- 1Urology and Nephrology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Department of Cell and Molecular Biology, Faculty of Life Sciences and Technology, Shahid Beheshti University G.C., Tehran, Iran
- 3Malopolska Centre of Biotechnology of the Jagiellonian University, Kraków, Poland
- 4Urogenital Stem Cell Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Renal cell carcinoma (RCC) includes 2.2% of all diagnosed cancers and 1.8% of cancer-related mortalities. The available biomarkers or screening methods for RCC suffer from lack of sensitivity or high cost, necessitating identification of novel biomarkers that facilitate early diagnosis of this cancer especially in the susceptible individuals. MicroRNAs (miRNAs) have several advantageous properties that potentiate them as biomarkers for cancer detection. Expression profile of miRNAs has been assessed in biological samples from RCC patients. Circulatory or urinary levels of certain miRNAs have been proposed as markers for RCC diagnosis or follow-up. Moreover, expression profile of some miRNAs has been correlated with response to chemotherapy, immunotherapy or targeted therapeutic options such as sunitinib. In the current study, we summarize the results of studies that assessed the application of miRNAs as biomarkers, therapeutic targets or modulators of response to treatment modalities in RCC patients.
Introduction
Renal cell carcinoma (RCC) is the 15th most frequent cancer, based on the statistics provided by GLOBOCAN (1). This kind of cancer includes 2.2% of all diagnosed cancers and 1.8% of cancer-related mortalities (1). The incidence of this type of cancer is different in different regions. RCC is associated with numerous risk factors among them are smoking, obesity, and hypertension (2). The varied incident and mortality rates of RCC in different geographical regions necessitate enactment of regional screening programs and development of precise biomarkers (2). Among the screening methods for sporadic RCC, urine dipstick has yielded low level of accuracy impeding its clinical application (3). Moreover, none of the proposed serum and urine markers such as aquaporin 1, perilipin 2, and KIM1 had enough sensitivity or specificity to be applied in this regard (4). On the other hand, computed tomography and abdominal ultrasound suffer from high cost and low sensitivity for the identification of small tumors, respectively (2, 3). Therefore, development of effective non-invasive screening methods for RCC is a necessity. Recent investigations have potentiated microRNAs (miRNAs) as screening tools for several kinds of human malignancies (5). These transcripts contribute in the pathogenesis of human disorders. In this review, we clarify the main points of studies in RCC to judge the potential of miRNAs as biomarkers or therapeutic targets in this malignant condition.
miRNA Biogenesis and Function
miRNAs have sizes about 23 nucleotides and are present in different species. By acting as antisense transcripts, miRNAs post-transcriptionally decrease expression of their targets. Although the regulatory effects of each miRNA on the expression of its target gene is not great, the resultant interactive network between miRNAs, target genes and downstream effectors plays crucial impacts on the regulation of cellular functions (6). The majorities of these transcripts are transcribed from DNA templates into primary miRNAs and undergo a number of steps to be processed into the precursor and mature miRNAs, respectively (7). Two kinds of RNase III molecules, i.e., Drosha and Dicer proteins participate in the miRNA processing in the nuclear and cytoplasmic cellular compartments, respectively (7). The critical function of miRNAs in gene expression modulation is additionally highlighted by the point that an individual gene is concurrently regulated by several miRNAs, and each miRNA can modulate expression of several targets which have sequence complementarity with its seed region (8). About one-third of human genome and virtually all essential cell processes are expected to be regulated by miRNAs (9, 10). The role of miRNAs in the pathogenesis of human cancers has been vastly examined (11). These molecules have been reported to influence the main features of carcinogenic process such as sustained proliferative capacity, evasion from growth inhibitor signals, resistance to apoptosis, induction of invasive and metastatic programs, and enhancement of angiogenic processes (12). The importance of miRNAs in development of cancer has been firstly highlighted through the spotting miR-15a and miR-16-1 in a commonly deleted region in B-cell chronic lymphocytic leukemias (13). Subsequent investigations revealed other genomic alterations in a number miRNA coding genes in different cancers such as lung cancer (14), melanoma as well as ovarian and breast cancers (15). Moreover, well-known oncogenes such as c-Myc were shown to influence expression of oncogenic activates miRNAs including miR-17-92 (16) or inhibit expression of tumor suppressor miRNAs including miR-15a, miR-26, miR-29, miR-30, and let-7 (17). In RCC, quite a lot of investigation have measured expression profile of miRNAs in different biological samples to identify the pathogenic roles of these transcripts in the development of this type of cancer (18).
Dysregulated miRNAs in RCC
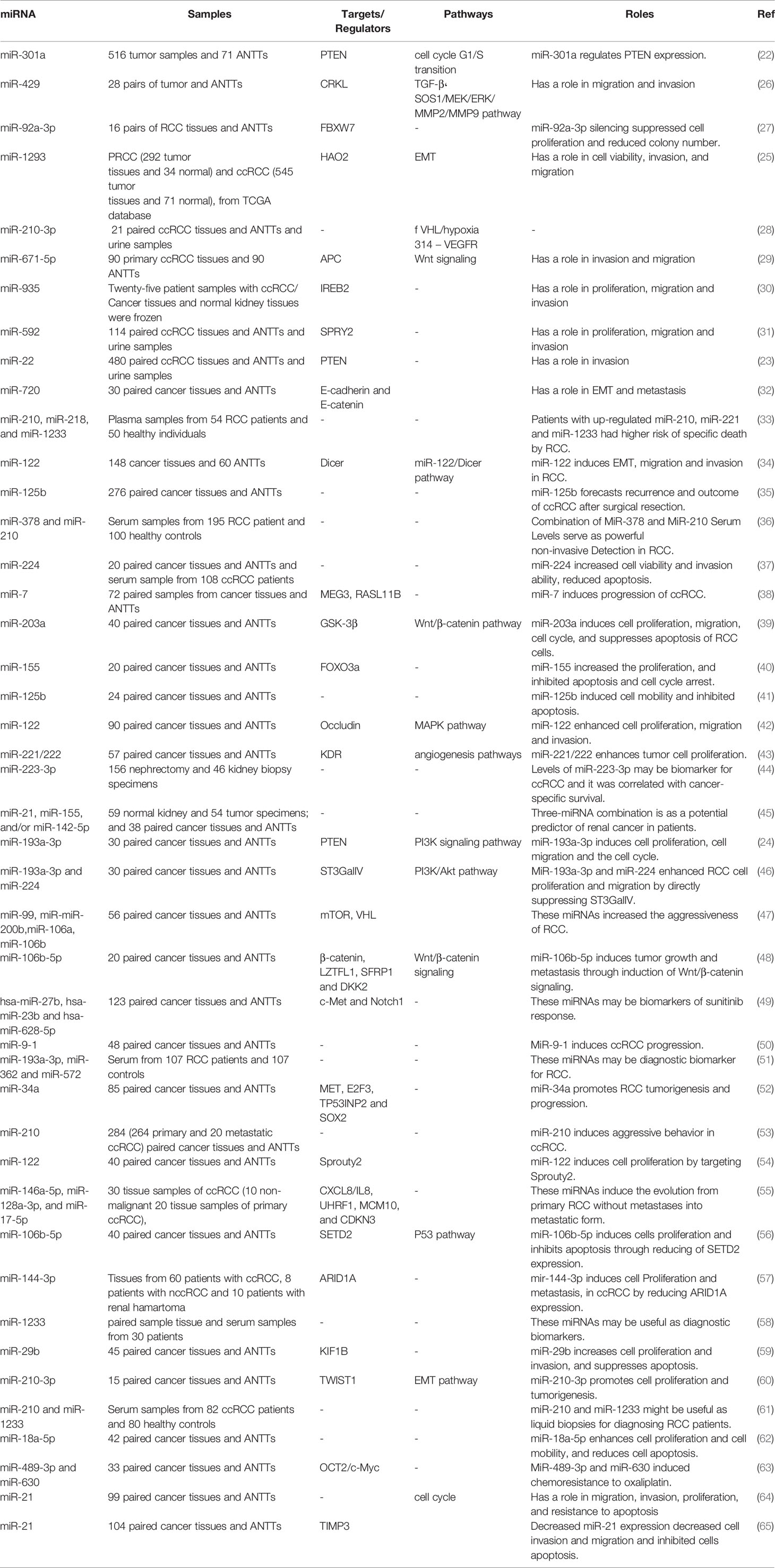
A number of studies have assessed differentially expressed miRNAs and their target genes in RCC samples and normal control. Using this approach, Li et al. have identified down-regulation of 521 genes and up-regulation of 473 genes in RCC samples. Protein-protein interaction network showed RHCG, RALYL, SLC4A1, UMOD, and CA9 as nodes with high degrees of interactions. The differentially expressed genes were enriched in cytokine and cytokine receptor pathway (19). Such approaches are useful in identification of biomarkers and therapeutic targets for RCC. Other studies have reported dysregulation of a number of miRNAs in RCC samples. Figure 1 shows a number of dysregulated miRNAs in RCC and their interaction with the PTEN tumor suppressor.
