- Department of Medical Ultrasound, Fudan University Shanghai Cancer Center, Shanghai, China
Purpose: The positivity of sentinel lymph node (SLN) metastasis is relatively low in ductal carcinoma in situ (DCIS) patients. The aim of this study was to investigate factors associated with SLN metastasis and build a model to predict the potential risk of SLN metastasis in patients with a preoperative diagnosis of DCIS.
Patients and Methods: Core needle biopsy-proved DCIS patients who underwent SLN biopsy and breast surgery were retrospectively reviewed and selected. Univariate analysis was used to identify the variables correlated with SLN metastasis. A model to predict SLN metastasis was developed using a multivariate logistic regression in the training set and then validated in an internal set.
Results: A total of 407 patients with a preoperative diagnosis of DCIS were included. Upstaging to invasive/microinvasive cancer occurred in 225 patients after surgery. SLN metastasis was found in 42 patients, including 32 patients upstaging to invasive disease, 8 to microinvasive disease, and 2 pure DCIS. Tumor size based on US examination, axillary ultrasound finding, multifocality, surgery, upstaging, and Ki-67 expression were significantly related to SLN metastasis. The model incorporating tumor size, axillary ultrasound finding and multifocality yielded an AUC of 0.805 (95% CI: 0.715–0.895, p<0.001) in the training set, and 0.729 (95% CI: 0.547–0.911, p=0.013) in the testing set.
Conclusion: A simple model was developed to predict SLN metastasis in patients with a preoperative diagnosis of DCIS. With good discriminatory power, this model should be helpful for surgeons to decide if SLN biopsy could be safely avoided in certain patients.
Introduction
Breast cancer is the most frequently diagnosed cancer among females in China as in most other countries (1, 2). Ductal carcinoma in situ (DCIS) is a non-invasive breast cancer which represents approximately 20-30% of all new breast cancer diagnoses (3, 4). Unlike invasive disease, DCIS is confined in the milk ducts and, theoretically, lacks the ability of metastasis. Therefore, sentinel lymph node (SLN) biopsy is just recommended for patients with preoperatively diagnosed pure DCIS undergoing mastectomy, in case when invasive disease is found in the surgical specimen, a second SLN procedure becomes mandatory but is no more possible (5). However, in clinical practice, a substantial proportion of patients with a preoperative diagnosis of DCIS still undergo SLN biopsy during breast conserving surgery (6–10). This discrepancy between guidelines and clinical practice may be largely attributed to the reluctance to return to the operating room when occult invasive disease is identified in the surgical specimen.
Notably, the proportion of SLN metastasis in DCIS patients undergoing SLN biopsy during breast surgery (breast conserving surgery or mastectomy) is reported to be 0.6-13.4% (6, 8, 11–17). Given the low positivity and the potential harms the SLN procedure may cause, the role of SLN biopsy in most DCIS patients has been controversial in recent years (18). Consequently, it is of clinical significance to identify DICS patients with high potential of axillary lymph node metastasis who will be benefited from the SLN procedure. However, little progress has been made until now (19). Therefore, the aim of this study was to investigate factors associated with SLN metastasis and build a model to predict the potential risk of SLN metastasis in patients with a preoperative diagnosis of DCIS.
Patients and Methods
Patients
Patients with DCIS diagnosed by ultrasound-guided core needle biopsy (CNB) between January 2017 and December 2018 in our hospital were included in this retrospective study. The inclusion criteria included: 1) first diagnosed as breast cancer, 2) undergoing SLN biopsy and breast conserving surgery or mastectomy, 3) without therapy before surgery. The data of ultrasound examination, mammography and pathological evaluation were collected from the medical records. The procedure and interpretation of the results of SLN biopsy and ultrasound assessment were illustrated in a prior study (20). Briefly, the largest metastases in the SLN with a maximal diameter >2 mm, 0.2–2 mm, and ≤0.2 mm were classified as macrometastases, micrometastases, and isolated tumor cell, respectively. The axillary ultrasound was considered positive when an abnormal node with at least one of the following suspicious findings was recorded: diffuse cortical thickening of ≥3 mm; focal cortical bulge; eccentric cortical thickening; rounded hypoechoic node; complete or partial effacement of the fatty hilum; nonhilar cortical blood flow on color Doppler images; complete or partial replacement of the node with an ill-defined or irregular mass; or microcalcifications in the node. Otherwise, it was considered negative. Multifocality of primary tumor was determined on the basis of ultrasound examination, with two or more lesions that completely separate from each other considering as multifocal.
