
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 16 April 2021
Sec. Pharmacology of Anti-Cancer Drugs
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.562315
 Shixue Chen1,2†
Shixue Chen1,2† Zhibo Zhang3†
Zhibo Zhang3† Xuan Zheng1,2
Xuan Zheng1,2 Haitao Tao1
Haitao Tao1 Sujie Zhang1
Sujie Zhang1 Junxun Ma1
Junxun Ma1 Zhefeng Liu1
Zhefeng Liu1 Jinliang Wang1
Jinliang Wang1 Yuanyu Qian1
Yuanyu Qian1 Pengfei Cui1,2
Pengfei Cui1,2 Di Huang1,4
Di Huang1,4 Ziwei Huang1,4
Ziwei Huang1,4 Zhaozhen Wu1,4
Zhaozhen Wu1,4 Yi Hu1*
Yi Hu1*Background: Immune checkpoint inhibitors targeting the PD-1/PD-L1 pathway have demonstrated promise in treating a variety of advanced cancers; however, little is known regarding their efficacy under various clinical situations, including different cancer types, treatment lines, drug combinations, and therapeutic regimens.
Methods: Published articles and conference abstracts (in English) in PubMed, Embase, the Cochrane Central Register, and Web of Science were searched up to February 10, 2020. The data were analyzed by the meta-analysis program in Stata.
Results: A total of 16,400 patients from 91 clinical trials were included in this meta-analysis. PD-1/PD-L1 inhibitors had a mean ORR of 19.56% (95% CI: 15.09–24.03), a median TTR of 2.05 months (m) (95%CI: 1.85–2.26), and a median DOR of 10.65 m (95%CI: 7.78–13.52). First-line treatment had a higher ORR (36.57% vs. 13.18%) but a shorter DOR (9.00 m vs. 13.42 m) compared to the second-line or subsequent treatment. Immunotherapy combined with chemotherapy (I+C) (46.81% [95%CI: 36.02–57.60]) had a statistically significant higher ORR compared to immunotherapy (I) (17.75% [95%CI: 14.47–21.03]) or immunotherapy combined with immunotherapy (I+O) (12.25% [95%CI: 1.56–22.94]), while I+C (8.09 m [95%CI: 6.86–9.32]) appeared to reduce the DOR compared to I (12.39 m [95%CI: 7.60–17.18]). PD-1 inhibitors were associated with better ORR (21.65% vs. 17.60%) and DOR (11.26 m vs. 10.03 m) compared to PD-L1 inhibitors. There were no significant differences in TTR under different situations.
Conclusions: PD-1/PD-L1 inhibitors were promising immunotherapeutic agents to achieve satisfactory response efficacies with different cancer types, treatment lines, drug combinations, and therapeutic regimens. This comprehensive summary of the response efficacy of PD-1/PD-L1 inhibitors serves as a reference for clinicians to make evidence-based decisions.
Cancer continues to be one of the most threatening diseases to human health and a leading cause of mortality worldwide (1). Some cancers are refractory to chemotherapy or targeted therapy. Blocking the immune checkpoint of programmed cell death-1 (PD-1) or programmed cell death-ligand 1 (PD-L1) receptor have led to great improvements in disease outcomes, and PD-1/PD-L1 inhibitors have emerged as frontline treatments for various cancers such as non-small cell lung cancer, metastatic melanoma, and renal cell carcinoma. PD-1 is a negative regulator with increased expression on reactive anti-tumor T cells, and its ligand PD-L1 is mainly expressed on the surface of tumor cells; the binding of PD-1 and PD-L1 can turn off the anti-tumor effect of T cells. Thus, PD-1/PD-L1 inhibitors can relieve immune suppression of anti-tumor T cells, which results in T cell proliferation, infiltration into the tumor microenvironment, and the induction of an anti-tumor response (2, 3). Unlike traditional anti-tumor treatments, PD-1/PD-L1 inhibitors have been reported to have a long-lasting anti-tumor response with reactivation of the immune system. While PD-1 and PD-L1 inhibitors have shown promise in advanced cancer immunotherapy, only a subset of patients can respond to this therapy, and the majority do not benefit. Thus, it is critical to understand the response efficacy of these drugs under various clinical situations.
Currently, two PD-1 inhibitors (nivolumab and pembrolizumab) and three PD-L1 inhibitors (atezolizumab, avelumab, and durvalumab) have been approved for the first-line or subsequent therapy of various cancer types. Hundreds of clinical trials of PD-1/PD-L1 inhibitors have reported response efficacy, including objective response rate (ORR: the proportion of patients experiencing complete response or partial response per RECIST v1.1 at any time during the study), time to response (TTR: the time from initiation of the treatment to the date of first documented complete or partial response), and duration of response (DOR: the time from first documented complete or partial response to disease progression or death). However, the reported response efficacies had substantial variations due to distinct clinical situations, such as different cancer types, drugs, treatment lines, and management practices among studies.
We therefore conducted a meta-analysis to comprehensively compare the response efficacy of PD-1/PD-L1 inhibitors in patients with a variety of cancer types, therapeutic regimens, treatment lines, and management strategies among published clinical trials.
We identified published clinical trials of PD-1/PD-L1 inhibitors that reported the response efficacy (ORR, TTR, and DOR) of advanced tumors from PubMed, Embase, the Cochrane Central Register, and Web of Science from their inceptions to February 10, 2020. The abstracts from major conference proceedings of the World Conference on Lung Cancer (WCLC), the American Society of Clinical Oncology (ASCO), the American Association for Cancer Research (AACR), and the European Society of Medical Oncology (ESMO) were also reviewed, with the search terms PD-1, PD-L1, PD-1 inhibitor, PD-L1 inhibitor, nivolumab, pembrolizumab, atezolizumab, avelumab, and durvalumab. We evaluated all search results according to the Preferred Reporting Items for Meta-Analyses (PRISMA) statement (4). Studies eligible for inclusion met all of the following criteria: (1) Clinical trials for advanced cancer therapy; (2) Patients were treated with a PD-1 or PD-L1 inhibitor; (3) Studies that reported at least one outcome of interest (ORR, TTR, or DOR); (4) Studies that were restricted to the English language. Letters, case reports, review articles, editorials, commentary articles, and expert opinions were excluded.
Data were extracted independently by three investigators (S.X. Chen, Z.B. Zhang, and X. Zheng) with a predefined information sheet. Any disagreements were discussed and resolved with a third investigator (P.F. Cui).
The data extracted from each included trial were the trial name or first author’s name, year of publication, name of publication, phase, cancer type, treatment line, stage, number of enrolled patients and responders, PD-1 and PD-L1 inhibitor used, drug management, ORR, TTR, DOR, and median follow-up time.
The study outcomes measured for response efficacy were ORR, TTR, and DOR. The treatment response in each clinical trial was determined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. ORR was recorded as a percentage. The time durations for TTR and DOR were recorded in unit of month.
Response efficacy (ORR, TTR, and DOR) among different cancer types, treatment lines, drug combinations, and therapeutic regimens.
