- Department of Urology, The Third Medical Centre, Chinese PLA General Hospital, Beijing, China
Background: To evaluate the efficacy and safety of everolimus, a mTOR inhibitor, on invasive malignant renal epithelioid angiomyolipoma (EAML).
Materials and Methods: From Oct 2014 to May 2019, we collected data from seven patients with a definite (clinical and pathological) diagnosis of EAML received everolimus in our hospital. Targeted sequence capture array technique with next-generation of high throughput sequencing (NGS) were performed to detect mutations of TSC1/2 genes. All patients had received surgery and everolimus. The clinical efficacy and safety of the therapy were evaluated.
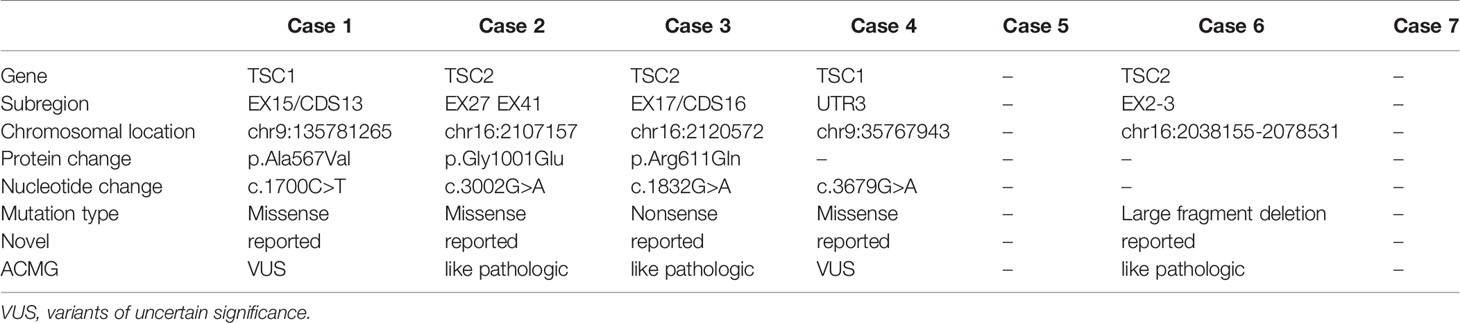
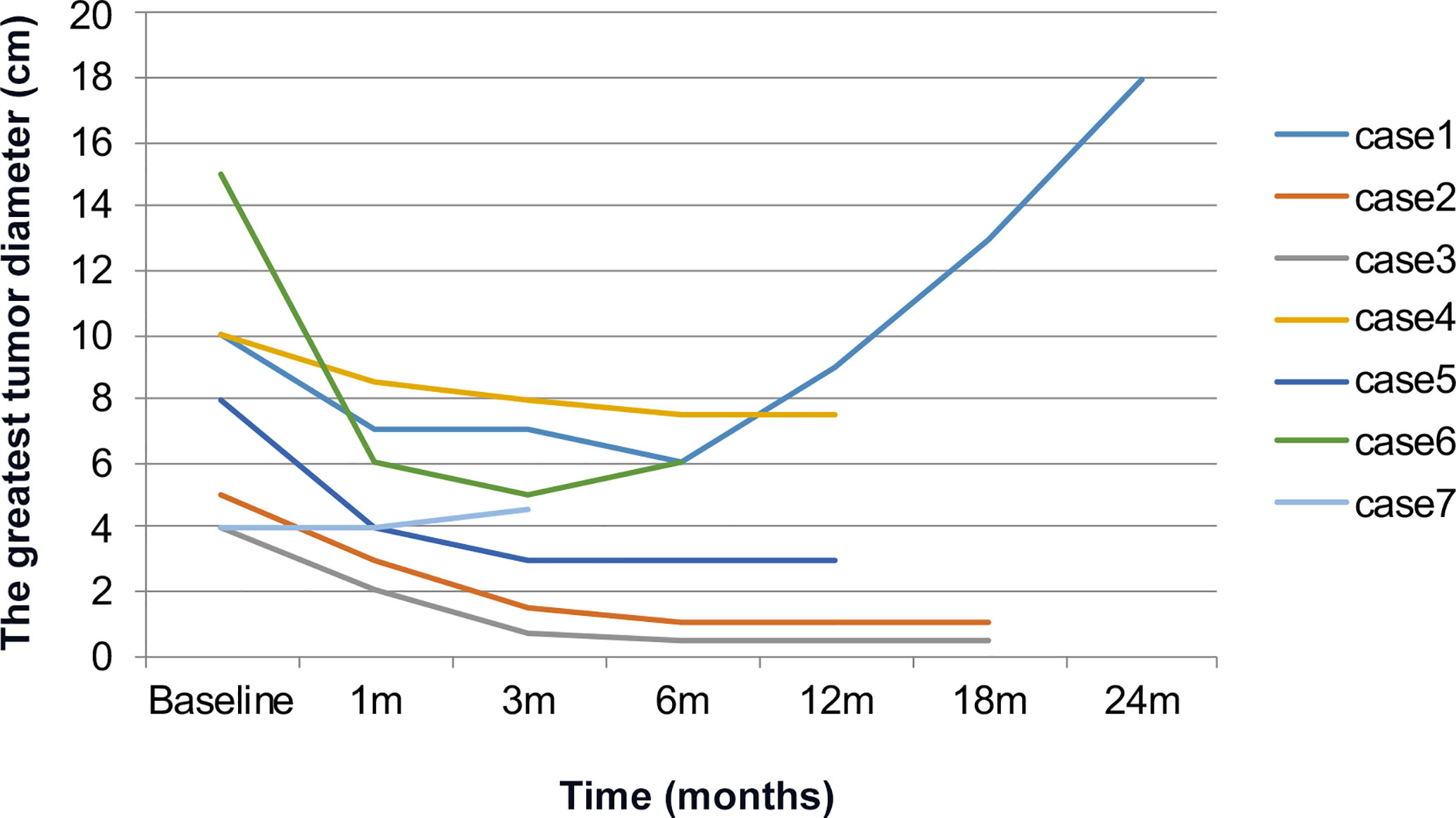
Results: Mutations of TSC1 and TSC2 were detected in two and three patients though targeted sequence capture array technique with NGS, respectively. Among seven patients, three had missense mutations, one had nonsense mutation, and one had the large fragment deletion mutation. Five patients accompanied with tuberous sclerosis complex (TSC) were identified. All patients were administered 10mg everolimus once daily, the treatment duration lasted for 3 to 28 months. The objective response was assessed 3 months later, five partial response, two stable disease (SD), the mean greatest tumor diameter of all patients decreased from 9.6 to 5.2cm. Six patients stayed SD and one patient died during follow up. Patients accompanying with TSC had better responses to everolimus compared with non-TSC.
Conclusion: The mTOR inhibitor can be an effective treatment for patients with invasive malignant renal EAML. Patients with TSC may benefit more from the therapy.
Introduction
