- 1State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China
- 2Department of Gastric Surgery, Sun Yat-sen University Cancer Center, Guangzhou, China
- 3Department of Medical Melanoma and Sarcoma, Sun Yat-sen University Cancer Center, Guangzhou, China
Objective: The purpose of this retrospective study was to identify the prognostic significance of time to local recurrence (TLR) with regard to overall survival (OS) and survival after local recurrence (SAR) in patients with soft tissue sarcoma (STS) of the extremity and abdominothoracic wall.
Methods: We identified 477 patients who underwent R0 resection for localized STS of the extremity and abdominothoracic wall, from January 1995 to December 2016, of whom 190 patients developed local recurrence as their first recurrent event. Based on TLR, patients were divided into two groups: early local recurrence (ELR, <12 months) and late local recurrence (LLR, ≥12 months). The Kaplan–Meier method and Cox regression analysis were used to estimate the OS and SAR, and to identify factors associated with patient outcomes.
Results: The median follow-up time for the entire cohort was 118.4 months, and was 118.5 months for the 190 patients who developed local recurrence. Deep tumor location (HR 1.73, 95% CI 1.27–2.37, P = 0.001) and tumor grade ≥2 (G2 vs. G1: HR 1.75, 95% CI 1.21–2.53, G3 vs. G1: HR 2.57, 95% CI 1.66–3.98, P < 0.001) were associated with a higher rate of local recurrence. There were 99 patients in the ELR group and 91 in the LLR group, with a median TLR of 10.8 months for the entire cohort. Patients from the ELR group had a shorter OS and a lower 5-year OS rate than the LLR group. Univariate and multivariate analyses demonstrated TLR as an independent prognostic factor for SAR and OS, in addition to tumor grade. Also, surgical treatment and absence of metastasis after local recurrence were associated with longer SAR.
Conclusions: In patients with STS of the extremity and abdominothoracic wall, ELR after R0 resection indicated a worse prognosis than those with LLR, and TLR can be considered an independent prognostic factor for OS and SAR. Furthermore, local recurrence was significantly influenced by the depth and the histopathological grading of the primary tumor, and reoperation after local recurrence could improve survival, which means salvage surgery may still be the preferred treatment when there are surgical indications after recurrence.
Introduction
Soft tissue sarcomas (STSs) are a heterogeneous group of malignancies with a low incidence, accounting for approximately 1% of all adult malignancies (1). STSs may arise in different body sites, including the head or neck, extremity, trunk, retroperitoneum, or chest wall, with local aggressiveness. Among all of STS, about 80% of tumors locate in the extremities and superficial trunk. There are more than 50 different histologic subtypes identified, each with distinct biologic behavior and clinical manifestation. The anatomic sites and pathologic subtypes of these tumors are crucial for their treatments and outcomes. Despite the established role of radical or wide surgical resection as a standard of treatment, 15%–40% of patients with localized STS tumors develop recurrence and have a dismal 5-year survival rate ranging between 55% and 70% (2, 3). Thus, tumor local relapse remains one of the major problems in managing STS, and can be defined as early or late recurrence. In breast adenocarcinoma, renal cell carcinoma, and gastric cancer, it was previously reported that patients with late recurrence had better prognosis than those with early recurrence (4–6). However, to the best of our knowledge, neither significant factors affecting the survival after recurrence (SAR) for STS patients nor information concerning the prognostic significance of time to local recurrence (TLR) in STS patients have been reported.
Therefore, we performed this retrospective study to determine the clinicopathological factors affecting local recurrence (LR), and the prognostic significance of TLR, with regard to overall survival (OS) and SAR, in patients with STS of the extremity and abdominothoracic wall.
Methods
Study Population
The data of 769 patients who underwent R0 resection for primary STS at the Sun Yat-sen University Cancer Center (SYSUCC, Guangzhou, China), from January 1995 to December 2016, were retrieved. As there is no clear standard for defining radical or extensive resection of STS, due to the existing different tumor types, tumor volume, and location, here, we used the standardized classifications (R0, R1, R2) of the International Union Against Cancer (UICC) for surgery to classify the radicality of the surgical resections performed (7). R0 was defined as the microscopic absence of malignant cells at the resection margin. Patients with R1 or R2 resection were excluded as they comprised of a very small proportion of the retrieved cases. Seventy-seven of the 769 (10%) patients were lost to follow-up and were excluded. Patients with inadequate medical records (5 patients) and distant metastasis at the time of initial diagnosis (82 patients) were also excluded. Although the proportion is low, patients who received the adjuvant treatments (23.9%), including chemotherapy (mostly doxorubicin-based), radiotherapy, or chemoradiotherapy, were included for the analysis. All adjuvant treatments were planned based on the patients’ disease stage and willingness to abide to treatment, and the regimen prescribed was based on the treating oncologist’s discretion. Finally, 477 patients were included in this study (Additional File 1: Figure S1).
Local recurrence was defined as tumor relapse in the operative field following R0 resection according to follow-up radiographic evidence, physical exam, or self-reported symptoms. Among the 477 patients, 190 patients were diagnosed with local recurrence as their first recurrent event, which was then histologically confirmed. Most of the patients with local recurrences underwent secondary resection, except for a small percentage of patients who received chemotherapy (n = 6) or radiotherapy (n = 1) only. The 190 patients were then classified into two groups according to their TLR, which was calculated from the date of R0 resection to the date of initial local recurrence. Patients who were diagnosed with TLR within 12 months (n = 99) were grouped into an early local recurrence (ELR) group while those diagnosed with TLR no less than 12 months (n = 91) were included in a late local recurrence (LLR) group. As there is no standard definition for early and late local recurrence, the 12 months cutoff value was determined based on published literatures (8, 9).
This study was approved by the institutional review board of SYSUCC (No. B2020-008-01), and the ethics committee waived the need for informed consent as this was retrospective study. All patients’ data used was anonymously analyzed.
Data Collection
Clinical and pathological data of the included patients were retrospectively obtained from the patient’s medical records. Tumor stage was classified using the AJCC 8th Edition (10), and the tumors were graded according to the Fédération Française des Centres de Lutte Contre le Cancer (FNCLCC) grading system (11).
