- 1Division of Autism Spectrum Disorders and Related Conditions, Department of Psychiatry, Lausanne University Hospital, Lausanne, Switzerland
- 2Institute of Higher Education and Research in Healthcare, University of Lausanne, Lausanne, Switzerland
- 3Department of Oncology, Lausanne University Hospital, Lausanne, Switzerland
- 4Pediatric Hematology-Oncology Unit, Division of Pediatrics, Department “Woman-Mother-Child”, Lausanne University Hospital, Lausanne, Switzerland
- 5Division of Medical Oncology, Department of Oncology, Lausanne University Hospital, Lausanne, Switzerland
- 6Department “Woman-Mother-Child”, Lausanne University Hospital, Lausanne, Switzerland
Delivering optimal cancer care to children, adolescents and adults with ASD has recently become a healthcare priority and represents a major challenge for all providers involved. In this review, and after consideration of the available evidence, we concisely deliver key information on this heterogenous group of neurodevelopmental disorders, as well as recommendations and concrete tools for the enhanced oncological care of this vulnerable population of patients.
Introduction
Providing care to children, adolescents and adults with cancer requires communicating effectively in an age- and development-specific way. With the increase in the complexity of healthcare systems and sophistication of available cancer therapies, individuals with neurodevelopmental challenges, including autism spectrum disorders (ASD), constitute a particularly vulnerable group for disparities in access and outcomes. Oncology providers need to become progressively more educated to the challenges posed by caring for the ASD population and have the necessary tools to implement effective communication and favorable environments for screening, diagnosis and therapy of cancer in this setting.
Therefore, our multidisciplinary expert panel of oncologists, nurses, neurologists and autism mental health specialists (cf. Supplementary Material 1) reviewed the available evidence and provide a) a concise synthesis of the evidence relevant for the optimal oncological care of patients with ASD and b) a discussion of recommendations and strategies and on how teams can enhance their care and provide support for these patients and their families/caregivers in their cancer trajectory.
Definition, Prevalence, and Causes of ASD: Required Basic Knowledge for All Oncology Teams
ASD is a neurodevelopmental condition characterized by social communication deficits, restricted, repetitive and stereotypical patterns of behavior, interests, or activities, and sensory atypicalities (1). It is associated with various levels of intellectual and motor functioning and verbal skills, anxiety, and often with symptoms of other neurodevelopmental disorders, such as Attention Deficit Hyperactivity Disorder (ADHD) (2, 3). A subgroup of patients with ASD without intellectual disability (ID) were referred to/diagnosed as having Asperger’s syndrome prior to the revision leading to the DSM-5 classification. In this population, where a diagnosis is often made later in life, autism without ID remains unrecognized because clinical manifestations can a) overlap with other comorbidities of ASD or other psychiatric disorders, and b) patients, in majority females, can display sophisticated autism compensatory strategies (e.g., social camouflaging) (4, 5).
ASD is a most likely complex polygenic condition, with both de novo and rare inherited variants acting on a background of common genetic polymorphisms. Twin and family studies support the existence of heritable risk factors in ASD (6–8). Associations have been found in genes involved in early brain development (incl. synapse formation/stabilization and neurotransmission), in particular in the gamma-aminobutyric acid (GABA)-ergic system (9). Numerous pre-/perinatal risk factors and environmental exposures have also been suggested (10–13). Two studies also showed an increased prevalence of cancer among children with ASD, and recent genome/exome-wide sequencing studies of de novo and recurrent copy number variations in ASD and cancer have suggested an overlap in genes conferring risk for autism and cancer (14–16).
On an epidemiological level, prevalence of ASD has been steadily increasing over the last two decades, and it is now estimated at 16·8/1,000 (i.e., 1/59) in children of 8 years of age in the United States, with a shift in gender ratios (M:F) recently dropping from 8:1 to of 3:1 (17–19). The increase in prevalence has been attributed, but not exclusively, to a growth in awareness of the disorder with younger ages at diagnosis (20, 21). Additionally, a 2013 revision of the diagnostic criteria part of the “Diagnostic and Statistical Manual of Mental Disorders” (DSM-5) classification has led to an increase of diagnoses of individuals with less severe symptoms (1, 22). Thus, in the past decade, ASD prevalence increased almost 3.5 fold among children aged 2–17 years, mainly accounted for by an eightfold increase of ASD without intellectual disability (17). Alternatively, the increase prevalence has been speculated to be linked to a combination of genetic and environmental risk factors (23, 24). ASD sex ratio variations have been associated with the use of more accurate diagnostic criteria or could be related to sexually dimorphic neuroimmune system activation and the microbiome (25, 26).
Healthcare and Cancer Care for Individuals With ASD
Within the population of individuals with high mental health needs, those with ASD represent a heterogeneous and challenging group in virtually every healthcare area (4, 27). During their lifetime, 70% of patients with ASD will be diagnosed with a medical or psychiatric comorbidity, with a negative impact on educational/employment outcomes (28, 29). In comparison to the general population, individuals with ASD have higher prevalence rates for almost all medical and psychiatric conditions, especially dyslipidemia, obesity, hypertension, gastrointestinal disorders, autoimmune conditions, asthma, allergies, infections, epilepsy, sleep disorders, depression, and visual and hearing impairments (30–35). How these comorbidities influence the risk of developing cancer, how they impact its early detection and the delivery of oncological care, remains unknown.
A recent study based on the United States National Survey of Children’s Health identified an approximately four times higher odds of unmet health care needs in children with ASD when compared to children without disabilities, disproportionately to children with other disabilities (36). Compiling data from research across the last decade, two recent reviews underline the limited availability of specific healthcare services for families with a child with ASD, and the lack of ASD-specific training in healthcare providers (37, 38).
