- 1Department of Radiotherapy, Guangxi Medical University Cancer Hospital, Nanning, China
- 2Department of Medical Oncology, Ruikang Hospital Affiliated to Guangxi University of Chinese Medical, Nanning, China
- 3Department of Medical Oncology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 4Department of Oncology, Wuming Hospital of Guangxi Medical University, Nanning, China
Background: The efficacy of induction chemotherapy (IC) followed by concurrent chemoradiotherapy (CCRT) in locoregionally advanced nasopharyngeal cancer (LA-NPC) is controversial. In this paper, we conduct a meta-analysis based on relevant studies to provide strong evidence for clinical strategies.
Materials and Methods: We searched the MEDLINE, Embase, Cochrane, PubMed, and Web of Science databases for studies that stratified patients based on a high or low plasma Epstein–Barr virus deoxyribonucleic acid (EBV-DNA) load before treatment and compared the clinical efficacy of IC+CCRT vs. CCRT alone in LA-NPC. We tested for heterogeneity of studies and conducted sensitivity analysis. Subgroup analysis was performed for overall survival (OS), progression-free survival (PFS), distant metastasis-free survival (DMFS), and locoregional relapse-free survival (LRFS).
Results: Seven studies with a total of 5289 cases were finally included in the meta-analysis. The heterogeneity test revealed the homogeneity of OS (I2 = 0.0%, p=0.794), PFS (I2 = 0.0%, p=0.778), DMFS (I2 = 0.0%, p=0.997), and LRFS (I2 = 0.0%, p=0.697) in patients with EBV-DNA loads of ≥4000 copies/ml in both the IC+CCRT and CCRT groups. The results reveal that IC+CCRT significantly extended the OS (HR 0.70 [95% CI 0.58-0.83], p=0.000), PFS (HR 0.83 [95% CI 0.70-0.99], p=0.033), and DMFS (HR 0.79 [95% CI 0.69-0.9], p=0.000) of patients compared with the CCRT group, but there were no beneficial effects on LRFS (HR 1.07 [95% CI 0.80-1.42], p=0.647). The heterogeneity test found that there was no significant heterogeneity of PFS (I2 = 0.0%, p=0.564), DMFS (I2 = 0.0%, p=0.648), LRFS (I2 = 22.3%, p=0.257), and OS (I2 = 44.6%, p=0.164) in patients with EBV-DNA loads of <4000 copies/ml. The results show that IC+CCRT prolonged DMFS (HR 0.57 [95% CI 0.39-0.85], p=0.006) of patients without significant improvements in OS (HR 0.88 [95% CI 0.55-1.26], p=0.240), PFS (HR 0.98 [95% CI 0.74-1.31], p=0.908), and LRFS (HR 0.98 [95% CI 0.54-1.77], p=0.943).
Conclusions: Pretreatment plasma EBV-DNA can be considered a promising effective marker for the use of IC in LA-NPC patients. The addition of IC could improve the OS and PFS of patients with EBV-DNA load ≥4000 copies/ml, but we saw no efficacy in patients with EBV-DNA load <4000 copies/ml. Moreover, regardless of the EBV-DNA load, IC could improve DMFS, but there was no effect on LRFS.
Introduction
Nasopharyngeal cancer (NPC) is an epithelial malignancy featuring a regional incidence as evidenced by the prevalence in Guangdong and Guangxi provinces in South China, Southeast Asia, and North Africa (1, 2). Non-keratinizing NPC is the main pathological type in high-incidence areas, and almost all patients have been infected with Epstein–Barr virus (EBV). Studies demonstrate that EBV is involved in the development of this malignancy and is considered a key pathogenic factor (3, 4). Therefore, EBV-related molecules are ideal biomarker candidates for NPC, of which plasma EBV-deoxyribonucleic acid (DNA) possesses high specificity and sensitivity and exhibits prominent perspectives in screening, diagnosis, and prognostic prediction of the disease (5).
According to the 7th or 8th edition of American Joint Committee on Cancer (AJCC), stage III–IVB NPC (AJCC 7th) or stage III–IVA NPC (AJCC 8th) is the most common advanced type with a high proportion of 70%–90% in newly diagnosed cases (6, 7). Because of its high radiosensitivity, intensity modulated radiation therapy (IMRT)-based concurrent chemoradiotherapy is confirmed as the main treatment for locoregionally advanced NPC (LA-NPC), providing 3- and 5-year overall survival (OS) rates of 76% and 72.3%, respectively (8–10). However, about 20%–30% of patients still develop relapses and metastases after standard treatment (11). Instead, concurrent chemoradiotherapy (CCRT)-based intensive chemotherapy (IC) is regarded as an effective method to lengthen the OS and to reduce progression risks for these patients (12, 13).
Currently, the National Comprehensive Cancer Network (NCCN) guidelines recommend CCRT in combination with IC for advanced NPC, supported by level IIA evidence (14). However, the application of IC has subsequently become a controversial focus for the treatment of LA-NPC—the main dissent is the increasing toxicity without benefit for the OS of patients (15). As a result, researchers make great efforts to develop reliable tools to address this concern. It is of particular note that, because the proposal of pretreatment EBV-DNA load for the guidance of IC, many studies have explored and uncovered that this biomarker, which sensitively reflects tumor burden, might point out a direction for intensification therapy in LA-NPC (16). This study aimed to investigate the role of pretreatment plasma EBV-DNA load in IC using a meta-analysis, hoping to provide evidence for the use of IC in clinical practice.
Materials and Methods
Literature Screening and Search Strategy
All methods were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (17, 18). We thoroughly searched all relevant studies updated to May 12, 2020, in PubMed (https://www.ncbi.nlm.nih.gov/pubmed), MEDLINE (https://www.nlm.nih.gov/bsd/medline), Cochrane (https://www.cochranelibrary.com), Embase (https://www.embase.com), and Web of Science (https://www.webofknowledge.com). The search strategy was as follows: ((nasopharyngeal carcinoma) OR (nasopharyngeal cancer) OR (nasopharyngeal neoplasms)) AND ((induction chemotherapy) OR (neo adjuvant chemotherapy) OR (neoadjuvant chemotherapy) OR (new adjuvant chemotherapy) OR (new supplementary chemotherapy) OR (inductive chemotherapy) OR (induced chemotherapy)) AND ((Epstein-Barr Virus DNA) OR (EBV-DNA)). Two authors independently conducted the literature search and initially picked out relevant studies by reading titles and abstracts. Studies describing irrelevant subjects were excluded in the first step. The remaining studies were further screened via reading the full texts, and ineligible studies were discarded.
Inclusion and Exclusion Criteria
Studies could be included if they met the following criteria: (i) subjects were pathologically diagnosed with LA-NPC without distant metastasis or other major complications and received initial treatment; (ii) subjects were assigned to the IC+CCRT and CCRT groups according to pretreatment EBV-DNA load levels, and the efficacy of the two treatment modes was evaluated; (iii) the survival endpoint was OS, progression-free survival (PFS), distant metastasis-free survival (DMFS), or locoregional relapse-free survival (LRFS); (iv) randomized controlled intervention studies and observational studies, such as case-control and cohort studies; (v) all studies were original English-language papers that had already been published with full texts. Studies were excluded if they were reporting (i) literature reviews, case reports, comments, conference proceedings, or animal experiments; (ii) without the data of efficacy between IC+CCRT and CCRT; (iii) without important data (e.g., plasma EBV-DNA loads) for analysis.