The authenticity of this article was validated by uploading the key raw data to the Research Data Deposit public platform (www.researchdata.org.cn) with the approval RDD number of RDDA2019001332.
Follow-Up
All patients were routinely followed with physical examination, computerized tomography or magnetic resonance imaging every 3 to 6 months for the first 2 years after resection, then annually via outpatient visits or telephone interviews by the independent follow-up department of SYSUCC. The minimum follow-up time was 6 months. The final survival follow-up time was considered the latest follow-up date of this study (October 1, 2019) or death. OS was defined as the time between the R0 resection and death of any cause or the last follow-up. SAR was defined as the time from the date of diagnosis of local recurrence to the last follow-up date or the date of death.
Statistical Analysis
The chi-square test of independence was used to test the distributive correlations between the clinicopathological variables and local recurrence. Survival curves were analysed by Kaplan-Meier method, and differences between survival rates were compared by using the log-rank test (12). The Cox proportional hazard model with the stepwise forward selection algorithm was used to find out independent prognostic variables associated with LR, OS, and SAR, and the results are presented as hazard ratios (HR) and 95% confidence intervals (95% CI). Two-sided P values < 0.05 were considered statistically significant. All data were analyzed using the IBM SPSS software, version 20.0 (SPSS, Inc., and IBM Company, Armonk, New York).
Results
Baseline Patient Characteristics
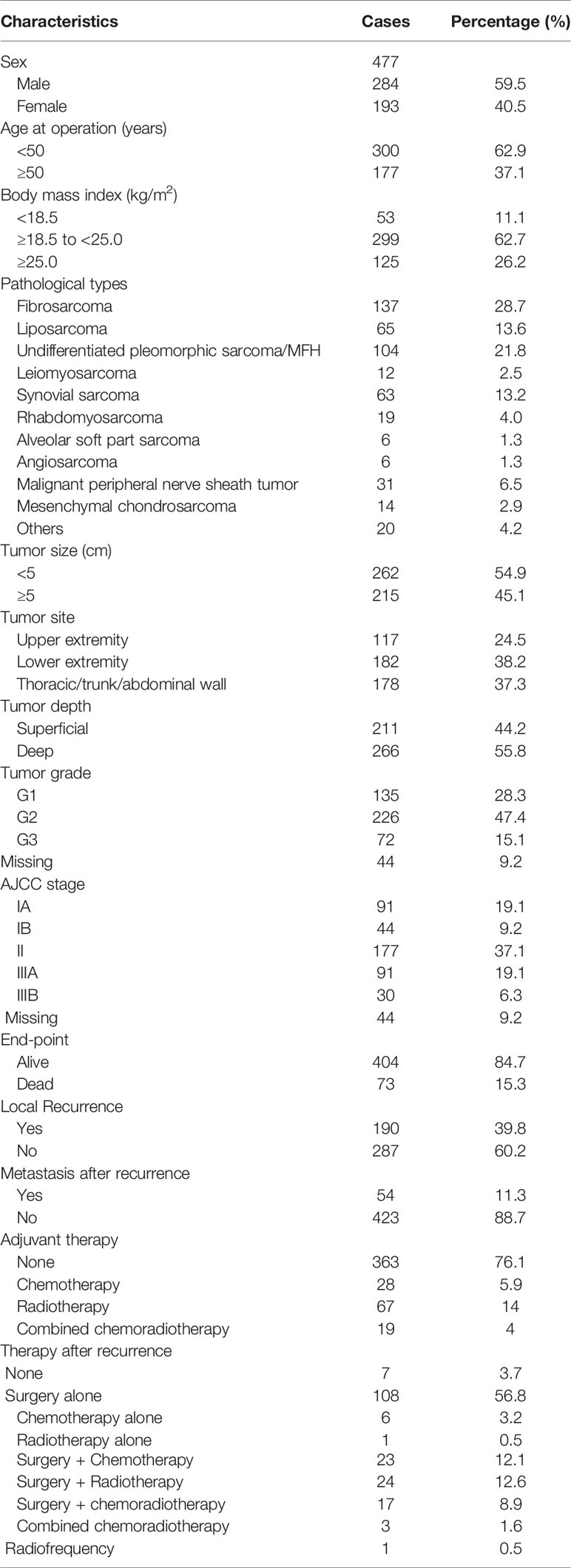
The patients’ baseline characteristics are shown in Table 1. The median age of the 477 patients was 42 years (range: 6–85 years). There were 284 male patients and 193 female patients in a ratio of 1.47:1. Fibrosarcoma (137, 28.7%) and undifferentiated pleomorphic sarcoma (104, 21.8%) were the most common pathological types. G1 tumors were identified in 135 (28.3%) patients, G2 in 226 (47.4%) patients, and G3 in 72 (15.1%) patients. Most patients had stage II disease (177, 37.1%). In addition, 135 (28.3%) patients had stage I disease and 121 (25.4%) had stage III disease. Due to the lack of understanding of the disease and standard treatment, only a small percentage of the STS patients received postoperative therapy, including chemotherapy (28, 5.9%), radiotherapy (67,14.0%) and chemoradiotherapy (19, 4.0%), spanning a period of 21 years. By comparisons, patients with deep tumor depth, G2-G3 tumor grade and II-III AJCC stage are more likely to receive adjuvant therapy (all P < 0.001).
