- Department of Urology, Affiliated Jiang-yin Hospital of the Southeast University Medical College, Jiang-yin, China
Objective: This systematic study aimed to assess and compare the comprehensive evidence regarding the impact of neoadjuvant hormone therapy (NHT) on surgical and oncological outcomes of patients with prostate cancer (PCa) before radical prostatectomy (RP).
Methods: Literature searches were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Using PubMed, Web of Science, Chinese National Knowledge Infrastructure, and Wanfang databases, we identified relevant studies published before July 2020. The pooled effect sizes were calculated in terms of the odds ratios (ORs)/standard mean differences (SMDs) with 95% confidence intervals (CIs) using the fixed or random-effects model.
Results: We identified 22 clinical trials (6 randomized and 16 cohort) including 20,199 patients with PCa. Our meta-analysis showed no significant differences in body mass index (SMD = 0.10, 95% CI: −0.08–0.29, p = 0.274) and biopsy Gleason score (GS) (OR = 1.33, 95% CI: 0.76–2.35 p = 0.321) between the two groups. However, the NHT group had a higher mean age (SMD = 0.19, 95% CI: 0.07–0.31, p = 0.001), preoperative prostate-specific antigen (OR = 0.47, 95% CI: 0.19–0.75, p = 0.001), and clinic tumor stage (OR = 2.24, 95% CI: 1.53–3.29, p < 0.001). Compared to the RP group, the NHT group had lower positive surgical margins (PSMs) rate (OR = 0.44, 95% CI: 0.29–0.67, p < 0.001) and biochemical recurrence (BCR) rate (OR = 0.47, 95% CI: 0.26–0.83, p = 0.009). Between both groups, there were no significant differences in estimated blood loss (SMD = −0.06, 95% CI: −0.24–0.13, p = 0.556), operation time (SMD = 0.20, 95% CI: −0.12–0.51, p = 0.219), pathological tumor stage (OR = 0.76, 95% CI: 0.54–1.06, p = 0.104), specimen GS (OR = 0.91, 95% CI: 0.49–1.68, p = 0.756), and lymph node involvement (OR = 0.76, 95% CI: 0.40–1.45, p = 0.404).
Conclusions: NHT prior to RP appeared to reduce the tumor stage, PSMs rate, and risk of BCR in patients with PCa. According to our data, NHT may be more suitable for older patients with higher tumor stage. Besides, NHT may not increase the surgical difficulty of RP.
Introduction
Prostate cancer (PCa), which generated from an androgen-dependent tumor cell, is the most common malignancy in men worldwide (1). PCa therapy aims to block androgen-dependent growth of tumor cells (2, 3). Theoretically, neoadjuvant hormone therapy (NHT) could offer treatment benefits not only for reducing the size of the prostate volume but also eliminating the invasion of tumor microenvironment (4, 5). NHT before radical prostatectomy (RP) has been reported in several trials involving patients with locally advanced PCa. In several non-randomized trials, NHT plus RP have demonstrated improvements in local control of PCa (6–9).
To date, the use of NHT prior to RP has been explored in multiple studies. The current European Association of Urology guidelines recommend the use of NHT only for patients with intermediate or high-risk PCa if they receive radiation therapy (10). However, due to the lack of solid evidence in survival benefit, the current international guidelines lack clear recommendations for the use of NHT before RP. Some studies have shown favorable effects with respect to tumor stage reduction and decline in rates of positive surgical margins (PSMs), seminal vesicle invasion, and lymph node involvement (LNI) in patients with PCa who received NHT followed by RP (11, 12). However, in numerous trials, the use of NHT before RP has failed to show a definitive benefit in terms of biochemical recurrence (BCR), overall survival (OS), or cancer-specific survival (CSS) (13–15). The aim of the present study was to assess the pathological outcomes of patients who received NHT prior to RP. We attempted to identify which populations of patients with PCa might benefit from NHT and whether NHT might increase the surgical difficulty of RP.
Materials and Methods
Search Strategy
A systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (16). A literature search was performed to identify relevant studies comparing the surgical and oncological outcomes of NHT using PubMed, Web of Science, Chinese National Knowledge Infrastructure (CNKI), and Wanfang databases. The search terms were [prostate cancer] AND [radical prostatectomy] AND [neoadjuvant hormone therapy] AND [surgical outcomes] OR [oncological outcomes]. The search language was restricted to English and Chinese. The reference lists from the retrieved articles were manually searched to identify additional studies. The latest date of this search was July 12, 2020. Ethical approval was not required because we did not conduct clinical research in this meta-analysis.
Inclusion and Exclusion Criteria
Our search was limited to randomized or observational controlled studies published as full papers. According to the PRISMA guidelines, the selected studies fulfilled the following criteria: (1) literature comparing NHT with non-NHT before RP in patients with pathologically confirmed PCa; (2) randomized controlled trials (RCTs) or retrospective comparative studies in English or Chinese with full text available; (3) evaluation of at least one of the surgical or oncological outcomes; and (4) sufficient data provided for comparison. Exclusion criteria were as follows: (1) non-human research; (2) editorial, case report, review, meta-analysis, and conference abstract; (3) studies that did not analyze patients with PCa; and (4) studies that could not obtain sufficient data to estimate odds ratios (ORs)/standard mean differences (SMDs) and 95% confidence intervals (CIs). When studies were reported on the same population and by the same authors, the study that was well designed and reported more relevant information was used.
Data Extraction and Study Quality
According to the inclusion criteria, two investigators (HZ and ZZ) independently extracted available data from the eligible studies, and discrepancies were resolved by discussion with a third reviewer (BW). All of the extracted information was recorded according to standardized protocol. The extracted elements included year of publication, first author’s name, study’s country of origin, study design, number of patients, mean age, surgical outcomes (estimated blood loss (EBL) and operation time [OT]), histopathological information (tumor staging, pathological grading, lymph nodes, and surgical margin status), and follow-up data (median/mean follow-up duration, BCR risk).
The methodological quality of RCTs was calculated using the Cochrane risk-of-bias tool (17), which consists of five domains: random sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, and selective reporting. The methodological quality of cohort studies was assessed using the Newcastle Ottawa Scale (NOS) (18). The quality assessment of NOS was evaluated using three broad domains: patient selection, comparability of the study groups, and assessment of outcomes. A score of 0 to 9 was allocated to each study. Studies that received a score of >6 stars were considered to be of high quality.
Statistical Analysis
The continuous outcome variables were pooled as SMDs with 95% CIs, and the dichotomous variables were pooled as ORs with 95% CIs. Statistical heterogeneity across included studies was calculated by the chi-square-based Q test and I2. A p < 0.10 or an I2 > 50% was considered as significant between-study heterogeneity. When significant heterogeneity was found in this meta-analysis, the pooled effect was calculated using a random Der-Simonian and Laird effects model; otherwise, the fixed Mantel–Haenszel effects model was used. We performed a subgroup analysis to explore clinical heterogeneity: geographic region (Asia vs. non-Asia), year of publication (≥2014 vs. <2014), number of patients (≥200 vs. <200), and study design (prospective vs. retrospective). Publication bias was evaluated by visual inspection of funnel plots, and Begg’s test was used to further assess publication bias. To explore the stability of our results, a sensitivity analysis was performed by removing one study at a time. All p values were two-tailed, and p < 0.05 was considered as statistically significant. A statistical analysis was conducted using Review Manager Version 5.3 (The Cochrane Collaboration, Oxford, London, UK) and Stata Version 12.0 software (Stata Corp, College Station, TX, USA).