Angiomyolipoma (AML) is one of the most common benign solid tumors in the kidney (1). Histologically, it is composed of different proportions of fat, blood vessels, and smooth muscle (2). However, epithelioid angiomyolipoma (EAML), is an uncommon subtype of renal AML, has been defined as a mesenchymal neoplasm derived from perivascular epithelioid cells (3, 4). Unlike typical AMLs, these lesions are usually predominantly epithelioid and demonstrate little or no macroscopic fat (5). It was reported that patients with EAML were significantly younger compared to AML (4). The reported malignant potential and high-aggressive biologic behaviors of renal EAML, as local recurrence, distant metastasis or death, were primarily based on case reports (6).
The standard treatment for EAML is still unknown at present, surgical resection is effective, and complete tumor resection can improve the outcome. Moreover, Kenerson et al. (7) reported abnormal activation of mTOR pathway may contribute to renal EAML growth and progression. In some patients, mTOR inhibitors such as rapamycin and everolimus reduced the size of the tumors, suggested that mTOR inhibitors may be therapeutic option for EAML. In this study, we described our experience of the comprehensive therapy including surgery and everolimus in seven patients with invasive malignant renal EAML. This comprehensive therapy strategy showed great clinical efficacy, which reflected the potential benefit for invasive malignant renal EAML.
Materials and Methods
The study was approved by Medical Ethics Committee of our hospital, and informed consent was acquired from each patient. The protocol for this study was registered on Chinese Clinical Trial Registry (ChiCTR-OPN-16008236).
Patients
From October 2014 to May 2019, seven patients with invasive malignant renal EAML were treated in our center, the related data were extracted from a prospectively maintained database. Invasive malignant renal EAML was defined as patients with renal EAML experienced recurrent or metastatic disease. Clinical presentations, tumor size, pathological and radiological features, surgical methods, and follow-up information were recorded.