Pathological Assessment
The expression of estrogen receptor (ER), progesterone receptor (PR) and Ki-67 was measured by immunohistochemical staining in the experimental specimens. Any staining of 1% of tumor cells or more was considered positive for both ER and PR. The case with staining of 14% or less tumor cells was considered low expression for Ki-67. The measurement of HER2 in the experimental specimens was determined according to the ASCO/CAP guidelines (21).
Statistical Analysis
Quantitative data were presented as median and range, while qualitative data as frequency and percentage. Univariate analysis was used to identify the variables correlated with SLN metastasis. Mann–Whitney U-test was used for the analysis of quantitative data, while the Chi-squared test for qualitative data. Patients were then randomly divided into a training set and a testing set with a ratio of 7:3. A model to predict SLN metastasis was developed using a multivariate logistic regression backward stepwise method in the training set. All variables determined preoperatively with p<0.2 in the univariate analysis were put in the logistic regression analysis. Variables with p<0.05 in the analysis were included in the final predictive model, which was validated using the testing set. Area under the receiver operating characteristic curves (AUCs) were calculated to quantify the ability of the model to rank patients according to risk. All tests were two-sided, and p<0.05 indicated statistical significance. The SPSS version 19.0 (IBM Corp., Armonk, NY, USA) software was used for all statistical analysis.
Results
