
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 29 January 2021
Sec. Cancer Epidemiology and Prevention
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.596355
This article is part of the Research TopicThe Role of Diet and Nutrition in Cancer Epidemiology: Recent Advancements and Future DirectionView all 5 articles
Background: Chronic gastritis along with Helicobacter pylori (H. pylori) infection has been implicated in inflammatory response-related genes linked to the causation of gastric cancer. Glutathione S-transferase Pi (GSTP1) plays a role in regulating oxidative stress and detoxification against carcinogenesis. In this study, we aimed to determine whether an antioxidant-rich diet is associated with gastric cancer risk and identify how this association could be altered by GSTP1 genetic variants.
Methods: This study included 1,245 participants (415 cases and 830 controls) matched for age and sex. The dietary antioxidant capacity was estimated based on the oxygen radical absorbance capacity (ORAC) incorporated with a semiquantitative food frequency questionnaire. Five single nucleotide polymorphisms (SNPs) of GSTP1 (rs1695, rs749174, rs1871042, rs4891, and rs947895) were selected among the exome array genotype data.
Results: High dietary ORAC was inversely associated with gastric cancer (hydrophilic ORAC OR T3 vs. T1, 95% CI = 0.57, 0.39–0.82, P = 0.004; lipophilic ORAC = 0.66, 0.45–0.95, P = 0.021; total phenolics = 0.57, 0.39–0.83, P = 0.005). The polymorphism rs1871042 increased the risk of gastric cancer (OR, 95% CI = 1.55, 1.10–2.16, P = 0.01, CT+TT vs. CC). A remarkably reduced risk of gastric cancer was observed among those who had a high dietary ORAC according to rs1871042 polymorphism (hydrophilic ORAC OR T3 vs. T1, 95% CI = 0.36, 0.17–0.78, P for trend = 0.013; lipophilic ORAC = 0.58, 0.37–0.93, P for trend = 0.021; total phenolics = 0.38, 0.17–0.83, P for trend = 0.019).
Conclusions: Our findings indicate that dietary ORAC intake may be inversely associated with the risk of gastric cancer altered by genetic variants of GSTP1, providing new intervention strategies for gastric cancer patients.
Gastric cancer (GC) was the leading cause of cancer death and the fifth most common cancer worldwide in 2018 (1). Although the global incidence rates of GC have declined, the incidence of GC in East Asia, including Korea, remains high (1, 2). There are several major risk factors for the development of GC, including Helicobacter pylori (H. pylori) infection, smoking, alcohol consumption, obesity, and excess sodium intake (2). Generally, H. pylori infection is a known carcinogen and a strong risk factor for non-cardia GC by the classical histopathologic Correa cascade, consequently resulting in GC (3–5). Helicobacter pylori infection is particularly associated with an increased risk of not only non-cardia GC but also cardia GC according to several studies targeting East Asian countries, such as Korea (5, 6). In addition, evidence suggests that gastritis derived from chronic inflammation of normal mucosa may be linked to various other dietary factors, such as a high intake of salted or preserved foods and grilled or processed meats and a low intake of fruits (7, 8).
Dietary effects have been reported to mediate the risk of cancer by playing a role in either the prevention of cellular carcinoma or diet-induced carcinogenesis (9, 10). Helicobacter pylori infection is required to consider the causes of GC derived from either direct or indirect inflammation in the gastric mucosa (11, 12). Cumulative studies have reported that multiple H. pylori-induced inflammatory responses from reactive oxygen species (ROS) can be suppressed by bioactive compounds abundant in fruits and vegetables (13, 14). Moreover, current studies have consistently demonstrated that a variety of bioactive compounds, such as phytochemicals, play pivotal roles in sympathetic activation ranging from the inhibition of cellular proliferation to the suppression of metastasis in gastric carcinoma cells (15–17). Given that the risk of GC is linked to dietary factors and the inflammatory response associated with H. pylori infection, this study focused on exploring the integrated and synergistic effects of antioxidants on GC using the oxygen radical absorbance capacity (ORAC) of a diet. The ORAC is an experimental value representing the total antioxidant capacity (TAC) and is used to indicate the capacity to scavenge free radicals from food components indicating hydrophilic ORAC (H-ORAC), lipophilic ORAC (L-ORAC), and total phenolics (TPs) (18). The use of ORAC to assess dietary effects on disease has the advantage of exploring the antioxidant activity of food rather than that of a specific nutrient (19). Based on the benefits of using ORAC, an examination of the antioxidant capacity of a diet using this metric in the context of GC risk with inflammation and H. pylori infection is needed.
Among the inflammation- and oxidative stress-related genes, glutathione S-transferase Pi (GSTP1) is a cytosolic detoxifying enzyme that encodes Pi-class glutathione S-transferases (GST) and is involved in phase II xenobiotic metabolism by conjugating glutathione with hydrophobic and electrophilic substrates (20–22). The deregulation of GSTP1 contributes to inducing oxidative stress by producing excessive ROS, leading to various types of tumors, including esophageal, stomach, lung, breast, and colorectal cancer (23–31). Regarding the risk of GC, studies exploring genetic polymorphisms of GSTP1 revealed the role of GSTP1 in and relevance of GSTP1 for GC susceptibility (26, 27). Some epidemiological studies have reported a significant association between GSTP1 rs1695 and the risk of GC (32, 33). One genetic polymorphism of GSTP1, namely, c.313 A > C (rs1695) in exon 5, results in an amino acid change (A to G) of isoleucine (Ile) to valine (Val), leading to impaired detoxification and catalytic activity (34). Furthermore, different genotypes of GSTP1 rs1695 have significant interaction effects with environmental factors, including H. pylori infection, smoking, and alcohol consumption on the risk of GC (35–37). However, evidence regarding the associations between dietary factors and GSTP1 polymorphisms in the context of GC risk based on the effects of antioxidants and imbalanced oxidative stress mechanisms is insufficient.
Given these points, we selected five single nucleotide polymorphisms (SNPs; rs1695, rs749174, rs1871042, rs4891, and rs947895) in GSTP1 that were found among the 713,348 SNPs assayed in a Korean population based on quality control (QC) criteria in genome-wide association studies (GWASs), determined the association between ORAC and GC, and examined whether this association was modified by GSTP1. The aim of this study was to identify how dietary ORAC intake is associated with GC risk alterations by GSTP1 genetic variants. We evaluated whether dietary ORAC intake affects the risk of GC. Additionally, we explored the associations between GC risk and ORAC intake according to GSTP1 genotypes.
This case-control study was conducted at the Center for Gastric Cancer (CGC) and the Center for Cancer Prevention & Detection (CCPD) of the National Cancer Center (NCC) in Korea between March 2011 and December 2014. The cases were recruited among patients who were diagnosed with early GC within the preceding 3 months with confirmed invasive carcinoma in the CGC. Individuals with diabetes mellitus, severe systemic or mental disease, or a history of cancer and women who were pregnant or breastfeeding were excluded. The control group comprised individuals who underwent health screening check-ups at the CCPD at the same hospital. Participants in the control group who had diabetes mellitus, gastric or duodenal ulcers, a history of cancer, or previous H. pylori treatment were excluded. Of these initial 1,727 participants who were enrolled in this study, 56 individuals with an incomplete semiquantitative food frequency questionnaire (SQFFQ) and 15 individuals with an implausible total energy intake (< 500 or > 4,000 kcal/day) were excluded. Among the remaining 1,656 participants, the cases and controls were frequency-matched at a ratio of 1:2 (case: control) by 5-year age groups and sex. Regarding the genetic variants of GSTP1, we excluded low-quality samples and markers from the cases and controls as follows: rs1695 (n = 38 and n = 74), rs749174 (n = 50 and n = 113), rs1871042 (n = 50 and n = 113), rs4891 (n = 44 and n = 91), and rs947895 (n = 50 and n = 113). Consequently, the total population included the final analysis was as follows: rs1695 (n = 1,133), rs749174 (n = 1,082), rs1871042 (n = 1,082), rs4891 (n = 1,110), and rs947895 (n = 1,082) (Figure 1). This study was approved by the Institutional Review Board (IRB) of NCC (IRB number: NCCNCS-11-438), and written informed consent was obtained from all participants.
