- Department of Urology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: Several recent publications have evaluated the prognostic value of preoperative hydronephrosis (HN) in patients with upper tract urinary carcinoma (UTUC). The aim of this meta-analysis was to explore the pooled effect of preoperative HN on the prognosis of UTUC patients treated with radical nephroureterectomy (RNU) based on current evidence.
Methods: We performed a systematic search of Pubmed, Cochrane library, and Web of Science databases from inception to June 2020. The outcomes of interest included overall survival (OS), cancer-special survival (CSS), disease-free survival (DFS), and intravesical recurrence-free survival (IVRFS).
Results: Twenty-two studies with a total of 7,542 patients satisfied the eligibility criteria and were finally included in this meta-analysis. The percent of patients with preoperative HN varied in the eligible studies, ranging from 18 to 81%. The pooled results showed that preoperative HN was significantly associated with worse OS (P = 0.004), CSS (P < 0.001), and DFS (P = 0.005), but not IVRFS (P = 0.12). No obvious publication bias was detected by Begg’s test in all the analyses.
Conclusions: The results drawn in our meta-analysis suggest that the presence of preoperative HN is associated with worse prognosis in patients treated with RNU for UTUC. Therefore, closer surveillance and more aggressive therapy may be needed for UTUC patients present with preoperative HN. Well-designed prospective studies are necessary to substantiate the prognostic value of HN in UTUC.
Introduction
Upper tract urothelial carcinoma (UTUC) is a relatively uncommon malignancy, accounting for only 5 to 10% of all urothelial carcinomas (1, 2). UTUC can arise anywhere along the urinary tract epithelium from renal calyces to ureteral orifice; most of the tumors are located in the renal pelvis, and only 30% occur in the ureter (3). In spite of advances in minimally invasive treatments, radical nephroureterectomy (RNU) with bladder cuff removal still remains the ‘gold-standard’ treatment for UTUC, mainly via either an open or a laparoscopic approach (1). However, the prognosis of UTUC is generally not good after radical surgery due to the high possibility of invasive diseases at diagnosis.
Currently, many studies have discussed the prognostic factors for UTUC undergoing RNU, and accumulating knowledge of the factors would help the urologists better evaluate the outcome of UTUC, for a more effective therapy overall. Neutrophil-to-lymphocyte ratio (NLR), a blood-based biomarker, was reported to correlate with worse outcomes in various malignant tumors; a previous meta-analysis summarized that high preoperative blood-based NLR was obviously associated with poorer OS, RFS, and CSS in UTUC patients who underwent RNU (4). Ku et al. also reported that lymphovascular invasion (LVI) could be used as a potential predictor of mortality in UTUC (5). In addition, tumor stage, tumor grade, and lymph node (LN) status have been established as the major prognostic factors for UTUC, demonstrating the heterogeneity and aggressiveness of this type of cancer (6–8).
Preoperative hydronephrosis (HN) status can be detected in patients with bladder cancer or UTUC. For bladder cancer patients, a meta-analysis summarized that the presence of preoperative HN was significantly correlated with worse OS and CSS after radical cystectomy (9). Recently, several studies also have indicated that preoperative HN may be a potential prognostic predictor for UTUC after RNU, but its effect has not been fully understood and their results are still in controversies (10, 11). As it is easily available for the detection of preoperative HN in clinical practice, a better understanding of HN may improve the oncological outcomes for UTUC patients after RNU. Thus, we performed a literature review and meta-analysis to further explore the generalized impact of preoperative HN on the prognosis of patients who underwent RNU for UTUC.
Materials and Methods
Search Strategy
This work was reported in line with PRISMA (Preferred Reporting Items for Systematic Review and Meta-analyses) (12). Published studies were identified from Pubmed, Cochrane library, and Web of Science databases (last search date: June 2020), and additional articles were found by screening the reference lists of the retrieved records. Only original studies written in English and published after January 2010 were included. The following terms were combined to perform the electronic search: ‘upper tract urothelial cancer’ or ‘transitional cell carcinoma of the upper urinary tract’, ‘radical nephroureterectomy’, ‘prognosis’ or ‘survival’ or ‘oncological outcome’, and ‘hydronephrosis’. Two independent investigators (TY and XQY) screened the titles and abstracts of retrieved articles, and disagreements were settled by negotiation. The protocol was registered in the International Prospective Register of Systematic Reviews database (PROSPERO: CRD42019132011).
Inclusion and Exclusion Criteria
Studies satisfying the following criteria were included: (i) studies that evaluated the association between preoperative HN and oncological outcomes in patients who underwent RNU for UTUC; (ii) studies that directly reported hazard ratios (HRs) with their corresponding 95% CIs of overall survival (OS), cancer-special survival (CSS), disease-free survival (RFS), and intravesical recurrence-free survival (IVRFS) in multivariable logistic regression analysis; (iii) studies that enrolled more than 100 participants, and (iv) the median follow-up >12 months. The exclusion criteria were as follows: (i) the literatures were non-original articles, comments, reviews, or meta-analysis; (ii) studies that included patients with recurrent UTUC, metastatic carcinoma, or receiving neoadjuvant chemotherapy were excluded; and (iii) (potentially) overlapping study populations were reported for the same outcome.
Data Extraction and Quality Assessment
A standardized-items form was used by two independent reviewers (TY and HL) to extract usable data from eligible full length articles. In our meta-analysis, OS and CSS were defined as the interval between surgery and any cause death and cancer-caused death, respectively; DFS was defined as the interval between surgery and local relapse or distant metastasis, excluding the recurrence in bladder, and these studies in which DFS was defined as recurrence both in bladder and non-bladder lesions were not considered for DFS analysis; IVRFS was defined as the interval between surgery and the recurrence in the bladder. The following information was recorded: first author’s name, publication year, country/region of origin, study period, study design, sample size, the number of patients with HN, patient characteristics (age and gender), follow-up duration, outcomes (OS, CSS, DFS, and IVRFS), tumor characteristics (location, size, pT stage, pN stage, and grade), and treatment management. Newcastle-Ottawa Scale was applied to determine the quality of the included studies (13). The score ≥6 was considered high-quality. Disagreements were discussed to reach a consensus.
Statistical Analysis
Stata 12.0 statistical software (Stata Corp., College Station, TX) was applied to perform all the data analyses. HRs with their 95% CIs of OS, CSS, DFS, and IVRFS in multivariable logistic regression analysis from each study were used to obtain the combined HRs. A random-effect or fixed-effect model was chosen to pool the results based on the between-study heterogeneity. I2 statistics >50% and chi-squared test P value <0.1 demonstrated notable heterogeneity. A Galbraith plot was performed and a leave-one-out analysis was conducted to identify studies causing heterogeneity and evaluate their influence on the combined HRs (14). Sensitivity analysis was conducted to examine the stability of the final results and Begg’s test to determine the risk of publication bias among the included studies. P-value lower than 0.05 was considered statistically significant.
