
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 23 February 2021
Sec. Cancer Imaging and Image-directed Interventions
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.585257
This article is part of the Research Topic Image-guided Ablation of Tumors View all 17 articles
Background: Reninoma is a rare renal endocrine tumor that can cause secondary hypertension, characterized by hypertension, hypokalemia, high renin and aldosterone with normal aldosterone renin ratio (ARR), and occurs more in young female. Mainstream treatment option is surgery, but is less suitable for small or deep lesions, which makes ablation a promising alternative.
Case presentation: Two young female with typical manifestations of reninoma, including hypertension, hypokalemia, high renin, high aldosterone and normal ARR, were treated successfully with real-time contrast-enhanced ultrasound guided radiofrequency ablation, and contrast-enhanced ultrasound was also performed before and after treatment for diagnosis and postoperative assessment. Afterward, their blood pressure and laboratory tests became normal and remained steady during the follow-up of 32 and 6 months, respectively.
Conclusion: Contrast-enhanced ultrasound guided radiofrequency ablations is a promising alternative for reninoma treatment with comparable safety and efficacy with surgery, and has advantages especially in small or deep lesions.
Reninoma, also called juxtaglomerular cell tumor, is a rare cause of secondary hypertension due to the oversecreting renin of juxtaglomerular apparatus. Since the first case reported by Robertson et al. at 1967 (1), nearly 200 cases were described worldwide, and the incidence rate is 0.023% in hypertensive patients at initial diagnosis (2). It often occurs in young adults, especially female, and is characterized by hypertension, hypokalemia, high renin and aldosterone with normal aldosterone renin ratio (ARR). In cases with severe or chronic hypertension, target organ damage was observed, such as proteinuria, cardiomyopathy, and retinopathy (2). Most cases of reninoma were considered benign, though metastases and recurrence cases had been reported (3, 4), and pathological evidence as well as FDG-PET/CT did indicate malignancy sometimes (5, 6). The gold standard of diagnosis is histopathological examination, while clinical evidence combined with laboratory tests and imaging can also lead to diagnosis preoperatively. Treatment modalities include surgery, ablation, and medical therapy. Surgery is the main option, while the effect of medical therapy is very uncertain, and ablation is not widely used but considered a potential alternative (2, 7, 8).
Here we presented two reninoma cases admitted in our hospital in Feb. 2018 and Mar. 2020. Their lesions were tackled successfully with real-time contrast-enhanced ultrasound (CEUS) guided radiofrequency ablation (RFA), and CEUS was also performed before and after ablation for diagnosis and postoperative assessment. The treatment outcomes were followed for 32 and 6 months, respectively. To our knowledge, these two cases were the first to use real-time CEUS guided RFA in reninoma treatment and filled the blank of long-term efficacy of ablation.
A 17-year-old female was admitted to our hospital in Feb. 2018 with paroxysmal headache, nausea and vomiting for over one year, accompanied with marked hypertension (the highest blood pressure was 190/120 mm Hg) and hypokalemia (serum potassium 2.45–3.18 mmol/L). She tried various antihypertensive regimens such as Spironolactone 40 mg, Benazepril 10 mg combined with Amlodipine Besylate 5 mg; Indapamide 1.5 mg combined with Arolol 10 mg. When taking drugs, her blood pressure was among 120–140/80–100 mm Hg. She discontinued all medication for one month for diagnostic demand. Contrast-enhanced magnetic resonance (CEMR) and CEUS revealed a 10-mm diameter cortex lesion in the upper pole of the left kidney (Figure 1). And the key endocrine parameters were summarized in Table 1. Renal veins sampling was performed but failed to detect lateralization. Target organ damage evaluation indicated bilateral ocular fundus arteriosclerosis and moderate proteinuria. We gave her Spironolactone 60 mg, 10% Potassium chloride 45 ml (oral) and Adalat 30 mg after admission. The clinical timeline of Patient 1 was organized in Supplementary Figure 1.
