
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 24 February 2021
Sec. Surgical Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.609844
This article is part of the Research Topic Transplant Oncology of Liver Malignancies View all 6 articles
Backgrounds: Inadequate liver volume and weight is a major source of morbidity and mortality after adult living donor liver transplantation (LDLT). The purpose of our study was to investigate HCC recurrence, graft failure, and patient survival according to change in right liver graft weight after histidine-tryptophan-ketoglutarate (HTK) solution perfusion in LDLT.
Methods: Two hundred twenty-eight patients underwent LDLT between 2013 and 2017. We calculated the change in graft weight by subtracting pre-perfusion graft weight from post-perfusion graft weight. Patients with increased graft weight were defined as the positive group, and patients with decreased graft weight were defined as the negative group.
Results: After excluding patients who did not meet study criteria, 148 patients underwent right or extended right hepatectomy. The negative group included 89 patients (60.1%), and the positive group included 59 patients (39.9%). Median graft weight change was -28 g (range; -132–0 g) in the negative group and 21 g (range; 1–63 g) in the positive group (P<0.001). Median hospitalization time was longer for the positive group than the negative group (27 days vs. 23 days; P=0.048). There were no statistical differences in tumor characteristics, postoperative complications, early allograft dysfunction, or acute rejection between the two groups. Disease-free survival, death-censored graft survival, and patient survival were lower in the positive group than the negative group. Additionally, the positive group showed strong association with HCC recurrence, death-censored graft survival, and patient survival in multivariate analysis.
Conclusion: This study suggests that positive graft weight change during HTK solution perfusion indicates poor prognosis in LDLT.
In Asia, where there is a shortage of deceased donors, living donor liver transplantation (LDLT) is frequently performed in hepatocellular carcinoma (HCC) patients using right or left hemi-liver graft from living donors. Grafts from living donors are higher quality than livers from deceased donors. Numerous factors have been identified as predictors of short-term outcomes of LDLT such as age, model for end-stage liver disease (MELD) score, cold ischemic time, graft-to-recipient weight ratio (GRWR), transfusion demands, and laboratory test findings (1). Among these, graft volume and weight directly affect recipient recovery and graft survival because the volume of the implanted partial liver graft is less than the metabolic demand (2).
Histidine-Tryptophan-Ketoglutarate (HTK) solution is the only organ preservation solution used in Korea (3). HTK solution has a low viscosity index and low potassium and sodium levels (4). The low viscosity in HTK solution favors efficient liver graft washout because it flushes rapidly, and diffusion of preservation solution to the hepatocytes arrests hepatocellular damage. Additionally, HTK solution contains histidine acting as a buffer, tryptophan as a membrane stabilizer, and ketoglutarate acting as a substrate during ischemia (4). Because HTK solution is safe and effective, ICU stay, primary dysfunction rate, and biliary complication after liver transplantation are lower when HTK solution is used than when other preservation solutions are used (4).
Partial liver grafts are flushed with a chilled HTK solution through the portal vein in the back-table procedure. After this process, the liver graft weight decreases after perfusion compared to before perfusion. However, some patients have increased graft weight after perfusion. No previous study has investigated the effect of right liver graft weight change during organ preservation solution perfusion on recipient outcomes in LDLT patients. Herein, we investigated HCC recurrence, graft failure, and patient survival according to right liver graft weight change in adult LDLT patients.
Two hundred twenty-eight patients underwent LDLT between January 2013 and March 2017 at Samsung Medical Center, Korea. There were 155 HCC patients who received LDLT with HCC. The present study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (SMC-2020-07-027). The need for patient consent was waived by the IRB because this was a retrospective observational study of data that used patient medical records. We reviewed all patients’ medical records and excluded those who did not have records of graft weight (n=4), patients without HCC (n=48), multiple organ transplantation (n=2), re-transplantation (n=3), received a left-side graft (n=10), pediatric liver transplantation patients (n=8), or were lost during follow-up or had incomplete medical records (n=5). Finally, 148 HCC patients were identified in the study and underwent right or extended right hepatectomy.
