- 1Department of Ultrasonography, Fudan University Shanghai Cancer Center, Shanghai, China
- 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
- 3Department of Breast Surgery, Fudan University Shanghai Cancer Center, Shanghai, China
- 4Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai, China
Background: The rate of carcinoma upgrade for atypical ductal hyperplasia (ADH) diagnosed on core needle biopsy (CNB) is variable on open excision. The purpose of the present study was to develop and validate a simple-to-use nomogram for predicting the upgrade of ADH diagnosed with ultrasound (US)-guided core needle biopsy in patients with US-detected breast lesions.
Methods: Two retrospective sets, the training set (n = 401) and the validation set (n = 186), from Fudan University Shanghai Cancer Center between January 2014 and December 2019 were retrospectively analyzed. Clinicopathological and US features were selected using univariate and multivariable logistic regression, and the significant features were incorporated to build a nomogram model. Model discrimination and calibration were assessed in the training set and validation set.
Results: Of the 587 ADH biopsies, 67.7% (training set: 267/401, 66.6%; validation set: 128/186, 68.8%) were upgraded to cancers. In the multivariable analysis, the risk factors were age [odds ratio (OR) 2.739, 95% confidence interval (CI): 1.525–5.672], mass palpation (OR 3.008, 95% CI: 1.624–5.672), calcifications on US (OR 4.752, 95% CI: 2.569–9.276), ADH extent (OR 3.150, 95% CI: 1.951–5.155), and suspected malignancy (OR 4.162, CI: 2.289–7.980). The model showed good discrimination, with an area under curve (AUC) of 0.783 (95% CI: 0.736–0.831), and good calibration (p = 0.543). The application of the nomogram in the validation set still had good discrimination (AUC = 0.753, 95% CI: 0.666–0.841) and calibration (p = 0.565). Instead of surgical excision of all ADHs, if those categorized with the model to be at low risk for upgrade were surveillanced and the remainder were excised, then 63.7% (37/58) of surgeries of benign lesions could have been avoided and 78.1% (100/128) malignant lesions could be treated in time.
Conclusions: This study developed a simple-to-use nomogram by incorporating clinicopathological and US features with the overarching goal of predicting the probability of upgrade in women with ADH. The nomogram could be expected to decrease unnecessary surgery by nearly two-third and to identify most of the malignant lesions, helping guide clinical decision making with regard to surveillance versus surgical excision of ADH lesions.
Introduction
Approximately 20% of the suspicious breast lesions diagnosed by core needle biopsy (CNB) demonstrate atypical ductal hyperplasia (ADH) (1). Morphologically, ADH is similar to low-grade ductal carcinoma in situ (DCIS) and their differentiation is based only on the lesion size whereby a lesion that restricted to ducts and ductules and ≤2 mm in maximum diameter is classified as ADH and a larger lesion is classified as low-grade DCIS. Therefore, potential for underestimation of malignant lesion may exist in ADH lesions obtained with CNB. For this reason, the current standard of care is to excise ADH found at percutaneous biopsy to exclude co-existing cancer even if the lesions seems to be completely excised by vacuum-assisted biopsy (2). The underestimated rates have been reported to range from 0 to 84%, although most studies found upgrade rates of approximately 25% (2, 3). Therefore, surveillance instead of surgical excision might be appropriate in patients with low risk of upgrade (4–6). This emphasizes the need to find approaches to identify those women who are more likely to have a cancer, which may contribute to the early detection of breast cancer. Thereby, low-risk patients will be advised to safely forgo surgical treatment (7).
Previous studies have attempted to identify features which are correlated with the likelihood of ADH upgrade, including age, lesion size, type of biopsy gauge, number of cores, and extent of ADH (5, 6, 8). However, none of these features can reliably stratify patients into different treatment subgroups (8–10). The diversity or even conflict in risk factors might be due to the study design because the investigated potential risk factors varied widely and most of the studies were single institution studies with a limited number of cases. Some studies have suggested that combining imaging features with biopsy and clinical characteristics maybe necessary for improving the preoperative prediction of final pathological diagnosis for ADH (6, 8, 11–14). The ultrasound (US) features such as margin, size of the lesions, and echogenicity features, are vital for the detection and discrimination of benign and malignant lesions (15), and also help in prediction of axillary lymph node metastasis in breast cancer (16). US has been widely used in imaging-guided breast CNB, especially in developing countries, with the advantage of real-time visualization, less expensive, not using ionizing radiation, and not limited to breast density (15, 17). However, only a limited number of studies have investigated the use of US features for predicting the upgrade of ADH (18). Therefore, the main aim of this study was to ascertain clinicopathological and US features with the goal of developing and validating a nomogram that will be able to predict the risk of pathologic upgrade in patients with ADH diagnosed with CNB.
Methods
Patient Selection
This retrospective study was approved by the review board of Fudan University Shanghai Cancer Center. The training set for this study comprised of 401 patients who were diagnosed with ADH by using US-guided core biopsy and underwent surgical resection at our institution from January 2014 to December 2018. A validation set of 186 consecutive patients was then established from January to December 2019 using the same criteria. All patients underwent percutaneous US-guided CNB using the 14-gauge automated gun method (Gallini, Mantova, Italy or Bard Technologies, Covington, USA), and approximately five cores were taken from each lesion by the surgeon.
Inclusion and Exclusion Criteria
This study included patients who were diagnosed with ADH using US-guided CNB, without DCIS or invasive carcinomas in the same biopsy, and no lymph node metastases found preoperatively. We excluded lesions diagnosed as columnar cell changes with atypia, flat epithelial atypia, or atypical lobular hyperplasia (ALH) if ADH was not also present. Ipsilateral breast cancer was also excluded because the aim of the study was to determine isolated imaging findings associated with the underestimation of these lesions and ensure that the postoperative specimens were independent of other breast lesions. The flow chart of patient inclusion and exclusion is shown in Figure 1.
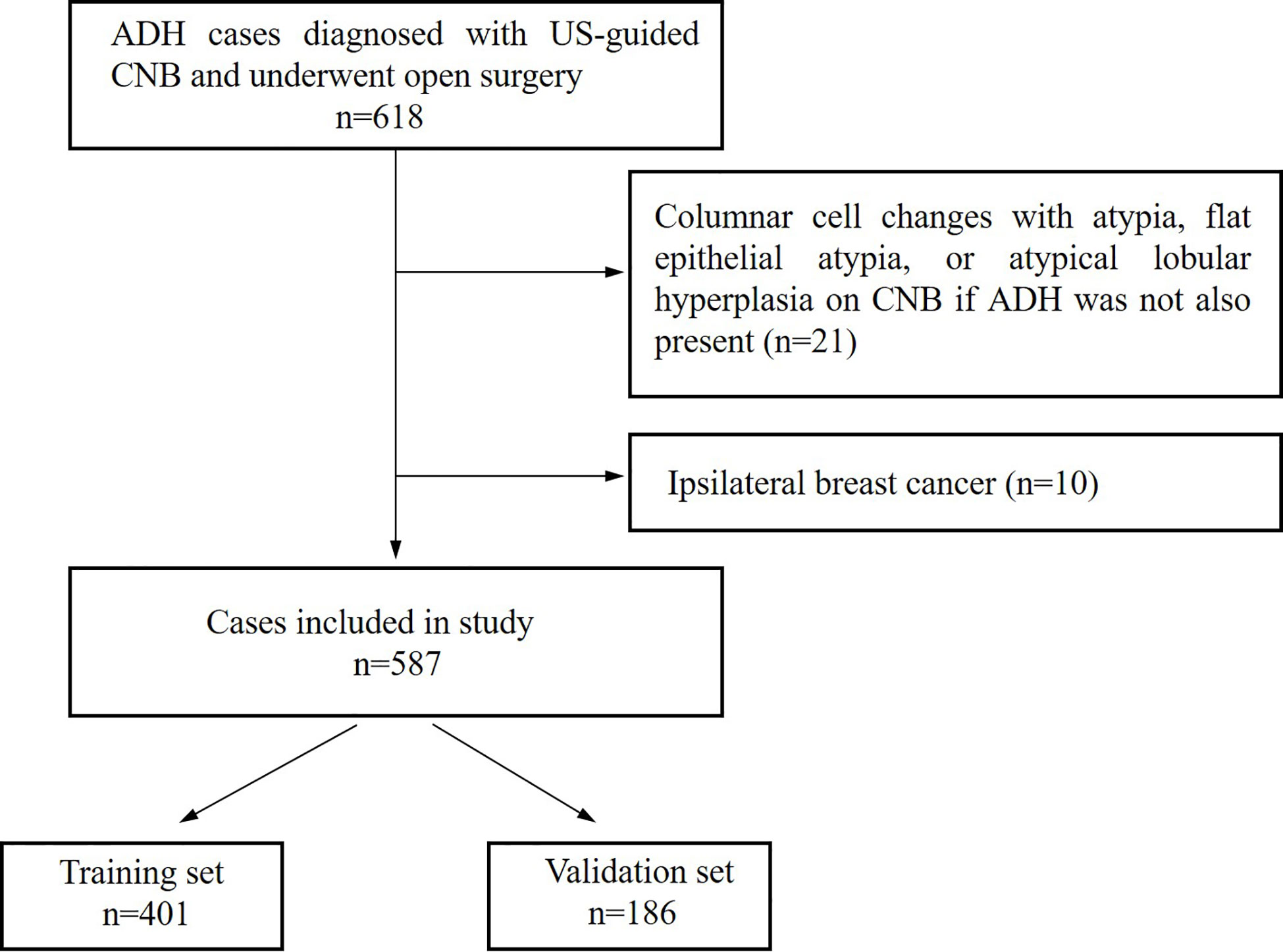
Figure 1 Flow chart of cases selection from atypical ductal hyperplasia (ADH) cases diagnosed between January 2014 and December 2019.