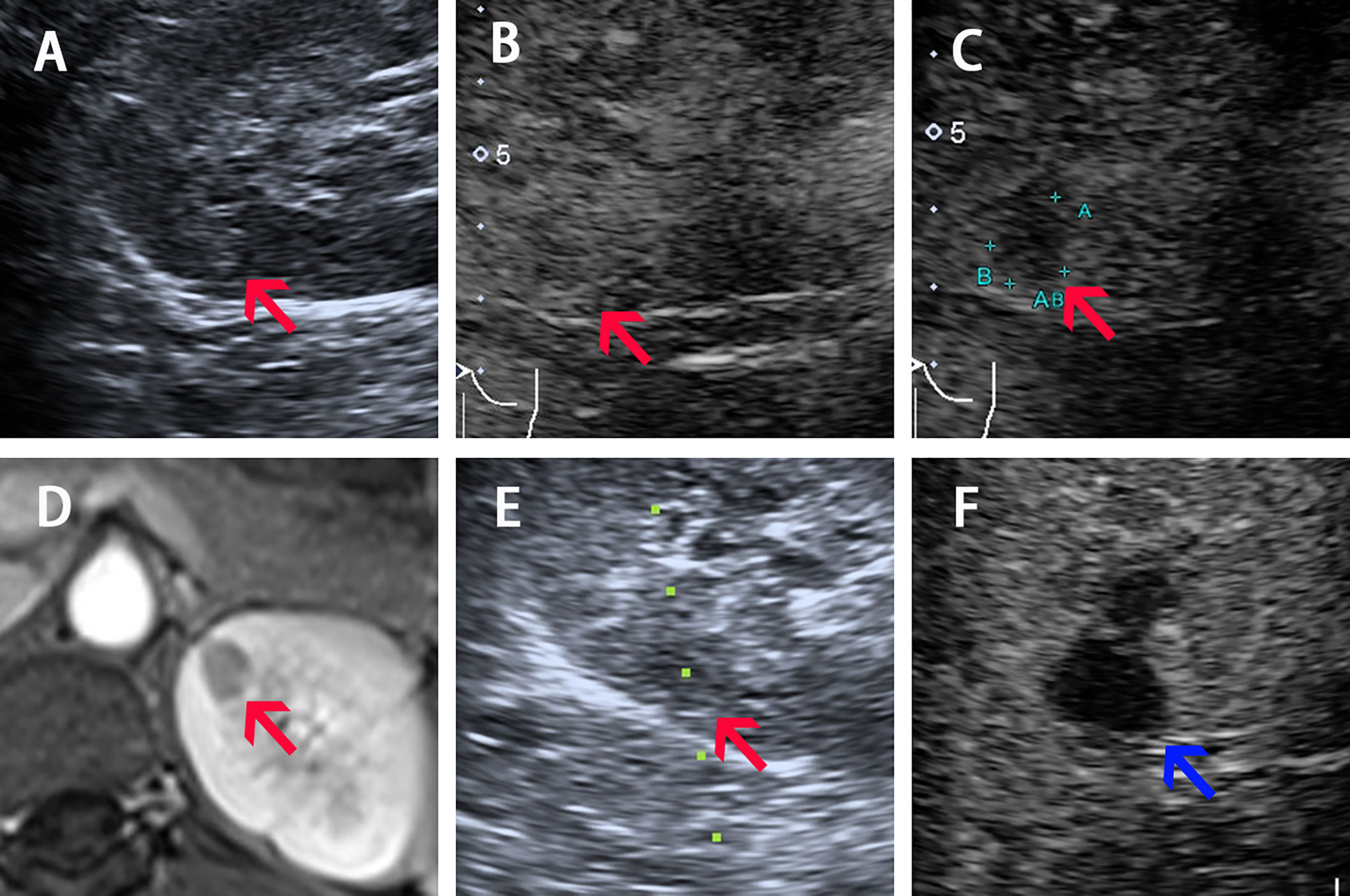
Figure 1 Imaging of Patient 1 before, during, and after ablation procedure. (A–C) Ultrasound image acquired in prone position before ablation shows a slight hyperechoic nodule of 10-mm diameter with iso-enhancement in the cortical phase (30 s) and hypo-enhancement in the medulla phase (90 s) (pointed by red arrow). (D) Arterial phase of four phases MR before ablation shows a 10-mm diameter nodule (pointed by red arrow) located in the upper pole of left kidney. (E) The 17G electrode was implanted into the lesions under real-time CEUS guidance during the ablation (red arrow shows the lesion and the green dotted line shows the puncture path). (F) CEUS showed non-enhancement during both cortical and medulla phases postprocedure (pointed by blue arrow).
