- 1Department of Otolaryngology and Laryngological Oncology, Poznan University of Medical Sciences, Poznan, Poland
- 2Department of Clinical Pathology, Poznan University of Medical Sciences, Poznan, Poland
Objective: Pleomorphic adenomas (PAs) with divergent clinical behavior, differing from the vast majority of PAs, were distinguished. “Fast” PAs are characterized by an unexpectedly short medical history and relatively rapid growth. The reference group consisted of “slow” PAs with very stable biology and long-term progression. We divide the PA group as a whole into three subsets: “fast,” “normal,” and “slow” tumors. Our goal is a multifactorial analysis of the “fast” and “slow” PA subgroups.
Methods: Consecutive surgeries in a tertiary referral center, the Department of Otolaryngology and Laryngological Surgery, Poznan University of Medical Sciences, Poland, were carried out between 2002 and 2011. Out of 1,154 parotid tumors, 636 (55.1%) were PAs. The data were collected prospectively in collaboration with the Polish National Registry of Benign Salivary Gland Tumors. The main outcome measure was the recurrence rate in “fast” and “slow” PA subgroups. All surgical qualifications and surgeries were performed by two experienced surgeons.
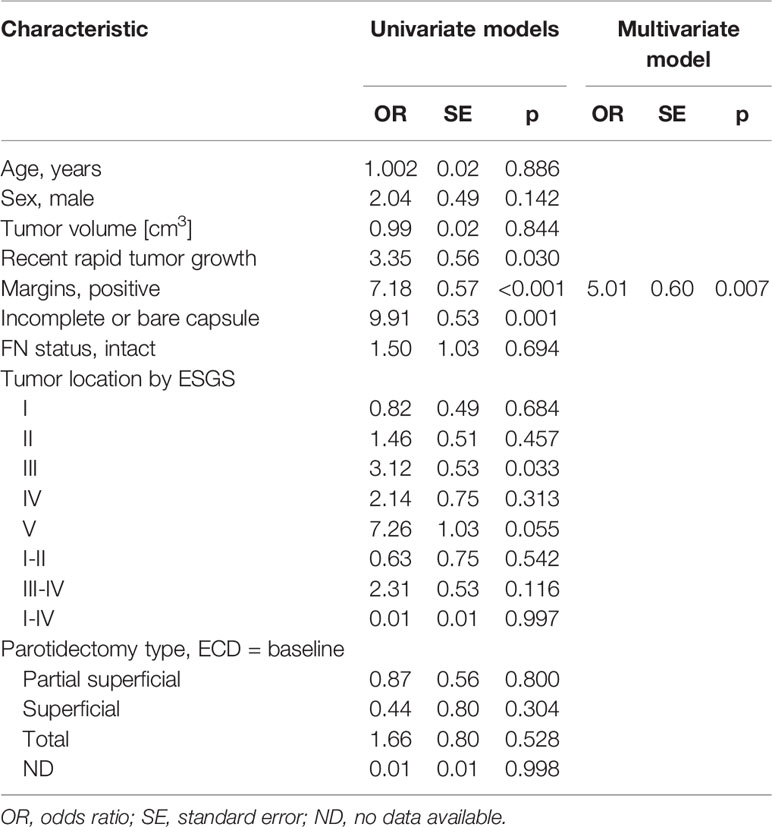
Results: Slow PAs, compared to fast PAs, presented in older patients (53.25 ± 15.29 versus 47.92 ± 13.44 years). Multifactor logistic regression analysis with recurrence (yes/no) as the outcome variable, fast/slow as the predictor variable and age, gender, margin, FN status as covariates showed that fast PAs were significantly predicting recurrence vs. slow PAs (p = 0.035). Fast PAs were increasing the risk of PAs 10-fold vs. slow PAs, exp β = 10.20, CI95 [1.66; 197.87]. The variables impacting relapse were recent accelerated growth of the tumor OR = 3.35 (SE = 0.56), p = 0.030, positive margins OR = 7.18 (SE = 0.57), p < 0.001, incomplete or bare capsule OR = 9.91 (SE = 0.53), p = 0.001 and location III OR = 3.12 (SE = 0.53), p = 0.033. In the multivariate model only positive margin was selected as the best predictor of relapse, OR = 5.01 (SE = 0.60), p = 0.007.
Conclusions: The simple clinical aspect of slow or fast PA progression is of great practical importance and can constitute a surrogate of the final histopathological information that is derived from the surgical specimen. The slow or fast nature of the PA to some extent indicates prognostic features such as recurrence risk. This finding requires correlation with histological and molecular features in further stages of research.
Introduction
Pleomorphic adenomas (PA) are the most common parotid tumors and their trend of incidence is increasing (1, 2). These tumors are slow-growing and can remain asymptomatic and unrecognized, or unobtrusive enough that the patient decides not to undergo treatment. Though they may reach significant size over a period of years, some of them present misleadingly short histories constituting rather rapid development (3, 4).
It is important to accurately establish the histology of all benign salivary tumors in order to predict their clinical behavior, and this is particularly true in the case of PA due to its histological variants, different tumor entities, and the possibility of treatment failure (5, 6). The post-operative incidence of PA recurrence is significant and varies largely because of differences in surgical technique (1, 7, 8), as well as other factors including multi-nodularity and pseudopodia, tumor diameter, the age of the patient, and cellular and molecular changes (9–12). The risk of malignant transformation to carcinoma ex pleomorphic adenoma (Ca ex PA) occurs in only 1.8–6.2% of cases (13, 14), with a prevalence rate of 5.6 cases per 100,000 malignant tumors and an incidence rate of 0.17 tumors per million persons (15).
