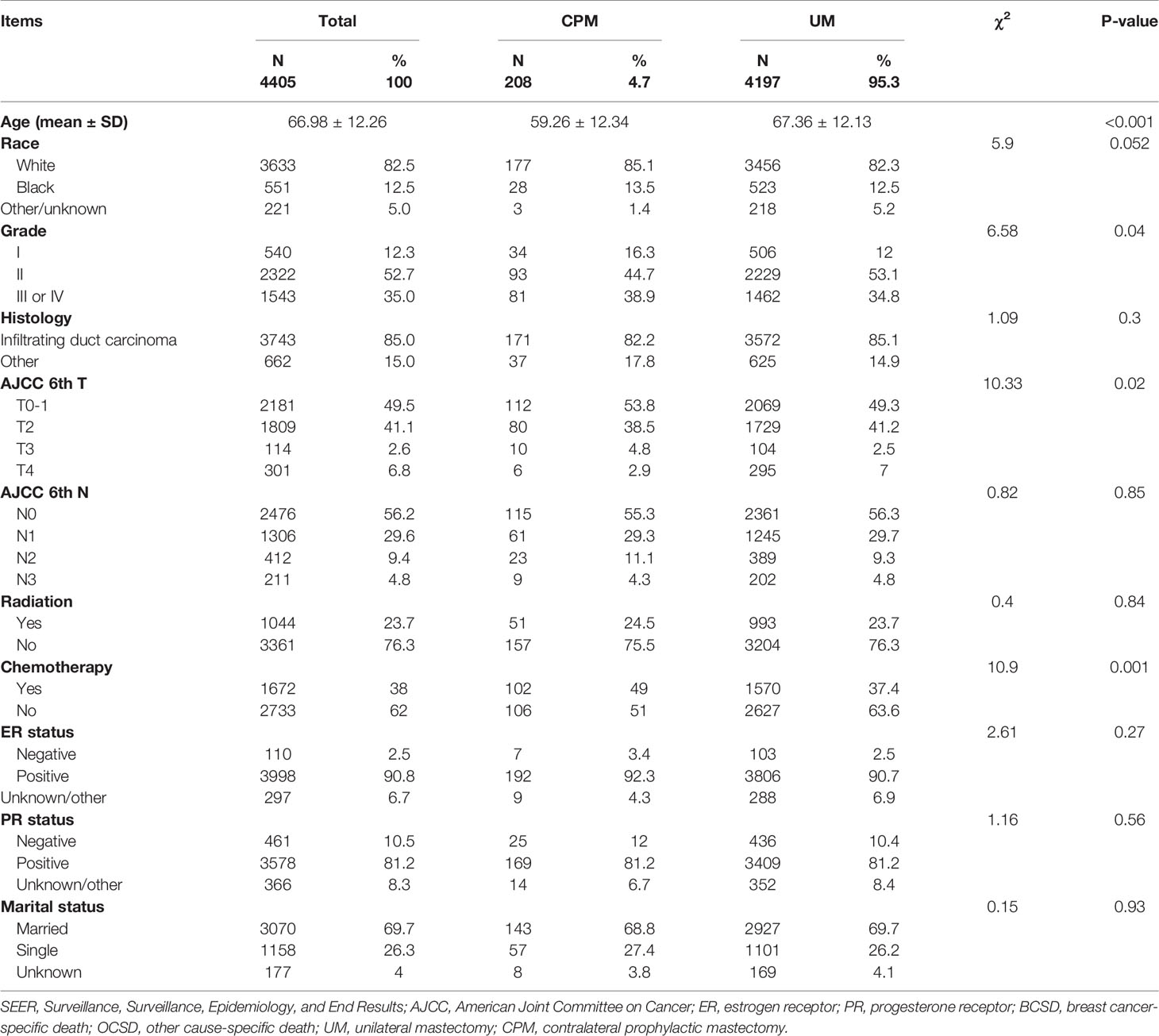
Table 1 The baseline characteristics of patients with different surgery procedures in the SEER database.
Kaplan–Meier Analysis of OS and BCSS
A total of 1,757 (39.89%) patients died in this cohort study, and 30.05% (528/1,757) of them had a breast cancer‐specific death, while 69.95% (1,229/1,757) did not. The OS after three, five, and eight years was 93.3%, 85.9%, and 75.7% in the CPM group, respectively; and 84.9%, 73.3% and 59.4% in the UM group, respectively (Figure 2A). The BCSS after three, five, and eight years was 98.5%, 95.1%, and 92.1% in the CPM group, respectively; and 93.7%, 87.3% and 79.8% in the UM group, respectively (Figure 2B).
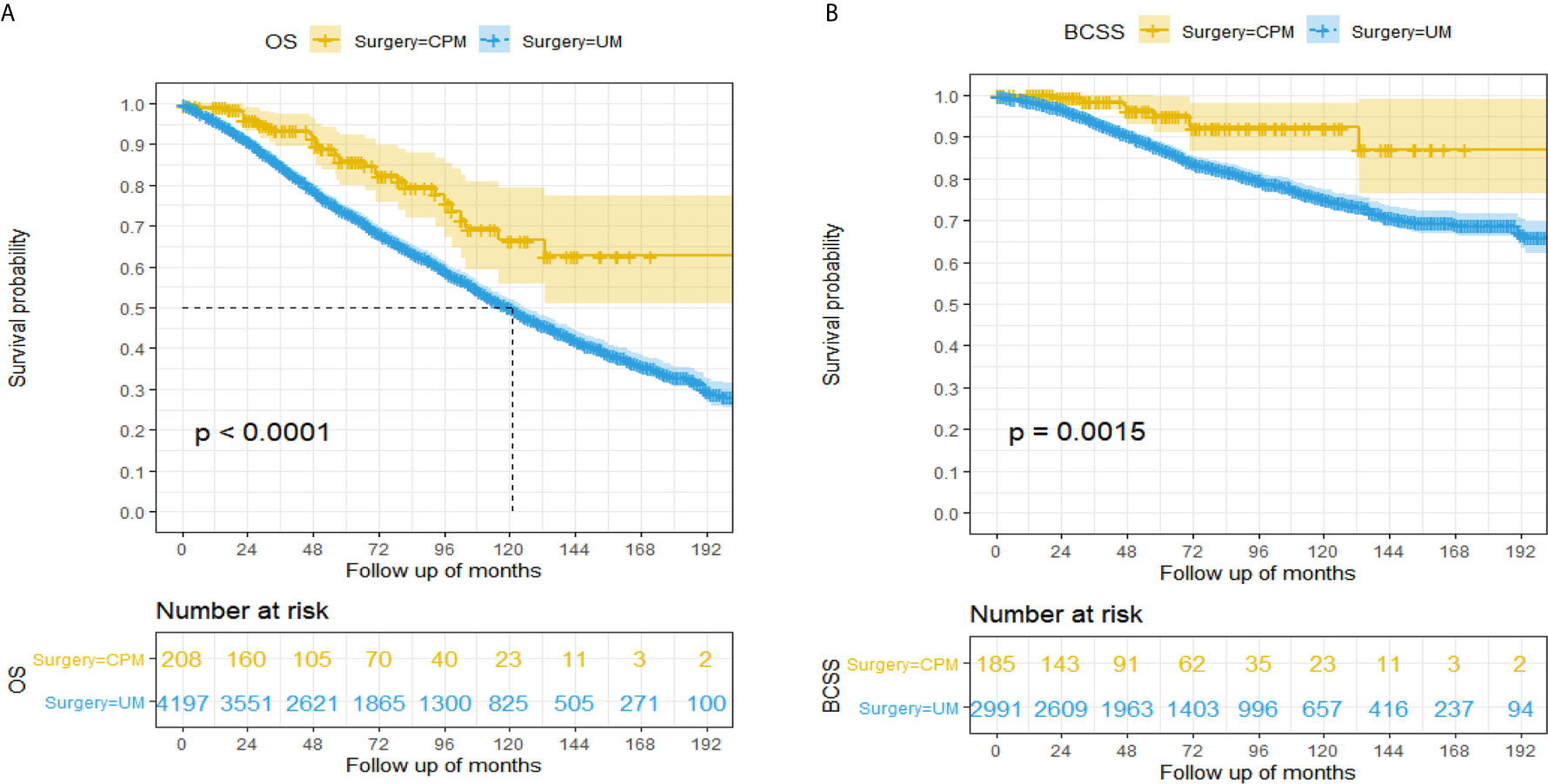
Figure 2 Kaplan-Meier survival analysis for male breast cancer patients. (A) Overall survival curves in the CPM group and UM group. (B) Breast cancer-specific survival curves in the CPM group and UM group.