A 27-year-old female with a 5-year history of poorly controlled hypertension was referred to our hospital in Mar. 2020. The highest blood pressure was 179/99 mm Hg. She intermittently used antihypertensive regimens (including Fosinopril 10 mg combined with Metoprolol 47.5 mg; Telmisarta 40 mg combined with Spironolactone 40 mg), and her blood pressure was among 120–140/80–90 mm Hg while taking medication, but returned to 170/100 mm Hg after withdrawal, and she stopped taking any medicine for four months for diagnostic need according to the advice of her doctor. Contrast-enhanced computed tomography (CECT) and CEUS revealed a 6-mm diameter cortex lesion in her right kidney (Figure 2). Key endocrine parameters were summarized in Table 1. Target organ damage evaluation found nothing except slight proteinuria. We gave her Terazosin Hydrochloride 4 mg, Diltiazem Hydrochloride 90 mg and oral potassium supplement after admission. The clinical timeline of Patient 2 was organized in Supplementary Figure 2.
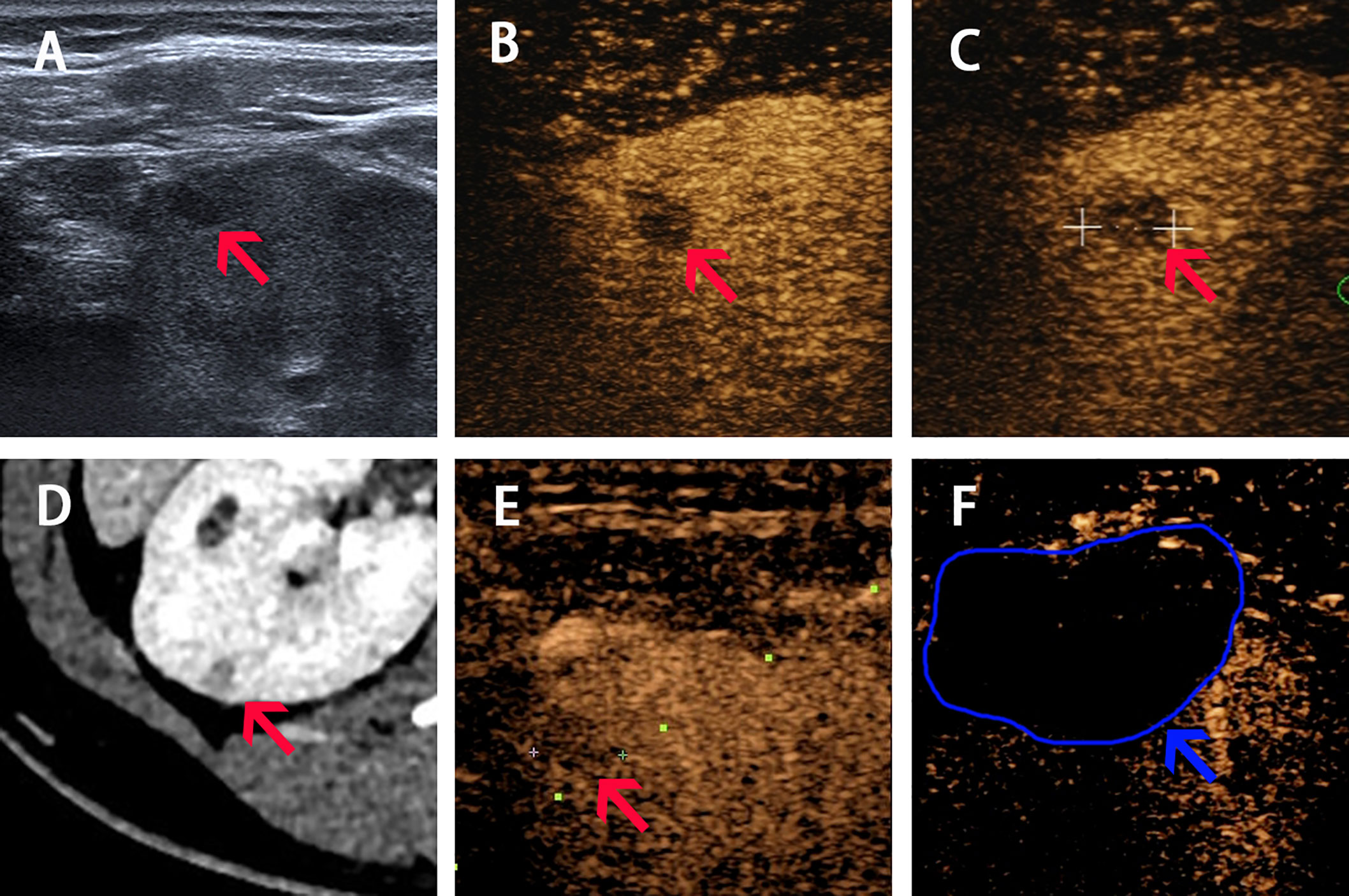
Figure 2 Imaging of Patient 2 before, during and after ablation procedure. (A–C) Ultrasound image acquired in prone position before ablation shows a slight hypoechoic nodule of 6-mm diameter with hypo-enhancement in both cortical phase (24 s) and medulla phase (70 s) (pointed by red arrow). (D) Arterial phase of CT before procedure shows a 6-mm diameter nodule located in the right kidney (pointed by red arrow). (E) The real-time CEUS guided the locating of nodule during ablation (red arrow shows the lesion and the green dotted line shows the puncture path). (F) CEUS showed non-enhancement during both cortical and medulla phases postprocedure (pointed by blue arrow and curve).