The histological diagnosis of the majority of PAs is straightforward. The tumor is usually well-circumscribed, encapsulated with a bosselated outer surface, and often presents with tongue-like protrusions or sometimes satellite nodules. Morphological patterns vary, with typically the following three components present: (1) epithelial and (2) myoepithelial cells, with (3) areas of mesenchymal differentiation. There are varying proportions of tubules, duct-like structures, and mesenchymal tissues (16) and different histological patterns of myoepithelial cells, which may appear as plasmacytoid, spindle, epithelioid, clear, or stellate (16, 17). Metaplastic changes and the foci of squamous cells are an integral feature of PAs, however extensive squamous metaplasia is uncommon and can easily be misinterpreted as squamous cell carcinoma (18).
Morphologic and genetic studies on PAs are scarce and there are still gaps in the knowledge concerning variations in clinical behavior and adverse outcomes (19). Furthermore, no pathological features of the tumor are available prior to surgery. We know only the tumor’s dimensions and the duration and speed of its growth. Our experience with 1,154 benign salivary gland tumors over a 10-year period has prompted us to distinguish a small group of PA tumors with clinical behavior that differs from the vast majority of PA. Progression, recurrence, and malignant transformation are well-known PA behavior, but the unusually fast growth of this benign tumor has always surprised clinicians. The impact of this phenomenon on the treatment failure rate is unknown. Our goal is a multifactorial analysis of fast versus slow PA tumors, with the main end result being recurrence and the main outcome measure being the correlation of this failure with the clinical nature of the tumor (slow/fast), tumor size, tumor volume, and additional factors such as age, gender, margins, and facial nerve (FN) status.
Materials and Methods
In total, 1,154 benign parotid tumors were consecutively operated on in a tertiary referral center, the Department of Otolaryngology and Laryngological Surgery, Poznan University of Medical Sciences, Poland, between 2002 and 2011. Of these, 636 (55.1%) were PA. The data were initially collected prospectively from a local database and, from 2015 onwards, from the Polish National Registry of Benign Salivary Gland Tumors. There were 224 (35.2%) men, 412 (64.8%) women, with ages ranging from 13 to 86 years, mean 47.93 ± 14.93 years and median 48 years. All patients were operated on by two experienced surgeons (MW, TK).
This study was conducted in accordance with a protocol approved by the Bioethics Committee of Poznan University of Medical Sciences (Resolution No. 781/16), and written consent was obtained from each patient.
Clinically “Fast” and “Slow” Tumors
The PA group was divided into three subsets: “fast,” “normal/stable,” and “slow” tumors, based on several clinical and radiological features. Three different criteria were used to categorize tumors. Objective criteria were history-based growth time and growth rate, determined by tumor increment in percent by volume, as per the patient’s description. The subjective criteria were the radiological features assessed by the doctor in one of the imaging modalities, predominantly ultrasonography. “Slow” tumors had over 10 years’ history and exhibited slow growth (<5% of tumor size over the last 10 years). “Stable” tumors constitute the vast majority of PA and are characterized by anamnesis >=3 years, stable size of the tumor or its slow growth (<5% of tumor size over the last 6 months); a well-visualized tumor capsule in the radiological investigation, and tumor homogeneity. The “fast” tumors are characterized by an unexpectedly short medical history and relatively rapid growth. The criteria were as follows: anamnesis <3 years; >5% growth of the tumor size within six months; and multi-polycyclic outline, heterogenic echostucture and loss of capsule echogenicity in radiological investigation. To accurately and unequivocally categorize a tumor as “fast,” all the criteria had to be obtained.
Variables Collected for PAs
The variables age, sex, place of residence, time between first symptoms and surgery, tumor location, margins, FN status after surgery, and recurrence were collected.
Tumor location was presented according to the European Salivary Gland Society’s (ESGS) classification of salivary gland surgeries (2, 20). The ESGS operative report includes the level removed, designated by the Roman numerals I to V in ascending order, and non-glandular structures removed, each identified through the use of specified acronyms.
Surgical approach. The classification of salivary gland surgeries was presented according to the ESGS (2, 20) and distinguishes two types of surgery: extracapsular dissection and parotidectomy. The ESGS operative report includes the glandular parenchyma level removed, designated by Roman numerals I to V. Extracapsular dissection, partial superficial parotidectomy, superficial parotidectomy, and total parotidectomy were noted.
Margins. In benign salivary gland tumors, there is no concept of positive or negative margins as there would be in malignant cancers. Positive margins were categorized by the following adverse findings: capsular rupture and intra-operative tumor spillage, the presence of incomplete or bare capsule or absence of encapsulation in the pathology specimen, and satellite nodules as distinct tumor nodules.
FN status. Function of the facial nerve using the House-Brackmann scale was recorded at 1 week, 1 month, and 12 months.
Follow up. Routine follow-up is based on ultrasonography performed once a year. In cases with a higher risk of recurrence, ultrasound is performed twice a year, and an additional MRI once a year if needed.
Furthermore, tumor features such as growth rate, capsule visualization in pre-operative imaging, and tumor homogeneity were taken into consideration. The main predictive value was categorization into “fast,” “normal,” and “slow” PA.
The outcome measure was the correlation of recurrence with tumor size, volume, and of recurrence with PA nature (“fast,” “normal,” and “slow”). The main outcome measure was the determination of whether tumor size, tumor nature (slow/fast), or the other variables influenced recurrence more. Subsequent multivariant analysis included additional factors such as age, gender, margins, and FN status.