Results
Summary of the Enrolled Studies
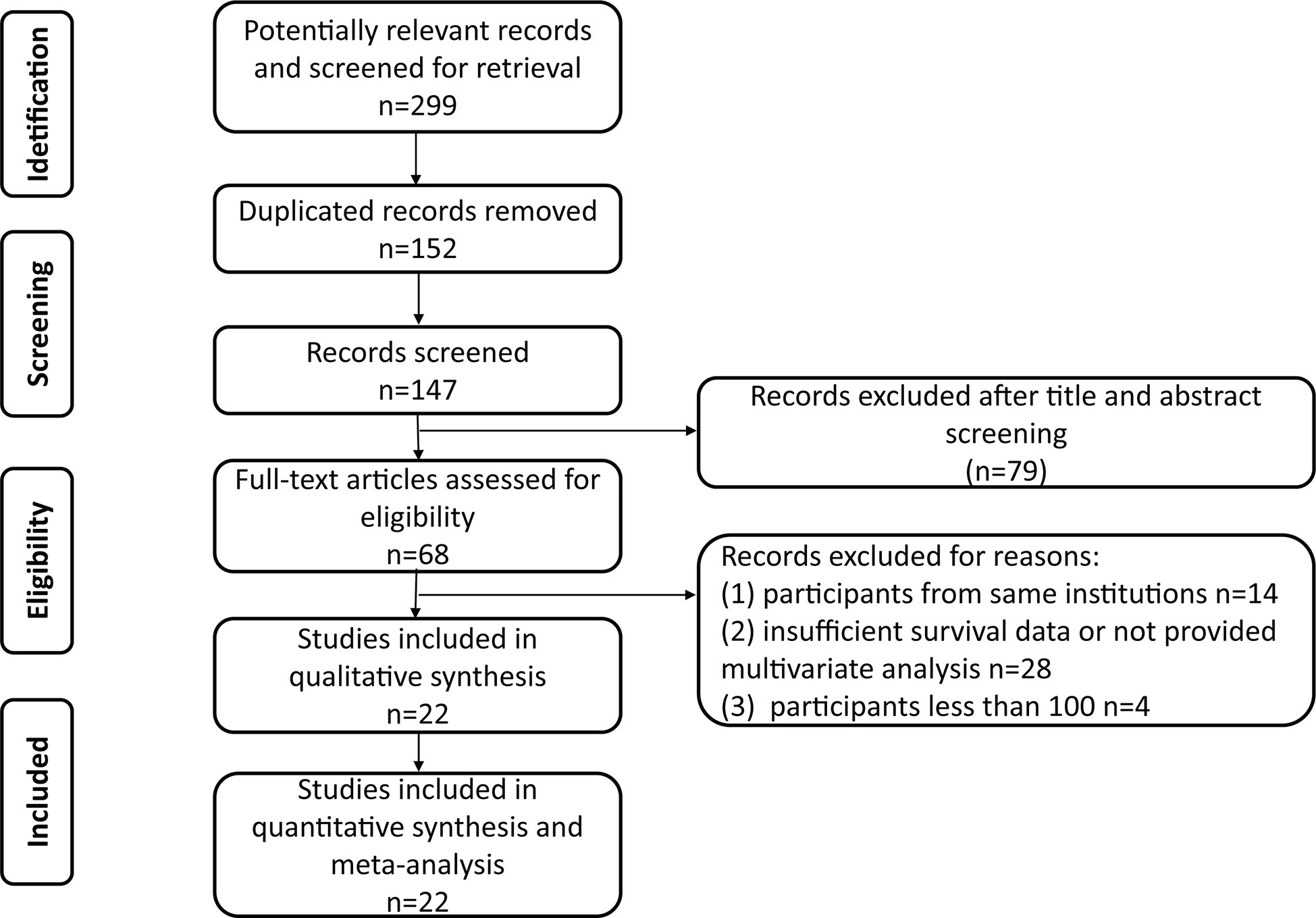
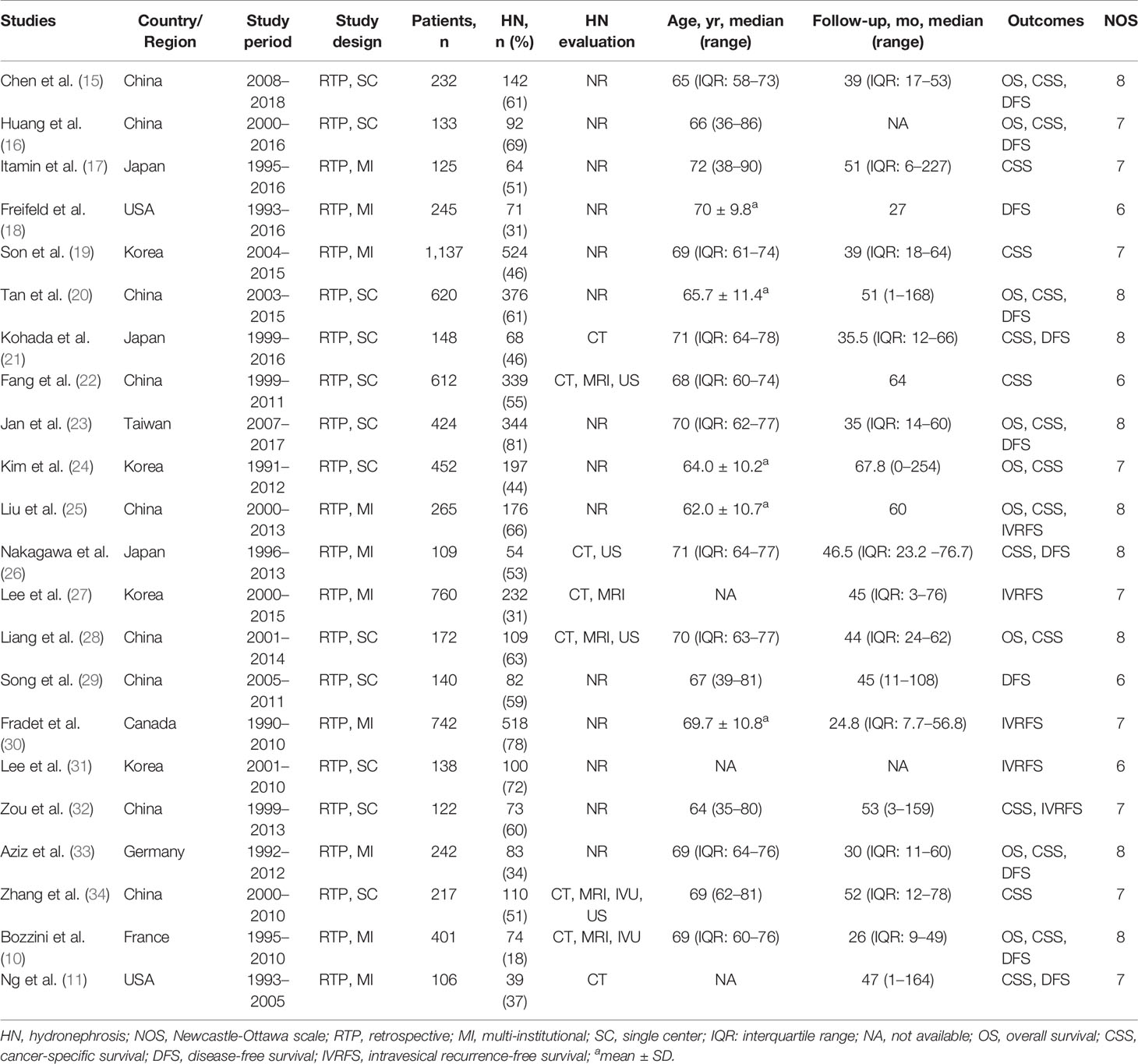
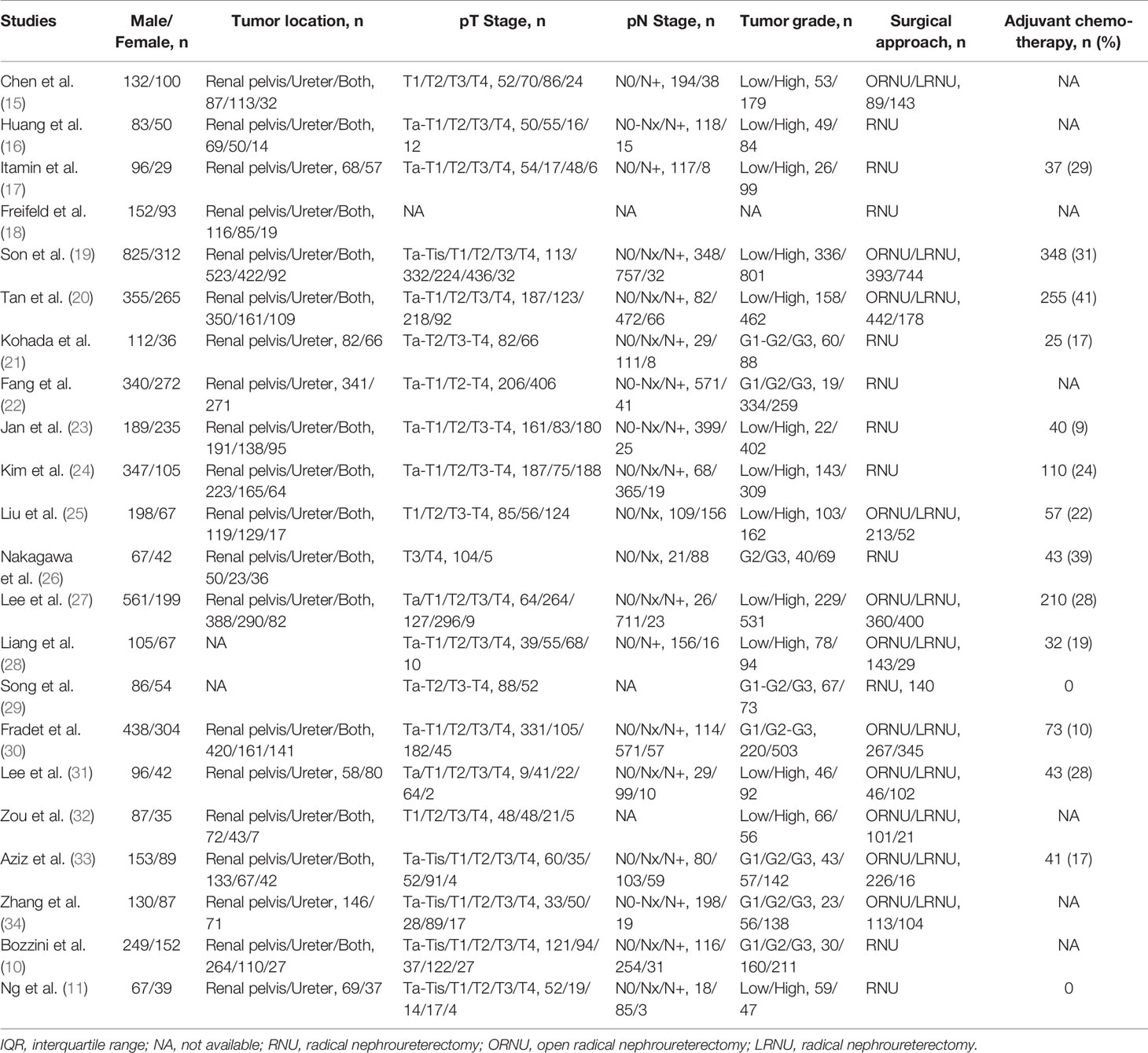
The PRISMA flow chart of the search strategy for eligible studies is summarized in Figure 1. We assembled a total of 299 potentially relevant records from the electronic databases, and finally 68 articles were retrieved for full texts. After final evaluation, only 22 studies were deemed fully eligible for this meta-analysis (10, 11, 15–34). The main characteristics and findings of the 22 eligible studies are summarized in Tables 1, 2. Among the 7,542 patients (4,868 males and 2,674 females), the status of HN was confirmed in 3,867 of them, and the percent of patients with preoperative HN in each study ranged from 18 to 81%. As for the HN evaluation method, eight studies reported directly, while the others were not in the published papers. All the included studies were retrospective design, with a wide recruitment period from 1990 to 2018. The enrolled UTUC patients were from different countries or regions (China, Japan, USA, Korea, Taiwan, Canada, Germany, and