As a possible consequence of underutilization of healthcare resources leading to unmet needs, an increased overall mortality has been observed in the ASD population (39–41). This is associated with complications related to different comorbidities including suicide, accidental death resulting from unsafe behaviors, use of supplemental medications such as atypical antipsychotics, poor nutrition, and insufficient/inappropriate use of healthcare resources (42–45). Specifically, the odds ratio of death from a neoplasm was estimated to be close to double in individuals with ASD (1.83 in females, 1.79 in males) when compared than non-ASD controls (41). This latter analysis was a matched case cohort study linking two nationwide population-based Swedish registers including 27’122 individuals with ASD (n=6240 low-functioning, n=20882 high functioning) and almost 3 million controls. Interestingly, individuals with low-functioning ASD had a higher OR of death from a neoplasm (2.12, 1.25–3.61 95% CI) than their high-functioning counterparts (1.75, 1.39–2.21 95% CI). As the incidence of cancer in ASD has been measured to be globally equal to that of the general population, differences in outcomes are most likely attributable to disparities in optimal cancer care, ranging from limited access to screening, delayed diagnosis and ineffective therapy.
While an early and correct diagnosis of ASD facilitates the deployment of adequate conditions for cancer care, this is possibly not sufficient. Oncology healthcare providers might lack a sufficient understanding of these neuro-developmental conditions, and/or be unaccustomed to the challenges caused by admitting an individual with ASD to their services (i.e., difficulties with emotional rigidity, inflexibility, misunderstanding/misinterpreting social situations, among others). Education and training in communication have been identified as a priority to improve cancer care for all individuals with limited intellectual abilities (46). This is particularly relevant for patients with ASD who generally are heavily dependent on their proxy-network (e.g., parents, siblings, family members in extenso, and other support providers) for their daily activities and health maintenance. National programs to improve education and communication skills of providers have been initiated in several countries, including the United Kingdom’s National Health Service’s “Right to be heard” campaign (47).
Delivering Cancer Care to Individuals With ASD: Potential Obstacles and Solutions
Oncology teams expect concrete strategies to improve their understanding of the challenges faced by in/out-patients with ASD, and to identify their individual needs and the necessary environmental arrangements. Raising awareness and providing specific education for all members of the interprofessional team is the required first step. The abovementioned recent United Kingdom national policy addresses the rationale for a mandatory nation-wide staff training in identifying and specifically supporting patients with autism/learning disabilities and their associated conditions (47). Among the innovative proposals is the concept that, because face-to-face educational opportunities trump e-learning, individuals with ASD should contribute to this training, by advocating for the community’s needs and challenges.
While we await that other implement similarly ambitious national strategies, it is key for oncologists and their teams to have access to information on “red flags”. We therefore summarized the main features hinting to ASD in Table 1, categorizing them by age group.
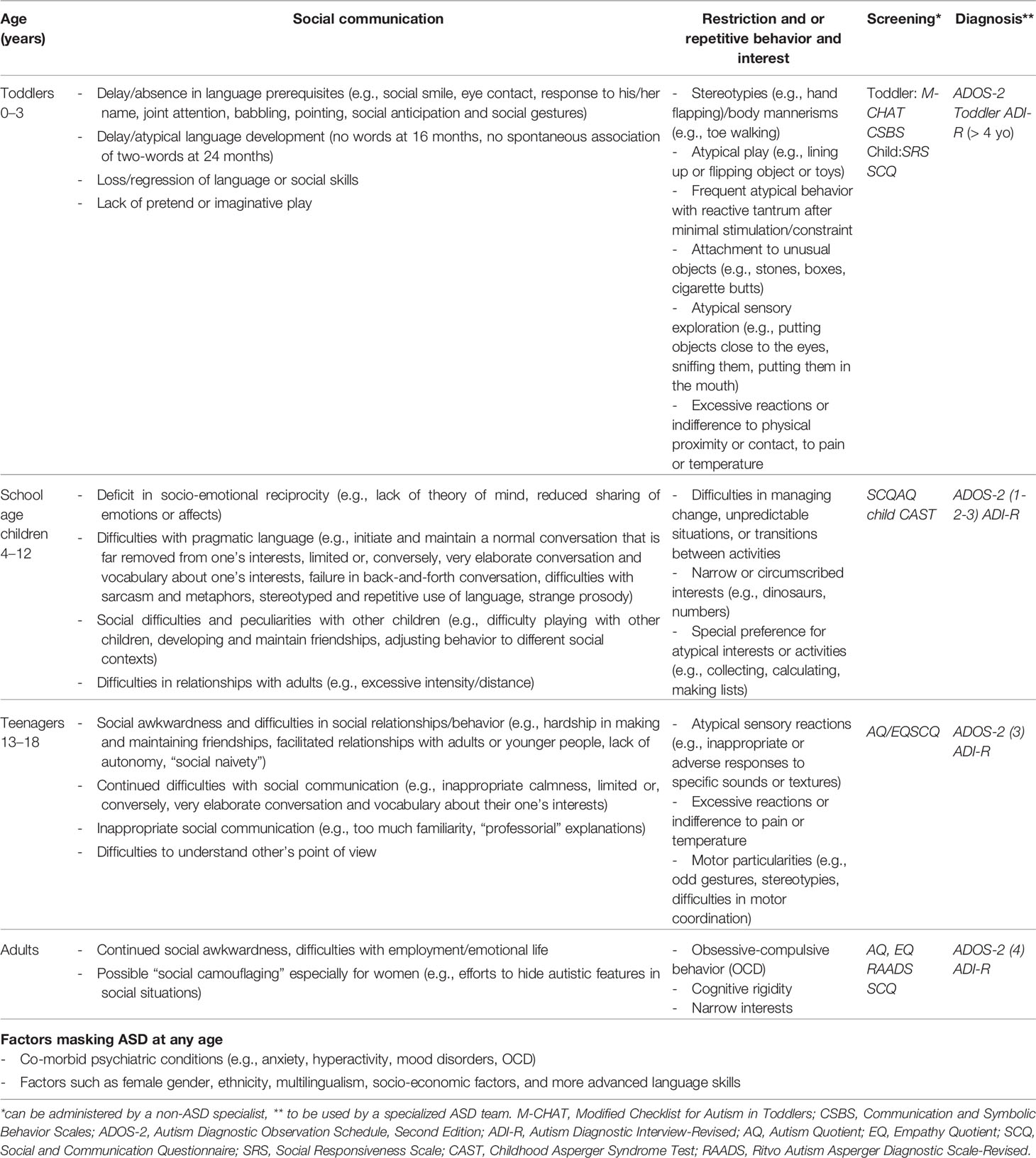
Table 1 Signs and symptoms of ASD at different ages; screening and diagnostic tools (adapted from (48, 49)).