Statistical Analysis
Analysis was conducted using R software version 3.5.1. Nominal variables are presented as n (% of group), and continuous variables as mean ± SD or median (Q1;Q3). Normality of distribution was validated using the Shapiro-Wilk test as well as a visual assessment of histograms, skewness, and kurtosis values. Comparison of fast and slow PA groups was conducted with a chi-square test or chi-square test with Yate’s correction for nominal variables and with t-test or Mann-Whitney U test for continuous variables, as appropriate. The mean/median difference (MD) with 95% confidence interval was calculated for continuous variables. To verify the impact of fast/slow PAs on recurrence, a multifactorial logistic regression model was calculated, with age, sex, margins, and FN status as covariates. Model assessment was conducted with the Hosmer–Lemeshow goodness of fit (GOF) test. Additionally, relapse-free survival (RFS) was calculated using Kaplan-Meier survival analysis, including 95% confidence interval. RFS stratified by independent variables (i.e., sex, location, margin, etc.) was compared with log-rank chi-square test. Cox regression model with Breslow method was used to identify parameters impacting relapse. First, univariate models were prepared for each of the independent variables, and based on those models, variables with p < 0.2 in Wald test were included to the final multivariate model. For location variables, due to their inter-dependence, location with the lowest p-value in univariate models was included in the final model. For the margins variable (positive/negative) and the reasons for positive variable: due to inter-dependence of both variables, the final model included the variable (positive/negative) that had a lower p-value in the univariate model. The final multivariate model was created using a stepwise approach. All tests were based on α = 0.05.
Results
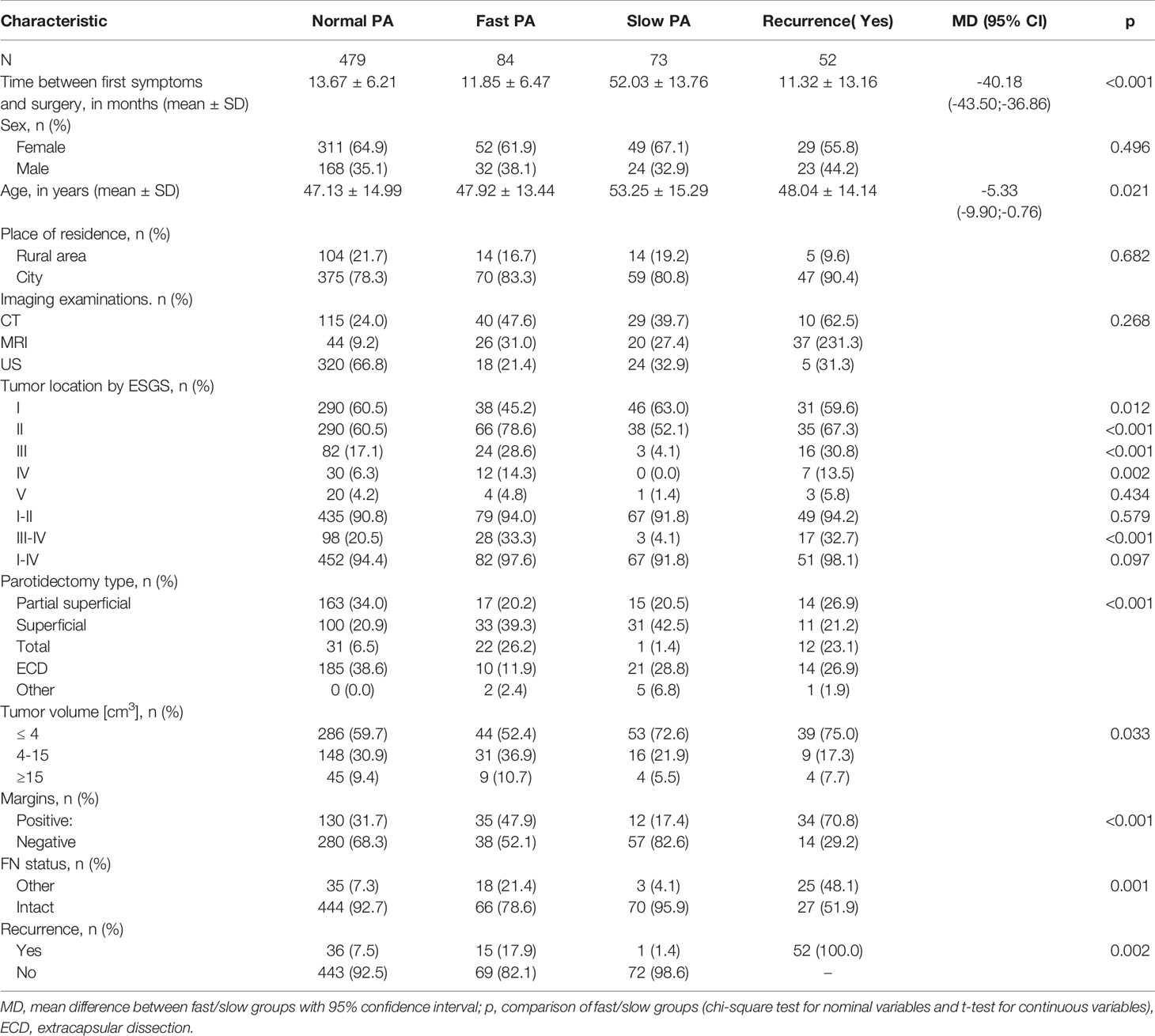
Of the 636 PAs over a 10-year period, there were 84 (13.2%) fast, 73 (11.5%) slow, and 479 (75.3%) normal/stable PAs. The recurrence rate was 8.2% (52/636). All recurrences were ipsilateral. There was no difference in the frequency distribution of individual groups over the years.