Data Collection
The clinical features including age, menopausal status, palpability, contralateral tumor, family history of breast cancer, and mammography calcification features were collected from the electronic medical record system. The pathology of CNB was designated ADH according to the ADH diagnostic criteria in the WHO guidelines (19), while the extent of ADH was classified as focal if the pathology report contained “focal ADH,” “focus ADH,” or “single duct of ADH.” In addition, suspected malignancy was coded “yes” in instances where the report contained the statement “suspicious for DCIS” or “suspicious for malignant.” An ADH case was considered “suspected malignancy” if the cytologic and/or architectural features were that of low grade DCIS but either the spectrum of changes present in the core biopsy made it difficult to distinguish if one area definitively qualified for a diagnosis of low grade DCIS or the extent of changes was considered too limited to classify definitively as DCIS on a CNB sample alone (20). The following additional data was also recorded: the co-diagnosis of ADH (adenosis, sclerosing adenosis, papilloma fibroepithelial lesions, and apocrine hyperplasia) and the presence of calcifications within ADH. At final pathological diagnosis, underestimation was defined as cancer found at open excision after a biopsy diagnosis of ADH. In addition, proliferative lesions (neoplasia/atypical lobular hyperplasia, columnar cell change) were classified as borderline lesions (5, 21). The US appearance and category of each lesion were characterized according to the fifth edition of the Breast Imaging Reporting and Data System (BI-RADS) (22). Meanwhile, the different lesion types were classified as mass and non-mass (ductal dilatation, complex cystic lesions, or low echo area) (23). Specific features noted on US examination included the presence or absence of calcifications, and their maximum sizes were measured. Two experienced sonographer (Y.L.C and Y.X.H, with more than 5 years of experience breast ultrasound) who were blinded to the study review the US images, and micro-calcifications with an indistinct oval round or irregular mass, micro-calcifications with a ductal change, and micro-calcifications with a speculated irregular mass were defined as calcification on US.
Establishment of a Predictive Model and Nomogram
Univariate analysis was used to assess the clinical and demographic parameters, and biopsy, pathological, and US features with the goal of identifying the most relevant predictors of the underestimated risks using Pearson’s Chi-square test or Fisher’s exact test in the training set. The features that had p-values of <0.1 after univariate analysis were used to perform multivariate logistic regression with the overarching goal of selecting the most useful variables (p < 0.05) via stepwise backward selection. In addition, multiple imputation was used in the multivariable logistic analysis to account for missing data (<5%). Twenty-three and 12 imputed data were generated in the training and validation set, respectively. Finally, multivariable logistic regression analysis was applied on the training set to build a nomogram, which is a visual tool for predicting the individualized underestimation risk of patients with biopsy-diagnosed ADH. Two models were built as follows: In the first model, clinical and pathological data were included. In the second model, US features were also added to the predict the upgrade of ADH.
Predictive Performance and Validation of the Nomogram
The area under curve (AUC) of the receiver operating characteristic (ROC) was used to evaluate the discrimination ability of the nomogram, and followed by calculation of the sensitivity, specificity, and accuracy with 95% confidence intervals (95% CIs). A calibration curve was then plotted to assess the calibration of the nomogram using the Hosmer-Lemeshow goodness-of-fit test. A P > 0.05 indicated non-significant deviance from the theoretical perfect calibration. The decision curve analysis was also evaluated. All statistical analysis were conducted using R software (V.3.6.2, http://www.r-project.org) and SPSS v25.0 (SPSS, Inc., IMB Company Chicago, IL, USA).
Results
Clinicopathological and US Characteristics
Among the 401 patients included in the training set, 267 (66.6%) were upgraded into malignancy, 126 (31.4%) were benign, and 8 (2.0%) were borderline. Furthermore, 142 (53.2%) of the underestimated cases with malignancy were DCIS, 69 (25.8%) were IDC, 43 (16.1%) were papillary carcinoma, and 3 (1.1%) were invasive lobular carcinoma (ILC). On the other hand, 39 (31.0%) of the benign cases were papilloma, 35 (27.8%) were adenosis, and 10 (7.9%) were fibroadenoma. Of the data in training and validation set, 21 patients had missing data for one or more potential parameters: 8 for family history of breast cancer, 2 for past or present contralateral breast cancer, 9 for mass palpation, 10 for BI-RADS score, and 13 for ADH extent.
The clinicopathological and US characteristics of patients in the underestimated group, non-upgraded group, training set and validation set are listed in Table 1. The obtained results indicated that there was no significant difference in the upgrade rate between the training and validation sets (66.6 vs. 68.9%, p = 0.326). In addition, there were no significant differences in the clinicopathological and US characteristics between the training and validation sets (all p > 0.05). Significant differences (p < 0.1) were found in age, menopausal status, mass palpation, US diameter, calcifications on US, BI-RADS category, ADH extent, suspected malignancy, and co-diagnosis of ADH.