Meta-analyses were conducted to synthesize the pooled ORR, TTR, and DOR by different clinical conditions, including cancer types, treatment lines, drug combinations, and therapeutic regimens. The heterogeneity of different outcomes was assessed by Q and I2. Both inverse-variance fixed- and random-effects meta-analyses were explored for each comparator. When P > 0.1 and I2 < 50%, it indicated that the studies were homogeneous, and a fixed effect model was selected. Otherwise, a random effect model was used (P < 0.1 or I2 > 50%). A Dersimonian-Laird random effects model was used to account for both within- and between-study heterogeneity when substantial heterogeneity was observed (5). Quality assessment of included studies was performed using the Cochrane Risk of Bias Tool for RCTs. Publication bias was accessed by funnel plot and Begg’s and Egger’s tests.
The ORR proportions were transformed to a logit scale (logit (z) = log (z)-log (1-z)) to calculate the 95% confidence interval (CI), and then transformed back to proportions. For TTR and DOR, we used elementary inequalities and approximations to estimate the mean and standard deviation from the median, range and sample size by verified formulas, which were distribution-free of the underlying data (6–8).
For TTR, DOR, and ORR, we utilized forest plots to describe the values or incidences and their 95% CIs by cancer type, treatment line, drug combination, and therapeutic regimen. The data were analyzed in Stata (version 15.0) using the “metafor” package. Non-overlapping 95% CIs of different subgroups were considered statistically significant from the forest plots.
A total of 6,619 relevant publications were identified from the database search, of which 6,373 were excluded for not meeting the inclusion criteria. Then, 246 publications were reviewed for full-text evaluation, of which 11 were duplicates and 121 reported neither available TTR nor DOR for analyses and were therefore excluded. In the end, a total of 91 clinical trials involving 16,400 patients were included in this meta-analysis (Figure 1) (9–98). The main characteristics of the 91 eligible trials are listed in Table S1. The numbers of trials for pembrolizumab, nivolumab, atezolizumab, avelumab, and durvalumab were 35, 33, 15, 4, and 4, respectively. The trials covered the treatments of various cancer types: 25 trials in lung cancer, 8 in melanoma, 19 in genitourinary cancer, 10 in head-and-neck cancer, 6 in breast cancer, 12 in gastrointestinal cancer, and 11 in other cancers (1 in skin cancer, 1 in brain cancer, 2 in Hodgkin lymphoma, 1 in soft-tissue sarcoma or bone sarcoma, 1 in thymic carcinoma, 2 in hepatocellular carcinoma, 1 in sarcoma, and 2 in malignant pleural mesothelioma). Among all selected trials, there were 19 trials for the first-line treatment, 51 for the second-line and subsequent treatments, and 23 trials covered the first-line as well as subsequent treatments. Eighty-three clinical trials were performed with single-agent PD-1/PD-L1 (I), 7 with PD-1/PD-L1 combined with chemotherapy (I+C), and 6 with PD-1/PD-L1 combined with other immune checkpoint inhibitors (I+O). In addition, 2 studies (17, 98) reported research data of first-line and non-first-line separately, and 5 studies (23, 26, 42, 54, 55) had both I and I+C therapy arms.
Based on the cancer type included in the 91 selected clinical trials, we divided these trials into 7 subgroups (Figure 2A). The subgroups with the highest ORR were observed in ‘others’ (32.34% [95%CI: 14.74–49.94]) and melanoma (29.03% [95%CI: 24.02–34.04]), followed by lung cancer (26.91% [95%CI: 15.44–38.38]) and genitourinary cancer (20.66% [95%CI: 16.87–24.45]). The mean ORR of melanoma was statistically significantly higher than that of the other three cancer types, gastrointestinal, head and neck, and breast cancer. Except for melanoma and head-and-neck cancer, the ORR of the first-line treatment was generally better than that of second-line or subsequent treatment, which had a statistically significant difference in ‘others’ and lung cancer. For the first-line treatment, the highest ORR was observed in ‘others’ (Merkel cell skin carcinoma, 62.07% [95%CI: 44.41–89.73]) and lung cancer (47.47% [95%CI: 39.66–55.27]), which was significantly higher than that of the other five cancer types.
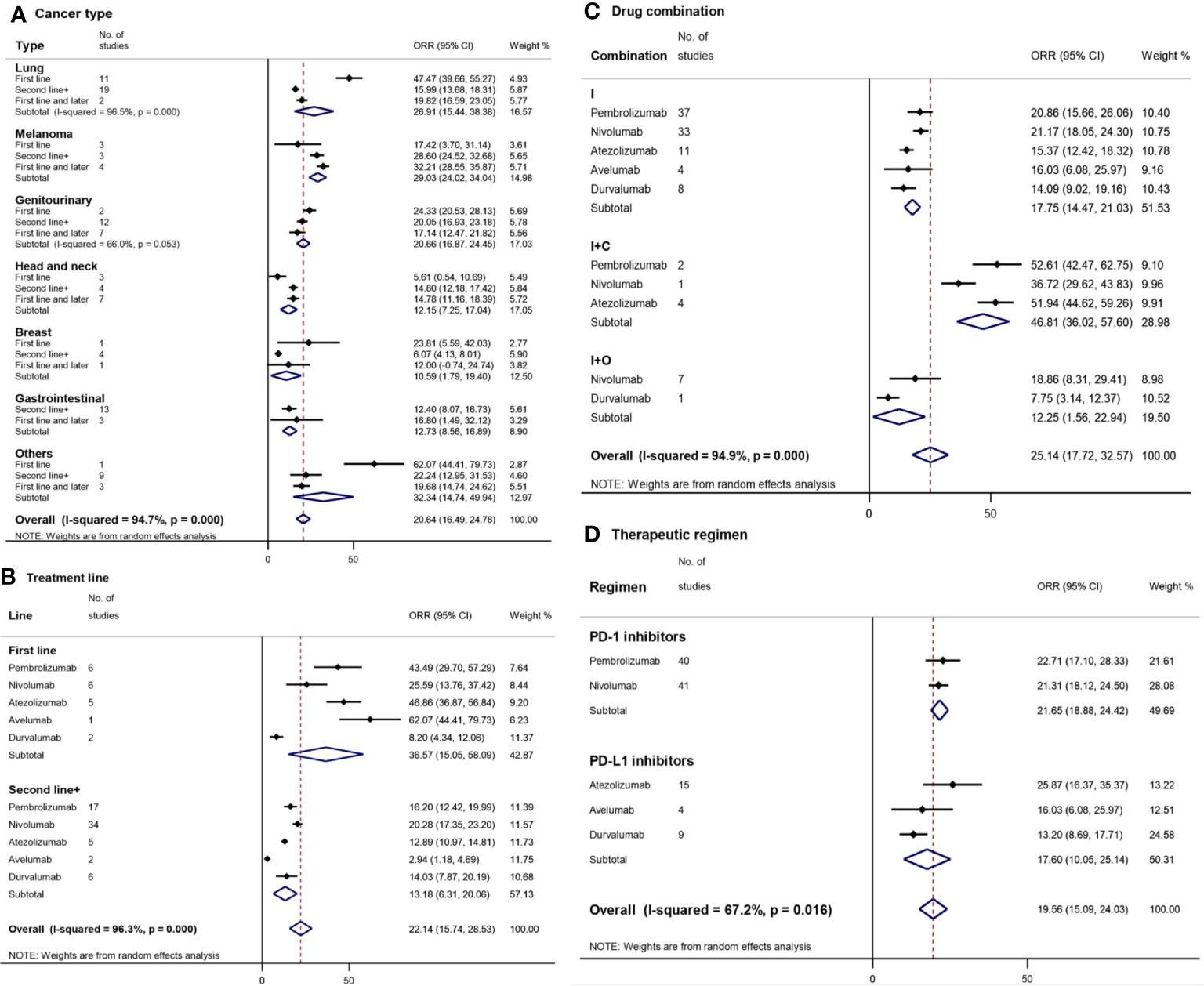
Figure 2 Forest plot for objective response rate (ORR) by cancer type (A), treatment line (B), drug combination (C), and therapeutic regimen (D). Squares represent the study-specific effect size (ORR). The area of the square is inversely proportional to the standard error of the study (and therefore indirectly to the sample size), and a larger area indicates greater weight in the calculation of the pooled effect size. The horizontal line crossing the square represents the 95% CI. The diamonds represent the estimated overall effect based on the meta-analysis. CI, confidence interval.