TSC1/2 Next-Generation Sequencing
Five milliliters of venous blood was drawn from subjects and controls, and genomic DNA was extracted by standard procedure (QIAamp DNA Blood Midi Kit, Qiagen, Hilden, Germany). Then the purified DNA fragments were subjected to an end repair, an addition of “A” and adapter reactions. Finally, the DNA database of individuals was established.
After obtaining raw reads from the Illumina Pipeline software (version 1.3.4), we started to analyze those. Firstly, evaluating the quality of sequencing, and remove low quality reads and reads contaminated by adapters. Then, performing sequence alignment by the BWA software (Burrows Wheeler Aligner) and HG19. In the meanwhile, evaluating the efficacy of sequence capture, and inquiring SNV (single nucleotide variant) and Indel (insertion and deletion) by SOAPsnp and SAMtools. Finally, generating target region base polymorphism results, comparing databases (NCBI dbSNP, HapMap, 1000 human genome dataset, and database of 100 Chinese healthy adults), and annotating and screening the suspicious mutations founded.
Everolimus Treatment and Evaluation
After the first operation, these seven patients received everolimus when disease recurrence or progression. All patients were treated with everolimus (Novartis Pharmaceuticals, Switzerland) at an initial dosage of 10 mg/day QD orally until disease progression or grade III/IV drug-related adverse events which requiring reduction or discontinuation. The clinical response, plasma drug concentration, and adverse events were collected regularly.
All patients performed spiral CT or MRI for baseline tumor lesion before receiving everolimus. The largest tumor was recognized as the target lesion. The response is evaluated by solid tumor’s effect evaluation criterion (RECIST version 1.1) (8) at 1, 3, 6, 12 months, and then every 6 months. Adverse events were evaluated every cycle and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE 4.0, May 2009) (9). The plasma concentration of everolimus was determined by measuring the serum concentration of rapamycin and converted with the reported formula (10).
Results
Clinical and Pathological Data
For all patients, three were male and four were female, and the median age was 35 (17–60) years. Five patients firstly presented with symptom of low back pain and other two with renal tumor identified by ultrasound during physical examination, and one of those patients accompanied with hematuria. The invasive renal EAML in four patients were unilateral on left side, other three were bilateral, two patients diagnosed as tuberous sclerosis by clinical while three patients diagnosed as tuberous sclerosis by gene test. Five patients underwent radical nephrectomy, and two underwent partial nephrectomy.