There was also a statistically significant relationship between fast/slow PAs and tumor volume (p = 0.033). Smaller tumors (≤ 4 cm3) were more frequent with slow PAs (72.6%) vs. fast PAs (52.4%). (Table 1).
Next, we analyzed the categories of fast/slow tumors and the correlations with patient epidemiological data and tumor features: tumor location in individual regions of the salivary gland, margins, and condition of the facial nerve after surgery.
The time elapsed between the first symptoms and surgery was significantly different between fast (11.85 ± 6.47 months) and slow (52.03 ± 13.76 months) PA, MD = -40.18 CI95 [-43.50; -36.86]; (p < 0.001). There was no significant relationship between slow vs. fast PA and sex or place of residence. Slow PAs presented in older patients (53.25 ± 15.29 years vs. 47.92 ± 13.44 for fast PAs), MD = -5.33 CI95 [-9.90; -0.76]; (p = 0.021).
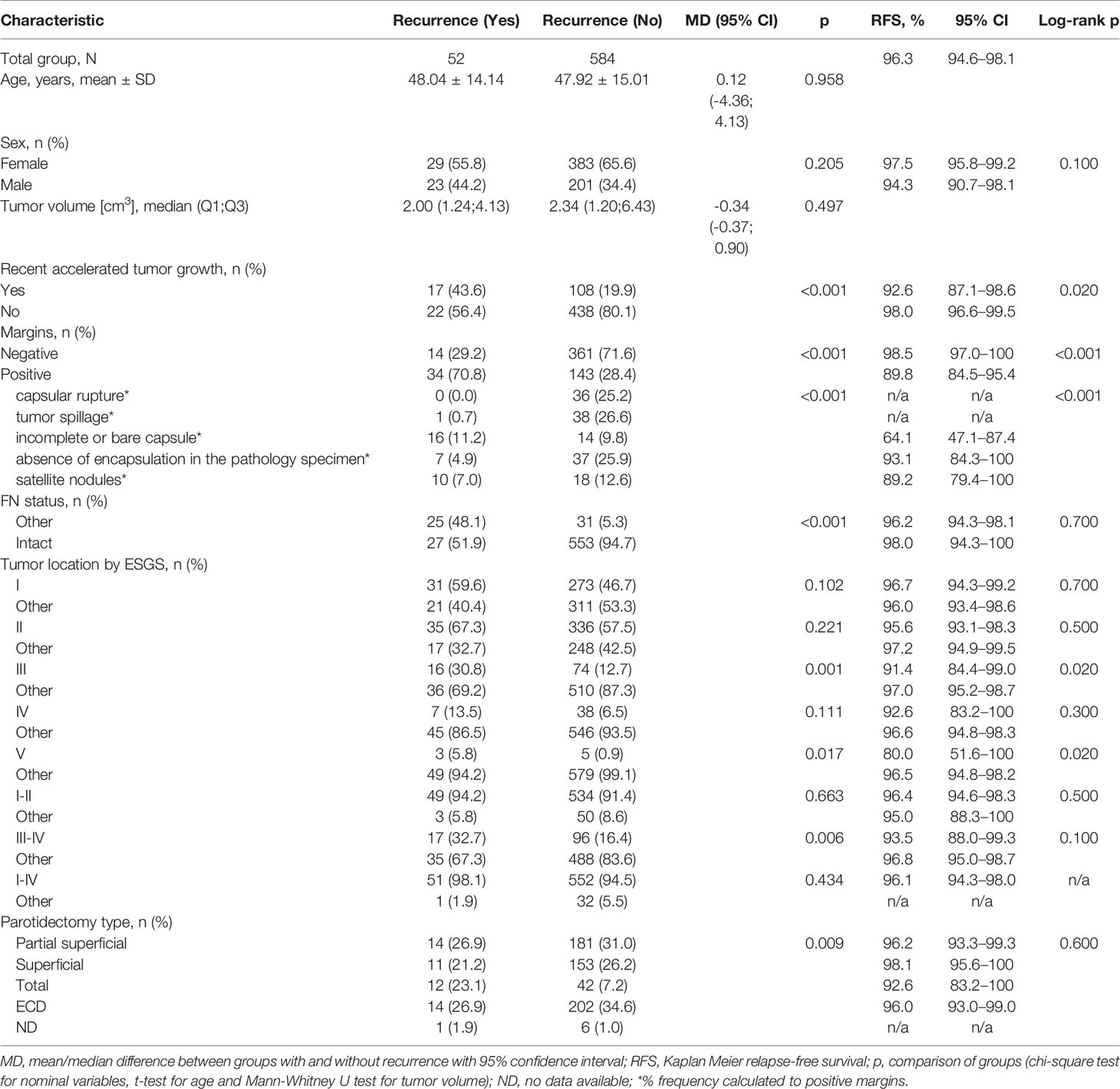
Relapse-free survival (RFS) for the whole group was 96.3% CI95 [94.6%; 98.1%]. RFS was significantly different in regard to the pace of recent rapid tumor growth (log-rank p = 0.020), positive/negative margins (log-rank p < 0.001), the reason for positive margin (log-rank p < 0.001), location of the tumor in area III (log-rank p = 0.020), and location in area V (log-rank p = 0.020). Log-rank test did not confirm statistically significant differences in RFS for the remaining variables (sex, FN status, location of the tumor in area I, II, IV, I–II, III–IV, parotidectomy type). Figure 1 demonstrates that the recurrence risk increased during the first 4.2 years after surgery and stabilized after this time.