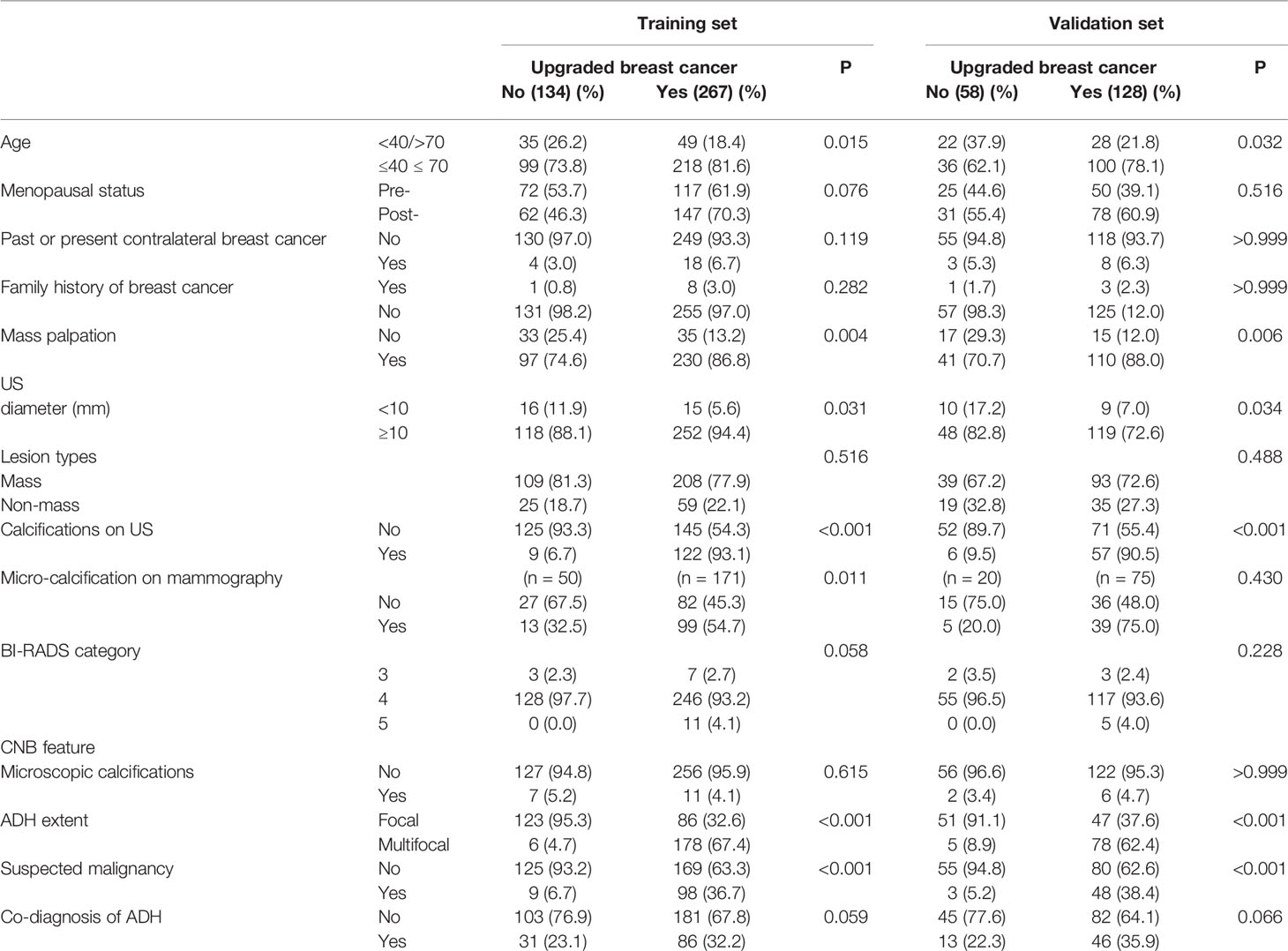
Table 1 Clinicopathological and US factors correlations with upgrades in the training and validation sets.
Development of the Predictive Nomogram
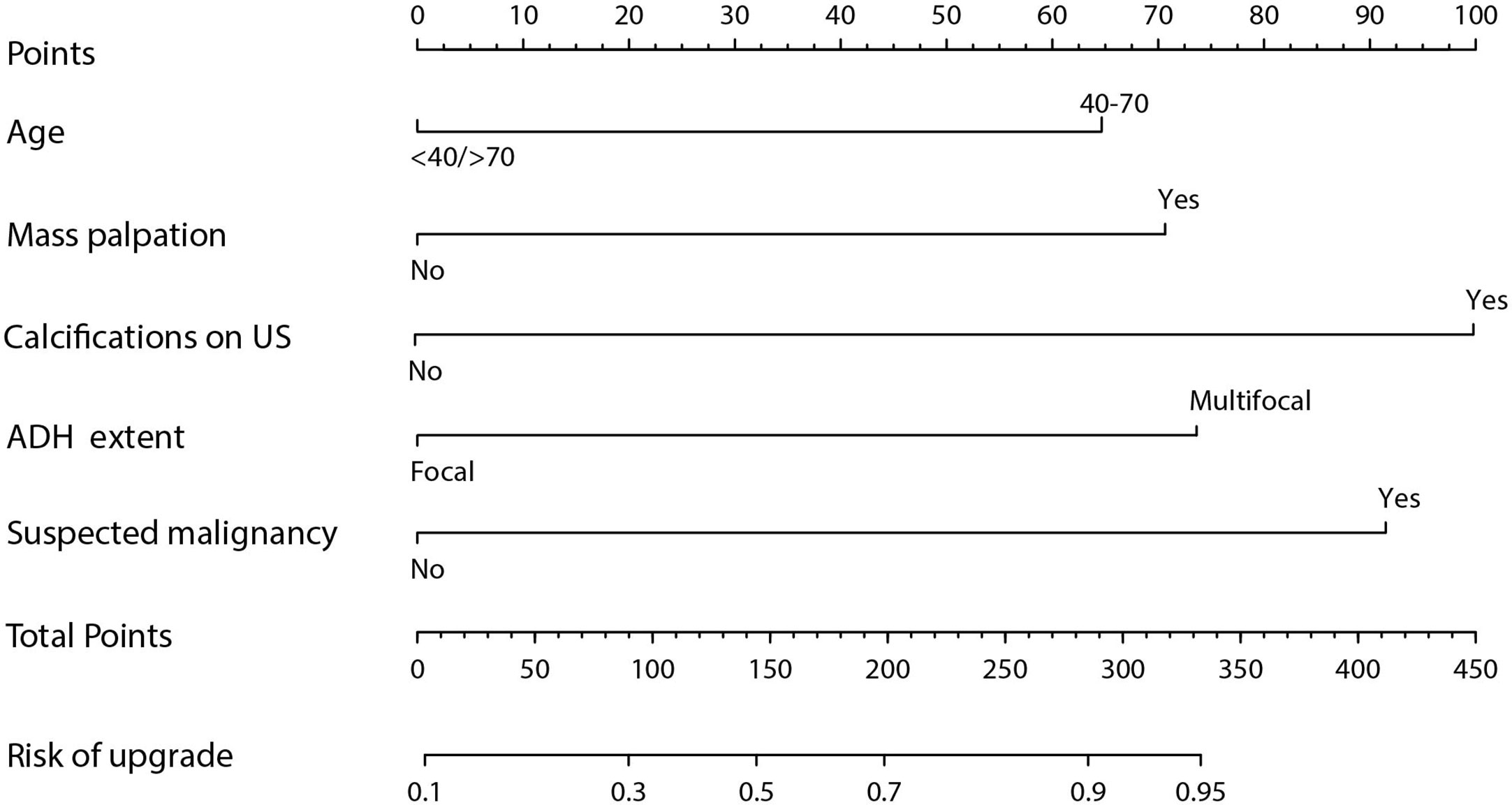
Results obtained after univariate logistic regression analysis indicated that age, menopausal status, mass palpation, calcifications on US, ADH extent, suspected malignant component, and co-diagnosis of ADH were significant risk factors. On the other hand, multivariate logistic regression analysis results showed that the risk factors for upgrade were age (40–70 years vs. <40/>70 years, odds ratio 2.739, 95% CI: 1.525–5.672, p = 0.01), mass palpation (yes vs. no, odds ratio 3.008, 95% CI: 1.624–5.672, p < 0.01), calcifications on US (yes vs. no, odds ratio 4.752, 95% CI: 2.569–9.276, p < 0.01), ADH extent (multifocal vs. focal, odds ratio 3.150, 95% CI: 1.951–5.155, p < 0.01), and suspicious malignancy (yes vs. no, odds ratio 4.162, 95% CI: 2.289–7.980, p < 0.01) (Table 2). Finally, a nomogram was developed by integrating the independent clinicopathological and US risk factors mentioned above (Figure 2).