All of the seven patients had disease progressed after the first operation (7–100 months). The median time to disease progression was 25.8 months. Three had local recurrence and four had multiple metastases; two relapsed patients underwent a secondary surgical resection, and one patient with distant metastases underwent a resection of the right scapula metastases.
All patients were pathologically diagnosed as malignant epithelioid renal angiomyolipoma and positive HMB45 staining. The positive rate of Ki-67 was more than 10% and mitotic figure was more than 2/10 HPF in five patients (Figure 1). For the other two patients, the positive rate of Ki-67 was 3% and the mitotic figure was 1–2/10 HPF (Table 1).
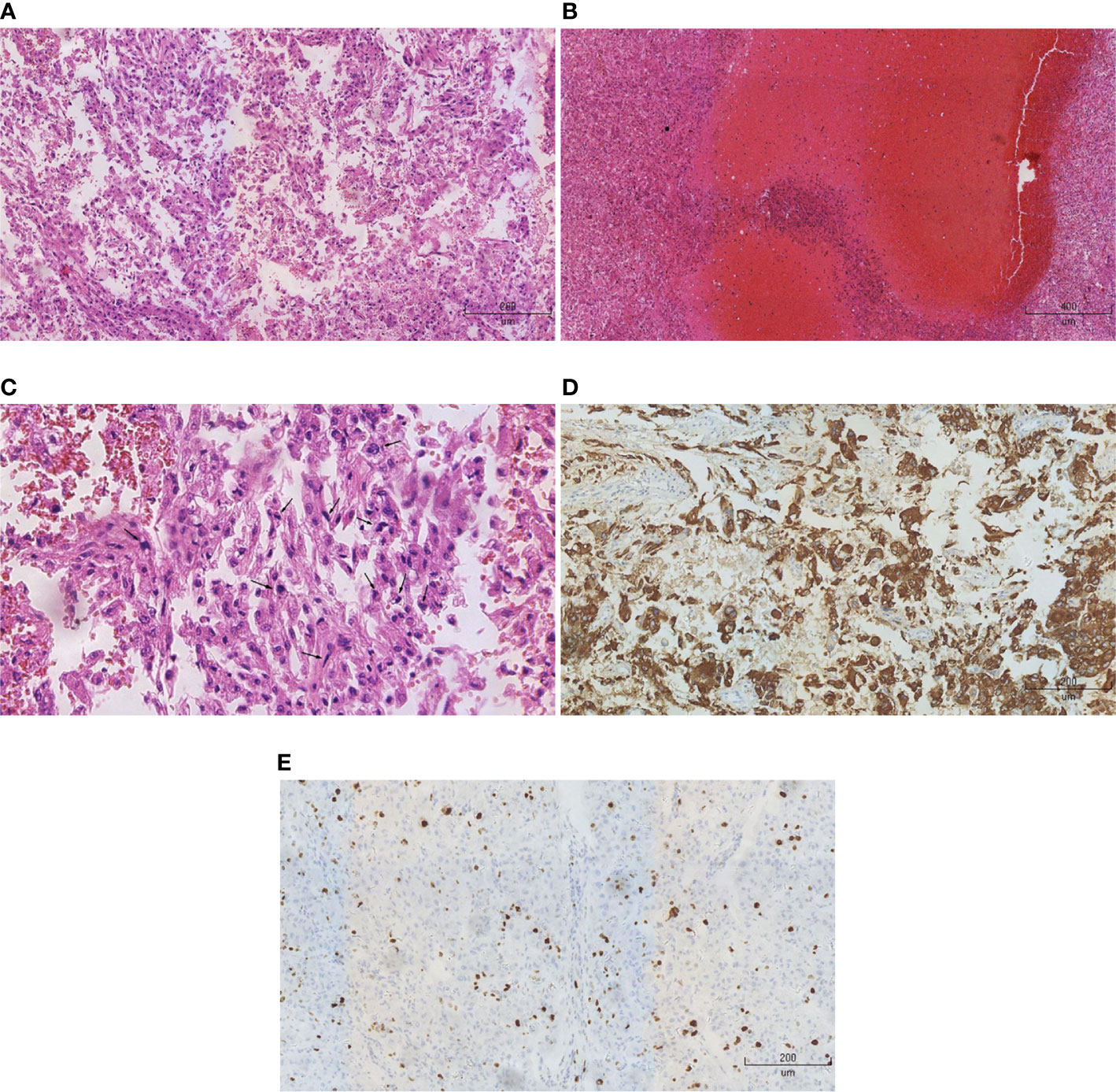
Figure 1 Pathological and immunohistochemical analysis. (A) HE staining (x10) showed that epithelioid cells were predominant in the tumors, but adipose tissue was insufficient. (B) HE staining (x5) showed a large number of necrotic lesions in the tumor tissues. (C) HE staining (x20) showed polymorphonuclear and heterokaryon tumor cells. (D) Immunohistochemistry (x10) showed that HMB45 was positive. (E) Immunohistochemistry (x10) showed that the rate of positive-Ki-67 was 20%.
TSC1/2 Next-Generation Sequencing
Sequence capture by the BWA software shows that the average base coverage of all NGS samples was 99.88%, and the minimum sample coverage was 99.44%. The average proportion of targeted area with average sequencing depth > 30X loci was 98.92%, the minimum value was 97.35%. The average sequencing depth of all samples was 280.4X, and the minimum sequencing depth was 168.4X. Moreover, the average and the median sequencing depth of each exon of TSC1 and TSC2 gene were similar, which indicated that the target sequence capturing next-generation sequencing had great randomness.
A total of two clinically meaningful TSC2 mutations and 2 TSC1 mutation were detected, including three missense mutations, one large fragment deletion mutation; one had point mutations without clinical meaning; two did not have mutations (Table 2). According to the previous literatures and the LOVD database MJ (11), c.1700C > T missense mutation, a mutation unconfirmed clinical meaning, in exon 15 of TSC1 was detected in case 1. The routine Sanger sequencing was used to verify the five NGS detected mutation sites in seven cases. The results were identical with those of target sequence capture sequencing, and the coincidence rate was 100%.