Results
Search Results
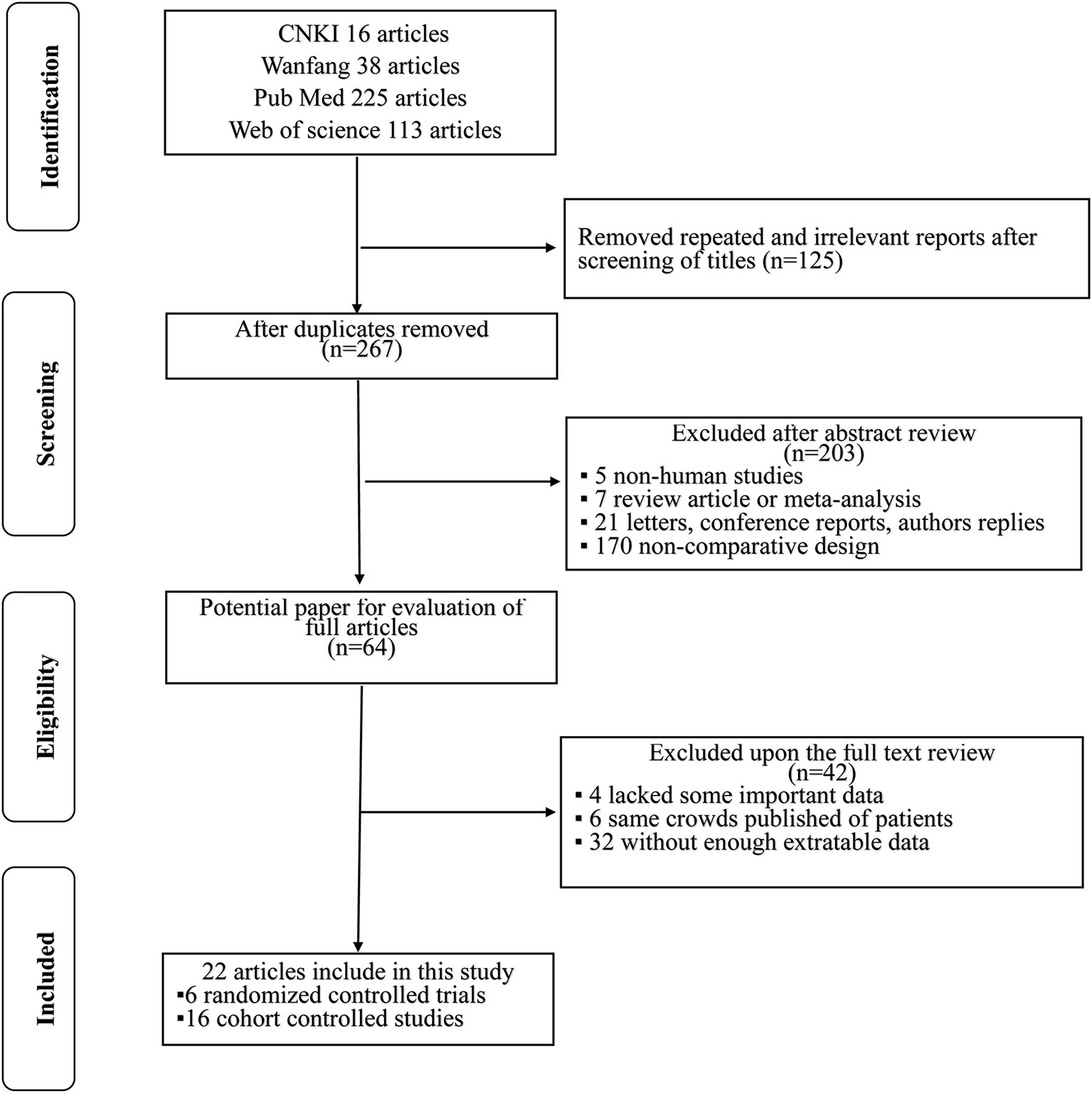
Altogether, 392 literature searches were initially identified employing the described search strategy. After screening the titles and abstracts, 328 articles were excluded for various reasons such as repeated and irrelevant reports, non-human studies, letters, case reports, conference reports, reviews, and non-comparative design studies. The remaining 64 articles were assessed in full text. Of those, 42 articles were excluded: 4 lacked key information; 6 duplicated cohorts; and 32 lacked sufficient extractable data. Finally, in accordance with the inclusion criteria, a total of 22 studies (4, 8, 11, 14, 15, 19–35) [6 were RCTs (4, 14, 19, 30, 33, 35) and 16 were retrospective non-randomized studies (8, 11, 15, 20–29, 31, 32, 34)] published from 1996 to 2020 were included in this meta-analysis. A flowchart of the study selection process is shown in Figure 1.
Characteristics of Included Studies
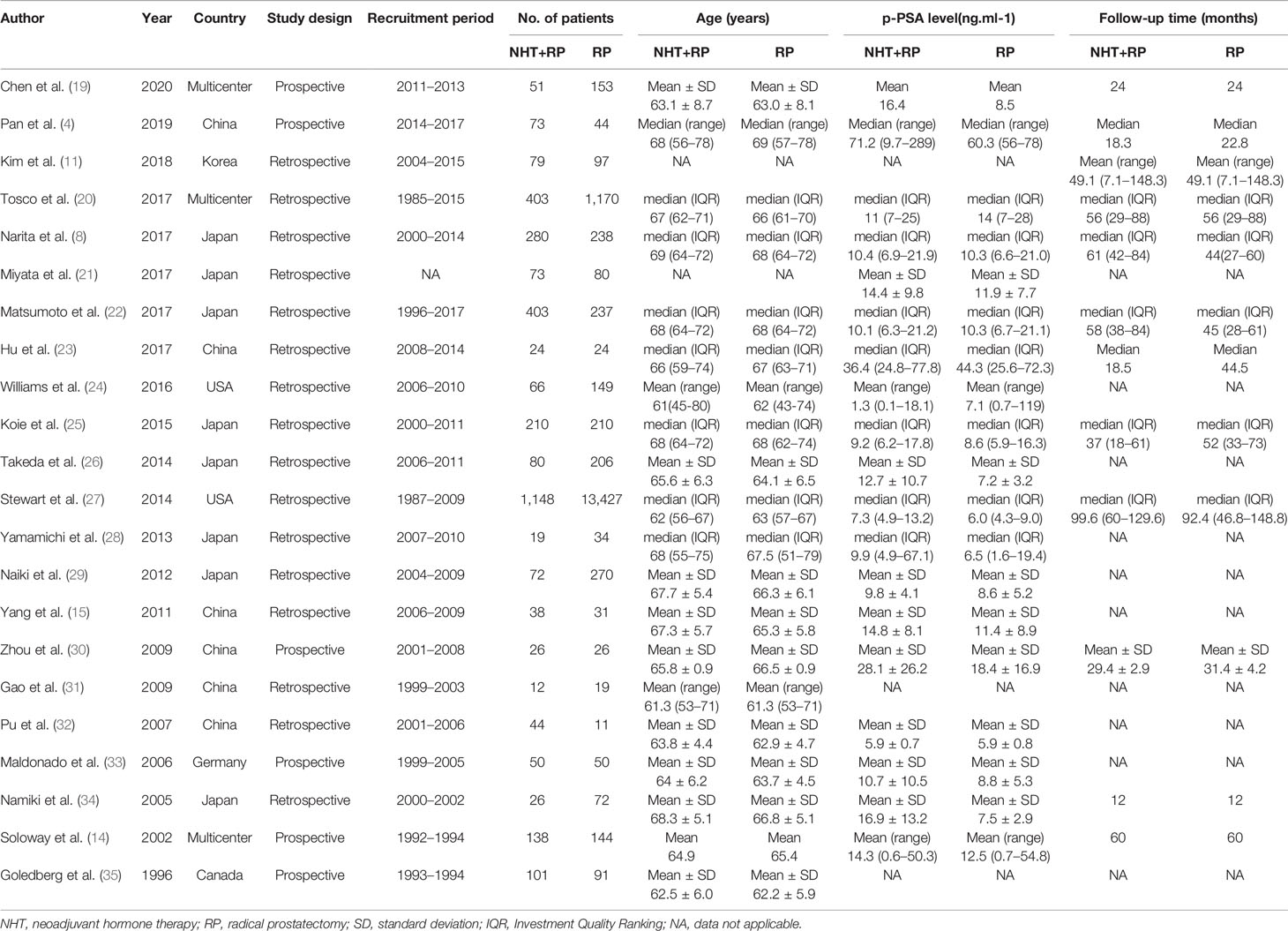
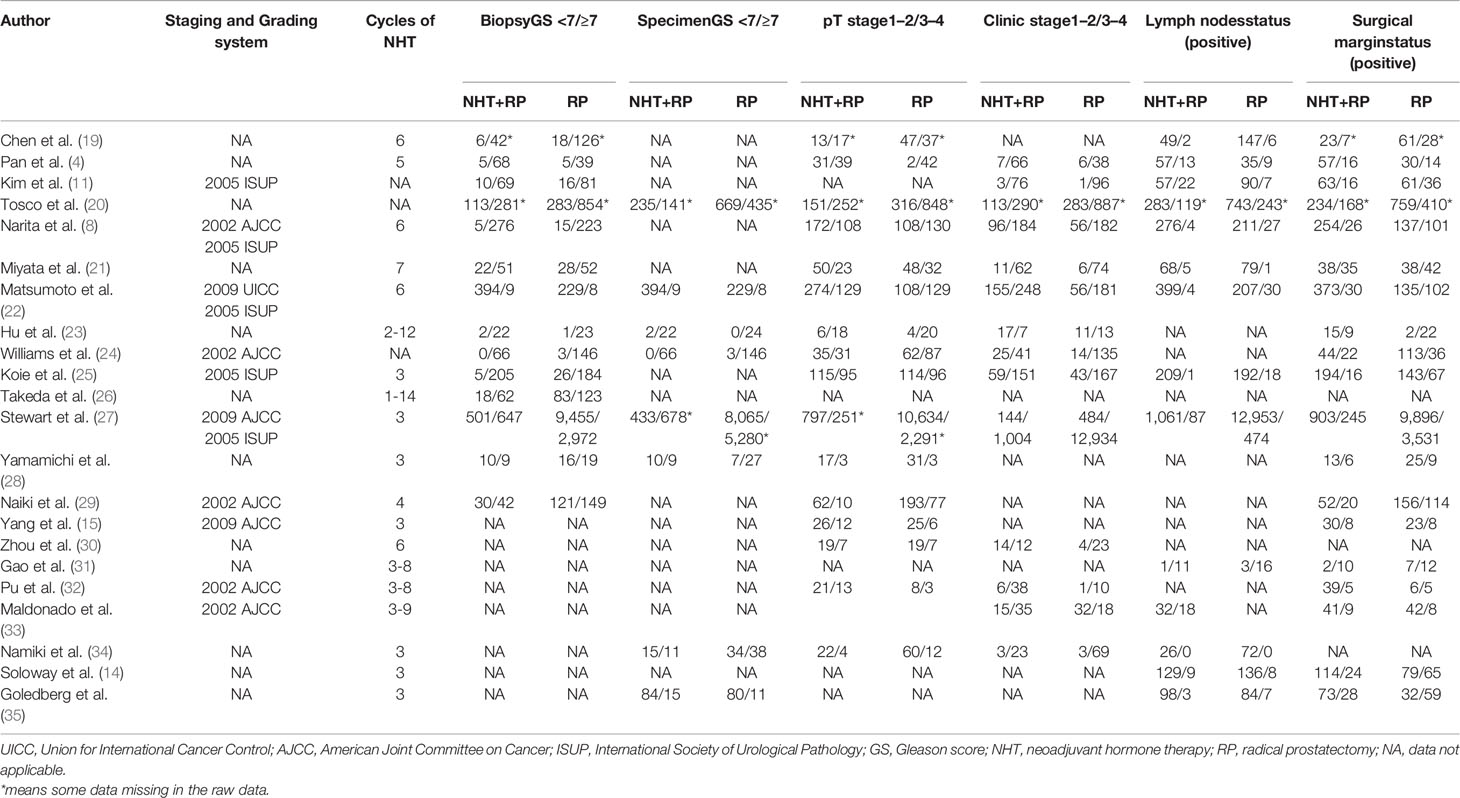
The baseline clinical and pathological characteristics of included studies are shown in Table 1. Our data revealed 20,199 patients with PCa (range: 31–14,575). In this study, 3,416 patients with PCa were treated with NHT before RP, whereas 16,783 patients with PCa were treated only with RP. The median or mean age of patients ranged from 61 to 69 years, and the median follow-up duration ranged from 12 months to 99.6 months. Among the studies, eight studies originated from Japan, six were from China, three were from multi-centers, two were from United States, one was from Germany, one was from Canada, and one was from Korea. Various pathological data were collected to compare the oncologic outcomes for the NHT and RP groups (Table 2).