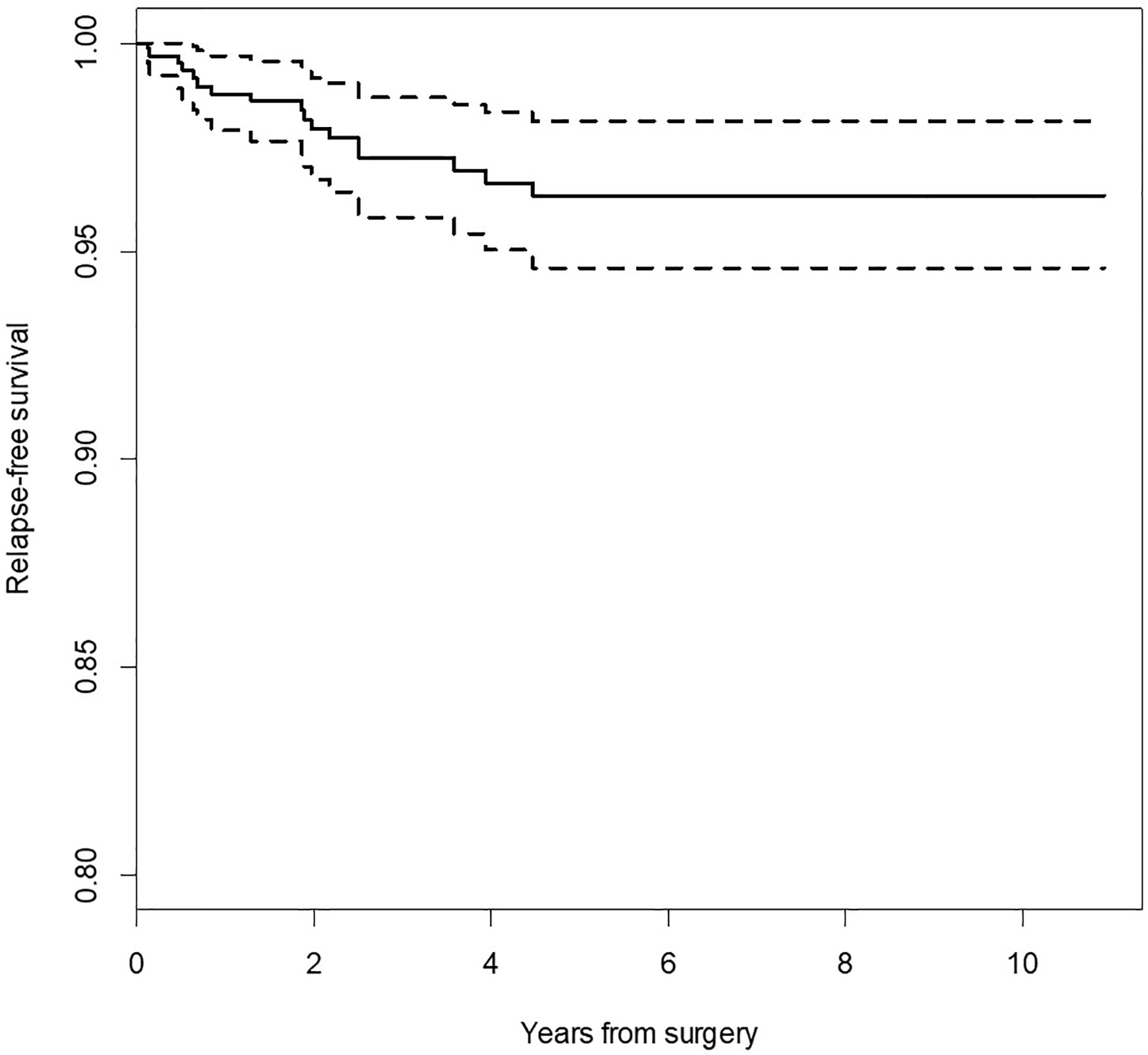
Figure 1 Kaplan-Meier survival curve for relapse-free survival (RFS). Dotted lines indicate 95% confidence interval for survival curve.
Localization of the tumor in area I, as designated by the ESGS classification, was significantly more frequent in slow PAs (63.0% vs. 45.2%, p = 0,012) while localization in areas II, III, and IV were more frequent in fast PAs (78.6%% vs. 52.1%, p < 0.001 for location II, 28.6% vs. 4.1%, p < 0.001 for location III, 14.3% vs. 0%, p = 0.002 for location IV). There was no significant relationship between location V and fast/slow PAs. Locations I and II combined, as well as locations I–IV combined were not significantly different when comparing fast vs slow PAs. However, locations III and IV combined were more frequent in fast PAs (33.3% vs. 4.1%, p < 0.001).
FN dysfunction of the marginal-mandibular branch occurred in 35 (7.3%) normal PAs, 18 (21.4%) fast Pas, and 3 (4.1%) slow PAs. Patients of 17 (3.5%) normal PAs, 9 (9.7%) fast Pas, and 2 (2.7%) slow PAs recovered facial function at 1 month; 12 (2.5%) normal PAs, 7 (8.3%) fast Pas, and 1 (1.4%) slow PA recovered facial function at 6 months, and 100% had recovered at 12 months. There were no cases of definitive involvement of FN.
The main outcome measure was the correlation of treatment failure, that is, recurrence with examined variables, with special regard to the fast/slow PA nature. Thus, the key question was, Which of the clinical parameters, age of onset, tumor volume, tumor growth rate, surgical approach, better correlated with a higher risk of recurrence? Based on univariate Cox regression models presented in Table 2, variables that were significantly impacting relapse were recent rapid tumor growth, OR = 3.35 (SE = 0.56), p = 0.030, positive margins vs. negative, OR = 7.18 (SE = 0.57), p < 0.001, incomplete or bare capsule vs. other reasons of positive margin, OR = 9.91 (SE = 0.53), p = 0.001 and location III vs. other, OR = 3.12 (SE = 0.53), p = 0.033. In the multivariate model only positive margin was selected as the best predictor of relapse, OR = 5.01 (SE = 0.60), p = 0.007.