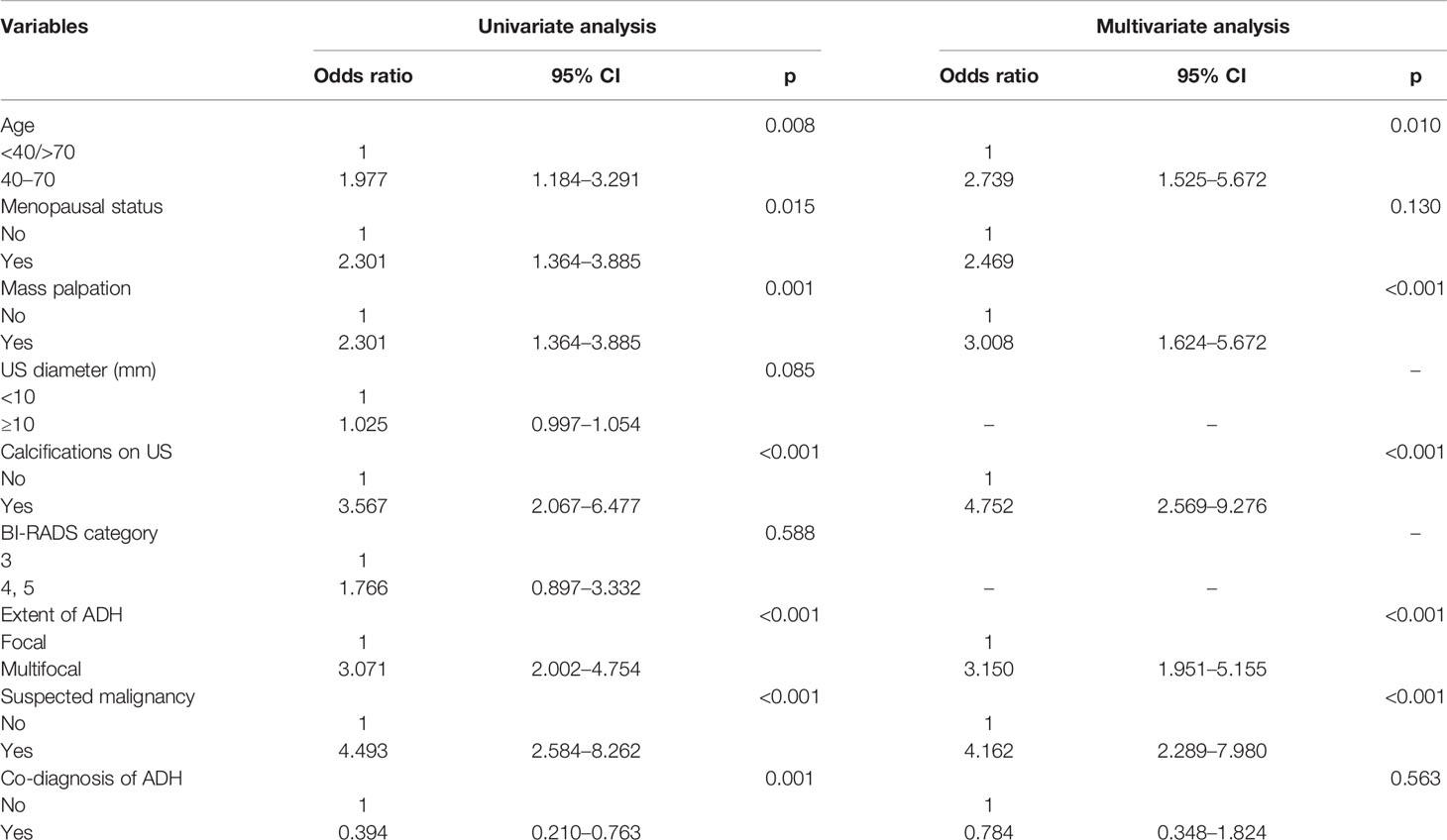
Table 2 Univariate and multivariate logistic regression analyses of factors predictive of upgrading.
Performance of the Nomogram in the Training and Validation Sets
The calibration curve of the nomogram for the probability of breast cancer upgrade demonstrated good agreement between the predictions and observations in the training and validation sets (p > 0.05, Figure 3). The Hosmer-Lemeshow test yielded non-significant results in both cohorts (p = 0.543 and p = 0.565), which suggested that there was no departure from a perfect fit. For predicting the upgrade of ADH, model 1 (clinicopathological features) yield an AUC, a sensitivity, a specificity, and a best cut-off of 0.747 (0.697–0.797), 67.9%, 75.9%, and 0.631, respectively, in the training set, and 0.681 (0.581–0.780), 62.3%, 72.2%, and 0.663 respectively, in the validation set. While model 2 (clinicopathological and US features) yielded an AUC, a sensitivity, a specificity, and a best cut-off of 0.783 (0.736–0.831), 80.9%, 66.2%, and 0.557, respectively, in the training set, 0.753 (0.666–0.841), 80.2%, 63.9%, and 0.574, respectively, in the validation set (Figure 4, Supplementary Table 1). The total points could be plotted into Table 3 to conclude the diagnosis values.
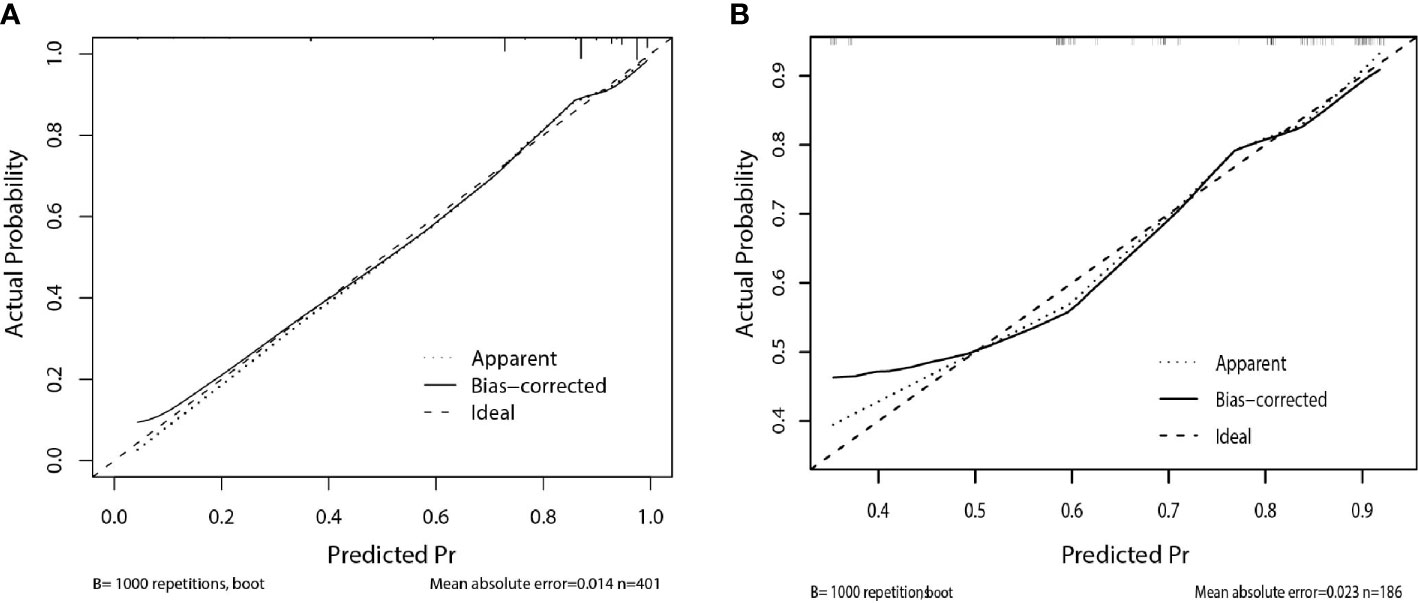
Figure 3 Calibration curve of the nomogram for predicting upgrade to breast cancer: (A) in the training set, (B) in the validation set. The solid line represents the ideal reference line that predicted ADH upgrade corresponds to the actual outcome, the short-dashed line represents the apparent prediction of nomogram, and the long-dashed line represents the ideal estimation. The prediction performance of the nomogram in training and validation set show closely to observed rates.