Regarding oncology-specific care recommendations, only very limited high-grade (i.e., prospective trial) evidence is unfortunately available on specific plans to adopt for the optimal oncological care of individuals with ASD at this time (50, 51). Nonetheless, we decided to summarize these expert-based recommendations in Table 2, as we feel these measures could improve care before more extensive evidence is at hand. We will discuss the main recommendations below.
First, oncology teams need to be particularly vigilant to children, adolescents and adults with ASD, as their unique physiological and neuro-psychological (i.e., behavioral, cognitive, and motor) profiles might cause difficulties in their adherence/compliance with the “standard of care” clinical strategies in place for other patients. ASD individuals with cancer have to cognitively and emotionally grasp the stakes of a serious illness, face invasive investigations and treatments while dealing with the struggles of a neurodevelopmental disorder (52). Therefore, oncology providers need to be aware that the behavioral manifestations and the communication deficits can pose a particularly significant threat to the provision of optimal care in this very heterogeneous population (53, 54). For example, cognitive inflexibility is a key difficulty that may manifest itself in the context of cancer care, including in individuals with ASD without ID or a more subtle phenotype. In this setting, oncology teams unaware of the signs of ASD might stigmatize patients as poorly adherent/compliant or “difficult”. Overall, if not addressed, the ASD-specific features of non-standard communication and behavior carry the risk of deleteriously impacting clinical service operations and lead to unforeseen morbidity and mortality.
Second, to meet the needs of individuals with ASD and cancer, clinicians must imperatively and effectively collaborate with parent/families and other caregivers with the aims to constantly adapt communication and monitor/manage the clinical environment. It cannot be stressed enough that working in partnership with the parents/caregivers is of key importance to prioritize and coordinate interventions and treatments, and to provide adequate supportive care to the patient and his/her family members/care-proxies. This collaboration needs to be re-evaluated on a regular basis for the effectiveness in assessing patient- and caregiver-reported symptoms and issues.
Third, regarding communication, as information is being mostly provided verbally, healthcare professionals must use appropriate strategies and tools adjusted to the characteristics of the patient to facilitate transmission and understanding of information. Regardless of age and cognitive functioning level, individuals with ASD generally present strengths in the visual processing of information whereas they are often “drowned” by the flood of information presented verbally, particularly in contexts perceived as stressful. Consequently, a coherent and systematic use of visual aids by the healthcare team (e.g., images, pictures, and graphics), short visual scenarios illustrating the steps of a treatment to come, a “thermometer of emotions” to indicate the level of perceived discomfort or even pre-established diagrams/drawings at the bedside can be means to reduce anxiety and prevent a potential crisis. Examples of visual support methods related to care protocols for children and adults with ASD have been developed (55). The Autism Speaks website is a very valuable resource and provides toolkits for communication in many situations (www.autismspeaks.org/tool-kit). Knowledge on the patient’s preferred communication system (e.g., use of pictograms, images, or electronic “tablet” devices) will allow the prompt identification of needs, facilitate the integration into new or unexpected environments (incl. new providers), and allow the establishment of routines.
Fourth, establishing an individualized global care plan and specific protocols prior to the start of any exam, treatment or procedure can help the healthcare team to anticipate potential disruptions. The development of these strategies should always involve the patient, the family and/or primary caregivers and, to the extent possible, the expertise of autism specialists and individuals who themselves present ASD (47). Critical information such as ASD-specific indicators of severity, of intellectual functioning, the sensory profile, somatic and psychiatric comorbidities and whether the individual is verbal or not will need to be gathered and communicated effectively within the entire team. Oncology providers need to be aware that caregiver reports about behavioral changes or changes in physiological patterns can inform the clinical team about potential problems more swiftly than any other means. Lastly, the comprehensive and personalized care plan should be used as a tool for communication between the healthcare team members in charge of the patient, particularly at shift changes or other sign-off timepoints. Table 2 provides an overview of our recommendations to plan, anticipate, react and report in case of behavioral crises in the clinical environment (Goal C-7). It will be important to always ensure the latter interventions can be activated swiftly in out- and inpatient settings.
Fifth, clinical environments rarely adapt to an individual sensory profile, and patients might associate oncology care with a particularly hostile experience. This is even more difficult in situations involving young children, where families play a central role as an interface. Taking the time to gradually expose the patient to the various instruments used during therapy (e.g., monitoring equipment, needles, bandages) and their sensory exploration could represent a mean to increase the chances of optimal collaboration. If, despite these arrangements, a crisis arises, the family/primary caregivers remain of crucial importance in determining the sources of discomfort. They will be able to provide insights on the warning signs of crisis and effective strategies to reduce anxiety used at home or elsewhere (e.g., stop talking, reducing noise, movement, and light, provide a sensory withdrawal room, use visual distraction, engage in the patient’s specific interests).