Local Recurrence Rate and Influencing Factors
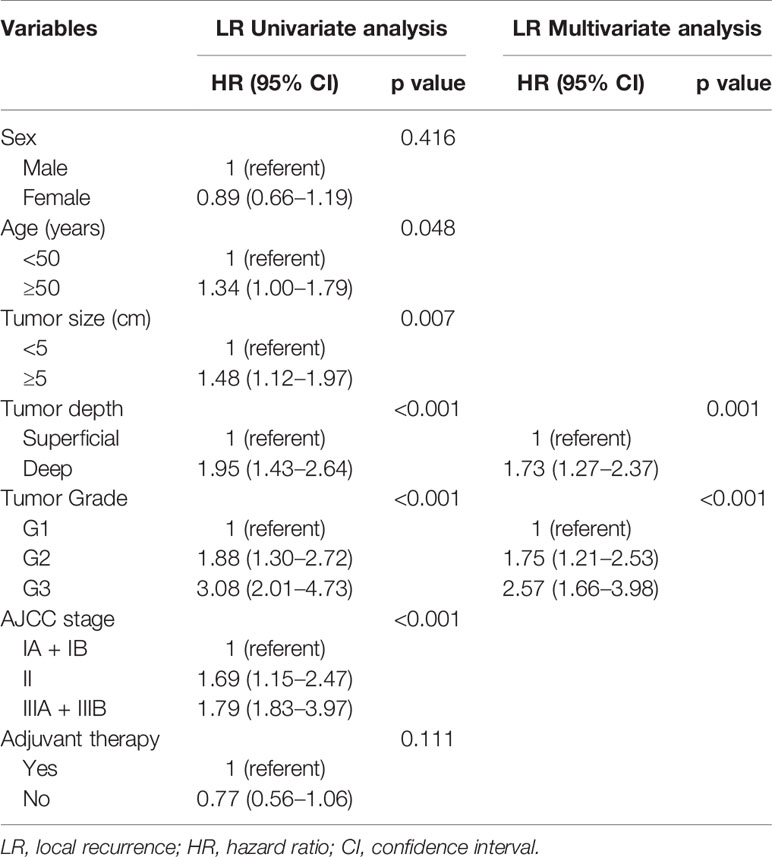
Over a median follow-up time of 118.4 months (range 9.6–368.8 months), 73 (15.3%) patients died, and 190 (39.8%) experienced local recurrence. Fifty-four (28.4%) of the 190 patients with local recurrence developed distant metastasis. A total of 46 patients had grade 3 sarcomas, 105 had grade 2, and 39 had grade 1. In 61 patients the depth of the tumor was superficial and in 129 it was deep. The recurrence rates observed in patients classified as stage I, II, and III were 29.6% (40/135), 44.1% (78/177), and 59.5% (72/121), respectively. However, there were no differences in the incidence of local recurrence between the patients with or without postoperative treatments (P = 0.096), and this might be on account of the small sample. Histological subtype (e.g., fibrosarcoma, undifferentiated pleomorphic sarcoma, liposarcoma and synovial sarcoma) did not affect the local recurrence in this study. Furthermore, the 5- and 10-year OS rates of patients who did not develop local recurrence were significantly higher than those who developed local recurrence (97.9% vs. 75.7%; 96.6% vs. 63.4%; p < 0.001; Figure 1A). Deep tumor location (deep vs. superficial: HR 1.73, 95% CI 1.27–2.37, P = 0.001) and tumor grade ≥ 2 (G2 vs. G1: HR 1.75, 95% CI 1.21–2.53, G3 vs. G1: HR 2.57, 95% CI 1.66–3.98, P < 0.001) were significantly associated with a higher rate of local recurrence (Table 2).
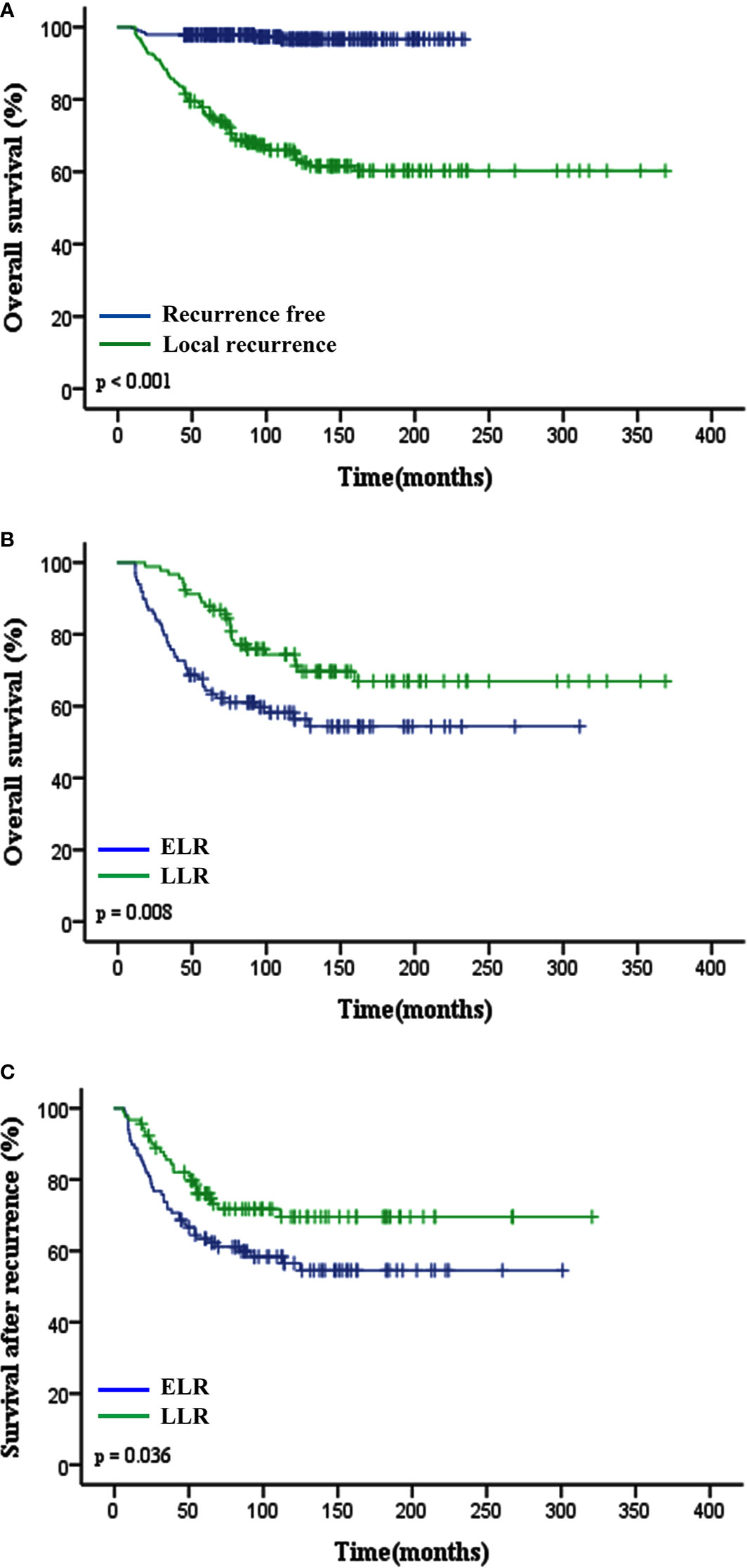
Figure 1 Impact of local recurrence and TLR (ELR vs. LLR) on clinical outcomes of patients with STS of extremity and abdominothoracic wall. (A) Overall survival in recurrent free and local recurrent patients (p < 0.001). (B) Overall survival (p = 0.008) and (C) Survival after recurrence (p = 0.036) curves showed that patients in the ELR group had a worse prognosis than those in the LLR group. TLR, time to local recurrence; ELR, early local recurrence (<12 months after primary surgery); LLR, late local recurrence (≥12 months after primary surgery).