As the two surgeons (MW, TK) performed all surgical qualifications as well as all surgeries, it can be assumed that such a standardization of surgical technique, considered for a given type of surgery, had a limited impact on the incidence of recurrence.
The analysis of fast and slow PA with special regard to recurrence is presented in Table 3. The relationship between fast/slow PAs and margins, condition of the FN after surgery, recurrence rate was significant. Positive margins were more frequent in fast PAs (47.9% vs. 17.4% of slow PAs, p < 0.001), and intact FN was also more frequent in fast PAs (21.4% vs. 4.1% of slow PAs, p = 0.001). PAs recurred in 17.9% of fast PAs vs. 1.4% of slow PAs (p = 0.002).
Then two entities were compared in Table 4: recurrent tumors (r-PA) and those successfully treated. Patients with recurrence demonstrated significantly faster tumor growth in the last few years (44% in patients with recurrence vs. 20% in patients without recurrence, p < 0.001). There was no significant difference in age and tumor volume between recurrence groups.
Thus, second, a multivariate analysis was performed. Using recurrence (yes/no) as the outcome variable, fast/slow categories as the predictor variable, and age, gender, margin, FN status as the covariates, multifactor logistic regression analysis showed that fast PAs significantly predicted recurrence vs. slow PAs (p = 0.035). Fast PAs were increasing the risk of recurrence 10-fold vs. slow PAs, exp β = 10.20, CI95 [1.66; 197.87]. Model assessment using Hosmer–Lemeshow GOF test (p = 0.743) confirmed good fit of the model to the data. Interpretation of logistic regression data for fast/slow categories indicates that in patients with fast PA, the risk of recurrence increases by 10.2-fold compared to patients with slow PA.
Discussion
PA progression rate, differences in tumor growth rate, and impact on recurrence still remain unclear. In this study, the authors aimed to show that one of the clinical parameters—tumor growth rate—significantly correlates with a higher risk of recurrence. Despite the progress in this field, the exact causes of PA recurrence remain elusive. It has been hypothesized that the various reasons for PA recurrence can be grouped into pathology-related (capsule thickness or lack of capsule (21, 22), pseudopodia, satellite nodules (23, 24), and multi-centricity) and surgery-related factors such as rupture of the tumor, spillage of tumor contents, insufficient margins of resection due to nerve branches, and inadequate excision related to the type of surgery (25). Conceptually, re-growth of the tumor as a result of inadequate initial resection could be defined as PA persistence rather than PA recurrence. Owing to the time frame between the initial surgery and recurrence, it is generally implied that the re-operation is performed by a different surgeon who tends to blame the first inadequate procedure (25). In our setting, we can abandon the hypothesis that tumor re-growth results from inadequate surgery, as the 1,154 benign salivary gland tumors observed over a 10-year period were operated on by only two experienced surgeons.
The initial medical interview allowed us to derive data concerning the speed of tumor progression, and it is on this basis that the patient was advised on the pressing necessity to undergo surgery. Thus, the surgeon was able to make short- or long-term considerations and plan the procedure precisely according to these indications. Clinical observation has led us to distinguish a small group of PAs demonstrating clinical behavior that differs from the vast majority of PAs.
Fast PAs are characterized by an unexpectedly short medical history and relatively rapid growth. Additionally, they exhibit imaging features that, while similar to other PAs, are extremely exaggerated, that is, presenting jagged fragments instead a smooth tumor capsule, with only polycyclic pseudopodia and satellites. In a diametrically different group, we distinguished from typical PAs a group of tumors demonstrating even calmer biology, with very slow, long-term progression. Thus, we divided the whole PA group into three subsets of “fast,” “normal,” and “slow” tumors. The criteria for such division were based on several clinical and radiological features that differed in this seemingly homogenous benign PA group (25–29).
So far, two clinical features—patient age and tumor size—have been associated with a higher risk of recurrence, and this finding is coherent with most conclusions in the literature. Larger PAs have a tendency to exhibit incomplete capsules and are additionally associated with more numerous satellite nodules (9, 24).
Based on fast/normal/slow PA categorization, we proved that this clinical aspect is of great practical importance. Not only does it allow for preliminary selection of patients for immediate surgery, they are under greater vigilance during surgery and are more frequently monitored for relapse. Surgical access can be potentially modified, such as forgoing extracapsular access in rapid tumors in favor of parotidectomy. One may also consider a lower threshold for postoperative RT in the event of tumor spillage. We conduct follow-up visits once a year for all PAs, while select tumors demonstrating adverse findings are followed up every six months for a period of 10 years. It is of note that tumor development over a shorter period is also very probable (1, 27, 30).
Our publication delineating the clinical aspect of the course and speed of PA development is innovative and unique. It measurably defines the clinical distinctiveness of PAs. Every experienced surgeon is aware of this problem and probably intuitively schedules earlier surgeries and closely monitors rapid tumors. Nevertheless, we have proven that this feature is statistically more significant than other features for the development of recurrence, and on this basis we recommend careful and longer monitoring of these patients.
The main limitations of our study include inconsistent imaging examinations in our patients. Magnetic resonance imaging (MRI), ultrasonography (US), and computed tomography (CT) are the most commonly ordered studies for PA because these protocols describe the precise location and size of the tumor (31). However, some of the patients received US or MRI while some received CT. Another limitation of this study is patient-reported symptom duration, where we can broadly assume that symptom duration was underestimated by a few months.
