- 1Department of Radiation Oncology, University of Verona Hospital Trust, Verona, Italy
- 2Department of Medical Physics, University of Verona Hospital Trust, Verona, Italy
- 3Department of Radiology, University of Verona Hospital Trust, Verona, Italy
- 4Department of General and Pancreatic Surgery, University of Verona Hospital Trust, Verona, Italy
- 5Department of Oncology, University of Verona Hospital Trust, Verona, Italy
Background and Objective: To assess the dosimetric feasibility of a stereotactic body radiotherapy (SBRT) dose escalated protocol, with a simultaneous integrated boost (SIB) and a simultaneous integrated protection (SIP) approach, in patients with locally advanced pancreatic cancer (LAPC).
Material and Methods: Twenty LAPC lesions, previously treated with SBRT at our Institution, were re-planned. The original prescribed and administered dose was 50/30/25 Gy in five fractions to PTVsib (tumor-vessel interface [TVI])/PTVt (tumor volume)/PTVsip (overlap area between PTVt and planning organs at risk volume [PRVoars]), respectively. At re-planning, the prescribed dose was escalated up to 60/40/33 Gy in five fractions to PTVsib/PTVt/PTVsip, respectively. All plans were performed using an inspiration breath hold (IBH) technique and generated with volumetric modulated arc therapy (VMAT). Well-established and accepted OAR dose constraints were used (D0.5cc < 33 Gy for luminal OARs and D0.5cc < 38 Gy for corresponding PRVoars). The primary end-point was to achieve a median dose equal to the prescription dose for the PTVsib with D98≥ 95% (95% of prescription dose is the minimum dose), and a coverage for PTVt and PTVsip of D95≥95%, with minor deviations in OAR dose constraints in < 10% of the plans.
Results: PTVsib median (± SD) dose/D95/conformity index (CI) were 60.54 (± 0.85) Gy/58.96 (± 0.86) Gy/0.99 (± 0.01), respectively; whilst PTVt median (± SD) dose/D95 were 44.51 (± 2.69) Gy/38.44 (± 0.82) Gy, and PTVsip median (± SD) dose/D95 were 35.18 (± 1.42) Gy/33.01 (± 0.84) Gy, respectively. With regard to OARs, median (± SD) maximum dose (D0.5cc) to duodenum/stomach/bowel was 29.31 (± 5.72) Gy/25.29 (± 6.90) Gy/27.03 (± 5.67) Gy, respectively. A minor acceptable deviation was found for a single plan (bowel and duodenum D0.5cc=34.8 Gy). V38 < 0.5 cc was achieved for all PRV luminal OARs.
Conclusions: In LAPC patients SBRT, with a SIB/SIP dose escalation approach up to 60/40/33 Gy in five fractions to PTVsib/PTVt/PTVsip, respectively, is dosimetrically feasible with adequate PTVs coverage and respect for OAR dose constraints.
Introduction
The results of standard dose radiation therapy (RT) in locally advanced, unresectable pancreatic cancer (LAPC) are unsatisfactory. Conventional RT strategies (conventionally fractionated radiation therapy [CFRT]) have a modest impact on long-term tumor control and survival. Indeed, the randomized LAP-07 Phase III trial failed to demonstrate improvement in overall survival (OS) in LAPC patients by adding CFRT (54 Gy/30 fractions with concurrent capecitabine) after neoadjuvant chemotherapy versus continuation of chemotherapy alone (1). However, RT was associated with a decrease in local progression (32% vs 46%, p = 0.03) without increasing grade ≥ 3 toxicity. Four other randomized trials have compared CFRT, concomitant with chemotherapy, versus chemotherapy alone in LAPC, with interlocutory results: two trials supported a chemo-radiation approach (2, 3), while two did not (4, 5).
Although metastatic disease represents the main cause of morbidity and mortality in LAPC, about one third of patients die from complications related to local tumor progression (6). Moreover, Crane et al. found that local tumor progression was the dominant cause of death in patients alive at more than 15 months (7). Thus, further studies with more effective RT strategies in LAPC are widely expected. In this context, stereotactic body radiation therapy (SBRT) has emerged as an effective component for the multimodal treatment of pancreatic cancer. According to recent studies, SBRT after systemic therapy can increase survival in LAPC compared to either chemotherapy alone or CFRT (8–10). At present, the optimal SBRT schedule has yet to be determined, but the administration of a higher biologically effective dose (BED) is essential to achieve durable tumor control and impact on survival (11). In addition, it can be postulated that SBRT, following induction chemotherapy, may improve the likelihood of resection also for LAPC, in the context of a total neoadjuvant therapy approach (12–14). In particular, the administration of ablative doses to the tumor-vessel interface (TVI), can sterilize the tumor boundaries involving peripancreatic vessels, and together with mass shrinkage, potentially allow surgery.
However, the administration of such high doses is challenging when tumors are close to critical organs at risk (OARs) such as the duodenum, stomach and bowel. A novel approach of SBRT with simultaneous integrated boost (SIB) and simultaneous integrated protection (SIP) has recently been described in an observational study, showing a promising local control (LC) rate of 75% in non-resected patients (versus 82.3% in resected patients; p=0.46) (15). After induction chemotherapy, SBRT was delivered in 5 consecutive daily fractions by administering 30 Gy to the planning target volume tumor (PTVt), while simultaneously delivering a 50 Gy SIB to the tumor-vessel interface (PTVsib). The SIP volume (PTVsip) was created by lowering the dose to 25 Gy on the overlap area between the PTVt and the planning organs at risk volume (PRVoars). No acute or late grade ≥ 3 adverse events related to SBRT were observed. Moreover, 34.4% of locally advanced patients received surgical resection. Nonetheless, the performed dosimetric evaluation showed a predominant incidence of in-field failure, with a progression median dose of 40.42 Gy. These data support the need to further investigate the possibility of administering higher doses of RT, using this SBRT approach, in order to improve oncological outcomes in LAPC.
Based on this background, we aimed at performing a dosimetric study to assess the feasibility of SBRT with a SIB/SIP dose escalated protocol in LAPC, to administer higher doses to the tumor, while preserving OAR dose constraints.