In order to compare the exact differences between the first-line treatment and the second-line or subsequent treatment, clinical trials (N = 23) simultaneously covering first-line as well as subsequent line treatment were excluded. The overall mean ORR of the first-line treatment (36.57% [95%CI: 15.05–58.09]) with PD-1/PD-L1 inhibitors was higher compared to the second-line or subsequent treatment (13.18% [95%CI: 6.31–20.06]) (Figure 2B). Pembrolizumab, atezolizumab, and avelumab had a statistically significantly higher ORR of the first-line treatment (43.49% [95%CI: 29.70–57.29], 46.86% [95%CI: 36.87–56.84], and 62.07% [95%CI: 44.41–79.73], respectively) compared to that of the second-line or subsequent treatment (16.20% [12.42–29.99], 12.89% [10.97–14.81], and 2.94% [1.18–4.69], respectively).
Avelumab and atezolizumab had the highest ORR among the first-line treatments, while nivolumab (20.28% [17.35–23.20]) and pembrolizumab had the highest ORR among the second-line or subsequent treatments.
I+C (46.81% [95%CI: 36.02–57.60]) had a statistically significantly higher ORR compared to I (17.75% [95%CI: 14.47–21.03]) or I+O (12.25% [95%CI: 1.56–22.94]) (Figure 2C). Among I+C therapies, atezolizumab (51.94% [95%CI: 44.62–59.26]) had a statistically significantly higher ORR compared to nivolumab (36.72% [95%CI: 29.62–43.83]). No statistically significant differences were found in ORR between different drugs in each subgroup.
The overall mean ORR of PD-1/PD-L1 inhibitors was 19.56% with a 95%CI of 15.09–24.03% (Figure 2D). The ORR of PD-1 inhibitors (21.65% [95%CI: 18.88–24.42]) was higher than that of PD-L1 inhibitors (17.60% [95%CI: 10.05–25.14]). Atezolizumab (25.67% [95%CI: 16.37–35.37]) and durvalumab (13.20% [95%CI: 8.69–17.71]) had the highest and lowest ORR, respectively.
The shortest and longest TTR were found in ‘others’ (1.66 months (m) with a 95%CI of 1.32–2.00 m) and breast cancer (2.46 m [95%CI: 0.83–4.09]), respectively (Figure 3A), which were similar to the TTR of other cancer types. There were also no significant differences in TTR either between the first-line treatment and the second-line or subsequent treatments of each cancer type.
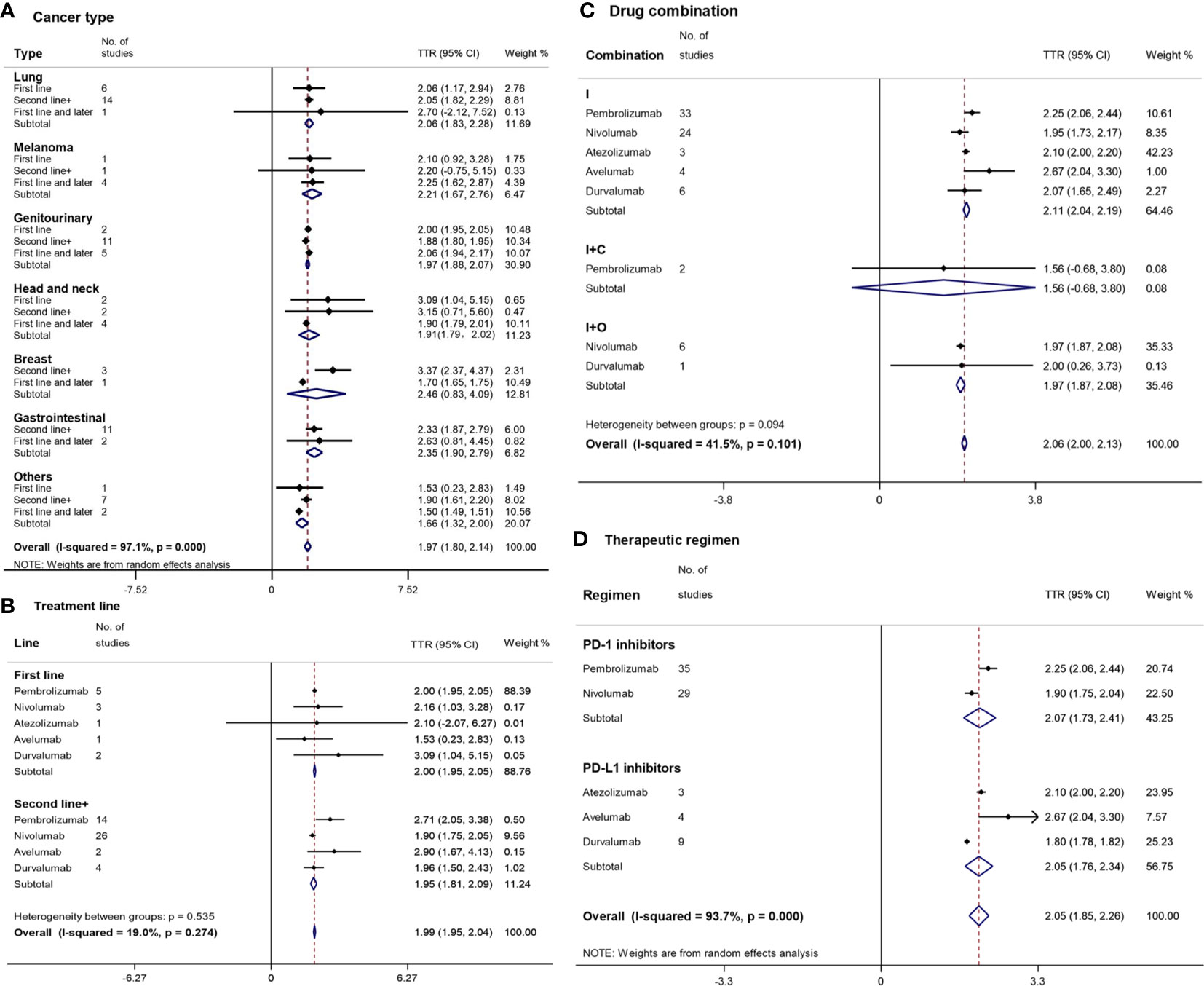
Figure 3 Forest plot for time to response (TTR) by cancer type (A), treatment line (B), drug combination (C), and therapeutic regimen (D). Squares represent the study-specific effect size (TTR). The area of a square is inversely proportional to the standard error of the study (and therefore indirectly to the sample size), and a larger area indicates greater weight in the calculation of the pooled effect size. The horizontal line crossing the square represents the 95% CI. The diamonds represent the estimated overall effect based on the meta-analysis. CI, confidence interval.