Sixth, oncology providers should be aware that their patients, regardless of age, might have an undiagnosed ASD and be without a definitive neurodevelopmental diagnosis at the time of the discovery of their neoplasm. In fact, even though ASD can be diagnosed as early as age 2 years, most children remain unidentified until after the age of 4 years (18). Particularly relevant for the pediatric oncologist, this is an age at which a peak in cancer diagnoses is observed. Of course, oncology teams should not be screening every patient for ASD, but consultation with in-house mental health providers or ASD specialists is indicated in cases of suspicion (see Table 1). As psychologists and other mental health professionals are an integral part of individuals with cancer, most oncology teams will have easy access to these resources. Nonetheless, while the first and most important reflex should always be the timely referral to specialized teams (i.e., psycho-oncology, social work, psychiatry, depending on the local setting), an improved awareness of front-line actors to the existence of standardized screening instruments (Table 1) could contribute to the earlier detection of ASD in situations where it does not seem apparent at first glance (56–61).
Seventh, patients with ASD often experience physical manifestations which overlap with common cancer- or cancer treatment-related symptoms, such as pain, sleep disturbances and fatigue, gastrointestinal (GI) problems or immune dysregulation (27). These symptoms can easily be exacerbated by the cancer-directed therapy or be misinterpreted. Furthermore, difficulties in communication in patients with ASD, and the lack of adapted and validated instruments, may cause difficulties in their early identification and monitoring (62). Five oncology-specific symptoms deserve special attention in individuals with ASD. They are: a) pain, b) cancer-related fatigue, c) neurological and behavioral toxicity, d) fever and e) gastro-intestinal issues. We will discuss them individually due to their high incidence/prevalence and importance for cancer-associated outcomes. Our aim for this section is mainly to provide awareness for the oncology team and hint at possible solutions to some of the challenges they can pose in individuals with ASD. More precise recommendations to manage these cancer-related symptoms in ASD specifically cannot be delineated due to the lack of evidence, and further research is urgently needed. We wish to underline that children with ASD are a) particularly at risk for misinterpretation/underestimation of these symptoms, and b) underserved by the tools available to measure them, due to many reasons whose discussion is beyond the scope of this review.
Pain
Pain has a prevalence ranging 30–60% in oncology patients, and its screening and assessment through validated instruments is an integral part of cancer care (63, 64). However, the perception of the “experience of pain” in individuals of all ages with ASD can be difficult to assess by conventional scale measurements and research in this area is ongoing (65–67). We strongly recommend that caregivers/parents are closely involved in the assessment of pain in individuals with ASD, and when possible, self-assessment should be performed by a visual scale [e.g., adaptation of the Face Pain Scale-Revised (68)]. If the individual with ASD is non-verbal or has ID, hetero-rating scales will help to objectify manifestations of pain, favoring recording of scores from close parents/family members/caregivers. Although adjusted scales and measuring techniques have been developed and validated for children with cognitive limitations, data are still needed to adapt them to the specificities of ASD (69). The use of the revised Face Legs Activity Cry Consolability (rFLACC) pain assessment tool or the Non-Communicating Children’s Pain Checklist Postoperative Version (NCCPC-PV) and the inclusion of parent/family member/caregiver input in the interpretation of signs indicating pain is the minimum standard (70). Taken as a whole, the incorrect interpretation of behavioral patterns typical of individuals with ASD can lead caregivers to assign the manifestations of pain incorrectly to either an “exaggeration” or cause an underestimation of the intensity of the pain experience.
Cancer-Related Fatigue (CRF)
Cancer-related figure (CRF) is the most prevalent symptom experienced during the cancer trajectory (incidence of 40% at diagnosis and 80–90% during chemo- and/or radiotherapy) (71). CRF is defined as a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion related to cancer or cancer treatment (72). No studies or reviews published addressing how to measure and manage CRF in the ASD population, which constitutes a major issue as it significantly impacts quality of life and other treatment outcomes (71, 72). Its perception and expression are most likely altered in children and adults with ASD. In fact, associated behavioral issues can be misleading in the interpretation of CRF symptoms/signs and the communication difficulties render the differentiation of fatigue vs. other symptoms (e.g., sleepiness, dizziness, confusion, disorientation) a challenging, if not an impossible task without the collaboration of parents/caregivers. Furthermore, there is evidence to suggest that maladaptive sleep patterns in this population are associated with increased baseline daytime sleepiness, creating an additional layer of complexity (73). As it is not clear whether the current diagnostic criteria and the routine CRF management approaches appropriately address the needs of patients with ASD, we recommend that caregivers/parents are closely involved in the assessments of fatigue in patients with ASD undergoing cancer therapy. To allow the delineation of a personalized CRF-expression pattern, these evaluations need to take the individual communication/behavioral patterns into account, be compared to baseline/prior signs/symptoms and be repeated in several contexts. Once suspected, monitoring using the CRF-expression pattern should occur on a regular basis.
Neurotoxicity
Neurological toxicity from cancer therapy has been widely recognized (i.e., headache, seizures, encephalitis, movement disorders, peripheral neuropathy) (74). With the development of immunotherapy, novel and complex patterns to identify have emerged (i.e., polyneuropathy, demyelination, leukoencephalopathy, aseptic meningitis). There is evidence of an enhanced proinflammatory profile linked with major depression, memory complaints and behavioral deficits, all of them potentially present or enhanced in the context of ASD. As neurological symptoms and cognitive deficiencies can be common side effects of cancer treatment, individuals with ASD could be at risk for underdiagnosis. Sometimes these symptoms can remain even after cessation severely impacting long-term quality of life. A similar approach to pain and fatigue should be used for neurotoxicity (see 4.1 and 4.2 above).
Fever
Fever is a key symptom in the practice of oncology, as it can indicate infectious complications resulting from chemotherapy-induced myelosuppression. There is evidence to suggest that the manifestation of fever is altered in ASD as children with this condition rarely present with fever, and certain individuals even display an improvement of the behavioral phenotype during hyperthermia (75). The behavioral-state changes associated with fever in ASD have been speculated to depend upon the selective normalization of key components of a functionally impaired locus coeruleus-noradrenergic system (76). Therefore, oncology providers need to gather information about which signs and symptoms could indicate fever in each individual with ASD and communicate about them effectively within their teams (e.g., on-call/night shifts), as this could prevent the underreporting or underdiagnosis of potential life-threatening complications.