Conclusion
The simple clinical aspect of slow or fast PA development is of great practical importance and can constitute a surrogate of the final histopathological result derived from the surgical specimen. The slow or fast nature of the PA to some extent indicates prognostic features such as recurrence risk. This finding requires correlation with histological and molecular features in further stages of research.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Bioethics Committee of Poznan University of Medical Sciences (Resolution No. 781/16). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization, KP, MW. Data curation, KP, PK. Formal analysis, PK, JC. Investigation, KP, EB, JC. Methodology, KP, EB, MW. Project administration, MW. Resources, KP, PK, MW. Software, EB, JC. Supervision, MW. Validation, KP, EB, MW. Visualization, KP, JC. Writing—original draft, KP, JC, MW. Writing—review and editing, KP, JC, MW. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Andreasen S, Therkildsen MH, Bjørndal K, Homøe P. Pleomorphic adenoma of the parotid gland 1985-2010: A Danish nationwide study of incidence, recurrence rate, and malignant transformation. Head Neck (2016) 38(Suppl 1):E1364–9. doi: 10.1002/hed.24228
2. Wierzbicka M, Piwowarczyk K, Nogala H, Błaszczyńska M, Kosiedrowski M, Mazurek C. Do we need a new classification of parotid gland surgery? Otolaryngol Pol (2016) 70:9–14. doi: 10.5604/00306657.1202390
3. Shome S, Shah N, Mahmud SA, Pal M. A miscellany of cribriform pattern, squamous metaplasia and clear cells in pleomorphic adenoma of upper lip: A diagnostic paradox. J Oral Maxillofac Pathol (2020) 24:46. doi: 10.4103/jomfp.JOMFP_354_19
4. Sharma S, Mehendiratta M, Chaudhary N, Gupta V, Kohli M, Arora A. Squamous Metaplasia in Pleomorphic Adenoma: A Diagnostic and Prognostic Enigma. J Pathol Transl Med (2018) 52:411–5. doi: 10.4132/jptm.2018.07.15
5. Hellquist H, Paiva-Correia A, Vander Poorten V, Quer M, Hernandez-Prera JC, Andreasen S, et al. Analysis of the Clinical Relevance of Histological Classification of Benign Epithelial Salivary Gland Tumours. Adv Ther (2019) 36:1950–74. doi: 10.1007/s12325-019-01007-3
6. Nonitha S, Yogesh TL, Nandaprasad S, Maheshwari BU, Mahalakshmi IP, Veerabasavaiah BT. Histomorphological comparison of pleomorphic adenoma in major and minor salivary glands of oral cavity: A comparative study. J Oral Maxillofac Pathol (2019) 23:356. doi: 10.4103/jomfp.JOMFP_91_19
7. Valstar MH, Andreasen S, Bhairosing PA, McGurk M. Natural history of recurrent pleomorphic adenoma: implications on management. Head Neck (2020) 42(8):2058-2066. doi: 10.1002/hed.26137
8. Riad MA, Abdel-Rahman H, Ezzat WF, Adly A, Dessouky O, Shehata M. Variables related to recurrence of pleomorphic adenomas: outcome of parotid surgery in 182 cases. Laryngoscope (2011) 121:1467–72. doi: 10.1002/lary.21830
9. Zbären P, Stauffer E. Pleomorphic adenoma of the parotid gland: histopathologic analysis of the capsular characteristics of 218 tumors. Head Neck (2007) 29:751–7. doi: 10.1002/hed.20569
10. Soares AB, Demasi APD, Altemani A, de Araújo VC. Increased mucin 1 expression in recurrence and malignant transformation of salivary gland pleomorphic adenoma. Histopathology (2011) 58:377–82. doi: 10.1111/j.1365-2559.2011.03758.x
11. Salzman R, Stárek I, Kučerová L, Skálová A, Hoza J. Neither expression of VEGF-C/D nor lymph vessel density supports lymphatic invasion as the mechanism responsible for local spread of recurrent salivary pleomorphic adenoma. Virchows Arch (2014) 464:29–34. doi: 10.1007/s00428-013-1502-5
12. de Souza AA, Altemani A, Passador-Santos F, Turssi CP, de Araujo NS, de Araújo VC, et al. Dysregulation of the Rb pathway in recurrent pleomorphic adenoma of the salivary glands. Virchows Arch (2015) 467:295–301. doi: 10.1007/s00428-015-1804-x
13. Antony J, Gopalan V, Smith RA, Lam AKY. Carcinoma ex pleomorphic adenoma: a comprehensive review of clinical, pathological and molecular data. Head Neck Pathol (2012) 6:1–9. doi: 10.1007/s12105-011-0281-z
14. Valstar MH, de Ridder M, van den Broek EC, Stuiver MM, van Dijk BAC, van Velthuysen MLF, et al. Salivary gland pleomorphic adenoma in the Netherlands: A nationwide observational study of primary tumor incidence, malignant transformation, recurrence, and risk factors for recurrence. Oral Oncol (2017) 66:93–9. doi: 10.1016/j.oraloncology.2017.01.004
15. Mariano FV, Noronha ALF, Gondak RO, de A.M. Altemani AM, de Almeida OP, Kowalski LP. Carcinoma ex pleomorphic adenoma in a Brazilian population: clinico-pathological analysis of 38 cases. Int J Oral Maxillofac Surg (2013) 42:685–92. doi: 10.1016/j.ijom.2013.02.012