The sociodemographic characteristics were collected from each participant using a self-administered questionnaire. The status of H. pylori infection was assessed histologically or serologically from at least a positive result on a rapid urease test (Pronto Dry, Medical Instruments Corporation, Solothurn, Switzerland). The dietary intake data were obtained using a validated and reliable 106-item SQFFQ administered by a well-trained interviewer (38). The daily nutrient intake was calculated based on a combination of average intake frequency (never or rarely, 1 time per month, 2–3 times per month, 1–2 times per week, 3–4 times per week, 5–6 times per week, one time per day, two times per day, and three times per day) and the portion size (small, medium, and large) using CAN-PRO 4.0 (computer aided nutritional analysis program, Korean Nutrition Society, Seoul, Korea).
To estimate the values of the dietary antioxidant capacity, the ORAC database from the USDA release 2 was incorporated into our dietary intake data according to the food description (39). Our previous study reported the associations between dietary ORAC intake and interleukin-6 levels regarding the risk of colorectal cancer (40). Briefly, the ORAC database contains the antioxidant activity level of 326 food items. To calculate the dietary ORAC of each participant, the 106-item SQFFQ was integrated into the ORAC database by common food items. Of these food sources, 56 food items, including mostly fruits and vegetables, from the SQFFQ were considered to have antioxidant compounds and selected for the analysis in this study. It reported H-ORAC and L-ORAC as μmol of trolox equivalents per 100 g (μmol TE/100 g) and TPs as mg gallic acid equivalents per 100 g (mg GAE/100 g). We calculated each daily index of ORAC through the same process applied in the daily nutrient intake.
In this study, the SNPs of GSTP1 were selected based on our previous study using exome array genotype data as described elsewhere (41). The genomic DNA samples were extracted from the peripheral blood leukocytes of all participants. The genotyping was performed using an Affymetrix Axiom® Exome 319 Array containing 318,983 SNPs (Affymetrix Inc., Santa Clara, CA, USA). In the QC procedure applied to genotype data, the samples and genetic markers were excluded according to the call rate (< 95%), deviation from HWE, and MAF (< 0.01) (41, 42). After genotype imputation with an Asian population of 1000 Genome haplotypes phase III, we selected five SNPs of GSTP1 (rs1695, rs749174, rs1871042, rs4891, and rs947895) for further analysis (Supplementary Table S1). The LD patterns of the SNPs were analyzed for the efficient selection of tag SNPs in GSTP1 according to the pairwise D’ and r2 using Haploview (43).
The differences in the sociodemographic, anthropometric, lifestyle factors, and total energy intake between the cases and controls were assessed by using the χ2 test for categorical variables and Student’s t-test for continuous variables. The dietary ORAC intake and foods contributing to each ORAC, covering up to 90% of the cumulative contribution of 56 food items, were compared between the cases and controls by using a Wilcoxon signed-rank test because of its distribution (Supplementary Tables S2–S4). Energy-adjusted ORAC values and their contributing foods adjusted by a residual method were used in the analyses (44). The values of H-ORAC, L-ORAC, and TPs were divided into three groups depending on the median value of the controls. To analyze the associations among dietary ORAC intake, GSTP1 polymorphisms and GC risk, unconditional logistic models were constructed to estimate the odds ratios (OR) and 95% confidence intervals (95% CI) of the risk of GC while considering potential confounding factors, such as age, BMI, education level, income, physical activity, smoking status, first-degree family history of GC, and total energy intake identified by the backward selection procedure in a stepwise regression analysis. The H. pylori infection status was additionally considered in the final statistical models. The analyses of the associations between dietary ORAC intake and GC risk were also stratified by GSTP1 SNPs, particularly in the dominant model. All tests were performed using the SAS package (SAS 9.4; SAS Institute Inc., Cary, NC, USA) with a two-sided P-value of 0.05 regarded as significant.
Table 1 shows the distribution of the sociodemographic characteristics and dietary ORAC intake in the cases and controls. The GC patients had a higher prevalence of positive H. pylori infection, a first-degree family history of GC and current smoking status, and a lower prevalence of regular exercise, education level, and income than the control group (P < 0.05). However, no differences in age, sex, BMI, or alcohol consumption were observed.
The cases had a higher intake of daily total energy than the controls (1,924.11 ± 612.91 kcal/day vs. 1,713.59 ± 545.52 kcal/day, P < 0.001). Regarding the three components of dietary ORAC intake, the mean and median values of H-ORAC, L-ORAC, and TPs in the cases were lower than those in the controls (mean intake; H-ORAC, 3,443.90 ± 2,988.95 μmol TE/day vs. 4,485.77 ± 3,371.96 μmol TE/day, P < 0.001; L-ORAC, 166.75 ± 97.75 μmol TE/day vs. 197.75 ± 117.55 μmol TE/day, P < 0.001; TPs, 307.85 ± 282.93 mg GAE/day vs. 423.17 ± 367.41 mg GAE/day, P < 0.001, cases vs. controls).
The associations between each index of dietary ORAC intake and GC risk are presented in Table 2. Compared to the lowest tertiles of H-ORAC, L-ORAC, and TPs, the highest tertiles of these three indices were significantly associated with GC risk after adjusting for all confounding factors. A decreased risk of GC was observed in those with a higher dietary ORAC intake as follows: H-ORAC (OR T3 vs. T1, 95% CI = 0.57, 0.39–0.82, P = 0.004); L-ORAC (OR T3 vs. T1, 95% CI = 0.66, 0.45–0.95, P = 0.021); and TPs (OR T3 vs. T1, 95% CI = 0.57, 0.39–0.83, P = 0.005).