France) with the median follow-up duration ranging from 24.8 to 67.8 months. The quality assessment of eligible studies by the Newcastle-Ottawa scale showed that all the included studies were high-quality with the scores ≥6 (Table 1).
Meta-Analysis of OS
Nine studies with 2,941 patients were performed to explore the impact of HN on the OS of patients with UTUC receiving RNU. Due to no significant heterogeneity observed (I2 = 23.1%), a fixed-effect model was used. The results showed that the patients with preoperative HN were subjected to unfavorable OS (HR = 1.26, 95% CI: 1.08 to 1.47, P = 0.004) (Figure 2A). Sensitivity analysis was conducted to evaluate the outcome stability, and the result verified the robustness of the meta-analysis of OS (Figure 2B). No obvious publication bias was detected by Begg’s test (P = 0.175) (Figure 2C).
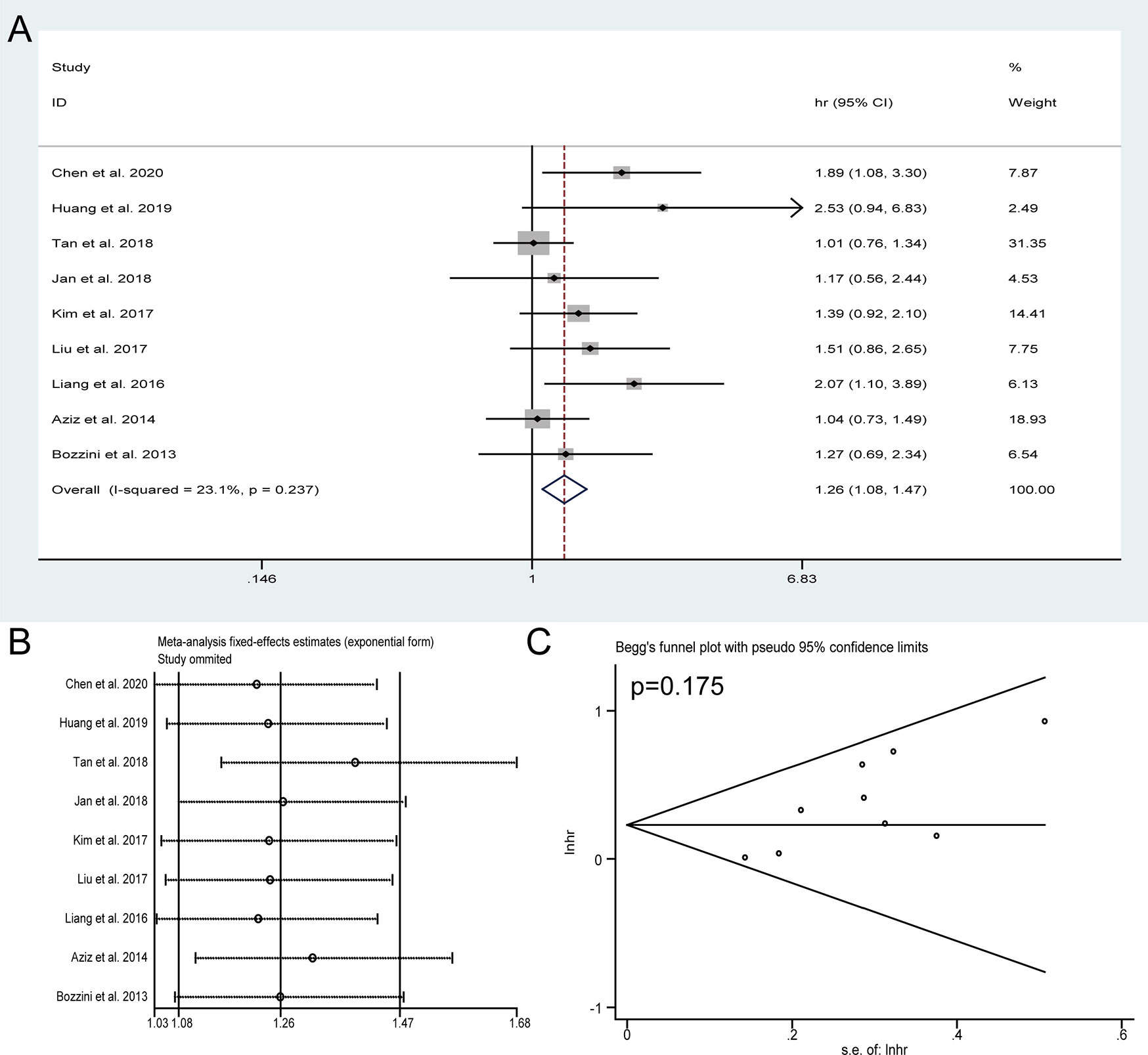
Figure 2 Forest plots of studies to evaluate the relation between preoperative hydronephrosis and overall survival (A). Sensitivity analysis for the meta-analysis of overall survival (B). Begg’s test for the meta-analysis of overall survival (C).