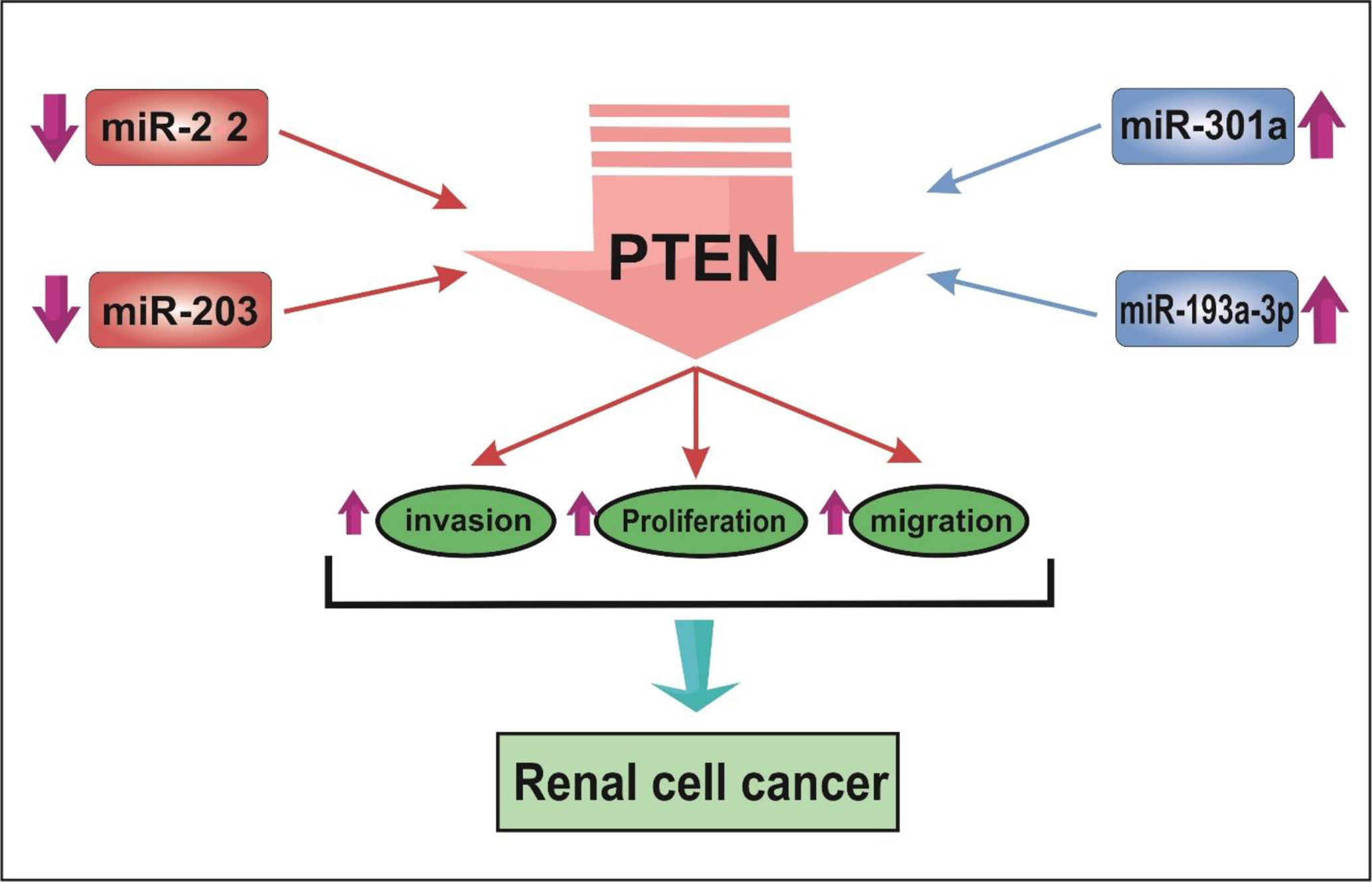
Figure 1 The schematic depiction of the interplay between miRNAs and tumor-suppressive gene PTEN in renal cell cancer. MiR-22 and miR-203 are decreased, while miR-301 and miR-193a-3p are up-regulated in RCC. miRNA expression changes result in reducing the expression of PTEN. Consequently, cell proliferation, invasive behavior, and migration are enhanced in RCC.
The following sections describe the function of these miRNAs.
Up-Regulated miRNAs in RCC
Numerous oncomiRs have been recognized in RCC. Gottardo et al. have described up-regulation of miR-28, miR-185, miR-27, and let-7f-2 in tissue samples obtained from RCC patients compared to normal kidney samples. Notably, these miRNAs were different from up-regulated miRNAs in bladder cancer samples in their cohort of patients, implying the presence of distinctive miRNA signature between these two cancers of the urogenital system (20). Wulfken et al. have investigated miRNA signature in both tissue and serum specimens of patients with RCC. They reported over-expression of 109 circulatory miRNAs in cancer patients; among them were 36 miRNAs that were up-regulated in tissue samples as well. Additional verification steps indicated up-regulation of miR-1233 in another cohort of RCC patients. Notably, expression patterns of this miRNA in patients with angiomyolipoma or oncocytoma was similar with RCC patients (21). miR-301a is another up-regulated miRNA in RCC cell lines and clinical samples. Over-expression of this miRNA has been associated with advanced stage and poor survival of RCC patients. Mechanistically, miR-301a has been displayed to target PTEN tumor suppressor (22). Two other oncomiRs, namely, miR-22 and miR-193a-3p also suppress expression of PTEN in RCC cells (23, 24). In addition, miR-1293 has been up-regulated in RCC cells enhancing viability of these cells their migratory potential and invasiveness. These effects are mediated through suppression of Hydrocyanic Oxidase 2 (25). Table 1 gives a summary of the roles of up-regulated miRNAs in RCC.
Down-Regulated miRNAs in RCC
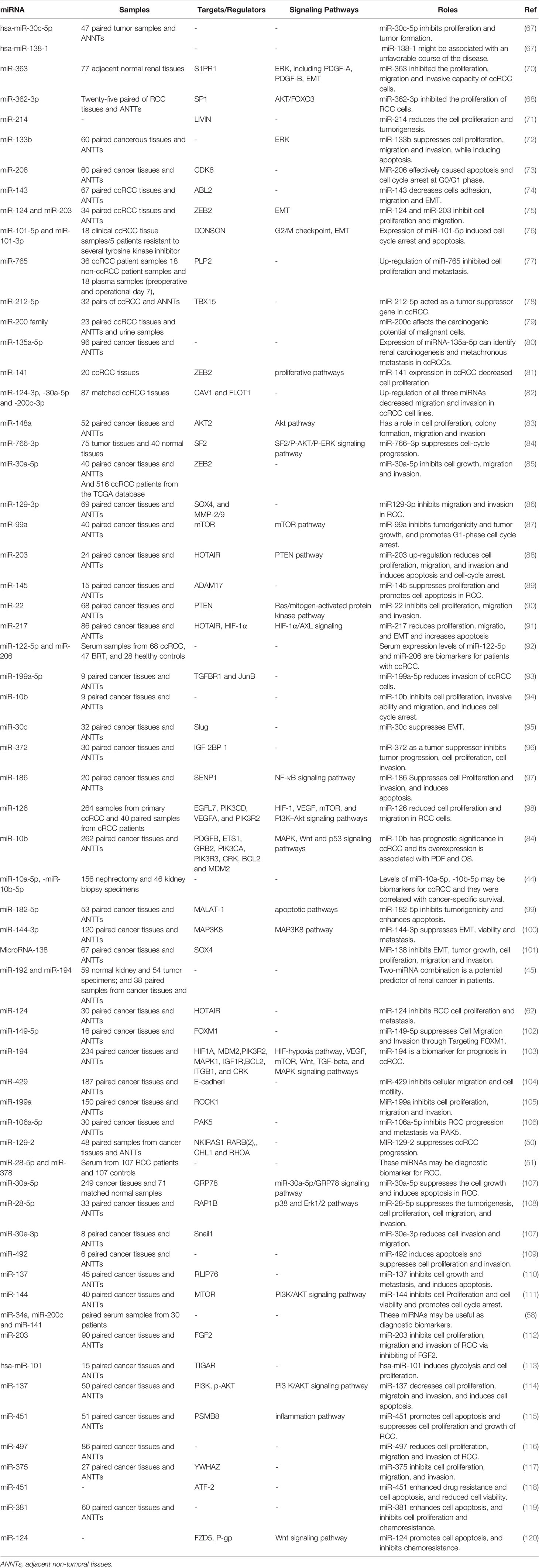
In a high throughput approach, Nakada et al. have assessed miRNA signature in clear cell carcinomas (CCCs)., and chromophobe RCC compared with normal kidney tissues. They reported down-regulation of 37 and 51 miRNAs in CCC and chromophobe RCC, respectively. As the number of up-regulated miRNAs in cancer tissues was significantly lower than the number of down-regulated ones, authors have deduced that expression of miRNAs have a tendency to be decreased in both histological types of RCC compared with normal renal samples. miR‐141 and miR‐200c were the most remarkably under-expressed miRNAs in CCC samples being down-regulated in all assessed samples of this type. In silico and functional studies indicated that decreased expression of miR‐141 and miR‐200c in CCCs may inhibit CDH1/E‐cadherin expression through increasing ZFHX1B levels (66). Two other tumor suppressor miRNAs, namely, miR-30c-5p and miR-138-1 levels, have been down-regulated in RCC samples even in the early stage tumors. Its expression has been lower in RCC samples of Fuhrman grade G3 + G4 compared with G2 (67). Another commonly down-regulated miRNA in RCC is miR-362-3p. Forced up-regulation of miR-362-3p resulted in the attenuation of cell proliferation, induction of cell cycle arrest and reduction of motility. These effects are exerted through modulation of AKT/FOXO3 signaling. SP1 has been identified as a direct target of miR-362-3p (68). Besides, expression of miR-200b has been reduced in RCC samples. Forced over-expression of miR-200b in the RCC cell lines has inhibited their migration and invasiveness and reduced cancer metastasis in xenograft models. Laminin subunit alpha 4 (LAMA4) has been predicted as a direct target of miR-200b (69). Table 2 summarizes the data about down-regulated miRNAs in RCC.