Patients Characteristics
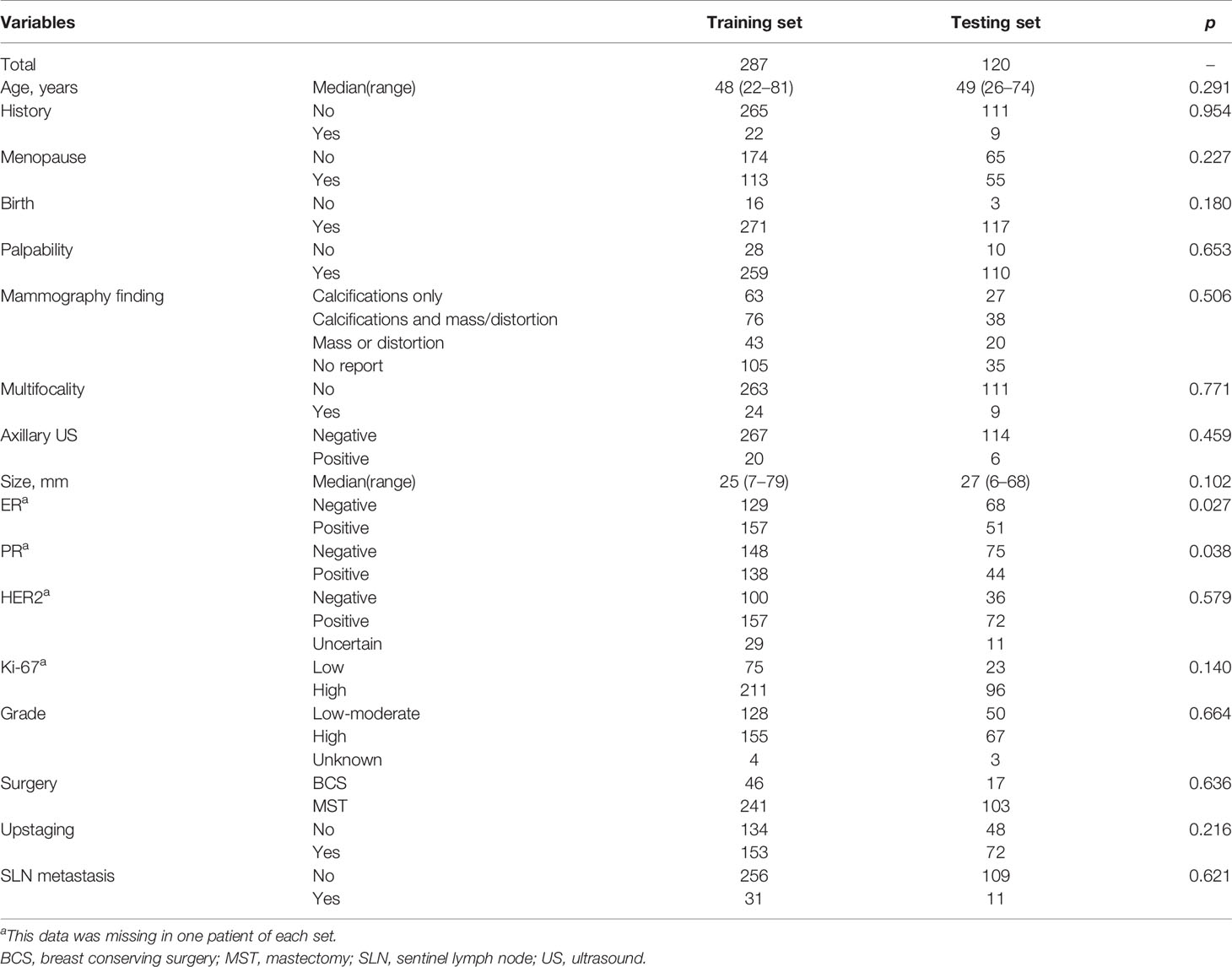
A total of 407 female patients with median age of 49 years (range 22–81 years) were eligible for this study (Table 1). Most of patients (84.5%, 344/407) underwent mastectomy. Upstaging occurred in 225 (55.3%) patients after surgery. Of them, invasive disease was identified in 103 patients, and 122 cases were accompanied with microinvasive disease. SLN metastasis was found in 42 (10.3%) patients, among which macrometastasis alone and micrometastasis alone both presented in 20 patients, respectively. The other two patients had both macrometastsis and micrometastasis in the SLNs. Thirty-two of 103 (31.1%) patients upstaging to invasive cancer had SLN metastasis including 18 cases of macrometastsis alone, 12 cases of micrometastasis alone and two cases of both macrometastsis and micrometastasis. Eight of 122 (6.7%) patients upstaging to microinvasive cancer had SLN metastasis (two cases of macrometastsis alone and six cases of micrometastasis alone). There were only two patients (1.1%, 2/182) who had SLN metastasis without upstaging after surgery, both of whom were micrometastasis alone. This study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center. Informed consent was waived due to its retrospective design and no identifiable information was disclosed.
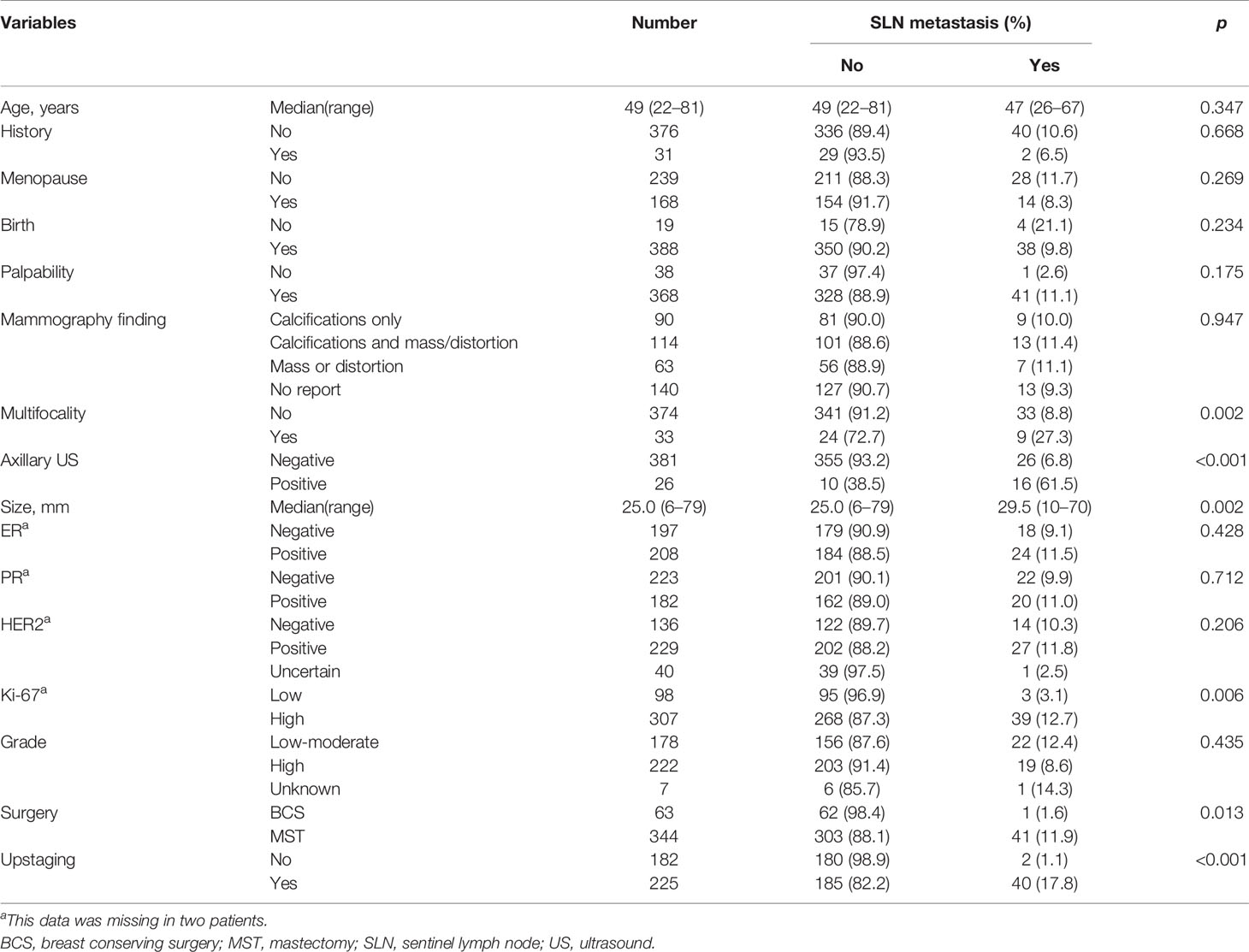
Table 1 Patients’ characteristics and univariate analysis of variables for sentinel lymph node metastasis.
Factors Correlated With Sentinel Lymph Node Metastasis