The hazard ratio (HR) summarized the risk of OS and BCSS. As shown in Figures 2A, B, the CPM group was significantly correlated with better OS (HR=0.48, 95%CI: 0.34-0.69, P<0.001) and BCSS (HR=0.34, 95%CI: 0.17-0.68, P<0.001) in comparison with the UM group.
Univariate and Multivariate Cox Regression Model Analysis of MaBC Patients
As shown in Table 2, through univariate Cox analysis, a total of nine variables, such as age, race, histology, tumor grade, T-stage, N-stage, surgery, receiving chemotherapy, and marital status, were significantly associated with OS and BCSS. To further explore the independent predictive consequences of OS and BCSS, multivariate Cox proportional hazard regression analyses were performed. After adjustment of the clinical features in the Cox model, CPM was only significantly correlated with better BCSS (HR=0.44, 95%CI: 0.22-0.89, P=0.02) and threshold value of OS (HR, 0.72, 95% CI: 0.51-1.02, P=0.07). In addition, race, tumor grade, histology, tumor T stage, tumor N stage, age, and marital status were independent predictive factors in OS and BCSS.
Nomogram Variable Screening by Competing Risk Analysis
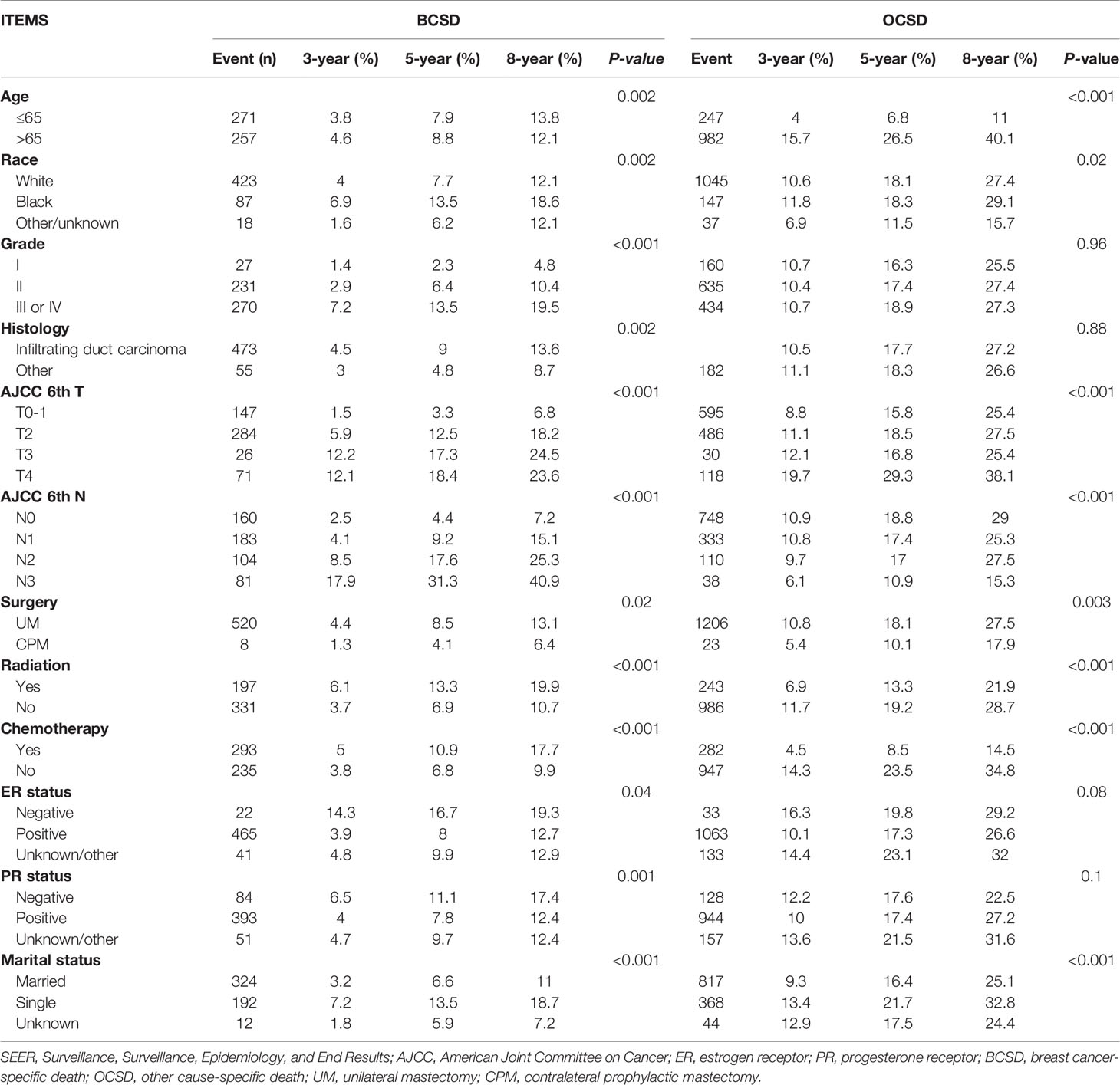
Of the 1,757 deaths from 4,405 patients, the whole cumulative incidence of BCSD was only 11.99% (528/4,405), but the cumulative OCSD (other cause-specific death) incidence was as high as 27.9% (1,229/4,405). In the univariate analysis by a competing risk model (Table 3), twelve variables (age, race, tumor grade, histology, T-stage, N-stage, radiation, chemotherapy, surgery, ER status, PR status, marital status), the P-value of which presented less than 0.05, were screened for competing risks regression analysis. Patients in the CPM group had both lower cumulative BCSD incidence (Gray’s test, P=0.02) and OCSD incidence (Gray’s test, P=0.003) than those in the UM group (Figure 3).