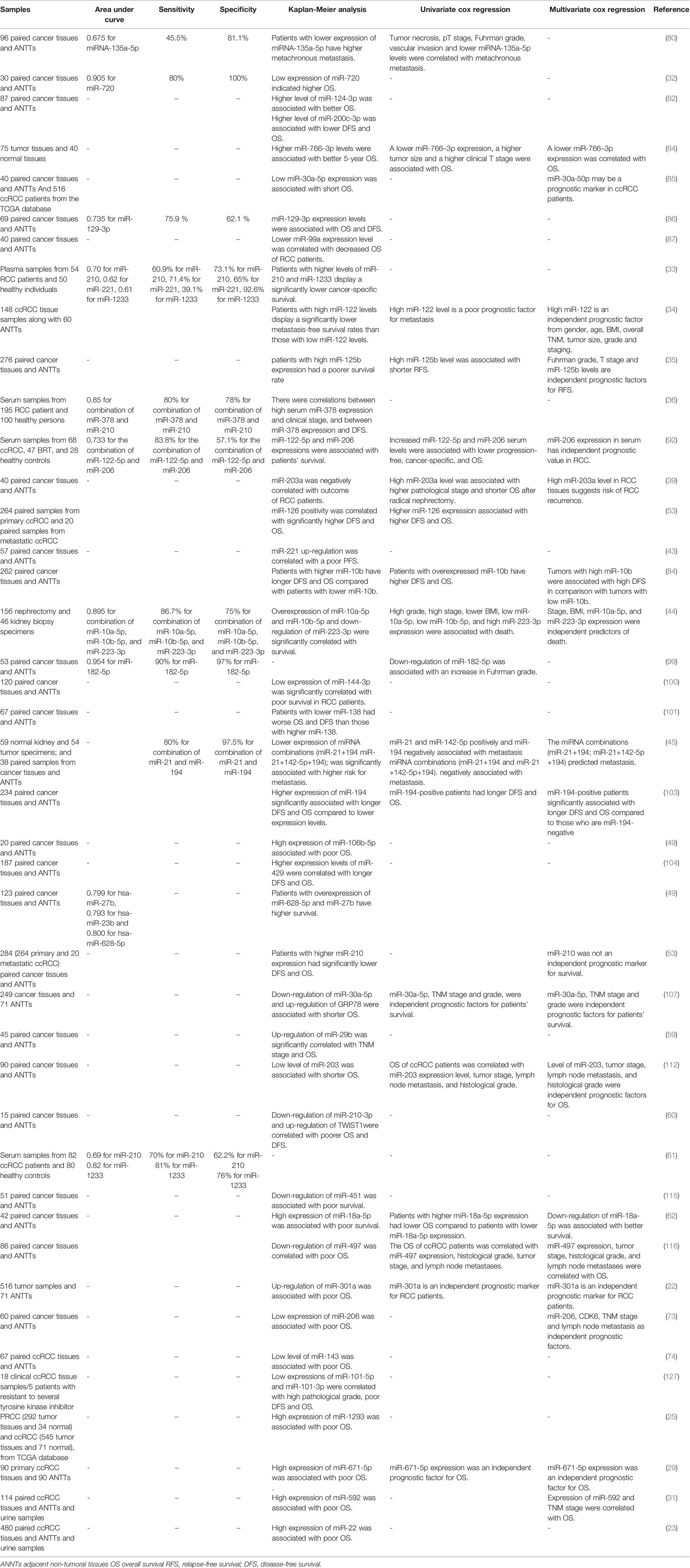
Diagnostic/Prognostic Value of miRNAs in RCC
Diagnostic and prognostic values of several miRNAs have been appraised in tissue samples, urine, or peripheral blood of RCC patients. A previous meta-analysis of available literature about miRNA signature in RCC tissues and their matching non-cancerous tissues has shown elevated levels of miR-21 and miR-210, while decreased levels of miR-141, miR-200c, and miR-429. Altered expressions of these miRNAs have been related with poor cancer-specific survival after tumor excision. Expression profile of these miRNA has been shown to be a suitable prognostic and predictive method for appraisal of survival of RCC patients particularly those with CCC (121).
An important application of miRNAs in the diagnostic process of RCC has been provided by their presence in the circulation of patients and their potential in liquid biopsy. Tusong et al. have reported over-expression of miR-21 and miR-106a in the serum samples of RCC patients compared with normal control samples. Notably, serum levels of these miRNAs have been decreased in patients a month after surgery suggesting their appropriateness as biomarkers for RCC (122). Wang et al. have reported consistent down-regulation of miR-200a in serum samples of patients with this kind of cancer, particularly in patients with stage I disease. Notably, level of this miRNA was commonly decreased in urine specimens of patients as well (123). A comprehensive assessment of miRNA profile in plasma specimens of ccRCC patients and healthy subjects has revealed the correlation between circulating miRNA signature and ccRCC stage. miRNA profiles were remarkably different between stage III/IV sections and both controls and early stage samples. Plasma levels of miR‐150 were considerably correlated with patients’ survival (124). A large-scale detection of formerly unannotated miRNA sequences in human renal specimens has led to identification of several miRNAs being dysregulated in ccRCC tumors and linked with poor survival of patients (125). Finally, experiments in a transgenic model of Xp11 RCC have shown higher amounts of miR‐204‐5p in urinary exosomes compared with control animals. Expression of this miRNA was also elevated in primary RCC cell lines created from transgenic mice indicating its role as a diagnostic marker for Xp11 tRCC (126).
Table 3 gives a brief record of studies which reported the diagnostic/prognostic role of miRNAs in RCC.
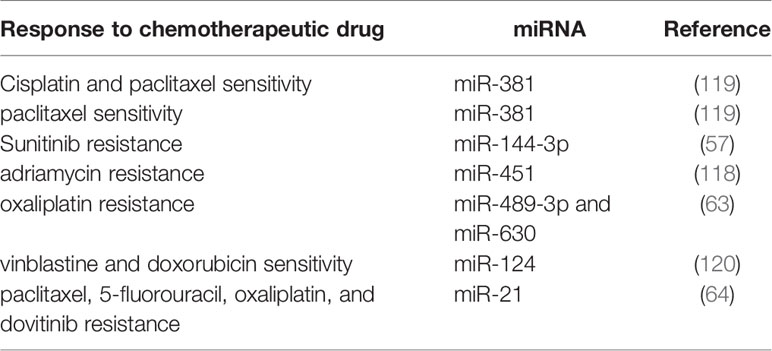
The Role of miRNAs in Determination of Response of RCC Patients to Treatment Modalities
Expression profile of a number of miRNAs correlates with response of RCC cells to chemotherapeutic agents. For instance, miR-381 has been shown to improve response of RCC cells to 5-fluorouracil through targeting WEE1 and enhancing activity of cyclin-dependent kinase 2 (128). Expression of miR-451 has been elevated in low multi-drug resistant (MDR);,;, cell line compared with the high MDR cell line. This miRNA has been shown to target ATF-2 and suppress its expression. Up-regulation of miR-451 has increased drug resistance, while its silencing improved response to chemotherapeutic agents (118). In the clinical settings, serum levels of miR-183 have been shown to predict response of RCC patients to cytotoxic effects of natural killer cells (129), implying the importance of miRNAs in immunotherapeutic options. A genome-wide miRNA profiling in RCC patients who received sunitinib showed lower levels of miR-141 in tumor samples of poor responders compared with good responders (81). Therefore, miRNAs modulate response of RCC patients to a wide range of treatment modalities. Table 4 summarizes the impact of miRNAs in resistance to therapeutic modalities in RCC.