Materials and Methods
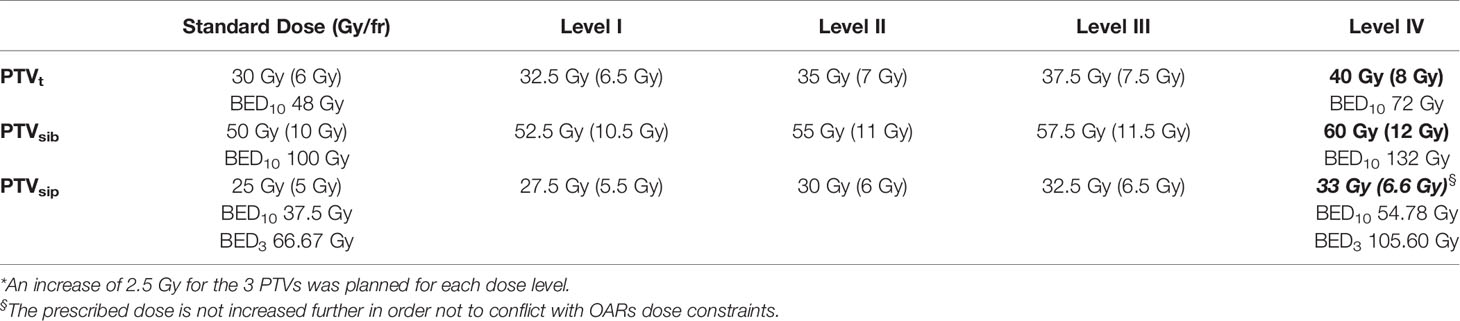
Study Design
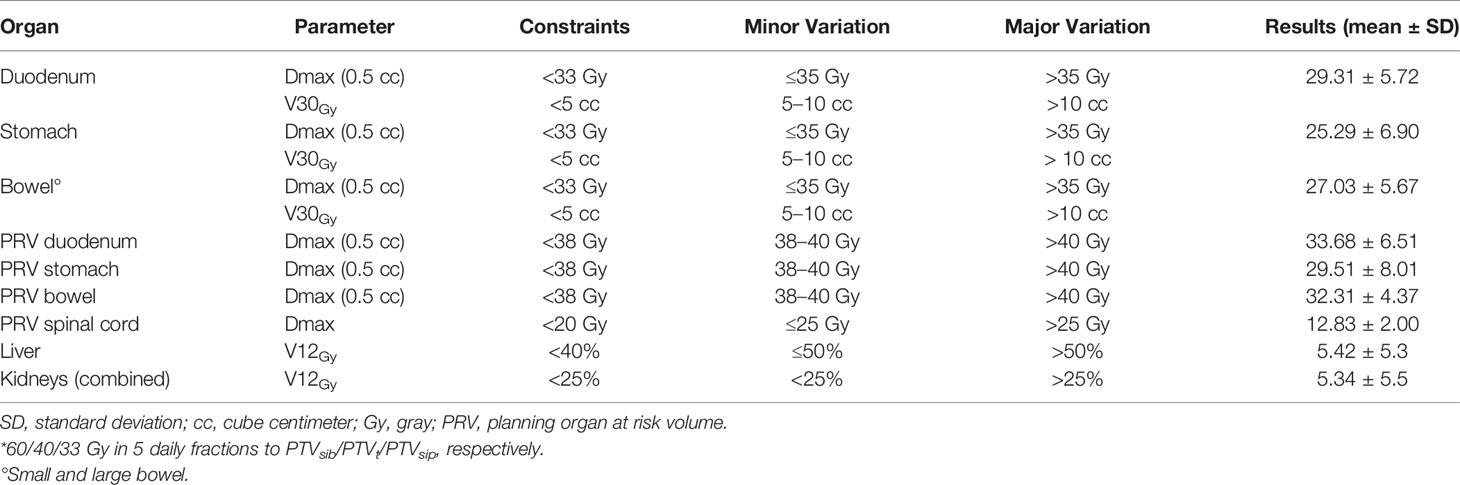
Twenty patients with LAPC, treated at our Institution with SBRT in 5 consecutive daily fractions using a SIB/SIP approach (50/30/25 Gy to PTVsib/PTVt/PTVsip, respectively) (15), were re-planned for a dose escalation proposal. Patients were randomly selected from a prospective collected database. The final goal of this dosimetric evaluation study was to escalate the dose up to 60/40/33 Gy in five fractions to PTVsib/PTVt/PTVsip, respectively (Table 1). If the planning objectives were not met at this dose level (level IV), the prescription dose would be progressively reduced to the inferior levels (level III, II or I), until the pre-established planning objectives were achieved. The biologically effective dose (BED) was used to compare the different dose levels among each other, and with other recommended fractionations adopted in the clinical practice. The BED was calculated using the linear quadratic formula: BED = nd × [1 + d/(α/β)], where n is the total number of fractions and d is the dose per fraction (Gy). Standard α/β ratio for tumors (α/β =10) and normal tissues (α/β =3) was chosen. Dose constraints to organs at risk (OARs) were selected according to recently published guidelines (16). In particular, a D0.5cc < 33 Gy for luminal OARs and a D0.5cc < 38 Gy for corresponding PRVs were adopted.
SBRT Protocol and Planning
Patients were immobilized in a supine position with arms over the head, on a custom-made Vac-Lok™ cushion to optimize set-up reproducibility. Fiducial markers (3–4 gold seeds), using an eco-endoscopic procedure (EUS), were placed prior to simulation computed tomography (CT). To manage breathing-induced tumor motion, an inspiration breath hold (IBH) technique was used. Briefly, patients were trained to maintain a regular respiratory cycle, using the real-time position management® system (RPM) (Varian Medical Systems, Palo Alto, CA) as visual guide. At a comfortable inspiration phase of the respiratory cycle, patients were asked to hold their breath (IBH) to allow CT scan acquisition. After a first unenhanced IBH scan, a multi-phase contrast-enhanced simulation CT was performed (Figure 1), including the acquisition of an additional 3 to 4 contrast-enhanced IBH scans.