Association Between TLR and Survival
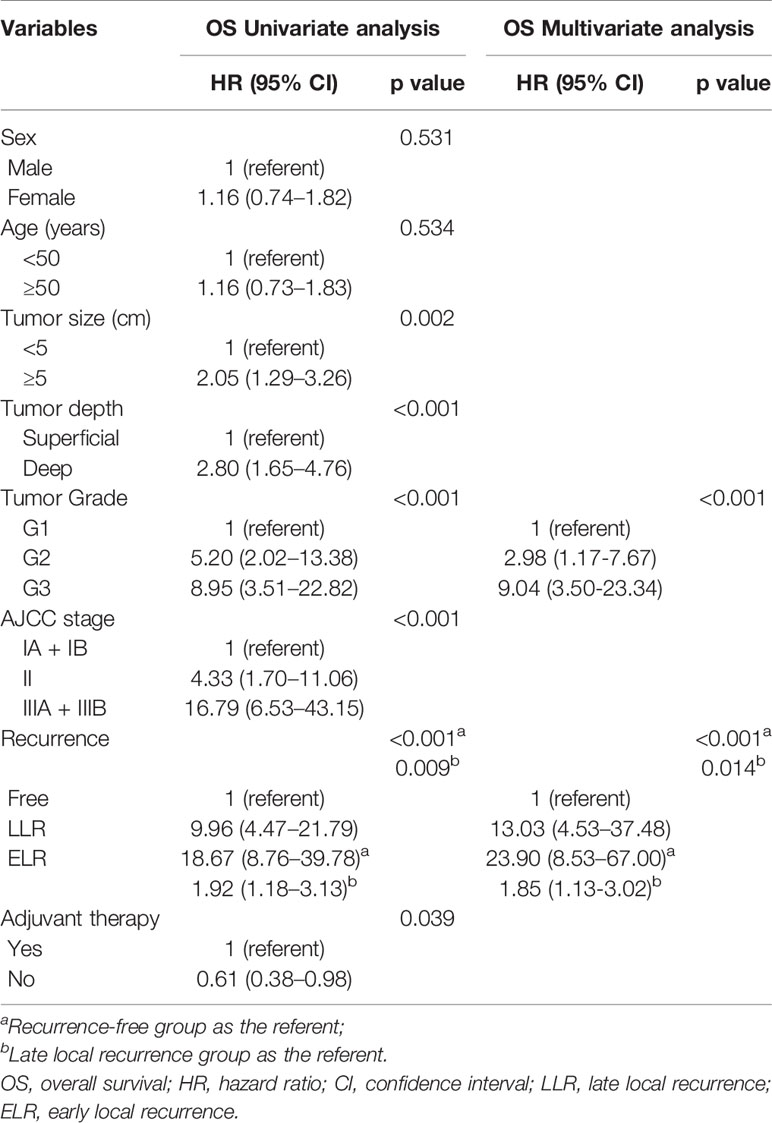
The median TLR was 10.8 months (range 1.4–190.7 months). Patients in the ELR group had a shorter median OS time and lower 5-year OS rate than those in the LLR group (P = 0.008; 64.4% vs. 87.9%, P < 0.001; Figure 1B). Patients with LLR had a longer SAR than patients with ELR (P = 0.036; Figure 1C).
To determine the factors affecting the prognosis of ELR and LLR patients, the prognostic relevance of TLR and the patients’ clinicopathological parameters were analyzed using the Cox proportional hazards model (Figure 2). Our results demonstrated that LLR patients had better OS than ELR patients in both gender (male, female), the presence of metastasis, and the performance of surgery after local recurrence. Furthermore, there were significant differences in OS between the two groups for patients with tumor grade ≥ 2 (G2: HR 2.21, 95% CI 1.02–4.79, P = 0.044; G3: HR 2.14, 95% CI 1.01–4.54, P = 0.047), with stage III disease (HR 2.99, 95% CI 1.40–6.38, P = 0.005), and without adjuvant therapy after initial R0 surgery (HR 3.01, 95% CI 1.55-5.84, P = 0.001) (Figure 2A).