Discussion
The oncogenic function of numerous miRNAs has been proved in RCC cells These oncomiRs have been shown to enhance cell proliferation and invasive features of RCC cells whilst decreasing apoptosis Notably some tumor suppressor genes such PTEN APC and MEG3 have been identified as targets of oncomiRs such as miR-301a miR-193a-3p miR-22 miR-671-5p, and miR-7, indicating a possible mechanism for their participation in the pathogenesis of RCC. Instead, tumor suppressor miRNAs which are down-regulated in RCC cells have potential roles in the activation of apoptotic pathways and arrestment of cell cycle transition. A number of these miRNAs target EMT-associated genes such as ZEB1, Slug, HOTAIR, and HIF-1α. Thus, their down-regulation is associated with the enhancement of EMT program. miRNAs are regarded as potential markers of different malignancies including RCC. These transcripts regulate several cancer-related cellular functions such as apoptosis, survival, migration and angiogenesis. Therefore, several miRNAs have similar functions and expression profiles in diverse cancers. Although aberrant expression of miRNAs in cancer patients is a useful tool for follow-up of patients, identification of tissue-specific pattern of their expression is necessary to differentiate between different cancers originating from a certain body system. In spite of extensive efforts for biomarker discovery, there is no consensus on miRNA panels that are specific for a certain type of cancer. A previous study has reported up-regulation of miR-28, miR-185, miR-27, and let-7f-2 in RCC samples, whereas expression of a different set of miRNAs including miR-223, miR-26b, miR-221, and miR-103-1was increased in bladder cancer samples. Based on these results, authors suggested the potential of miRNAs in differentiating between these two types of cancers (20). However, others have reported over-expression of bladder cancer-related miRNAs such as miR-223 and miR-221 in RCC samples (33, 130) casting doubt on the possibility of identification of tissue-specific miRNA signature in different cancers. Studies which appraised the biomarker role of miRNAs in RCC suffer from small sample size, inclusion of samples from diverse clinical stages and histologic subclasses as well as benign kidney lesions and validation in independent samples. Possibly, the most important limitation of miRNAs as diagnostic markers is their inability for differentiation between malignancies with diverse origins. Based on this limitation, they cannot be used for primary diagnosis of cancer but for patients’ follow-up. Another possible application of miRNAs in the RCC patients rises from their importance in the determination of patients’ response to chemotherapy. Therefore, a prior identification of miRNA profile in the biopsy samples might facilitate selection of the most appropriate therapeutic regimen in a personalized manner. Moreover, targeted suppression of certain miRNAs is a possible modality to enhance response of patients to chemotherapy. miR-21 represents a promising candidate in this regard, since it has been shown to be over-expressed in RCC samples in independent studies and its silencing has enhance response to multiple anti-cancer drugs such as paclitaxel, 5-fluorouracil, oxaliplatin, and dovitinib. Yet, miRNA-based therapies face a number of challenges such as design of specific formulations to avoid off-target effects and low efficacy of delivery methods (131).
Comparison of miRNA levels in serum and tissue samples of RCC patients and healthy subjects has led to identification of several dysregulated miRNAs in serum samples. Yet, only a fraction of these miRNAs have been dysregulated in tissue samples, implying that a minor portion of circulating miRNAs have been originated from the tumor tissues (21). Therefore, future studies are needed to explore the source of circulating miRNAs in RCC patients. Based on the results of recent investigations, both serum and urine samples of patients with RCC might be used as sources for discovery of miRNA levels, facilitating conduction of non-invasive methods for RCC diagnosis.
miRNA signature can be used for classification of RCC subtypes. The miRNA-based classification system developed by Youssef et al. could discriminate different subtypes of RCC such as clear cell, papillary, oncocytoma, and chromophobe RCC with sensitivity values between 97% and 100% (132). Moreover, miR-15a has been shown to have distinct expression pattern between RCC and oncocytoma being up-regulated in the former, yet down-regulated in the latter. Expression of this miRNA was similarly up-regulated in chromophobe carcinoma, while in the papillary RCC samples miR-15a expression was not such over-expressed. Over-expression of miR-15a was also detectable in urine samples of RCC patients. However, miR-15a was almost untraceable in oncocytoma, other tumors, and inflammatory disorders of the urinary tract (133). These results indicate the possibility of substitution of histopathological classification methods by molecular methods. The clinical implications of these findings should be confirmed in larger samples of patients.
Taken together, miRNAs participate in the pathogenesis of RCC and response of patients to diverse therapeutic modalities. Moreover, as they are traceable in circulation and urine samples of patients, they can be used as biomarkers for this kind of cancer. However, at the present time, there is no miRNA that can be widely applied as biomarker or treatment target in the clinical setting. This is partly because of the heterogeneous pattern of expression of miRNAs in RCC samples and circulation of patients. This research filed lacks comprehensive assessment of miRNA profiles in large cohorts of RCC patients. Therefore, future studies with these features are expected to facilitate design of suitable diagnostic panels containing miRNAs.
Author Contributions
MT and SG-F wrote the draft and revised it. ZS-F and WB designed the tables and study, and performed the data collection. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, et al. Epidemiology of Renal Cell Carcinoma. Eur Urol (2019) 75(1):74–84. doi: 10.1016/j.eururo.2018.08.036
3. Rossi SH, Klatte T, Usher-Smith J, Stewart GD. Epidemiology and screening for renal cancer. World J Urol (2018) 36(9):1341–53. doi: 10.1007/s00345-018-2286-7
4. Morrissey JJ, Mobley J, Figenshau RS, Vetter J, Bhayani S, Kharasch ED. Urine aquaporin 1 and perilipin 2 differentiate renal carcinomas from other imaged renal masses and bladder and prostate cancer. Mayo Clin Proc (2015) 90(1):35–42. doi: 10.1016/j.mayocp.2014.10.005
5. Wang H, Peng R, Wang J, Qin Z, Xue L. Circulating microRNAs as potential cancer biomarkers: the advantage and disadvantage. Clin Epigenet (2018) 10(1):1–10. doi: 10.1186/s13148-018-0492-1
6. Bracken CP, Scott HS. Goodall GJ. A network-biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet (2016) 17(12):719–32. doi: 10.1038/nrg.2016.134
7. Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol (2014) 15(8):509–24. doi: 10.1038/nrm3838
8. Kehl T, Backes C, Kern F, Fehlmann T, Ludwig N, Meese E, et al. About miRNAs, miRNA seeds, target genes and target pathways. Oncotarget (2017) 8(63):107167–75. doi: 10.18632/oncotarget.22363
9. Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature (2008) 455(7209):64–71. doi: 10.1038/nature07242
10. Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol (2007) 23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406
11. Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol (2012) 6(6):590–610. doi: 10.1016/j.molonc.2012.09.006
12. Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther (2016) 1(1):1–9. doi: 10.1038/sigtrans.2015.4
13. Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci (2002) 99(24):15524–9. doi: 10.1073/pnas.242606799
14. Calin GA, Croce C. MicroRNAs and chromosomal abnormalities in cancer cells. Oncogene (2006) 25(46):6202–10. doi: 10.1038/sj.onc.1209910
15. Zhang L, Huang J, Yang N, Greshock J, Megraw MS, Giannakakis A, et al. microRNAs exhibit high frequency genomic alterations in human cancer. Proc Natl Acad Sci (2006) 103(24):9136–41. doi: 10.1073/pnas.0508889103
16. O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature (2005) 435(7043):839–43. doi: 10.1038/nature03677
17. Chang T-C, Yu D, Lee Y-S, Wentzel EA, Arking DE, West KM, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet (2008) 40(1):43–50. doi: 10.1038/ng.2007.30
18. Mytsyk Y, Dosenko V, Skrzypczyk MA, Borys Y, Diychuk Y, Kucher A, et al. Potential clinical applications of microRNAs as biomarkers for renal cell carcinoma. Cent Eur J Urol (2018) 71(3):295–303. doi: 10.5173/ceju.2018.1618
19. Li J, Huang J-H, Qu Q-H, Xia Q, Wang D-S, Jin L, et al. Evaluating the microRNA-target gene regulatory network in renal cell carcinomas, identification for potential biomarkers and critical pathways. Int J Clin Exp Med (2015) 8(5):7209–19.