Data Extraction
Two authors independently extracted data from all included studies using standardized forms. Any disagreement was addressed through discussions between them or by a more experienced senior researcher if necessary. The extracted data mainly were clinical characteristics, including publication year, study design, location, sample size, and subjects as well as primary outcomes between IC+CCRT and CCRT, such as the corresponding hazard ratios (HRs) and 95% confidence intervals (CIs) for OS, PFS, DMFS, and LRFS. The two authors directly extracted HRs and 95% CIs from studies or extracted survival rates from survival curves using Engauge Digitizer 6.1 software and calculated HRs and 95% CIs using Tierney’s Excel (19, 20).
Literature Quality Assessment
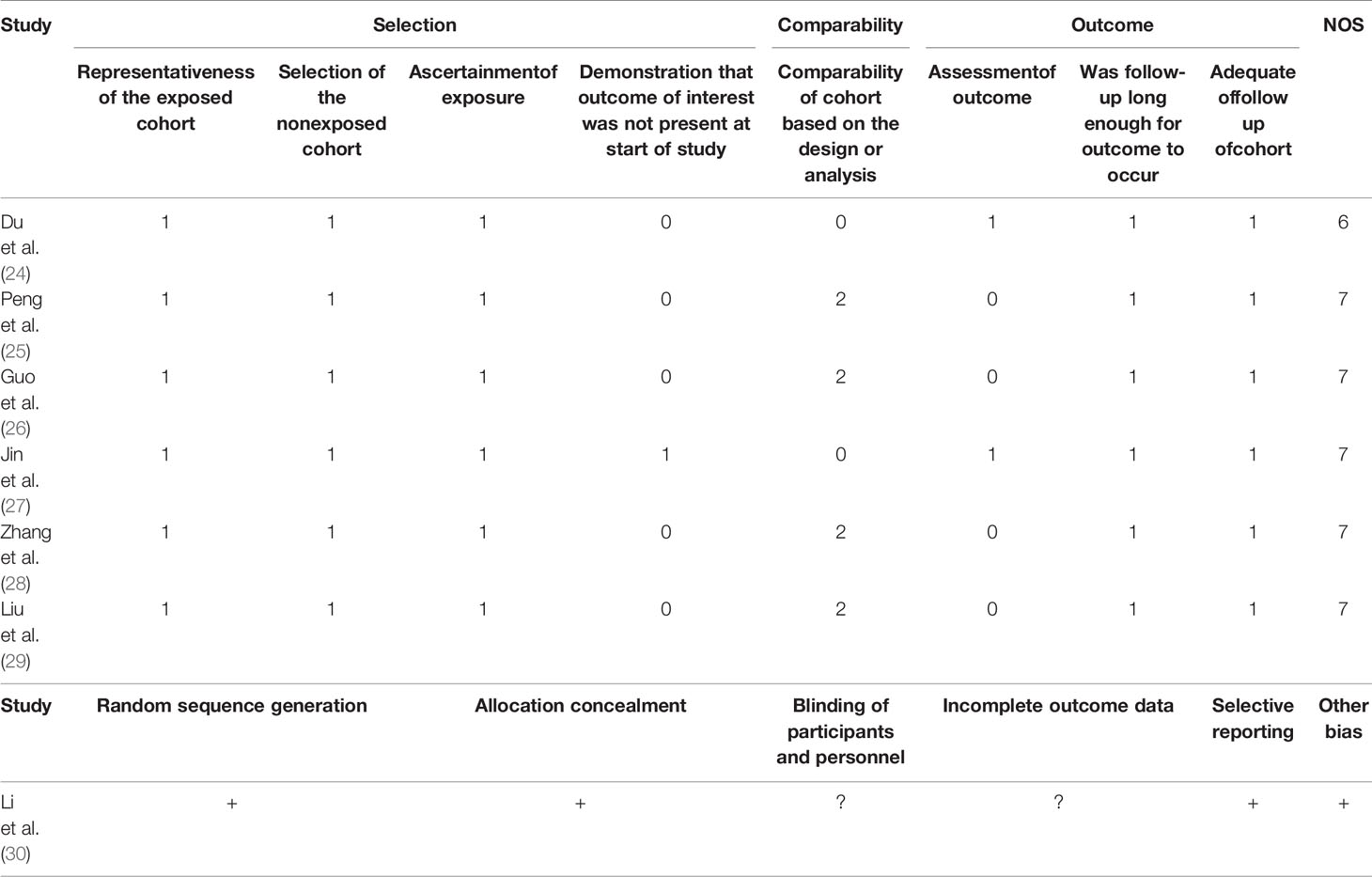
Two authors independently assessed the quality of the included studies. Any disagreement was settled through discussion until consensus was reached. Unsettled disagreements were addressed by consultations with the corresponding author. The quality of nonrandomized studies was evaluated using the Newcastle–Ottawa scale (NOS) (21). The quality of randomized controlled trials (RCTs) was assessed using the Cochrane bias assessment tool from the following six dimensions: random allocation, allocation concealment, blinding, research integrity, selective reporting bias, and other bias (22).
Statistical Analysis
Statistical analysis was performed using Stata 11.0 software (Stata Corporation, College Station, TX), and a p-value < 0.05 of summary statistics was considered significant. Heterogeneity was assessed by I2 statistics, and the p-value of the chi-square test. I2 values of 25%, 50%, and 75% were considered as low, moderate, and high heterogeneity, respectively (23). If heterogeneity was not significant (I2 ≤ 50% and p>0.1), a fixed-effects model was applied; if heterogeneity was significant (I2>50% and p ≤ 0.1), a random-effects model was used to merge the data and assess effect-size indicators. Sensitivity analyses (no less than 3 studies) were repeated by removing one study each time to estimate the effect of a single study on the overall risk. Publication bias was assessed by the funnel plot, and funnel plot asymmetry (no less than 9 studies) was analyzed using Egger’s test.