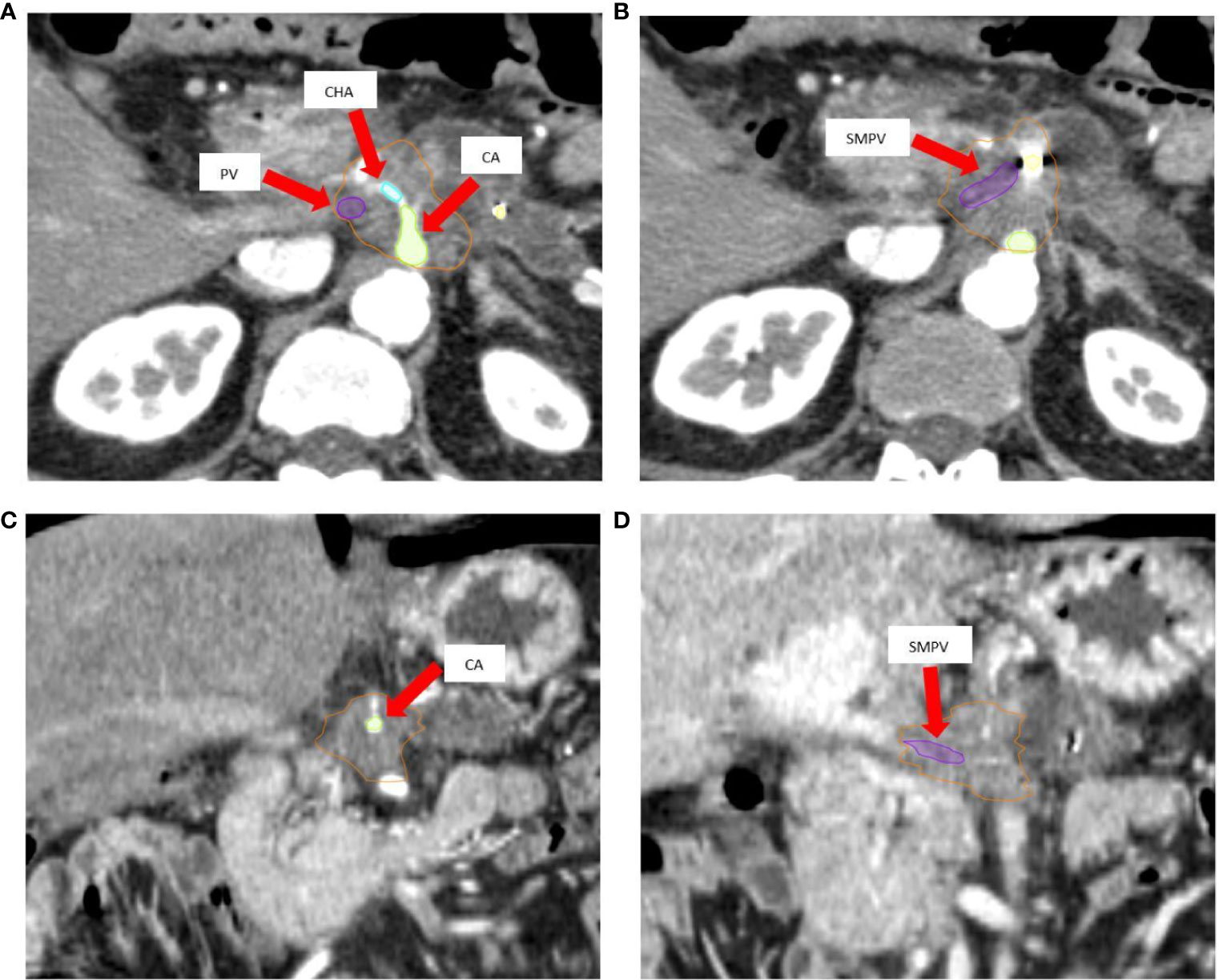
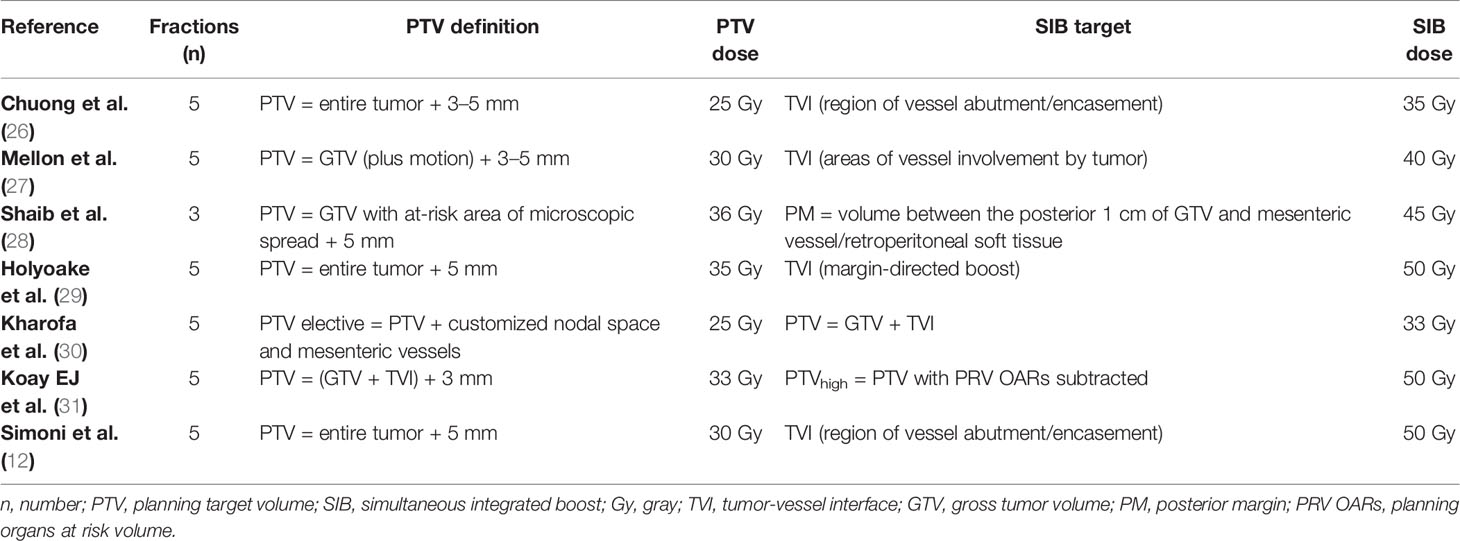
Figure 1 (A, B) Axial pancreatic CT-simulation phase images show hypovascular body mass of pancreas, delineated as gross tumor volume (GTV, orange) with encasement of celiac axis (CA, green), common hepatic artery (CHA, cyan), and superior mesenteric-portal venous confluence (SMPV, violet). (C) Arterial coronal CT-simulation image shows lesion encasing CA. (D) Coronal CT-simulation image highlights the SMPV system occlusion and portal vein infiltration.