The median TTR of PD-1/PD-L1 inhibitors in the first-line treatment was similar to that in the second-line treatment (Figure 3B). Except for nivolumab (1.90 m [95%CI: 1.75–2.05]), which had a shorter TTR than pembrolizumab (2.71 m [95%CI: 2.05–3.38]), there was no other statistically significant difference in TTR among various drugs and treatment lines. The shortest and longest TTR were found in avelumab (1.53 m [95%CI: 0.23–2.83]) and durvalumab (3.09 m [95%CI: 1.04–5.15]) of the first-line treatments, respectively.
I+C (1.56 m [95%CI: 0.68–3.80]) had a shorter TTR than I+O (1.97 m [95%CI: 1.87–2.08]) and I (2.11 m [95%CI: 2.04–2.19]) without a statistically significant difference (Figure 3C). Nivolumab (1.95 m [95%CI: 1.73–2.17]) had the shortest TTR in the monotherapy subgroup.
The overall median TTR of PD-1/PD-L1 inhibitors was 2.05 m with a 95%CI of 1.85 m to 2.26 m (Figure 3D). PD-1 inhibitors (2.07 m [95%CI: 1.73–2.41]) and PD-L1 inhibitors (2.05 m [95%CI: 1.85–2.26]) had a similar TTR. The TTRs of durvalumab (1.80 m [95%CI: 1.78–1.82]) and nivolumab (1.90 m [95%CI: 1.75–2.04]) were statistically significantly shorter than those of pembrolizumab (2.25 m [95%CI: 2.06–2.44]) and avelumab (2.67 m [95% CI: 2.04–3.30]).
The longest DORs were observed for breast cancer (15.80 m [95%CI: 9.52–22.08]) and melanoma (14.58 m [95%CI: 0.14–29.30]), followed by genitourinary cancer (13.30 m [95%CI: 10.13–16.46]), ‘others’ (12.17 m [95%CI: 2.67–21.67]), head-and-neck cancer (11.08 m [95%CI: 6.16–16.01]), gastrointestinal cancer (10.85 m [95%CI: 7.63–14.07]), and lung cancer (8.41 m [95%CI: 7.23–9.59]) (Figure 4A). There were no statistically significant differences in DOR between various cancer types except for the difference between genitourinary cancer and lung cancer. In addition, no statistically significant differences were found between different treatment lines in each cancer type.
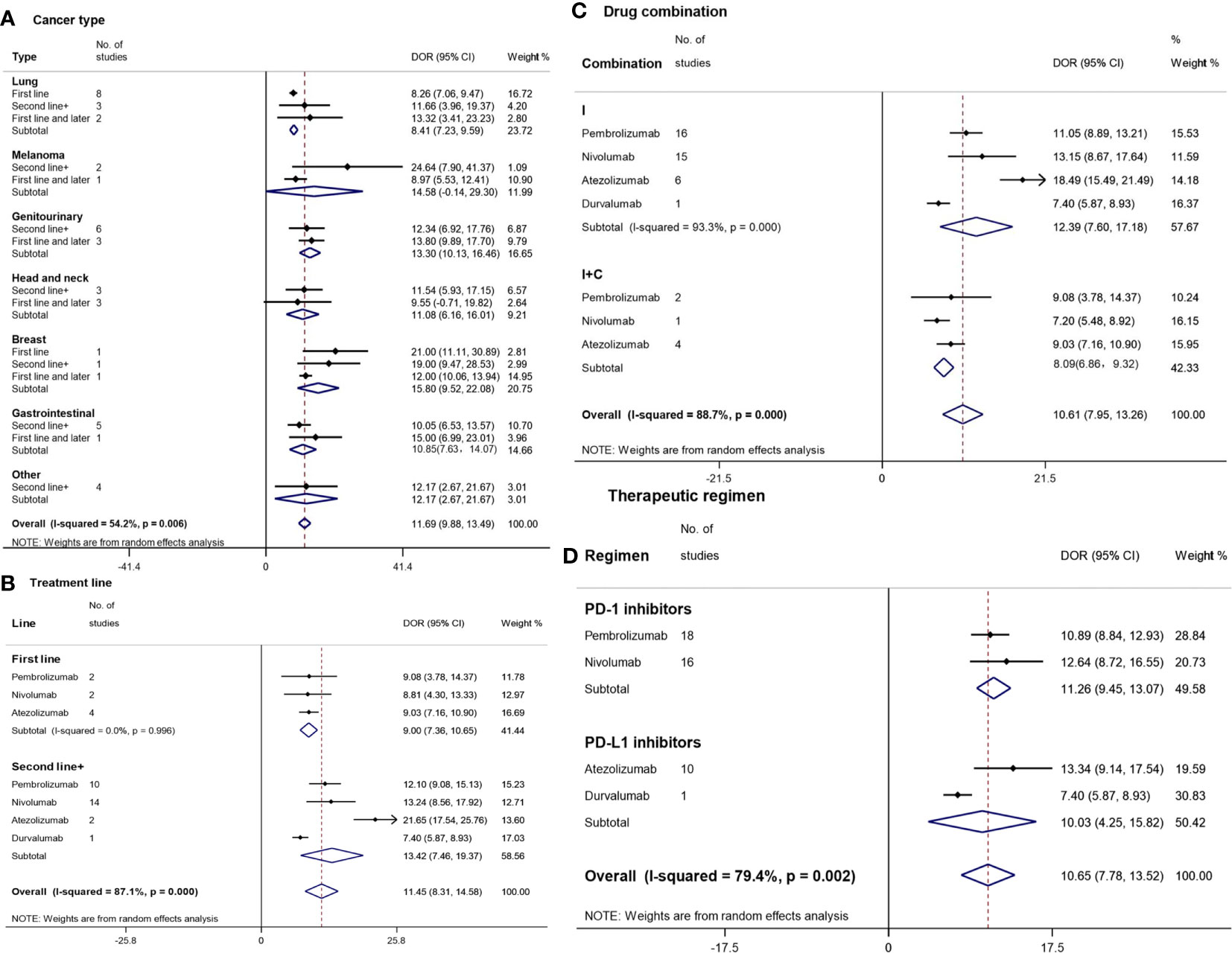
Figure 4 Forest plot for duration of response (DOR) by cancer type (A), treatment line (B), drug combination (C), and therapeutic regimen (D). Squares represent study-specific effect sizes (DOR). The area of a square is inversely proportional to the standard error of the study (and therefore indirectly to the sample size), and a larger area indicates greater weight in the calculation of the pooled effect size. The horizontal line crossing the square represents the 95% CI. The diamonds represent the estimated overall effect based on the meta-analysis. CI, confidence interval.
The DOR of the second and subsequent treatments (13.42 m [95%CI: 7.46–21.67]) was unexpectedly longer than that of the first-line treatment (9.00 m [95%CI: 7.36–10.65]) (Figure 4B). The longest and shortest TTRs were found in atezolizumab (21.65 m [95%CI: 17.54–25.76]) and durvalumab (7.40 m [95%CI: 5.87–8.93]) among the second and subsequent treatments, respectively, which had a statistically significant difference.