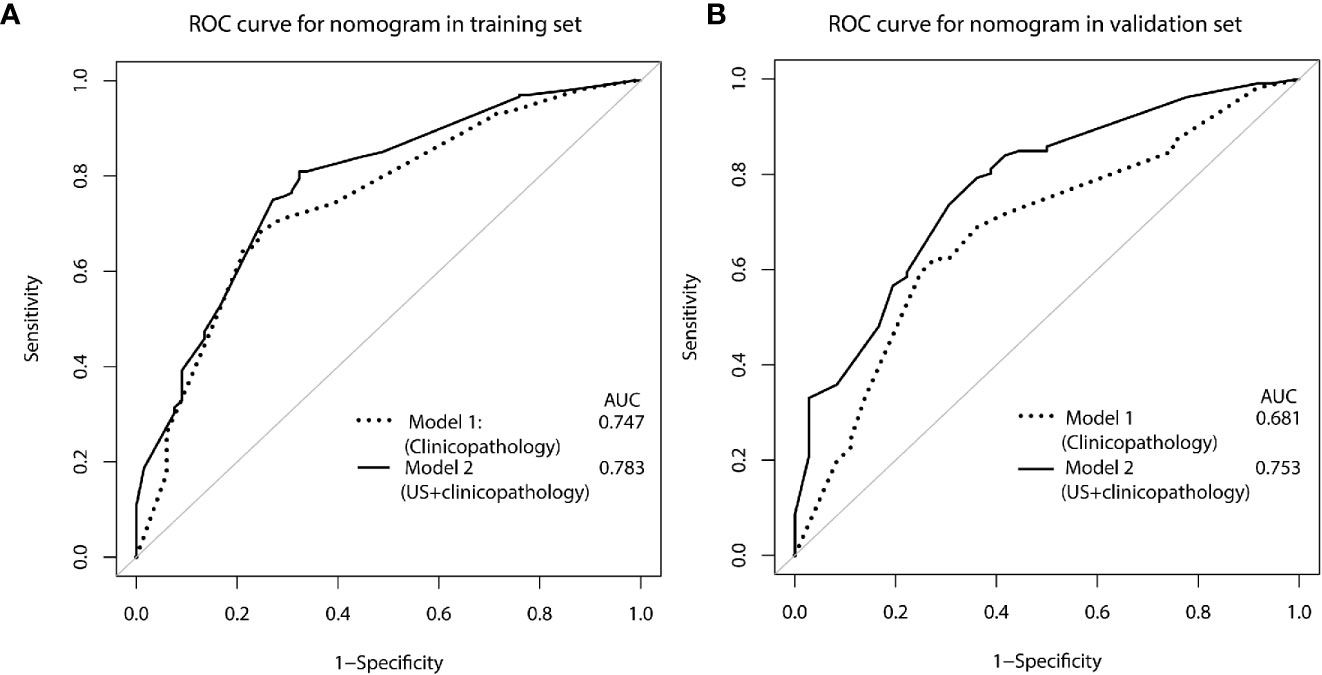
Figure 4 ROC curve for predicting ADH upgrades: (A) in the training set, (B) in the validation set. Model 1, clinicopathological features; Model 2, US +clinicopathological features.
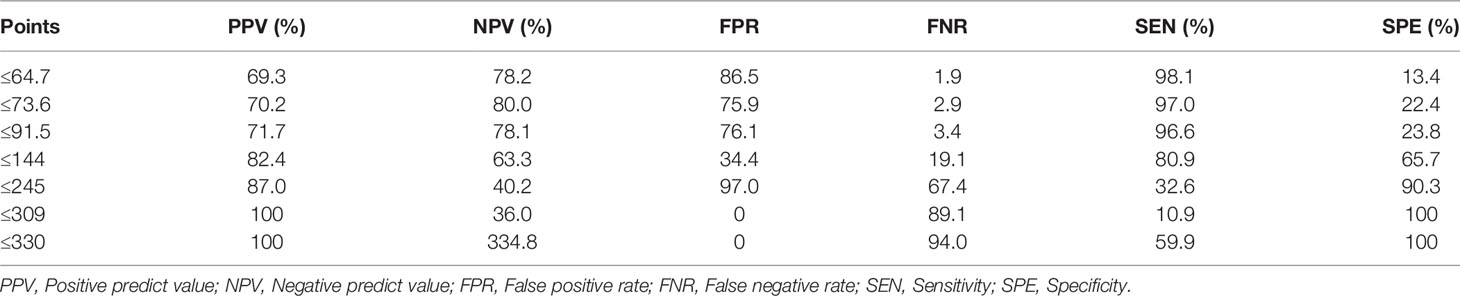
Table 3 Corresponding mammogram showed grouping and indeterminate micro-calcifications in CC position (B) and MLO position (C).
Clinical Application of the Model
The decision curve analysis for the constructed nomogram were presented in Figures 5A, B. The plot indicated that the model-based decision showed a more net benefit than either the treat-none-patients scheme or the treat-all scheme for the predicted probability thresholds between 0 and 90%.
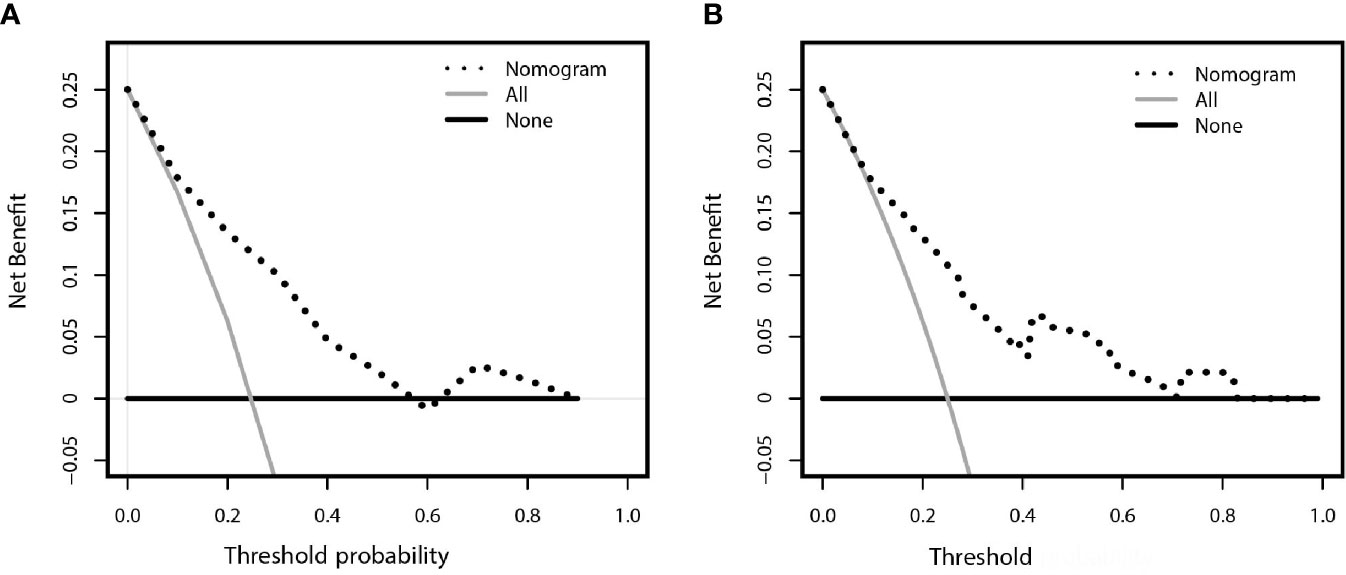
Figure 5 Decision curve analysis comparing the net-benefit of using the nomogram (black clashed line) depicted in (A) training set (B) validation set. Black solid line: net benefit when all breast cancer patients are considered as not upgrade with ADH; gray solid line: net benefit when all ADH patients are considered as upgraded to breast cancer. The ideal model is the model with highest net benefit at any given threshold.
Discussion
Despite open-excision being consistently recommended in all the ADH lesions diagnosed by CNB (2), it remains elusive that whether the patients might or might not benefit from the surgery or the surveillance. Therefore, this study developed and validated a US-based nomogram for predicting ADH upgrade in 587 patients (training set, n = 401; validation set, n = 186) under US-guided breast biopsy. The nomogram incorporating two clinical features (age and palpation), one US sign (calcifications), and two pathological features (ADH extent and suspected malignancy). The developed nomogram can be widely applied to facilitate efficient treatment decisions and rational resource allocation, especially in developing countries, because all predicting factors are routinely available before surgery.