An integrated gross tumor volume (iGTV) was defined as the envelope of the GTVs delineated on each CT scan. An iGTV-to-PTV margin of 3 mm was applied to generate the PTV tumor (PTVt). For critical OARs such as the duodenum, stomach and bowel, a 3 mm expansion PRVoars was defined. The simultaneous protection volume (PTVsip) was generated by the intersection of the PTVt and the PRVoars. A PTV high dose (PTVsib) was generated to encompass the tumor-vessel interface (TVI). Critical vessels (e.g. superior mesenteric artery/vein, portal vein, celiac artery) inside the iGTV were contoured for the whole circumference and then expanded by 3 mm to generate the PTVsib. If necessary, this PTVsib was contracted to respect a minimal distance of 5 mm from the PTVsip (Figures 2A, B).
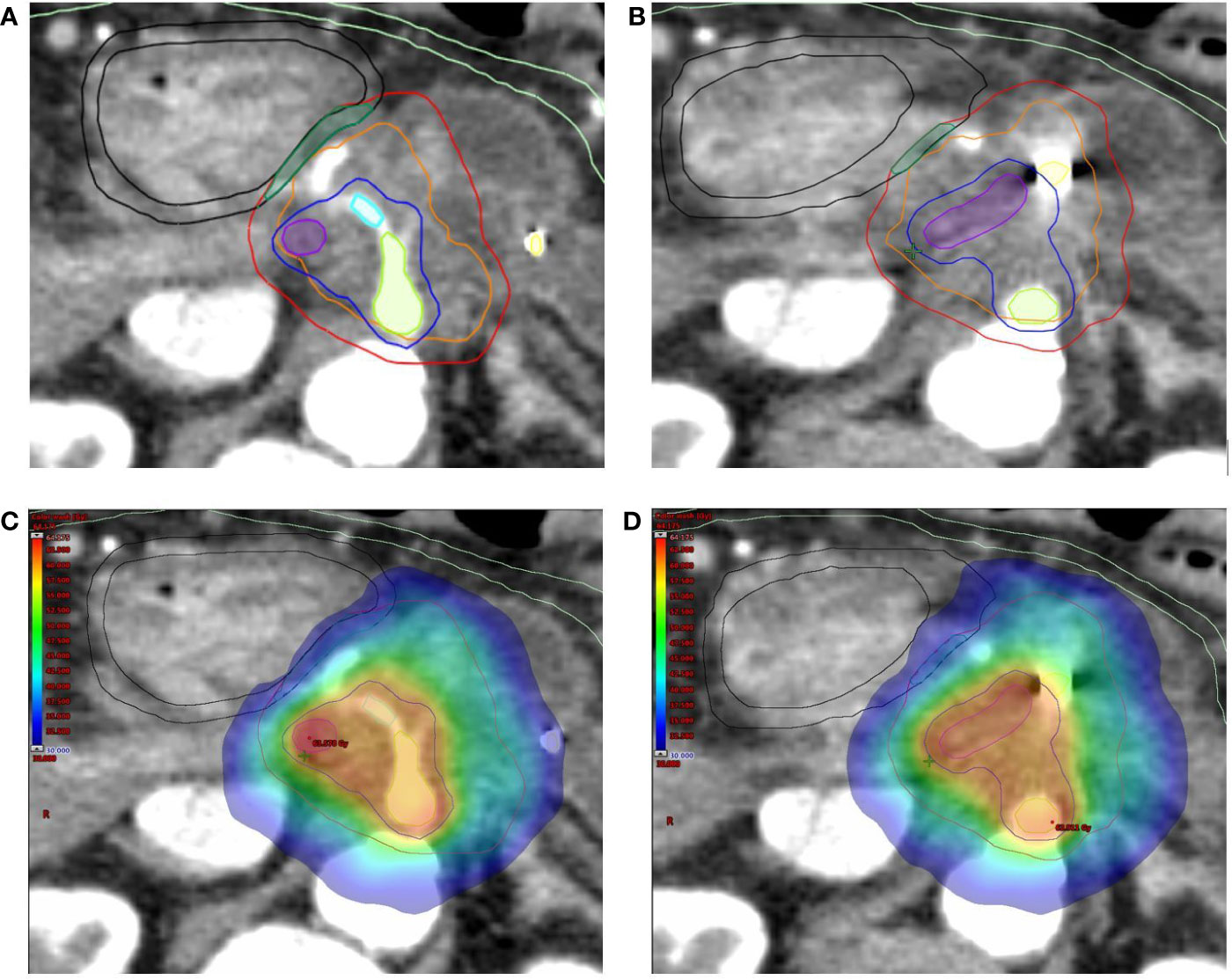
Figure 2 (A, B) Target volumes delineation. The high-dose planning target volume (PTVsib, blue) encompasses the tumor-vessel interface (celiac axis [green], common hepatic artery (cyan) and superior mesenteric-portal venous confluence [violet] + 3 mm expansion) inside the tumor planning target volume (PTVt, red). Respect to organ at risk constraints is guaranteed by the simultaneous protection volume (PTVsip, dark green). The following Organ at Risk (OARs) are shown: duodenum (black), and bowel (light green), as well as the fiducial markers (yellow). The Planning at Risk Volume (PRVoars) are generated by 3 mm expansion from corresponding OARs (shown with same color on axial images). (C, D) Typical dose distribution (color wash) for SBRT plan with Simultaneous Integrated Boost (SIB) and Simultaneous Integrated Protection (SIP). The prescription dose is 60/40/33 Gy in 5 daily fractions to PTVsib/PTVt/PTVsip, respectively. The sample plan demonstrates excellent PTVs coverage with appropriate respect of OARs.
All SBRT plans were calculated for a TrueBeam® medical linac (Varian Medical System, Palo Alto, CA) equipped with a high definition multileaf collimator (HDMLC-120) and using a Volumetric Modulated Arc Therapy (VMAT) technique (Figures 2C, D). A photon energy of 6MV, flattening filter free (FFF) technique, dose rate 1400 MU/min, three arc configuration and anisotropic analytic algorithm (AAA) were used for planning and dose calculation in the Eclipse® treatment planning system (Varian Medical Systems, Palo Alto, CA). All the plans have been calculated using the standard inverse optimization process, based on Dose-Volume Histogram (DVH) parameters.
All the plans were prepared to be managed, during the delivery phase, using an IBH respiratory gating system (RPM® Varian Medical Systems, Palo Alto, CA) with daily IBH cone-beam CT (CBCT) image registration. IBH-CBCTs acquisition allows high quality daily scans with minimized motion artefacts, that, along with the presence of fiducial markers, improves the day-to-day target position verification and reduces inter and intra-fractions errors.