Results
Study Characteristics
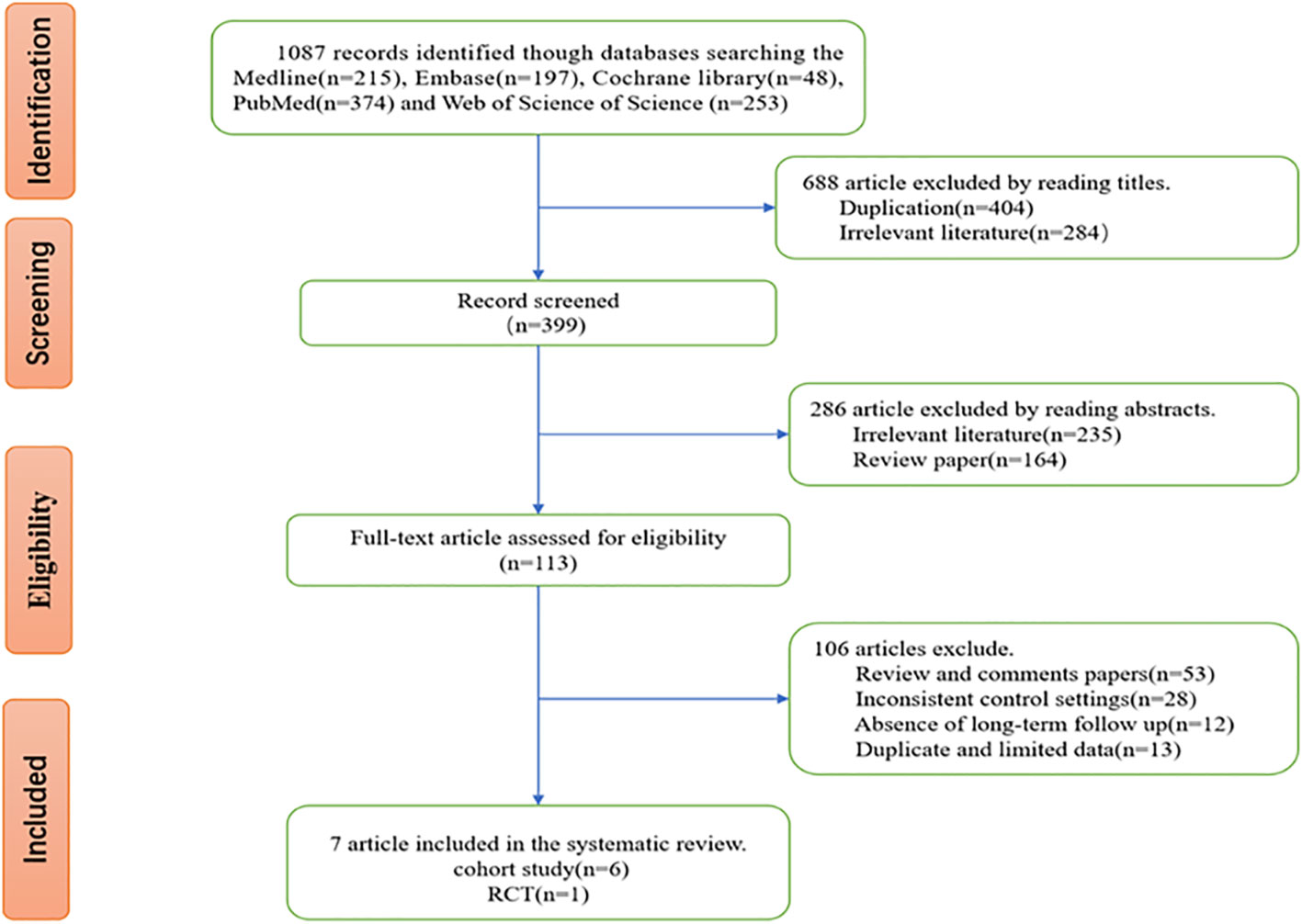
After we searched the five databases, we initially acquired 1087 relevant studies, including 215 studies from Medline, 197 studies from Embase, 48 studies from the Cochrane Library, 374 studies from PubMed, and 253 studies from Web of Science. After screening, 7 studies that met the inclusion and exclusion criteria and whose data were extractable were finally included in our meta-analysis. The flowchart of study selection processes is shown in Figure 1.
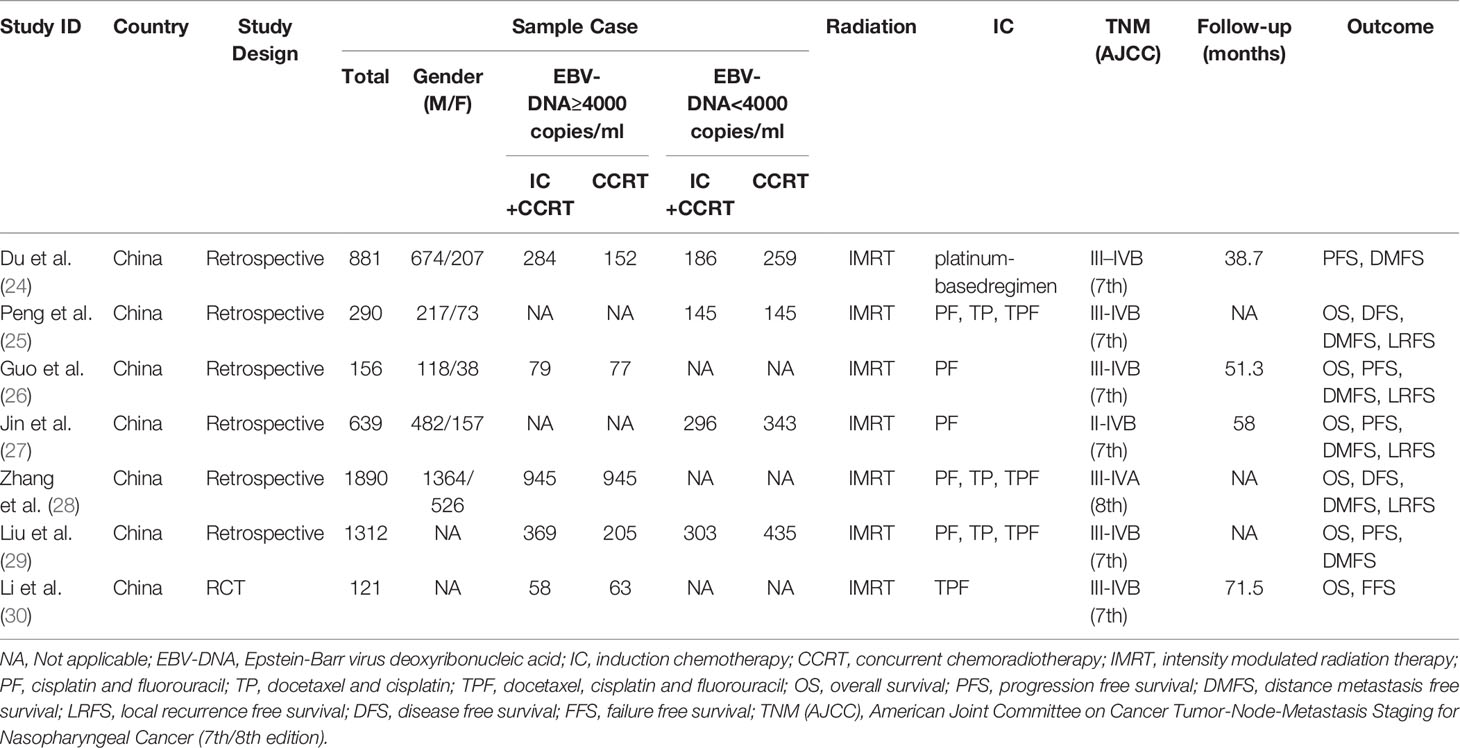
Of the 7 included studies, 6 were observational studies, and 1 was an RCT. All studies were conducted in China with a total sample size of 5289 cases. The minimum sample size of investigation was 121 cases, and the maximum sample size was 1890 cases. Two studies possessed a sample size of more than 1000 cases. All subjects were stage III–IVB NPC (AJCC 7th) or III–IVA NPC (AJCC 8th) patients who received IMRT in combination with cisplatin-based chemotherapy or IC + cisplatin-based CCRT. The pretreatment plasma EBV-DNA load was quantitated using reverse transcription polymerase chain reaction (RT-PCR) analysis in all studies and had distinct cutoff values (0, 1550, 4000, 4650, and 6000 copies/ml). Most studies did not provide detailed illustration of the PCR method. Some studies indicate that the target gene fragment of PCR detection is EBV BamHI-W region. For the convenience of data extraction and merge, the cutoff value at 4000 copies/ml was selected to stratify patients and merge their outcomes. The median time of follow-up varied from 38.7 to 71.5 months (Table 1).