Methodological Quality Assessment
The quality assessment for the six RCTs included in this meta-analysis is shown in Supplementary Figure S1. According to the Cochrane risk-of-bias tool, all of the trials were rated with low risk of bias. For the 16 retrospective observational studies, we assessed the quality following the NOS guidelines. The quality of the studies varied from a score of 6 to 8, with a mean of 6 (Supplementary Table S1). Therefore, all 22 studies were included in the subsequent analysis.
Meta-Analysis of Perioperative Variables
The pooled data from the included studies that reported body mass index (BMI) (SMD = 0.10, 95% CI: −0.08–0.29, p = 0.274, Figure 2A) and biopsy Gleason score (GS) (OR = 1.33, 95% CI: 0.76–2.35, p = 0.321, Figure 2B) showed no significant differences between the NHT and RP groups. However, the NHT group had a higher mean age (SMD = 0.19, 95% CI: 0.07–0.31, p = 0.001, Figure 3A), preoperative prostate-specific antigen (p-PSA) (OR = 0.47, 95% CI: 0.19–0.75, p = 0.001, Figure 3B), and clinic tumor stage (OR = 2.24, 95% CI: 1.53–3.29, p < 0.001, Figure 3C).
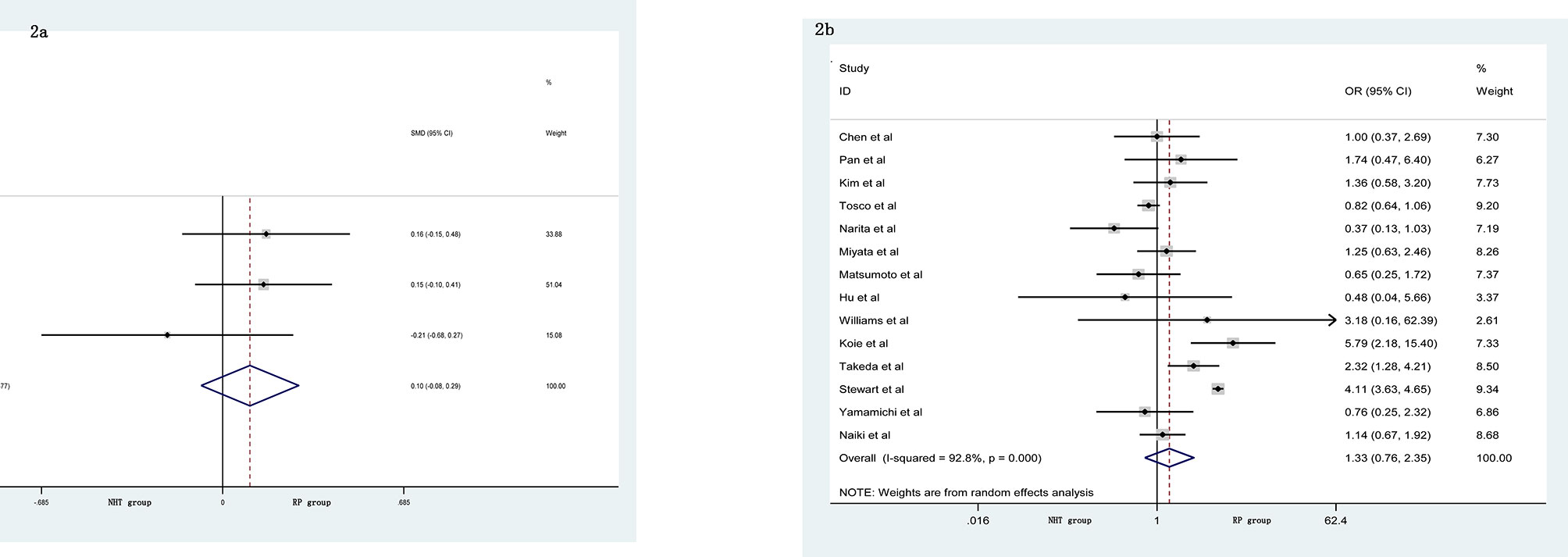
Figure 2 Forest plot of pooled hazard ratios for perioperative variables: (A) BMI; (B) biopsy GS. No significant differences between the NHT and RP groups were found.
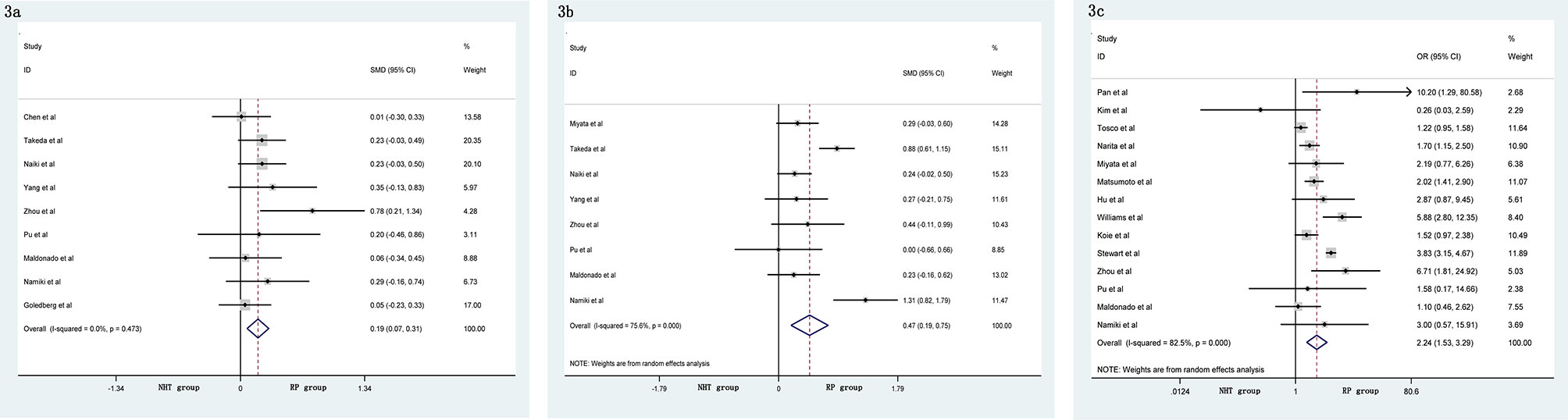
Figure 3 Forest plot of pooled hazard ratios for perioperative variables, NHT group had a higher: (A) advanced age; (B) p-PSA; (C) clinic tumor stage than RP group.