The risk factors found in this study are partly similar to those reported in the literature (Supplementary Table 2). The difference could be attributed to the sample size and combination of available data. For example, we had 587 cases and 395 events, whereas most published studies had 45 to 422 cases with up to 133 events. With regard to the age of patients, several studies reported various risks in the age category where some found no increase in the risk (24, 25), others reported significant risks in univariable analysis but not in the multivariable analysis (10, 11), and some had significant in multivariable analysis (8). The results obtained in this study indicated that the upgrade rate was higher in middle age years (40–70) and lower at the extremes of age (<40/>70), which was consistent with the findings of another study with a large sample size (9). The higher risk at middle ages can be attributed the fact that the breast gland undergoes tissue remodeling during perimenopausal years, which likely presents a permissive microenvironment for the premalignant epithelium to progress to cancer (9). Similar to the previous studies. We also found that the upgrade was more associated with multifocal ADH than the focal one, which was consistent with previous studies (5, 6, 8, 10, 12, 26). This is probably due to the fact that the foci of ADH may be present at the periphery of DCIS (27). This study also confirmed the report in previous studies that the palpability of lesions is as a risk factor (12, 14, 28). Only a limited number of studies have reported that suspected malignancy a high risk for upgrading biopsies with a suspected component (20).
Despite the clinical and pathological factors, we found that US features may help predict the possibility of breast cancer upgrade. Specifically, calcifications on the US were more discriminative than any other risk factor in the model, with a high positive predictive value because they were present in 93% of cases having positive surgical excisions (Figures 6 and 7). This association between the likelihood of malignancy and calcifications on mammography is not unexpected, because several previous studies reported that the extent of calcifications clusters (>15mm) is a determinant feature for malignancy on mammography, and incomplete removal of calcifications was considered to be correlated with the upgrade of ADH (12, 25, 29). Although mammography is the standard method for evaluating breast calcification, however, it is expensive to perform in developing countries. It is worth noting that US is also able to detect most microcalcifications (with a maximum diameter of 1 mm) that correlates well with mammography (30–32). To our knowledge, calcifications on US has never been demonstrated in the prediction of ADH before. One study observed that calcifications was associated with upgrade rate under ultrasound-guided CNB, but whether the calcifications on US was the same as it on MG was not clear (12). It has been though that breast tumor cells acquired osteoblast-like phenotype through epithelial-mesenchymal transition and drive pathophysiological microcalcifications, but little has been understood about the precise underlying molecular mechanism (33). A higher specificity (86.9%) has been reported in a nomogram (11) incorporating nine clinical, radiological, and pathological variables, but with a relatively small sample size (n = 203) without a validation set. On the other hand, Ko et al. (12) proposed a scoring system based on clinical, imaging, and pathological features with a diagnostic power of 0.903. This system was externally validated by Bendifallah et al. (13), which resulted in a low reproducibility of 0.510 and specificity of 0.22.
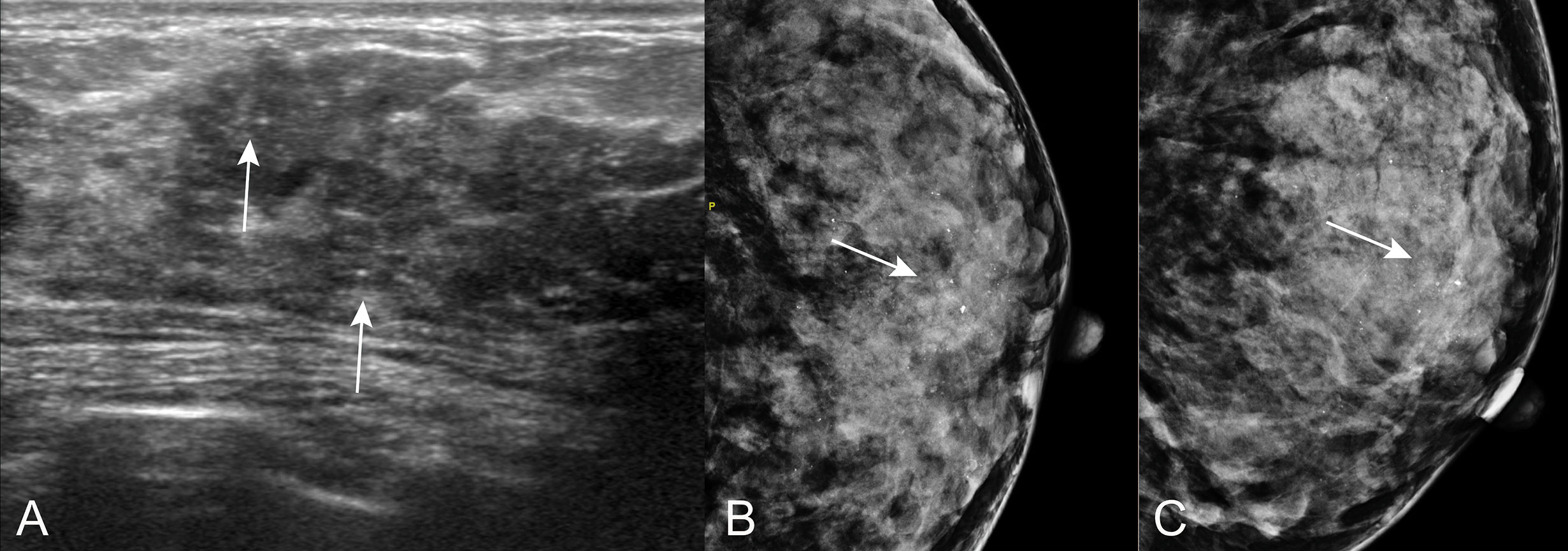
Figure 6 Calcifications on US in patients with ADH on CNB. A 44 years old woman diagnosed with sclerosing adenosis at final pathologic examination. (A) US showed sparse calcifications (arrow) at hypoechoic hypoechoic non-mass of breast lesion. Corresponding mammogram showed regional microcalcifications in CC position (B) and MLO position (C).
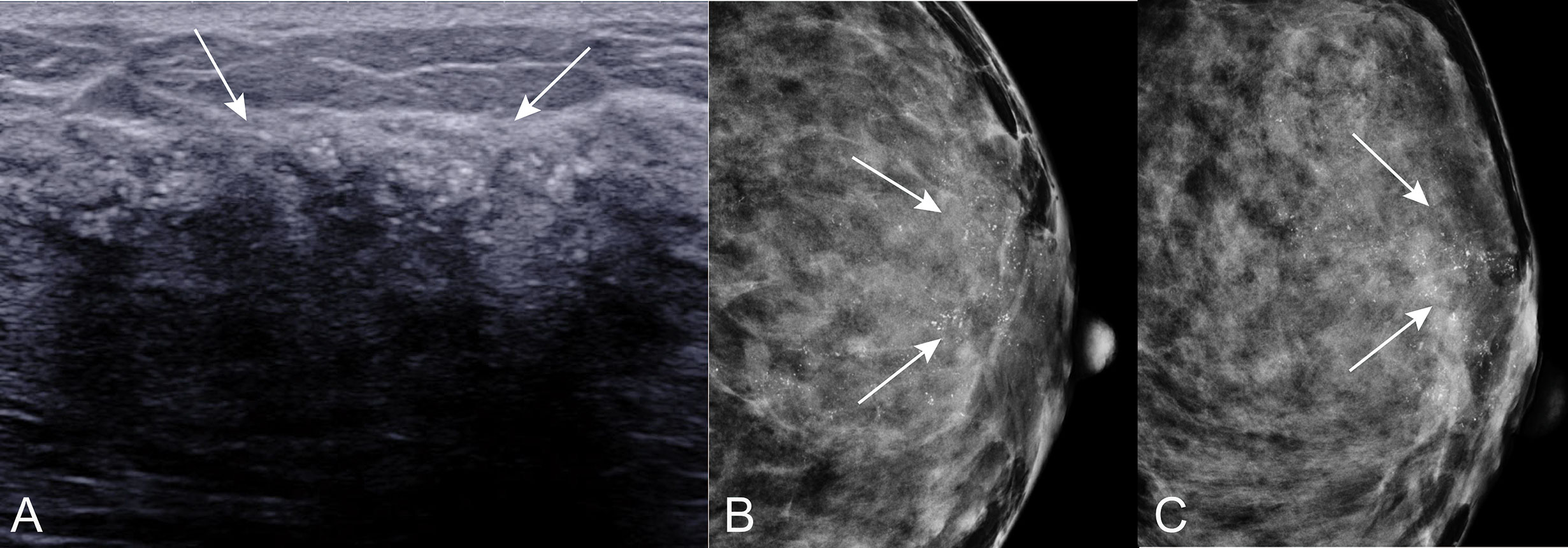
Figure 7 Calcifications on US in patients with ADH on CNB. A 55 years old woman diagnosed with DCIS at final pathologic examination. (A) US showed calcifications (arrow) around with hypoechoic non-mass of breast lesion. Corresponding mammogram showed grouping and indeterminate microcalcifications in CC position (B) and MLO position (C).