Our immunosuppressive regimen has been described in previous studies (5). Basiliximab was administered at a dose of 20 mg/day at the time of operation and on postoperative day 4. All patients received triple immunosuppressive drugs consisting of tacrolimus, mycophenolate mofetil (MMF), and methylprednisolone. Tacrolimus dose was adjusted to maintain whole-blood trough levels at 8–10 ng/ml for 1–2 months postoperatively and at 6–8 ng/ml thereafter. MMF was used at 500-1,000 mg/day postoperatively and adjusted according to white blood cell count. Methylprednisolone therapy was initiated on the operation day at a dose of 500 mg/day and then tapered to 4–8 mg/day within 1–2 months postoperatively. In case of ABO-incompatible LDLT, rituximab prophylaxis and frequent total plasma exchange were conducted for desensitization (6). When HCC recurs, tacrolimus trough level was lowered compared to patients without HCC recurrence and everolimus was added.
At the beginning living liver donor surgery, liver wedge resection is performed in segment 4 to check for hepatic steatosis. The portal vein of the right liver graft is clamped using a vascular clamp, but the hepatic artery and hepatic vein are not clamped during liver graft extraction. The right liver graft comes out of the body immediately after right hepatic vein division and the vascular clamp is released. We manually and gently press the right liver graft to remove blood within the graft. The graft was then perfused with 3L of HTK solution via the portal vein.
We routinely measured the right liver graft weight twice before and after HTK preservation solution perfusion in the back-table procedure: (1) immediately after procurement after the blood was drained (blood-free graft weight) and (2) after perfusion with HTK solution (graft weight after perfusion). We calculated the change in liver graft weight by subtracting pre-perfusion graft weight from post-perfusion graft weight.
Patients with increased graft weight were defined as the positive group and patients with decreased graft weight as the negative group. GRWR was calculated by dividing graft weight by recipient body weight before and after perfusion. EAD was defined on the basis of abnormal increases in total bilirubin, international normalized ratio (INR), and aminotransferase within 7 days after LDLT (7).
To compare the differences between the positive and negative groups, Fisher’s exact test for categorical variables and the Mann-Whitney U-test for continuous variables were used. Continuous variables are expressed as median and range and categorical variables are expressed as number and percentage. Disease-free survival (DFS), death-censored graft survival, and patient survival (PS) were calculated as the duration from the starting date of LDLT to the date when a new event was first detected or, if the entire follow-up period was event-free, to the date of the last follow-up visit. DFS rates, death-censored graft survival rates, and PS rates between the positive and negative groups were estimated using the Kaplan-Meier method, and survival curves for those rates were compared with the log-rank test. Significant variables in univariate analyses (P < 0.05) were entered into a Cox multivariate proportional hazards model to determine which factors independently predicted DFS, death-censored graft survival, and patient survival.
All statistical analyses were performed using SPSS 24.0 for Windows (IBM Corp., Armonk, NY, USA). P < 0.05 was considered statistically significant and all statistical tests were evaluated as two-sided.
There were 89 people (60.1%) in the negative group and 59 people (39.9%) in the positive group. Baseline characteristics are summarized in Table 1. Sex, age, and body mass index (BMI) in living liver donors were not significantly different between the two groups. Hepatitis B virus (HBV) was the most common cause of HCC in recipients, accounting for 79 cases (88.8%) in the negative group and 47 cases (79.7%) in the positive group. However, alcoholic damage (n=6, 10.2%) and HCV (n=5, 8.5%) were more common causes among the positive group than the negative group. There were no statistically significant differences in sex, age, body mass index, hypertension, diabetes, Child-Pugh class, MELD score, history of radiation, operation, radiofrequency ablation, and/or transarterial chemoembolization before LDLT, alpha-fetoprotein (AFP), and protein induced by vitamin K absence-II (PIVKA-II) between the two groups.
Perioperative and pathologic characteristics are outlined in Table 2. Extended right hepatectomy was performed in two patients in the negative group and one patient in the positive group. All other patients underwent right hepatectomy. The incidence of laparoscopic liver resection in living liver donors was 33.7% (n=30) in the negative group and 28.8% (n=17) in the positive group. Median macrosteatosis and microsteatosis was 5% in both groups. The median donor operation time in both groups was also very similar (351 min in the negative group vs. 351 min in the positive group; P=0.893). For right lobe liver grafts extracted from a living donor, graft weight and GRWR before perfusion in the negative group were significantly greater than in the positive group (P=0.009 and P=0.020, respectively), but graft weight and GRWR after perfusion were no different between the two groups. Median graft weight change was -28 g (range; -132–0 g) in the negative group and 21 g (range; 1–63 g) in the positive group (P<0.001).