Meta-Analysis of Postoperative Variables
A meta-analysis did not show significant differences in EBL (SMD = −0.06, 95% CI: −0.24–0.13, p = 0.556, Figure 4A), OT (SMD = 0.20, 95% CI: −0.12–0.51, p = 0.219, Figure 4B), pathological tumor stage (OR = 0.76, 95% CI: 0.54–1.06, p = 0.104, Figure 4C), LNI (OR = 0.76, 95% CI: 0.40–1.45, p = 0.404, Figure 4D), and specimen GS (OR = 0.91, 95% CI: 0.49–1.68, p = 0.756, Figure 4E) between the NHT and RP groups. Compared with the RP group, the NHT group had lower PSMs rate (OR = 0.44, 95% CI: 0.29–0.67, p < 0.001, Figure 5A) and BCR risk (OR = 0.47, 95% CI: 0.26–0.83, p = 0.009, Figure 5B).
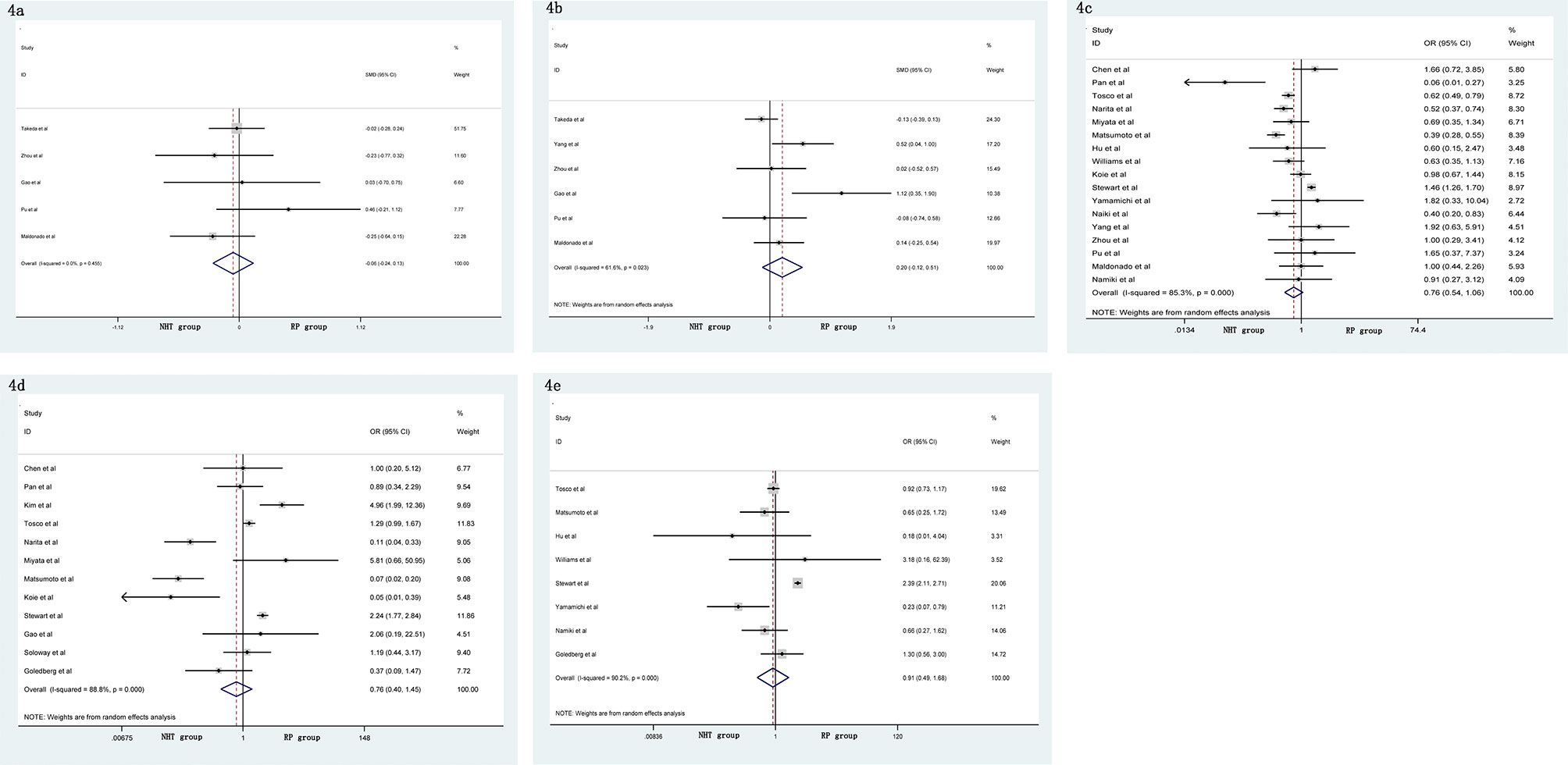
Figure 4 Forest plot of pooled hazard ratios for postoperative variables: (A) EBL; (B) OT; (C) pathological tumor stage; (D) LNI; (E) specimen GS. No significant differences between the NHT and RP groups were found.
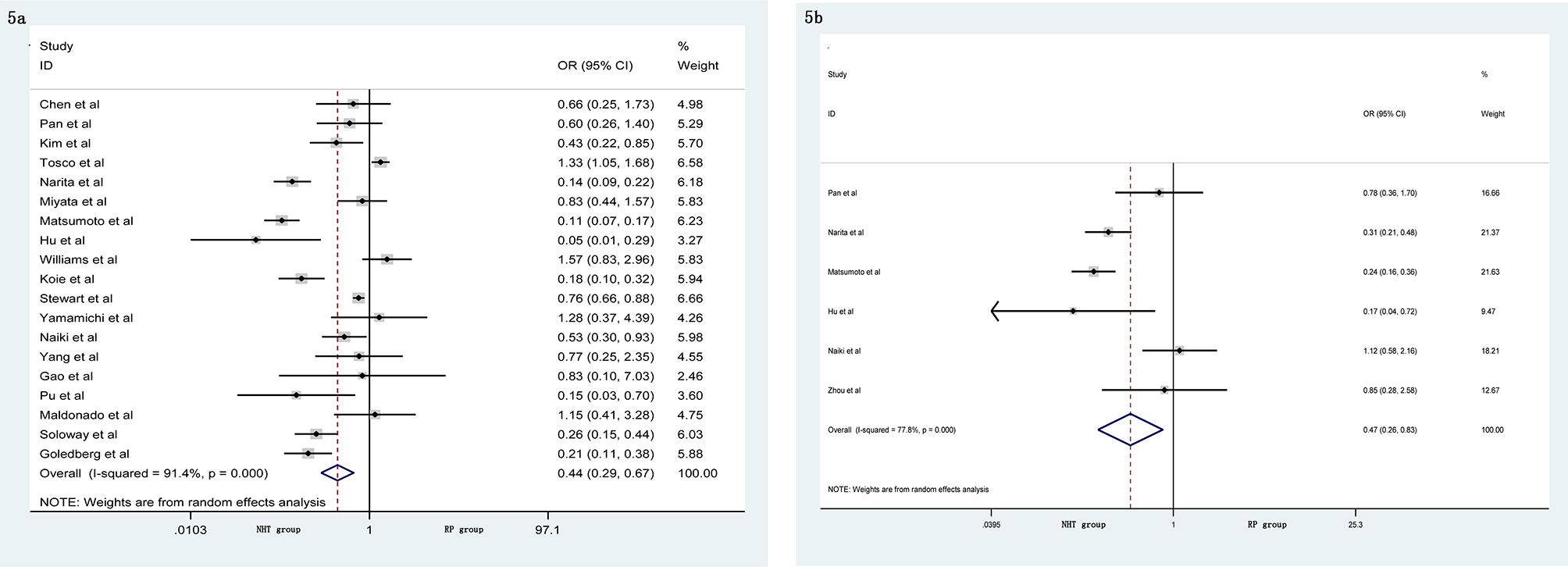
Figure 5 Forest plot of pooled hazard ratios for postoperative variables, NHT group had a lower (A) PSMs; (B) BCR than RP group.