Factors including age, family history, menopause status, birth history, palpability, mammography finding, nuclear grade, ER expression, PR expression, and HER2 expression were not correlated with SLN metastasis (Table 1). However, tumor size based on US examination, axillary ultrasound finding, multifocality, surgery, upstaging, and Ki-67 expression were significantly related to SLN metastasis (Table 1). Specifically, SLN metastasis was more likely to happen in patients with larger tumor size, positive axillary ultrasound, multifocal lesions. Patients who underwent mastectomy, upstaged or had cancer with higher Ki-67 expression had a higher possibility to have metastasis in SLNs.
Model to Predict Sentinel Lymph Node Metastasis
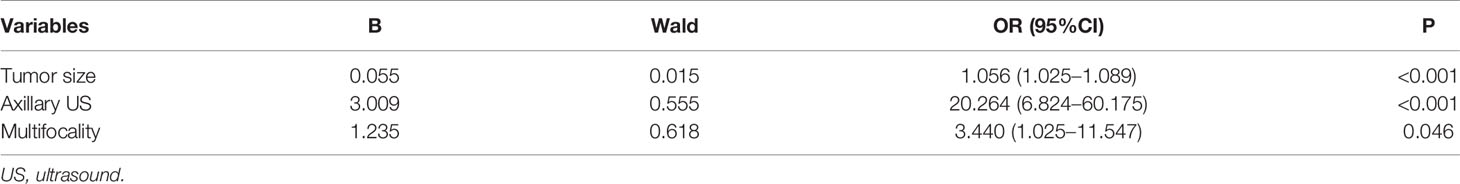
Two hundred and eighty-seven patients were allocated in the training set, and 120 patients in the testing set. They were comparable in patients’ characteristics except that the training set had little higher proportion of patients with ER/PR positive than the testing set (Table 2). Based on the results of univariate analysis, preoperative variables including palpability, tumor size, axillary ultrasound finding and multifocality were put in the logistic regression analysis, which turned out that only the last three variables remained significant (Table 3). Thus, a model to predict SLN metastasis in DCIS patients diagnosed by CNB was developed with the three variables:
in which p indicated the probability of SLN metastasis. The AUC of this model was 0.805 (95% CI: 0.715–0.895, p<0.001) (Figure 1). When applying to the testing set, this model yielded an AUC of 0.729 (95% CI: 0.547–0.911, p=0.013) (Figure 2).