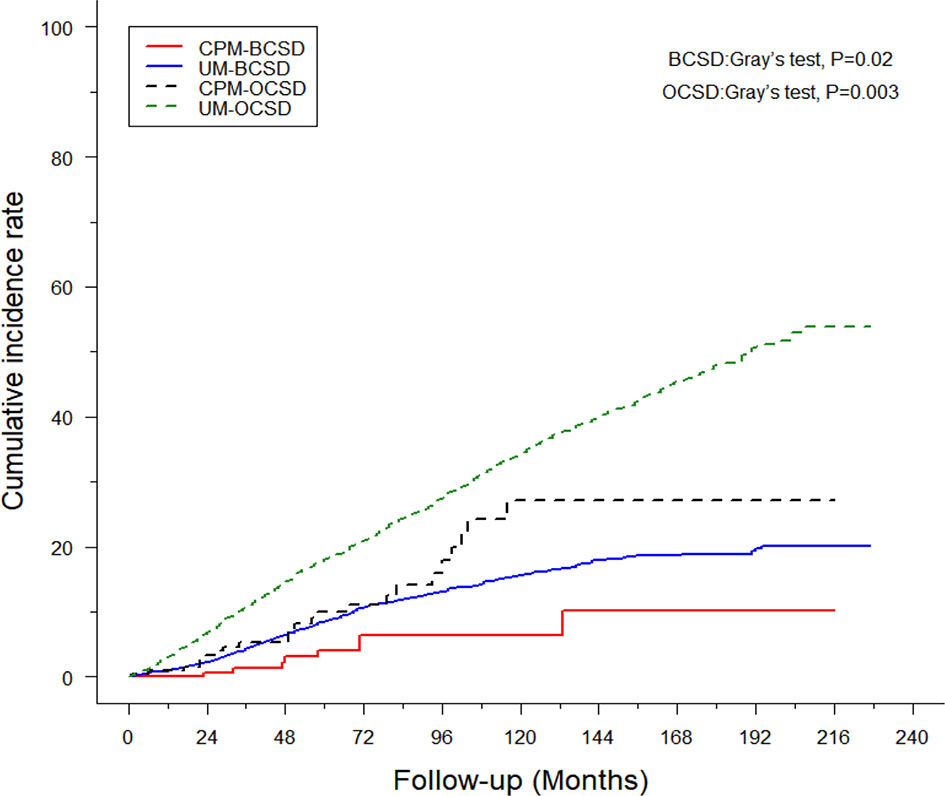
Figure 3 Cumulative incidence of breast cancer-specific death (BCSD) and other cause-specific death (OCSD) in the CPM group and UM group.
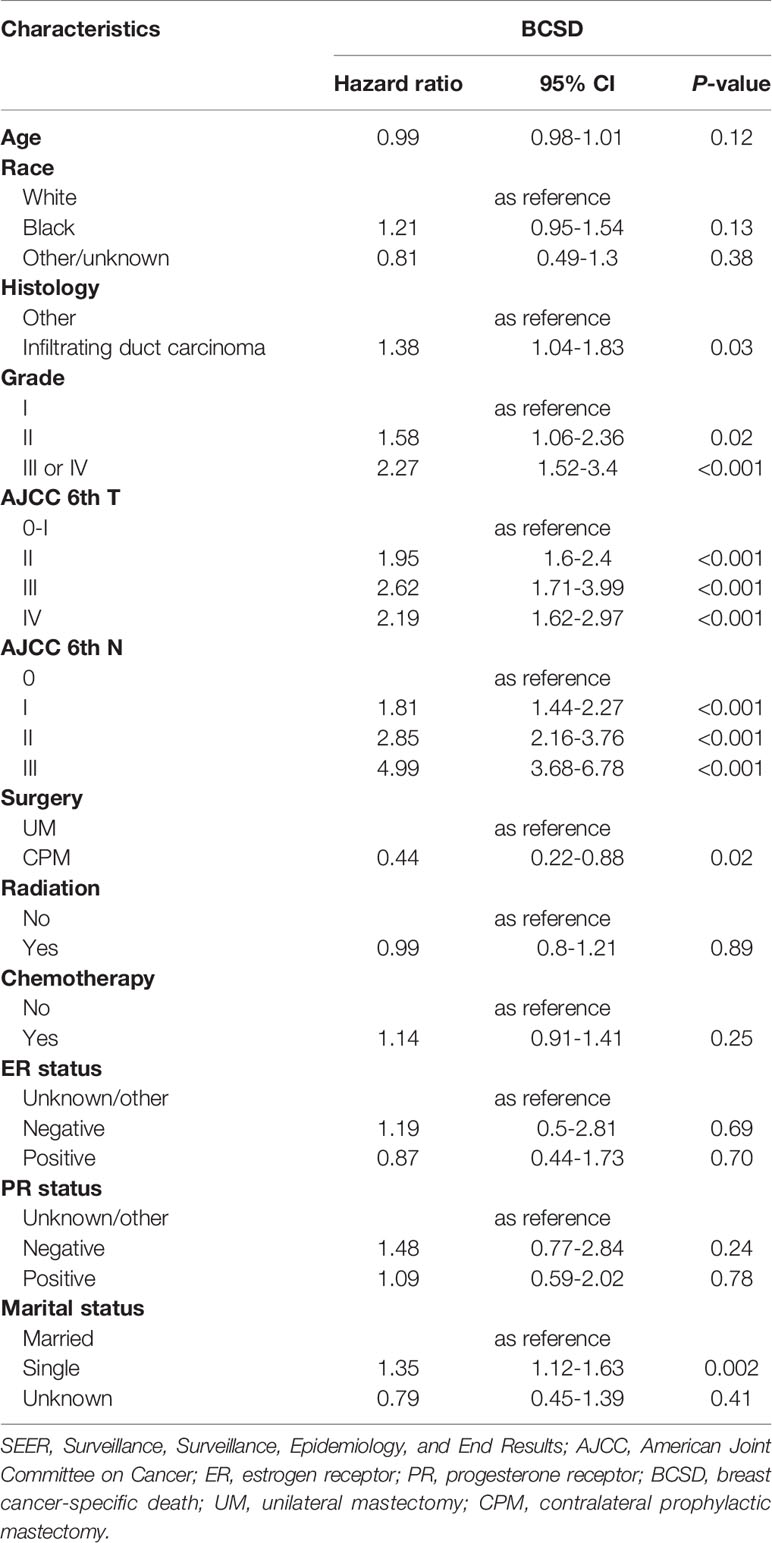
In the multivariate analysis by competing risks regression, the results suggested that histology and six variables (tumor grade, T-stage, N-stage, surgery, and marital status) were still the independent predictive factors of BCSD (Table 4). Results showed that CPM was significantly associated with better BCSD (HR=0.44, 95%CI: 0.22-0.88, P=0.02). In addition, patients with highly differentiated (grade I), T0-I stage, and N0 stage tumors, other histology, and those who were married tended to have significantly better BCSD than the corresponding group (P<0.05).