MDT discussion was held by endocrinologists, urologists and interventional ultrasound doctors for two patients. Both patients were young female with hypertension, hypokalemia, high renin, high aldosterone and normal ARR. Secondary hypertension caused by endocrine diseases were considered, including pheochromocytoma, Cushing syndrome, congenital adrenal hyperplasia, primary aldosteronism and reninoma. Pheochromocytoma was firstly excluded according to the absence of paroxysmal hypertension or sweating and negative Regitine test in both patients. Cushing syndrome was then ruled out since there were no signs of Cushing appearance and abnormal cortisol level. Congenital adrenal hyperplasia (usually appear as high renin, low aldosterone and decreased ARR) and primary aldosteronism (usually appear as low renin, high aldosterone and increased ARR) were also excluded. Consensus of reninoma diagnosis was finally achieved according to clinical evidence. Both patients refused preoperative biopsy. Surgery and ablation could be the treatment options for both lesions. However, considering that the lesions were rather small and may have difficulty in intraoperative locating, which could prolong the operation time and thus cause more tissue injury and finally damage renal function, percutaneous RFA guided by real-time CEUS was recommended over surgery. And both patients were aware of MDT discussion recommendation and agreed to receive ablation.
The CEUS was performed before RFA. CEUS using a low mechanical index (MI) mode (0.07–0.08) can provide a real-time evaluation of tumor enhancement and location. For case 1, the tumor indicates iso-enhancement and hypo-enhancement during cortical phase and medulla phase. For case 2, the tumor indicates hypo-enhancement in both cortical and medulla phases (Figures 1 and 2).
RFA was performed under real-time CEUS guidance using the Toshiba ultrasound system. Two physicians who had 10 years of experience performing RFA for renal tumors performed all the procedures. A 20-cm-long, 17-gauge Cool-tip radiofrequency electrode with a 2-cm-long exposed tip (Covidien Valleylab, Boulder, CO, United States) was inserted into the targeted tumor. RFA was performed under local anesthesia. The temperature of the ablated tissue was increased to above 60°C and the ablation duration was 12 min for both patients. Immediate CEUS after ablation was done for Patient 2 as shown in Supplementary Figure 3. The next day, we performed CEUS for both patients and found the ablation area indicated non-enhancement throughout both cortical and medulla phases, and completely covered the former nodules with satisfactory ablation margin. No intraoperative adverse events occurred and the vital signs remained stable in perioperative period.
The laboratory tests 1 to 2 days after RFA were also summarized in Table 1. As for Patient 1, the result was delightful, blood pressure and serum tests became normal 12 h after the procedure without any drugs. Patient 2 had a significant decrease of serum renin and aldosterone, her blood pressure and serum potassium remained abnormal without medication, but indeed better than before. Both patients were discharged 3 days after ablation with no discomfort or complications, Patient 2 got take-home medicine (Valsarta 160 mg and Spironolactone 20 mg) while Patient 1 did not.