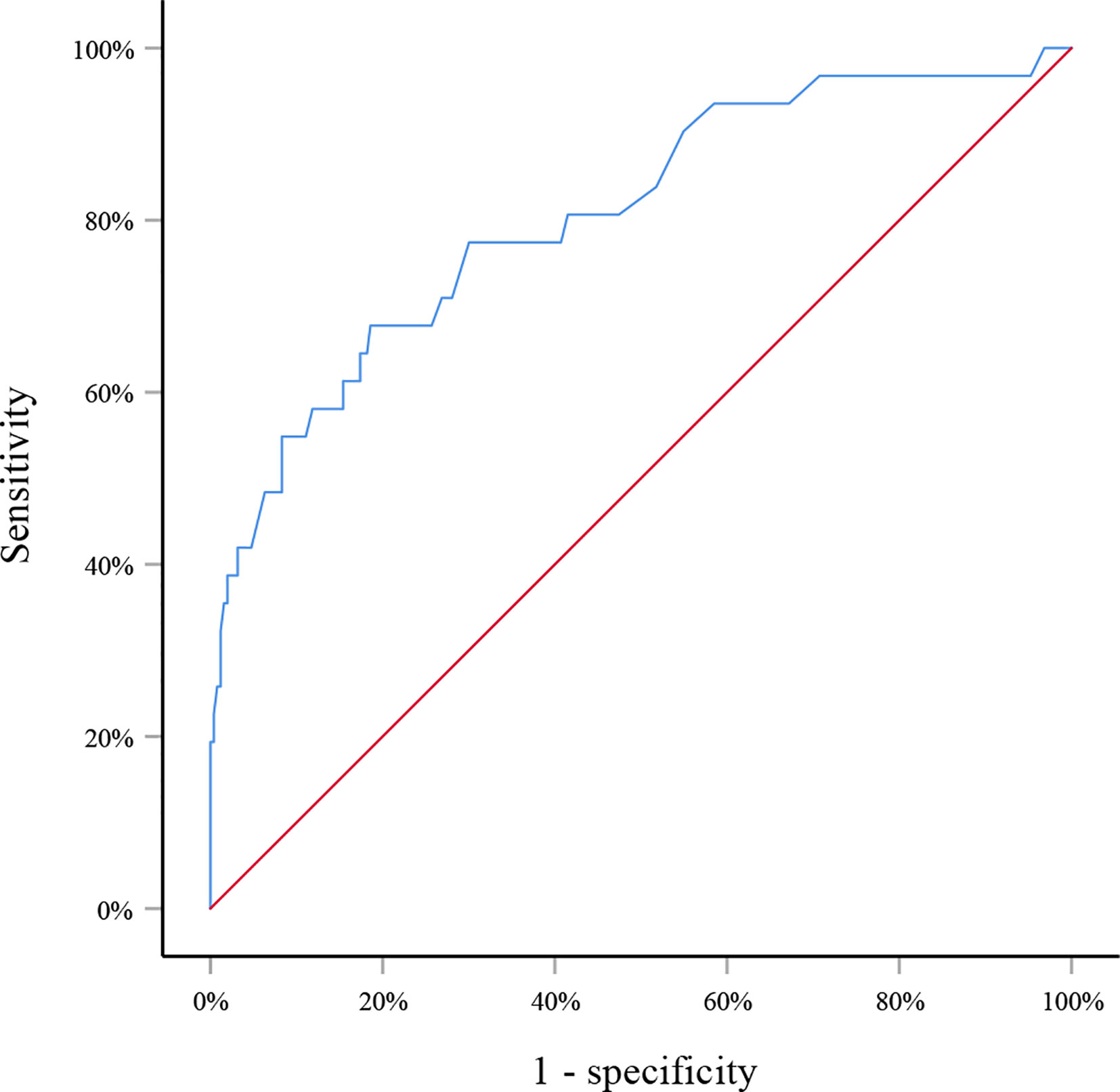
Figure 1 Receiver operating curve for the training set. The area under the curve was 0.805 (95% CI: 0.715–0.895, p<0.001).