Quality Assessment
Of all included studies, 6 observational studies received NOS scores of ≥6, and one RCT was at moderate risk of bias. Generally, most studies were high quality in our analysis (Table 2).
Meta-Analysis and Heterogeneity Test
A cutoff value of 4000 copies/ml of pretreatment EBV-DNA was used to define a high (≥4000 copies/ml) or low (<4000 copies/ml) load. The OS, PFS, DMFS, and LRFS of patients after IC+CCRT or CCRT treatment were separately merged for the heterogeneity test.
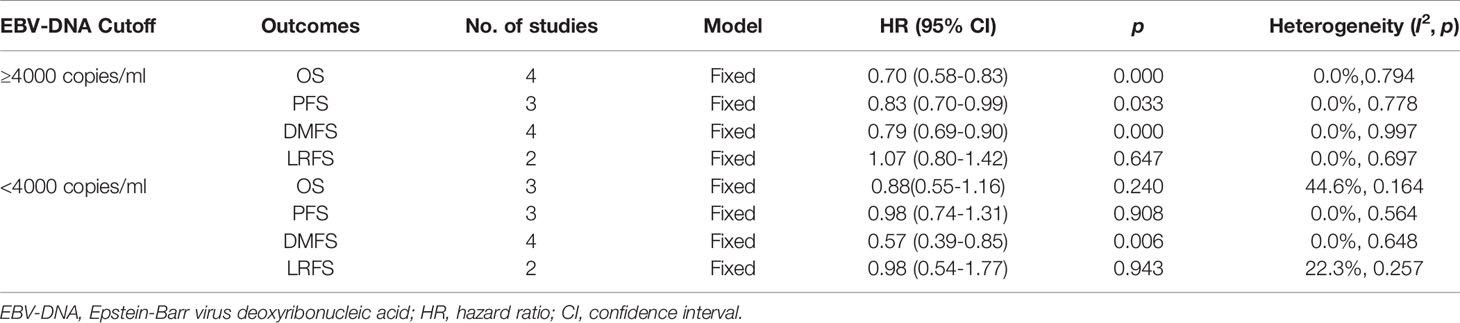
The heterogeneity test for the efficacy outcome revealed the homogeneity of OS (I2 = 0.0%, p=0.794), PFS (I2 = 0.0%, p=0.778), DMFS (I2 = 0.0%, p=0.997), and LRFS (I2 = 0.0%, p=0.697) in patients with pretreatment EBV-DNA loads of ≥4000 copies/ml in both the IC+CCRT and CCRT groups (Figures 2A–D). A fixed-effects model was used for the meta-analysis of the efficacy outcome. The results show that IC+CCRT significantly extended the OS (HR 0.70 [95% CI 0.58-0.83], p=0.000), PFS (HR 0.83 [95% CI 0.70-0.99], p=0.033), and DMFS (HR 0.79 [95% CI 0.69-0.9], p=0.000) of patients compared with the CCRT alone, but there were no benefits to LRFS (HR 1.07 [95% CI 0.80-1.42], p=0.647) (Table 3).
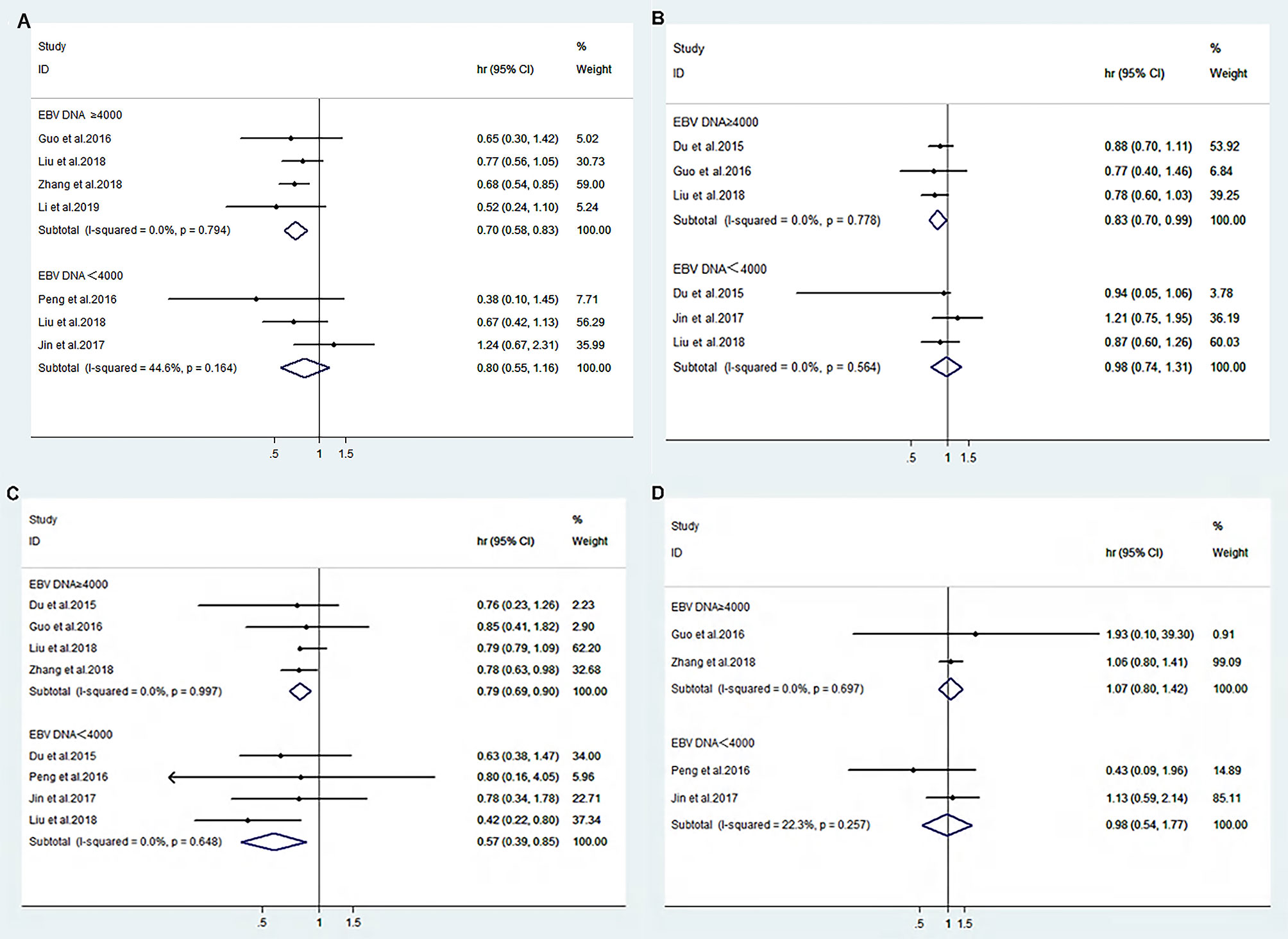
Figure 2 Forest plot of overall survival (A), progression-free survival (B), distance metastasis-free survival (C), and locoregional recurrence-free survival (D) between IC+CCRT and CCRT based on EBV-DNA ≥4000 copies/ml or EBV-DNA <4000 copies/ml.