For Patient 1, during the 32-month follow-up after RFA, her blood pressure (100–120/60–80 mm Hg), serum potassium and other endocrine tests were totally normal without any medication, urinary protein turned negative, and headache never occurred. Patient 2 stopped Spironolactone 2 weeks after RFA, her blood pressure remains in normal range (100–120/75–85 mm Hg) with Valsarta 160 mg according to the latest follow-up (6 months after ablation). Her serum potassium, other endocrine tests and renal ultrasound showed completely normal. Long-term effect still demands follow-up for Patient 2.
Reninoma is an uncommon but curable renal endocrine tumor which can cause secondary hypertension. Although it is often considered a benign disease, malignant potentials had shown sometimes (3–6).
To the best of our knowledge, these two cases from our institute were one of the few practices using RFA to treat reninoma, and this is the first report to use real-time CEUS as guidance. Both patients went through RFA successfully, and the postoperative results were delightful. We provided a follow-up of more than 2 years after ablation, which filled the blank of long-term efficacy of RFA. Furthermore, we introduced CEUS into the detecting and follow-up routine, which showed great potential in locating small tumors and indicating the ablation region.
Currently, treatment options for reninoma include surgery, ablation and medical therapy. Medication, such as angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, beta-blockers and Spironolactone combined with oral potassium can help control blood pressure and serum potassium (2). However, the use of antihypertensive medications before diagnosis can mask hypokalemia and make reninoma more difficult to recognize (9). And the effect varies from person to person. It is hard to control the blood pressure over years without tackling the oversecreting of renin. Thus, medication is only considered an adjuvant therapy.
Surgery procedures, including nephron sparing partial nephrectomy for superficial or small lesions and radical nephrectomy for deep or large lesions, are the mainstream treatment options. The safety and long-term efficacy have been proved with numerous evidences (2, 7, 8). However, when it comes to small nodules with difficulty in intraoperative locating, surgery may lead to prolonged warm ischemic time as well as operation time, and renal dysfunction consequently. Moreover, using radical nephrectomy to remove deep lesions is in cost of renal dysfunction along with more tissue injury (10, 11).
For these small or deep reninoma, ablation can be an alternative. Ablation for reninoma is not regularly performed currently. Less than five cases of RFA and only one cryoablation have been reported, and none of them provided evidence of long-term efficacy (12–15). However, ablation is actually widely used in other renal tumors, including renal cell carcinoma (RCC), Wilms tumor, adenoma and angioleiomyoma (11, 16). It is considered a type of nephron sparing procedure and recommended mainly in benign tumor or RCC of T1a stage (17). But no randomized controlled trial has been conducted yet to compare ablation and nephron sparing partial nephrectomy in renal tumors, and the long-term follow-up data lacks in ablation cases.
There are several types of ablations, including RFA, cryoablation and microwave ablation (MWA). RFA is used most frequently and the ablation shape can be controlled precisely. It is preferred to surgery for patients with nodules less than 4 cm or intolerable to surgery, and those requiring better postoperative renal function in cases of solitary kidney, bilateral lesions, or chronic renal insufficiency (17). Cryoablation has less application, according to recent literatures, it may be effective for renal tumors larger than 4 cm, but is more time-consuming (18). MWA has never been reported in reninoma treatment, but it is a potential option with many advantages, such as less affected by vascular heat-sink effect and tissue carbonization, more effective in large nodules, and more uniform thermal field distribution compared to RFA (19).
As we mentioned above, RFA has advantages for small or deep lesions. The reasons are as follows: for small lesion, the image-guidance allows quick and precise locating that leads to appropriate needle implanted angle and satisfactory ablation range; for deep lesions, it can preserve better renal function by less tissue injury. What is more, compared to surgery, RFA has less complications, less postoperative pain, shorter hospitalization time with comparable safety and efficacy (10, 11, 16, 20). The limitations of RFA application include nodules in dangerous sites and tumors larger than 4 cm (17). For nodules near the renal hilum, RFA have higher risk of renal pelvis injury, bleeding or infection, and incomplete ablation due to the heat-sink effect (21).
Up to now, all the reninoma cases reported using RFA were under guidance of CT (12–14). This is the first report to use real-time CEUS during procedure. With superiority in real-time, non-radiant and high sensitivity in blood supply detection facilitated by the pure blood pool contrast agents, CEUS shows more potential in intraoperative guidance and postoperative surveillance than CT. What is more, the use of CEUS through the diagnosis, treatment and follow-up routine makes it convenient and easy to compare the change of lesions.