Gastro-Intestinal Issues
Digestive manifestations accompanying the cancer journey include both lower (i.es., constipation, diarrhea) and upper gastro-intenstinal (GI) symptoms (i.e., nausea/vomiting, abdominal pain) with a prevalence ranging from 5–90% depending on treatment modalities (77–80). In children with ASD, diarrhea and constipation are among the most frequently reported (62, 81–85). Moreover, medications administered to patients with ASD (β-blockers and α2 agonists, dopamine-receptor blockers, opioid antagonists, anticonvulsants, etc.) can also influence gut function. Given the communication difficulties in individuals with ASD, any atypical behavior (sleep disorders, irritability, food intolerance, self-injurious behavior, posturing, grimacing, holding the abdomen, squeezing the legs together, or walking around with a narrow gait to hold the stool in) should trigger an evaluation for constipation. Furthermore, little is known about the subjective experience of chemotherapy-induced nausea/vomiting (CINV) in individuals with ASD. For oncology providers, excessive nausea/vomiting to minimally or even non-emetogenic drugs could constitute a “red flag” for ASD in a patient with other suggestive signs (see Table 1).
Research on the Delivery of Cancer Care in Individuals With ASD
There is an urgent need for studies on the optimal cancer care for patients with ASD. Clinical investigation should focus on a) delineating the impact of cancer- and its therapy (incl. supportive care) on these individuals specifically, b) designing and testing adapted instruments and/or strategies to measure symptoms (e.g., CRF), c) adjusting the clinical environment to facilitate care, and d) targeting providers and healthcare systems for the delivery of effective training. Prospective trials should be conducted with the participation and close collaboration of direct caregivers (parents, families, etc.).
Limitations and Conclusions
The main limitation in our review is the lack of study-based evidence on the cancer care of children, adolescents and adults with ASD. Thus, an extensive, systematic review of the evidence is not feasible at this time. As the number of pediatric and adult patients with ASD in our oncology practices increases, our recommendations at this time largely remain extrapolations of available data from other clinical settings.
Nonetheless, we firmly believe that this should not hinder the discussion of concrete measures that, if implemented, can immediately benefit patients with ASD undergoing cancer treatment. Giving providers (medical, nursing and affiliated staff) the opportunity of improving their care of individuals with ASD is a global priority. In the absence of higher-grade evidence, they are intended as a call for vigilance of the particularities of these highly vulnerable individuals. Further research is urgently needed in order to improve our understanding of the impact of cancer- and its therapy (incl. supportive care) on individuals with ASD, which in turn shall help to develop instruments/strategies reliably measuring quality and outcomes in this population during their cancer care trajectory.
Author Contributions
DV, SC-L, P-AF, and RR reviewed the available evidence and took the lead in writing the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.595734/full#supplementary-material
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association (2013).
2. McVey AJ. The neurobiological presentation of anxiety in autism spectrum disorder: A systematic review. Autism Res (2019) 12(3):346–69. doi: 10.1002/aur.2063
3. Shephard E, Bedford R, Milosavljevic B, Gliga T, Jones EJH, Pickles A, et al. Early developmental pathways to childhood symptoms of attention-deficit hyperactivity disorder, anxiety and autism spectrum disorder. J Child Psychol Psychiatry (2019) 60(9):963–74. doi: 10.1111/jcpp.12947
4. Lai MC, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry (2015) 2(11):1013–27. doi: 10.1016/s2215-0366(15)00277-1
5. Happé F. “What does research tell us about girls on the autism spectrum?” In: Girls and Autism: Educational, Family and Personal Perspectives. Routledge (2019).
6. Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry (2011) 68(11):1095–102. doi: 10.1001/archgenpsychiatry.2011.76
7. Tick B, Bolton P, Happe F, Rutter M, Rijsdijk F. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J Child Psychol Psychiatry (2016) 57(5):585–95. doi: 10.1111/jcpp.12499
8. Sandin S, Lichtenstein P, Kuja-Halkola R, Hultman C, Larsson H, Reichenberg A. The Heritability of Autism Spectrum Disorder. JAMA (2017) 318(12):1182–4. doi: 10.1001/jama.2017.12141
9. Wang X, Kery R, Xiong Q. Synaptopathology in autism spectrum disorders: Complex effects of synaptic genes on neural circuits. Prog Neuropsychopharmacol Biol Psychiatry (2018) 84(Pt B):398–415. doi: 10.1016/j.pnpbp.2017.09.026
10. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet (2017) 174(6):651–60. doi: 10.1002/ajmg.b.32567
11. Qin Y, Wade PA. Crosstalk between the microbiome and epigenome: messages from bugs. J Biochem (2018) 163(2):105–12. doi: 10.1093/jb/mvx080
12. Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, et al. The epidemiology of autism spectrum disorders. Annu Rev Public Health (2007) 28:235–58. doi: 10.1146/annurev.publhealth.28.021406.144007
13. Kim YS, Leventhal BL. Genetic epidemiology and insights into interactive genetic and environmental effects in autism spectrum disorders. Biol Psychiatry (2015) 77(1):66–74. doi: 10.1016/j.biopsych.2014.11.001
14. Wen Y, Herbert MR. Connecting the dots: Overlaps between autism and cancer suggest possible common mechanisms regarding signaling pathways related to metabolic alterations. Med Hypotheses (2017) 103:118–23. doi: 10.1016/j.mehy.2017.05.004
15. Alabaf S, Gillberg C, Lundstrom S, Lichtenstein P, Kerekes N, Rastam M, et al. Physical health in children with neurodevelopmental disorders. J Autism Dev Disord (2019) 49(1):83–95. doi: 10.1007/s10803-018-3697-4
16. Chiang HL, Liu CJ, Hu YW, Chen SC, Hu LY, Shen CC, et al. Risk of cancer in children, adolescents, and young adults with autistic disorder. J Pediatr (2015) 166(2):418–23.e1. doi: 10.1016/j.jpeds.2014.10.029
17. Idring S, Lundberg M, Sturm H, Dalman C, Gumpert C, Rai D, et al. Changes in prevalence of autism spectrum disorders in 2001-2011: findings from the Stockholm youth cohort. J Autism Dev Disord (2015) 45(6):1766–73. doi: 10.1007/s10803-014-2336-y
18. Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ (2018) 67(6):1–23. doi: 10.15585/mmwr.ss6706a1
19. Loomes R, Hull L, Mandy WPL. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J Am Acad Child Adolesc Psychiatry (2017) 56(6):466–74. doi: 10.1016/j.jaac.2017.03.013
20. Hertz-Picciotto I, Delwiche L. The rise in autism and the role of age at diagnosis. Epidemiology (2009) 20(1):84–90. doi: 10.1097/EDE.0b013e3181902d15
21. Atladottir HO, Schendel DE, Henriksen TB, Hjort L, Parner ET. Gestational Age and Autism Spectrum Disorder: Trends in Risk Over Time. Autism Res (2016) 9(2):224–31. doi: 10.1002/aur.1525
22. Whitehouse AJ, Cooper MN, Bebbington K, Alvares G, Lin A, Wray J, et al. Evidence of a reduction over time in the behavioral severity of autistic disorder diagnoses. Autism Res (2017) 10(1):179–87. doi: 10.1002/aur.1740
23. Bolte S, Girdler S, Marschik PB. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life Sci (2019) 76(7):1275–97. doi: 10.1007/s00018-018-2988-4
24. Emberti Gialloreti L, Mazzone L, Benvenuto A, Fasano A, Alcon AG, Kraneveld A, et al. Risk and Protective Environmental Factors Associated with Autism Spectrum Disorder: Evidence-Based Principles and Recommendations. J Clin Med (2019) 8(2):1–23. doi: 10.3390/jcm8020217
25. Vemuri R, Sylvia KE, Klein SL, Forster SC, Plebanski M, Eri R, et al. The microgenderome revealed: sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin Immunopathol (2019) 41(2):265–75. doi: 10.1007/s00281-018-0716-7
26. Young H, Oreve MJ, Speranza M. Clinical characteristics and problems diagnosing autism spectrum disorder in girls. Arch Pediatr (2018) 25(6):399–403. doi: 10.1016/j.arcped.2018.06.008
27. Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet (2014) 383(9920):896–910. doi: 10.1016/s0140-6736(13)61539-1
28. Mannion A, Leader G. Comorbidity in autism spectrum disorder: A literature review. Autism Spec Disord (2013) 7(12):1595–616. doi: 10.1016/j.rasd.2013.09.006
29. Kohane IS, McMurry A, Weber G, MacFadden D, Rappaport L, Kunkel L, et al. The co-morbidity burden of children and young adults with autism spectrum disorders. PLoS One (2012) 7(4):e33224. doi: 10.1371/journal.pone.0033224
30. Tye C, Runicles A, Whitehouse AJO, Alvares GA. Corrigendum: Characterizing the Interplay Between Autism Spectrum Disorder and Comorbid Medical Conditions: An Integrative Review. Front Psychiatry (2019) 10:438. doi: 10.3389/fpsyt.2019.00438
31. Croen LA, Zerbo O, Qian Y, Massolo ML, Rich S, Sidney S, et al. The health status of adults on the autism spectrum. Autism (2015) 19(7):814–23. doi: 10.1177/1362361315577517
32. Doshi-Velez F, Ge Y, Kohane I. Comorbidity clusters in autism spectrum disorders: an electronic health record time-series analysis. Pediatrics (2014) 133(1):e54–63. doi: 10.1542/peds.2013-0819
33. Antshel KM, Zhang-James Y, Wagner KE, Ledesma A, Faraone SV. An update on the comorbidity of ADHD and ASD: a focus on clinical management. Expert Rev Neurother (2016) 16(3):279–93. doi: 10.1586/14737175.2016.1146591
34. Vohra R, Madhavan S, Sambamoorthi U. Comorbidity prevalence, healthcare utilization, and expenditures of Medicaid enrolled adults with autism spectrum disorders. Autism (2017) 21(8):995–1009. doi: 10.1177/1362361316665222
35. Rydzewska E, Hughes-McCormack LA, Gillberg C, Henderson A, MacIntyre C, Rintoul J, et al. Prevalence of long-term health conditions in adults with autism: observational study of a whole country population. BMJ Open (2018) 8(8):e023945. doi: 10.1136/bmjopen-2018-023945
36. Karpur A, Lello A, Frazier T, Dixon PJ, Shih AJ. Health Disparities among Children with Autism Spectrum Disorders: Analysis of the National Survey of Children’s Health 2016. J Autism Dev Disord (2019) 49(4):1652–64. doi: 10.1007/s10803-018-3862-9
37. McBain RK, Kareddy V, Cantor JH, Stein BD, Yu H. Systematic Review: United States Workforce for Autism-Related Child Healthcare Services. J Am Acad Child Adolesc Psychiatry (2020) 59(1):113–39. doi: 10.1016/j.jaac.2019.04.027
38. Straus J, Coburn S, Maskell S, Pappagianopoulos J, Cantrell K. Medical Encounters for Youth With Autism Spectrum Disorder: A Comprehensive Review of Environmental Considerations and Interventions. Clin Med Insights Pediatr (2019) 13:1179556519842816. doi: 10.1177/1179556519842816
39. Bilder D, Botts EL, Smith KR, Pimentel R, Farley M, Viskochil J, et al. Excess mortality and causes of death in autism spectrum disorders: a follow up of the 1980s Utah/UCLA autism epidemiologic study. J Autism Dev Disord (2013) 43(5):1196–204. doi: 10.1007/s10803-012-1664-z
40. Woolfenden S, Sarkozy V, Ridley G, Coory M, Williams K. A systematic review of two outcomes in autism spectrum disorder - epilepsy and mortality. Dev Med Child Neurol (2012) 54(4):306–12. doi: 10.1111/j.1469-8749.2012.04223.x
41. Hirvikoski T, Mittendorfer-Rutz E, Boman M, Larsson H, Lichtenstein P, Bolte S. Premature mortality in autism spectrum disorder. Br J Psychiatry (2016) 208(3):232–8. doi: 10.1192/bjp.bp.114.160192
42. Richa S, Fahed M, Khoury E, Mishara B. Suicide in autism spectrum disorders. Arch Suicide Res (2014) 18(4):327–39. doi: 10.1080/13811118.2013.824834
43. Mouridsen SE. Mortality and factors associated with death in autism spectrum disorders-A review. Am J Autism (2013) 1:17–25. doi: 10.7726/aja.2013.1002
44. Barrett B, Byford S, Sharac J, Hudry K, Leadbitter K, Temple K, et al. Service and wider societal costs of very young children with autism in the UK. J Autism Dev Disord (2012) 42(5):797–804. doi: 10.1007/s10803-011-1306-x
45. Tregnago MK, Cheak-Zamora NC. Systematic review of disparities in health care for individuals with autism spectrum disorders in the United States. Res Autism Spectr Disord (2012) 6(3):1023–31. doi: 10.1016/j.rasd.2012.01.005
46. Witham G, Haigh C. A narrative literature review examining cancer treatment issues for patients living with intellectual disabilities. Eur J Oncol Nurs (2018) 36:9–15. doi: 10.1016/j.ejon.2018.07.004
47. H. G. The Department of Health & Social Care, U.K. Right to be heard": The Government"s response to the consultation on learning disability and autism training for health and care staff. U. K.: HM Government (2019). Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/844356/autism-and-learning-disability-training-for-staff-consultation-response.pdf.