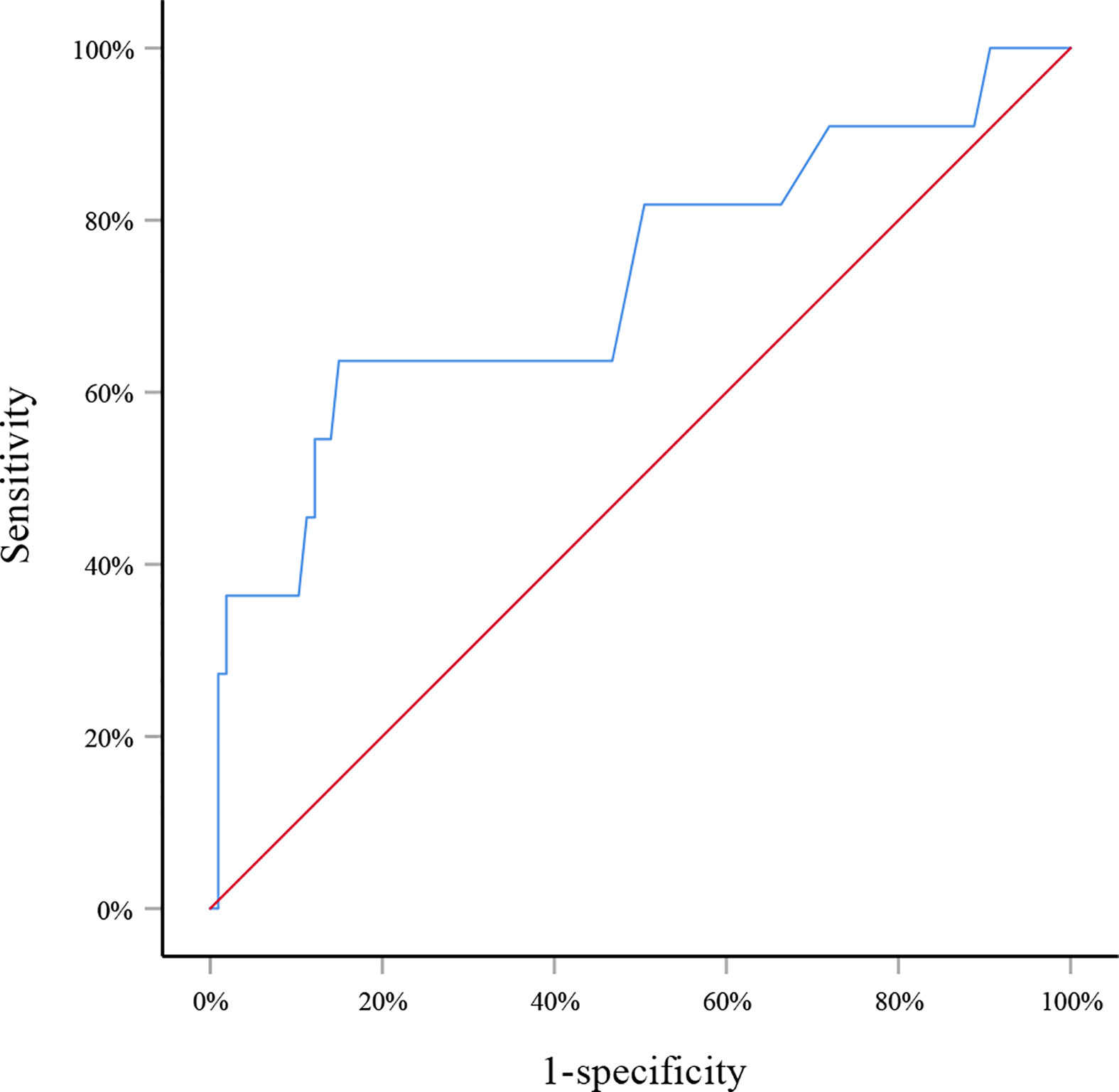
Figure 2 Receiver operating curve for the testing set. The area under the curve was 0.729 (95% CI: 0.547–0.911, p=0.013).