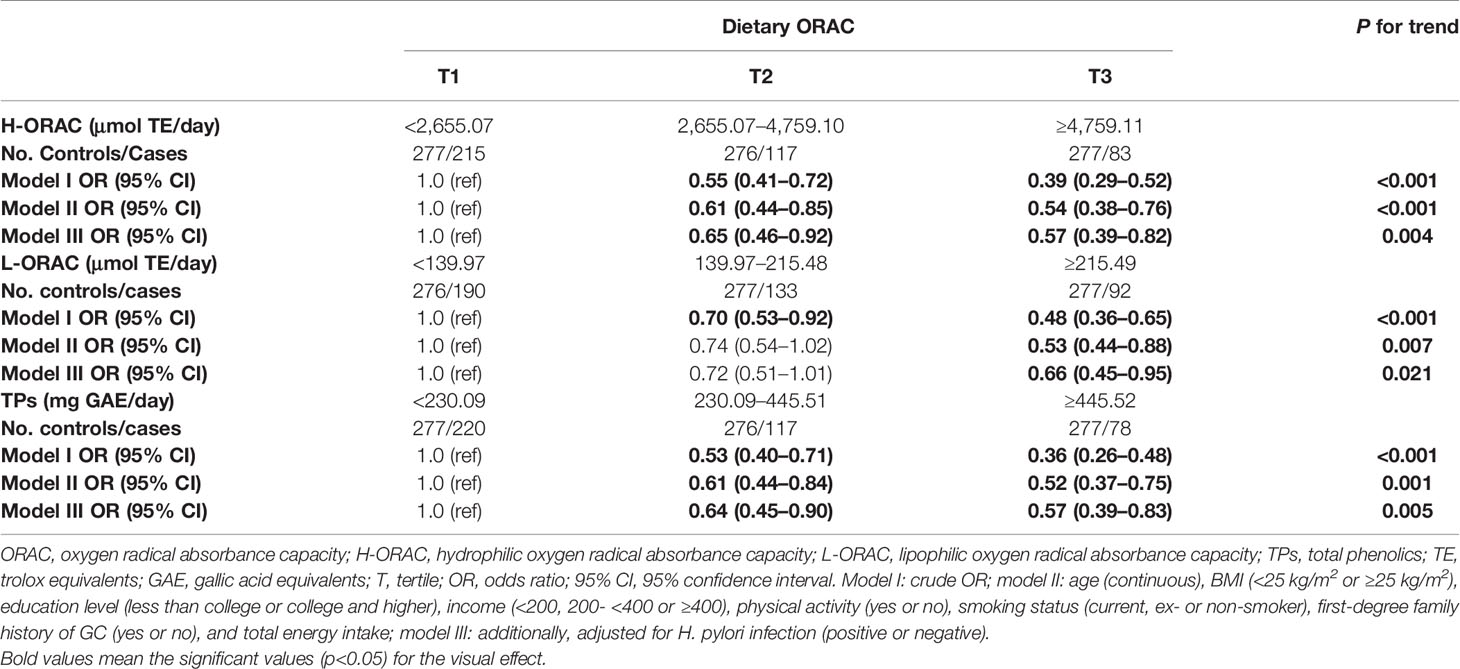
Table 2 Association between dietary oxygen radical absorbance capacity (ORAC) intake and gastric cancer (GC) risk.
The minor allele frequencies of the five SNPs (rs1695, rs749174, rs1871042, rs4891, and rs947895) were common (minor allele frequency, MAF > 5%), and the genotype frequencies of the SNPs were consistent with Hardy-Weinberg equilibrium (HWE) (Supplementary Table S1). We identified the linkage disequilibrium (LD) structure of the five SNPs using a pairwise LD test (Figure 2). We observed that all five SNPs of GSTP1 gene were in the same block with high LD, supporting the strong correlation among the five SNPs in this study. According to the LD patterns with r2 > 0.8, tag SNPs (rs1695 and rs1871042) in GSTP1 were selected. Table 3 presents the associations between GSTP1 variants and GC risk. Apart from H. pylori infection, an increased risk of GC was found among those with heterozygous variants of GSTP1, namely, rs1871042 (OR, 95% CI = 1.60, 1.14–2.23, P = 0.006, CT vs. CC). When genetic models of each SNP were compared, the dominant model showed significant associations, but the recessive model did not. In the dominant model, an increased risk of GC was observed in those who carried T allele of rs1871042 (OR, 95% CI = 1.56, 1.13–2.16, P = 0.007, CT+TT vs. CC). After adjusting for confounders, a heterozygous variant of rs1871042 showed a significant association with GC risk (OR, 95% CI = 1.58, 1.12–2.25, P = 0.01, CT vs. CC). In the comparisons within the dominant genetic model, a modest and borderline association was observed between rs1871042 of GSTP1 and GC risk (OR, 95% CI = 1.55, 1.10–2.16, P = 0.010, CT+TT vs. CC). However, there was no association between rs1695 polymorphism and GC risk. Additionally, the remaining SNPs in GSTP1 showed associations with GC risk in the dominant genetic model as follows: rs749174 (OR, 95% CI = 1.55, 1.11–2.17, P = 0.010, GA+AA vs. GG); rs4891 (OR, 95% CI = 1.52, 1.09–2.10, P = 0.012, TC+CC vs. TT); and rs947895 (OR, 95% CI = 1.55, 1.10–2.16, P = 0.011, CA+AA vs. CC) (Supplementary Table S5).
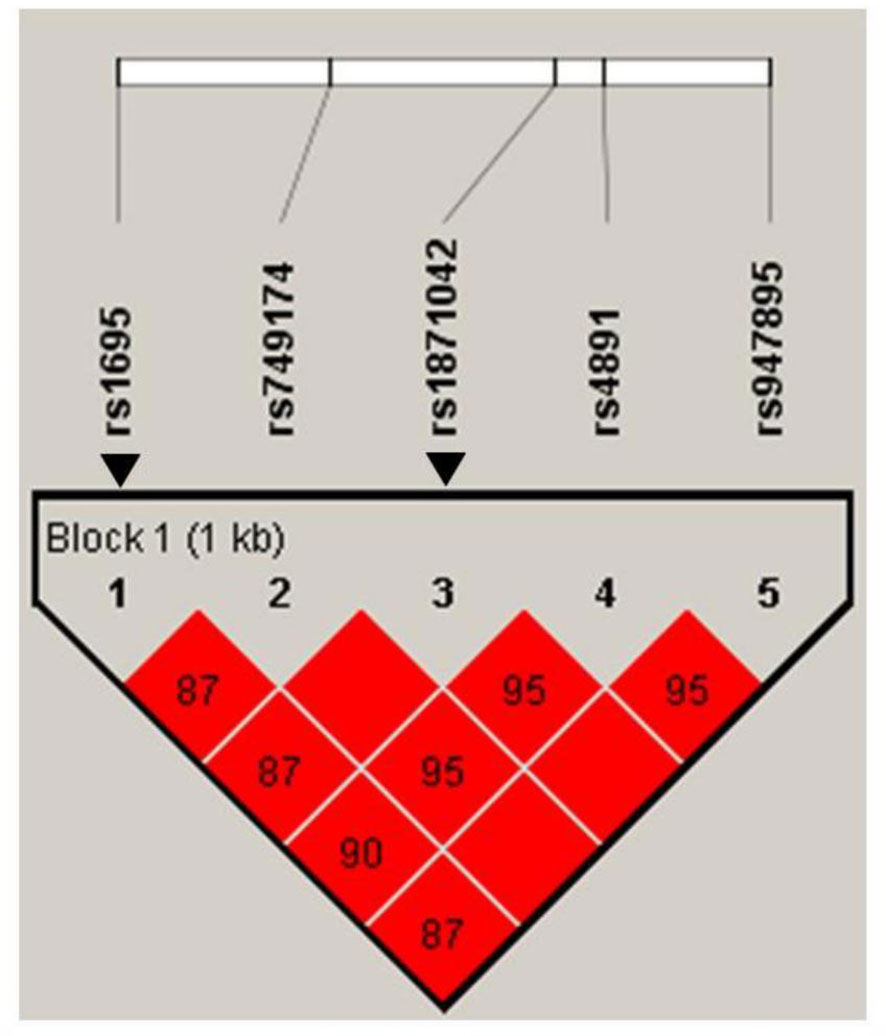
Figure 2 Haploview linkage disequilibrium (LD) patterns of GSTP1 polymorphisms in chromosome 11. Pairwise LD is expressed as D’ (colors) and r2 (numbers). Arrows indicate Tag SNPs of GSTP1 gene.