Subgroup Analysis
To discover the source of heterogeneity, a subgroup analysis was applied with a random effects model. Considering the potential factors, we performed a subgroup analysis by geographic region, year of publication, number of patients, and study design. Because there was no significant heterogeneity detected for advanced age, BMI, and EBL, a subgroup analysis was not performed. For the perioperative variables, subgroup analyses showed that the NHT group had significantly higher p-PSA and clinic tumor stage in the prospective study compared to the RP group (Table 3). For the postoperative variables, no significant changes occurred in the subgroup analyses for OT, pathological tumor stage, specimen GS, LNI, and PSMs (Table 4). However, the results varied in some subgroup analyses. The reason for the above difference may be related to the small number of studies included in the subgroup analysis.
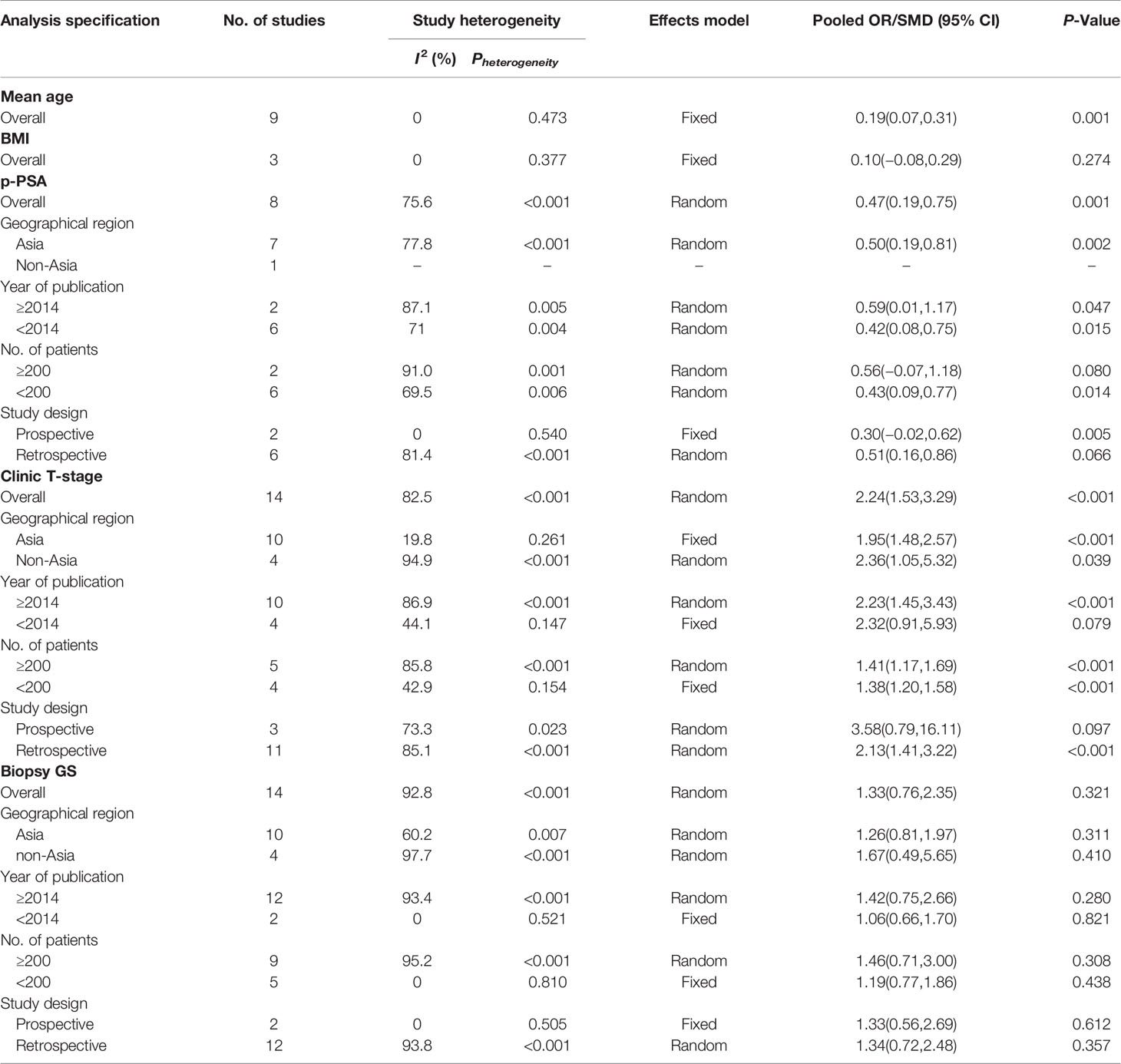
Table 3 Summary and subgroup analysis of preoperative clinicopathological features for the PCa patients treated with NHT before RP.
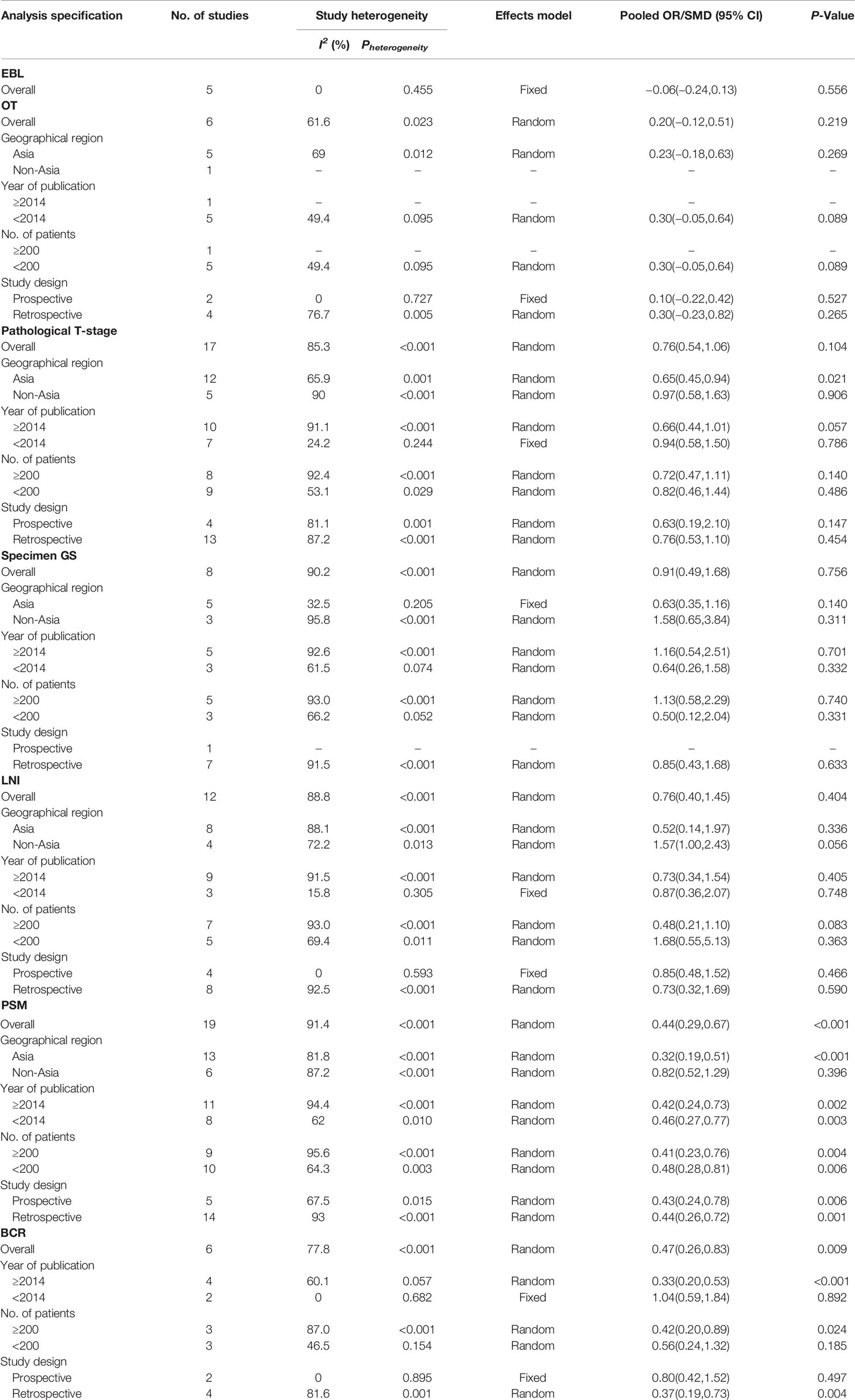
Table 4 Summary and subgroup analysis of postoperative clinicopathological features for the PCa patients treated with NHT before RP.