20. Gottardo F, Liu CG, Ferracin M, Calin GA, Fassan M, Bassi P, et al. Micro-RNA profiling in kidney and bladder cancers. Urol Oncol (2007) 25(5):387–92. doi: 10.1016/j.urolonc.2007.01.019
21. Wulfken LM, Moritz R, Ohlmann C, Holdenrieder S, Jung V, Becker F, et al. MicroRNAs in renal cell carcinoma: diagnostic implications of serum miR-1233 levels. PloS One (2011) 6(9):e25787. doi: 10.1371/journal.pone.0025787
22. Li J, Jiang D, Zhang Q, Peng S, Liao G, Yang X, et al. MiR-301a Promotes Cell Proliferation by Repressing PTEN in Renal Cell Carcinoma. Cancer Manag Res (2020) 12:4309–20. doi: 10.2147/CMAR.S253533
23. Gong X, Zhao H, Saar M, Peehl DM, Brooks JD. miR-22 Regulates Invasion, Gene Expression and Predicts Overall Survival in Patients with Clear Cell Renal Cell Carcinoma. Kidney Cancer (Clifton Va) (2019) 3(2):119–32. doi: 10.3233/KCA-190051
24. Liu L, Li Y, Liu S, Duan Q, Chen L, Wu T, et al. Downregulation of miR-193a-3p inhibits cell growth and migration in renal cell carcinoma by targeting PTEN. Tumor Biol (2017) 39(6):1010428317711951. doi: 10.1177/1010428317711951
25. Liu XL, Pan WG, Li KL, Mao YJ, Liu SD, Zhang RM. miR-1293 Suppresses Tumor Malignancy by Targeting Hydrocyanic Oxidase 2: Therapeutic Potential of a miR-1293/Hydrocyanic Oxidase 2 Axis in Renal Cell Carcinoma. Cancer Biother Radiopharm (2020) 35(5):377–86. doi: 10.1089/cbr.2019.2957
26. Wang J, Wang C, Li Q, Guo C, Sun W, Zhao D, et al. miR-429-CRKL axis regulates clear cell renal cell carcinoma malignant progression through SOS1/MEK/ERK/MMP2/MMP9 pathway. BioMed Pharmacother (2020) 127:110215. doi: 10.1016/j.biopha.2020.110215
27. Zeng R, Huang J, Sun Y, Luo J. Cell proliferation is induced in renal cell carcinoma through miR-92a-3p upregulation by targeting FBXW7. Oncol Lett (2020) 19(4):3258–68. doi: 10.3892/ol.2020.11443
28. Petrozza V, Costantini M, Tito C, Giammusso LM, Sorrentino V, Cacciotti J, et al. Emerging role of secreted miR-210-3p as potential biomarker for clear cell Renal Cell Carcinoma metastasis. Cancer Biomark (2020) 27(2):181–8. doi: 10.3233/CBM-190242
29. Chi XG, Meng XX, Ding DL, Xuan XH, Chen YZ, Cai Q, et al. HMGA1-mediated miR-671-5p targets APC to promote metastasis of clear cell renal cell carcinoma through Wnt signaling. Neoplasma (2020) 67(1):46–53. doi: 10.4149/neo_2019_190217N135
30. Liu F, Chen Y, Chen B, Liu C, Xing J. MiR-935 Promotes Clear Cell Renal Cell Carcinoma Migration and Invasion by Targeting IREB2. Cancer Manag Res (2019) 11:10891–900. doi: 10.2147/CMAR.S232380
31. Lv X, Shen J, Guo Z, Kong L, Zhou G, Ning H. Aberrant Expression of miR-592 Is Associated with Prognosis and Progression of Renal Cell Carcinoma. Onco Targets Ther (2019) 12:11231–9. doi: 10.2147/OTT.S227834
32. Bhat NS, Colden M, Dar AA, Saini S, Arora P, Shahryari V, et al. MicroRNA-720 Regulates E-cadherin–αE-catenin Complex and Promotes Renal Cell Carcinoma. Mol Cancer Ther (2017) 16(12):2840–8. doi: 10.1158/1535-7163.MCT-17-0400
33. Dias F, Teixeira AL, Ferreira M, Adem B, Bastos N, Vieira J, et al. Plasmatic miR-210, miR-221 and miR-1233 profile: potential liquid biopsies candidates for renal cell carcinoma. Oncotarget (2017) 8(61):103315–26. doi: 10.18632/oncotarget.21733
34. Fan Y, Ma X, Li H, Gao Y, Huang Q, Zhang Y, et al. miR-122 promotes metastasis of clear-cell renal cell carcinoma by downregulating Dicer. Int J cancer (2018) 142(3):547–60. doi: 10.1002/ijc.31050
35. Fu Q, Liu Z, Pan D, Zhang W, Xu L, Zhu Y, et al. Tumor miR-125b predicts recurrence and survival of patients with clear-cell renal cell carcinoma after surgical resection. Cancer Sci (2014) 105(11):1427–34. doi: 10.1111/cas.12507
36. Fedorko M, Stanik M, Iliev R, Redova-Lojova M, Machackova T, Svoboda M, et al. Combination of MiR-378 and MiR-210 Serum Levels Enables Sensitive Detection of Renal Cell Carcinoma. Int J Mol Sci (2015) 16(10):23382–9. doi: 10.3390/ijms161023382
37. Fujii N, Hirata H, Ueno K, Mori J, Oka S, Shimizu K, et al. Extracellular miR-224 as a prognostic marker for clear cell renal cell carcinoma. Oncotarget (2017) 8(66):109877–88. doi: 10.18632/oncotarget.22436
38. He H, Dai J, Zhuo R, Zhao J, Wang H, Sun F, et al. Study on the mechanism behind lncRNA MEG3 affecting clear cell renal cell carcinoma by regulating miR-7/RASL11B signaling. J Cell Physiol (2018) 233(12):9503–15. doi: 10.1002/jcp.26849
39. Hu G, Lai P, Liu M, Xu L, Guo Z, Liu H, et al. miR-203a regulates proliferation, migration, and apoptosis by targeting glycogen synthase kinase-3β in human renal cell carcinoma. Tumor Biol (2014) 35(11):11443–53. doi: 10.1007/s13277-014-2476-x
40. Ji H, Tian D, Zhang B, Zhang Y, Yan D, Wu S. Overexpression of miR-155 in clear-cell renal cell carcinoma and its oncogenic effect through targeting FOXO3a. Exp Ther Med (2017) 13(5):2286–92. doi: 10.3892/etm.2017.4263
41. Jin L, Zhang Z, Li Y, He T, Hu J, Liu J, et al. miR-125b is associated with renal cell carcinoma cell migration, invasion and apoptosis. Oncol Lett (2017) 13(6):4512–20. doi: 10.3892/ol.2017.5985
42. Jingushi K, Kashiwagi Y, Ueda Y, Kitae K, Hase H, Nakata W, et al. High miR-122 expression promotes malignant phenotypes in ccRCC by targeting occludin. Int J Oncol (2017) 51(1):289–97. doi: 10.3892/ijo.2017.4016
43. Khella HWZ, Butz H, Ding Q, Rotondo F, Evans KR, Kupchak P, et al. miR-221/222 Are Involved in Response to Sunitinib Treatment in Metastatic Renal Cell Carcinoma. Mol Ther J Am Soc Gene Ther (2015) 23(11):1748–58. doi: 10.1038/mt.2015.129
44. Kowalik CG, Palmer DA, Sullivan TB, Teebagy PA, Dugan JM, Libertino JA, et al. Profiling microRNA from nephrectomy and biopsy specimens: predictors of progression and survival in clear cell renal cell carcinoma. BJU Int (2017) 120(3):428–40. doi: 10.1111/bju.13886
45. Lokeshwar SD, Talukder A, Yates TJ, Hennig MJP, Garcia-Roig M, Lahorewala SS, et al. Molecular Characterization of Renal Cell Carcinoma: A Potential Three-MicroRNA Prognostic Signature. Cancer Epidemiol Biomarkers Prevent (2018) 27(4):464–72. doi: 10.1158/1055-9965.EPI-17-0700
46. Pan Y, Hu J, Ma J, Qi X, Zhou H, Miao X, et al. MiR-193a-3p and miR-224 mediate renal cell carcinoma progression by targeting alpha-2,3-sialyltransferase IV and the phosphatidylinositol 3 kinase/Akt pathway. Mol Carcinogenesis (2018) 57(8):1067–77. doi: 10.1002/mc.22826
47. Oliveira RdC, Ivanovic RF, Leite KRM, Viana NI, Pimenta RCA, Junior JP, et al. Expression of micro-RNAs and genes related to angiogenesis in ccRCC and associations with tumor characteristics. BMC Urol (2017) 17(1):113. doi: 10.1186/s12894-017-0306-3