The Efficacy and Safety of Everolimus
Seven patients were treated with everolimus 10 mg once daily for 3 to 28 months. The clinical response was evaluated after 1-month treatment: five PR, 2 SD, the mean longest tumor diameter of all patients decreased from 9.6 to 6.4 cm. After 3-month treatment: five PR, 2 SD, the mean greatest tumor diameter was 5.2 cm (Figure 2), and there was no significant change of tumor size at 6 months (Figure 3). After 3–28 months’ follow-up, six patients were SD; one patient suffered progression after 16 months and died after 26 months. The efficacy evaluation showed five patients with tuberous sclerosis were PR, in contrast, patients without tuberous sclerosis were SD.
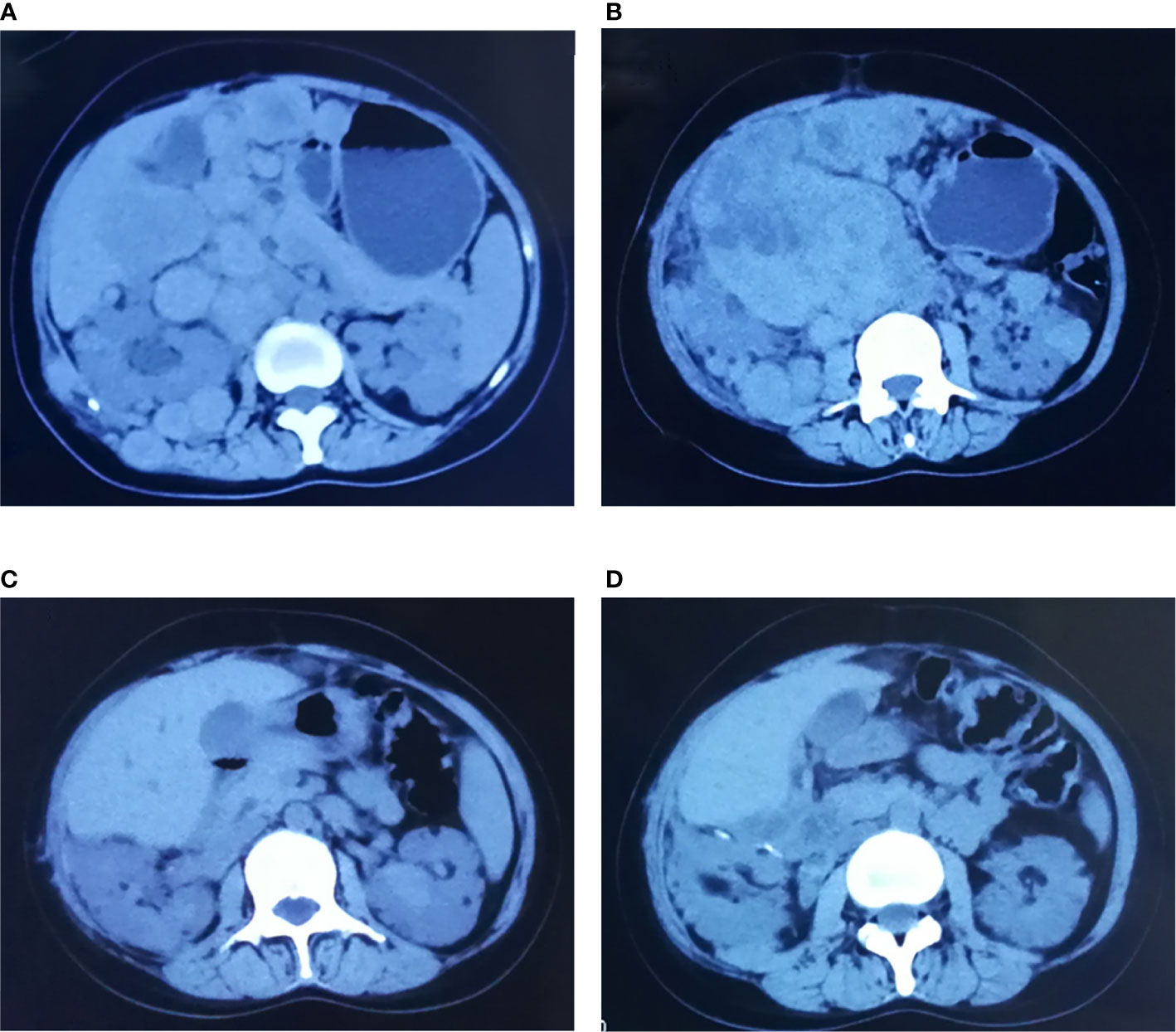
Figure 2 Comparisons of CT before and after treatment with everolimus (case 5). (A, B) Before the treatment, CT scan: right retroperitoneal and abdominal cavity multiple space occupied lesion. (C, D) After 3-month treatment of everolimus, CT scan: space-occupying lesions in retroperitoneal and abdominal cavity mostly disappeared, the lesions shrank significantly.