There are also limitations of this report. First, no histopathology evidence was obtained due to patients’ unwillingness; but still, MDT discussion had reached in consensus of reninoma after differential diagnosis. Second, the long-term efficacy of Patients 2 still requires to be further confirmed.
In summary, we presented two typical cases of reninoma here, which was the first report of utilizing CEUS guided RFA in treating reninoma worldwide. We showed that real-time CEUS guided RFA is safe and effective in reninoma treatment, and it is a promising alternative to surgery especially in small or deep lesions. More data are still warranted to further confirm the long-term efficacy of RFA compared to surgery.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) and minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Case report design: all authors. Data collecting and patients’ follow-up: RZ and MX. Drafting of the manuscript: RZ and MX. Critical revision of the manuscript: MX and X-YX. All authors contributed to the article and approved the submitted version.
Our work is supported by the National Natural Science Foundation of China (81501489)
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to express their gratitude to Guangliang Huang and Manxia Lin, PhD from the Department of Medical Ultrasonics, Institute of Diagnostic and Interventional Ultrasound, The First Affiliated Hospital of Sun Yat-sen University, for their valuable contributions to the data description of our study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.585257/full#supplementary-material
Supplementary Figure 1 | Timeline for Patient 1.
Supplementary Figure 2 | Timeline for Patient 2.
Supplementary Figure 3 | Immediate CEUS after ablation for Patient 2, showed non-enhancement area during both cortical and medulla phases, which completely covered the former 6mm-diameter nodule with satisfactory ablation margin (pointed by blue arrow and curve).
ARR, aldosterone renin ratio; CEUS, contrast-enhanced ultrasound; RFA, radiofrequency ablation; CEMR, contrast-enhanced magnetic resonance; CECT, contrast-enhanced computed tomography; MDT, multiple disciplinary team; MI, mechanical index; ACE, angiotensin-converting enzyme; RCC, renal cell carcinoma; MWA, microwave ablation.
1. Robertson PW, Klidjian A, Harding LK, Walters G, Lee MR, Robb-Smith AH. Hypertension due to a renin-secreting renal tumour. Am J Med (1967) 43(6):963–76. doi: 10.1016/0002-9343(67)90256-2
2. Wong L, Hsu TH, Perlroth MG, Hofmann LV, Haynes CM, Katznelson L. Reninoma: case report and literature review. J Hypertension (2008) 26(2):368–73. doi: 10.1097/HJH.0b013e3282f283f3
3. Duan X, Bruneval P, Hammadeh R, Fresco R, Eble JN, Clark JI, et al. Metastatic juxtaglomerular cell tumor in a 52-year-old man. Am J Surg Pathol (2004) 28(8):1098–102. doi: 10.1097/01.pas.0000126722.29212.a7
4. Shera AH, Baba AA, Bakshi IH, Lone IA. Recurrent malignant juxtaglomerular cell tumor: A rare cause of malignant hypertension in a child. J Indian Assoc Pediatr Surgeons (2011) 16(4):152–4. doi: 10.4103/0971-9261.86876
5. Kuroda N, Gotoda H, Ohe C, Mikami S, Inoue K, Nagashima Y, et al. Review of juxtaglomerular cell tumor with focus on pathobiological aspect. Diagn Pathol (2011) 6:80. doi: 10.1186/1746-1596-6-80
6. Jiang Y, Hou G, Zhu Z, Zang J, Cheng W. Increased FDG Uptake on Juxtaglomerular Cell Tumor in the Left Kidney Mimicking Malignancy. Clin Nucl Med (2020) 45(3):252–54. doi: 10.1097/RLU.0000000000002924
7. Liu K, Wang B, Ma X, Li H, Zhang Y, Li J, et al. Minimally Invasive Surgery-Based Multidisciplinary Clinical Management of Reninoma: A Single-Center Study. Med Sci Monit: Int Med J Exp Clin Res (2019) 25:1600–10. doi: 10.12659/MSM.913826
8. Mete UK, Niranjan J, Kusum J, Rajesh LS, Goswami AK, Sharma S. Reninoma treated with nephron-sparing surgery. Urology (2003) 61(6):1259. doi: 10.1016/S0090-4295(03)00104-3
9. Sierra JT, Rigo D, Arancibia A, Mukdsi J, Nicolai S. Reninoma Masked by the Use of an Angiotensin Receptor Blocker. Iranian J Kidney Dis (2016) 10(6):413–15.