Construction of Competing Risks Regression Nomogram Model
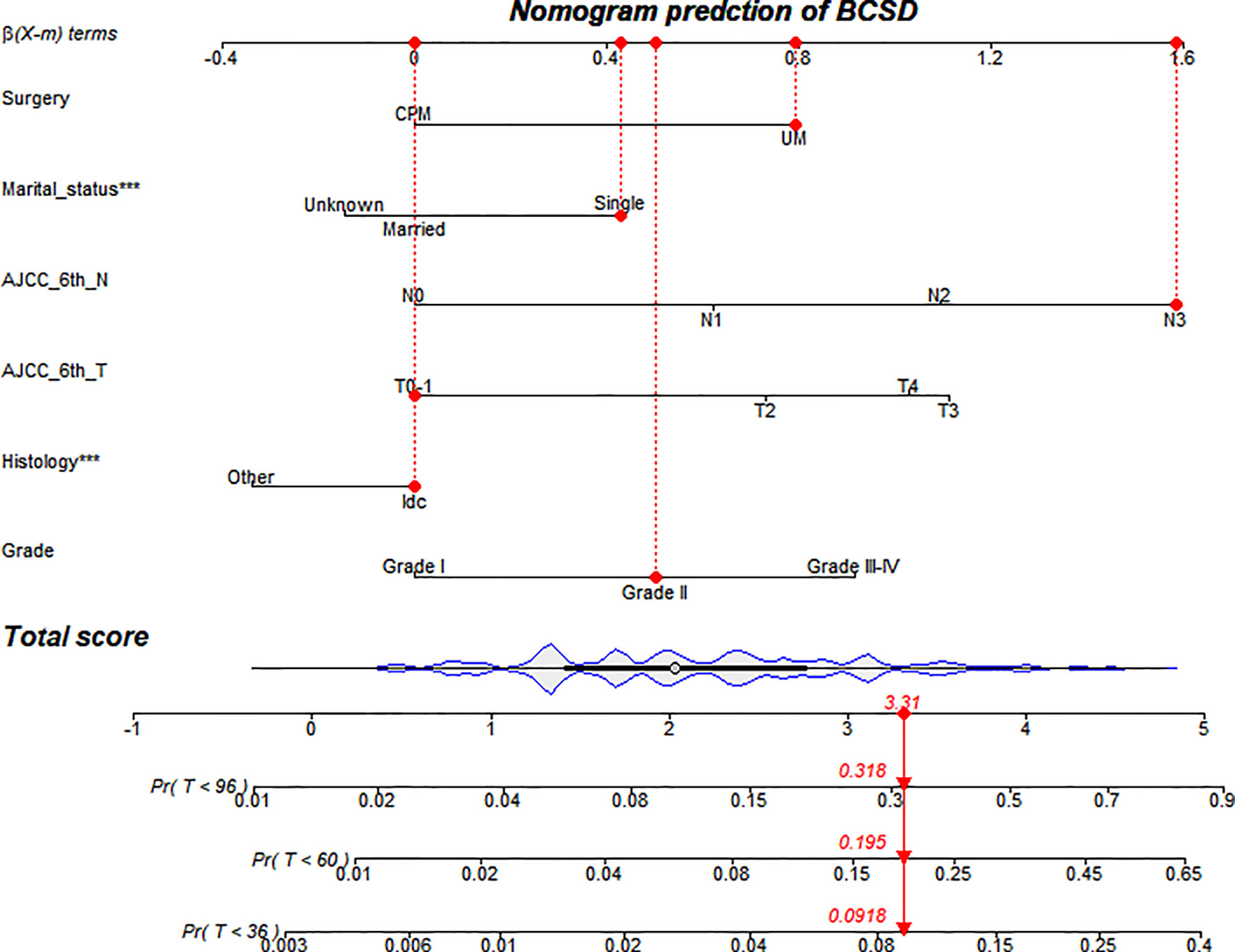
Based on screening variables, the nomogram model established by competing risks regression models was used for forecasting the BCSD of every patient after three, five, and eight years, adjusted variables pointed to a score deriving from the scale, then we could get a total score by adding up all scores (Figure 4). The predictive cumulative probabilities of BCSD after three, five, and eight years could be evaluated by the total score according to the bottom scale. By using the nomogram, we forecasted a given patient after three, five, and eight years a BCSD of 9.2%, 19.5%, and 31.8%, respectively.
Clinical Value of the Nomogram Compared With the AJCC-TNM Stage
A portion of the cohort (30%) was chosen at random for internal validation. As shown in Table 5, the C-index was 0.76 (95%CI: 0.75-0.77) in the training cohort and 0.75 (95%CI: 0.74-0.77) in the validation cohort, implying improved prediction capability compared with AJCC-TNM stage (training cohort: 0.72, 95%CI, 0.71-0.73; validation cohort: 0.69, 95% CI, 0.69-0.72, respectively). Calibration curves also reflected the favorable consistency between nomogram-predicted and observed BCSD at 3-year, 5-year, and 8-year intervals (Figures 5A, B).
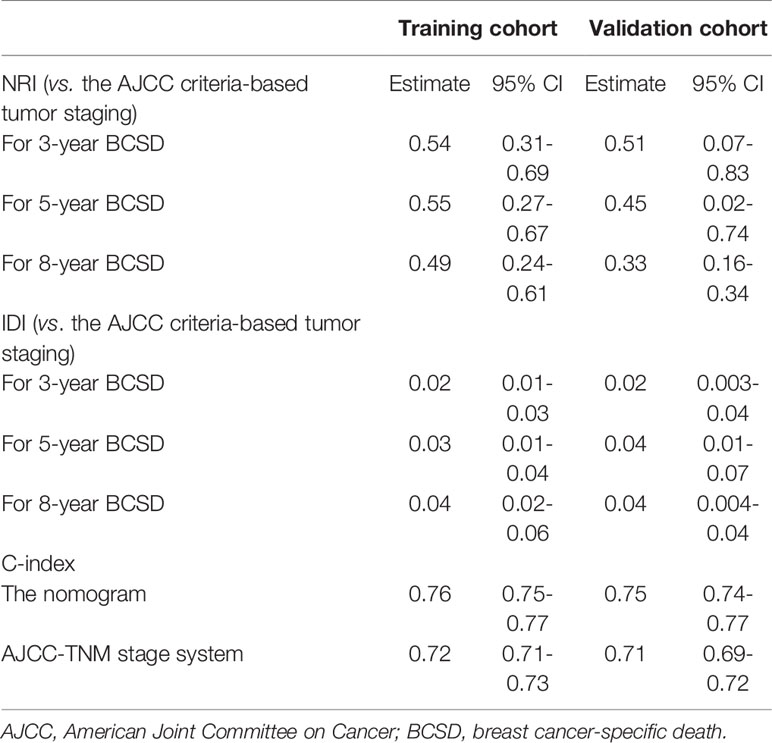
Table 5 C-index, NRI, and IDI of the nomogram and AJCC-TNM stage system in BCSD prediction for MaBC patients.