Publication Bias and Sensitivity Analysis
The Begg’s tests and funnel plots were adopted to detect potential publication bias in the present meta-analysis. As shown in Figure 6, the funnel plots indicated that the included studies had no evident asymmetry. Furthermore, the results from the Begg’s test for the included studies assessing the perioperative variables were as follows: advanced age (p = 0.223, Figure 6A), p-PSA (p = 0.873, Figure 6B), and clinic tumor stage (p = 0.821, Figure 6C); and the postoperative variables were as follows: EBL (p = 0.682, Figure 7A), OT (p = 0.129, Figure 7B), PSMs (p = 0.241, Figure 7C), BCR (p = 0.300, Figure 7D), pathological tumor stage (p = 0.315, Figure 7E), specimen GS (p = 0.181, Figure 7F), and LNI (p = 0.749, Figure 7G). Although we found a slight publication bias in biopsy GS (p = 0.047), no significant publication bias was found in the other parameters.
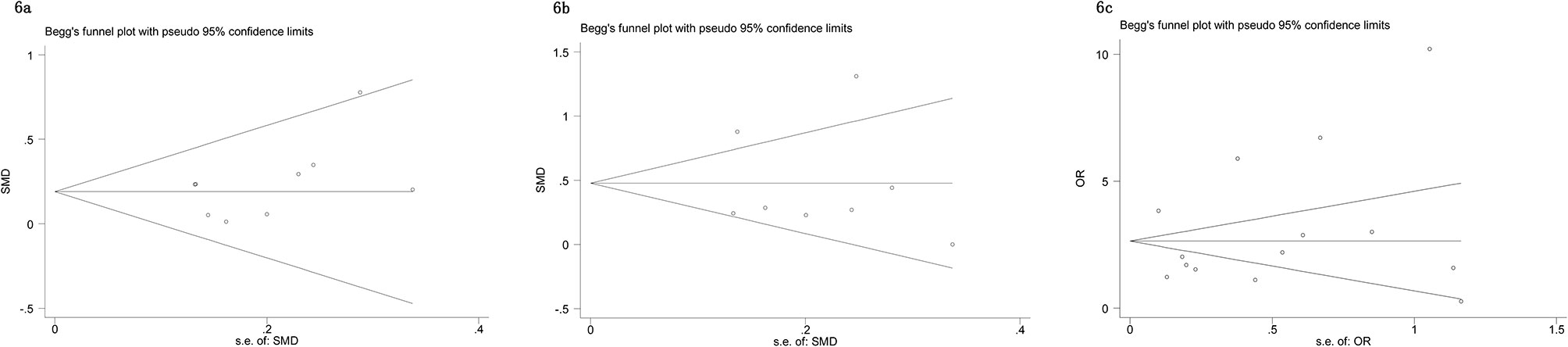
Figure 6 Funnel plots to explore publication bias in the estimates of perioperative variables: (A) advanced age; (B) p-PSA; (C) clinic tumor stage. No significant publication bias was found.
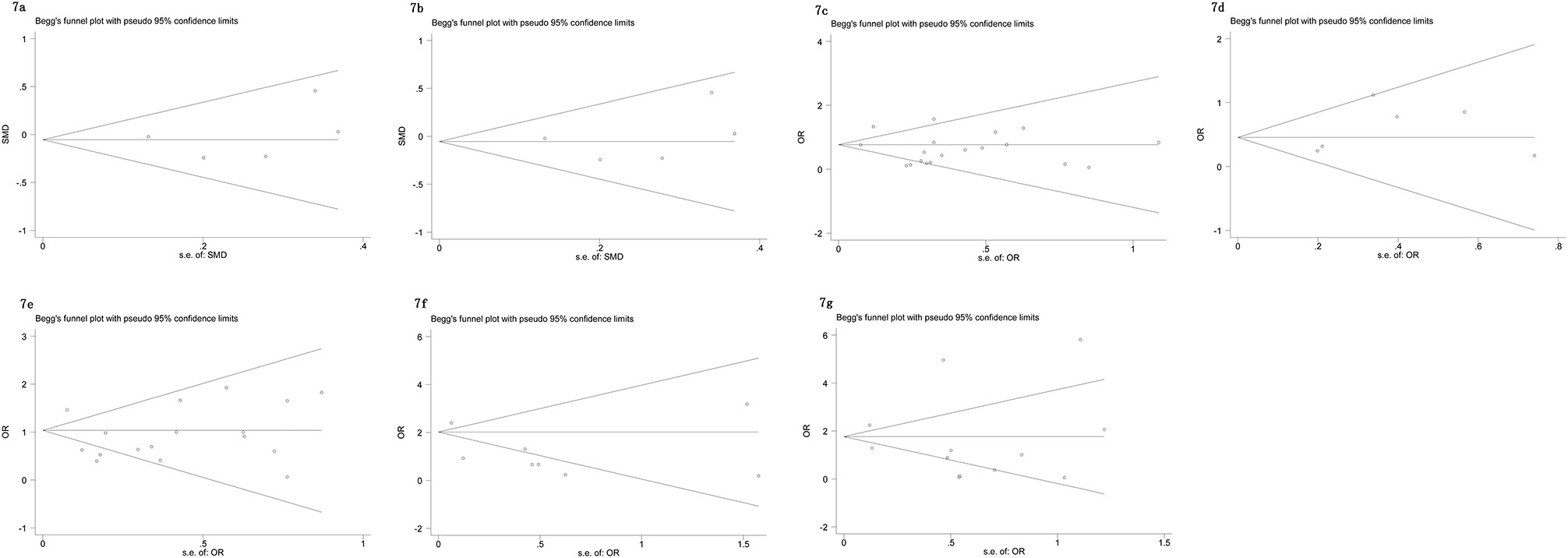
Figure 7 Funnel plots to explore publication bias in the estimates of postoperative variables: (A) EBL; (B) OT; (C) PSMs; (D) BCR; (E) pathological tumor stage; (F) specimen GS; (G) LNI. No significant publication bias was found.
A sensitivity analysis was conducted using the leave-one-out approach to examine the stability of the current study. The selected studies were sequentially omitted to investigate whether any individual study influenced the results (data not shown). The results showed no significant changes in our pooled results, suggesting the robustness of the pooled results. Funnel plot and sensitivity analysis for BMI were not conducted because of the small size.
Discussion
PCa is the most commonly diagnosed malignancy in men and the second most common cause of cancer-related death in the USA (36). Although controversy exists regarding the definitive treatment recommendations (radiation therapy, RP, or expectant management) for localized and high-risk PCa, RP has long remained the preferred therapeutic option for localized PCa (37). The ultimate goal of RP is the complete removal of all cancer cells. Unfortunately, this is not always possible, not even in carefully selected patients. As a result, incomplete resection of PCa cancer cells may result in a higher risk of local or distant metastasis (38). Presently, the systematic treatment of high-risk localized PCa has been the consensus of many clinicians (39, 40).
Given the advances in PCa systemic therapy, much interest centers on the application of NHT. NHT is defined as a systemic therapy that is administered before commencing definitive locoregional therapy (41). The proposed mode of NHT is based on the hypothesis that androgen blockade may induce PCa cell death (apoptosis) by inhibiting the growth of hormone sensitive cells, which will downstage the tumor prior to RP (42). By theoretically increasing the likelihood of organ confined tumors, the efficacy of RP is augmented. Considering these findings, NHT prior to RP has been widely performed.
The reason for using NHT in PCa was to decrease the size of the prostate volume (43). Decreasing prostate size might help the urologist to remove the prostate more efficiently and easily, with fewer intraoperative comorbidities during RP (24). Use of NHT before RP was assessed in different RCTs showing higher rates of downstaging and lower rates of PSMs compared to RP alone (14, 19, 32). In contrast, NHT administered before RP has yet to show a definitive survival benefit. This stems from the numerous trials that have demonstrated a lack of statistically significant improvement in CSS and OS (13, 15, 44). However, why NHT fails to improve survival is unclear. One possible reason is that androgen-resistant tumor cells may exist in early PCa. Another potential reason is that NHT cannot sufficiently suppress androgen levels in prostate tissue, thereby significantly destroying tumor cells. Despite the international guidelines advising against its use (37), the administration of NHT prior to RP remains controversial. Presently, many urologists incorporate NHT and RP in their clinical practice.
No clear evidence shows that NHT is beneficial in patients with PCa. Some studies have indicated that patients treated with NHT have fewer PSMs but without improving BCR after RP. Naiki et al. (29) and Soloway et al. (14) found that pre-surgical NHT is beneficial for PCa control by suppressing BCR. In this study, the efficacy of NHT was statistically significant in reducing the tumor stage after RP, PSMs rates, and BCR risk. However, no significant differences were detected between the two groups regarding biopsy GS, specimen GS, and LNI. This meta-analysis demonstrated that the NHT group comprised patients who were significantly older and were classified at a high clinic tumor stage, which means that NHT tends to be used more often in elderly patients and patients with non-localized PCa. The subgroup analyses of the perioperative variables, however, demonstrated that the NHT group had significantly higher p-PSA and higher clinic tumor stage in the prospective study. We speculate that the reasons for the above differences may come from the study population, pathological backgrounds, and methods of NHT.