16. Dardick I, van Nostrand AW, Jeans MT, Rippstein P, Edwards V. Pleomorphic adenoma, I: Ultrastructural organization of “epithelial” regions. Hum Pathol (1983) 14:780–97. doi: 10.1016/s0046-8177(83)80301-3
17. Palmer RM, Lucas RB, Langdon JD. Ultrastructural analysis of salivary gland pleomorphic adenoma, with particular reference to myoepithelial cells. Histopathology (1985) 9:1061–76. doi: 10.1111/j.1365-2559.1985.tb02785.x
18. Lim S, Cho I, Park J-H, Lim S-C. Pleomorphic Adenoma with Exuberant Squamous Metaplasia and Keratin Cysts Mimicking Squamous Cell Carcinoma in Minor Salivary Gland. Open J Pathol (2013) 2013:113–5. doi: 10.4236/ojpathology.2013.33020
19. Thielker J, Weise A, Othman MAK, Carreria IM, Melo JB, von Eggeling F, et al. Molecular cytogenetic pilot study on pleomorphic adenomas of salivary glands. Oncol Lett (2020) 19:1125–30. doi: 10.3892/ol.2019.11198
20. Quer M, Guntinas-Lichius O, Marchal F, Poorten VV, Chevalier D, León X, et al. Classification of parotidectomies: A proposal of the European Salivary Gland Society. Eur Arch Otorhinolaryngol (2016) 273(10):3307–12. doi: 10.1007/s00405-016-3916-6
21. Bankamp DG, Bierhoff E. Proliferative activity in recurrent and nonrecurrent pleomorphic adenoma of the salivary glands. Laryngorhinootologie (1999) 78:77–80. doi: 10.1055/s-2007-996835
22. Stennert E, Guntinas-Lichius O, Klussmann JP, Arnold G. Histopathology of pleomorphic adenoma in the parotid gland: a prospective unselected series of 100 cases. Laryngoscope (2001) 111:2195–200. doi: 10.1097/00005537-200112000-00024
23. Orita Y, Hamaya K, Miki K, Sugaya A, Hirai M, Nakai K, et al. Satellite tumors surrounding primary pleomorphic adenomas of the parotid gland. Eur Arch Otorhinolaryngol (2010) 267:801–6. doi: 10.1007/s00405-009-1149-7
24. Li C, Xu Y, Zhang C, Sun C, Chen Y, Zhao H, et al. Modified partial superficial parotidectomy versus conventional superficial parotidectomy improves treatment of pleomorphic adenoma of the parotid gland. Am J Surg (2014) 208(1)112-118. doi: 10.1016/j.amjsurg.2013.08.036
25. Dulguerov P, Todic J, Pusztaszeri M, Alotaibi NH. Why Do Parotid Pleomorphic Adenomas Recur? A Systematic Review of Pathological and Surgical Variables. Front Surg (2017) 4:26 1-5. doi: 10.3389/fsurg.2017.00026
26. Kanatas A, Ho MWS, Mücke T. Current thinking about the management of recurrent pleomorphic adenoma of the parotid: a structured review. Br J Oral Maxillofac Surg (2018) 56:243–8. doi: 10.1016/j.bjoms.2018.01.021
27. Abu-Ghanem Y, Mizrachi A, Popovtzer A, Abu-Ghanem N, Feinmesser R. Recurrent pleomorphic adenoma of the parotid gland: Institutional experience and review of the literature. J Surg Oncol (2016) 114:714–8. doi: 10.1002/jso.24392
28. Rowley H, Murphy M, Smyth D, O’Dwyer TP. Recurrent pleomorphic adenoma: uninodular versus multinodular disease. Ir J Med Sci (2000) 169:201–3. doi: 10.1007/BF03167696
29. Witt RL, Eisele DW, Morton RP, Nicolai P, Poorten VV, Zbären P. Etiology and management of recurrent parotid pleomorphic adenoma. Laryngoscope (2015) 125:888–93. doi: 10.1002/lary.24964
30. Wittekindt C, Streubel K, Arnold G, Stennert E, Guntinas-Lichius O. Recurrent pleomorphic adenoma of the parotid gland: analysis of 108 consecutive patients. Head Neck (2007) 29:822–8. doi: 10.1002/hed.20613
Keywords: mixed tumor, parotid gland tumor, recurrence, surgery, progression, facial nerve
Citation: Piwowarczyk K, Bartkowiak E, Kosikowski P, Chou JT-T and Wierzbicka M (2021) Salivary Gland Pleomorphic Adenomas Presenting With Extremely Varied Clinical Courses. A Single Institution Case-Control Study. Front. Oncol. 10:600707. doi: 10.3389/fonc.2020.600707
Received: 30 August 2020; Accepted: 23 November 2020;
Published: 08 January 2021.
Edited by:
Alberto Paderno, University of Brescia, ItalyReviewed by:
Shilpi Sharma, Narayana Superspeciality Hospital, IndiaIain James Nixon, National Health Service Scotland, United Kingdom
Copyright © 2021 Piwowarczyk, Bartkowiak, Kosikowski, Chou and Wierzbicka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Krzysztof Piwowarczyk, a3J6eXN6dG9mcGl3b3dhcmN6eWsyQGdtYWlsLmNvbQ==
†This paper is dedicated to the memory of Tomasz Kopec, MD, PhD
 Krzysztof Piwowarczyk
Krzysztof Piwowarczyk Ewelina Bartkowiak
Ewelina Bartkowiak Paweł Kosikowski2
Paweł Kosikowski2 Jadzia Tin-Tsen Chou
Jadzia Tin-Tsen Chou Małgorzata Wierzbicka
Małgorzata Wierzbicka