10. Hui GC, Tuncali K, Tatli S, Morrison PR, Silverman SG. Comparison of percutaneous and surgical approaches to renal tumor ablation: metaanalysis of effectiveness and complication rates. J Vasc Intervent Radiol: JVIR (2008) 19(9):1311–20. doi: 10.1016/j.jvir.2008.05.014
11. Krokidis ME, Orsi F, Katsanos K, Helmberger T, Adam A. CIRSE Guidelines on Percutaneous Ablation of Small Renal Cell Carcinoma. Cardiovasc Intervent Radiol (2017) 40(2):177–91. doi: 10.1007/s00270-016-1531-y
12. Branger N, Maurin C, Daniel L, André M, Coulange C, Vacher-Coponnat H, et al. [Treatment by radiofrequency ablation for a renin-secreting juxtaglomerular tumour: a case report]. Progres en urologie J l’Association francaise d’urologie la Societe francaise d’urologie (2014) 24(6):349–52. doi: 10.1016/j.purol.2013.10.001
13. Gil NS, Han JY, Ok SH, Shin IW, Lee HK, Chung YK, et al. Anesthetic management for percutaneous computed tomography-guided radiofrequency ablation of reninoma: a case report. Korean J Anesthesiol (2015) 68(1):78–82. doi: 10.4097/kjae.2015.68.1.78
14. Pedicini V, Gennaro N, Muglia R, Saita A, Casale P, Negro A, et al. Renin-dependent hypertension cured with percutaneous radiofrequency ablation. J Hypertension (2019) 37(3):653–56. doi: 10.1097/HJH.0000000000002035
15. Maiolino G, Battistel M, Barbiero G, Bisogni V, Rossi GP. Cure With Cryoablation of Arterial Hypertension Due to a Renin-Producing Tumor. Am J Hypertension (2018) 31(5):537–40. doi: 10.1093/ajh/hpx213
16. McCarthy CJ, Gervais DA. Decision Making: Thermal Ablation Options for Small Renal Masses. Semin Intervent Radiol (2017) 34(2):167–75. doi: 10.1055/s-0037-1602708
17. Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernández-Pello S, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur Urol (2019) 75(5):799–810. doi: 10.1016/j.eururo.2019.02.011
18. Miao D, Margolis CA. Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Vasc Interv Radiol (2018) 50: (9):1271–81. doi: 10.1016/j.jvir.2013.05.030
19. Crocetti L, Bozzi E, Faviana P, Cioni D, Della Pina C, Sbrana A, et al. Thermal ablation of lung tissue: in vivo experimental comparison of microwave and radiofrequency. Cardiovasc Intervent Radiol (2010) 33(4):818–27. doi: 10.1007/s00270-010-9869-z
20. Xing M, Kokabi N, Zhang D, Ludwig JM, Kim HS. Comparative Effectiveness of Thermal Ablation, Surgical Resection, and Active Surveillance for T1a Renal Cell Carcinoma: A Surveillance, Epidemiology, and End Results (SEER)-Medicare-linked Population Study. Radiology (2018) 288(1):81–90. doi: 10.1148/radiol.2018171407
21. Veltri A, Calvo A, Tosetti I, Pagano E, Genovesio A, Virzì V, et al. Experiences in US-guided percutaneous radiofrequency ablation of 44 renal tumors in 31 patients: analysis of predictors for complications and technical success. Cardiovasc Intervent Radiol (2006) 29(5):811–8. doi: 10.1007/s00270-005-0261-3
Keywords: reninoma, case report, secondary hypertension, radiofrequency ablation, contrast-enhanced ultrasound
Citation: Zhang R, Xu M and Xie X-y (2021) The Role of Real-Time Contrast-Enhanced Ultrasound in Guiding Radiofrequency Ablation of Reninoma: Case Report and Literature Review. Front. Oncol. 11:585257. doi: 10.3389/fonc.2021.585257
Received: 20 July 2020; Accepted: 06 January 2021;
Published: 23 February 2021.
Edited by:
Wei Yang, Peking University Cancer Hospital, ChinaReviewed by:
Guolin Ma, China-Japan Friendship Hospital, ChinaCopyright © 2021 Zhang, Xu and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Xu, eHVtaW5nOEBtYWlsLnN5c3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.