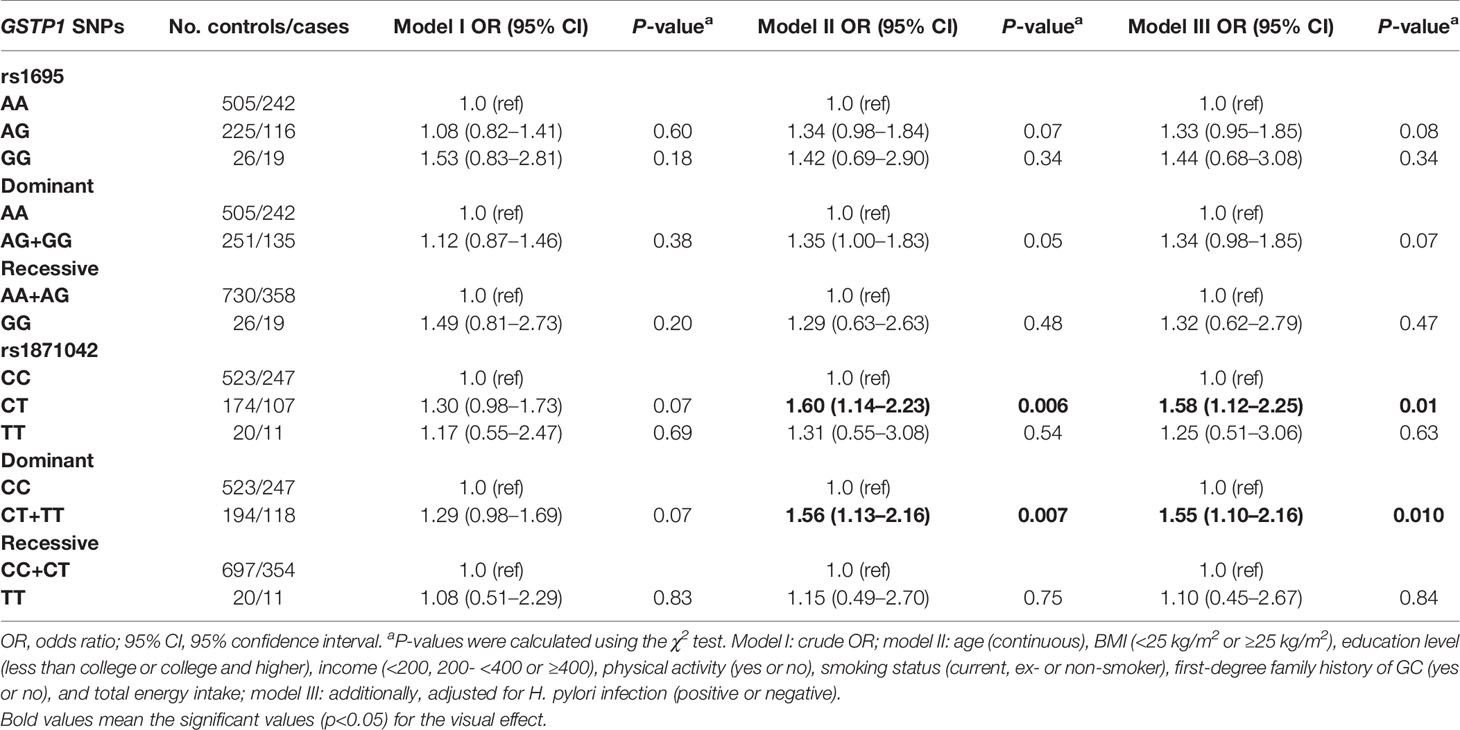
Table 3 Association between tag single nucleotide polymorphisms (SNPs) of GSTP1 gene and gastric cancer (GC) risk.
Table 4 shows the associations between dietary intake and GC risk in the dominant model of rs1871042 GSTP1 polymorphism. Inverse associations between dietary H-ORAC intake and GC risk were observed among those with the rs1871042 T allele after fully adjusting for potential confounders (OR T3 vs. T1, 95% CI = 0.36, 0.17–0.78, P = 0.013). The analysis showed a similar pattern between dietary TPs intake and GC risk (OR T3 vs. T1, 95% CI = 0.38, 0.17–0.83, P = 0.019). However, a high intake of dietary L-ORAC decreased the GC risk with a homozygous variant of CC genotype (OR T3 vs. T1, 95% CI = 0.58, 0.37–0.93, P = 0.021). Without considering H. pylori infection status, higher H-ORAC and TPs intake were consistently associated with a decreased risk of GC with rs1871042 T allele (H-ORAC OR T3 vs. T1, 95% CI = 0.31, 0.15–0.64, P = 0.003; TPs OR T3 vs. T1, 95% CI = 0.34, 0.16–0.71, P = 0.005). Moreover, higher L-ORAC and TPs intake was associated with a reduced risk of GC in those with the minor allele (L-ORAC OR T3 vs. T1, 95% CI = 0.58, 0.37–0.91, P = 0.016; TPs OR T3 vs. T1, 95% CI = 0.61, 0.38–0.97, P = 0.046). The remaining SNPs (rs749174, rs4891, and rs947895) in GSTP1 showed similar patterns of association with dietary ORAC intake on GC risk (Supplementary Table S6). Regarding the interaction effect between dietary ORAC intake and GSTP1 rs1871042 polymorphism on gastric cancer risk, each dietary ORAC intake was divided into low and high groups based on the median level of the intake of controls (Supplementary Table S7). Although there are no interaction effects between dietary ORAC intake and GSTP1 rs1871042 polymorphism on gastric cancer, a high intake of ORAC significantly reduced the risk of gastric cancer in patients homozygous for CC at rs1871042 after adjusting for potential confounding factors (OR, 95% CI: H-ORAC = 0.63, 0.44–0.91; L-ORAC = 0.60, 0.42–0.87; TPs = 0.68, 0.47-0.99). However, a low intake of H-ORAC and TPs while carrying a T allele (CT+TT) increased the risk of gastric cancer compared with that observed in the CC homozygous patients (OR, 95% CI: H-ORAC = 1.64, 1.08–2.49; TPs = 1.67, 1.11–2.53).