Study End-Points
The main objective of the study was to ensure adequate coverage of the PTVsib, simultaneously respecting dose constraints to OARs. In particular, coverage goals for the targets were:
-median dose equal (± 2%) to the prescription dose for PTVsib
-D98≥95% (95% of prescription dose is the minimum dose) for PTVsib
-maximum point dose of 107% inside PTVsib
-D95≥95% for PTVt and PTVsip
The goal for OARs was a minor deviation in dose constraints in < 10% of the plans.
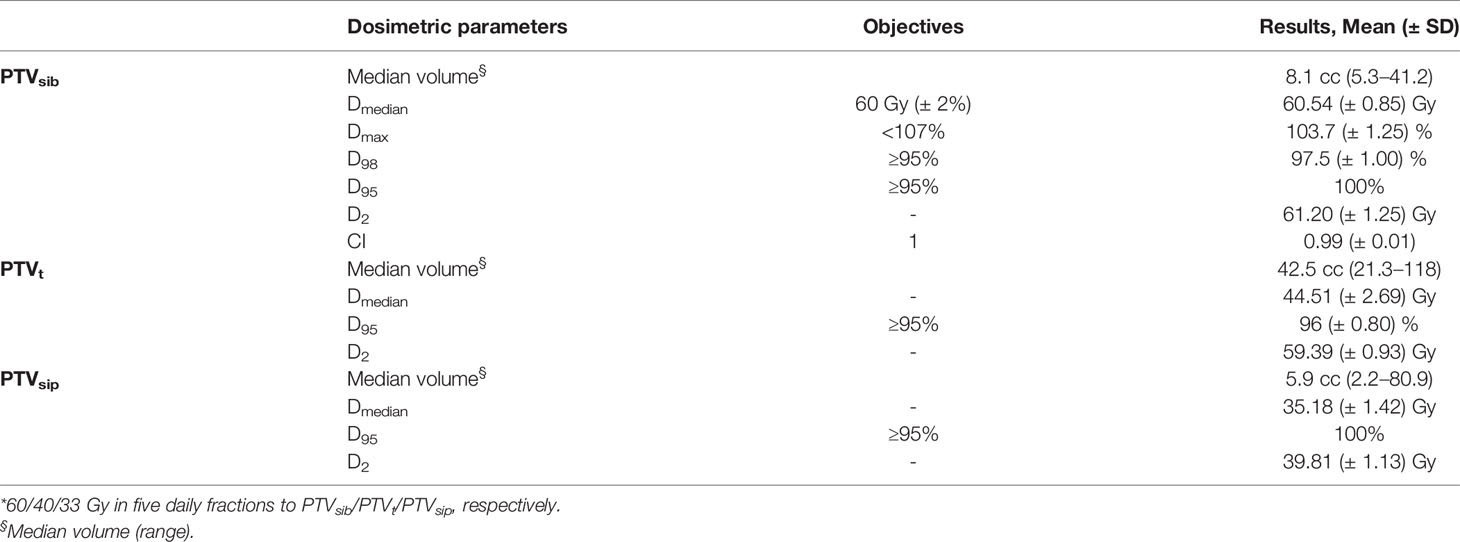
Dose volume histograms (DVHs) were generated for each plan, and multiple dosimetric parameters for PTVs (PTVt, PTVsib, and PTVsip) and OARs (duodenum, stomach, small and large bowel, spinal cord, liver, kidneys, and PRVs) were evaluated. The conformity index (CI) was defined as the volume encompassed by the 95% isodose divided by the PTV volume. CI was evaluated for PTVsib alone, since this index is formulated based on the paradigm of uniform dose prescription, which is not the case for this SBRT treatment with SIB/SIP approach.
Results
Study Population
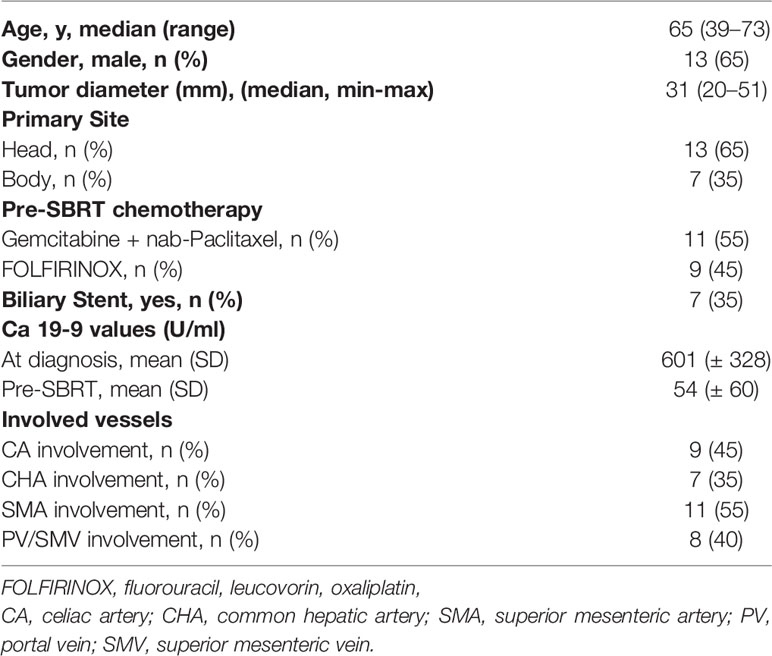
Baseline characteristics of the 20 patients included in this study are outlined in Table 2. All patients had locally advanced pancreatic carcinoma (LAPC), and were considered unresectable due to vascular involvement.
SBRT Planning and PTVs Coverage
All the SBRT plans met the predetermined target coverage objectives. Table 3 describes the results of the treatment plan analysis of the dose escalation proposal (60/40/33 Gy in five fractions to PTVsib/PTVt/PTVsip, respectively). PTVsib median dose, D95, and CI were 60.54 Gy (± SD 0.85), 58.96 Gy (± SD 0.85), and 0.99 (± SD 0.01), respectively. The median dose was 44.51 Gy (± SD 2.69) for PTVt, and 35.18 Gy (± SD 1.42) for PTVsip. For PTVsib, a D100≥95% was reached in 18 (90%) plans, while D98≥95% was obtained in all cases (100%). A maximum dose of less than 107% for PTVsib was maintained in every plan.
OAR Constraints
With regard to OARs, mean maximum dose (D0.5cc) to duodenum/stomach/bowel was 29.31 Gy (± SD 5.72)/25.29 Gy (± SD 6.90)/27.03 Gy (± SD 5.67), respectively. Table 4 describes treatment plans analysis for OARs. A minor acceptable deviation was observed in a single plan, with bowel and duodenum D0.5cc = 34.8 Gy (Figure 3). V38 < 0.5 cc was achieved for all PRV luminal OARs.