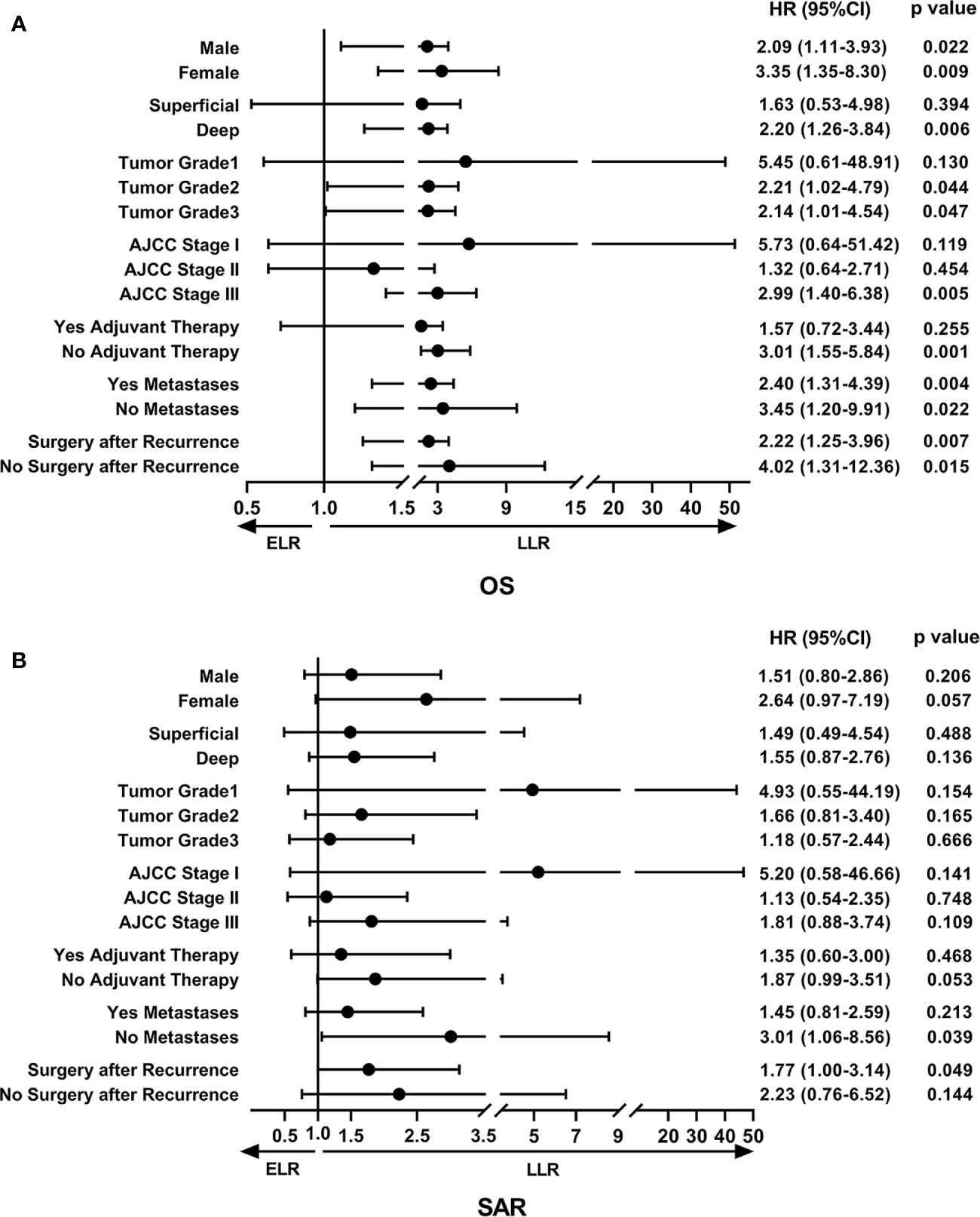
Figure 2 The forest plot of prognostic relevance of TLR and relevant clinicopathological parameters using the Cox proportional hazards model. (A) Overall survival curve showed that there were significant differences between the ELR and LLR groups for patients with tumor grade ≥ 2, stage III disease and without adjuvant therapy after initial R0 surgery. (B) Survival after recurrence curve showed that patients without metastases or with surgery after local recurrence in the LLR group exhibited a better SAR than those in the ELR group. OS, overall survival; SAR, survival after recurrence; ELR, early local recurrence (<12 months after primary surgery); LLR, late local recurrence (≥12 months after primary surgery); HR, hazard ratio; CI, confidence interval.
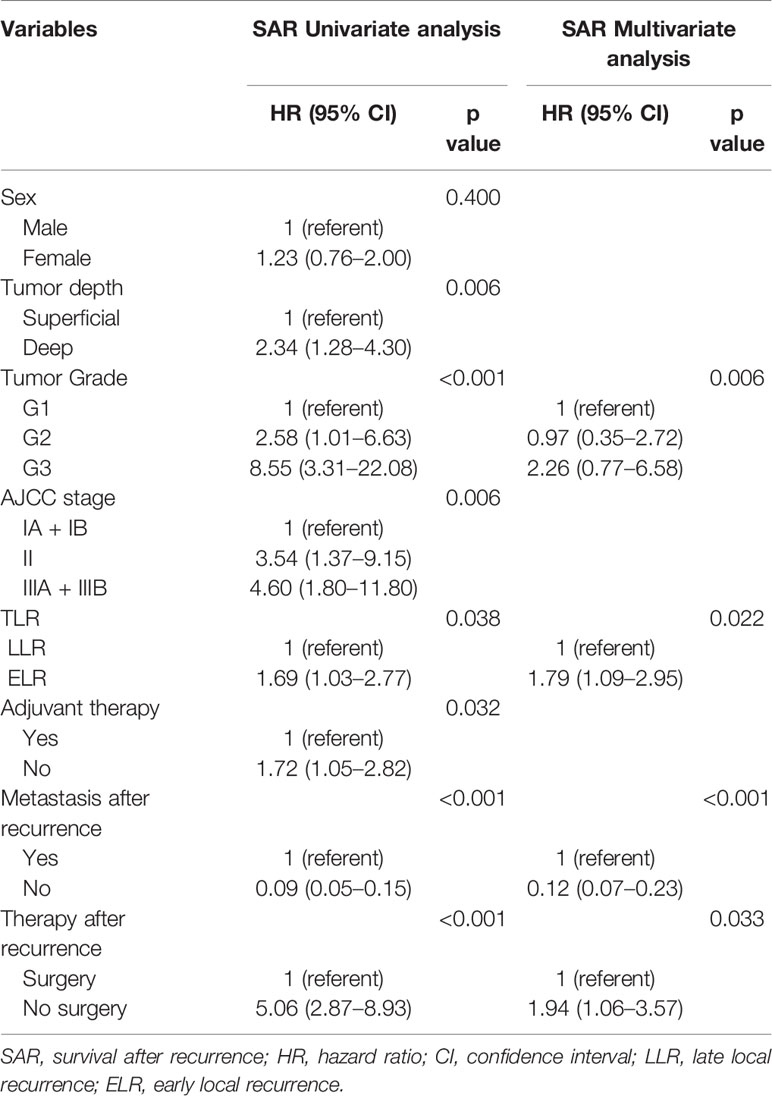
In addition, there were no statistically significant differences in SAR between the two groups regardless of sex, tumor depth, tumor grade, AJCC stage, and adjuvant therapies. However, it was worth noting that patients without metastases (HR 3.01, 95% CI 1.06–8.56, P = 0.039) or with surgery (HR 1.77, 95% CI 1.00–3.14, P = 0.015) after local recurrence in the LLR group exhibited a better SAR than those in the ELR group (Figure 2B).