The heterogeneity test for the efficacy displayed nonsignificant heterogeneity of OS (I2 = 44.6%, p=0.164), PFS (I2 = 0.0%, p=0.564), DMFS (I2 = 0.0%, p=0.648), and LRFS (I2 = 22.3%, p=0.257) in patients with pretreatment EBV-DNA loads of <4000 copies/ml (Figures 2A–D). Therefore, a fixed-effects model was selected for the meta-analysis, and the results suggest that IC+CCRT prolonged DMFS (HR 0.57 [95% CI 0.39-0.85], p=0.006) without significant improvements in OS (HR 0.88 [95% CI 0.55-1.26], p=0.240), PFS (HR 0.98 [95% CI 0.74-1.31], p=0.908), and LRFS (HR 0.98 [95% CI 0.54-1.77], p=0.943) of patients compared with CCRT (Table 3).
Sensitivity Analysis and Publication Bias
We performed a sensitivity analysis to measure the stability and reliability of the data. The results demonstrate that IC+CCRT had more stable efficacy than CCRT in OS, PFS, DMFS, and LRFS of patients with pretreatment plasma EBV-DNA ≥4000 copies/ml (Figures 3A–D). In addition, IC+CCRT exhibited more reliable efficacy than CCRT in OS, PFS, DMFS, and LRFS of patients with pretreatment plasma EBV-DNA <4000 copies/ml (Figures 4A–D). The assessment of publication bias was unavailable as the number of included studies for each meta-analysis was less than 9.
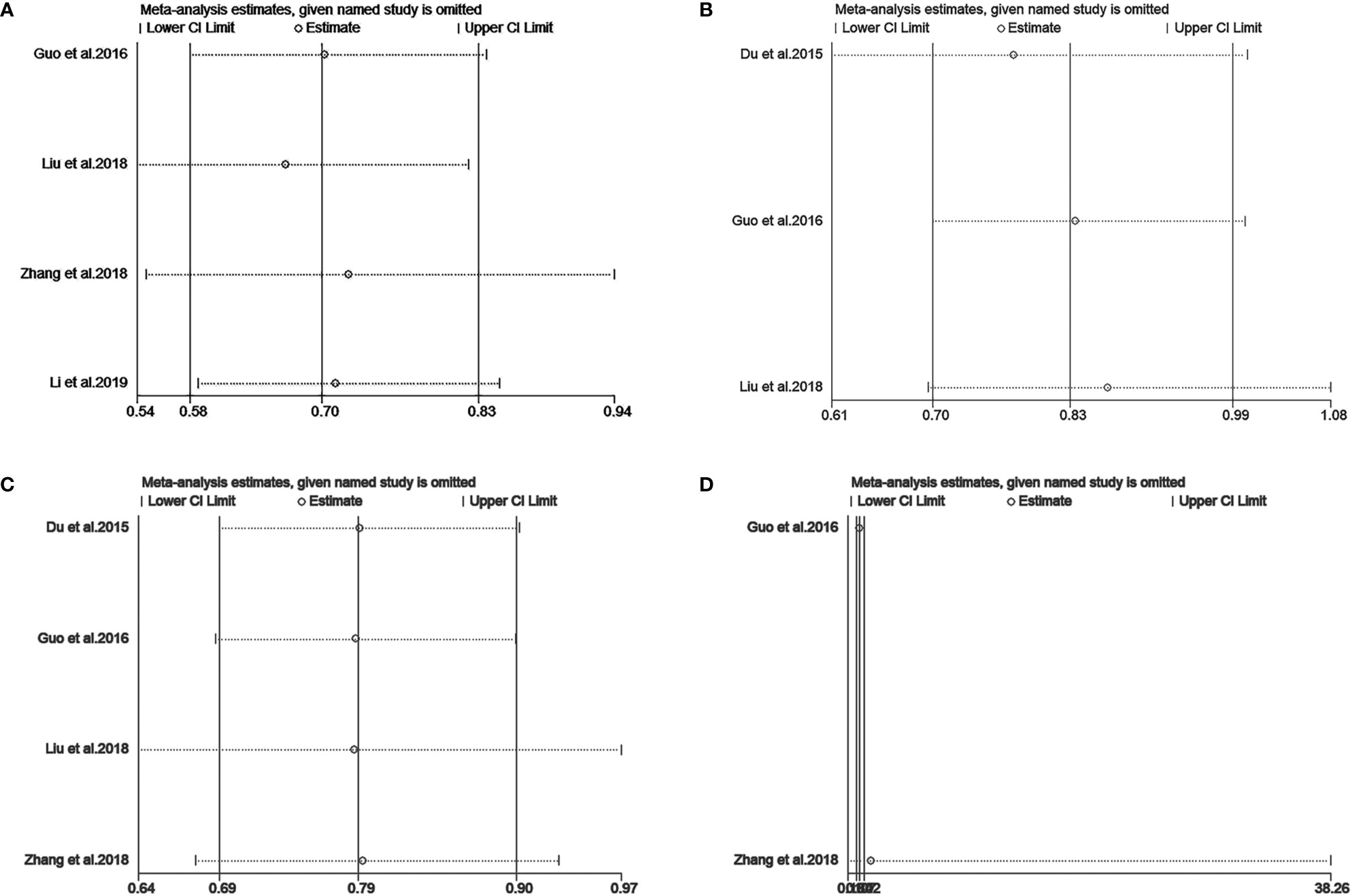
Figure 3 Sensitivity analysis of overall survival (A), progression-free survival (B), distance metastasis-free survival (C), and locoregional recurrence-free survival (D) between IC+CCRT and CCRT based on EBV-DNA ≥4000 copies/ml.