Meta-Analysis of CSS
Seventeen studies with 5,517 patients reported the CSS assessed by multivariate analysis. In view of significant heterogeneity (I2 = 64%) in the included studies, a random-effect model was used. The pooled HRs of these studies indicated that the presence of preoperative HN was obviously associated with shorter CSS (HR = 1.71, 95% CI: 1.33 to 2.19, P < 0.001) (Figure 3A). The sensitivity analysis confirmed the robustness of the results (Figure 3B) and the Begg’s test revealed no publication bias among the included studies (P = 0.174) (Figure 3C).
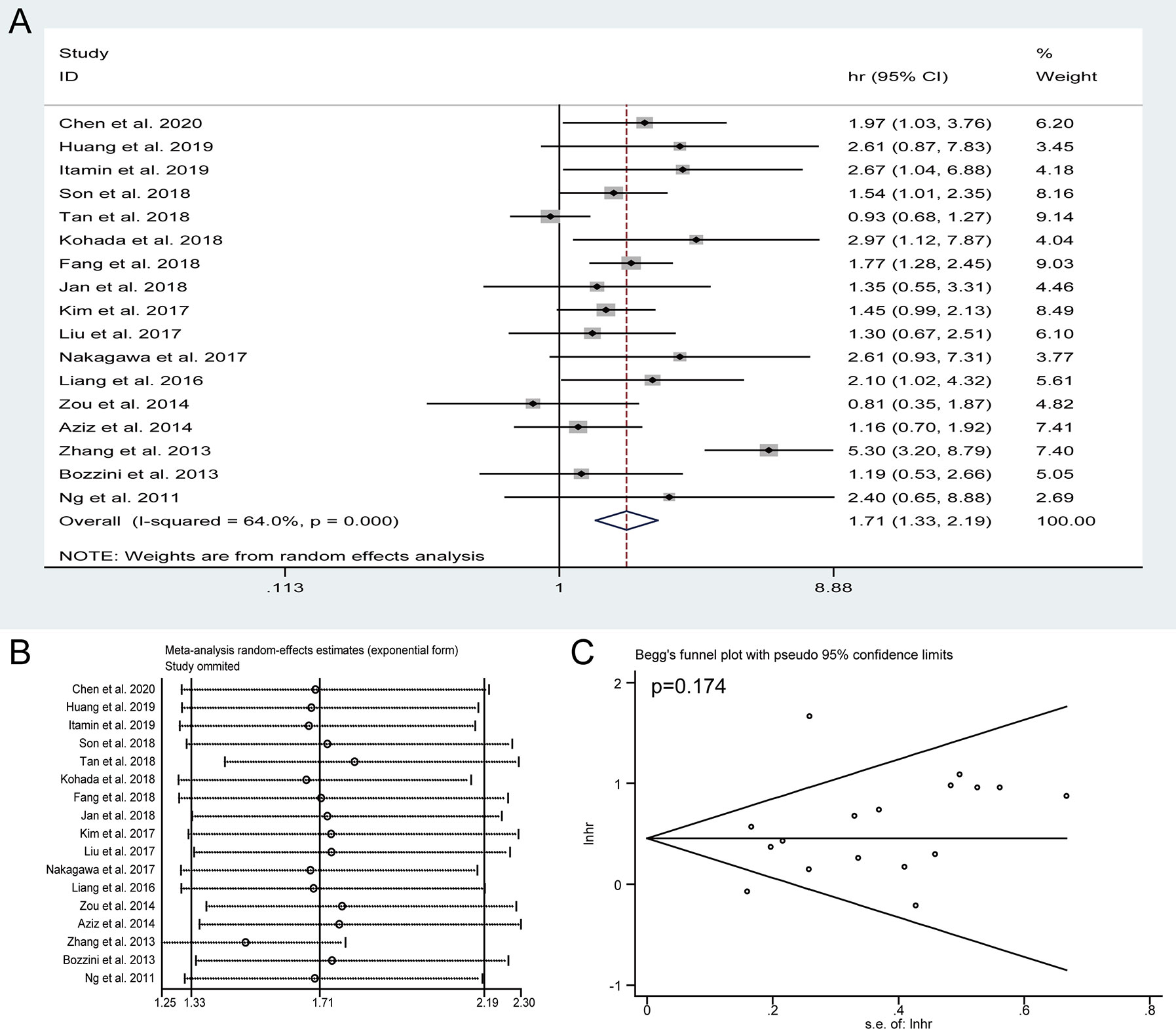
Figure 3 Forest plots of studies to evaluate the relation between preoperative hydronephrosis and cancer-specific survival (A). Sensitivity analysis for the meta-analysis of cancer-specific survival (B). Begg’s test for the meta-analysis of cancer-specific survival (C).