Median hospitalization time was longer in the positive group than the negative group (27 days vs. 23 days; P=0.048). Total bilirubin, AST, ALT, and INR, tested repeatedly up to 30 days after LDLT, did not differ between the two groups (Figure 1). None of the following measures were significantly different between the two groups: ABO-incompatibility, cold and warm ischemic times, recipient operation time, early allograft dysfunction, intensive care unit stay, incidence of values beyond Milan criteria for tumor pathology, tumor size, tumor number, presence of total tumor necrosis, tumor grade 3 or 4, encapsulation, presence of portal vein tumor thrombosis (PVTT), intrahepatic metastasis, and multicentric occurrence.
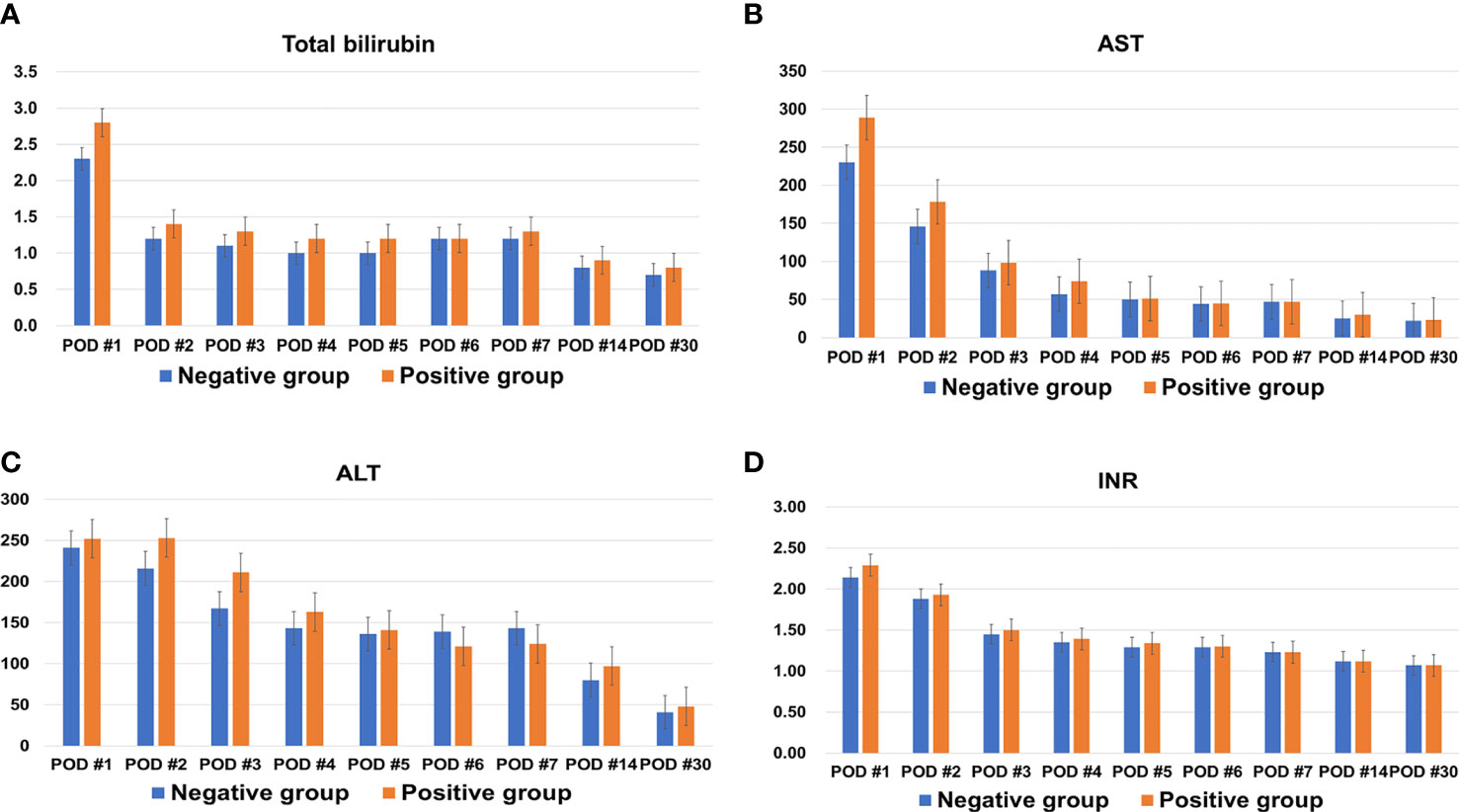
Figure 1 Changes in (A) total bilirubin, (B) aspartate transaminase (AST), (C) alanine transaminase (ALT), and (D) international normalized ratio (INR) of both groups within one month after living donor liver transplantation.