Patients treated with I (12.39 m [95%CI: 7.60–17.18]) had a longer DOR compared with those treated with I+O (8.09 m [95%CI: 6.86–9.32]), though there was no significant difference (Figure 4C). In the monotherapy subgroup, the longest DOR was observed for patients treated with atezolizumab (21.65 m [95%CI: 17.54–25.76]), followed by those treated with nivolumab (13.15 m [95%CI: 8.67–17.64]), pembrolizumab (11.05 m [95%CI: 8.89–13.21]), and durvalumab (7.40 m [95%CI: 5.87–8.93]).
The overall median DOR of PD-1/PD-L1 inhibitors was 10.65 m (95%CI: 7.78–13.52) (Figure 4D). PD-1 inhibitors (11.26 m [95%CI: 9.45–13.07]) and PD-L1 inhibitors (10.03 m [95%CI: 4.25–15.82]) had similar TTRs. The longest DOR was observed for atezolizumab (13.34 m [95%CI: 9.14–17.54]), followed by nivolumab (12.64 m [95%CI: 8.72–16.55]), pembrolizumab (10.89 m [95%CI: 8.84–12.93]), and durvalumab (7.40 m [95%CI: 5.87–8.93]).
Quality assessment of the 28 included RCTs was performed using the Cochrane Risk of Bias Tool (Figure S1). Due to lack of appropriate evaluation tools, the risk of bias of the 63 single arm clinical trials was not estimated. Funnel plots (Figures S2–4), Begg’s tests, and Egger’s tests (Table S2) for the majority of treatments demonstrated symmetrical results (P > 0.05), while a few treatments were asymmetrical due to the heterogeneity of the drugs and doses, intention to treat populations, or lack of contrast cohorts in some studies. However, this meta-analysis is still suggested to be sufficiently effective considering the following: (1) it included a large number of high-quality studies; (2) we conducted subgroup analysis under various clinical situations; and (3) we adopted statistical methods with high test efficiencies. Further prospective, randomized control trials are warranted to reduce publication bias and for validation.
Targeting the PD-1 and PD-L1 pathway is an important new approach to cancer therapy. PD-1/PD-L1 inhibitors can reactivate T cells to work against cancer cells, though they depend on pre-existing anti-tumor T cells. The mechanism of response involves infiltrating T cells and engaging their receptors to recognize tumor antigens and trigger the expression of PD-1 on T cells and PD-L1 on cancer cells; this can be simply reversed by blocking PD-1/PD-L1 (99). Anti-PD-1/PD-L1 treatments are optional for cancer patients, and only a portion of patients benefit from this immunotherapy. Patients may lack immune activation factors such as tumor antigens and pre-existing anti-tumor T cells (CD8+T cells) in the tumor, which may weaken the anti-tumor effect of PD-1/PD-L1 inhibitors. PD-1 blockade is unlikely to work if there are no CD8+T cells to be inhibited by PD-1 and PD-L1 interaction in the tumor microenvironment (100, 101).
Immune-combination therapies are often used to improve anti-tumor efficacy. In addition to the combination of chemotherapy or targeted therapy with PD-1/PD-L1 inhibitors, promising therapeutic options, such as the combination of oncolytic viruses, have shown anti-tumor activity for patients who fail to respond or achieve durable responses following immunotherapy (102–104). Oncolytic viruses are tumor specific and have the advantage of triggering anti-tumor immune responses in the tumor microenvironment, so the combination of oncolytic viruses and PD-1/PD-L1 inhibitors may be a useful strategy for future cancer treatment (102–104).
Due to the reactivation of the immune system and the prolonged response once immunotherapy works, clinicians are interested in response efficacy metrics (ORR, TTR, and DOR) other than overall survival (OS, time from initiation of the treatment to death from any cause) and progression-free survival (PFS, time from initiation of the treatment to disease progression or death from any cause, whichever occurred first). The emerging use of PD-1/PD-L1 inhibitors in clinical practice highlights an urgent need for evidence-based analysis to understand their exact response efficacy. Data from multiple clinical trials suggest that the wide range of response efficacy can be attributed to either variability in therapeutic regimens or differences due to drug combinations. Other significant factors are the heterogeneity in distinct cancer types and treatment lines. This is the first meta-analysis to study the response efficacy of PD-1/PD-L1 inhibitors under various clinical situations, including cancer type, treatment line, therapeutic regimen, and medication combination. This comprehensive response efficacy analysis provides clinicians with an important reference and guidelines for making clinical decisions.
When pooling quantitative data in a meta-analysis, it is necessary to know the mean and standard deviation in every single study. However, obtaining original individual patient data from numerous clinical trials in order to calculate the means and standard deviations is difficult. Thus, this meta-analysis introduced a method of estimating the mean and standard deviation based on the median, range, and sample size (6, 7). Therefore, quantitative data without means and standard deviations can also be used in a meta-analysis without loss of relative accuracy.
Our pooled analysis showed that PD-1/PD-L1 inhibitors had a mean ORR of 19.56%, a median TTR of 2.05 m, and a median DOR of 10.65 m overall. The mean ORR ranged from 10.59% to 32.34% among various cancer types, while ‘others’, melanoma, and lung cancer had relatively high ORRs of about 30%. The highest ORR was 62% observed in ‘others’ from a study of Merkel cell skin carcinoma (27). The DORs for breast cancer and melanoma lasted about 15 months, approximately twice the DOR for lung cancer (8.41 m), but was not significantly different due to the overlapping 95% CI. These results indicate that PD-1/PD-L1 inhibitors can induce effective anti-tumor responses in a wide range of tumor types.
PD-1/PD-L1 inhibitors had a higher ORR for the first-line treatment compared to the second-line or subsequent treatment (36.57% vs. 13.18%). In the first-line treatment, avelumab (62.07%), atezolizumab (46.86%), and pembrolizumab (43.49%) showed favorable ORRs of over 40%. In addition, the inclusion of clinical studies using immunotherapy combined with chemotherapy as the first-line treatment was one reason for the improvement of ORR. Unlike ORR, the DOR of second-line or subsequent treatments was unexpectedly 4.42 m better than that of the first-line treatment (13.42 m vs. 9.00 m). This suggests that immunotherapy can sustain a prolonged response regardless of the treatment lines. However, the optimal time to initiate the PD-1/PD-L1 inhibitors requires further study. The longest DOR reached 21.65 m for atezolizumab as a second-line or subsequent treatment.
The addition of other therapies, such as chemotherapy, radiotherapy, or immunotherapy, is intended to improve the response efficiency of PD-1/PD-L1 monotherapy in clinical trials (105, 106). We found that being combined with chemotherapy (I+C) could significantly improve the ORR compared to monotherapy (I) by 29% (46.81% vs. 17.75%), while the addition of other immunotherapies (I+O) reduced the ORR compared with monotherapy (I) by 5% (12.25% vs. 17.75%). This difference might be partly due to the cytotoxicity of chemotherapy, which enabled immune cells to be activated by the increased antigens released by tumor cells. I+C (1.56 m) also appeared to reduce the time to response with a shorter TTR compared to I+O (1.97 m) and I (2.11 m), although there were no significant differences among the three groups. However, the addition of chemotherapy (I+C) significantly shortened the DOR compared to I by 4.3 m, but there was no pooled DOR of I+O due to the unavailability of relevant studies. Chemotherapy drugs can damage immune cells while killing tumor cells, which may be one reason for the shorter duration. Thus, the combination of immunotherapy and other therapies should be further studied in terms of treatment dosing, cycle, and medication order, so as to find the best way to achieve a synergistic effect.