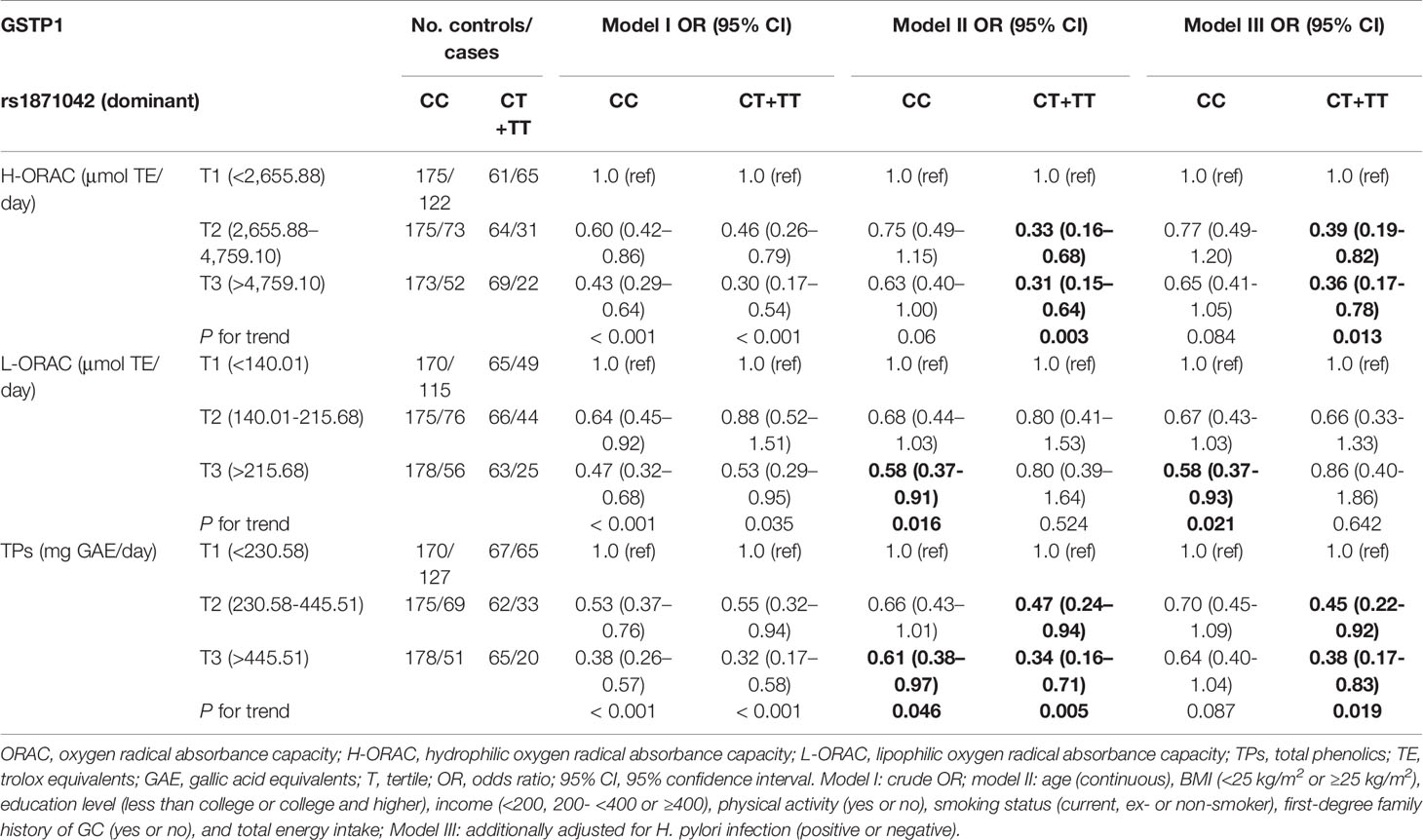
Table 4 Association between dietary oxygen radical absorbance capacity (ORAC) intake and gastric cancer (GC) risk by rs1871042 polymorphism of GSTP1 gene.
The present study aimed to determine the association between dietary ORAC intake and GC risk according to GSTP1 genetic variants. A high dietary intake of ORAC was significantly associated with a decreased risk of GC in a Korean population. Regarding the genetic variants of GSTP1 gene, dietary ORAC intake was inversely associated with GC risk according to GSTP1 rs1871042 genotypes.
Gastric adenocarcinoma occurs when normal mucosa cells are continuously exposed to a variety of carcinogens that lead to uncontrolled cell proliferation in the gastric mucosa membrane (45). The following two major mechanisms are linked to the development of GC with H. pylori infection: (1) epigenetic alterations in gastric epithelial cells by H. pylori infection and (2) H. pylori-induced inflammation in the gastric mucosa (46). Many studies have shown that persistent inflammation, through cytokines, chemokines, growth factors, and oxygen-derived free radicals is responsible for GC risk associated with H. pylori infection (46, 47). The role of oxidative stress from inflammation in GC has been determined, suggesting the importance of a balance between radical production and the antioxidant defense system (48). Numerous studies have reported that the intake of fruits and vegetables is inversely associated with GC risk, while some studies found no such associations (49–53). Specifically, a high intake of fruits by H. pylori-negative subjects decreased the risk of GC compared to a low intake of fruits by H. pylori-positive subjects, indicating that the intake of fruits and vegetables may play a role in preventing H. pylori-induced gastric carcinogenesis (52–54). In contrast, data regarding the effects of vitamin A, vitamin C, vitamin E, and carotenoids on GC risk were inconsistent or conflicting due to the different doses used (55–57). In this study, we examined the antioxidant capacity of food and determined the antioxidant effects of ORAC on gastric carcinogenesis. A recent meta-analysis reported inverse associations between cancer risk and dietary TAC by using multiple methods, including ORAC (58). Other previous studies found inverse associations between ORAC intake and risk of other cancers but not GC (59–62). We observed similar findings between GC risk and three indices of dietary ORAC, namely, H-ORAC, L-ORAC, and TPs, after adjusting for H. pylori infection and other potential confounding factors. Furthermore, in the comparisons of the food items that highly contribute to the ORAC level, the food items with the highest ORAC were brewed green tea and fruits for H-ORAC, spicy red, or black pepper for L-ORAC, and canned tomato juice for TPs (Supplementary Tables S2–S4).
The major function of GSTP1 is to detoxify exogenous or endogenous factors involved in carcinogenesis by regulating cell death and DNA damage (21, 63). Additionally, GSTP1 plays a role as a modifier gene in the regulation of the molecular expression and activation of enzymes from other GST subfamilies and their effects on cancer, and GSTP1 expression regulates cellular redox homeostasis in carcinogenesis (20, 64, 65). Although many studies have shown associations between GSTP1 polymorphisms and various types of cancer, the results of a few studies investigating the associations between GC risk and GSTP1 genetic variants are inconsistent across geographic areas and diverse populations. In a Chinese population, the Val allele of GSTP1, namely, the Val/Val genotype, was significantly associated with an increased risk of GC (37, 66, 67). However, GSTP1 Ile105Val (rs1695) and GSTP1 Val114Ala (rs1138272) polymorphisms were not associated with the risk of GC in either a South European or an Indian population (68, 69). In a Korean population, we observed that five GSTP1 polymorphisms (rs1695, rs749174, rs1871042, rs4891, and rs947895) located in the same block with a strong correlation with high LD had a tendency to increase GC risk, although the risk increase with rs1695 polymorphism was not statistically significant. These conflicting results suggest that ethnic differences in GSTP1 genetic susceptibility may affect the development of GC with epigenetic interactions of environmental factors and that the relevance of GSTP1 genetic variants to GC risk needs to be confirmed in future studies. Among five GSTP1 polymorphisms examined in this study, four polymorphisms (rs749174, rs1871042, rs4891, and rs947895) have been investigated in only a few studies in the context of lung cancer and asthma, and to date, their associations with GC risk have not been determined (70–72).
In this study, we observed an association between a high intake of dietary ORAC and a reduced GC risk according to GSTP1 rs1871042 polymorphism. Our findings can be explained by the interconnections between dietary TAC and the role of GSTP1 gene in the regulation of oxidative stress and detoxification of the immune response against gastric carcinogenesis-induced chronic inflammation by H. pylori infection. Imbalanced oxidative stress plays an obligatory role in gastric carcinogenesis by increasing the level of ROS induced by H. pylori infection, leading to DNA damage and tumor progression (4, 73). A high intake of dietary ORAC is responsible for the scavenging substances produced by H. pylori-infected gastric cells and, thus, may protect against the promotion of gastric carcinogenesis. More than half of H. pylori strains produce various cytotoxins, such as Cag-A, which can damage gastric mucosal cell membranes and trigger local immune responses (74). Previous studies have shown that vitamin C protects against H. pylori infection-related GC by neutralizing free radicals and directly modifying the anticancer immune response against malignant progression (75, 76). In addition to the role of GSTP1 gene, the specific allele of GSTP1 is able to regulate oxidative stress and detoxification against carcinogenesis (21, 65). Moreover, the impact of H. pylori infection on the relationship between GSTP1 genetic polymorphisms and GC risk varies, suggesting that H. pylori infection may have different oncogenic effects depending on GSTP1 genetic polymorphism, including controlling the activation of the detoxification system, thereby resulting in gastric carcinogenesis (36, 77). A high intake of dietary ORAC may synergistically interact with GSTP1 rs1871042 polymorphism by detoxifying and eradicating excessive ROS, eventually leading to protection against the development of GC.