Meta-Analysis of DFS
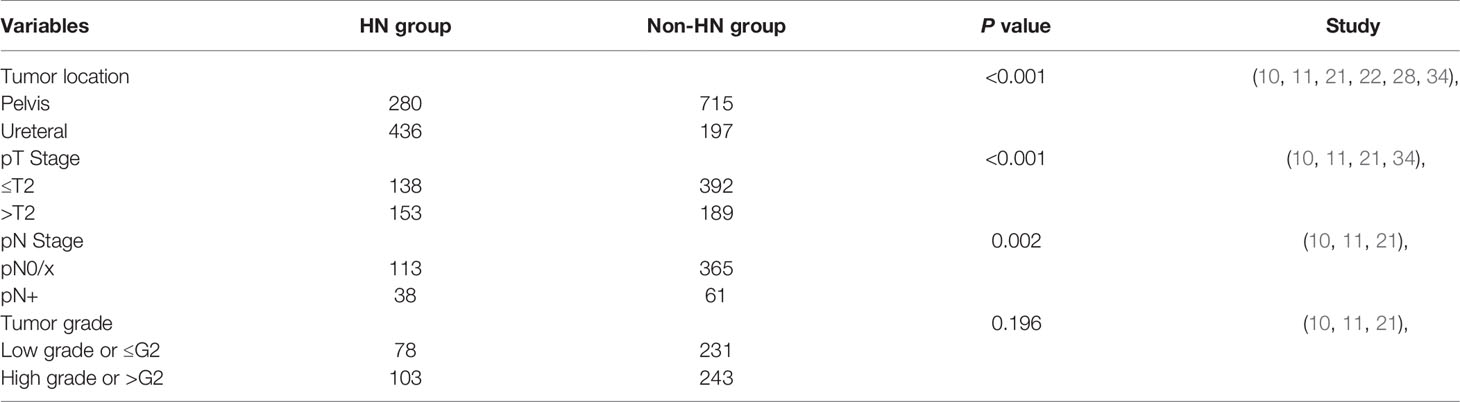
Pooled HRs and 95% CIs of DFS were collected in 11 studies with 2,800 patients. No obvious heterogeneity was detected among studies (I2 = 31.7%), and a fixed-effect model was applied to obtain the pooled HRs and corresponding 95% CIs. The result suggested that the presence of preoperative HN predicted a poor outcome of DFS (HR = 1.25, 95% CI: 1.07 to 1.47, P = 0.005) (Figure 4A). The sensitivity analysis showed that the pooled result of DFS were robust (Figure 4B) and Begg’s tests indicated no obvious publication bias in the meta-analysis of DFS (P = 0.062) (Figure 4C). To discuss the source of significantly worse OS, CSS, and DFS in these patients with preoperative HN, we compared the differences in tumor location, pathologic T stage, lymph node status, and tumor grade between the HN group patients and non-HN group patients, thereby identifying the features between the groups using chi-squared tests for categorical variables (Table 3). The results revealed remarkable significant differences in tumor location (P < 0.001), pathologic T stage (P < 0.001), and lymph node status (P = 0.002) between the HN and non-HN groups based on the included studies. However, no obvious difference was found in tumor grade between the two groups (P = 0.196)
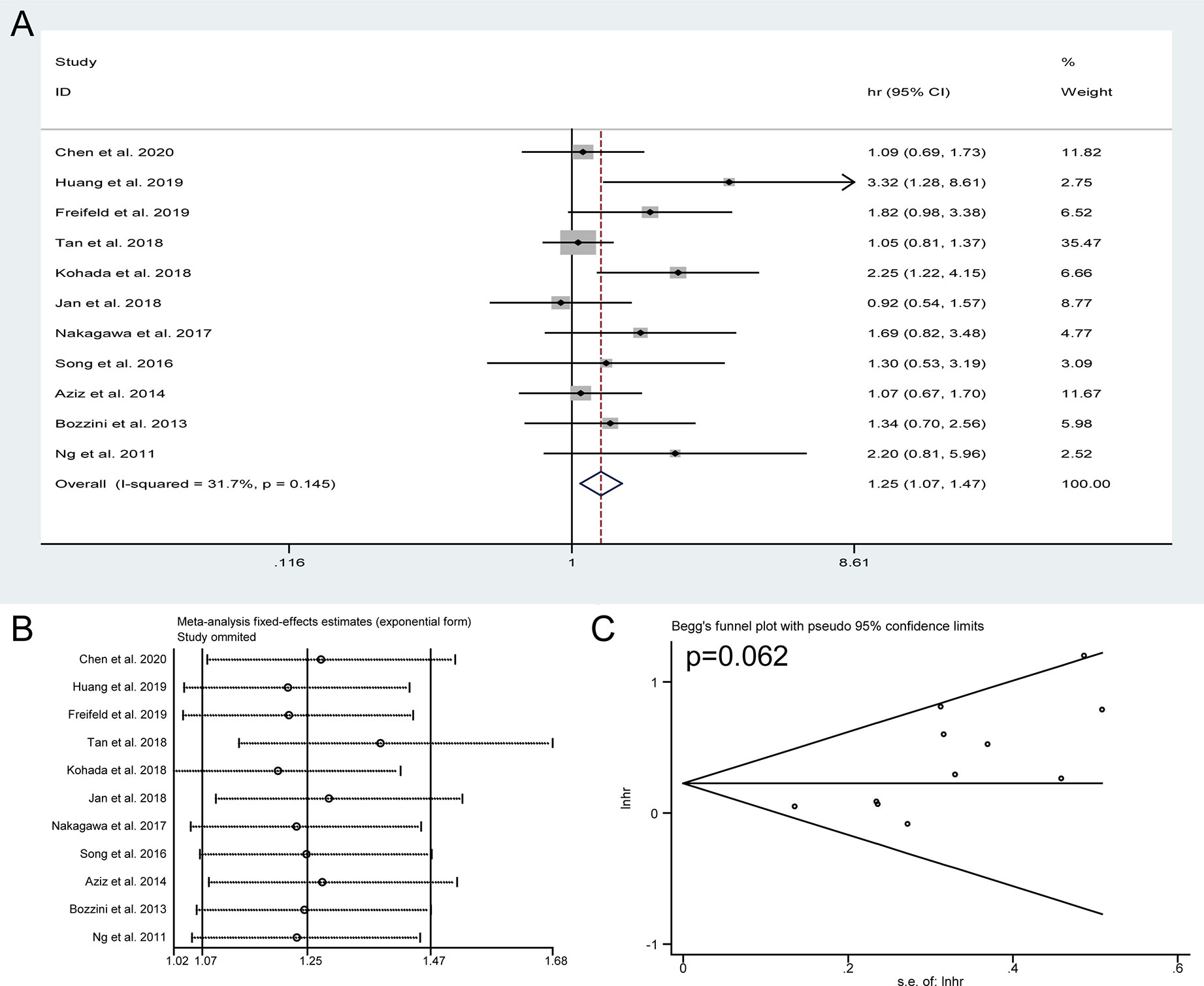
Figure 4 Forest plots of studies to evaluate the relation between preoperative hydronephrosis and disease-free survival (A). Sensitivity analysis for the meta-analysis of disease-free survival (B). Begg’s test for the meta-analysis of disease-free survival (C).
Meta-Analysis of IVRFS
Five studies incorporating 2,027 patients investigated the actual impact of preoperative HN on intravesical recurrence for UTUC after RNU. As present in Figure 5A, no significant association was observed between preoperative HN and IVRFS (HR = 1.43, 95% CI: 0.91 to 2.24, P = 0.12). The sensitivity analysis showed that the pooled HRs of IVRFS were stable (Figure 5B) and Begg’s plot showed no significant evidence of publication bias (Figure 5C). These results suggested that preoperative HN could not be used to predict intravesical recurrence for UTUC after RNU based on these included studies.