Multivariate analyses revealed that TLR and tumor grade were independent prognostic factors for both OS (P = 0.014, P < 0.001) and SAR (P = 0.006, P = 0.022). Moreover, for the 190 patients with local recurrence, non-surgical treatment and metastases after recurrence were negative prognostic factors for SAR, with HRs of 1.94 (95% CI 1.06–3.57, P = 0.033) and 0.12 (95% CI 0.07–0.23, P < 0.001), respectively (Tables 3 and 4).
Discussion
Local recurrence is a common reason for treatment failure after R0 surgery in STS (13). To assess the effects of local recurrence and other clinicopathological factors on survival, especially TLR, in patients with STS of the extremity and abdominothoracic wall, we performed a retrospective study based on data from 477 patients from the SYSUCC. This study is—to our best knowledge—the largest study to analyze the association between the TLR and survival in patients with STS.
In this study, a high local recurrence rate was found in STS (n = 190, 39.8%), which was slightly above other relevant literatures (14–16). There can be several reasons for this. First, although the tumor treatment condition has been improved in the past years in China, patients usually come to the hospital in a comparatively later stage or when their symptoms have been aggravated. Most of the patients included in this study had advanced tumor grade (G2-G3) or stage (II-III) with deep location at the time of initial diagnosis. And also, the percentage (only 23.9%) of patients who received postoperative adjuvant treatment was low due to clinical, financial, or personal reasons. Second, all of the patients included in this study had received the R0 resection, but the distance between the tumor and the surgical margins was not clear completely owing to the long retrospective span. As we all know that those patients who presented with a surgical margin of 2 mm or less might have a worse survival and a higher local recurrence rate (9). Moreover, this article involved a cohort of patients with long follow-up time (some for more than 15 years), based on which the risk for local recurrence was observed to increase accordingly.
Tumor recurrence is a well-known factor for poor prognosis of STS. Zhao et al. (17) and Eilber et al. (18) reported respectively in 133 and 753 STS patients groups that there was a lower 5-year OS rate in the local recurrence group than in the no local recurrence group. Posch et al. (19) observed that patients with local recurrence were more likely to develop distant metastasis (HR = 8.4; 95% CI, 4.3–16.5; P < 0.001). Another study demonstrated that 17% of patients with extremity STS after R1 resection died of local recurrence without any distant metastasis (20). All of these studies illustrated that local recurrence had a negative effect on the survival of patients with STS, which was in accordance with our study findings.
Some investigators reported that prognostic factors such as surgical margin and location played an important role in local control and were associated with the local recurrence in STS (15, 21, 22). In addition, high tumor grade, larger tumor size, and deep tumor location were also considered as predictors of local recurrence in STS (17, 19, 23, 24). Consistent with other studies (20), tumor depth and tumor grade were identified as significant prognostic factors affecting local recurrence by multivariate analysis in our study. Since early diagnosis of STS recurrence is important to offer the patient a realistic second treatment chance, an adequate identification of patients at higher risk, those with a deep tumor location and higher tumor grade, can promote the development of individualized surveillance programs. These patients may require more extensive resection and closer postoperative follow-up, and may be considered for additional preoperative therapy or more intense adjuvant chemotherapy to reduce the risk of recurrence. However, AJCC stage were not independent predictors of local recurrence, OS and SAR in our analysis, which is similar to a previous large-scale study (25). This could be due to the staging defects of human subjectivity and the heterogeneity of STS. The 8th AJCC stage system illustrated an unprecedented change for risk stratification by redefining the T-stage categories (26), which disregarding the independent prognostic information provided by tumor depth. Superficial tumors are associated with better outcomes than deep ones, even after controlling for tumor size and histologic grade (27, 28). Our data suggest that the system still needs further investigation to improve risk stratification.
TLR as a predictior for survival in patients with various cancers has been researched with divergent results. Several studies have suggested that TLR is a prognostic factor for survival in primary breast sarcoma, renal cell carcinoma and gastric cancer (4, 6, 29), while others have not found TLR of significant importance (8, 30). Sugiura et al. found the survival rate was lower in STS patients with local recurrence developing within 2 years than after 2 years (46% vs. 83%, P = 0.01) (31). Our study confirmed that TLR in patients with STS of the extremity and abdominothoracic wall was associated with survival and was considered an independent prognostic factor for OS and SAR, and patients with ELR (TLR within 12 months) indicated worse prognosis compared with those with LLR (TLR no less than 12 months). In addition, our research included 54 patients who developed distant metastasis after the local recurrence. We found that patients without metastases after local recurrence in the LLR group also exhibited a better SAR than those in the ELR group, but there was no difference in patients with distant metastases between two groups. The study from Posch (19) demonstrated that patients who suffered a local recurrence were more likely to develop distant matastasis and patients with distant metastasis after a long tumor-free interval did not show a better survival prognosis compared to those with distant metastasis occurring early after primary surgery, which had similar views with our research.
Moreover, though there is no concrete proof, it is generally accepted that STS patients with local recurrence need another resection with a goal of negative margins. A previous study reported that local recurrence in retroperitoneal STS patients was amenable to surgery, which could improve survival (32). Our results confirmed that operation after recurrence was also strongly associated with better SAR in STS of the extremity and abdominothoracic wall, indicating that salvage surgery may still be the preferred treatment when there are surgical indications after recurrence. This observation supports the mainstream view nowadays.