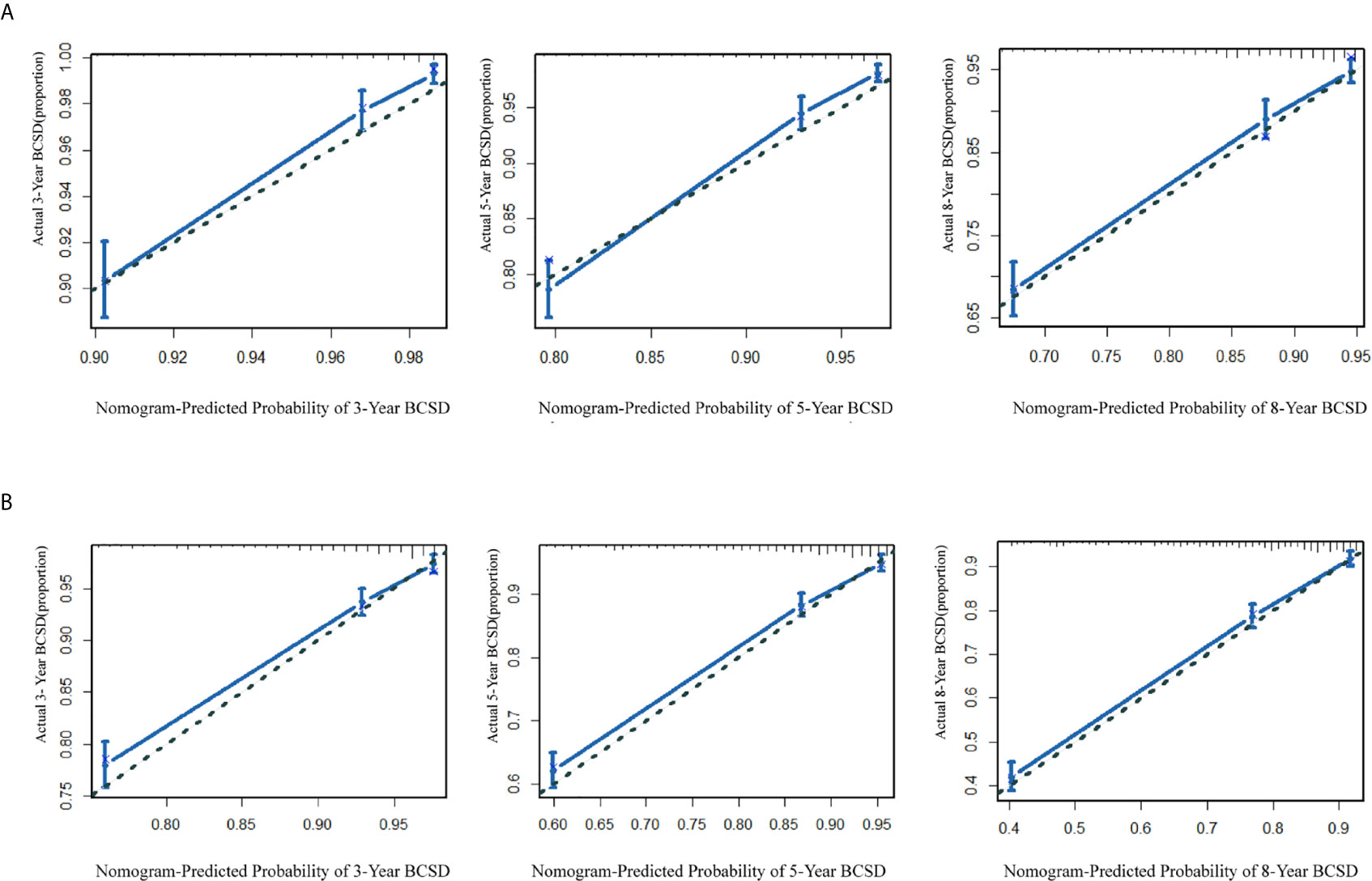
Figure 5 (A) The calibration curve for predicting patient BCSD after three, five, and eight years in the training cohort; (B) the calibration curve for predicting patient BCSD after three, five, and eight years in the internal validation cohort.
The NRI and IDI were also performed to compare the efficiency between the nomogram and AJCC-TNM stage (Table 5). In the training cohort, the NRI values for the 3-year, 5-year, and 8-year BCSD were 0.54 (95%CI: 0.31-0.69), 0.55 (95%CI: 0.27-0.67), and 0.49 (95%CI: 0.24-0.61), respectively, the IDI values for the 3-year, 5-year, and 8-year BCSD were 0.02 (95%CI: 0.01-0.03), 0.03 (95%CI: 0.01-0.04), and 0.04 (95%CI: 0.02-0.06), respectively. While using the nomogram in the validation cohort, the NRI values for the 3-year, 5-year, and 8-year BCSD were 0.51 (95%CI: 0.07-0.83), 0.45 (95%CI: 0.02-0.74), and 0.33 (95%CI: 0.16-0.34), respectively, the IDI values for the 3-year, 5-year, and 8-year BCSD were 0.02 (95%CI: 0.003-0.04), 0.04 (95%CI: 0.01-0.07), and 0.04 (95%CI: 0.004-0.04), respectively. In summary, the abovementioned results suggested that the competing risks regression nomogram model had significantly enhanced precision and reliability for BCSD prediction compared with the TNM stage system.
Discussion
In this retrospective study, we conducted Cox regression models and competing risk analysis based on 4,405 male patients with non-metastatic breast cancer in the SEER database from 1998 to 2015. The application was significantly associated with better BCSS and BCSD. Based on the corresponding parameters by competing risks regression, we built a nomogram to predict the 3-year, 5-year, and 8-year breast cancer-specific death (BCSD). To our knowledge, this was the first and largest population-based nomogram model to predict the impact of CPM on MaBC by competing risk analysis.In our study, surgery procedure was associated with improvement in BCSS and OS, which were objective and bias-free measurements for patients with MaBC. In the Kaplan-Meier curve analysis, significant improvements in BCSS and OS were observed in the CPM group rather than the UM group. To reduce the estimation bias and further investigate the efficiency of CPM on BCSS and OS for patients with MaBC, the multivariate Cox regression models analysis was performed. After adjusting for demographic, clinicopathological, and therapeutic variables, we found that administration of CPM could prolong BCSS, but had the threshold value of benefit in OS in comparison with UM. These findings were inconsistent with the previous trials where the application of CPM played a vital role in MaBC treatment (18–20). Multiple single and multi-institution studies reported the CPM’s positive effect on OS and disease-free survival (DFS). Four single (21, 22) and three multi-institution (23–25) studies demonstrated that CPM could have benefit in DFS, while two single (21, 26) and three multi-institution (23–25) studies indicated an OS benefit. A recent review study showed that patients who received CPM might be more healthy and had access to more advanced treatments than patients who did not undergo CPM (27).