The incidences of bacterial, viral, and surgical complications within 90 days after LDLT were not different between the two groups. Accordingly, the severity of Clavien-Dingo grade was not different between the two groups.
Mean follow-up duration was 44.5 ± 17.6 months in the negative group and 38.8 ± 19.2 months in the positive group. The incidence of acute cellular rejection across the follow-up period was 14.6% (n=13) in the negative group and 10.2% (n=6) in the positive group. Only one patient in each group had antibody-mediated rejection.
The incidence of HCC recurrence was 14.6% (n=13) in the negative group and 25.4% (n=15) in the positive group. The 1-, 2-, and 3-year cumulative disease-free survival rates were 92.0%, 86.2%, and 86.2%, respectively, in the negative group and 82.0%, 76.3%, and 71.5% in the positive group (P=0.053; Figure 2A). Three patients (3.4%) in the negative group and nine patients (15.3%) in the positive group developed graft failure during the follow-up period. The 1-, 2-, and 3-year cumulative death-censored graft failure rates were 97.8%, 97.8%, and 96.4%, respectively, in the negative group and 91.4%, 85.5%, and 82.1% in the positive group (P=0.007; Figure 2B). The incidence of death was 14.6% (n=13) in the negative group and 28.8% (n=17) in the positive group. The 1-, 2-, and 3-year cumulative overall survival rates were 96.6%, 91.0%, and 88.5%, respectively, in the negative group and 88.1%, 81.2%, and 75.1% in the positive group (P=0.017; Figure 2C).
Risk factors for HCC recurrence, death-censored graft failure, and death are summarized in Supplement Tables 1, 2, and 3. Multivariate analysis showed that young recipient age, the positive group, being beyond the Milan criteria, and intrahepatic metastasis were predisposing factors for HCC recurrence. Death-censored graft failure and death were strongly associated with the positive group (Table 3).
Because graft quality affects recipient’ outcome, numerous studies have investigated the effects of graft volume, fatty changes in donor liver, and GRWR on recipient outcome after LDLT (2). However, no previous study has investigated the effect of change in graft weight after perfusion on LDLT outcome.
In previous studies, Graft weight measurement timing is not mentioned or measured at various times and measured immediately after procurement, or after preservation solution, or after back-table procedure (8–10). Previous studies showed that careful interpretation of liver graft weight is required because graft weight after back-bench surgery can decrease to 90% of the initial graft weight when using University of Wisconsin (UW) solution for perfusion because the solution is lighter than blood. However, LDLT in our study used HTK solution, and one-third of patients experienced liver graft weight increases after procurement, which is inconsistent with previous findings.
Passive volume changes according to HTK solution perfusion would be expected to be quite small. Individual liver graft compliance was clinically observed as the discrepancy between perfused liver graft weight and non-perfused liver graft weight at the time of retrieval. In this study, median graft weight decreased by 28 g in the negative group and increased 21 g in the positive group. Weight change was very small because the liver has a large capacitance reservoir and very low venous resistance.