48. Lu J, Wei J-H, Feng Z-H, Chen Z-H, Wang Y-Q, Huang Y, et al. miR-106b-5p promotes renal cell carcinoma aggressiveness and stem-cell-like phenotype by activating Wnt/β-catenin signalling. Oncotarget (2017) 8(13):21461–71. doi: 10.18632/oncotarget.15591
49. Puente J, Laínez N, Dueñas M, Méndez-Vidal MJ, Esteban E, Castellano D, et al. Novel potential predictive markers of sunitinib outcomes in long-term responders versus primary refractory patients with metastatic clear-cell renal cell carcinoma. Oncotarget (2017) 8(18):30410–21. doi: 10.18632/oncotarget.16494
50. Pronina IV, Klimov EA, Burdennyy AM, Beresneva EV, Fridman MV, Ermilova VD, et al. Methylation of the genes for the microRNAs miR-129-2 and miR-9-1, changes in their expression, and activation of their potential target genes in clear cell renal cell carcinoma. Mol Biol (2017) 51(1):61–71. doi: 10.1134/S0026893316060169
51. Wang C, Hu J, Lu M, Gu H, Zhou X, Chen X, et al. A panel of five serum miRNAs as a potential diagnostic tool for early-stage renal cell carcinoma. Sci Rep (2015) 5:7610–0. doi: 10.1038/srep07610
52. Toraih EA, Ibrahiem AT, Fawzy MS, Hussein MH, Al-Qahtani SAM, Shaalan AAM. MicroRNA-34a: A Key Regulator in the Hallmarks of Renal Cell Carcinoma. Oxid Med Cell Longevity (2017) 2017:3269379–3269379. doi: 10.1155/2017/3269379
53. Samaan S, Khella HW, Girgis A, Scorilas A, Lianidou E, Gabril M, et al. miR-210 is a prognostic marker in clear cell renal cell carcinoma. J Mol Diagnostics JMD (2015) 17(2):136–44. doi: 10.1016/j.jmoldx.2014.10.005
54. Wang Z, Qin C, Zhang J, Han Z, Tao J, Cao Q, et al. MiR-122 promotes renal cancer cell proliferation by targeting Sprouty2. Tumor Biol (2017) 39(2):1010428317691184. doi: 10.1177/1010428317691184
55. Wotschofsky Z, Gummlich L, Liep J, Stephan C, Kilic E, Jung K, et al. Integrated microRNA and mRNA Signature Associated with the Transition from the Locally Confined to the Metastasized Clear Cell Renal Cell Carcinoma Exemplified by miR-146-5p. PloS One (2016) 11(2):e0148746–e0148746. doi: 10.1371/journal.pone.0148746
56. Xiang W, He J, Huang C, Chen L, Tao D, Wu X, et al. miR-106b-5p targets tumor suppressor gene SETD2 to inactive its function in clear cell renal cell carcinoma. Oncotarget (2015) 6(6):4066–79. doi: 10.18632/oncotarget.2926
57. Xiao W, Lou N, Ruan H, Bao L, Xiong Z, Yuan C, et al. Mir-144-3p Promotes Cell Proliferation, Metastasis, Sunitinib Resistance in Clear Cell Renal Cell Carcinoma by Downregulating ARID1A. Cell Physiol Biochem (2017) 43(6):2420–33. doi: 10.1159/000484395
58. Yadav S, Khandelwal M, Seth A, Saini AK, Dogra PN, Sharma A. Serum microRNA Expression Profiling: Potential Diagnostic Implications of a Panel of Serum microRNAs for Clear Cell Renal Cell Cancer. Urology (2017) 104:64–9. doi: 10.1016/j.urology.2017.03.013
59. Xu Y, Zhu J, Lei Z, Wan L, Zhu X, Ye F, et al. Expression and functional role of miR-29b in renal cell carcinoma. Int J Clin Exp Pathol (2015) 8(11):14161–70.
60. Yoshino H, Yonemori M, Miyamoto K, Tatarano S, Kofuji S, Nohata N, et al. microRNA-210-3p depletion by CRISPR/Cas9 promoted tumorigenesis through revival of TWIST1 in renal cell carcinoma. Oncotarget (2017) 8(13):20881–94. doi: 10.18632/oncotarget.14930
61. Xu Y, Deng W, Zhang W. Long non-coding RNA TUG1 protects renal tubular epithelial cells against injury induced by lipopolysaccharide via regulating microRNA-223. Biomed Pharmacother (2018) 104:509–19. doi: 10.1016/j.biopha.2018.05.069
62. Zhou L, Li Z, Pan X, Lai Y, Quan J, Zhao L, et al. Identification of miR-18a-5p as an oncogene and prognostic biomarker in RCC. Am J Trans Res (2018) 10(6):1874–86.
63. Chen L, Chen L, Qin Z, Lei J, Ye S, Zeng K, et al. Upregulation of miR-489-3p and miR-630 inhibits oxaliplatin uptake in renal cell carcinoma by targeting OCT2. Acta Pharm Sin B (2019) 9(5):1008–20. doi: 10.1016/j.apsb.2019.01.002
64. Gaudelot K, Gibier JB, Pottier N, Hémon B, Van Seuningen I, Glowacki F, et al. Targeting miR-21 decreases expression of multi-drug resistant genes and promotes chemosensitivity of renal carcinoma. Tumour Biol J Int Soc Oncodevelopmental Biol Med (2017) 39(7):1010428317707372. doi: 10.1177/1010428317707372
65. Chen J, Gu Y, Shen W. MicroRNA-21 functions as an oncogene and promotes cell proliferation and invasion via TIMP3 in renal cancer. Eur Rev Med Pharmacol Sci (2017) 21(20):4566–76.
66. Nakada C, Matsuura K, Tsukamoto Y, Tanigawa M, Yoshimoto T, Narimatsu T, et al. Genome-wide microRNA expression profiling in renal cell carcinoma: significant down-regulation of miR-141 and miR-200c. J Pathol: A J Pathol Soc Great Britain Ireland (2008) 216(4):418–27. doi: 10.1002/path.2437
67. Onyshchenko KV, Voitsitskyi TV, Grygorenko VM, Saidakova NO, Pereta LV, Onyschuk AP, et al. Expression of micro-RNA hsa-miR-30c-5p and hsa-miR-138-1 in renal cell carcinoma. Exp Oncol (2020) 42(2):115–9. doi: 10.32471/exp-oncology.2312-8852.vol-42-no-2.14632
68. Zhu H, Wang S, Shen H, Zheng X, Xu X. SP1/AKT/FOXO3 Signaling Is Involved in miR-362-3p-Mediated Inhibition of Cell-Cycle Pathway and EMT Progression in Renal Cell Carcinoma. Front Cell Dev Biol (2020) 8:297. doi: 10.3389/fcell.2020.00297
69. Li Y, Guan B, Liu J, Zhang Z, He S, Zhan Y, et al. MicroRNA-200b is downregulated and suppresses metastasis by targeting LAMA4 in renal cell carcinoma. EBioMedicine (2019) 44:439–51. doi: 10.1016/j.ebiom.2019.05.041
70. Xie Y, Chen L, Gao Y, Ma X, He W, Zhang Y, et al. miR-363 suppresses the proliferation, migration and invasion of clear cell renal cell carcinoma by downregulating S1PR1. Cancer Cell Int (2020) 20:227. doi: 10.1186/s12935-020-01313-9
71. Xu H, Wu S, Shen X, Shi Z, Wu D, Yuan Y, et al. Methylation-mediated miR-214 regulates proliferation and drug sensitivity of renal cell carcinoma cells through targeting LIVIN. J Cell Mol Med (2020) 24(11):6410–25. doi: 10.1111/jcmm.15287
72. Xu Y, Ma Y, Liu XL, Gao SL. miR−133b affects cell proliferation, invasion and chemosensitivity in renal cell carcinoma by inhibiting the ERK signaling pathway. Mol Med Rep (2020) 22(1):67–76. doi: 10.3892/mmr.2020.11125
73. Guo Z, Jia H, Ge J. MiR-206 suppresses proliferation and epithelial-mesenchymal transition of renal cell carcinoma by inhibiting CDK6 expression. Hum Cell (2020) 33(3):750–8. doi: 10.1007/s13577-020-00355-5
74. Xu B, Wang C, Wang YL, Chen S-Q, Wu J-P, Zhu W-D, et al. miR-143 inhibits renal cell carcinoma cells metastatic potential by suppressing ABL2. Kaohsiung J Med Sci (2020) 36(8). doi: 10.1002/kjm2.12207