Figure 3 (A) Dose distribution (color wash) and (B) dose-volume histogram (DVH) for the single SBRT plan showing a minor acceptable deviation for bowel and duodenum (D0.5cc = 34.8 Gy). As shown in panel A, the anatomy for this lesion is rather unfavorable, with the simultaneous protection volume (PTVsip, dark green), surrounded for almost two thirds of the circumference by the PRVs. The following structures are shown: tumor planning target volume (PTVt, red), high-dose planning target volume (PTVsib, blue), duodenum (black), and bowel (light green). The Planning at Risk Volume (PRVoars) are shown with same color on axial image.
Discussion
Stereotactic body radiation therapy (SBRT) has demonstrated promising results in locally advanced, unresectable pancreatic cancer (LAPC). However, durable local control (LC) remains challenging, and higher biologically effective doses (BED10) are suggested to achieve tumor ablation. Advances in radiation delivery techniques, image-guidance (IGRT) and treatment planning, may allow for dose escalation to levels not previously achievable, potentially improving LC and survival. The results of the present study demonstrate that for LAPC, a 5-fraction SBRT with a SIB/SIP dose escalation protocol up to 60/40/33 Gy to PTVsib/PTVt/PTVsip, respectively, is dosimetrically feasible with adequate PTVs coverage and respect for OAR dose constraints.
The clinical rationale for this dosimetric study derives from our current experience with SBRT in pancreatic cancer. In a series of 59 patients treated with SBRT with SIB/SIP at our Institution, we found no G3 toxicities, but a predominant incidence of in-field failures (15). Based on these results, we evaluated the opportunity and feasibility of a dose escalation protocol, with the aim of improving the clinical outcomes of the aforementioned SBRT approach. In this regard, a recent MDACC study provided a remarkable roadmap to achieve a dose escalation up to 60 Gy in SBRT for LAPC (17).
The currently recommended dose in pancreatic SBRT is 33–40 Gy in five fractions (BED10 = 54.78–72 Gy) (18), instead BED10 of not less than 100 Gy is generally advocated to maximize the RT therapeutic effect and improve oncological outcomes (19). These doses are presumably necessary if the goal of SBRT is to achieve results comparable to surgery. However, when SBRT is applied to pancreatic tumors, the prescription of such high doses is challenging due to the proximity of critical OARs (e.g. duodenum, stomach and bowel), and serious late toxicity, such as perforation, stenosis, and ulcer with bleeding, could be expected (14). In our experience, the use of the SIP in pancreatic SBRT had presumably prevented serious damage to OARs. Considering that less than 10% of patients with LAPC are suitable for surgery after neoadjuvant therapy (20), the administration of such high doses should be as safe as possible. Thus, the use of this 3-dose level SBRT approach can certainly allow to maximize the therapeutic window. Indeed, a clinically acceptable plan was obtained for all patients, with an excellent PTVsib coverage (D98≥95% reached in all plans) and adequate respect for OAR dose constraints (a minor acceptable deviation was observed in a single plan for bowel and duodenum D0.5cc = 34.8 Gy), even when the prescribed dose corresponds to 60 Gy and 33 Gy to the TVI and SIP volume, respectively. Noteworthy, the median dose of PTV tumor (PTVt) at level IV of the dose escalation proposal was 44.51 (± 2.69) Gy, corresponding to a BED10 of 84.13 (± 2.83) Gy. Therefore, as a consequence of the high dose gradient within the tumor target, the PTVt absorbed dose would be consistently higher than the one expected (40 Gy for a BED10 of 72 Gy), potentially further increasing the final local effect of the SBRT.
Organ motion control is crucial for a dose escalation proposal. Our standard approach involves the use of the abdominal compressor (15). Indeed, in a study evaluating the effect of abdominal compression in pancreatic cancer, it was observed that with the use of this technique a cranio-caudal (CC) margin of 5 mm was adequate to encompass the tumor target motion in more than 90% of the patients (21). More recently, Campbell et al. compared compression and gating for pancreatic SBRT: the average motion in CC direction was 8.5 mm with abdominal compression, and 5.5 mm with respiratory gating (22). Similarly, the use of a breath hold technique can minimize the required PTV expansion compared with treatment during free breathing or with the use of abdominal compression (23). As a whole, these results suggest that respiratory gating and breath hold may be the best choice for organ motion management in pancreatic SBRT, in particular if a dose escalated approach is planned. Nevertheless, pancreatic region‐dependent variations in respiratory induced organ motion, and their effects on motion control approach, have been described (24). In particular, motion mitigation techniques resulted less effective in the tail region, with no difference between the use of abdominal compression versus respiratory gating, probably due to the larger positional error in the tail region based on the abdominal wall surrogate. Taking this into consideration, in the present study no tail lesions were included for the dose escalated proposal, hence the results are not applicable to the tumors of this pancreatic region. In the near future, the use of Magnetic Resonance-guided Radiation Therapy (MRgRT) will allow a daily online adaptation of the treatment plan, immediately before each fraction delivery, to optimize the dose distribution based on target and OAR anatomy, as well as a real-time management of the organ motion (25).
Another point of discussion is SBRT target delineation. Recent guidelines provide a clear definition for the primary GTV and tumor-vessel interface (TVI), aiming to standardize treatment volumes (16). Moreover, in our and other experiences reported in the literature, a SIB technique was used for clinical dose painting to deliver higher doses to a specific area of the tumor (15, 26–31). Table 5 summarizes studies describing the use of SBRT with a SIB approach in pancreatic cancer, underlining the variability among authors in the definition of the SIB volume. In the present study, the PTVsib was generated to encompass the TVI, in order to simplify the comparison with the plans evaluated and approved in our clinical practice. Furthermore, the vascular encasement, represented by the TVI is the main obstacle to plan and achieve a curative resection for LAPC. Since the SBRT technique can be easily integrated into a total neoadjuvant therapy (TNT), an ablative boost to the TVI could maximize the possibility of a conversion to surgery. In this regard, to better define TVI, the integration of MRI images with a contrast-enhancement CT-simulation scan, could offer a higher definition of tumor relationship with neighboring vessels and a greater accuracy of target delineation (32).