This study showed no significant difference in donor characteristics between the two groups. On the recipient side, hepatitis C virus (HCV) and alcoholic patients in the positive group were slightly more prevalent in the negative group, and median hospitalization in the positive group was longer than that in the negative group. There were no statistically significant differences in tumor characteristics, postoperative complications, EAD, or acute rejection between the two groups. Interestingly, disease-free survival, death-censored graft survival, and patient survival were lower in the positive group than the negative group. Additionally, the positive group was strongly associated with HCC recurrence, death-censored graft survival, and patient survival in multivariate analysis.
We suspect that the increase in liver graft weight after HTK solution perfusion was due to deficiency in liver graft elasticity, which is thought to have adverse effects the patient after graft implantation. The increase in kidney graft weight during perfusion showed vascular disruption by endothelial disruption and inflammatory infiltration (11). The volume HTK solution in the liver vascular bed has not been well examined in our study. The increase in liver graft weight after HTK solution perfusion that could reflect hepatocyte parenchymal edema. Liver graft edema may destroy the endothelial surface layer along with the inevitable damage caused by surgical trauma (such as mechanical stress and ischemia-reperfusion injury), leading to pathologic shifts of fluid and protein towards the interstitium (12). Disruption of electrolyte cell membrane gradients due to sodium-potassium membrane pumps damage results in cellular edema, with free calcium influx, and subsequent activation of enzyme cascade leading to cell death (13). Furthermore, pathologic inflammation and endothelial dysfunction are known to promote angiogenesis, fibrogenesis, cirrhosis, and increased hepatic resistance, ultimately resulting in portal hypertension and decreased effective hepatocyte perfusion with the risk of liver failure (14).
After graft implantation, hepatocyte injury after reperfusion is mediated by release of reactive oxygen species with subsequent oxidative stress (8). In addition, when Kupffer cells and sinusoidal endothelial cells in the partial liver graft are exposed to high portal venous blood flow, shear stress caused by high portal venous blood flow induces hepatic regeneration (15). Decreased liver graft elasticity might affect early outcomes after LDLT. Therefore, we tested liver function tests and INR repeatedly until one month after LDLT to compare levels between the two groups because the most appropriate time to evaluate graft regeneration in the early postoperative period is during the second postoperative week (15, 16). However, there was no difference in aspartate transaminase (AST), alanine transaminase (ALT), total bilirubin, or INR between the two groups. Additionally, there were no significant differences in EAD, acute rejection, and postoperative surgical complications between the two groups. Positive liver graft weight change does not seem to have a significant early effect after LDLT.
HCC recurrence after LDLT increases the likelihood of death in liver transplant patients. The most common risk factors for HCC recurrence after liver transplantation were tumor size and tumors, tumor differentiation, tumor markers included AFP or PIVKA-II, vascular invasion, and neutrophil-lymphocyte ratio (17). The AFP model was a well-validated preoperative risk model for stratifying patients into high- and low-risk groups (18).
HCC patients may have a higher probability of HCC recurrence after LDLT than DDLT. When partial liver graft is implanted, growth factor and vascular inflow increase for regeneration of liver graft (19). This process may contribute to progression and seeding in any organs of tumor cells in blood. Additionally, small size grafts are more likely to induce HCC recurrence due to cell adhesion, angiogenesis, and migration caused by acute phase graft injury and rapid graft regeneration (20). Hepatic fibrosis reflects liver function and shows the possibility of cirrhosis and HCC development in patients with chronic liver disease (21). Elastography-based imaging techniques have emerged as an accurate method for assessing liver fibrosis. High liver stiffness and transient elastography results were significantly correlated with HCC occurrence in chronic liver disease patients without a history of HCC (22). An increase in liver graft weight is likely to be related to liver graft elasticity, which may have a synergistic effect on HCC recurrence through the various factors described above that affect HCC recurrence in LDLT. Our study showed that the positive group had a higher likelihood of death-censored graft survival and patient survival in multivariate analysis; therefore, liver graft elasticity may eventually affect graft survival and patient survival.