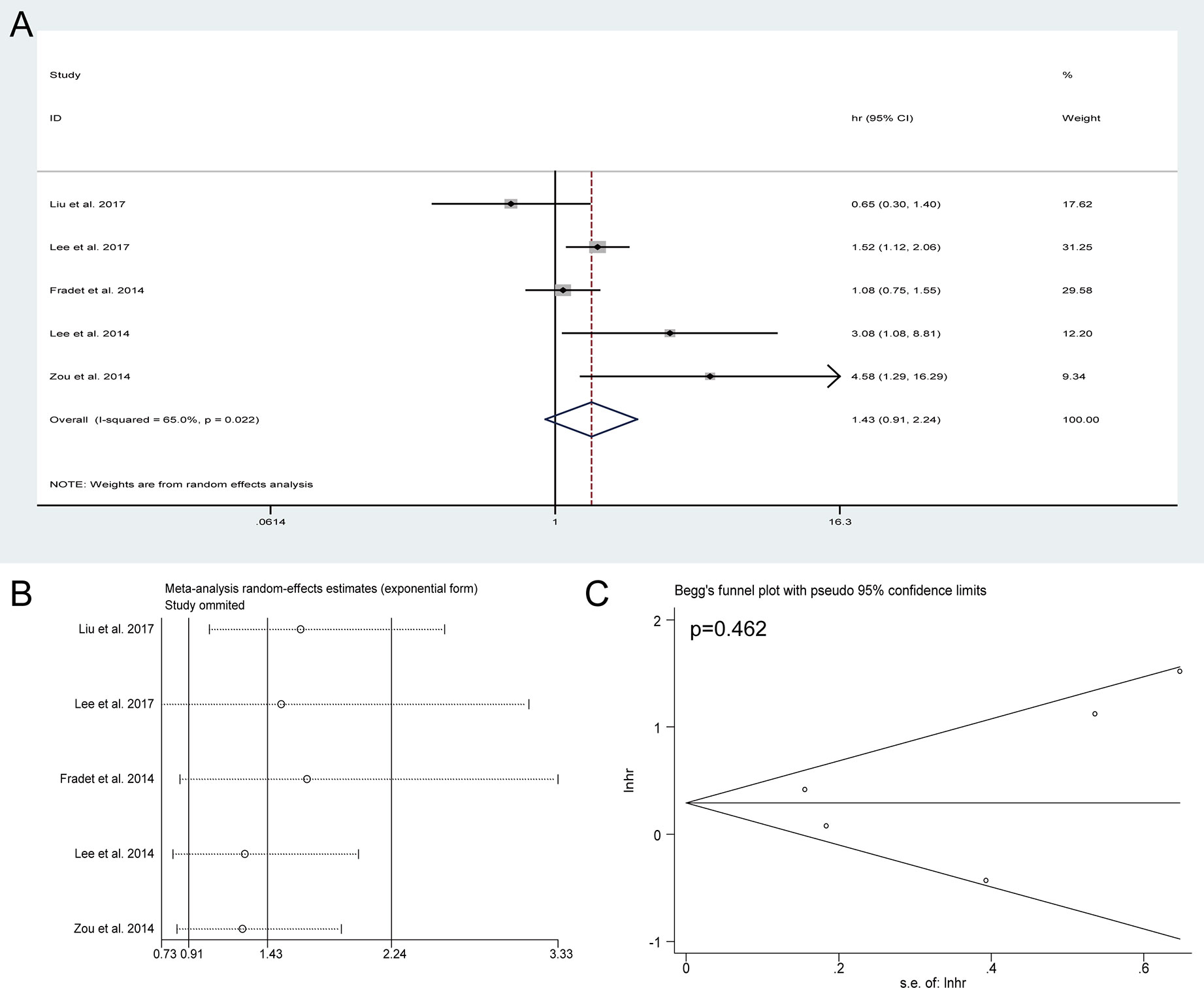
Figure 5 Forest plots of studies to evaluate the relation between preoperative hydronephrosis and intravesical recurrence-free survival (A). Sensitivity analysis for the meta-analysis of intravesical recurrence-free survival (B). Begg’s test for the meta-analysis of intravesical recurrence-free survival (C).
Discussion
The association between preoperative HN and oncological outcomes in patients who underwent RNU for UTUC has been widely discussed; however, the results reported by relevant studies were still controversial. Our present meta-analysis of 22 studies including 7,542 patients aimed to investigate the impact of preoperative HN on oncological outcomes for these UTUC patients. The final results revealed the independent predictor status of preoperative HN on OS, CSS, and DFS for patients who underwent RNU for UTUC. However, no significant difference was found when discussing the effect of HN on IVRFS. Besides, we summarized the related data in the eligible studies and found that preoperative HN was significantly associated with ureteral tumors, advanced pT stage, and positive lymph node status, which might contribute to the significant correlation between HN and poor outcomes in UTUC. This correlation will be helpful for guiding decisions about the administration of neoadjuvant chemotherapy.
Recent years, identifying valuable predictors for tumor stage, survival and recurrence of UTUC has attracted extensive interest. Many studies have explored the potential individual or combined implications of various preoperative and postoperative parameters (35). Meta-analysis performed by Wu et al. enrolled 17 articles with 12,094 participants and indicated that ureteral and multifocal tumors could be a prognostic predictor of disease progression and cancer-specific survival for UTUC patients (36). Macroscopic sessile tumor architecture also has been reported to be independently correlated with worse survival after RNU for UTUC (37). Moreover, other valuable prognostic factors also have been identified, and the 2017 EAU guidelines recommended that tumor multifocality, grade on biopsy or cytology, ureteral location, patient age, smoking status, obesity (BMI >30), ECOG-PS ≧1, and delayed surgery (more than 3 months) were valuable preoperative prognosticators (38). However, it remains not very clear for the effect of these potential prognosticators on UTUC patients’ survival. A better understanding of these prognostic factors for tumor progression and patients’ survival would allow more accurate prognostic assessment and more effective therapy approach.
The preoperative pathologic tumor stage, grade, lymph node (LN), and distant metastasis are used as the most crucial prognostic factors for most kinds of malignancies, including UTUC. Despite great advances in medical imaging techniques, its ability to predict preoperative staging and degree of local invasion still has limited accuracy (39), while the preoperative HN status is easily diagnosed for UTUC patients by multiple upper-tract imaging methods, such as CT, MRI, intravenous pyelography, and renal ultrasonography. Several studies have recommended that HN could serve as a potential indicator for invasive disease (>pT2) (40, 41) or non-organ-confined (NOC) disease (pT3–4) (42). Furthermore, it has been identified as a valuable predictor for advanced stage and poor prognosis in transitional cell carcinoma of bladder (43, 44).
Cho et al. reported that the grade of HN on preoperative imaging was a valuable predictor for advanced pathologic tumor stage and worse CSS for ureteral carcinoma (41). Furthermore, both Ng et al. and Zhang et al. similarly found that ureter tumors were more likely to develop HN compared with diseases in renal pelvis, and both the ureter tumor and renal pelvic tumor patients with preoperative HN were correlated with higher tumor stage than patients without HN (11, 34). In addition, several studies revealed that tumor location could be used as an independent prognostic factor of CSS for UTUC patients, and ureter tumors showed worse oncologic outcomes than renal pelvic tumors (34, 45). However, some different voice was also noted. Bozzini et al. conducted a multi-institutional study in France on 401 patients with non-metastatic UTUC and observed no difference in 5 year CSS between HN group (80.1%) and no HN group (83.6%) (P > 0.05); moreover, a trend of association between HN and pN stage, not pT stage, was found (P = 0.052) (10). Using logistic regression analysis, Liang et al. found preoperative HN had a significant relationship with decreased renal function and LN not LVI; importantly, the Cox analysis results also revealed that HN could serve as an independent risk factor for OS and CSS (28). Otherwise, study by Chung et al. also indicated that HN could predict worse survival for patients with high grade UTUC, not those with low grade UTUC (46).
Overall, preoperative HN can be detected in 37–55% of UTUC patients, while the present knowledge regarding the effect of HN on UTUC prognosis is still inadequate. Our study revealed a significant association of preoperative HN with ureteral tumors, bigger tumor’s size, and positive lymph node status, which might affect the UTUC patients’ prognosis. In addition, it was speculated that HN might cause the outward expansion of high-pressure renal pelvic or ureter wall and increase outward centrifugal pressure, resulting in counter flow in lymphatics and vasculature, which might promote the cancer cells’ seeding to nearby or distant organs (46). To identify the prognostic value of preoperative HN, we performed this meta-analysis and enrolled 22 eligible studies to discuss the pooled effects of HN on oncologic outcomes of UTUC patients after RNU. Preoperative evaluation of advanced stage disease on the basis of HN status will allow urologists make more effective pre- or postoperative treatment strategies. A study about a phase 3, open-label, randomized controlled trial reported that gemcitabine–platinum combination chemotherapy could significantly improve disease-free survival in patients with locally advanced UTUC after nephroureterectomy (47). Earlier use of valid adjuvant therapy will contribute to the improvement in the prognosis for locally advanced UTUC.
Inevitably, our meta-analysis suffered from several limitations. First, all the enrolled studies were retrospectively designed. In spite of the fact that we reached the results by pooling the HRs in multivariate models, some underlying selection bias still existed, and we could not control all the confounding factors. Second, the classification and imaging evaluation method of HN were different: some studies classified HN as “none versus present”, but others as “none or mild versus severe”; in addition, the definite grade of HN and the influence of different HN grades on prognosis of UTUC patients were not reported in these included studies, which might limit the clinical application of preoperative HN as a adjunctive modality to ureteroscopic biopsy and urinary cytology when counseling UTUC patients on medical and surgical therapies. Third, due to the limited number of included studies, this meta-analysis failed to confirm the value of preoperative HN as a predictor of intravesical recurrence for UTUC after RNU. Finally, this study was performed using the pooled data reported by the eligible studies but not individual patient data, so it is not an individual patient-level meta-analysis. Despite these limitations, our study was the first meta-analysis to identify HN as a potential preoperative prognosticator for UTUC patients undergoing RNU. Well-designed prospective studies are still warranted to further substantiate the prognostic value of preoperative HN in patients with UTUC.