The potential surgical advantages of NHT during RP remain a subject of debate. Some researchers have stated that NHT decreases the operative parameters and thereby facilitates the surgical procedure (26, 29, 45). Others have reported no differences in OT, EBL, and complication rate in patients who received or did not receive NHT prior to RP (15, 33). Narita et al. (8) reported that the patients who received NHT required a longer OT and a higher transfusion rate than the non-NHT patients. However, Hu et al. (23) found that the OT was significantly shorter and that the EBL was significantly less in the NHT group. Our data did not show that use of NHT resulted in increased OT or greater EBL, which means that NHT may not increase the surgical difficulty of RP. These results could be useful for surgeons, but additional large-scale RCT studies are warranted to draw more definitive conclusions on NHT.
Several potential limitations in this study should be acknowledged. First, except for six RCTs, all of the studies included were observational. Although we have conducted a quality evaluation of all the included literature, the quality of eligible studies is a concern because the included studies were conducted with different modes and levels of surgical expertise. Second, obvious heterogeneity among studies was identified in several analyses. Different study design, patients’ baseline features, surgical approach, adequacy of follow-up, and measurement of outcomes might be potential contributors to the heterogeneity. Therefore, the random effect model was used to reduce the impact of heterogeneity but could not completely eliminate it. Third, the data included in these documents span a lengthy time period. Therefore, selection bias of the original articles remains a significant limitation in the current study. It is reasonable to believe that the recent technical developments in PCa make the NHT of today more complete than it was 30 years ago. Finally, the follow-up duration was quite short in several included studies, and we did not synthesize the evidence to assess the relative differences in the long-term survival outcomes because of the limited number of studies that provided the data.
Despite these limitations, there are several strengths in our study. Our research was conducted at an appropriate time because this problem urgently needs to be resolved and sufficient clinical research has accumulated in recent decades to permit analysis. In the present study, relying on the PRISMA, we strictly adhered to the established inclusion and exclusion criteria, carefully assessed the quality of the included literature, and further performed subgroup and sensitivity analyses to minimize heterogeneity differences. Therefore, the reliability and the stability of our results are guaranteed.
Conclusion
This meta-analysis showed that NHT prior to RP appeared to reduce the pathological tumor stage, PSMs rate, and risk of BCR in patients with PCa. Altogether, based on our data, NHT may be more suitable for older patients with high PCa clinical stages. The clinical application of NHT on PCa should carefully consider potential risks and benefits to ensure maximizing treatment benefits. Given the inherent limitations of the included studies, additional well-designed RCTs with proper inclusion and exclusion criteria are required to confirm our findings.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
LZ: Project development and manuscript writing. BW: Data management. HZ: Data collection. ZZ: Data collection. JY: Data analysis, data management. YF: Data analysis, data management. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.615801/full#supplementary-material
Supplementary Figure 1 | Risk of bias graph (up: risk of bias graph; down: risk of bias summary), all of the trials were rated with low risk of bias.
Supplementary Table 1 | Quality assessment of cohort studies included in this meta-analysis.
References
1. Rebbeck TR. Prostate Cancer Genetics: Variation by Race, Ethnicity, and Geography. Semin Radiat Oncol (2017) 27(1):3–10. doi: 10.1016/j.semradonc.2016.08.002
2. Oksala R, Moilanen A, Riikonen R, Rummakko P, Karjalainen A, Passiniemi M, et al. Discovery and development of ODM-204: A Novel nonsteroidal compound for the treatment of castration-resistant prostate cancer by blocking the androgen receptor and inhibiting CYP17A1. J Steroid Biochem Mol Biol (2019) 192:105115. doi: 10.1016/j.jsbmb.2018.02.004
3. Tafuri A, Cerruto MA, Antonelli A. Neoadjuvant strategies before radical prostatectomy for high risk prostate cancer in the era of new hormonal agents. Curr Drug Targets (2020) 22(1):68–76. doi: 10.2174/1389450121666200621194409
4. Pan J, Chi C, Qian H, Zhu Y, Shao X, Sha J, et al. Neoadjuvant chemohormonal therapy combined with radical prostatectomy and extended PLND for very high risk locally advanced prostate cancer: A retrospective comparative study. Urol Oncol (2019) 37(12):991–8. doi: 10.1016/j.urolonc.2019.07.009
5. Enokida H, Yamada Y, Tatarano S, Yoshino H, Yonemori M, Sakaguchi T, et al. Oncological outcome of neoadjuvant low-dose estramustine plus LHRH agonist/antagonist followed by extended radical prostatectomy for Japanese patients with high-risk localized prostate cancer: a prospective single-arm study. Jpn J Clin Oncol (2020) 50(1):66–72. doi: 10.1093/jjco/hyz138
6. Aliberti A, Bada M, Rapisarda S, Natoli C, Schips L, Cindolo L. Adherence to hormonal deprivation therapy in prostate cancer in clinical practice: a retrospective, single-center study. Minerva Urol Nefrol (2019) 71(2):181–4. doi: 10.23736/S0393-2249.18.03109-0
7. Sayyid RK, Evans A, Hersey K, Maloni R, Hurtado-Coll A, Kulkarni G, et al. A Phase II, Randomized, Open-Label Study of Neoadjuvant Degarelix versus LHRH Agonist in Prostate Cancer Patients Prior to Radical Prostatectomy. Clin Cancer Res (2017) 23(8):1974–80. doi: 10.1158/1078-0432.CCR-16-1790
8. Narita T, Koie T, Ookubo T, Mitsuzuka K, Narita S, Yamamoto H, et al. The impact of extended lymph node dissection versus neoadjuvant therapy with limited lymph node dissection on biochemical recurrence in high-risk prostate cancer patients treated with radical prostatectomy: a multi-institutional analysis. Med Oncol (2017) 34(1):1. doi: 10.1007/s12032-016-0859-0
9. Hata S, Shin T, Abe S, Kawano K, Sato R, Kai T, et al. Degarelix as a neoadjuvant hormonal therapy for acute urinary tract toxicity associated with external beam radiotherapy for intermediate- and high-risk prostate cancer: a propensity score matched analysis. Jpn J Clin Oncol (2020) 2:hyaa163. doi: 10.1093/jjco/hyaa163
10. Rijksen BLT, Pos FJ, Hulshof MCCM, Vernooij RWM, Jansen H, van Andel G, et al. Variation in the Prescription of Androgen Deprivation Therapy in Intermediate- and High-risk Prostate Cancer Patients Treated with Radiotherapy in the Netherlands, and Adherence to European Association of Urology Guidelines: A Population-based Study. Eur Urol Focus (2019) S2405-4569(19) 30346–3. doi: 10.1016/j.euf.2019.11.005
11. Kim SH, Park EY, Joo J. Effect of Neoadjuvant Hormone Therapy on Resection Margin and Survival Prognoses in Locally Advanced Prostate Cancer after Prostatectomy Using Propensity-Score Matching. Biomed Res Int (2018) 2018:4307207. doi: 10.1155/2018/4307207
12. McKay RR, Montgomery B, Xie W, Zhang Z, Bubley GJ, Lin DW, et al. Post prostatectomy outcomes of patients with high-risk prostate cancer treated with neoadjuvant androgen blockade. Prostate Cancer Prostatic Dis (2018) 21(3):364–72. doi: 10.1038/s41391-017-0009-6
13. Aus G, Abrahamsson PA, Ahlgren G, Hugosson J, Lundberg S, Schain M, et al. Three-month neoadjuvant hormonal therapy before radical prostatectomy: a 7-year follow-up of a randomized controlled trial. BJU Int (2002) 90(6):561–6. doi: 10.1046/j.1464-410X.2002.02982.x
14. Soloway MS, Pareek K, Sharifi R, Wajsman Z, McLeod D, Wood DP Jr, et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol (2002) 167(1):112–6. doi: 10.1016/S0022-5347(05)65393-1