We divided PD-1/PD-L1 inhibitors into PD-1 and PD-L1 groups to observe their response efficacy according to their different functional targets. PD-1 inhibitors were associated with better ORR (21.65% vs. 17.60%) and DOR (11.26 m vs. 10.03 m) compared to PD-L1 inhibitors. Among all the PD-1/PD-L1 inhibitors, atezolizumab had the best ORR (25.87%) and DOR (13.34 m), while durvalumab had the poorest ORR (13.20%) and DOR (7.40 m). These results demonstrate that the efficiency of different drugs could vary greatly even when they act on the same target, which should be considered carefully when selecting drugs in the clinic. As for TTR, there were no significant differences among different cancer types, treatment lines, drug combinations, or therapeutic regimens. The median TTR was about 2 months.
A strength of this work is that we estimated the response efficacy of nearly 100 clinical trials and comprehensively analyzed their different clinical situations. We collected all available high-quality clinical trials for PD-1/PD-L1 from phases I to III. Thus, this meta-analysis can overcome the problem of inadequate power of each individual trial by pooling data together and minimizing inter-study heterogeneity. Furthermore, it is difficult to analyze TTR or DOR because a majority of clinical trials only provided the median and 95% CI without showing hazard ratios (HR) or individual patient data. Thus, we used a method of estimating the mean and standard deviation based on the median, range, and sample size. It has been reported that quantitative data without available means and standard deviations can be used in a meta-analysis without loss of accuracy (6, 7).
There are several limitations in this meta-analysis. First, the analysis was based on published results rather than on individual patient data. The estimation of means by using the median values will inevitably reduce precision. Second, the heterogeneity of many indicators can cause inaccuracy and publication biases existed among some outcomes. Third, we still lacked data from head-to-head comparisons, although the subgroups were divided in the meta-analysis. Therefore, caution should be exercised in interpreting the results of this study. Further prospective, randomized control trials are warranted for validation.
This systematic meta-analysis determined the response efficacy of PD-1/PD-L1 inhibitors with different cancer types, treatment lines, drug combinations, and therapeutic regimens. PD-1/PD-L1 inhibitors are promising treatment modalities for various cancers with the potential for long-term clinical benefits. This large-scale meta-analysis of response efficacy can serve as a reference for clinicians to make evidence-based decisions.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
YH conceived and designed research. Data collection and extraction was performed by SC, ZZ, and XZ, and verified by PC. Statistical analysis was performed by HT, SZ, DH, ZH, ZW, and JM. ZL, JW, and YQ participated in drafting article. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.562315/full#supplementary-material
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science (2018) 359(6382):1350–5. doi: 10.1126/science.aar4060
3. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol (2018) 8:86. doi: 10.3389/fonc.2018.00086
4. Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Cranio Maxill Surg (2011) 39(2):91–2. doi: 10.1016/j.jcms.2010.11.001
5. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials (1986) 7(3):177–88. doi: 10.1016/0197-2456(86)90046-2
6. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol (2005) 5(1):13. doi: 10.1186/1471-2288-5-13
7. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol (2014) 14(1):135. doi: 10.1186/1471-2288-14-135
8. Chen C, Zhang F, Zhou N, Gu Y-M, Zhang Y-T, He Y-D, et al. Efficacy and safety of immune checkpoint inhibitors in advanced gastric or gastroesophageal junction cancer: a systematic review and meta-analysis. OncoImmunology (2019) 8(5):e1581547. doi: 10.1080/2162402X.2019.1581547
9. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med (2018) 379(21):2040–51. doi: 10.1056/nejmoa1810865
10. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
11. Cappuzzo F, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter H, et al. LBA53 IMpower130: progression-free survival (PFS) and safety analysis from a randomised Phase III study of carboplatin+ nab-paclitaxel (CnP) with or without atezolizumab (atezo) as first-line (1L) therapy in advanced non-squamous NSCLC. Ann Oncol (2018) 29(suppl_8):mdy424. 065. doi: 10.1093/annonc/mdy424.065
12. Jotte RM, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Abreu DR, Hussein MA, et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab+ carboplatin+ paclitaxel or nab-paclitaxel vs carboplatin+ nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol (2018) 36(18 suppl):LBA9000. doi: 10.1200/JCO.2018.36
13. Papadimitrakopoulou V, Cobo M, Bordoni R, Dubray-Longeras P, Szalai Z, Ursol G, et al. IMPOWER132: PFS and safety results with 1L atezolizumab+ carboplatin/cisplatin+ pemetrexed in stage IV non-squamous NSCLC. J Thorac Oncol (2018) 13(10):S332–33. doi: 10.1016/j.jtho.2018.08.262
14. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med (2018) 378(24):2288–301. doi: 10.1056/nejmoa1716948
15. Borghaei H, Hellmann MD, Paz-Ares LG, Ramalingam SS, Reck M, O’Byrne KJ, et al. Nivolumab (Nivo)+ platinum-doublet chemotherapy (Chemo) vs chemo as first-line (1L) treatment (Tx) for advanced non-small cell lung cancer (NSCLC) with< 1% tumor PD-L1 expression: Results from CheckMate 227. ASCO (2018) 36(15_suppl):9001. doi: 10.1200/JCO.2018.36.15_suppl.9001
16. Hellmann MD, Ciuleanu T-E, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med (2018) 378(22):2093–104. doi: 10.1056/NEJMoa1801946
17. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med (2015) 372(21):2018–28. doi: 10.1056/NEJMoa1501824
18. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med (2015) 372(4):320–30. doi: 10.1056/NEJMoa1412082
19. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774
20. Mok TS, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (2019) 393(10183):1819–30. doi: 10.1016/S0140-6736(18)32409-7
21. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non–small-cell lung cancer. N Engl J Med (2017) 376(25):2415–26. doi: 10.1097/01.COT.0000525227.51402.c5
22. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (2017) 389(10088):2492–502. doi: 10.1016/S0140-6736(17)31046-2
23. Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol (2018) 19(11):1480–92. doi: 10.1016/S1470-2045(18)30700-9
24. Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol (2017) 18(11):1483–92. doi: 10.1016/S1470-2045(17)30616-2
25. Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet (2017) 389(10064):67–76. doi: 10.1016/S0140-6736(16)32455-2
26. Siu LL, Even C, Mesía R, Remenar E, Daste A, Delord J-P, et al. Safety and Efficacy of Durvalumab With or Without Tremelimumab in Patients With PD-L1–Low/Negative Recurrent or Metastatic HNSCC: The Phase 2 CONDOR Randomized Clinical Trial. JAMA Oncol (2019) 5(2):195–203. doi: 10.1001/jamaoncol.2018.4628