To eliminate the estimation bias from other causes of death and further investigate the efficacy of CPM on BCSD, competing multivariable regression models analysis which is common in oncology research was performed (28–31). After performing competing risks regression, we found that the patients in the CPM group had better BCSD in comparison with the UM group. The main reasons might be that most of the research involving CPM was conducted in patients with FBC rather than patients with MaBC. Several studies concentrated on the prevention of contralateral breast cancers (CBCs) through the administration of CPM (20, 23, 32, 33). And BRCA mutation carriers, who had a high risk of CBCs, also obtained a survival benefit from CPM (34–36). Many patients consequently tended to select CPM to reduce the risk of CBCs. Many studies have shown that CBCs tend to have more favorable tumor features, and patients who develop CBCs in a short interval from their primary cancer have worse prognosis than those who develop CBCs at a longer interval, especially in young patients with large tumors, and those who are node-positive (37–41). However, it is controversial whether worse survival is caused by the CBCs, which represents the aggressive biology of the primary tumor, distant metastatic disease, and older, inferior systemic treatments.
In addition, MaBC and FBC have different biological characteristics, such as the rate of ER-positive tumors and age at diagnosis. In our study, the rate of ER and PR-positive tumors were as high as 90.8% and 81.2%, respectively, but the percentage of ER-positive tumors and PR-positive tumors in FBC patients were only 78% and 64% in a previous study (42, 43). The majority of male cases who developed BC were older than those in FBC. Previous research reported that MaBC tended to have a 1.75 times higher risk of distant metastasis than FBC (7% vs. 4%) (44, 45). Furthermore, patients with MaBC were likely to have a higher mutation rate of CHEK2 c.1100delC and BRCA2, which play a particularly prominent role in metastasis and the prognosis of disease, than those in FBC (11, 12, 46–49). In brief, MaBC patients were more likely to have poorer differentiated grade, were older, a higher node-positive, higher rates of lymphovascular invasion, and estrogen receptor (ER+) tumors. Therefore, there are differences in treatment procedures, for example chemotherapy/radiotherapy and the corresponding prognosis between MaBC and FBC.
Meanwhile, this study set up a nomogram model to predict BCSD in patients with MaBC. After integrating the demographic and clinicopathological characteristics, the nomogram model could be more precise than the conventional TNM stage system, such as the AJCC stage system. In the traditional sense, the AJCC-TNM stage system was the preferred alternative for predicting the prognosis of patients with carcinoma. In general, the stages of this system were strongly correlated with BCSD (50). Inevitably, patients at the same stage often had different prognoses. The underlying reasons might be the vagueness in the TNM-stage system and the variables which were not included in the sociodemographic characteristics, such as age, marital status, and so on. Actually, in our study, married patients with a well-differentiation level and T0-1 stage and N0 stage tumors tended to have better prognostic indicators for BCSD. These results are consistent with previous reports (18–20, 35, 46, 47) and indicate that both demographic and clinicopathological characteristics, such as marital status and tumor differentiation level, were objective and reliable prognostic indicators in men with breast carcinoma. Then, the NRI value and IDI value of the nomogram confirmed that the nomogram had better prediction power than the AJCC-TNM stage system. Furthermore, the favorable results were replicated well in the validation cohort. In summary, the nomogram could provide precise and personalized prediction of the cumulative risk in patients with MaBC after CPM.
Our subject indeed has limitations, as shown below: Firstly, studies that randomly assigned patients into different groups by treatment methods were needed. The retrospective study could not prove causation and may be subject to selection bias and uncontrolled confounding factors, even with the administration of competing risks regression models. Secondly, we were unable to avoid the possibility that the observed risks reduction might exclude the influence of potential confounders, such as family history, insurance coverage, comorbidities, health status, MRI application, patient anxiety, BRCA gene status, counseling, and so on. These data greatly impacted the clinical decisions and even breast cancer prognosis (18–20, 34–36, 46, 47). Thirdly, there was a big gap between CPM and UM that may have some bias to the data, and the study sample might be insufficient to uncover some differences in the abovementioned phenomenon. Next, the proportion of T1 stage (49.5%), T2 stage (41.1%), N0 stage (56.2%), and N1 stage (29.6%) may have been too high in our study, this statistical bias from the SEER database might lead to the result that the efficacy of radiotherapy and chemotherapy were limited in our study. Randomized controlled clinical and multicenter-clinical trials with long follow-up periods are still needed to further confirm this. Lastly, P value <0.05 was used to possess the statistics sense, and no adjustment was made for multiple analysis; the chance of falsely rejecting a null hypothesis may exceed 0.05.
Conclusion
The administration of CPM was associated with the decrease in risk of BCSD in patients with MaBC. The nomogram could provide precise and personalized prediction of the cumulative risk in patients with MaBC after CPM. Randomized controlled clinical and multicenter-clinical trials with long follow-up time are still needed to further confirm the effects of CPM on BCSD and the prediction efficacy of the nomogram.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://seer.cancer.gov.
Author Contributions
KL and BW drafted the manuscript and analyzed data, ZY, HC, and YL generated the figure, and RY performed the background research. CZ and JH edited the manuscript. All authors have read and approved the content of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (NSFC 81502413 to CZ and NSFC 81702633 to BW) and the Shan’xi Provincial Natural Science Foundation of China (SNSFC 2019SF-145 to CZ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are thankful the Surveillance, Epidemiology, and End Results Program (National Cancer Institute) for the development of the SEER database. We wish to thank all our colleagues in the Departments of Breast Surgery, First Affiliate Hospital of Xi’an Jiaotong University.