This study has several imitations. First, we do not know the degree of liver stiffness of the liver donor, so it is difficult to measure liver elasticity precisely. Thus, we do not know the relationship between liver graft weight change and liver elasticity. Second, we were not able to control for confounding factors because of the retrospective nature of this observational study. In addition, it is difficult to completely remove blood and HTK solution in the graft and exactly measure the liver graft weight. The study population was small and enrolled from a single center, making it difficult to obtain high statistical power. Third, biological factors before and after perfusion were unknown in present study. Prospective research on peripheral blood, perfusate solutions, and liver biopsy before and after perfusion is required. Fourth, we are not sure how positive liver graft weights change contribute to HCC recurrence, graft survival, or patient death. Randomized controlled prospective studies are required to validate our findings.
This study showed that positive graft weight change during HTK solution perfusion strongly contributed to HCC recurrence, graft failure, and patient death. However, this change in graft weight change has no effect initially after LDLT. Our study revealed that positive graft weight change during HTK solution perfusion is an indicator of poor prognosis in LDLT patients. However, we do not know the exact mechanism between graft weight change and poor prognosis. More research is needed to clarify this mechanism or relationship.
The datasets generated for this study are available on request to the corresponding author.
This study was approved by the Samsung Medical Center Institutional Review Board (IRB) (SMC-2020-07-027). Patient consent was waived by the IRB. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JMK designed the study, conducted the literature search, acquired, analyzed, and interpreted the data and wrote the manuscript. J-WJ and YJC designed the study and interpreted the data. SK analyzed the data. JR and G-SC acquired and analyzed the data. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.609844/full#supplementary-material
HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease; GRWR, graft-to-recipient ratio; HTK, Histidine-Tryptophan-Ketoglutarate; EAD, early allograft dysfunction; DFS, disease-free survival; PS, patient survival; BMI, body mass index; HBV, hepatitis B virus; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence-II; HCV, hepatitis C virus; AST, aspartate transaminase; ALT, alanine transaminase.
1. Bolondi G, Mocchegiani F, Montalti R, Nicolini D, Vivarelli M, De Pietri L. Predictive factors of short term outcome after liver transplantation: A review. World J Gastroenterol (2016) 22:5936–49. doi: 10.3748/wjg.v22.i26.5936
2. Ikegami T, Kim JM, Jung D-H, Soejima Y, Kim D-S, Joh J-W, et al. Conceptual changes in small-for-size graft and small-for-size syndrome in living donor liver transplantation. Korean J Transplant (2019) 33:65–73. doi: 10.4285/jkstn.2019.33.4.65
3. Ko JS, Kim GS, Gwak MS, Yang M, Kim HK, Shin BS, et al. Greater hemodynamic instability with histidine-tryptophan-ketoglutarate solution than University of Wisconsin solution during the reperfusion period in living donor liver transplantation. Transplant Proc (2008) 40:3308–10. doi: 10.1016/j.transproceed.2008.04.022
4. Rao F, Yang J, Gong C, Huang R, Wang Q, Shen J. Systematic review of preservation solutions for allografts for liver transplantation based on a network meta-analysis. Int J Surg (2018) 54:1–6. doi: 10.1016/j.ijsu.2018.04.024
5. Kim JM, Kwon CHD, Joh JW, Han S, Yoo J, Kim K, et al. ABO-incompatible Living Donor Liver Transplantation With Rituximab and Total Plasma Exchange Does Not Increase Hepatocellular Carcinoma Recurrence. Transplantation (2018) 102:1695–701. doi: 10.1097/TP.0000000000002154
6. Oh J, Kim JM. Immunologic strategies and outcomes in ABO-incompatible living donor liver transplantation. Clin Mol Hepatol (2020) 26:1–6. doi: 10.3350/cmh.2019.0023
7. Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl (2010) 16:943–9. doi: 10.1002/lt.22091
8. Gilbo N, Catalano G, Salizzoni M, Romagnoli R. Liver graft preconditioning, preservation and reconditioning. Dig Liver Dis (2016) 48:1265–74. doi: 10.1016/j.dld.2016.06.031
9. Radtke A, Sotiropoulos GC, Nadalin S, Molmenti EP, Schroeder T, Lang H, et al. Preoperative volume prediction in adult living donor liver transplantation: how much can we rely on it? Am J Transplant (2007) 7:672–9. doi: 10.1111/j.1600-6143.2006.01656.x
10. Lemke AJ, Brinkmann MJ, Schott T, Niehues SM, Settmacher U, Neuhaus P, et al. Living donor right liver lobes: preoperative CT volumetric measurement for calculation of intraoperative weight and volume. Radiology (2006) 240:736–42. doi: 10.1148/radiol.2403042062
11. Wilson CH, Gok MA, Shenton BK, Balupuri S, Gupta AJ, Asher J, et al. Weight increase during machine perfusion may be an indicator of organ and in particular, vascular damage. Ann Transplant (2004) 9:31–2.