27. D’Angelo SP, Russell J, Lebbé C, Chmielowski B, Gambichler T, Grob J-J, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol (2018) 4(9):e180077–e. doi: 10.1001/jamaoncol.2018.0077
28. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
29. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med (2015) 373(2):123–35. doi: 10.1056/NEJMoa1504627
30. Wu Y-L, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J Thorac Oncol (2019) 14(5):867–75. doi: 10.1016/j.jtho.2019.01.006
31. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet (2016) 387(10030):1837–46. doi: 10.1016/S0140-6736(16)00587-0
32. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, Von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (2017) 389(10066):255–65. doi: 10.1016/S0140-6736(16)32517-X
33. Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, et al. Overall survival and long-term safety of nivolumab (anti–programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non–small-cell lung cancer. J Clin Oncol (2015) 33(18):2004–12. doi: 10.1200/JCO.2014.58.3708
34. Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol (2015) 16(3):257–65. doi: 10.1016/S1470-2045(15)70054-9
35. Lee JS, Lee KH, Cho EK, Kim DW, Kim SW, Kim JH, et al. Nivolumab in advanced non-small-cell lung cancer patients who failed prior platinum-based chemotherapy. Lung Cancer (2018) 122:234–42. doi: 10.1016/j.lungcan.2018.05.023
36. Horn L, Gettinger SN, Gordon MS, Herbst RS, Gandhi L, Felip E, et al. Safety and clinical activity of atezolizumab monotherapy in metastatic non-small-cell lung cancer: final results from a phase I study. Eur J Cancer (2018) 101:201–9. doi: 10.1016/j.ejca.2018.06.031
37. Garassino MC, Cho B-C, Kim J-H, Mazières J, Vansteenkiste J, Lena H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol (2018) 19(4):521–36. doi: 10.1016/S1470-2045(18)30144-X
38. Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol (2014) 32(10):1020–30. doi: 10.1200/JCO.2013.53.0105
39. McDermott DF, Drake CG, Sznol M, Choueiri TK, Powderly JD, Smith DC, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol (2015) 33(18):2013–20. doi: 10.1200/JCO.2014.58.1041
40. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med (2015) 373(19):1803–13. doi: 10.1056/NEJMoa1510665
41. Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol (2015) 33(13):1430–7. doi: 10.1200/JCO.2014.59.0703
42. Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol (2016) 17(7):883–95. doi: 10.1016/S1470-2045(16)30098-5
43. Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med (2016) 375(19):1856–67. doi: 10.1056/NEJMoa1602252
44. Sharma P, Callahan MK, Bono P, Kim J, Spiliopoulou P, Calvo E, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol (2016) 17(11):1590–8. doi: 10.1016/S1470-2045(16)30496-X
45. Weber J, Gibney G, Kudchadkar R, Yu B, Cheng P, Martinez AJ, et al. Phase I/II study of metastatic melanoma patients treated with nivolumab who had progressed after ipilimumab. Cancer Immunol Res (2016) 4(4):345–53. doi: 10.1158/2326-6066.CIR-15-0193
46. Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol (2016) 34(21):2460–7. doi: 10.1200/JCO.2015.64.8931
47. Kang Y-K, Boku N, Satoh T, Ryu M-H, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2017) 390(10111):2461–71. doi: 10.1016/S0140-6736(17)31827-5
48. Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol (2017) 18(5):631–9. doi: 10.1016/S1470-2045(17)30181-X
49. Larkin J, Minor D, D’Angelo S, Neyns B, Smylie M, Miller WH, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol (2018) 36(4):383–90. doi: 10.1200/JCO.2016.71.8023
50. Ready N, Farago AF, de Braud F, Atmaca A, Hellmann MD, Schneider JG, et al. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol (2019) 14(2):237–44. doi: 10.1016/j.jtho.2018.10.003
51. Maruyama D, Hatake K, Kinoshita T, Fukuhara N, Choi I, Taniwaki M, et al. Multicenter phase II study of nivolumab in Japanese patients with relapsed or refractory classical Hodgkin lymphoma. Cancer Sci (2017) 108(5):1007–12. doi: 10.1111/cas.13230
52. Morris VK, Salem ME, Nimeiri H, Iqbal S, Singh P, Ciombor K, et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study. Lancet Oncol (2017) 18(4):446–53. doi: 10.1016/S1470-2045(17)30104-3
53. Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol (2018) 36(14):1428–39. doi: 10.1200/JCO.2017.76.0793
54. D’Angelo SP, Mahoney MR, Van Tine BA, Atkins J, Milhem MM, Jahagirdar BN, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol (2018) 19(3):416–26. doi: 10.1016/S1470-2045(18)30006-8
55. Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J Clin Oncol (2018) 36(28):2836–44. doi: 10.1200/JCO.2017.76.6212
56. Ma BB, Lim W-T, Goh B-C, Hui EP, Lo K-W, Pettinger A, et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an international, multicenter study of the Mayo Clinic phase 2 consortium (NCI-9742). J Clin Oncol (2018) 36(14):1412–8. doi: 10.1200/JCO.2017.77.0388
57. Quispel-Janssen J, van der Noort V, de Vries JF, Zimmerman M, Lalezari F, Thunnissen E, et al. Programmed death 1 blockade with nivolumab in patients with recurrent malignant pleural mesothelioma. J Thorac Oncol (2018) 13(10):1569–76. doi: 10.1016/j.jtho.2018.05.038
58. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med (2015) 372(26):2509–20. doi: 10.1056/NEJMoa1500596
59. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med (2015) 372(26):2521–32. doi: 10.1056/NEJMoa1503093
60. Chow LQ, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol (2016) 34(32):3838–45. doi: 10.1200/JCO.2016.68.1478
61. Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol (2016) 17(6):717–26. doi: 10.1016/S1470-2045(16)00175-3
62. Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol (2016) 17(7):956–65. doi: 10.1016/S1470-2045(16)30066-3
63. Alley EW, Lopez J, Santoro A, Morosky A, Saraf S, Piperdi B, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol (2017) 18(5):623–30. doi: 10.1016/S1470-2045(17)30169-9
64. Bellmunt J, De Wit R, Vaughn DJ, Fradet Y, Lee J-L, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med (2017) 376(11):1015–26. doi: 10.1056/NEJMoa1613683
65. Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, et al. Pembrolizumab for platinum-and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol (2017) 35(14):1542–9. doi: 10.1200/JCO.2016.70.1524
66. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz H-J, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol (2017) 18(9):1182–91. doi: 10.1016/S1470-2045(17)30422-9
67. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol (2017) 18(3):312–22. doi: 10.1016/S1470-2045(17)30065-7
68. Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, et al. Safety and Antitumor Activity of the Anti–Programmed Death-1 Antibody Pembrolizumab in Patients With Advanced Esophageal Carcinoma. J Clin Oncol (2018) 36(1):61–7. doi: 10.1200/JCO.2017.74.9846
69. Frenel J-S, Le Tourneau C, O’Neil B, Ott PA, Piha-Paul SA, Gomez-Roca C, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1–positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol (2017) 35(36):4035–41. doi: 10.1200/JCO.2017.74.5471
70. Hsu C, Lee S-H, Ejadi S, Even C, Cohen RB, Le Tourneau C, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1–positive nasopharyngeal carcinoma: Results of the KEYNOTE-028 study. J Clin Oncol (2017) 35(36):4050–6. doi: 10.1200/JCO.2017.73.3675