Nevertheless, some limitations should be noted. First, selection and recall bias should be considered; the controls were recruited among patients who visited the clinic for a health check-up and may have been more health conscious than the patients with GC. To reduce the selection bias, controls who were confirmed to be cancer-free by linking to the Korea Central Cancer Registry database were recruited. However, it may be that the individuals who chose to visit a health check-up program may have a healthier lifestyle than those who did not choose to undergo a check-up. Moreover, the participants provided the structured questionnaire and validated SQFFQ by a well-trained interviewer to reduce the recall bias. The SQFFQ includes the average intake frequency and the portion size during the year preceding the interview. Second, the food items included in our food database were insufficient to cover the entire United States Department of Agriculture (USDA) ORAC database. Additionally, the antioxidant capacity from ORAC is based on in vitro antioxidant assays, which are limited to measuring the absorption rate in the body. Third, the sample size in each tertile of the case group is relatively small. Further prospective studies are needed to confirm and extend our findings with a larger sample size.
In conclusion, this study examined whether the associations between dietary ORAC and GC risk were modified by GSTP1 polymorphisms. We found associations between the risk of GC and dietary ORAC intake, including fruits, vegetables, spices, and nuts, depending on the genetic variants of GSTP1. Considering the highest incidence rates of GC with H. pylori infection in East Asia, the associations among dietary ORAC intake, GSTP1 polymorphisms, and GC risk may provide an effective strategy for the primary prevention of GC in Asian populations.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB) NCC (IRB number: NCCNCS-11-438), and written informed consent was obtained from all participants. The patients/participants provided their written informed consent to participate in this study.
We greatly appreciate the efforts of those who contributed to this study. The authors’ responsibilities were as follows: JMK, HK, and JSK: designed the research. JMK, HK, JL, IC, Y-IK, and JSK: conducted the research. JMK and HK: analyzed the data. JMK, HK, and JSK wrote the paper and are primarily responsible for the final content. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Cancer Center (No. 1410260, 1810980, and 1910330) and National Research Foundation funded by the South Korean Government (2018R1D1A1A09083876).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are thankful to all participants involved in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.596355/full#supplementary-material
BMI, body mass index; CCPD, Center for Cancer Prevention and Detection; CGC, Center for Gastric Cancer; CI, confidence intervals; GC, gastric cancer; GST, glutathione S-transferases; GSTP1, glutathione S-transferase Pi; GWASs, genome-wide association studies; H-ORAC, hydrophilic oxygen radical absorbance capacity; H. pylori, Helicobacter pylori; HWE, Hardy-Weinberg equilibrium; LD, linkage disequilibrium; L-ORAC, lipophilic oxygen radical absorbance capacity; MAF, minor allele frequency; NCC, National Cancer Center; OR, odds ratios; ORAC, oxygen radical absorbance capacity; QC, quality control; ROS, reactive oxygen species; SNP, single nucleotide polymorphism; SQFFQ, semiquantitative food frequency questionnaire; TAC, total antioxidant capacity; TPs, total phenolics.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol (2019) 14(1):26–38. doi: 10.5114/pg.2018.80001
3. Humans IWGotEoCRt. Schistosomes, liver flukes and Helicobacter pylori. Lyon, 7-14 June 1994. France: Lyon International Agency for Research on Cancer (1994). 1994/01/01 ed.
4. Moss SF. The clinical evidence linking Helicobacter pylori to gastric cancer. Cell Mol Gastroenterol Hepatol (2017) 3(2):183–91. doi: 10.1016/j.jcmgh.2016.12.001
5. Cavaleiro-Pinto M, Peleteiro B, Lunet N, Barros H. Helicobacter pylori infection and gastric cardia cancer: systematic review and meta-analysis. Cancer Causes Control (2011) 22(3):375–87. doi: 10.1007/s10552-010-9707-2
6. Bae JM, Kim EH. Helicobacter pylori Infection and risk of gastric cancer in Korea: a quantitative systematic review. J Prev Med Public Health (2016) 49(4):197–204. doi: 10.3961/jpmph.16.024
7. Clinton SK, Giovannucci EL, Hursting SD. The world cancer research fund/American institute for cancer research third expert report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr (2019) 150(4):663–71. doi: 10.1093/jn/nxz268
8. Vingeliene S, Chan DS, Aune D, Vieira AR, Polemiti E, Stevens C, et al. An update of the WCRF/AICR systematic literature review on esophageal and gastric cancers and citrus fruits intake. Cancer Causes Control (2016) 27(7):837–51. doi: 10.1007/s10552-016-0755-0
9. Donaldson MS. Nutrition and cancer: a review of the evidence for an anti-cancer diet. Nutr J (2004) 3:19. doi: 10.1186/1475-2891-3-19
10. Sugimura T. Nutrition and dietary carcinogens. Carcinogenesis (2000) 21(3):387–95. doi: 10.1093/carcin/21.3.387
11. Sepulveda AR. Helicobacter, inflammation, and gastric cancer. Curr Pathobiol Rep (2013) 1(1):9–18. doi: 10.1007/s40139-013-0009-8
12. Qadri Q, Rasool R, Gulzar GM, Naqash S, Shah ZA. H. pylori infection, inflammation and gastric cancer. J Gastrointest Cancer (2014) 45(2):126–32. doi: 10.1007/s12029-014-9583-1
13. De R, Kundu P, Swarnakar S, Ramamurthy T, Chowdhury A, Nair GB, et al. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob Agents Chemother (2009) 53(4):1592–7. doi: 10.1128/aac.01242-08