Conclusions
In this meta-analysis, our results showed that preoperative HN was significantly correlated with worse prognosis in patients with UTUC after RNU, and preoperative HN might serve as an independent prognostic factor. The determination of preoperative HN status might be helpful to identify UTUC patients at higher risk of worse oncological outcomes who could benefit from more aggressive preoperative planning and postoperative therapy.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
TY and XY analyzed the data and wrote the manuscript. PL and HL searched and collected the literatures. TY and HL contributed to statistical analysis. TY and ZY designed the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester RJ, Burger M, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma: 2015 Update. Eur Urol (2015) 68(5):868–79. doi: 10.1016/j.eururo.2015.06.044
2. Redrow GP, Matin SF. Upper tract urothelial carcinoma: epidemiology, high risk populations and detection. Minerva Urol Nefrol (2016) 68(4):350–8.
3. Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol (2000) 164(5):1523–5. doi: 10.1016/S0022-5347(05)67019-X
4. Vartolomei MD, Kimura S, Ferro M, Vartolomei L, Foerster B, Abufaraj M, et al. Is neutrophil-to-lymphocytes ratio a clinical relevant preoperative biomarker in upper tract urothelial carcinoma? A meta-analysis of 4385 patients. World J Urol (2018) 36(7):1019–29. doi: 10.1007/s00345-018-2235-5
5. Ku JH, Byun SS, Jeong H, Kwak C, Kim HH, Lee SE. Lymphovascular invasion as a prognostic factor in the upper urinary tract urothelial carcinoma: a systematic review and meta-analysis. Eur J Cancer (2013) 49(12):2665–80. doi: 10.1016/j.ejca.2013.04.016
6. Cha EK, Shariat SF, Kormaksson M, Novara G, Chromecki TF, Scherr DS, et al. Predicting clinical outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol (2012) 61(4):818–25. doi: 10.1016/j.eururo.2012.01.021
7. Petrelli F, Yasser Hussein MI, Vavassori I, Barni S. Prognostic Factors of Overall Survival in Upper Urinary Tract Carcinoma: A Systematic Review and Meta-analysis. Urology (2017) 100:9–15. doi: 10.1016/j.urology.2016.07.036
8. Leow JJ, Orsola A, Chang SL, Bellmunt J. A contemporary review of management and prognostic factors of upper tract urothelial carcinoma. Cancer Treat Rev (2015) 41(4):310–9. doi: 10.1016/j.ctrv.2015.02.006
9. Zhu Z, Zhao J, Li Y, Pang C, Zhu Z, Zhang X. Prognostic value of preoperative hydronephrosis in patients with bladder cancer undergoing radical cystectomy: A meta-analysis. PLoS One (2019) 14(9):e0222223. doi: 10.1371/journal.pone.0222223
10. Bozzini G, Nison L, Colin P, Ouzzane A, Yates DR, Audenet F, et al. Influence of preoperative hydronephrosis on the outcome of urothelial carcinoma of the upper urinary tract after nephroureterectomy: the results from a multi-institutional French cohort. World J Urol (2013) 31(1):83–91. doi: 10.1007/s00345-012-0964-4
11. Ng CK, Shariat SF, Lucas SM, Bagrodia A, Lotan Y, Scherr DS, et al. Does the presence of hydronephrosis on preoperative axial CT imaging predict worse outcomes for patients undergoing nephroureterectomy for upper-tract urothelial carcinoma. Urol Oncol (2011) 29(1):27–32. doi: 10.1016/j.urolonc.2008.10.023
12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg (2010) 8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007
13. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
14. Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol (2009) 9:80. doi: 10.1186/1471-2288-9-80
15. Chen X, Ji H, Wang J, Zhao G, Zheng B, Niu Z, et al. Prognostic Value of the Preoperative Plasma D-Dimer Levels in Patients with Upper Tract Urothelial Carcinoma in a Retrospective Cohort Study. Onco Targets Ther (2020) 13:5047–55. doi: 10.2147/OTT.S254514
16. Huang Y, Cen J, Wei J, Chen Z, Fang Y, Feng Z, et al. Impact of AIB1 expression on the prognosis of upper tract urothelial carcinoma after radical nephroureterectomy. Cancer Biomark (2019) 25(2):151–60. doi: 10.3233/CBM-182020
17. Itami Y, Miyake M, Tatsumi Y, Gotoh D, Hori S, Morizawa Y, et al. Preoperative predictive factors focused on inflammation-, nutrition-, and muscle-status in patients with upper urinary tract urothelial carcinoma undergoing nephroureterectomy. Int J Clin Oncol (2019) 24(5):533–45. doi: 10.1007/s10147-018-01381-y
18. Freifeld Y, Ghandour R, Singla N, Woldu S, Clinton T, Kulangara R, et al. Preoperative predictive model and nomogram for disease recurrence following radical nephroureterectomy for high grade upper tract urothelial carcinoma. Urol Oncol (2019) 37(10):758–64. doi: 10.1016/j.urolonc.2019.06.009
19. Son S, Hwang EC, Jung SI, Kwon DD, Choi SH, Kwon TG, et al. Prognostic value of preoperative systemic inflammation markers in localized upper tract urothelial cell carcinoma: a large, multicenter cohort analysis. Minerva Urol Nefrol (2018) 70(3):300–9. doi: 10.23736/S0393-2249.18.02914-4
20. Tan P, Xie N, Liao H, Zou L, Xu H, Yang L, et al. Prognostic impact of preoperative anemia on upper tract urothelial carcinoma. Med (Baltimore) (2018) 97(37):e12300. doi: 10.1097/MD.0000000000012300
21. Kohada Y, Hayashi T, Goto K, Kobatake K, Abdi H, Honda Y, et al. Preoperative risk classification using neutrophil-lymphocyte ratio and hydronephrosis for upper tract urothelial carcinoma. Jpn J Clin Oncol (2018) 48(9):841–50. doi: 10.1093/jjco/hyy084
22. Fang D, He S, Xiong G, Singla N, Cao Z, Zhang L, et al. Comparison of clinicopathologic characteristics, epigenetic biomarkers and prognosis between renal pelvic and ureteral tumors in upper tract urothelial carcinoma. BMC Urol (2018) 18(1):22. doi: 10.1186/s12894-018-0334-7
23. Jan HC, Yang WH, Ou CH. Combination of the Preoperative Systemic Immune-Inflammation Index and Monocyte-Lymphocyte Ratio as a Novel Prognostic Factor in Patients with Upper-Tract Urothelial Carcinoma. Ann Surg Oncol (2019) 26(2):669–84. doi: 10.1245/s10434-018-6942-3
24. Kim JK, Moon KC, Jeong CW, Kwak C, Kim HH, Ku JH, et al. Variant histology as a significant predictor of survival after radical nephroureterectomy in patients with upper urinary tract urothelial carcinoma. Urol Oncol (2017) 35(7):458.e9–458.e15. doi: 10.1016/j.urolonc.2017.02.010
25. Liu JY, Dai YB, Zhou FJ, Long Z, Li YH, Xie D, et al. Laparoscopic versus open nephroureterectomy to treat localized and/or locally advanced upper tract urothelial carcinoma: oncological outcomes from a multicenter study. BMC Surg (2017) 17(1):8. doi: 10.1186/s12893-016-0202-x