The main adverse effects (AEs) including oral mucositis in five patients, rash in two patients, anemia in two patients, menstrual disorder in two patients and hepatic dysfunction in one patient. Most of the AEs were grade 1 or 2, no need to dosage reduction or discontinuation, and can be alleviated after symptomatic treatment. One patient had grade 3 liver dysfunction after 9 months’ treatment. The minimum blood concentration of rapamycin was 15.18 ng/ml, which converted to the blood concentration of everolimus was 19.7 ng/ml; it was relieved after everolimus suspension and symptomatic treatment. And then, this patient restarted everolimus treatment with an adjusted dose of 5 mg once daily. The blood concentration of everolimus was 7.4 ng/ml in re-examination.
Discussion
Renal EAML is a rare renal neoplasm, represents less than 1% of all renal tumors, 4.6% in all kinds of renal angiomyolipoma (3). AMLs are benign and lack of specific clinical manifestations, when the lesions are small, most cases are asymptomatic and incidentally diagnosed. Renal EAMLs are always diagnosed with imaging examination, however, the imaging characteristics of renal EAML are diverse. Some tend to be more aggressive in appearance with invasion of adjacent organs or distant metastases, which lead to misdiagnosed as other types of renal tumors. In our study, the clinical presentation including pain in five patients and hematuria in one patient, MRI showed fat-deficient lesion. Two patients were diagnosed as tuberous sclerosis by clinical presentation.
Renal EAML has unique biological behaviors, predictors for malignant diagnosis and patient’s prognosis had been explored by other investigators. In 2015, Lei et al. have proposed predictive model based on tumor specimens and pathological features: 1) tumor size >9 cm, 2) tumor thrombus formation in the vein, 3) epithelioid cells >70% or atypia cells >60%, and 4) necrosis. A lesion that met three or more of the above features was predicted to have an increased risk of malignant behavior (12). Varma et al. have reported that P53-positive in renal EAML tumors indicating a tendency of malignant transformation, Ki67 and P53 staining were helpful in the distinguish of benign or malignant tumors (13).
In our study, all patients were HMB45 staining positive. Five of them had multiple metastases, their common characteristics included that the positive rate of Ki-67 was more than 10%, and the mitotic figure was more than 2/10 HPF. For the other two patients, the positive rate of Ki-67 was 3%, and the mitotic figure was 1–2/10HPF. A young female patient in our study developed tumor progression rapidly within a short period which inducing abortion. It is presumed that this phenomenon may be associated with high expression of estrogen and progesterone receptors in this disease (14). Tsai et al. have showed family tuberous sclerosis or tumors in other sites can be detected in more than half of renal EAML patients (15). The proportion of tuberous sclerosis in renal EMAL was significantly higher than AML. About 20–30% of young renal EAML patients were accompanied with tuberous sclerosis (16). All patients in our study underwent TSC1/2 gene test, four patients had mutations in pathogenic TSC1 or TSC2 genes, five patients met the clinical or genetic diagnostic criteria for tuberous sclerosis, accounting for 35.7% (5/14) of EAML patients diagnosed in our hospital during the same period.
There is no standard treatment for renal EAML, surgical resection is effective and the complete tumor resection can improve the outcome (17). Referring to selecting surgical methods, we should consider the size, site, invasion of surrounding tissues, and distant metastasis of the tumors comprehensively. According to our results, five patients experienced local recurrences which reflected the importance of complete resection. Some investigators have investigated the benefit of treatment with rapamycin, cyclophosphamide, and cisplatin for renal EAML; while some cases report showed that renal EAML was not sensitive to radiotherapy, chemotherapy, or molecular targeted therapy (18–20). Kenerson et al. (7) reported abnormal activation of mTOR pathway may contribute to renal EAML growth and progression. In some patients, mTOR inhibitors such as rapamycin and everolimus reduced the size of the tumors, suggested that mTOR inhibitors may be therapeutic option for EAML. In a prospective, randomized, multicenter clinical study (EXIST-2), 79 AML patients diagnosed TSC received 6-month treatment of everolimus, the proportion of renal AML patients achieving ≥50% reductions in everolimus cohort was 42%, and 0 in control cohort (21). However, the use of everolimus in invasive EAML has been reported only in some cases (22–24).