14. Mahady GB, Pendland SL, Yun G, Lu ZZ. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res (2002) 22(6c):4179–81.
15. Batool S, Joseph TP, Hussain M, Vuai MS, Khinsar KH, Din SRU, et al. LP1 from lentinula edodes C91-3 induces autophagy, apoptosis and reduces metastasis in human gastric cancer cell line SGC-7901. Int J Mol Sci (2018) 19(10):2986. doi: 10.3390/ijms19102986
16. Park HS, Kim GY, Choi IW, Kim ND, Hwang HJ, Choi YW, et al. Inhibition of matrix metalloproteinase activities and tightening of tight junctions by diallyl disulfide in AGS human gastric carcinoma cells. J Food Sci (2011) 76(4):T105–11. doi: 10.1111/j.1750-3841.2011.02114.x
17. Zhu X, Jiang X, Li A, Sun Y, Liu Y, Sun X, et al. S-allylmercaptocysteine suppresses the growth of human gastric cancer xenografts through induction of apoptosis and regulation of MAPK and PI3K/Akt signaling pathways. Biochem Biophys Res Commun (2017) 491(3):821–6. doi: 10.1016/j.bbrc.2017.06.107
18. Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic Biol Med (1993) 14(3):303–11. doi: 10.1016/0891-5849(93)90027-r
19. Cao G, Prior RL. Measurement of oxygen radical absorbance capacity in biological samples. Methods Enzymol (1999) 299:50–62. doi: 10.1016/s0076-6879(99)99008-0
20. Di Pietro G, Magno LA, Rios-Santos F. Glutathione S-transferases: an overview in cancer research. Expert Opin Drug Metab Toxicol (2010) 6(2):153–70. doi: 10.1517/17425250903427980
21. Schnekenburger M, Karius T, Diederich M. Regulation of epigenetic traits of the glutathione S-transferase P1 gene: from detoxification toward cancer prevention and diagnosis. Front Pharmacol (2014) 5:170:170. doi: 10.3389/fphar.2014.00170
22. Henderson CJ, Wolf CR. Disruption of the glutathione transferase Pi class genes. Methods Enzymol (2005) 401:116–35. doi: 10.1016/s0076-6879(05)01007-4
23. Tan X, Chen M. Association between glutathione S-transferases P1 Ile105Val polymorphism and susceptibility to esophageal cancer: evidence from 20 case-control studies. Mol Biol Rep (2015) 42(2):399–408. doi: 10.1007/s11033-014-3781-6
24. Wang S, Zhang J, Jun F, Bai Z. Glutathione S-transferase Pi 1 variant and squamous cell carcinoma susceptibility: a meta-analysis of 52 case-control studies. BMC Med Genet (2019) 20(1):22. doi: 10.1186/s12881-019-0750-x
25. Ogino S, Konishi H, Ichikawa D, Matsubara D, Shoda K, Arita T, et al. Glutathione S-transferase Pi 1 is a valuable predictor for cancer drug resistance in esophageal squamous cell carcinoma. Cancer Sci (2019) 110(2):795–804. doi: 10.1111/cas.13896
26. Mocellin S, Verdi D, Pooley KA, Nitti D. Genetic variation and gastric cancer risk: a field synopsis and meta-analysis. Gut (2015) 64(8):1209–19. doi: 10.1136/gutjnl-2015-309168
27. Xu Z, Zhu H, Luk JM, Wu D, Gu D, Gong W, et al. Clinical significance of SOD2 and GSTP1 gene polymorphisms in Chinese patients with gastric cancer. Cancer (2012) 118(22):5489–96. doi: 10.1002/cncr.27599
28. Liu WZ, Sun Y, Feng X, Bi XH, Liu T, Zhou HF. An updated meta-analysis for association of glutathione S-transferase P1 gene polymorphism with the susceptibility of lung cancer. J Cancer Res Ther (2018) 14(Supplement):S1084–s90. doi: 10.4103/0973-1482.199455
29. Liu HX, Li J, Ye BG. Correlation between gene polymorphisms of CYP1A1, GSTP1, ERCC2, XRCC1, and XRCC3 and susceptibility to lung cancer. Genet Mol Res (2016) 15(4):gmr15048813. doi: 10.4238/gmr15048813
30. Egan KM, Cai Q, Shu XO, Jin F, Zhu TL, Dai Q, et al. Genetic polymorphisms in GSTM1, GSTP1, and GSTT1 and the risk for breast cancer: results from the Shanghai Breast Cancer Study and meta-analysis. Cancer Epidemiol Biomarkers Prev (2004) 13(2):197–204. doi: 10.1158/1055-9965.epi-03-0294
31. Economopoulos KP, Sergentanis TN. GSTM1, GSTT1, GSTP1, GSTA1 and colorectal cancer risk: a comprehensive meta-analysis. Eur J Cancer (2010) 46(9):1617–31. doi: 10.1016/j.ejca.2010.02.009
32. de Araujo RM, de Melo CF, Neto FM, da Silva JN, Soares LF, de Arruda Cardoso Smith M, et al. Association study of SNPs of genes IFNGR1 (rs137854905), GSTT1 (rs71748309), and GSTP1 (rs1695) in gastric cancer development in samples of patient in the northern and northeastern Brazil. Tumour Biol (2014) 35(5):4983–6. doi: 10.1007/s13277-014-1656-z
33. Ghosh S, Ghosh S, Bankura B, Saha ML, Maji S, Ghatak S, et al. Association of DNA repair and xenobiotic pathway gene polymorphisms with genetic susceptibility to gastric cancer patients in West Bengal, India. Tumour Biol (2016) 37(7):9139–49. doi: 10.1007/s13277-015-4780-5
34. Romero A, Martin M, Oliva B, de la Torre J, Furio V, de la Hoya M, et al. Glutathione S-transferase P1 c.313A > G polymorphism could be useful in the prediction of doxorubicin response in breast cancer patients. Ann Oncol (2012) 23(7):1750–6. doi: 10.1093/annonc/mdr483
35. Ghatak S, Yadav RP, Lalrohlui F, Chakraborty P, Ghosh S, Ghosh S, et al. Xenobiotic pathway gene polymorphisms associated with gastric cancer in high risk Mizo-Mongoloid Population, Northeast India. Helicobacter (2016) 21(6):523–35. doi: 10.1111/hel.12308
36. Zhang Y, Sun LP, Xing CZ, Xu Q, He CY, Li P, et al. Interaction between GSTP1 Val allele and H. pylori infection, smoking and alcohol consumption and risk of gastric cancer among the Chinese population. PloS One (2012) 7(10):e47178. doi: 10.1371/journal.pone.0047178
37. Chen ZH, Xian JF, Luo LP. Association between GSTM1, GSTT1, and GSTP1 polymorphisms and gastric cancer risk, and their interactions with environmental factors. Genet Mol Res (2017) 16(1):gmr16018877. doi: 10.4238/gmr16018877
38. Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, et al. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr (2007) 61(12):1435–41. doi: 10.1038/sj.ejcn.1602657
39. Haytowitz DB, Bhagwat S. USDA database for the oxygen radical absorbance capacity (ORAC) of selected foods, release 2 United States Department of Agriculture. USA: Agricultural Research Service (2010).
40. Kim J, Lee J, Oh JH, Chang HJ, Sohn DK, Shin A, et al. Circulating interleukin-6 level, dietary antioxidant capacity, and risk of colorectal cancer. Antioxidants (Basel) (2019) 8(12):595. doi: 10.3390/antiox8120595
41. Kim W, Woo HD, Lee J, Choi IJ, Kim YW, Sung J, et al. Dietary folate, one-carbon metabolism-related genes, and gastric cancer risk in Korea. Mol Nutr Food Res (2016) 60(2):337–45. doi: 10.1002/mnfr.201500384
42. Yang S, Lee J, Choi IJ, Kim YW, Ryu KW, Sung J, et al. Effects of alcohol consumption, ALDH2 rs671 polymorphism, and Helicobacter pylori infection on the gastric cancer risk in a Korean population. Oncotarget (2017) 8(4):6630–41. doi: 10.18632/oncotarget.14250
43. Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics (2005) 21(2):263–5. doi: 10.1093/bioinformatics/bth457
44. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr (1997) 65(4 Suppl):1220S–8S; discussion 9S-31S. doi: 10.1093/ajcn/65.4.1220S
45. Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: classification, histology and application of molecular pathology. J Gastrointest Oncol (2012) 3(3):251–61. doi: 10.3978/j.issn.2078-6891.2012.021
46. Valenzuela MA, Canales J, Corvalan AH, Quest AF. Helicobacter pylori-induced inflammation and epigenetic changes during gastric carcinogenesis. World J Gastroenterol (2015) 21(45):12742–56. doi: 10.3748/wjg.v21.i45.12742