There were some limitations of the analyses in this study that should be noted. First, this was a retrospective study which could have inherent sources of transfer bias (i.e., loss to follow-up) and selection bias (i.e., clinical decision based on economic condition by patients), and we enrolled consecutive patients to reduce the influence of possible selection bias. Second, all patients enrolled in this study were selected from one hospital, the SYSUCC and therefore, the investigated patients’ characteristics and the study results may not be generalizable to other populations. Our conclusions should be verified in a larger population of STS patients from multiple centers. In addition, the clinicopathological data of some patients, such as data on AJCC stage in 44 patients, and the details of adjuvant therapy, were incomplete owing to the huge spans of time, thus we were unable to provide more information about the effects of the chemotherapy and/or radiotherapy. It seems reasonable that tumors with different subtypes may exhibit different clinical behaviors and altered survivals, and this is a topic that requires further investigation to figure out the relationship between the histologic subtypes and local recurrence.
Despite these limitations, this is the largest study based on a heterogeneous group of patients to demonstrate the prognostic values of TLR in STS of the extremity and abdominothoracic wall, the conclusions postulated remain highly reasonable.
Conclusion
Our results showed that local recurrence was significantly associated with a decreased OS in patients with STS of the extremity and abdominothoracic wall, and those with deeply located initial tumor or a higher tumor grade were more likely to experience local recurrence than their counterparts. Surgery after local recurrence could prolong the OS and SAR of the patients as compared to other treatments. Furthermore, ELR after R0 resection indicated a worse prognosis than those with LLR, and TLR can be considered an independent prognostic factor for OS and SAR. If substantiated in a larger, multicenter study, the observations from this pilot study might provide the rationale to develop individualized surveillance programs for the patients at higher risk, providing an earlier diagnosis and better second treatment chance in the case of a recurrence.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the institutional review board of Sun Yat-sen University Cancer Center. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s) and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
YL, TG, and DH collected the patients’ data. YL and TG did the data analysis. All authors designed the study, and YL, TG, and DH finished the original manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the National Natural Science Foundation of China (no. 81902736).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.599097/full#supplementary-material
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: A Cancer J Clin (2018) 68(1):7–30. doi: 10.3322/caac.21442
2. Kraybill WG, Harris J, Spiro IJ, Ettinger DS, DeLaney TF, Blum RH, et al. Long-term results of a phase 2 study of neoadjuvant chemotherapy and radiotherapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. Cancer (2010) 116(19):4613–21. doi: 10.1002/cncr.25350
3. Chowdhary M, Chowdhary A, Sen N, Zaorsky NG, Patel KR, Wang D. Does the addition of chemotherapy to neoadjuvant radiotherapy impact survival in high-risk extremity/trunk soft-tissue sarcoma? Cancer (2019) 125(21):3801–9. doi: 10.1002/cncr.32386
4. Hu Q-C, Mei X, Feng Y, Ma J-L, Yang Z-Z, Shao Z-M, et al. Early Local Recurrence Presents Adverse Effect on Outcomes of Primary Breast Sarcoma. Medicine (2016) 95(1):e2422. doi: 10.1097/md.0000000000002422
5. Rieken M, Kluth LA, Fajkovic H, Capitanio U, Briganti A, Krabbe LM, et al. Predictors of Cancer-specific Survival After Disease Recurrence in Patients With Renal Cell Carcinoma: The Effect of Time to Recurrence. Clin Genitourin Cancer (2018) 16(4):e903–e8. doi: 10.1016/j.clgc.2018.03.003
6. Li H, Jin X, Liu P, Hong W. Time to local recurrence as a predictor of survival in unrecetable gastric cancer patients after radical gastrectomy. Oncotarget (2017) 8(51):89203–13. doi: 10.18632/oncotarget.19038
7. Wittekind C, Compton CC, Greene FL, Sobin LH. TNM residual tumor classification revisited. Cancer (2002) 94(9):2511–6. doi: 10.1002/cncr.10492.abs
8. Westberg K, Palmer G, Johansson H, Holm T, Martling A. Time to local recurrence as a prognostic factor in patients with rectal cancer. Eur J Surg Oncol (2015) 41(5):659–66. doi: 10.1016/j.ejso.2015.01.035
9. Novais EN, Demiralp B, Alderete J, Larson MC, Rose PS, Sim FH. Do surgical margin and local recurrence influence survival in soft tissue sarcomas? Clin Orthop Relat Res (2010) 468(11):3003–11. doi: 10.1007/s11999-010-1471-9
10. Cates JMM. AJCC eighth edition for soft tissue sarcoma of the extremities and trunk. Ann Oncol Off J Eur Soc Med Oncol (2018) 29(9):2023. doi: 10.1093/annonc/mdy247
11. Neuville A, Chibon F, Coindre JM. Grading of soft tissue sarcomas: from histological to molecular assessment. Pathology (2014) 46(2):113–20. doi: 10.1097/PAT.0000000000000048
12. In J, Lee DK. Survival analysis: Part I - analysis of time-to-event. Korean J Anesthesiol (2018) 71(3):182–91. doi: 10.4097/kja.d.18.00067
13. Daigeler A, Zmarsly I, Hirsch T, Goertz O, Steinau HU, Lehnhardt M, et al. Long-term outcome after local recurrence of soft tissue sarcoma: a retrospective analysis of factors predictive of survival in 135 patients with locally recurrent soft tissue sarcoma. Brit J Cancer (2014) 110(6):1456–64. doi: 10.1038/bjc.2014.21