The nomogram we developed could support the clinical decision of ADH diagnosed on CNB. For example, point 91.5 indicated a FNR less than 5%, this might indicate a lower risk for upgraded according to the consensus that overall underestimation rates of ADH should not exceed 5% for invasive cancer and 10% for DCIS (2). Corresponding to the predictive model with specificity of 0.639, use of the nomogram could have avoided surgery in more than 60% of the patients with finally confirmed benign diagnosis. On the other hand, the upgrade rate of 66.6% (267/401) in our results indicates that excision of the ADH is essential for finding already existing breast cancers. Furthermore, we found that about one-fourth of the cancer were IDC, which was in line with or somewhat higher than the results reported in a previous study where one-fifth of the upgraded lesions were invasive breast cancer and non-low-grade DCIS (34). This might be related to the heterogeneity of breast cancer that DCIS is always co-existing with IDC (35). The higher rate of IDC in our study may indicate that ADH lesions were obtained neighboring malignancy with CNB (21). The necessity of the excision procedure was further strengthened by another study where multiple surgeries were reported in 29% of the patients diagnosed with ADH, including re-excision and mastectomy, to achieve clear margin in extensive DCIS cases (34).
Clinically, more breast lesion types are detectable and under direct vision by US-guided biopsy. MRI is superior to US and mammography because it has a high sensitivity in breast imaging diagnosis. However, the cost of MRI remains a great economic burden and not all the women can tolerate MRI scanning procedure. Application of the nomogram model the developed in this study can help high-risk patients receive prompt treatment with a valuable decrease in patient waiting anxiety. However, the potential for reducing costs for low-risk patients should be explored in a dedicated analysis considering long-interval follow-up recommendations and additional imaging supporting. Combining artificial intelligence and molecular methodology in a large number of cases, through collaborative and, multicenter studies has the potential to advance knowledge in this field and assist in appropriate design of trials of treatment decision for women diagnosed with ADH using CNB (36).
This study had the following limitations: Firstly, the nomogram was established and validated based on retrospective data. Therefore, subsequent prospective randomized trials should be conducted to determine whether the nomogram model improves current patient stratification approaches for clinical decision-making and the corresponding prognosis. Secondly, the calcifications detected by US were not confirmed by mammography, which is considered to have a more diagnostic value. Further studies using large datasets that compare and correlate predictive radiological features in multiple imaging guidance methods, including stereotactic (mammographic) guidance, US guidance, and MRI guidance, may help to facilitate the development of an individualized model as a promising tool for clinical use.
Conclusion
This study developed an easy-to-use nomogram model that incorporates clinicopathological and US features to predict the upgrade of ADH on CNB. Application of the nomogram model can provide information for clinical procedure planning and potentially increase clinical efficiency.
Data Availability Statement
The datasets used and analysed during the current study are readily available from the corresponding author upon reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committees of Fudan University Cancer Center. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
Y-XH and S-CZ contributed to the conception and design of the study, data analysis and interpretation, and drafting the manuscript. Y-LC, KZ, and S-PL collected and analyzed US and pathological data,while CC and J-PS contributed to the acquisition and assembly of data. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Science and Technology Commission of Shanghai Municipality (18411967400, 17411953400), Shanghai Municipal Commission of Health and Family Planning (20174Y0011), Shanghai Anticancer Association EYAS PROJECT (SACA-CY1C06), and Fudan University Shanghai Cancer Center Special Fund for Iconography (YX201804).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Lei Wang for the contribution to statistical assistance in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.609841/full#supplementary-material
References
1. Kader T, Hill P, Zethoven M, Goode DL, Elder K, Thio N, et al. Atypical ductal hyperplasia is a multipotent precursor of breast carcinoma. J Pathol (2019) 248:326–38. doi: 10.1002/path.5262
2. Rageth CJ, O’flynn E, Pinker K, Kubik-Huch RA, Mundinger A, Decker T, et al. Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res Treat (2019) 174:279–96. doi: 10.1007/s10549-018-05071-1
3. Schiaffino S, Calabrese M, Melani EF, Trimboli RM, Cozzi A, Carbonaro LA, et al. Upgrade Rate of Percutaneously Diagnosed Pure Atypical Ductal Hyperplasia: Systematic Review and Meta-Analysis of 6458 Lesions. Radiology (2020) 294:76–86. doi: 10.1148/radiol.2019190748
4. Brem RF. Management of Breast Atypical Ductal Hyperplasia: Now and the Future. Radiology (2020) 294:87–8. doi: 10.1148/radiol.2019192192
5. Chen LY, Hu J, Tsang JYS, Lee MA, Ni YB, Chan SK, et al. Diagnostic upgrade of atypical ductal hyperplasia of the breast based on evaluation of histopathological features and calcification on core needle biopsy. Histopathology (2019) 75:320–28. doi: 10.1111/his.13881
6. Pena A, Shah SS, Fazzio RT, Hoskin TL, Brahmbhatt RD, Hieken TJ, et al. Multivariate model to identify women at low risk of cancer upgrade after a core needle biopsy diagnosis of atypical ductal hyperplasia. Breast Cancer Res Treat (2017) 164:295–304. doi: 10.1007/s10549-017-4253-1
7. Bicchierai G, Nori J, De Benedetto D, Boeri C, Vanzi E, Bianchi S, et al. Follow-up of B3 breast lesions without residual microcalcifications post vacuum-assisted biopsy, can contrast-enhanced digital mammography help? Breast J (2020) 26:299–302. doi: 10.1111/tbj.13598
8. Salagean ED, Slodkowska E, Nofech-Mozes S, Hanna W, Parra-Herran C, Lu FI. Atypical ductal hyperplasia on core needle biopsy: Development of a predictive model stratifying carcinoma upgrade risk on excision. Breast J (2019) 25:56–61. doi: 10.1111/tbj.13155
9. Degnim AC, Winham SJ, Frank RD, Pankratz VS, Dupont WD, Vierkant RA, et al. Model for Predicting Breast Cancer Risk in Women With Atypical Hyperplasia. J Clin Oncol (2018) 36:1840–46. doi: 10.1200/JCO.2017.75.9480
10. Deshaies I, Provencher L, Jacob S, Cote G, Robert J, Desbiens C, et al. Factors associated with upgrading to malignancy at surgery of atypical ductal hyperplasia diagnosed on core biopsy. Breast (2011) 20:50–5. doi: 10.1016/j.breast.2010.06.004
11. Khoury T, Chen X, Wang D, Kumar P, Qin M, Liu S, et al. Nomogram to predict the likelihood of upgrade of atypical ductal hyperplasia diagnosed on a core needle biopsy in mammographically detected lesions. Histopathology (2015) 67:106–20. doi: 10.1111/his.12635
12. Ko E, Han W, Lee JW, Cho J, Kim EK, Jung SY, et al. Scoring system for predicting malignancy in patients diagnosed with atypical ductal hyperplasia at ultrasound-guided core needle biopsy. Breast Cancer Res Treat (2008) 112:189–95. doi: 10.1007/s10549-007-9824-0
13. Bendifallah S, Defert S, Chabbert-Buffet N, Maurin N, Chopier J, Antoine M, et al. Scoring to predict the possibility of upgrades to malignancy in atypical ductal hyperplasia diagnosed by an 11-gauge vacuum-assisted biopsy device: an external validation study. Eur J Cancer (2012) 48:30–6. doi: 10.1016/j.ejca.2011.08.011
14. Kim J, Han W, Go EY, Moon HG, Ahn SK, Shin HC, et al. Validation of a scoring system for predicting malignancy in patients diagnosed with atypical ductal hyperplasia using an ultrasound-guided core needle biopsy. J Breast Cancer (2012) 15:407–11. doi: 10.4048/jbc.2012.15.4.407
15. Hsu HH, Yu JC, Hsu GC, Yu CP, Chang WC, Tung HJ, et al. Atypical ductal hyperplasia of the breast diagnosed by ultrasonographically guided core needle biopsy. Ultraschall Med (2012) 33:447–54. doi: 10.1055/s-0029-1245877
16. Han P, Yang H, Liu M, Cheng L, Wang S, Tong F, et al. Lymph Node Predictive Model with in Vitro Ultrasound Features for Breast Cancer Lymph Node Metastasis. Ultrasound Med Biol (2020) 46:1395–402. doi: 10.1016/j.ultrasmedbio.2020.01.030
17. Hodorowicz-Zaniewska D, Brzuszkiewicz K, Szpor J, Kibil W, Matyja A, Dylag-Trojanowska K, et al. Clinical predictors of malignancy in patients diagnosed with atypical ductal hyperplasia on vacuum-assisted core needle biopsy. Wideochir Inne Tech Maloinwazyjne (2018) 13:184–91. doi: 10.5114/wiitm.2018.73528
18. Hong ZJ, Chu CH, Fan HL, Hsu HM, Chen CJ, Chan DC, et al. Factors predictive of breast cancer in open biopsy in cases with atypical ductal hyperplasia diagnosed by ultrasound-guided core needle biopsy. Eur J Surg Oncol (2011) 37:758–64. doi: 10.1016/j.ejso.2011.06.014
19. Lakhani S, Ellis IS S. World Health Organisation Classification of Tumors of the Breast. Lyon, France: International Agency for Research on Cancer (IARC (2012) p. 142–47.