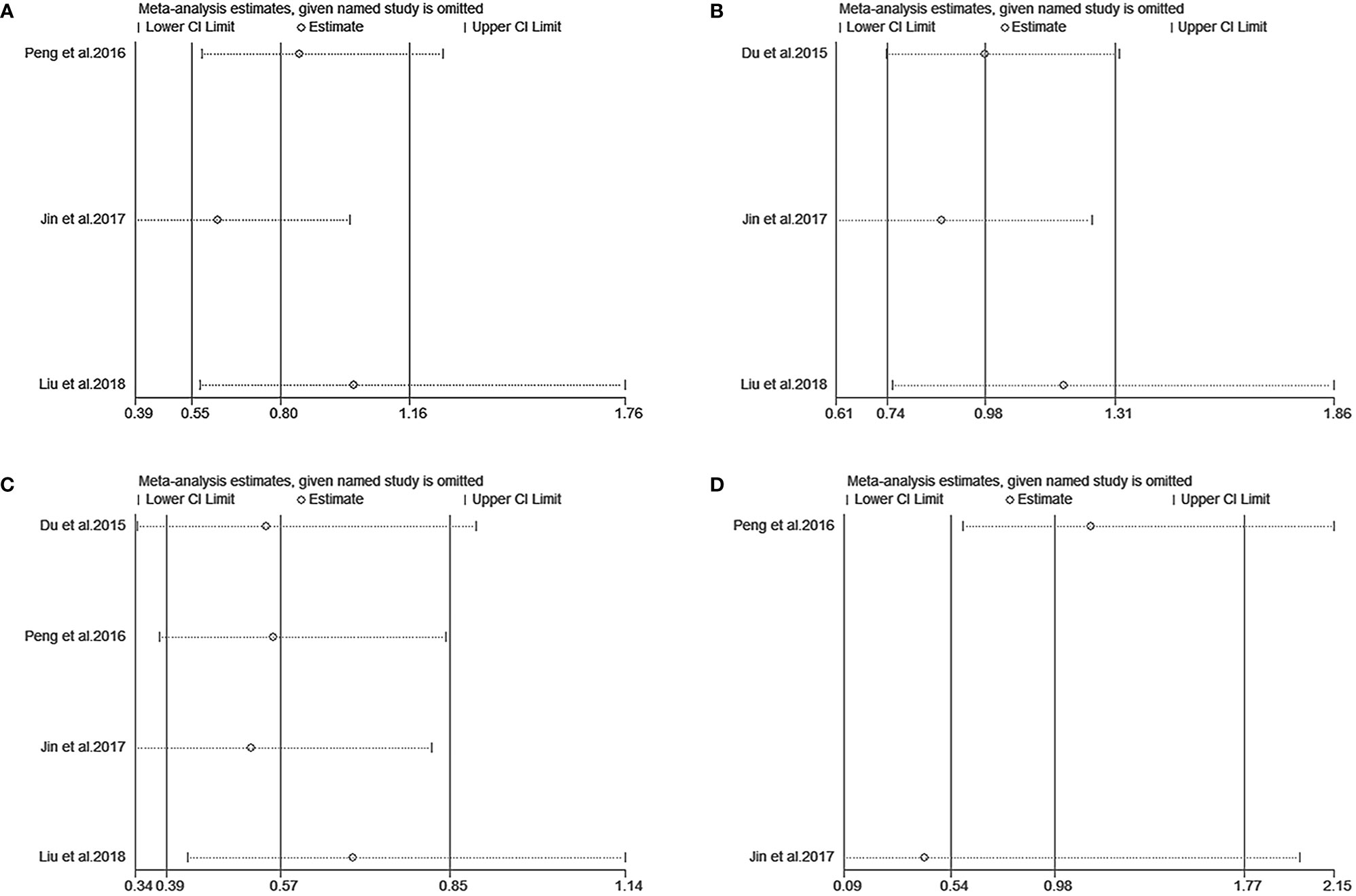
Figure 4 Sensitivity analysis of overall survival (A), progression-free survival (B), distance metastasis-free survival (C), and locoregional recurrence-free survival (D) between IC+CCRT and CCRT based on EBV-DNA <4000 copies/ml.
Discussion
This study contains LA-NPC patients classified as stage III–IVB NPC by AJCC 7th or stage III–IVA NPC by AJCC 8th and investigates the clinical value of the emerging biomarker pretreatment EBV-DNA load in the use of IC. EBV-DNA was detected by PCR methodologically in all of the included studies. We, for the first time, probe the role of pretreatment plasma EBV-DNA load as a guide for IC in LA-NPC through meta-analysis, and the results show that high-risk patients who had a pretreatment EBV-DNA load ≥4000 copies/ml were more likely to benefit from IC. However, low-risk patients who had a pretreatment EBV-DNA load <4000 copies/ml might not benefit from IC.
With the great improvement of focal therapy for cancer control in the era of IMRT, distant metastasis has become the major cause of treatment failure. Thus, studies on the long-term efficacy of NPC treatment have gradually turned to systemic drug therapy from focal therapy (31), and the addition of IC to the basic treatment of CCRT for LA-NPC has been applied in clinical practice (32, 33). However, it is very urgent to stratify the high-risk populations as the OS of all patients cannot be significantly ameliorated after IC (34). The prognostic value of the pretreatment EBV-DNA load in NPC has been confirmed by many researchers. The majority of studies show that high pretreatment EBV-DNA load is a predictor of poor OS, PFS, or DMFS and a risk factor for distant metastasis compared to low pretreatment EBV-DNA load (35, 36). Currently, anti-NPC regimens are adopted according to the sites of tumor invasion and TNM staging. However, tumor heterogeneity also affects the efficacy among patients at this stage (37). In addition, there are a number of studies on the prognostic value of plasma EBV-DNA levels in NPC. In 2000, researchers in Hong Kong confirmed that plasma EBV-DNA load was an independent predictor of metastasis within 1 year after treatment for NPC, and the load level was closely associated with the risk of cancer death; a 10-fold increase in the load level brought about a 1.6-fold increase in the risk of death (5). In 2004, Lin et al. found that the 2-year OS (100% vs. 88.8%) and RFS (83.4% vs. 66.4%) rates in patients who had a pretreatment EBV-DNA load <1500 copies/ml were higher than those in patients who had a pretreatment EBV-DNA load ≥1500 copies/ml (38). All of these findings provide a novel strategy to guide individualized treatment by using pretreatment EBV-DNA load.
In this meta-analysis, the combination of IC and CCRT offered longer PFS and OS for LA-NPC patients who had a pretreatment plasma EBV-DNA load ≥4000 copies/ml without heterogeneity of the efficacy outcome. Sensitivity analysis showed the stable efficacy of IC+CCRT. These results confirm that IC followed by CCRT is an indispensable strategy for patients with a high EBV-DNA load. For patients who had a pretreatment EBV-DNA load <4000 copies/ml, IC did not provide better OS or PFS. Although there was heterogeneity of merged effect sizes in terms of OS, the heterogeneity was nonsignificant (I2 <50%), and efficacy was stable after sensitivity analysis. These results suggest that IC+CCRT cannot improve the PFS and OS of patients with a low EBV-DNA load. Perhaps other combination treatments based on CCRT might be more effective for these low-risk patients. In addition, our findings may aid in guiding medical strategies in clinical practice. Overall, regardless of a pretreatment EBV-DNA load ≥4000 or <4000 copies/ml, the combination of IC and CCRT contributes to improved DMFS but not LRFS compared with CCRT alone. The reason may account for intensive chemotherapy, which mainly plays a role for distant metastasis and has less impact on the risk of local recurrence. This finding highlights the importance of IC for the control of distant metastasis and the optimization of the control of localized or regional lesions. Of note, the improvement of DMFS and LRFS after IC+CCRT has no relation to pretreatment plasma EBV-DNA loads. This outcome indicates that there is great room for investigating more sensitive and reliable prognostic markers based on pretreatment plasma EBV-DNA load.