71. Lukas RV, Rodon J, Becker K, Wong ET, Shih K, Touat M, et al. Clinical activity and safety of atezolizumab in patients with recurrent glioblastoma. J Neurooncol (2018) 140(2):317–28. doi: 10.1007/s11060-018-2955-9
72. Petrylak DP, Powles T, Bellmunt J, Braiteh F, Loriot Y, Morales-Barrera R, et al. Atezolizumab (MPDL3280A) monotherapy for patients with metastatic urothelial cancer: long-term outcomes from a phase 1 study. JAMA Oncol (2018) 4(4):537–44. doi: 10.1001/jamaoncol.2017.5440
73. Shimizu T, Seto T, Hirai F, Takenoyama M, Nosaki K, Tsurutani J, et al. Phase 1 study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced solid tumors. Invest New Drugs (2016) 34(3):347–54. doi: 10.1007/s10637-016-0347-6
74. Powles T, Durán I, Van Der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet (2018) 391(10122):748–57. doi: 10.1016/S0140-6736(17)33297-X
75. Apolo AB, Infante JR, Balmanoukian A, Patel MR, Wang D, Kelly K, et al. Avelumab, an anti–programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase ib study. J Clin Oncol (2017) 35(19):2117. doi: 10.1200/JCO.2016.71.6795
76. Bang Y-J, Ruiz EY, Van Cutsem E, Lee K-W, Wyrwicz L, Schenker M, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol (2018) 29(10):2052–60. doi: 10.1093/annonc/mdy264
77. Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau H-T, Forero-Torres A, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat (2018) 167(3):671–86. doi: 10.1007/s10549-017-4537-5
78. Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, et al. Safety and efficacy of durvalumab (MEDI4736), an anti–programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol (2016) 34(26):3119–25. doi: 10.1200/JCO.2016.67.9761
79. Powles T, O’donnell PH, Massard C, Arkenau H-T, Friedlander TW, Hoimes CJ, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol (2017) 3(9):e172411. doi: 10.1001/jamaoncol.2017.2411
80. O’Neil BH, Wallmark JM, Lorente D, Elez E, Raimbourg J, Gomez-Roca C, et al. Safety and antitumor activity of the anti–PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PloS One (2017) 12(12):e0189848. doi: 10.1371/journal.pone.0189848
81. Plimack ER, Bellmunt J, Gupta S, Berger R, Chow LQ, Juco J, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol (2017) 18(2):212–20. doi: 10.1016/S1470-2045(17)30007-4
82. Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol (2017) 18(11):1493–501. doi: 10.1016/S1470-2045(17)30624-1
83. Yamazaki N, Takenouchi T, Fujimoto M, Ihn H, Uchi H, Inozume T, et al. Phase 1b study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced melanoma (KEYNOTE-041). Cancer Chemother Pharmacol (2017) 79(4):651–60. doi: 10.1007/s00280-016-3237-x
84. Adams S, Schmid P, Rugo H, Winer E, Loirat D, Awada A, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol (2018) 30(3):397–404. doi: 10.1093/annonc/mdy517
85. Cohen RB, Delord J-P, Doi T, Piha-Paul SA, Liu SV, Gilbert J, et al. Pembrolizumab for the treatment of advanced salivary gland carcinoma: findings of the phase 1b KEYNOTE-028 study. Am J Clin Oncol (2018) 41(11):1083–8. doi: 10.1097/COC.0000000000000429
86. Cohen EE, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn M-J, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet (2019) 393(10167):156–67. doi: 10.1016/S0140-6736(18)31999-8
87. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol (2018) 4(5):e180013. doi: 10.1001/jamaoncol.2018.0013
88. Giaccone G, Kim C, Thompson J, McGuire C, Kallakury B, Chahine JJ, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol (2018) 19(3):347–55. doi: 10.1016/S1470-2045(18)30062-7
89. Hansen A, Massard C, Ott P, Haas N, Lopez J, Ejadi S, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol (2018) 29(8):1807–13. doi: 10.1093/annonc/mdy232
90. Rugo HS, Delord J-P, Im S-A, Ott PA, Piha-Paul SA, Bedard PL, et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer. Clin Cancer Res (2018) 24(12):2804–11. doi: 10.1158/1078-0432.CCR-17-3452
91. Shitara K, Özgüroğlu M, Bang Y-J, Di Bartolomeo M, Mandalà M, Ryu M-H, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet (2018) 392(10142):123–33. doi: 10.1016/S0140-6736(18)31257-1
92. Zhu A, Finn R, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol (2018) 19(7):940–52. doi: 10.1016/S1470-2045(18)30351-6
93. McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, et al. Atezolizumab, an anti–programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol (2016) 34(8):833–42. doi: 10.1200/JCO.2015.63.7421
94. Rosenberg JE, Hoffman-Censits J, Powles T, Van Der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (2016) 387(10031):1909–20. doi: 10.1016/S0140-6736(16)00561-4
95. Colevas A, Bahleda R, Braiteh F, Balmanoukian A, Brana I, Chau N, et al. Safety and clinical activity of atezolizumab in head and neck cancer: results from a phase I trial. Ann Oncol (2018) 29(11):2247–53. doi: 10.1093/annonc/mdy411
96. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7
97. Ott PA, Bang YJ, Berton-Rigaud D, Elez E, Pishvaian MJ, Rugo HS, et al. Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1-Positive Endometrial Cancer: Results From the KEYNOTE-028 Study. J Clin Oncol (2017) 35(22):2535–41. doi: 10.1200/JCO.2017.72.5952
98. Emens LA, Cruz C, Eder JP, Braiteh F, Chung C, Tolaney SM, et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol (2019) 5(1):74–82. doi: 10.1001/jamaoncol.2018.4224
99. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239
100. Ribas A. Adaptive Immune Resistance: How Cancer Protects from Immune Attack. Cancer Discov (2015) 5:915–9. doi: 10.1158/2159-8290.Cd-15-0563
101. Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med (2013) 5:200ra116. doi: 10.1126/scitranslmed.3006504
102. Kuryk L, Bertinato L, Staniszewska M, Pancer K, Wieczorek M, Salmaso S, et al. From Conventional Therapies to Immunotherapy: Melanoma Treatment in Review. Cancers (Basel) (2020) 12(10):3057. doi: 10.3390/cancers12103057
103. Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell (2017) 170(6):1109–19.e10. doi: 10.1016/j.cell.2017.08.027
104. Harrington K, Freeman DJ, Kelly B, Harper J, Soria JC. Optimizing oncolytic virotherapy in cancer treatment. Nat Rev Drug Discov (2019) 18(9):689–706. doi: 10.1038/s41573-019-0029-0
105. Chowdhury P, Chamoto K, Honjo T. Combination therapy strategies for improving PD-1 blockade efficacy: a new era in cancer immunotherapy. J Intern Med (2018) 283(2):110–20. doi: 10.1111/joim.12708
Keywords: PD-1/PD-L1 inhibitors, meta-analysis, response efficacy, objective response rate, time to response, duration of response
Citation: Chen S, Zhang Z, Zheng X, Tao H, Zhang S, Ma J, Liu Z, Wang J, Qian Y, Cui P, Huang D, Huang Z, Wu Z and Hu Y (2021) Response Efficacy of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-Analysis. Front. Oncol. 11:562315. doi: 10.3389/fonc.2021.562315
Received: 31 July 2020; Accepted: 19 March 2021;
Published: 16 April 2021.
Edited by:
Tzi Bun Ng, The Chinese University of Hong Kong, ChinaReviewed by:
Feng Wang, Affiliated Hospital of Nantong University, ChinaCopyright © 2021 Chen, Zhang, Zheng, Tao, Zhang, Ma, Liu, Wang, Qian, Cui, Huang, Huang, Wu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Hu, aHV5aTA0MDEzMDFAc2luYS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.