This study has potential limitations. The sample size is relatively small, and although a reasonable variety of locally advanced diseases was included, not all possible tumor characteristics and anatomical heterogeneity were represented. Therefore, some patients who meet the inclusion criteria for this dose escalated SBRT in the “dosimetric reality”, may not be suitable for the same dose escalation in “real life”. Furthermore, the dose constraints used are based on a commonly accepted consensus for SBRT, however validation in the clinical practice is necessary, thus the inclusion of patients in a clinical trial is strongly recommended. Finally, not all LAPC patients are candidates for SBRT. Exclusion criteria for SBRT are usually as follows: tumor > 6 cm in greatest dimension, nodal spread that cannot be included in the SBRT target volume, and tumors infiltrating the stomach or duodenum. For these patients, an alternative 15-fractions hypofractionated ablative radiation therapy approach may be investigated (Supplementary Material).
In conclusion, this study demonstrates that a SBRT dose escalation protocol with a SIB/SIP approach for LAPC up to 60/40/33 Gy in five fractions to PTVsib/PTVt/PTVsip, respectively, is feasible with adequate target coverage and without unacceptable increased OAR exposure. Based on this dosimetric analysis, a Phase II dose escalated trial is ongoing at our Institution.
Data Availability Statement
The datasets presented in this article are not readily available because the datasets generated for this study are available on request to the corresponding author, subject to approval by the Institutional Review Board. Requests to access the datasets should be directed to bmljb2xhLnNpbW9uaUBhb3ZyLnZlbmV0by5pdA==.
Ethics Statement
The studies involving human participants were reviewed and approved by Comitato etico per la Sperimentazione Clinica (CESC) delle Province di Verona e Rovigo. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Conceptualization, RMa and NS. Methodology, NS and SG. Validation, GR, RMi, GM, and SP. Plan study and preparation, SG, AP, and EZ. Plan evaluation, NS, GR, and RMi. Writing—original draft preparation, NS and SG. Writing—review and editing, RMi, EZ, and CC. Images curation: NS and RD. Supervision, RMa, RS, MM, and CB. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with several of the authors RM, NS.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.600940/full#supplementary-material
References
1. Hammel P, Huguet F, van Laethem JL, Goldstein D, Glimelius B, Artru P, et al. Effect of Chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic Cancer controlled after 4 months of gemcitabine with or without Erlotinib: the LAP07 randomized clinical trial. JAMA (2016) 315:1844–53. doi: 10.1001/jama.2016.4324
2. Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst (1988) 80:751–5. doi: 10.1093/jnci/80.10.751
3. Loehrer PJ Sr, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol (2011) 29:4105–12. doi: 10.1200/JCO.2011.34.8904
4. Klaassen DJ, MacIntyre JM, Catton GE, Engstrom PF, Moertel CG. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil—an Eastern Cooperative Oncology Group study. J Clin Oncol (1985) 3:373–8. doi: 10.1200/JCO.1985.3.3.373
5. Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouché O, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol (2008) 19:1592–9. doi: 10.1093/annonc/mdn281
6. Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol (2009) 27:1806–13. doi: 10.1200/JCO.2008.17.7188
7. Crane CH, Varadhachary GR, Yordy JS, Staerkel GA, Javle MM, Hobbs BD, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol (2011) 29:3037–43. doi: 10.1200/JCO.2010.33.8038
8. de Geus SWL, Eskander MF, Kasumova GG, Ng SC, Kent TS, Mancias JD, et al. Stereotactic body radiotherapy for unresected pancreatic cancer: A nationwide review. Cancer (2017) 123:4158–67. doi: 10.1002/cncr.30856
9. Zhong J, Patel K, Switchenko J, Cassidy RJ, Hall WA, Gillespie T, et al. Outcomes for patients with locally advanced pancreatic adenocarcinoma treated with stereotactic body radiation therapy versus conventionally fractionated radiation. Cancer (2017) 123:3486–93. doi: 10.1002/cncr.30706
10. Tchelebi LT, Lehrer EJ, Trifiletti DM, Sharma NK, Gusani NJ, Crane CH, et al. Conventionally fractionated radiation therapy versus stereotactic body radiation therapy for locally advanced pancreatic cancer (CRiSP): An international systematic review and meta-analysis. Cancer (2020) 126:2120–31. doi: 10.1002/cncr.32756
11. Arcelli A, Guido A, Buwenge M, Simoni N, Mazzarotto R, Macchia G, et al. Higher Biologically Effective Dose Predicts Survival in SBRT of Pancreatic Cancer: A Multicentric Analysis (PAULA-1). Anticancer Res (2020) 40:465–72. doi: 10.21873/anticanres.13975
12. Rajagopalan MS, Heron DE, Wegner RE, Zeh HJ, Bahary N, Krasinskas AM, et al. Pathologic response with neoadjuvant chemotherapy and stereotactic body radiotherapy for borderline resectable and locally-advanced pancreatic cancer. Radiat Oncol (2013) 8:254. doi: 10.1186/1748-717X-8-254
13. Moningi S, Dholakia AS, Raman SP, Blackford A, Cameron JL, Le DT, et al. The Role of Stereotactic Body Radiation Therapy for Pancreatic Cancer: A Single-Institution Experience. Ann Surg Oncol (2015) 22:2352–8. doi: 10.1245/s10434-014-4274-5
14. Suker M, Nuyttens JJ, Eskens FALM, Haberkorn BCM, Coene PPLO, Van Der Harst E, et al. Efficacy and feasibility of stereotactic radiotherapy after folfirinox in patients with locally advanced pancreatic cancer (LAPC-1 trial). EClinicalMedicine (2019) 19(17):100200. doi: 10.1016/j.eclinm.2019.10.013
15. Simoni N, Micera R, Paiella S, Guariglia S, Zivelonghi E, Malleo G, et al. Hypofractionated Stereotactic Body Radiation Therapy With Simultaneous Integrated Boost and Simultaneous Integrated Protection in Pancreatic Ductal Adenocarcinoma. Clin Oncol (R Coll Radiol) (2020) S0936–6555(20)30275-2. doi: 10.1016/j.clon.2020.06.019