Recently, more researchers have begun to explore the value of IC in LA-NPC on the basis of pretreatment EBV-DNA load. Du et al. (24) first recognized that pretreatment plasma EBV-DNA load could aid in decision making rather than TNM stage to guide the selection of IC. The authors believed that IC prolonged the DMFS and PFS of N2-3 stage NPC patients with an EBV-DNA load ≥4000 copies/ml. These patients were defined as a very high-risk population who benefited from introduction IC by Guo et al. (26). Subsequently, Peng et al. (25) defined patients with a pretreatment plasma EBV-DNA load <1500 copies/ml as low-risk populations and believed that the introduction of IC did not improve OS, DMFS, PFS, or LRFS of these patients. Similarly, Jin et al. (27) also selected low-risk patients with a pretreatment plasma EBV-DNA load of 0 copies/ml for analysis and yielded consistent results. Zhang et al. (28) first stratified LA-NPC patients by cutting off pretreatment plasma EBV-DNA. The results show that low-risk patients (EBV-DNA <4650 copies/ml) receiving IC obtained no amelioration in OS, DMFS, or LRFS, and high-risk patients (EBV-DNA ≥4650 copies/ml) could benefit from IC in terms of OS and DMFS. Regarding whether patients with a high EBV-DNA load may benefit from IC, a phase III RCT of the optimum IC shows that the OS in the subgroup of patients whose pretreatment plasma EBV-DNA >6000 copies/ml could be significantly improved after IC with docetaxel, cisplatin, and fluorouracil (TPF) regimens (30). However, a study reports that IC promoted DMFS but not OS or PFS in N0-1 of stage III–IVB (AJCC 7th) NPC patients with an EBV-DNA load <4000 copies/mL (the low-risk group). N2-3 patients who had EBV-DNA ≥4000 copies/ml (the high-risk group) did not benefit from IC in terms of OS, PFS, and DMFS (29). Although the cutoff value of pretreatment plasma EBV-DNA was distinct among the above studies, they highlight that pretreatment plasma EBV-DNA could serve as an effective marker in the stratification of a population suitable for IC.
For the first time, we conducted a meta-analysis of plasma EBV-DNA stratifying LA-NPC patients into high- (a high plasma EBV-DNA load) and low-risk (a low plasma EBV-DNA load) groups and compared the efficacy of IC+CCRT vs. CCRT alone in these patients. Our study provided evidence that plasma EBV-DNA had great potential in guiding the use of IC. However, there are some limitations in our study. First, the most included studies are case-control studies, and more prospective randomized controlled studies will be included to make our conclusion more reliable. Second, selection bias is inevitable in the literature-screening processes because unpublished and ongoing publication studies could not be included. Third, studies on the relevant topic are limited, and there is great room for further exploration in this area.
Conclusion
Pretreatment plasma EBV-DNA load can serve as a promising effective marker to guide the selection of IC based on current CCRT strategies for LA-NPC patients. In our study, IC could prolong OS and PFS among patients with a pretreatment plasma EBV-DNA load ≥4000 copies/ml but not in patients with a pretreatment plasma EBV-DNA load <4000 copies/ml. Moreover, our results demonstrate that, regardless of the pretreatment plasma EBV-DNA load, IC could improve DMFS and provides no benefit for LRFS, meaning that it may decrease the risk of distant metastasis and have no effect on reducing local or regional relapse. Hence, it is worth conducting prospective studies to explore and verify these findings further.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
XZ: study conception and design. LL, XYC, CZ, and LC: literature screening and quality assessment. LL, XYC, XSC, and GT: data extraction and data analysis. LL and XYC: manuscript writing and manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by National Natural Science Foundation of China (81760544), Key Research and Development Program Project of Guangxi Zhuang Autonomous Region (Grant No. GuikeAB18221007), Independent Project of Key Laboratory of Early Prevention & Treatment for Regional High‐Incidence‐Tumor (Grant No. GKE2019‐17), Self-Raised Scientific Research Fund of the Ministry of Health of Guangxi Province (No. z2015434), and Science Foundation for Distinguished Young Scholars of Guangxi University of Chinese Medicine (No. 2020JQ001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin (2015) 65(2):87–108. doi: 10.3322/caac.21262
2. Wei K-R, Zheng R-S, Zhang S-W, Liang Z-H, Li Z-M, Chen W-Q. Nasopharyngeal carcinoma incidence and mortality in China, 2013. Chin J Cancer (2017) 36(1):90. doi: 10.1186/s40880-017-0257-9
3. Hatton OL, Harris-Arnold A, Schaffert S, Krams SM, Martinez OM. The interplay between Epstein-Barr virus and B lymphocytes: implications for infection, immunity, and disease. Immunol Res (2014) 58(2-3):268–76. doi: 10.1007/s12026-014-8496-1
4. Chen Y-P, Chan ATC, Le Q-T, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet (2019) 394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0
5. Lo YM, Chan AT, Chan LY, Leung SF, Lam CW, Huang DP, et al. Molecular prognostication of nasopharyngeal carcinoma by quantitative analysis of circulating Epstein-Barr virus DNA. Cancer Res (2000) 60(24):6878–81.
6. Zong J, Lin S, Lin J, Tang L, Chen B, Zhang M, et al. Impact of intensity-modulated radiotherapy on nasopharyngeal carcinoma: Validation of the 7th edition AJCC staging system. Oral Oncol (2015) 51(3):254–9. doi: 10.1016/j.oraloncology.2014.10.012
7. Pan JJ, Ng WT, Zong JF, Chan LLK, O’Sullivan B, Lin SJ, et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer (2016) 122(4):546–58. doi: 10.1002/cncr.29795
8. Lin J-C, Jan J-S, Hsu C-Y, Liang W-M, Jiang R-S, Wang W-Y. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol (2003) 21(4):631–7. doi: 10.1200/JCO.2003.06.158
9. Chan ATC, Teo PML, Ngan RK, Leung TW, Lau WH, Zee B, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol (2002) 20(8):2038–44. doi: 10.1200/JCO.2002.08.149
10. Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol (1998) 16(4):1310–7. doi: 10.1200/JCO.1998.16.4.1310
11. Wu F, Wang R, Lu H, Wei B, Feng G, Li G, et al. Concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: treatment outcomes of a prospective, multicentric clinical study. Radiother Oncol (2014) 112(1):106–11. doi: 10.1016/j.radonc.2014.05.005
12. Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol (2015) 16(6):645–55. doi: 10.1016/S1470-2045(15)70126-9
13. Yang Q, Cao S-M, Guo L, Hua Y-J, Huang P-Y, Zhang X-L, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer (2019) 119:87–96. doi: 10.1016/j.ejca.2019.07.007