47. Peek RM Jr., Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol (2006) 208(2):233–48. doi: 10.1002/path.1868
48. Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev (2010) 4(8):118–26. doi: 10.4103/0973-7847.70902
49. Larsson SC, Bergkvist L, Wolk A. Fruit and vegetable consumption and incidence of gastric cancer: a prospective study. Cancer Epidemiol Biomarkers Prev (2006) 15(10):1998–2001. doi: 10.1158/1055-9965.Epi-06-0402
50. Epplein M, Shu XO, Xiang YB, Chow WH, Yang G, Li HL, et al. Fruit and vegetable consumption and risk of distal gastric cancer in the Shanghai Women’s and Men’s Health studies. Am J Epidemiol (2010) 172(4):397–406. doi: 10.1093/aje/kwq144
51. Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Int J Cancer (2011) 129(11):2681–93. doi: 10.1002/ijc.25928
52. Wang T, Cai H, Sasazuki S, Tsugane S, Zheng W, Cho ER, et al. Fruit and vegetable consumption, Helicobacter pylori antibodies, and gastric cancer risk: a pooled analysis of prospective studies in China, Japan, and Korea. Int J Cancer (2017) 140(3):591–9. doi: 10.1002/ijc.30477
53. Gonzalez CA, Lujan-Barroso L, Bueno-de-Mesquita HB, Jenab M, Duell EJ, Agudo A, et al. Fruit and vegetable intake and the risk of gastric adenocarcinoma: a reanalysis of the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study after a longer follow-up. Int J Cancer (2012) 131(12):2910–9. doi: 10.1002/ijc.27565
54. Machida-Montani A, Sasazuki S, Inoue M, Natsukawa S, Shaura K, Koizumi Y, et al. Association of Helicobacter pylori infection and environmental factors in non-cardia gastric cancer in Japan. Gastric Cancer (2004) 7(1):46–53. doi: 10.1007/s10120-004-0268-5
55. Kong P, Cai Q, Geng Q, Wang J, Lan Y, Zhan Y, et al. Vitamin intake reduce the risk of gastric cancer: meta-analysis and systematic review of randomized and observational studies. PloS One (2014) 9(12):e116060. doi: 10.1371/journal.pone.0116060
56. Wu Y, Ye Y, Shi Y, Li P, Xu J, Chen K, et al. Association between vitamin A, retinol intake and blood retinol level and gastric cancer risk: a meta-analysis. Clin Nutr (2015) 34(4):620–6. doi: 10.1016/j.clnu.2014.06.007
57. Zhou Y, Wang T, Meng Q, Zhai S. Association of carotenoids with risk of gastric cancer: a meta-analysis. Clin Nutr (2016) 35(1):109–16. doi: 10.1016/j.clnu.2015.02.003
58. Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer (2007) 10(2):75–83. doi: 10.1007/s10120-007-0420-0
59. Holtan SG, O’Connor HM, Fredericksen ZS, Liebow M, Thompson CA, Macon WR, et al. Food-frequency questionnaire-based estimates of total antioxidant capacity and risk of non-Hodgkin lymphoma. Int J Cancer (2012) 131(5):1158–68. doi: 10.1002/ijc.26491
60. Gifkins D, Olson SH, Paddock L, King M, Demissie K, Lu SE, et al. Total and individual antioxidant intake and risk of epithelial ovarian cancer. BMC Cancer (2012) 12:211. doi: 10.1186/1471-2407-12-211
61. Karimi Z, Bahadoran Z, Abedini S, Houshyar-Rad A, Rashidkhani B. Dietary total antioxidant capacity and the risk of breast cancer: a case-control study. East Mediterr Health J (2015) 21(8):564–71. doi: 10.26719/2015.21.8.564
62. Gifkins D, Olson SH, Demissie K, Lu SE, Kong AN, Bandera EV. Total and individual antioxidant intake and endometrial cancer risk: results from a population-based case-control study in New Jersey. Cancer Causes Control (2012) 23(6):887–95. doi: 10.1007/s10552-012-9958-1
63. Laborde E. Glutathione transferases as mediators of signaling pathways involved in cell proliferation and cell death. Cell Death Differ (2010) 17(9):1373–80. doi: 10.1038/cdd.2010.80
64. Marchewka Z, Piwowar A, Ruzik S, Dlugosz A. Glutathione S-transferases class Pi and Mi and their significance in oncology. Postepy Hig Med Dosw (Online) (2017) 71(0):541–50. doi: 10.5604/01.3001.0010.3835
65. Chatterjee A, Gupta S. The multifaceted role of glutathione S-transferases in cancer. Cancer Lett (2018) 433:33–42. doi: 10.1016/j.canlet.2018.06.028
66. Bao LD, Niu JX, Song H, Wang Y, Ma RL, Ren XH, et al. Association between the GSTP1 codon 105 polymorphism and gastric cancer risk: an updated meta-analysis. Asian Pac J Cancer Prev (2012) 13(8):3687–93. doi: 10.7314/apjcp.2012.13.8.3687
67. Wang ZY, Zhou J, Luo L, Huang YL, Dong PD. Predictive role of glutathione-S-transferase gene polymorphisms in the survival of gastric cancer cases. Asian Pac J Cancer Prev (2012) 13(4):1515–8. doi: 10.7314/apjcp.2012.13.4.1515
68. Garcia-Gonzalez MA, Quintero E, Bujanda L, Nicolas D, Benito R, Strunk M, et al. Relevance of GSTM1, GSTT1, and GSTP1 gene polymorphisms to gastric cancer susceptibility and phenotype. Mutagenesis (2012) 27(6):771–7. doi: 10.1093/mutage/ges049
69. Tripathi S, Ghoshal U, Ghoshal UC, Mittal B, Krishnani N, Chourasia D, et al. Gastric carcinogenesis: possible role of polymorphisms of GSTM1, GSTT1, and GSTP1 genes. Scand J Gastroenterol (2008) 43(4):431–9. doi: 10.1080/00365520701742930
70. Gu JD, Hua F, Mei CR, Zheng DJ, Wang GF, Zhou QH. HapMap-based study on the association between MPO and GSTP1 gene polymorphisms and lung cancer susceptibility in Chinese Han population. Acta Pharmacol Sin (2014) 35(5):636–44. doi: 10.1038/aps.2014.11
71. Timofeeva M, Kropp S, Sauter W, Beckmann L, Rosenberger A, Illig T, et al. Genetic polymorphisms of MPO, GSTT1, GSTM1, GSTP1, EPHX1 and NQO1 as risk factors of early-onset lung cancer. Int J Cancer (2010) 127(7):1547–61. doi: 10.1002/ijc.25175
72. Joubert BR, Reif DM, Edwards SW, Leiner KA, Hudgens EE, Egeghy P, et al. Evaluation of genetic susceptibility to childhood allergy and asthma in an African American urban population. BMC Med Genet (2011) 12:25. doi: 10.1186/1471-2350-12-25
73. Suzuki H, Nishizawa T, Tsugawa H, Mogami S, Hibi T. Roles of oxidative stress in stomach disorders. J Clin Biochem Nutr (2012) 50(1):35–9. doi: 10.3164/jcbn.11-115SR
74. Polk DB, Peek RM Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer (2010) 10(6):403–14. doi: 10.1038/nrc2857
75. Cheng XJ, Lin JC, Tu SP. Etiology and prevention of gastric cancer. Gastrointest Tumors (2016) 3(1):25–36. doi: 10.1159/000443995
76. Jakszyn P, Bingham S, Pera G, Agudo A, Luben R, Welch A, et al. Endogenous versus exogenous exposure to N-nitroso compounds and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study. Carcinogenesis (2006) 27(7):1497–501. doi: 10.1093/carcin/bgl019
Keywords: gastric cancer, oxygen radical absorbance capacity, glutathione S-transferase Pi, oxidative stress, antioxidants
Citation: Kim J, Kim H, Lee J, Choi IJ, Kim Y-I and Kim J (2021) Antioxidant-Rich Diet, GSTP1 rs1871042 Polymorphism, and Gastric Cancer Risk in a Hospital-Based Case-Control Study. Front. Oncol. 10:596355. doi: 10.3389/fonc.2020.596355
Received: 19 August 2020; Accepted: 14 December 2020;
Published: 29 January 2021.
Edited by:
Qi-Jun Wu, ShengJing Hospital of China Medical University, ChinaReviewed by:
Liping Sun, The First Affiliated Hospital of China Medical University, ChinaCopyright © 2021 Kim, Kim, Lee, Choi, Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeongseon Kim, anNraW1AbmNjLnJlLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.