16. Oar A, Lee M, Le H, Hruby G, Dalfsen R, Pryor D, et al. Australasian Gastrointestinal Trials Group (AGITG) and Trans-Tasman Radiation Oncology Group (TROG) Guidelines for Pancreatic Stereotactic Body Radiation Therapy (SBRT). Pract Radiat Oncol (2020) 10:e136–46. doi: 10.1016/j.prro.2019.07.018
17. Colbert LE, Rebueno N, Monigi S, Beddar S, Sawakuchi GO, Herman JM, et al. Dose escalation for locally advanced pancreatic cancer: How high can we go? Adv Radiat Oncol (2018) 3:693–700. doi: 10.1016/j.adro.2018.07.008
18. Palta M, Godfrey D, Goodman KA, Hoffe S, Dawson LA, Dessert D, et al. Radiation Therapy for Pancreatic Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract Radiat Oncol (2019) 9:322–32. doi: 10.1016/j.prro.2019.06.016
19. Bernard V, Herman JM. Pancreas SBRT: Who, What, When, Where, and How…. Pract Radiat Oncol (2020) 10:183–5. doi: 10.1016/j.prro.2019.11.005
20. Maggino L, Malleo G, Marchegiani G, Viviani E, Nessi C, Ciprani D, et al. Outcomes of Primary Chemotherapy for Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma. JAMA Surg (2019) 154:942. doi: 10.1001/jamasurg.2019.2278
21. Lovelock DM, Zatcky J, Goodman K, Yamada Y, Dawson LA, Dessert D, et al. The effectiveness of a pneumatic compression belt in reducing respiratory motion of abdominal tumors in patients undergoing stereotactic body radiation. Technol Cancer Res Treat (2004) 13:259–67. doi: 10.1016/j.prro.2019.06.016
22. Campbell WG, Jones BL, Schefter T, Goodman KA, Miften M. An evaluation of motion mitigation techniques for pancreatic SBRT. Radiother Oncol (2017) 124:168–73. doi: 10.1016/j.radonc.2017.05.013
23. Forbang Teboh R, Srinivasan S, Ng SP, Aliru ML, Herman JM. Setup Management for Stereotactic Body Radiation Therapy of Patients With Pancreatic Cancer Treated via the Breath-Hold Technique. Pract Radiat Oncol (2020) 10:e280–9. doi: 10.1016/j.prro.2019.10.012
24. Fujimoto K, Shiinoki T, Yuasa Y, Onizuka R, Yamane M. Evaluation of the effects of motion mitigation strategies on respiration-induced motion in each pancreatic region using cine-magnetic resonance imaging. J Appl Clin Med Phys (2019) 20:42–50. doi: 10.1002/acm2.12693
25. Placidi L, Romano A, Chiloiro G, Cusumano D, Boldrini L, Cellini F, et al. On-line adaptive MR guided radiotherapy for locally advanced pancreatic cancer: Clinical and dosimetric considerations. Tech Innov Patient Support Radiat Oncol (2020) 15:15–21. doi: 10.1016/j.tipsro.2020.06.001
26. Chuong MD, Springett GM, Freilich JM, Park CK, Weber JM, Mellon EA, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Bio Phys (2013) 86:516–22. doi: 10.1016/j.ijrobp.2013.02.022
27. Mellon EA, Hoffe SE, Springett GM, Frakes JM, Strom TJ, Hodul PJ, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol (2015) 54:979–85. doi: 10.3109/0284186X.2015.1004367
28. Shaib WL, Hawk N, Cassidy RJ, Chen Z, Zhang C, Brutcher E, et al. A Phase 1 Study of Stereotactic Body Radiation Therapy Dose Escalation for Borderline Resectable Pancreatic Cancer After Modified FOLFIRINOX (NCT01446458). Int J Radiat Oncol Bio Phys (2016) 96:296–303. doi: 10.1016/j.ijrobp.2016.05.010
29. Holyoake DLP, Ward E, Grose D, McIntosh D, Sebag-Montefiore D, Radhakrishna G, et al. A phase-I trial of pre-operative, margin intensive, stereotactic body radiation therapy for pancreatic cancer: the ‘SPARC’ trial protocol. BMC Cancer (2016) 16:728. doi: 10.1186/s12885-016-2765-4
30. Kharofa J, Mierzwa M, Olowokure O, Sussman J, Latif T, Gupta A, et al. Pattern of marginal local failure in a phase ii trial of neoadjuvant chemotherapy and stereotactic body radiation therapy for resectable and borderline resectable pancreas cancer. Am J Clin Oncol (2019) 42:247–52. doi: 10.1097/COC.0000000000000518
31. Koay EJ, Hanania AN, Hall WA, Taniguchi CM, Rebueno N, Myrehaug S, et al. Dose-Escalated Radiation Therapy for Pancreatic Cancer: A Simultaneous Integrated Boost Approach. Pract Radiat Oncol (2020) 13:S1879–8500(20)30035-7. doi: 10.1016/j.prro.2020.01.012
32. Caravatta L, Cellini F, Simoni N, Rosa C, Niespolo MR, Lupatelli M, et al. Magnetic resonance imaging (MRI) compared with computed tomography (CT) for interobserver agreement of gross tumor volume delineation in pancreatic cancer: a multi-institutional contouring study on behalf of the AIRO group for gastrointestinal cancers. Acta Oncol (2019) 58:439–47. doi: 10.1080/0284186X.2018.1546899
Keywords: stereotactic body radiotherapy, pancreatic cancer, locally advanced, dose escalation, ablative dose
Citation: Mazzarotto R, Simoni N, Guariglia S, Rossi G, Micera R, De Robertis R, Pierelli A, Zivelonghi E, Malleo G, Paiella S, Salvia R, Cavedon C, Milella M and Bassi C (2020) Dosimetric Feasibility Study of Dose Escalated Stereotactic Body Radiation Therapy (SBRT) in Locally Advanced Pancreatic Cancer (LAPC) Patients: It Is Time to Raise the Bar. Front. Oncol. 10:600940. doi: 10.3389/fonc.2020.600940
Received: 31 August 2020; Accepted: 17 November 2020;
Published: 17 December 2020.
Edited by:
Francesco Cellini, Catholic University of the Sacred Heart, ItalyReviewed by:
Angela Romano, Fondazione Policlinico Universitario “Agostino Gemelli” IRCCS, ItalyTsair-Fwu Lee, National Kaohsiung University of Science and Technology, Taiwan
Copyright © 2020 Mazzarotto, Simoni, Guariglia, Rossi, Micera, De Robertis, Pierelli, Zivelonghi, Malleo, Paiella, Salvia, Cavedon, Milella and Bassi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Simoni, bmljb2xhc2ltb25pODFAZ21haWwuY29t
 Renzo Mazzarotto
Renzo Mazzarotto Nicola Simoni
Nicola Simoni Stefania Guariglia
Stefania Guariglia Gabriella Rossi
Gabriella Rossi Renato Micera1
Renato Micera1 Salvatore Paiella
Salvatore Paiella Carlo Cavedon
Carlo Cavedon Michele Milella
Michele Milella Claudio Bassi
Claudio Bassi