14. Gill A, Givi B, Moore MG. AHNS Series - Do you know your guidelines?: Assessment and management of malnutrition in patients with head and neck cancer: Review of the NCCN Clinical Practice Guidelines In Oncology (NCCN Guidelines). Head Neck (2019) 41(3):577–83. doi: 10.1002/hed.24866
15. Sun X-S, Xiao B-B, Lu Z-J, Liu S-L, Chen Q-Y, Yuan L, et al. Stratification of Candidates for Induction Chemotherapy in Stage III-IV Nasopharyngeal Carcinoma: A Large Cohort Study Based on a Comprehensive Prognostic Model. Front Oncol (2020) 10:255. doi: 10.3389/fonc.2020.00255
16. Xu C, Zhang S, Li WF, Chen L, Mao YP, Guo Y, et al. Selection and Validation of Induction Chemotherapy Beneficiaries Among Patients With T3N0, T3N1, T4N0 Nasopharyngeal Carcinoma Using Epstein-Barr Virus DNA: A Joint Analysis of Real-World and Clinical Trial Data. Front Oncol (2019) 9:1–11. doi: 10.3389/fonc.2019.01343
17. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med (2009) 151(4):W65–94. doi: 10.1371/journal.pmed.1000100
18. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (2009) 339:b2535. doi: 10.1136/bmj.b2535
19. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med (1998) 17(24):2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8
20. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8(1):16. doi: 10.1186/1745-6215-8-16
21. Wells G. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. Ottawa Hospital Health Research Institute (2004). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
22. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
23. Higgins J, Thompson S, Deeks J, Altman D. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy (2002) 7(1):51–61. doi: 10.1258/1355819021927674
24. Du XJ, Tang LL, Chen L, Mao YP, Guo R, Liu X, et al. Neoadjuvant chemotherapy in locally advanced nasopharyngeal carcinoma: Defining high-risk patients who may benefit before concurrent chemotherapy combined with intensity-modulated radiotherapy. Sci Rep (2015) 5:16664. doi: 10.1038/srep16664
25. Peng H, Chen L, Li WF, Guo R, Zhang Y, Zhang F, et al. Prognostic Value of Neoadjuvant Chemotherapy in Locoregionally Advanced Nasopharyngeal Carcinoma with Low Pre-treatment Epstein-Barr Virus DNA: a Propensity-matched Analysis. J Cancer (2016) 7(11):1465–71. doi: 10.7150/jca.15736
26. Guo SS, Tang LQ, Chen QY, Zhang L, Liu LT, Guo L, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in stage III-IVb nasopharyngeal carcinoma patients with Epstein-Barr virus DNA ≥4000 copies/ml: A matched study. Oncotarget (2016) 7(20):29739–48. doi: 10.18632/oncotarget.8828
27. Jin YN, Yao JJ, Wang SY, Zhang WJ, Zhang F, Zhou GQ, et al. The Effect of Adding Neoadjuvant Chemotherapy to Concurrent Chemoradiotherapy in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma and Undetectable Pretreatment Epstein-Barr Virus DNA. Trans Oncol (2017) 10(4):527–34. doi: 10.1016/j.tranon.2017.03.007
28. Zhang J, Peng H, Li WF, Zhang Y, Liu LZ, Tian L, et al. Individualized induction chemotherapy by pre-treatment plasma Epstein-Barr viral DNA in advanced nasopharyngeal carcinoma. BMC Cancer (2018) 18(1):1276. doi: 10.1186/s12885-018-5177-9
29. Liu LT, Chen QY, Tang LQ, Guo SS, Guo L, Mo HY, et al. Neoadjuvant or Adjuvant Chemotherapy Plus Concurrent CRT Versus Concurrent CRT Alone in the Treatment of Nasopharyngeal Carcinoma: A Study Based on EBV DNA. J Natl Compr Canc Netw (2019) 17(6):703–10. doi: 10.6004/jnccn.2018.7270
30. Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, Sun Y, et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: long-term results of phase 3 randomized controlled trial. Int J Cancer (2019) 145(1):295–305. doi: 10.1002/ijc.32099
31. Mao Y-P, Tang L-L, Chen L, Sun Y, Qi Z-Y, Zhou G-Q, et al. Prognostic factors and failure patterns in non-metastatic nasopharyngeal carcinoma after intensity-modulated radiotherapy. Chin J Cancer (2016) 35(1):103. doi: 10.1186/s40880-016-0167-2
32. Tan TH, Soon YY, Cheo T, Ho F, Wong LC, Tey J, et al. Induction chemotherapy for locally advanced nasopharyngeal carcinoma treated with concurrent chemoradiation: A systematic review and meta-analysis. Radiother Oncol (2018) 129(1):10–7. doi: 10.1016/j.radonc.2018.02.027
33. Chen Y-P, Tang L-L, Yang Q, Poh S-S, Hui EP, Chan ATC, et al. Induction Chemotherapy plus Concurrent Chemoradiotherapy in Endemic Nasopharyngeal Carcinoma: Individual Patient Data Pooled Analysis of Four Randomized Trials. Clin Cancer Res (2018) 24(8):1824–33. doi: 10.1158/1078-0432.CCR-17-2656
34. Ribassin-Majed L, Marguet S, Lee AWM, Ng WT, Ma J, Chan ATC, et al. What Is the Best Treatment of Locally Advanced Nasopharyngeal Carcinoma? An Individual Patient Data Network Meta-Analysis. J Clin Oncol (2017) 35(5):498–505. doi: 10.1200/JCO.2016.67.4119
35. Lan X-W, Xiao Y, Zou X-B, Zhang X-M, OuYang P-Y, Xie F-Y. Outcomes of adding induction chemotherapy to concurrent chemoradiotherapy for stage T3N0-1 nasopharyngeal carcinoma: a propensity-matched study. Onco Targets Ther (2017) 10:3853–60. doi: 10.2147/OTT.S133917
36. Chen W-H, Tang L-Q, Guo S-S, Chen Q-Y, Zhang L, Liu L-T, et al. Prognostic Value of Plasma Epstein-Barr Virus DNA for Local and Regionally Advanced Nasopharyngeal Carcinoma Treated With Cisplatin-Based Concurrent Chemoradiotherapy in Intensity-Modulated Radiotherapy Era. Medicine (2016) 95(5):e2642. doi: 10.1097/MD.0000000000002642
37. Jin Y-N, Yao J-J, Zhang F, Wang S-Y, Zhang W-J, Zhou G-Q, et al. Is pretreatment Epstein-Barr virus DNA still associated with 6-year survival outcomes in locoregionally advanced nasopharyngeal carcinoma? J Cancer (2017) 8(6):976–82. doi: 10.7150/jca.18124
Keywords: pretreatment plasma EBV-DNA load, locoregionally advanced nasopharyngeal cancer, induction chemotherapy, concurrent chemoradiotherapy, meta-analysis
Citation: Lai L, Chen X, Zhang C, Chen X, Chen L, Tian G and Zhu X (2021) Pretreatment Plasma EBV-DNA Load Guides Induction Chemotherapy Followed by Concurrent Chemoradiotherapy in Locoregionally Advanced Nasopharyngeal Cancer: A Meta-Analysis. Front. Oncol. 10:610787. doi: 10.3389/fonc.2020.610787
Received: 29 September 2020; Accepted: 30 December 2020;
Published: 16 February 2021.
Edited by:
Paolo Bossi, University of Brescia, ItalyReviewed by:
Liting Liu, Sun Yat-sen University Cancer Center (SYSUCC), ChinaPatrizia Comoli, San Matteo Hospital Foundation (IRCCS), Italy
Nicola Alessandro Iacovelli, Istituto Nazionale dei Tumori (IRCCS), Italy
Copyright © 2021 Lai, Chen, Zhang, Chen, Chen, Tian and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaodong Zhu, emh1eGRvbmdneG11QDEyNi5jb20=
†These authors have contributed equally to this work
 Lin Lai
Lin Lai Xinyu Chen3†
Xinyu Chen3† Xiaodong Zhu
Xiaodong Zhu