14. Zaidi MY, Cardona K. Post-operative surveillance in soft tissue sarcoma: using tumorspecific recurrence patterns to direct approach. Chin Clin Oncol (2018) 7(4):45. doi: 10.21037/cco.2018.08
15. Gundle KR, Kafchinski L, Gupta S, Griffin AM, Dickson BC, Chung PW, et al. Analysis of Margin Classification Systems for Assessing the Risk of Local Recurrence After Soft Tissue Sarcoma Resection. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36(7):704–9. doi: 10.1200/jco.2017.74.6941
16. Ezuddin NS, Pretell-Mazzini J, Yechieli RL, Kerr DA, Wilky BA, Subhawong TK. Local recurrence of soft-tissue sarcoma: issues in imaging surveillance strategy. Skeletal Radiol (2018) 47(12):1595–606. doi: 10.1007/s00256-018-2965-x
17. Zhao R, Yu X, Feng Y, Yang Z, Chen X, Wand J, et al. Local recurrence is correlated with decreased overall survival in patients with intermediate high-grade localized primary soft tissue sarcoma of extremity and abdominothoracic wall. Asia-Pacific J Clin Oncol (2018) 14(2):e109–e15. doi: 10.1111/ajco.12807
18. Eilber FC, Rosen G, Nelson SD, Selch M, Dorey F, Eckardt J, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg (2003) 237(2):218–26. doi: 10.1097/01.SLA.0000048448.56448.70
19. Posch F, Leitner L, Bergovec M, Bezan A, Stotz M, Gerger A, et al. Can Multistate Modeling of Local Recurrence, Distant Metastasis, and Death Improve the Prediction of Outcome in Patients With Soft Tissue Sarcomas? Clin Orthop Relat Res (2017) 475(5):1427–35. doi: 10.1007/s11999-017-5232-x
20. Gronchi A, Lo Vullo S, Colombo C, Collini P, Stacchiotti S, Mariani L, et al. Extremity soft tissue sarcoma in a series of patients treated at a single institution: local control directly impacts survival. Ann Surg (2010) 251(3):506–11. doi: 10.1097/SLA.0b013e3181cf87fa
21. Fujiwara T, Stevenson J, Parry M, Tsuda Y, Tsoi K, Jeys L. What is an adequate margin for infiltrative soft-tissue sarcomas? Eur J Surg Oncol (2020) 46(2):277–81. doi: 10.1016/j.ejso.2019.10.005
22. Milovancev M, Tuohy JL, Townsend KL, Irvin VL. Influence of surgical margin completeness on risk of local tumour recurrence in canine cutaneous and subcutaneous soft tissue sarcoma: A systematic review and meta-analysis. Vet Comp Oncol (2019) 17(3):354–64. doi: 10.1111/vco.12479
23. Sugiura H, Nishida Y, Nakashima H, Yamada Y, Tsukushi S, Yamada K. Surgical procedures and prognostic factors for local recurrence of soft tissue sarcomas. J Orthop Sci (2014) 19(1):141–9. doi: 10.1007/s00776-013-0469-z
24. Maretty-Nielsen K, Aggerholm-Pedersen N, Safwat A, Jorgensen PH, Hansen BH, Baerentzen S, et al. Prognostic factors for local recurrence and mortality in adult soft tissue sarcoma of the extremities and trunk wall: a cohort study of 922 consecutive patients. Acta Orthop (2014) 85(3):323–32. doi: 10.3109/17453674.2014.908341
25. Cates JMM. AJCC eight edition for soft tissue sarcoma of the Extremities or Trunk: A Cohort Study of the SEER Database. J Natl Compr Cancer Netw JNCCN (2018) 16(2):144–52. doi: 10.6004/jnccn.2017.7042
26. Cates JMM. AJCC 8th edition for soft tissue sarcoma of the extremities and trunk. Ann Oncol (2018) 29(9):2023. doi: 10.1093/annonc/mdy/247
27. Brennan M, Antonescu C, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg (2014) 260:416–22. doi: 10.1097/SLA.0000000000000869
28. Maki R, Moraco N, Antonescu C. Toward better soft tissue sarcoma staging: building on American Joint Committee on Cancer staging systems versions 6 and 7. Ann Surg Oncol (2013) 20:3377–83. doi: 10.1245/s10434-013-3052-0
29. Brookman-May SD, May M, Shariat SF, Novara G, Zigeuner R, Cindolo L, et al. Time to recurrence is a significant predictor of cancer-specific survival after recurrence in patients with recurrent renal cell carcinoma–results from a comprehensive multi-centre database (CORONA/SATURN-Project). BJU Int (2013) 112(7):909–16. doi: 10.1111/bju.12246
30. Robbins JR, Yechieli R, Laser B, Mahan M, Rasool N, Elshaikh MA. Is time to recurrence after hysterectomy predictive of survival in patients with early stage endometrial carcinoma? Gynecol Oncol (2012) 127(1):38–42. doi: 10.1016/j.ygyno.2012.06.042
31. Sugiura H, Tsukushi S, Yoshida M, Nishida Y. What Is the Success of Repeat Surgical Treatment of a Local Recurrence After Initial Wide Resection of Soft Tissue Sarcomas? Clin Orthop Relat Res (2018) 476(9):1791–800. doi: 10.1007/s11999.0000000000000158
Keywords: time to local recurrence, soft tissue sarcoma, extremity and abdominothoracic wall, survival, prognostic factors
Citation: Liang Y, Guo T, Hong D, Xiao W, Zhou Z and Zhang X (2020) Time to Local Recurrence as a Predictor of Survival in Patients With Soft Tissue Sarcoma of the Extremity and Abdominothoracic Wall. Front. Oncol. 10:599097. doi: 10.3389/fonc.2020.599097
Received: 26 August 2020; Accepted: 09 October 2020;
Published: 04 November 2020.
Edited by:
Raymund E. Horch, University Hospital Erlangen, GermanyReviewed by:
Matthew Tristan Wallace, MedStar Franklin Square Medical Center, United StatesZiv Radisavljevic, Brigham and Women’s Hospital and Harvard Medical School, United States
Copyright © 2020 Liang, Guo, Hong, Xiao, Zhou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiwei Zhou, emhvdXpod0BzeXN1Y2Mub3JnLmNu; Xing Zhang, emhhbmd4aW5nQHN5c3VjYy5vcmcuY24=
†These authors have contributed equally to this work
 Yao Liang
Yao Liang Tianhui Guo
Tianhui Guo Dongchun Hong1,3†
Dongchun Hong1,3†