12. Jeong HW, Jung KW, Kim SO, Kwon HM, Moon YJ, Jun IG, et al. Early postoperative weight gain is associated with increased risk of graft failure in living donor liver transplant recipients. Sci Rep (2019) 9:20096. doi: 10.1038/s41598-019-56543-3
13. Tchilikidi KY. Liver graft preservation methods during cold ischemia phase and normothermic machine perfusion. World J Gastrointest Surg (2019) 11:126–42. doi: 10.4240/wjgs.v11.i3.126
14. Robinson MW, Harmon C, O’Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol (2016) 13:267–76. doi: 10.1038/cmi.2016.3
15. Byun SH, Yang HS, Kim JH. Liver graft hyperperfusion in the early postoperative period promotes hepatic regeneration 2 weeks after living donor liver transplantation: A prospective observational cohort study. Medicine (Baltimore) (2016) 95:e5404. doi: 10.1097/MD.0000000000005404
16. Park JK, Yang JI, Lee JK, Park JK, Lee KH, Lee KT, et al. Long-term Outcome of Endoscopic Retrograde Biliary Drainage of Biliary Stricture Following Living Donor Liver Transplantation. Gut Liver (2020) 14:125–34. doi: 10.5009/gnl18387
17. Al-Ameri AAM, Wei X, Wen X, Wei Q, Guo H, Zheng S, et al. Systematic review: risk prediction models for recurrence of hepatocellular carcinoma after liver transplantation. Transpl Int (2020) 33:697–712. doi: 10.1111/tri.13585
18. Rhu J, Kim JM, Choi GS, Kwon CHD, Joh JW. Validation of the alpha-fetoprotein Model for Hepatocellular Carcinoma Recurrence After Transplantation in an Asian Population. Transplantation (2018) 102:1316–22. doi: 10.1097/TP.0000000000002136
19. Shi JH, Huitfeldt HS, Suo ZH, Line PD. Growth of hepatocellular carcinoma in the regenerating liver. Liver Transpl (2011) 17:866–74. doi: 10.1002/lt.22325
20. Man K, Lo CM, Xiao JW, Ng KT, Sun BS, Ng IO, et al. The significance of acute phase small-for-size graft injury on tumor growth and invasiveness after liver transplantation. Ann Surg (2008) 247:1049–57. doi: 10.1097/SLA.0b013e31816ffab6XXX
21. Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, Ohki T, et al. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology (2009) 49:1954–61. doi: 10.1002/hep.22870
Keywords: living donors, partial liver graft, perfusion, outcomes, hepatocellular carcinoma
Citation: Kim JM, Chung YJ, Kim S, Rhu J, Choi G-S and Joh J-W (2021) Impact of Graft Weight Change During Perfusion on Hepatocellular Carcinoma Recurrence After Living Donor Liver Transplantation. Front. Oncol. 10:609844. doi: 10.3389/fonc.2020.609844
Received: 24 September 2020; Accepted: 04 December 2020;
Published: 24 February 2021.
Edited by:
Nicholas Syn, National University of Singapore, SingaporeReviewed by:
Shuji Isaji, Mie University Hospital, JapanCopyright © 2021 Kim, Chung, Kim, Rhu, Choi and Joh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jae-Won Joh, ancuam9oQHNhbXN1bmcuY29t
†ORCID: Jong Man Kim, orcid.org/0000-0002-1903-8354; Young Jae Chung, orcid.org/0000-0003-0725-1040; Sangjin Kim, orcid.org/0000-0002-0080-176X; Jinsoo Rhu, orcid.org/0000-0001-9809-8525; Gyu-Seong Choi, orcid.org/0000-0003-2545-3105; Jae-Won Joh, orcid.org/0000-0003-4823-6218
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.