The tumor size of five patients in our trial, diagnosed as EAML with tuberous sclerosis, significantly decreased after everolimus treatment; only one suffered progression and died. Two without tuberous sclerosis did not have a significant change in tumor size. We chose 10mg/day as an initial dose of everolimus based on previous treatment for renal angiomyolipoma with tuberous sclerosis. Most of the AEs were of grade 1–2, the incidence of grade 3–4 AEs was quite low. Regular follow up for safety and plasma concentration can minimize the risk of serious AEs. Dosage adjustment can be considered based on the plasma concentration, which will be helpful for patients’ safety and compliance to treatment.
In conclusion, invasive renal EAML is a type of tumor with malignant potential, and the complete tumor resection is a key factor for cure. Gene mutation analysis of TSC1 and TSC2 should be performed in the patient whose lesion can’t be resected completely in preoperative assessment, or progressed after surgery. The mTOR inhibitor can be an effective treatment for patients with invasive malignant renal EAML. Patients with TSC may benefit more from the therapy. Our finding still needs to be confirmed in a larger, prospective, randomized trial.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Chinese PLA General Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
Conception and design: GG, LG, XZ. Data collection or management: GG, LG. data analysis: GG, LG. manuscript writing/editing: GG, LG, XZ. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.610858/full#supplementary-material
References
1. Bhatt JR, Richard PO, Kim NS, Finelli A, Manickavachagam K, Legere L, et al. Natural History of Renal Angiomyolipoma (AML): Most Patients with Large AMLs >4cm Can Be Offered Active Surveillance as an Initial Management Strategy. Eur Urol (2016) 70(1):85–90. doi: 10.1016/j.eururo.2016.01.048
2. Bissler JJ, Kingswood JC. Renal angiomyolipomata. Kidney Int (2004) 66(3):924–34. doi: 10.1111/j.1523-1755.2004.00838.x
3. He W, Cheville JC, Sadow PM, Gopalan A, Fine SW, Al-Ahmadie HA, et al. Epithelioid angiomyolipoma of the kidney: pathological features and clinical outcome in a series of consecutively resected tumors. Mod Pathol (2013) 26(10):1355–64. doi: 10.1038/modpathol.2013.72
4. Aydin H, Magi-Galluzzi C, Lane BR, Sercia L, Lopez JI, Rini BI, et al. Renal angiomyolipoma: clinicopathologic study of 194 cases with emphasis on the epithelioid histology and tuberous sclerosis association. Am J Surg Pathol (2009) 33(2):289–97. doi: 10.1097/PAS.0b013e31817ed7a6
5. Raman SP, Hruban RH, Fishman EK. Beyond renal cell carcinoma: rare and unusual renal masses. Abdom Imaging (2012) 37(5):873–84. doi: 10.1007/s00261-012-9903-5
6. Delgado R, de Leon Bojorge B, Albores-Saavedra J. Atypical angiomyolipoma of the kidney: a distinct morphologic variant that is easily confused with a variety of malignant neoplasms. Cancer (1998) 83(8):1581–92. doi: 10.1002/(SICI)1097-0142(19981015)83:8<1581::AID-CNCR13>3.0.CO;2-R
7. Kenerson H, Folpe AL, Takayama TK, Yeung RS. Activation of the mTOR pathway in sporadic angiomyolipomas and other perivascular epithelioid cell neoplasms. Hum Pathol (2007) 38(9):1361–71. doi: 10.1016/j.humpath.2007.01.028
8. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
9. Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, Version 4.0, DCTD, NCI, NIH, DHHS May 28, 2009. (2009). Available at: http://ctep.cancer.gov.