48. Boyd BA, Shaw E. Autism in the Classroom: A Group of Students Changing in Population and Presentation. Prevent School Failure (2010) 54(4):211–9. doi: 10.1080/10459881003744552
49. Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J. Autism spectrum disorder. Lancet (Lond Engl) (2018) 392(10146):508–20. doi: 10.1016/S0140-6736(18)31129-2
50. Sakai C, Miller K, Brussa AK, MacPherson C, Augustyn M. Challenges of autism in the inpatient setting. J Dev Behav Pediatr (2014) 35(1):82–4. doi: 10.1097/dbp.0000000000000024
51. Dell DD, Feleccia M, Hicks L, Longstreth-Papsun E, Politsky S, Trommer C. Care of patients with autism spectrum disorder undergoing surgery for cancer. Oncol Nurs Forum (2008) 35(2):177–82. doi: 10.1188/08.Onf.177-182
52. Lappe M, Lau L, Dudovitz RN, Nelson BB, Karp EA, Kuo AA. The Diagnostic Odyssey of Autism Spectrum Disorder. Pediatrics (2018) 141(Suppl 4):S272–s279. doi: 10.1542/peds.2016-4300C
53. Olivie H. The medical care of children with autism. Eur J Pediatr (2012) 171(5):741–9. doi: 10.1007/s00431-011-1669-1
54. Venkat A, Jauch E, Russell WS, Crist CR, Farrell R. Care of the patient with an autism spectrum disorder by the general physician. Postgrad Med J (2012) 88(1042):472–81. doi: 10.1136/postgradmedj-2011-130727
55. Richards B. Caring for children with autism spectrum condition in paediatric emergency departments. Emerg Nurse (2017) 25(4):30–4. doi: 10.7748/en.2017.e1713
56. Robins DL, Fein D, Barton ML, Green JA. The Modified Checklist for Autism in Toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. J Autism Dev Disord (2001) 31(2):131–44. doi: 10.1023/a:1010738829569
57. Chandler S, Charman T, Baird G, Simonoff E, Loucas T, Meldrum D, et al. Validation of the social communication questionnaire in a population cohort of children with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry (2007) 46(10):1324–32. doi: 10.1097/chi.0b013e31812f7d8d
58. Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord (2001) 31(1):5–17. doi: 10.1023/a:1005653411471
59. Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord (2004) 34(2):163–75. doi: 10.1023/b:jadd.0000022607.19833.00
60. Stagg SD, Belcher H. Living with autism without knowing: receiving a diagnosis in later life. Health Psychol Behav Med (2019) 7(1):348–61. doi: 10.1080/21642850.2019.1684920
61. Garel N, Garel P. Diagnosis of Autism Spectrum Disorder in Adolescents with Complex Clinical Presentations: A Montreal Case Series. Adolesc Psychiatry (2019) 9(1):33–43. doi: 10.2174/2210676609666181204125951
62. Mannion A, Leader G. Gastrointestinal Symptoms in Autism Spectrum Disorder: A Literature Review. Rev J Autism Dev Disord (2013) 1(1):11–7. doi: 10.1007/s40489-013-0007-0
63. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol (2007) 18(9):1437–49. doi: 10.1093/annonc/mdm056
64. Fallon M, Giusti R, Aielli F, Hoskin P, Rolke R, Sharma M, et al. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann Oncol (2018) 29(Suppl 4):iv166–91. doi: 10.1093/annonc/mdy152
65. Ely E, Chen-Lim ML, Carpenter KM 2nd, Wallhauser E, E. Friedlaender. Pain Assessment of Children with Autism Spectrum Disorders. J Dev Behav Pediatr (2016) 37(1):53–61. doi: 10.1097/DBP.0000000000000240
66. Tordjman S, Anderson GM, Botbol M, Brailly-Tabard S, Perez-Diaz F, Graignic R, et al. Pain reactivity and plasma beta-endorphin in children and adolescents with autistic disorder. PLoS One (2009) 4(8):e5289. doi: 10.1371/journal.pone.0005289
67. Moore DJ. Acute pain experience in individuals with autism spectrum disorders: a review. Autism (2015) 19(4):387–99. doi: 10.1177/1362361314527839
68. Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain (2001) 93(2):173–83. doi: 10.1016/s0304-3959(01)00314-1
69. Solodiuk JC, Scott-Sutherland J, Meyers M, Myette B, Shusterman C, Karian VE, et al. Validation of the Individualized Numeric Rating Scale (INRS): a pain assessment tool for nonverbal children with intellectual disability. Pain (2010) 150(2):231–6. doi: 10.1016/j.pain.2010.03.016
70. Breau LM, Finley GA, McGrath PJ, Camfield CS. Validation of the Non-communicating Children’s Pain Checklist-Postoperative Version. Anesthesiology (2002) 96(3):528–35. doi: 10.1097/00000542-200203000-00004