20. Allison KH, Eby PR, Kohr J, Demartini WB, Lehman CD. Atypical ductal hyperplasia on vacuum-assisted breast biopsy: suspicion for ductal carcinoma in situ can stratify patients at high risk for upgrade. Hum Pathol (2011) 42:41–50. doi: 10.1016/j.humpath.2010.06.011
21. Masood S, Rosa M. Borderline breast lesions: diagnostic challenges and clinical implications. Adv Anat Pathol (2011) 18:190–8. doi: 10.1097/PAP.0b013e31821698cc
22. American College of Radiology, D'Orsi CJ. ACR BI-RADS Atlas: Breast Imaging Reporting and Data System; Mammography, Ultrasound, Magnetic Resonance Imaging, Follow-up and Outcome Monitoring, Data Dictionary. ACR, American College of Radiology (2013).
23. Ha GW, Yi MS, Lee BK, Youn HJ, Jung SH. Clinical outcome of magnetic resonance imaging-detected additional lesions in breast cancer patients. J Breast Cancer (2011) 14:213–8. doi: 10.4048/jbc.2011.14.3.213
24. Wagoner MJ, Laronga C, Acs G. Extent and histologic pattern of atypical ductal hyperplasia present on core needle biopsy specimens of the breast can predict ductal carcinoma in situ in subsequent excision. Am J Clin Pathol (2009) 131:112–21. doi: 10.1309/AJCPGHEJ2R8UYFGP
25. Jackman RJ, Birdwell LI, keda DM. Atypical ductal hyperplasia: can some lesions be defined as probably benign after stereotactic 11-gauge vacuum-assisted biopsy, eliminating the recommendation for surgical excision? Radiology (2002) 224:548–54. doi: 10.1148/radiol.2242011528
26. Forgeard C, Benchaib M, Guerin N, Thiesse P, Mignotte H, Faure C, et al. Is surgical biopsy mandatory in case of atypical ductal hyperplasia on 11-gauge core needle biopsy? A retrospective study of 300 patients. Am J Surg (2008) 196:339–45. doi: 10.1016/j.amjsurg.2007.07.038
27. Lennington WJ, Jensen RA, Dalton LW, Page DL. Ductal carcinoma in situ of the breast. Heterogeneity of individual lesions. Cancer (1994) 73:118–24. doi: 10.1002/1097-0142(19940101)73:1<118::aid-cncr2820730121>3.0.co;2-r
28. Co M, Kwong A, Shek T. Factors affecting the under-diagnosis of atypical ductal hyperplasia diagnosed by core needle biopsies - A 10-year retrospective study and review of the literature. Int J Surg (2018) 49:27–31. doi: 10.1016/j.ijsu.2017.11.005
29. Kohr JR, Eby PR, Allison KH, Demartini WB, Gutierrez RL, Peacock S, et al. Risk of upgrade of atypical ductal hyperplasia after stereotactic breast biopsy: effects of number of foci and complete removal of calcifications. Radiology (2010) 255:723–30. doi: 10.1148/radiol.09091406
30. Fischer T, Grigoryev M, Bossenz S, Diekmann F, Bick U, Slowinski T, et al. [Sonographic detection of microcalcifications - potential of new method]. Ultraschall Med (2012) 33:357–65. doi: 10.1055/s-0031-1299128
31. Kim Hy SB, Kim Hy, Yie a, Cho r, Seol Hy, Cha Sh, et al. Additional breast ultrasound examinations in clustered calcifications: for improving diagnostic performance. J Breast Cancer (2009) 12:142–50. doi: 10.4048/jbc.2009.12.3.142
32. Cho KR, Seo BK, Woo OH, Song SE, Choi J, Whang SY, et al. Breast Cancer Detection in a Screening Population: Comparison of Digital Mammography, Computer-Aided Detection Applied to Digital Mammography and Breast Ultrasound. J Breast Cancer (2016) 19:316–23. doi: 10.4048/jbc.2016.19.3.316
33. Sharma T, Radosevich JA, Pachori G, Mandal CC. A Molecular View of Pathological Microcalcification in Breast Cancer. J Mammary Gland Biol Neoplasia (2016) 21:25–40. doi: 10.1007/s10911-015-9349-9
34. Farshid G, Edwards S, Kollias J, Gill PG. Active surveillance of women diagnosed with atypical ductal hyperplasia on core needle biopsy may spare many women potentially unnecessary surgery, but at the risk of undertreatment for a minority: 10-year surgical outcomes of 114 consecutive cases from a single center. Mod Pathol (2018) 31:395–405. doi: 10.1038/modpathol.2017.114
35. Meurs CJC, Van Rosmalen J, Menke-Pluijmers MBE, Ter Braak BPM, De Munck L, Siesling S, et al. A prediction model for underestimation of invasive breast cancer after a biopsy diagnosis of ductal carcinoma in situ: based on 2892 biopsies and 589 invasive cancers. Br J Cancer (2018) 119:1155–62. doi: 10.1038/s41416-018-0276-6
Keywords: atypical ductal hyperplasia, breast, ultrasound, upgrade, prediction
Citation: Huang Y-X, Chen Y-L, Li S-P, Shen J-P, Zuo K, Zhou S-C and Chang C (2021) Development and Validation of a Simple-to-Use Nomogram for Predicting the Upgrade of Atypical Ductal Hyperplasia on Core Needle Biopsy in Ultrasound-Detected Breast Lesions. Front. Oncol. 10:609841. doi: 10.3389/fonc.2020.609841
Received: 24 September 2020; Accepted: 16 December 2020;
Published: 31 March 2021.
Edited by:
Hyung Seok Park, Yonsei University College of Medicine, South KoreaReviewed by:
Jeea Lee, Yonsei University, South KoreaVivian Park, Yonsei University College of Medicine, South Korea
Simone Schiaffino, IRCCS Policlinico San Donato, Italy
Copyright © 2021 Huang, Chen, Li, Shen, Zuo, Zhou and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi-Chong Zhou, c2N6aG91QGhvdG1haWwuY29t
 Yun-Xia Huang
Yun-Xia Huang Ya-Ling Chen
Ya-Ling Chen Shi-Ping Li
Shi-Ping Li Ju-Ping Shen
Ju-Ping Shen Ke Zuo
Ke Zuo Shi-Chong Zhou
Shi-Chong Zhou Cai Chang
Cai Chang