75. Chen J, Zhong Y, Li L. miR-124 and miR-203 synergistically inactivate EMT pathway via coregulation of ZEB2 in clear cell renal cell carcinoma (ccRCC). J Transl Med (2020) 18(1):69. doi: 10.1186/s12967-020-02242-x
76. Yamada Y, Nohata N, Uchida A, Kato M, Arai T, Moriya S, et al. Replisome genes regulation by antitumor miR-101-5p in clear cell renal cell carcinoma. Cancer Sci (2020) 111(4):1392–406. doi: 10.1111/cas.14327
77. Xiao W, Wang C, Chen K, Wang T, Xing J, Zhang X, et al. MiR-765 functions as a tumour suppressor and eliminates lipids in clear cell renal cell carcinoma by downregulating PLP2. EBioMedicine (2020) 51:102622. doi: 10.1016/j.ebiom.2019.102622
78. Deng JH, Zheng GY, Li HZ, Ji ZG. MiR-212-5p inhibits the malignant behavior of clear cell renal cell carcinoma cells by targeting TBX15. Eur Rev Med Pharmacol Sci (2019) 23(24):10699–707. doi: 10.26355/eurrev_201912_19770
79. Gilyazova IR, Klimentova EA, Bulygin KV, et al. MicroRNA-200 family expression analysis in metastatic clear cell renal cell carcinoma patients. Cancer Gene Ther (2019). doi: 10.1038/s41417-019-0149-z
80. Shiomi E, Sugai T, Ishida K, Osakabe M, Tsuyukubo T, Kato Y, et al. Analysis of Expression Patterns of MicroRNAs That Are Closely Associated With Renal Carcinogenesis. Front Oncol (2019) 9:431. doi: 10.3389/fonc.2019.00431
81. Berkers J, Govaere O, Wolter P, Beuselinck B, Schöffski P, Kempen LCv, et al. A Possible Role for MicroRNA-141 Down-Regulation in Sunitinib Resistant Metastatic Clear Cell Renal Cell Carcinoma Through Induction of Epithelial-to-Mesenchymal Transition and Hypoxia Resistance. J Urol (2013) 189(5):1930–8. doi: 10.1016/j.juro.2012.11.133
82. Butz H, Szabó PM, Khella HW, Nofech-Mozes R, Patocs A, Yousef GM. miRNA-target network reveals miR-124as a key miRNA contributing to clear cell renal cell carcinoma aggressive behaviour by targeting CAV1 and FLOT1. Oncotarget (2015) 6(14):12543–57. doi: 10.18632/oncotarget.3815
83. Cao H, Liu Z, Wang R, Zhang X, Yi W, Nie G, et al. miR-148a suppresses human renal cell carcinoma malignancy by targeting AKT2. Oncol Rep (2017) 37(1):147–54. doi: 10.3892/or.2016.5257
84. Khella HWZ, Daniel N, Youssef L, Scorilas A, Nofech-Mozes R, Mirham L, et al. miR-10b is a prognostic marker in clear cell renal cell carcinoma. J Clin Pathol (2017) 70(10):854–9. doi: 10.1136/jclinpath-2017-204341
85. Chen Z, Zhang J, Zhang Z, Feng Z, Wei J, Lu J, et al. The putative tumor suppressor microRNA-30a-5p modulates clear cell renal cell carcinoma aggressiveness through repression of ZEB2. Cell Death Disease (2017) 8(6):e2859–9. doi: 10.1038/cddis.2017.252
86. Chen X, Ruan A, Wang X, Han W, Wang R, Lou N, et al. miR-129-3p, as a diagnostic and prognostic biomarker for renal cell carcinoma, attenuates cell migration and invasion via downregulating multiple metastasis-related genes. J Cancer Res Clin Oncol (2014) 140(8):1295–304. doi: 10.1007/s00432-014-1690-7
87. Cui L, Zhou H, Zhao H, Zhou Y, Xu R, Xu X, et al. MicroRNA-99a induces G1-phase cell cycle arrest and suppresses tumorigenicity in renal cell carcinoma. BMC Cancer (2012) 12(1):546. doi: 10.1186/1471-2407-12-546
88. Dasgupta P, Kulkarni P, Majid S, Shahryari V, Hashimoto Y, Bhat NS, et al. MicroRNA-203 Inhibits Long Noncoding RNA HOTAIR and Regulates Tumorigenesis through Epithelial-to-mesenchymal Transition Pathway in Renal Cell Carcinoma. Mol Cancer Ther (2018) 17(5):1061–9. doi: 10.1158/1535-7163.MCT-17-0925
89. Doberstein K, Steinmeyer N, Hartmetz A-K, Eberhardt W, Mittelbronn M, Harter PN, et al. MicroRNA-145 Targets the Metalloprotease ADAM17 and Is Suppressed in Renal Cell Carcinoma Patients. Neoplasia (2013) 15(2):218–IN231. doi: 10.1593/neo.121222
90. Fan W, Huang J, Xiao H, Liang Z. MicroRNA-22 is downregulated in clear cell renal cell carcinoma, and inhibits cell growth, migration and invasion by targeting PTEN. Mol Med Rep (2016) 13(6):4800–6. doi: 10.3892/mmr.2016.5101
91. Hong Q, Li O, Zheng W, Xiao W-z, Zhang L, Wu D, et al. LncRNA HOTAIR regulates HIF-1α/AXL signaling through inhibition of miR-217 in renal cell carcinoma. Cell Death Disease (2017) 8(5):e2772–2. doi: 10.1038/cddis.2017.181
92. Heinemann FG, Tolkach Y, Deng M, Schmidt D, Perner S, Kristiansen G, et al. Serum miR-122-5p and miR-206 expression: non-invasive prognostic biomarkers for renal cell carcinoma. Clin Epigenetics (2018) 10(1):11. doi: 10.1186/s13148-018-0444-9
93. He H, Wang L, Zhou W, Zhang Z, Wang L, Xu S, et al. MicroRNA expression profiling in clear cell renal cell carcinoma: identification and functional validation of key miRNAs. PloS One (2015) 10(5):e0125672. doi: 10.1371/journal.pone.0125672
94. He C, Zhao X, Jiang H, Zhong Z, Xu R. Demethylation of miR-10b plays a suppressive role in ccRCC cells. Int J Clin Exp Pathol (2015) 8(9):10595–604.
95. Huang J, Yao X, Zhang J, Dong B, Chen Q, Xue W, et al. Hypoxia-induced downregulation of miR-30c promotes epithelial-mesenchymal transition in human renal cell carcinoma. Cancer Sci (2013) 104(12):1609–17. doi: 10.1111/cas.12291
96. Huang X, Huang M, Kong L, Li Y. miR-372 suppresses tumour proliferation and invasion by targeting IGF2BP1 in renal cell carcinoma. Cell Proliferation (2015) 48(5):593–9. doi: 10.1111/cpr.12207
97. Jiao D, Wu M, Ji L, Liu F, Liu Y. MicroRNA-186 Suppresses Cell Proliferation and Metastasis Through Targeting Sentrin-Specific Protease 1 in Renal Cell Carcinoma. Oncol Res (2018) 26(2):249–59. doi: 10.3727/096504017X14953948675430
98. Khella HW, Scorilas A, Mozes R, Mirham L, Lianidou E, Krylov SN, et al. Low expression of miR-126 is a prognostic marker for metastatic clear cell renal cell carcinoma. Am J Pathol (2015) 185(3):693–703. doi: 10.1016/j.ajpath.2014.11.017
99. Kulkarni P, Dasgupta P, Bhat NS, Shahryari V, Shiina M, Hashimoto Y, et al. Elevated miR-182-5p Associates with Renal Cancer Cell Mitotic Arrest through Diminished MALAT-1 Expression. Mol Cancer Res (2018) 16(11):1750–60. doi: 10.1158/1541-7786.MCR-17-0762
100. Liu F, Chen N, Xiao R, Wang W, Pan Z. miR-144-3p serves as a tumor suppressor for renal cell carcinoma and inhibits its invasion and metastasis by targeting MAP3K8. Biochem Biophys Res Communications (2016) 480(1):87–93. doi: 10.1016/j.bbrc.2016.10.004
101. Liu F, Wu L, Wang A, Xu Y, Luo X, Liu X, et al. MicroRNA-138 attenuates epithelial-to-mesenchymal transition by targeting SOX4 in clear cell renal cell carcinoma. Am J Trans Res (2017) 9(8):3611–22.