10. Dasgupta A, Moreno V, Balark S, Smith A, Sonilal M, Tejpal N, et al. Rapid estimation of whole blood everolimus concentrations using architect sirolimus immunoassay and mathematical equations: comparison with everolimus values determined by liquid chromatography/mass spectrometry. J Clin Lab Anal (2011) 25(3):207–11. doi: 10.1002/jcla.20459
11. Piras R, Chiappe F, Torraca IL, Buers I, Usala G, Angius A, et al. Expanding the mutational spectrum of CRLF1 in Crisponi/CISS1 syndrome. Hum Mutat (2014) 35(4):424–33. doi: 10.1002/humu.22522
12. Lei JH, Liu LR, Wei Q, Song TR, Yang L, Yuan HC, et al. A Four-Year Follow-up Study of Renal Epithelioid Angiomyolipoma: A Multi-Center Experience and Literature Review. Sci Rep (2015) 5:10030. doi: 10.1038/srep10030
13. Varma S, Gupta S, Talwar J, Forte F, Dhar M. Renal epithelioid angiomyolipoma: a malignant disease. J Nephrol (2011) 24(1):18–22. doi: 10.5301/JN.2010.5451
14. El-Hashemite N, Walker V, Kwiatkowski DJ. Estrogen enhances whereas tamoxifen retards development of Tsc mouse liver hemangioma: a tumor related to renal angiomyolipoma and pulmonary lymphangioleiomyomatosis. Cancer Res (2005) 65(6):2474–81. doi: 10.1158/0008-5472.CAN-04-3840
15. Tsai CC, Wu WJ, Li CC, Wang CJ, Wu CH, Wu CC. Epithelioid angiomyolipoma of the kidney mimicking renal cell carcinoma: a clinicopathologic analysis of cases and literature review. Kaohsiung J Med Sci (2009) 25(3):133–40. doi: 10.1016/S1607-551X(09)70052-X
16. Froemming AT, Boland J, Cheville J, Takahashi N, Kawashima A. Renal epithelioid angiomyolipoma: imaging characteristics in nine cases with radiologic-pathologic correlation and review of the literature. AJR Am J Roentgenol (2013) 200(2):W178–86. doi: 10.2214/AJR.12.8776
17. Luo J, Liu B, Wang Y, Li J, Wang P, Chen J, et al. Comprehensive clinical and pathological analysis of aggressive renal epithelioid angiomyolipoma: report of three cases. Onco Targets Ther (2014) 7:823–7. doi: 10.2147/OTT.S61524
18. Shitara K, Yatabe Y, Mizota A, Sano T, Nimura Y, Muro K. Dramatic tumor response to everolimus for malignant epithelioid angiomyolipoma. Jpn J Clin Oncol (2011) 41(6):814–6. doi: 10.1093/jjco/hyr035
19. Park HK, Zhang S, Wong MK, Kim HL. Clinical presentation of epithelioid angiomyolipoma. Int J Urol (2007) 14(1):21–5. doi: 10.1111/j.1442-2042.2006.01665.x
20. Cibas ES, Goss GA, Kulke MH, Demetri GD, Fletcher CD. Malignant epithelioid angiomyolipoma (‘sarcoma ex angiomyolipoma’) of the kidney: a case report and review of the literature. Am J Surg Pathol (2001) 25(1):121–6. doi: 10.1097/00000478-200101000-00014
21. Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet (2013) 381(9869):817–24. doi: 10.1016/S0140-6736(12)61767-X
22. Espinosa M, Roldán-Romero JM, Duran I, de Álava E, Apellaniz-Ruiz M, Cascón A, et al. Advanced sporadic renal epithelioid angiomyolipoma: case report of an extraordinary response to sirolimus linked to TSC2 mutation. BMC Cancer (2018) 18(1):561. doi: 10.1186/s12885-018-4467-6
23. Chuang CK, Lin HCA, Tasi HY, Lee KH, Kao Y, Chuang FL, et al. Clinical presentations and molecular studies of invasive renal epithelioid angiomyolipoma. Int Urol Nephrol (2017) 49(9):1527–36. doi: 10.1007/s11255-017-1629-4
Keywords: renal epithelioid angiomyolipoma, everolimus, tuberous sclerosis complex, mutation, next-generation sequencing
Citation: Guo G, Gu L and Zhang X (2021) Everolimus in Invasive Malignant Renal Epithelioid Angiomyolipoma. Front. Oncol. 10:610858. doi: 10.3389/fonc.2020.610858
Received: 27 September 2020; Accepted: 30 November 2020;
Published: 26 January 2021.
Edited by:
Janice P. Dutcher, Cancer Research Foundation, United StatesReviewed by:
Xinan Sheng, Peking University Cancer Hospital, ChinaLothar Bergmann, University Hospital Frankfurt, Germany
Copyright © 2021 Guo, Gu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Zhang, eHpoYW5nQGZveG1haWwuY29t
†These authors have contributed equally to this work
 Gang Guo†
Gang Guo† Liangyou Gu
Liangyou Gu Xu Zhang
Xu Zhang