71. Fabi A, Bhargava R, Fatigoni S, Guglielmo M, Horneber M, Roila F, et al. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol (2020) 31(6):713–23. doi: 10.1016/j.annonc.2020.02.016
72. Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, et al. Cancer-Related Fatigue, Version 2.2015. J Natl Compr Canc Netw (2015) 13(8):1012–39. doi: 10.6004/jnccn.2015.0122
73. Baker E, Richdale A, Short M, Gradisar M. An investigation of sleep patterns in adolescents with high-functioning autism spectrum disorder compared with typically developing adolescents. Dev Neurorehabil (2013) 16(3):155–65. doi: 10.3109/17518423.2013.765518
74. Stone JB, DeAngelis LM. Cancer-treatment-induced neurotoxicity–focus on newer treatments. Nat Rev Clin Oncol (2016) 13(2):92–105. doi: 10.1038/nrclinonc.2015.152
75. Niehus R, Lord C. Early medical history of children with autism spectrum disorders. J Dev Behav Pediatr (2006) 27(2 Suppl):S120–7. doi: 10.1097/00004703-200604002-00010
76. Mehler MF, Purpura DP. Autism, fever, epigenetics and the locus coeruleus. Brain Res Rev (2009) 59(2):388–92. doi: 10.1016/j.brainresrev.2008.11.001
77. Larkin PJ, Cherny N II, La Carpia D, Guglielmo M, Ostgathe C, Scotte F, et al. Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Ann Oncol (2018) 29(Suppl 4):iv111–25. doi: 10.1093/annonc/mdy148
78. Bossi P, Antonuzzo A, Cherny N II, Rosengarten O, Pernot S, Trippa F, et al. Diarrhoea in adult cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol (2018) 29(Suppl 4):iv126–42. doi: 10.1093/annonc/mdy145
79. Classen J, Belka C, Paulsen F, Budach W, Hoffmann W, Bamberg M. Radiation-induced gastrointestinal toxicity. Pathophysiology, approaches to treatment and prophylaxis. Strahlenther Onkol (1998) 174(Suppl 3):82–4. doi: 10.1007/BF03040229
80. Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2018) 29(Suppl 4):iv264–6. doi: 10.1093/annonc/mdy162
81. Kuddo T, Nelson KB. How common are gastrointestinal disorders in children with autism? Curr Opin Pediatr (2003) 15(3):339–43. doi: 10.1097/00008480-200306000-00020
82. Buie T, Fuchs GJ, Furuta GT, Kooros K, Levy J, Lewis JD, et al. Recommendations for evaluation and treatment of common gastrointestinal problems in children with ASDs. Pediatrics (2010) 125: (Supplement 1):S19–29. doi: 10.1542/peds.2009-1878D
83. Buie T, Campbell DB, Fuchs GJ, Furuta GT, Levy J, VandeWater J, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics (2010) 125: (Supplement 1):S1–S18. doi: 10.1542/peds.2009-1878C
84. Furuta GT, Williams K, Kooros K, Kaul A, Panzer R, Coury DL, et al. Management of constipation in children and adolescents with autism spectrum disorders. Pediatrics (2012) 130(Suppl 2):S98–105. doi: 10.1542/peds.2012-0900H
Keywords: outcome disparities, autism (autism spectrum disorders), cancer, neurodevelopmental disorders, supportive care, oncology (general), medical oncology, pediatric oncology
Citation: Vuattoux D, Colomer-Lahiguera S, Fernandez P-A, Jequier Gygax M, Choucair M-L, Beck-Popovic M, Diezi M, Manificat S, Latifyan S, Ramelet A-S, Eicher M, Chabane N and Renella R (2021) Cancer Care of Children, Adolescents and Adults With Autism Spectrum Disorders: Key Information and Strategies for Oncology Teams. Front. Oncol. 10:595734. doi: 10.3389/fonc.2020.595734
Received: 17 August 2020; Accepted: 07 December 2020;
Published: 19 January 2021.
Edited by:
Dana Kristjansson, Norwegian Institute of Public Health (NIPH), NorwayReviewed by:
Nancy Cheak-Zamora, University of Missouri, United StatesChia-Liang Tsai, National Cheng Kung University, Taiwan
Ann M. Neumeyer, Massachusetts General Hospital and Harvard Medical School, United States
Copyright © 2021 Vuattoux, Colomer-Lahiguera, Fernandez, Jequier Gygax, Choucair, Beck-Popovic, Diezi, Manificat, Latifyan, Ramelet, Eicher, Chabane and Renella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raffaele Renella, UmFmZmFlbGUuUmVuZWxsYUBjaHV2LmNo
 Delphine Vuattoux
Delphine Vuattoux Sara Colomer-Lahiguera
Sara Colomer-Lahiguera Pierre-Alain Fernandez1
Pierre-Alain Fernandez1 Marine Jequier Gygax
Marine Jequier Gygax Marie-Louise Choucair
Marie-Louise Choucair Maja Beck-Popovic
Maja Beck-Popovic Manuel Diezi
Manuel Diezi Anne-Sylvie Ramelet
Anne-Sylvie Ramelet Manuela Eicher
Manuela Eicher Nadia Chabane
Nadia Chabane Raffaele Renella
Raffaele Renella