References
1. Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing Use of Contralateral Prophylactic Mastectomy for Breast Cancer Patients: A Trend Toward More Aggressive Surgical Treatment. J Clin Oncol (2007) 25:5203–9. doi: 10.1200/JCO.2007.12.3141
2. Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing Use of Contralateral Prophylactic Mastectomy Despite No Improvement in Long-term Survival for Invasive Breast Cancer. Ann Surg (2017) 265:581–9. doi: 10.1097/SLA.0000000000001698
3. Yao K, Stewart AK, Winchester DJ, Winchester DP. Trends in Contralateral Prophylactic Mastectomy for Unilateral Cancer: A Report From the National Cancer Data Base, 1998-2007. Ann Surg Oncol (2010) 17:2554–62. doi: 10.1245/s10434-010-1091-3
4. Portschy PR, Kuntz KM, Tuttle TM. Survival Outcomes After Contralateral Prophylactic Mastectomy: A Decision Analysis. J Natl Cancer Inst (2014) 106(8):dju160. doi: 10.1093/jnci/dju160
5. King TA, Sakr R, Patil S, Gurevich I, Stempel M, Sampson M, et al. Clinical Management Factors Contribute to the Decision for Contralateral Prophylactic Mastectomy. J Clin Oncol (2011) 29:2158–64. doi: 10.1200/JCO.2010.29.4041
6. Duma N, Hoversten KP, Ruddy KJ. Exclusion of Male Patients in Breast Cancer Clinical Trials. JNCI Cancer Spectr (2018) 2:y18. doi: 10.1093/jncics/pky018
7. Hodgson NC, Button JH, Franceschi D, Moffat FL, Livingstone AS. Male Breast Cancer: Is the Incidence Increasing? Ann Surg Oncol (2004) 11:751–5. doi: 10.1245/ASO.2004.01.001
8. Stang A, Thomssen C. Decline in Breast Cancer Incidence in the United States: What About Male Breast Cancer? Breast Cancer Res Treat (2008) 112:595–6. doi: 10.1007/s10549-007-9882-3
9. Chen Z, Xu L, Shi W, Zeng F, Zhuo R, Hao X, et al. Trends of Female and Male Breast Cancer Incidence At the Global, Regional, and National Levels, 1990-2017. Breast Cancer Res Treat (2020) 180:481–90. doi: 10.1007/s10549-020-05561-1
10. Abdelwahab YA. Male Breast Cancer: Epidemiology and Risk Factors. Semin Oncol (2017) 44:267–72. doi: 10.1053/j.seminoncol.2017.11.002
11. Anderson WF, Althuis MD, Brinton LA, Devesa SS. Is Male Breast Cancer Similar or Different Than Female Breast Cancer? Breast Cancer Res Treat (2004) 83:77–86. doi: 10.1023/B:BREA.0000010701.08825.2d
12. Cardoso F, Bartlett J, Slaets L, van Deurzen C, van Leeuwen-Stok E, Porter P, et al. Characterization of Male Breast Cancer: Results of the EORTC 10085/Tbcrc/Big/Nabcg International Male Breast Cancer Program. Ann Oncol (2018) 29:405–17. doi: 10.1093/annonc/mdx651
13. Pritzlaff M, Summerour P, McFarland R, Li S, Reineke P, Dolinsky JS, et al. Male Breast Cancer in a Multi-Gene Panel Testing Cohort: Insights and Unexpected Results. Breast Cancer Res Treat (2017) 161:575–86. doi: 10.1007/s10549-016-4085-4
14. Wingo PA, Jamison PM, Hiatt RA, Weir HK, Gargiullo PM, Hutton M, et al. Building the Infrastructure for Nationwide Cancer Surveillance and Control–a Comparison Between the National Program of Cancer Registries (NPCR) and the Surveillance, Epidemiology, and End Results (Seer) Program (United States). Cancer Causes Control (2003) 14:175–93. doi: 10.1023/A:1023002322935
15. Berger M, Schmid M, Welchowski T, Schmitz-Valckenberg S, Beyersmann J. Subdistribution Hazard Models for Competing Risks in Discrete Time. BIOSTATISTICS (2020) 21:449–66. doi: 10.1093/biostatistics/kxy069
16. Scrucca L, Santucci A, Aversa F. Regression Modeling of Competing Risk Using R: An in Depth Guide for Clinicians. Bone Marrow Transplant (2010) 45:1388–95. doi: 10.1038/bmt.2009.359
17. de Wreede LC, Fiocco M, Putter H. The Mstate Package for Estimation and Prediction in non- and Semi-Parametric Multi-State and Competing Risks Models. Comput Methods Programs BioMed (2010) 99:261–74. doi: 10.1016/j.cmpb.2010.01.001
18. Jemal A, Lin CC, DeSantis C, Sineshaw H, Freedman RA. Temporal Trends in and Factors Associated With Contralateral Prophylactic Mastectomy Among Us Men With Breast Cancer. JAMA Surg (2015) 150:1192–4. doi: 10.1001/jamasurg.2015.2657
19. Alaofi RK, Nassif MO, Al-Hajeili MR. Prophylactic Mastectomy for the Prevention of Breast Cancer: Review of the Literature. Avicenna J Med (2018) 8:67–77. doi: 10.4103/ajm.AJM_21_18
20. Kurian AW, Canchola AJ, Ma CS, Clarke CA, Gomez SL. Magnitude of Reduction in Risk of Second Contralateral Breast Cancer With Bilateral Mastectomy in Patients With Breast Cancer: Data From California, 1998 Through 2015. Cancer-Am Cancer Soc (2020) 126:958–70. doi: 10.1002/cncr.32618
21. Brewster AM, Bedrosian I, Parker PA, Dong W, Peterson SK, Cantor SB, et al. Association Between Contralateral Prophylactic Mastectomy and Breast Cancer Outcomes by Hormone Receptor Status. Cancer-Am Cancer Soc (2012) 118:5637–43. doi: 10.1002/cncr.27574
22. Boughey JC, Hoskin TL, Degnim AC, Sellers TA, Johnson JL, Kasner MJ, et al. Contralateral Prophylactic Mastectomy is Associated With a Survival Advantage in High-Risk Women With a Personal History of Breast Cancer. Ann Surg Oncol (2010) 17:2702–9. doi: 10.1245/s10434-010-1136-7
23. Herrinton LJ, Barlow WE, Yu O, Geiger AM, Elmore JG, Barton MB, et al. Efficacy of Prophylactic Mastectomy in Women With Unilateral Breast Cancer: A Cancer Research Network Project. J Clin Oncol (2005) 23:4275–86. doi: 10.1200/JCO.2005.10.080
24. Yao K, Winchester DJ, Czechura T, Huo D. Contralateral Prophylactic Mastectomy and Survival: Report From the National Cancer Data Base, 1998-2002. Breast Cancer Res Treat (2013) 142:465–76. doi: 10.1007/s10549-013-2745-1
25. Kruper L, Kauffmann RM, Smith DD, Nelson RA. Survival Analysis of Contralateral Prophylactic Mastectomy: A Question of Selection Bias. Ann Surg Oncol (2014) 21:3448–56. doi: 10.1245/s10434-014-3930-0
26. Boughey JC, Hoskin TL, Hartmann LC, Johnson JL, Jacobson SR, Degnim AC, et al. Impact of Reconstruction and Reoperation on Long-Term Patient-Reported Satisfaction After Contralateral Prophylactic Mastectomy. Ann Surg Oncol (2015) 22:401–8. doi: 10.1245/s10434-014-4053-3
27. Yao K, Sisco M, Bedrosian I. Contralateral Prophylactic Mastectomy: Current Perspectives. Int J Womens Health (2016) 8:213–23. doi: 10.2147/IJWH.S82816
28. Haller B, Schmidt G, Ulm K. Applying Competing Risks Regression Models: An Overview. Lifetime Data Anal (2013) 19:33–58. doi: 10.1007/s10985-012-9230-8