15. Yang SW, Song KH, Lim JS, Sul CK. Neoadjuvant hormonal therapy preceding radical prostatectomy for clinically localized prostate cancer: early postoperative complications and biochemical recurrence. Korean J Urol (2011) 52(1):19–23. doi: 10.4111/kju.2011.52.1.19
16. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical Res ed.) (2009) 339:b2700. doi: 10.1136/bmj.b2700
17. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical Res ed.) (2011) 343:d5928. doi: 10.1136/bmj.d5928
18. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
19. Chen MC, Kilday PS, Elliott PA, Artenstein D, Slezak J, Jacobsen SJ, et al. Neoadjuvant Leuprolide Therapy with Radical Prostatectomy: Long-term Effects on Health-related Quality of Life. Eur Urol Focus (2020) S2405-4569(20):30072–9. doi: 10.1016/j.euf.2020.03.001
20. Tosco L, Laenen A, Briganti A, Gontero P, Karnes RJ, Albersen M, et al. The survival impact of neoadjuvant hormonal therapy before radical prostatectomy for treatment of high-risk prostate cancer. Prostate Cancer Prostatic Dis (2017) 20(4):407–12. doi: 10.1038/pcan.2017.29
21. Miyata Y, Nakamura Y, Yasuda T, Matsuo T, Ohba K, Furusato B, et al. Neoadjuvant hormonal therapy for low-risk prostate cancer induces biochemical recurrence after radical prostatectomy via increased lymphangiogenesis-related parameters. Prostate (2017) 77(14):1408–15. doi: 10.1002/pros.23402
22. Matsumoto T, Hatakeyama S. Cost-effectiveness comparison between neoadjuvant chemohormonal therapy and extended pelvic lymph node dissection in high-risk prostate cancer patients treated with radical prostatectomy: a multi-institutional analysis. Med Oncol (2017) 34: (12):190. doi: 10.1007/s12032-017-1050-y
23. Hu JC, Hung SC, Ou YC. Assessments of Neoadjuvant Hormone Therapy Followed by Robotic-Assisted Radical Prostatectomy for Intermediate- and High-Risk Prostate Cancer. Anticancer Res (2017) 37(6):3143–50. doi: 10.21873/anticanres.11672
24. Williams SB, Davis JW, Wang X, Achim MF, Zurita-Saavedra A, Matin SF, et al. Neoadjuvant Systemic Therapy Before Radical Prostatectomy in High-Risk Prostate Cancer Does Not Increase Surgical Morbidity: Contemporary Results Using the Clavien System. Clin Genitourin Cancer (2016) 14(2):130–8. doi: 10.1016/j.clgc.2015.10.008
25. Koie T, Mitsuzuka K, Yoneyama T, Narita S, Kawamura S, Kaiho Y, et al. Neoadjuvant luteinizing-hormone-releasing hormone agonist plus low-dose estramustine phosphate improves prostate-specific antigen-free survival in high-risk prostate cancer patients: a propensity score-matched analysis. Int J Clin Oncol (2015) 20(5):1018–25. doi: 10.1007/s10147-015-0802-y
26. Takeda T, Miyajima A, Kaneko G, Kikuchi E, Nakagawa K, Oya M. Androgen deprivation therapy improves pneumoperitoneum time during laparoscopic radical prostatectomy in Japanese patients with enlarged prostates. Asian J Endosc Surg (2014) 7(2):145–51. doi: 10.1111/ases.12084
27. Stewart SB, Cheville JC, Sebo TJ, Frank I, Boorjian SA, Thompson RH, et al. Gleason grading after neoadjuvant hormonal therapy retains prognostic value for systemic progression following radical prostatectomy. Prostate Cancer Prostatic Dis (2014) 17(4):332–7. doi: 10.1038/pcan.2014.30
28. Yamamichi F, Shigemura K, Morishita S, Yamanaka K, Tanaka K, Miyake H, et al. Significance of neoadjuvant hormonal therapy in radical retropubic prostatectomy: a retrospective single-surgeon study. Yonsei Med J (2013) 54(2):410–5. doi: 10.3349/ymj.2013.54.2.410
29. Naiki T, Kawai N, Okamura T, Nagata D, Kojima Y, Akita H, et al. Neoadjuvant hormonal therapy is a feasible option in laparoscopic radical prostatectomy. BMC Urol (2012) 12:36. doi: 10.1186/1471-2490-12-36
30. Li-qun Z, Kun Y, Lin C, Gang S, Yi S, Li-ning C, et al. Effect of neoadjuvant hormone therapy on pathological parameters and PSA relapse-free survival of localized prostate cancer patients. Chin J Urol (2009) 30(11):765–8. doi: 10.3760/cma.j.issn.1000-6702.2009.11.014
31. Gao X, Zhou T, Tang YJ, Lu X, Sun YH. Neoadjuvant hormonal deprivation for patients undergoing radical prostatectomy. Asian J Androl (2009) 11(1):127–30. doi: 10.1038/aja.2008.16
32. Pu XY, Wang XH, Wu YL, Wang HP. Comparative study of the impact of 3- versus 8-month neoadjuvant hormonal therapy on outcome of laparoscopic radical prostatectomy. J Cancer Res Clin Oncol (2007) 133(8):555–62. doi: 10.1007/s00432-007-0204-2
33. Maldonado-Valadez R, Teber D, Erdogru T, Safi KC, Frede T, Rassweiler J. The impact of neoadjuvant hormonal therapy on the outcome of laparoscopic radical prostatectomy: a matched pair analysis. J Urol (2006) 175(6):2092–6. doi: 10.1016/S0022-5347(06)00260-6
34. Namiki S, Saito S, Tochigi T, Kuwahara M, Ioritani N, Yoshimura K, et al. Impact of hormonal therapy prior to radical prostatectomy on the recovery of quality of life. Int J Urol (2005) 12(2):173–81. doi: 10.1111/j.1442-2042.2005.01014.x
35. Goldenberg SL, Klotz LH, Srigley J, Jewett MA, Mador D, Fradet Y, et al. Randomized, prospective, controlled study comparing radical prostatectomy alone and neoadjuvant androgen withdrawal in the treatment of localized prostate cancer. Canadian Urologic Oncology Group. J Urol (1996) 156(3):873–7. doi: 10.1016/S0022-5347(01)65645-3
36. DeSantis CE, Miller KD. Cancer statistics for African Americans, 2019. CA Cancer J Clin (2019) 69(3):211–33. doi: 10.3322/caac.21555
37. Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J Urol (2018) 199(3):683–90. doi: 10.1016/j.juro.2017.11.095
38. Zhang L, Wu B, Zha Z, Zhao H, Yuan J, Jiang Y, et al. Surgical margin status and its impact on prostate cancer prognosis after radical prostatectomy: a meta-analysis. World J Urol (2018) 36(11):1803–15. doi: 10.1007/s00345-018-2333-4
39. Teo MY, Rathkopf DE, Kantoff P. Treatment of Advanced Prostate Cancer. Annu Rev Med (2019) 70:479–99. doi: 10.1146/annurev-med-051517-011947
40. Hu J, Hsu J, Bergerot PG, Yuh BE, Stein CA, Pal SK. Preoperative therapy for localized prostate cancer: a comprehensive overview. Maturitas (2013) 74(1):3–9. doi: 10.1016/j.maturitas.2012.10.012
41. Tosco L, Laenen A, Gevaert T, Salmon I, Decaestecker C, Davicioni E, et al. Neoadjuvant degarelix with or without apalutamide followed by radical prostatectomy for intermediate and high-risk prostate cancer: ARNEO, a randomized, double blind, placebo-controlled trial. BMC Cancer (2018) 18(1):354. doi: 10.1186/s12885-018-4275-z
42. McAllister MJ, Underwood MA, Leung HY, Edwards J. A review on the interactions between the tumor microenvironment and androgen receptor signaling in prostate cancer. Trans Res J Lab Clin Med (2019) 206:91–106. doi: 10.1016/j.trsl.2018.11.004
43. Langenhuijsen JF, van Lin EN, Hoffmann AL, Spitters-Post I, Alfred Witjes J, Kaanders JH, et al. Neoadjuvant androgen deprivation for prostate volume reduction: the optimal duration in prostate cancer radiotherapy. Urol Oncol (2011) 29(1):52–7. doi: 10.1016/j.urolonc.2009.03.024
44. Gandaglia G, Sun M, Trinh QD. Survival benefit of definitive therapy in patients with clinically advanced prostate cancer: estimations of the number needed to treat based on competing-risks analysis. BJU Int (2014) 114(6b):E62–9. doi: 10.1111/bju.12645
Keywords: prostate cancer, radical prostatectomy, neoadjuvant hormone therapy, meta-analysis, clinical research
Citation: Zhang L, Zhao H, Wu B, Zha Z, Yuan J and Feng Y (2021) The Impact of Neoadjuvant Hormone Therapy on Surgical and Oncological Outcomes for Patients With Prostate Cancer Before Radical Prostatectomy: A Systematic Review and Meta-Analysis. Front. Oncol. 10:615801. doi: 10.3389/fonc.2020.615801
Received: 09 October 2020; Accepted: 14 December 2020;
Published: 08 February 2021.
Edited by:
Marco Borghesi, University of Genoa, ItalyReviewed by:
Guangjie Ji, Peking University First Hospital, ChinaHiroaki Matsumoto, Yamaguchi University, Japan
Copyright © 2021 Zhang, Zhao, Wu, Zha, Yuan and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijin Zhang, c3R6bGo5MTM3Mjk1NTNAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Lijin Zhang
Lijin Zhang Hu Zhao
Hu Zhao Bin Wu
Bin Wu Zhenlei Zha
Zhenlei Zha Jun Yuan
Jun Yuan Yejun Feng
Yejun Feng