102. Okato A, Arai T, Yamada Y, Sugawara S, Koshizuka K, Fujimura L, et al. Dual Strands of Pre-miR-149 Inhibit Cancer Cell Migration and Invasion through Targeting FOXM1 in Renal Cell Carcinoma. Int J Mol Sci (2017) 18(9):1969. doi: 10.3390/ijms18091969
103. Nofech-Mozes R, Khella HWZ, Scorilas A, Youssef L, Krylov SN, Lianidou E, et al. MicroRNA-194 is a Marker for Good Prognosis in Clear Cell Renal Cell Carcinoma. Cancer Med (2016) 5(4):656–64. doi: 10.1002/cam4.631
104. Machackova T, Mlcochova H, Stanik M, Dolezel J, Fedorko M, Pacik D, et al. MiR-429 is linked to metastasis and poor prognosis in renal cell carcinoma by affecting epithelial-mesenchymal transition. Tumor Biol (2016) 37(11):14653–8. doi: 10.1007/s13277-016-5310-9
105. Qin Z, Wei X, Jin N, Wang Y, Zhao R, Hu Y, et al. MiR-199a targeting ROCK1 to affect kidney cell proliferation, invasion and apoptosis. Artif Cells Nanomed Biotechnol (2018) 46(8):1920–5. doi: 10.1080/21691401.2017.1396224
106. Pan Y-J, Wei L-L, Wu X-J, Huo F-C, Mou J, Pei D-S. MiR-106a-5p inhibits the cell migration and invasion of renal cell carcinoma through targeting PAK5. Cell Death Disease (2017) 8(10):e3155–5. doi: 10.1038/cddis.2017.561
107. Wang D, Zhu C, Zhang Y, Zheng Y, Ma F, Su L, et al. MicroRNA-30e-3p inhibits cell invasion and migration in clear cell renal cell carcinoma by targeting Snail1. Oncol Lett (2017) 13(4):2053–8. doi: 10.3892/ol.2017.5690
108. Wang C, Wu C, Yang Q, Ding M, Zhong J, Zhang C-Y, et al. miR-28-5p acts as a tumor suppressor in renal cell carcinoma for multiple antitumor effects by targeting RAP1B. Oncotarget (2016) 7(45):73888–902. doi: 10.18632/oncotarget.12516
109. Wu A, Wu K, Li M, Bao L, Shen X, Li S, et al. Upregulation of microRNA-492 induced by epigenetic drug treatment inhibits the malignant phenotype of clear cell renal cell carcinoma in vitro. Mol Med Rep (2015) 12(1):1413–20. doi: 10.3892/mmr.2015.3550
110. Wang M, Gao H, Qu H, Li J, Liu K, Han Z. MiR-137 suppresses tumor growth and metastasis in clear cell renal cell carcinoma. Pharmacol Rep (2018) 70(5):963–71. doi: 10.1016/j.pharep.2018.04.006
111. Xiang C, S-p C, Ke Y. MiR-144 inhibits cell proliferation of renal cell carcinoma by targeting MTOR. J Huazhong Univ Sci Technol [Medical Sciences] (2016) 36(2):186–92. doi: 10.1007/s11596-016-1564-0
112. Xu M, Gu M, Zhang K, Zhou J, Wang Z, Da J. miR-203 inhibition of renal cancer cell proliferation, migration and invasion by targeting of FGF2. Diagn Pathol (2015) 10:24–4. doi: 10.1186/s13000-015-0255-7
113. Xu X, Liu C, Bao J. Hypoxia-induced hsa-miR-101 promotes glycolysis by targeting TIGAR mRNA in clear cell renal cell carcinoma. Mol Med Rep (2017) 15(3):1373–8. doi: 10.3892/mmr.2017.6139
114. Zhang H, Li H. miR-137 inhibits renal cell carcinoma growth in vitro and in vivo. Oncol Lett (2016) 12(1):715–20. doi: 10.3892/ol.2016.4616
115. Zhu S, Huang Y, Su X. Mir-451 Correlates with Prognosis of Renal Cell Carcinoma Patients and Inhibits Cellular Proliferation of Renal Cell Carcinoma. Med Sci Monit Int Med J Exp Clin Res (2016) 22:183–90. doi: 10.12659/MSM.896792
116. Zhao X, Zhao Z, Xu W, Hou J, Du X. Down-regulation of miR-497 is associated with poor prognosis in renal cancer. Int J Clin Exp Pathol (2015) 8(1):758–64.
117. Zhang X, Xing N-D, Lai C-J, Liu R, Jiao W, Wang J, et al. MicroRNA-375 Suppresses the Tumor Aggressive Phenotypes of Clear Cell Renal Cell Carcinomas through Regulating YWHAZ. Chin Med J (2018) 131(16):1944–50. doi: 10.4103/0366-6999.238153
118. Sun X, Lou L, Zhong K, Wan L. MicroRNA-451 regulates chemoresistance in renal cell carcinoma by targeting ATF-2 gene. Exp Biol Med (Maywood NJ) (2017) 242(12):1299–305. doi: 10.1177/1535370217701625
119. Chan Y, Yu Y, Wang G, Wang C, Zhang D, Wang X, et al. Inhibition of MicroRNA-381 Promotes Tumor Cell Growth and Chemoresistance in Clear-Cell Renal Cell Carcinoma. Med Sci Monit Int Med J Exp Clin Res (2019) 25:5181–90. doi: 10.12659/MSM.915524
120. Long Q-Z, Du Y-F, Liu X-G, Li X, He D-L. miR-124 represses FZD5 to attenuate P-glycoprotein-mediated chemo-resistance in renal cell carcinoma. Tumor Biol (2015) 36(9):7017–26. doi: 10.1007/s13277-015-3369-3
121. Tang K, Xu H. Prognostic value of meta-signature miRNAs in renal cell carcinoma: an integrated miRNA expression profiling analysis. Sci Rep (2015) 5:10272. doi: 10.1038/srep10272
122. Tusong H, Maolakuerban N, Guan J, Rexiati M, Wang WG, Azhati B, et al. Functional analysis of serum microRNAs miR-21 and miR-106a in renal cell carcinoma. Cancer Biomark (2017) 18(1):79–85. doi: 10.3233/CBM-160676
123. Wang C, Ding M, Zhu Y-Y, Hu J, Zhang C, Lu X, et al. Circulating miR-200a is a novel molecular biomarker for early-stage renal cell carcinoma. ExRNA (2019) 1(1):25. doi: 10.1186/s41544-019-0023-z
124. Chanudet E, Wozniak MB, Bouaoun L, Byrnes G, Mukeriya A, Zaridze D, et al. Large-scale genome-wide screening of circulating microRNAs in clear cell renal cell carcinoma reveals specific signatures in late-stage disease. Int J cancer (2017) 141(9):1730–40. doi: 10.1002/ijc.30845
125. Sage AP, Minatel BC, Marshall EA, Martinez VD, Stewart GL, Enfield KSS, et al. Expanding the miRNA transcriptome of human kidney and renal cell carcinoma. Int J Genomics (2018) 2018. doi: 10.1155/2018/6972397
126. Kurahashi R, Kadomatsu T, Baba M, Hara C, Itoh H, Miyata K, et al. MicroRNA-204-5p: A novel candidate urinary biomarker of Xp11. 2 translocation renal cell carcinoma. Cancer Sci (2019) 110(6):1897. doi: 10.1111/cas.14026
127. Nakamura T, Iwamoto T, Nakamura HM, Shindo Y, Saito K, Yamada A, et al. Regulation of miR-1-mediated connexin 43 expression and cell proliferation in dental epithelial cells. Front Cell Dev Biol (2020) 8:156. doi: 10.3389/fcell.2020.00156
128. Chen B, Duan L, Yin G, Tan J, Jiang X. miR-381, a novel intrinsic WEE1 inhibitor, sensitizes renal cancer cells to 5-FU by up-regulation of Cdc2 activities in 786-O. J Chemother (2013) 25(4):229–38. doi: 10.1179/1973947813Y.0000000092
129. Zhang Q, Di W, Dong Y, Lu G, Yu J, Li J, et al. High serum miR-183 level is associated with poor responsiveness of renal cancer to natural killer cells. Tumour Biol J Int Soc Oncodevelopmental Biol Med (2015) 36(12):9245–9. doi: 10.1007/s13277-015-3604-y
130. Xiao W, Wang X, Wang T, Xing J. MiR-223-3p promotes cell proliferation and metastasis by downregulating SLC4A4 in clear cell renal cell carcinoma. Aging (2019) 11(2):615–33. doi: 10.18632/aging.101763
131. Baumann V, Winkler J. miRNA-based therapies: strategies and delivery platforms for oligonucleotide and non-oligonucleotide agents. Future Med Chem (2014) 6(17):1967–84. doi: 10.4155/fmc.14.116
132. Youssef YM, White NM, Grigull J, Krizova A, Samy C, Mejia-Guerrero S, et al. Accurate molecular classification of kidney cancer subtypes using microRNA signature. Eur Urol (2011) 59(5):721–30. doi: 10.1016/j.eururo.2011.01.004
133. von Brandenstein M, Pandarakalam JJ, Kroon L, Loeser H, Herden J, Braun G, et al. MicroRNA 15a, inversely correlated to PKCα, is a potential marker to differentiate between benign and malignant renal tumors in biopsy and urine samples. Am J Pathol (2012) 180(5):1787–97. doi: 10.1016/j.ajpath.2012.01.014
Keywords: miRNA, renal cell carcinoma, expression, cancer, biomarker
Citation: Ghafouri-Fard S, Shirvani-Farsani Z, Branicki W and Taheri M (2020) MicroRNA Signature in Renal Cell Carcinoma. Front. Oncol. 10:596359. doi: 10.3389/fonc.2020.596359
Received: 19 August 2020; Accepted: 22 October 2020;
Published: 30 November 2020.
Edited by:
Massimiliano Berretta, Centro di Riferimento Oncologico di Aviano (IRCCS), ItalyReviewed by:
Luciana N. S. Andrade, Universidad de São Paulo, BrazilMarco Sciacovelli, University of Cambridge, United Kingdom
Copyright © 2020 Ghafouri-Fard, Shirvani-Farsani, Branicki and Taheri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Taheri, bW9oYW1tYWRfODIzQHlhaG9vLmNvbQ==; bW9oYW1tYWQudGFoZXJpQHNibXUuYWMuaXI=
 Soudeh Ghafouri-Fard1
Soudeh Ghafouri-Fard1 Zeinab Shirvani-Farsani
Zeinab Shirvani-Farsani Mohammad Taheri
Mohammad Taheri