26. Nakagawa T, Komemushi Y, Kawai T, Otsuka M, Miyakawa J, Uemura Y, et al. Efficacy of post-nephroureterectomy cisplatin-based adjuvant chemotherapy for locally advanced upper tract urothelial carcinoma: a multi-institutional retrospective study. World J Urol (2017) 35(10):1569–75. doi: 10.1007/s00345-017-2032-6
27. Lee CH, Ku JY, Jeong CW, Ku JH, Kwak C, Kim HH, et al. Predictors for Intravesical Recurrence Following Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A National Multicenter Analysis. Clin Genitourin Cancer (2017) 15(6):e1055–61. doi: 10.1016/j.clgc.2017.07.009
28. Liang C, Chi R, Huang L, Wang J, Liu H, Xu D, et al. Upper Tract Urothelial Carcinomas Accompanied by Previous or Synchronous Nonmuscle-Invasive Bladder Cancer and Preoperative Hydronephrosis Might Have Worse Oncologic Outcomes After Radical Nephroureterectomy. Clin Genitourin Cancer (2016) 14(5):e469–77. doi: 10.1016/j.clgc.2016.02.008
29. Song X, Zhang GM, Ma XC, Luo L, Li B, Chai DY, et al. Comparison of preoperative neutrophil-lymphocyte, lymphocyte-monocyte, and platelet-lymphocyte ratios in patients with upper urinary tract urothelial carcinoma undergoing radical nephroureterectomy. Onco Targets Ther (2016) 9:1399–407. doi: 10.2147/OTT.S97520
30. Fradet V, Mauermann J, Kassouf W, Rendon R, Jacobsen N, Fairey A, et al. Risk factors for bladder cancer recurrence after nephroureterectomy for upper tract urothelial tumors: results from the Canadian Upper Tract Collaboration. Urol Oncol (2014) 32(6):839–45. doi: 10.1016/j.urolonc.2014.04.006
31. Lee JN, Kwon SY, Choi GS, Kim HT, Kim TH, Kwon TG, et al. Impact of surgical wait time on oncologic outcomes in upper urinary tract urothelial carcinoma. J Surg Oncol (2014) 110(4):468–75. doi: 10.1002/jso.23589
32. Zou L, Zhang L, Zhang H, Jiang H, Ding Q. Comparison of post-operative intravesical recurrence and oncological outcomes after open versus laparoscopic nephroureterectomy for upper urinary tract urothelial carcinoma. World J Urol (2014) 32(2):565–70. doi: 10.1007/s00345-013-1160-x
33. Aziz A, Fritsche HM, Gakis G, Kluth LA, Hassan FA, Engel O, et al. Comparative analysis of comorbidity and performance indices for prediction of oncological outcomes in patients with upper tract urothelial carcinoma who were treated with radical nephroureterectomy. Urol Oncol (2014) 32(8):1141–50. doi: 10.1016/j.urolonc.2014.04.008
34. Zhang X, Zhu Z, Zhong S, Xu T, Shen Z. Ureteral tumours showing a worse prognosis than renal pelvis tumours may be attributed to ureteral tumours more likely to have hydronephrosis and less likely to have haematuria. World J Urol (2013) 31(1):155–60. doi: 10.1007/s00345-012-0885-2
35. Verhoest G, Shariat SF, Chromecki TF, Raman JD, Margulis V, Novara G, et al. Predictive factors of recurrence and survival of upper tract urothelial carcinomas. World J Urol (2011) 29(4):495–501. doi: 10.1007/s00345-011-0710-3
36. Wu Y, Dong Q, Liu L, Han P, Wei Q. The impact of tumor location and multifocality on prognosis for patients with upper tract urothelial carcinoma: a meta-analysis. Sci Rep (2014) 4:6361. doi: 10.1038/srep06361
37. Fan B, Hu B, Yuan Q, Wen S, Liu T, Bai S, et al. Impact of tumor architecture on disease recurrence and cancer-specific mortality of upper tract urothelial carcinoma treated with radical nephroureterectomy. Tumour Biol (2017) 39(7):1010428317710822. doi: 10.1177/1010428317710822
38. Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester RJ, Burger M, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update. Eur Urol (2018) 73(1):111–22. doi: 10.1016/j.eururo.2017.07.036
39. Scolieri MJ, Paik ML, Brown SL, Resnick MI. Limitations of computed tomography in the preoperative staging of upper tract urothelial carcinoma. Urology (2000) 56(6):930–4. doi: 10.1016/S0090-4295(00)00800-1
40. Ito Y, Kikuchi E, Tanaka N, Miyajima A, Mikami S, Jinzaki M, et al. Preoperative hydronephrosis grade independently predicts worse pathological outcomes in patients undergoing nephroureterectomy for upper tract urothelial carcinoma. J Urol (2011) 185(5):1621–6. doi: 10.1016/j.juro.2010.12.035
41. Cho KS, Hong SJ, Cho NH, Choi YD. Grade of hydronephrosis and tumor diameter as preoperative prognostic factors in ureteral transitional cell carcinoma. Urology (2007) 70(4):662–6. doi: 10.1016/j.urology.2007.06.1106
42. Messer JC, Terrell JD, Herman MP, Ng CK, Scherr DS, Scoll B, et al. Multi-institutional validation of the ability of preoperative hydronephrosis to predict advanced pathologic tumor stage in upper-tract urothelial carcinoma. Urol Oncol (2013) 31(6):904–8. doi: 10.1016/j.urolonc.2011.07.011
43. Kim DS, Cho KS, Lee YH, Cho NH, Oh YT, Hong SJ. High-grade hydronephrosis predicts poor outcomes after radical cystectomy in patients with bladder cancer. J Korean Med Sci (2010) 25(3):369–73. doi: 10.3346/jkms.2010.25.3.369
44. Resorlu B, Baltaci S, Resorlu M, Ergun G, Abdulmajeed M, Haliloglu AH, et al. Prognostic significance of hydronephrosis in bladder cancer treated by radical cystectomy. Urol Int (2009) 83(3):285–8. doi: 10.1159/000241668
45. Ouzzane A, Colin P, Xylinas E, Pignot G, Ariane MM, Saint F, et al. Ureteral and multifocal tumours have worse prognosis than renal pelvic tumours in urothelial carcinoma of the upper urinary tract treated by nephroureterectomy. Eur Urol (2011) 60(6):1258–65. doi: 10.1016/j.eururo.2011.05.049
46. Chung PH, Krabbe LM, Darwish OM, Westerman ME, Bagrodia A, Gayed BA, et al. Degree of hydronephrosis predicts adverse pathological features and worse oncologic outcomes in patients with high-grade urothelial carcinoma of the upper urinary tract. Urol Oncol (2014) 32(7):981–8. doi: 10.1016/j.urolonc.2014.02.018
Keywords: preoperative hydronephrosis, prognostic value, upper tract urinary carcinoma, radical nephroureterectomy, meta-analysis
Citation: Ye T, Yang X, Lv P, Liu H and Ye Z (2020) Prognostic Value of Preoperative Hydronephrosis in Patients Undergoing Radical Nephroureterectomy for Upper Tract Urinary Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 10:600511. doi: 10.3389/fonc.2020.600511
Received: 30 August 2020; Accepted: 27 October 2020;
Published: 11 December 2020.
Edited by:
Marco Borghesi, University of Genoa, ItalyReviewed by:
Andrea Mari, University of Florence, ItalyGuru Sonpavde, Dana–Farber Cancer Institute, United States
Copyright © 2020 Ye, Yang, Lv, Liu and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhangqun Ye, emhhbmdxdW5feWVAMTYzLmNvbQ==; orcid.org/0000-0003-4492-8399
 Tao Ye
Tao Ye Xiaoqi Yang
Xiaoqi Yang Zhangqun Ye
Zhangqun Ye