29. Dignam JJ, Zhang Q, Kocherginsky M. The Use and Interpretation of Competing Risks Regression Models. Clin Cancer Res (2012) 18:2301–8. doi: 10.1158/1078-0432.CCR-11-2097
30. Shen W, Sakamoto N, Yang L. Model to Predict Cause-Specific Mortality in Patients With Head and Neck Adenoid Cystic Carcinoma: A Competing Risk Analysis. Ann Surg Oncol (2017) 24:2129–36. doi: 10.1245/s10434-017-5861-z
31. Roobol MJ, Heijnsdijk EA. Propensity Score Matching, Competing Risk Analysis, and a Competing Risk Nomogram: Some Guidance for Urologists may be in Place. Eur Urol (2011) 60:931–4. doi: 10.1016/j.eururo.2011.07.039
32. Goldflam K, Hunt KK, Gershenwald JE, Singletary SE, Mirza N, Kuerer HM, et al. Contralateral Prophylactic Mastectomy. Predictors of Significant Histologic Findings. Cancer-Am Cancer Soc (2004) 101:1977–86. doi: 10.1002/cncr.20617
33. McDonnell SK, Schaid DJ, Myers JL, Grant CS, Donohue JH, Woods JE, et al. Efficacy of Contralateral Prophylactic Mastectomy in Women With a Personal and Family History of Breast Cancer. J Clin Oncol (2001) 19:3938–43. doi: 10.1200/JCO.2001.19.19.3938
34. Heemskerk-Gerritsen BA, Rookus MA, Aalfs CM, Ausems MG, Collee JM, Jansen L, et al. Improved Overall Survival After Contralateral Risk-Reducing Mastectomy in BRCA1/2 Mutation Carriers With a History of Unilateral Breast Cancer: A Prospective Analysis. Int J Cancer (2015) 136:668–77. doi: 10.1002/ijc.29032
35. Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, et al. Cancer Risks for BRCA1 and BRCA2 Mutation Carriers: Results From Prospective Analysis of EMBRACE. J Natl Cancer Inst (2013) 105:812–22. doi: 10.1093/jnci/djt095
36. van der Kolk DM, de Bock GH, Leegte BK, Schaapveld M, Mourits MJ, de Vries J, et al. Penetrance of Breast Cancer, Ovarian Cancer and Contralateral Breast Cancer in BRCA1 and BRCA2 Families: High Cancer Incidence At Older Age. Breast Cancer Res Treat (2010) 124:643–51. doi: 10.1007/s10549-010-0805-3
37. Liederbach E, Piro R, Hughes K, Watkin R, Wang CH, Yao K. Clinicopathologic Features and Time Interval Analysis of Contralateral Breast Cancers. SURGERY (2015) 158:676–85. doi: 10.1016/j.surg.2015.03.059
38. Font-Gonzalez A, Liu L, Voogd AC, Schmidt MK, Roukema JA, Coebergh JW, et al. Inferior Survival for Young Patients With Contralateral Compared to Unilateral Breast Cancer: A Nationwide Population-Based Study in the Netherlands. Breast Cancer Res Treat (2013) 139:811–9. doi: 10.1007/s10549-013-2588-9
39. Vichapat V, Garmo H, Holmberg L, Fentiman IS, Tutt A, Gillett C, et al. Prognosis of Metachronous Contralateral Breast Cancer: Importance of Stage, Age and Interval Time Between the Two Diagnoses. Breast Cancer Res Treat (2011) 130:609–18. doi: 10.1007/s10549-011-1618-8
40. Vichapat V, Garmo H, Holmqvist M, Liljegren G, Warnberg F, Lambe M, et al. Tumor Stage Affects Risk and Prognosis of Contralateral Breast Cancer: Results From a Large Swedish-population-based Study. J Clin Oncol (2012) 30:3478–85. doi: 10.1200/JCO.2011.39.3645
41. Liederbach E, Sisco M, Wang C, Pesce C, Sharpe S, Winchester DJ, et al. Wait Times for Breast Surgical Operations, 2003-2011: A Report From the National Cancer Data Base. Ann Surg Oncol (2015) 22:899–907. doi: 10.1245/s10434-014-4086-7
42. Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male Breast Cancer: A Population-Based Comparison With Female Breast Cancer. J Clin Oncol (2010) 28:232–9. doi: 10.1200/JCO.2009.23.8162
43. Gucalp A, Traina TA, Eisner JR, Parker JS, Selitsky SR, Park BH, et al. Male Breast Cancer: A Disease Distinct From Female Breast Cancer. Breast Cancer Res Treat (2019) 173:37–48. doi: 10.1007/s10549-018-4921-9
44. Xie J, Ying YY, Xu B, Li Y, Zhang X, Li C. Metastasis Pattern and Prognosis of Male Breast Cancer Patients in US: A Population-Based Study From SEER Database. Ther Adv Med Oncol (2019) 11:432463253. doi: 10.1177/1758835919889003
45. Harlan LC, Zujewski JA, Goodman MT, Stevens JL. Breast Cancer in Men in the United States: A Population-Based Study of Diagnosis, Treatment, and Survival. Cancer-Am Cancer Soc (2010) 116:3558–68. doi: 10.1002/cncr.25153
46. Mueller C, Haymond A, Davis JB, Williams A, Espina V. Protein Biomarkers for Subtyping Breast Cancer and Implications for Future Research. Expert Rev Proteomics (2018) 15:131–52. doi: 10.1080/14789450.2018.1421071
47. O’Brien KM, Mooney T, Fitzpatrick P, Sharp L. Screening Status, Tumour Subtype, and Breast Cancer Survival: A National Population-Based Analysis. Breast Cancer Res Treat (2018) 172:133–42. doi: 10.1007/s10549-018-4877-9
48. Gilbert SF, Soliman AS, Karkouri M, Quinlan-Davidson M, Strahley A, Eissa M, et al. Clinical Profile, BRCA2 Expression, and the Androgen Receptor CAG Repeat Region in Egyptian and Moroccan Male Breast Cancer Patients. Breast Dis (2011) 33:17–26. doi: 10.3233/BD-2010-0323
49. Hallamies S, Pelttari LM, Poikonen-Saksela P, Jekunen A, Jukkola-Vuorinen A, Auvinen P, et al. Chek2 c.1100delC Mutation is Associated With an Increased Risk for Male Breast Cancer in Finnish Patient Population. BMC Cancer (2017) 17:620. doi: 10.1186/s12885-017-3631-8
Keywords: male breast cancer, contralateral prophylactic mastectomy, SEER, competing risk analysis, nomogram
Citation: Li K, Wang B, Yang Z, Yu R, Chen H, Li Y, He J and Zhou C (2021) Nomogram Predicts the Role of Contralateral Prophylactic Mastectomy in Male Patients With Unilateral Breast Cancer Based on SEER Database: A Competing Risk Analysis. Front. Oncol. 11:587797. doi: 10.3389/fonc.2021.587797
Received: 27 July 2020; Accepted: 01 April 2021;
Published: 29 April 2021.
Edited by:
Gianluca Franceschini, Catholic University of the Sacred Heart, ItalyReviewed by:
Armando Orlandi, Agostino Gemelli University Polyclinic, ItalyZiv Radisavljevic, Brigham and Women’s Hospital and Harvard Medical School, United States
Copyright © 2021 Li, Wang, Yang, Yu, Chen, Li, He and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianjun He, Y2hpbmFoampAMTYzLmNvbQ==; Can Zhou, emhvdWNhbnoyMDA1QDEyNi5jb20=
†These authors have contributed equally to this work
 Kunlong Li1,2†
Kunlong Li1,2† Bin Wang
Bin Wang Ren Yu
Ren Yu Yijun Li
Yijun Li Jianjun He
Jianjun He Can Zhou
Can Zhou