Discussion
The controversy on the role of SLN biopsy in patients preoperatively diagnosed as pure DCIS has been for a long time. The arguments for or against it, as introduced previously, mainly focus on the potential of upstaging to invasive cancer after surgery and low rate of SLN metastasis. However, what should not be neglected is that SLN metastasis could be found in some DCIS patients without upstaging after surgery, though in a very low rate (4%, 71/1787) (22). Also in this study, SLN metastasis was found in two patients with final pathology-proved pure DCIS (1.1%, 2/182). The mechanism of this phenomenon is beyond the scope of this study. But it does strengthen the clinical relevance of SLN biopsy in patients with a preoperative diagnosis of DCIS. And before the detrimental influence of axillary lymph node metastasis on the survival of patients can be certainly excluded, it is quite of clinical significance to perform SLN biopsy in highly selective patients with a preoperative diagnosis of DCIS because of low positivity of SLN metastasis.
SLN metastasis was found in 10.3% (42/407) of patients with preoperative DCIS in this study, a little higher than that reported over the latest five years (6, 8, 11, 12). This may be attributed to the relatively high total upstaging rate (55.3%, 225/407) of this study. About a quarter (25.5%, 103/407) of patients with invasive cancer were underestimated as DCIS in initial CNB, as well as another portion (30.0%, 122/407) with microinvasive cancer. These patients are more likely to have metastasis in SLNs, so the more they were included in the group, the higher the total rate of SLN metastasis was. And the main reason for relatively high upstaging rate in this study may be that in our center CNB specimens were stained to determine whether there were cancer cells but not mandatorily further stained to determine whether there was micoinvasiveness or invasiveness, which cannot be determined without further immunohistochemical staining.
Several prior studies have investigated the risk factors of SLN metastasis in patients with preoperative DCIS (12, 15, 17, 22). Factors such as age, tumor size, palpability, multifocality and upstaging have been proved to be correlated to SLN metastasis. In this study, we demonstrated that tumor size, multifocality and upstaging, but not age and palpability, were significantly related to SLN metastasis. In addition, we also found that positive axillary ultrasound finding, mastectomy and higher Ki-67 expression were significantly associated with more SLN metastasis.
However, what is most concerned by clinicians is to precisely predict SLN metastasis before surgery. So far, there have been plenty of models to predict axillary lymph node metastasis in breast cancer patients (23–25). Unfortunately, to the best of our knowledge no model has been developed to predict SLN metastasis in patients with a preoperative diagnosis of DCIS. Therefore, we built a model with factors that could be determined before surgery and were significantly correlated with SLN metastasis. This simple model incorporated factors including tumor size, axillary ultrasound finding and multifocality and yielded an AUC of 0.805 in the training set, suggesting good discrimination of SLN metastasis. The internal testing set was comparable with the training set in patients’ characteristics except for the ER/PR expression. Since they were determined after surgery and not correlated with SLN metastasis in the univariate analysis, no adjustment about ER/PR expression was applied in the logistic regression analysis. Internal validation of this model with the testing set achieved an AUC of 0.729, demonstrating relatively reliable discriminatory power of this model.
There were several limitations that should be noticed in this study. Firstly, this model did not incorporate any molecular information because it was not available before surgery in our hospital. In this study, Ki-67 expression was proved to be correlated with SLN metastasis in the univariate analysis. Therefore, the performance of this model may be improved if molecular information could be added. Secondly, this model was not validated externally. Thirdly, the retrospective nature of this study would inevitably lead to bias in the selection of patients.
Conclusion
A simple model incorporating tumor size, axillary ultrasound finding and multifocality was developed to predict SLN metastasis in patients with a preoperative diagnosis of DCIS. With good discriminatory power, this model should be helpful for surgeons to decide if SLN biopsy could be safely avoided in certain patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Fudan University Shanghai Cancer Center. The ethics committee waived the requirement of written informed consent for participation.
Author Contributions
CC and KZ conceived and designed the study. KZ and LQ wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81627804, 81830058).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, et al. Breast cancer in China. Lancet Oncol (2014) 15(7):e279–89. doi: 10.1016/S1470-2045(13)70567-9
2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin (2015) 65(2):87–108. doi: 10.3322/caac.21262
3. Sorum R, Hofvind S, Skaane P, Haldorsen T. Trends in incidence of ductal carcinoma in situ: the effect of a population-based screening programme. Breast (2010) 19(6):499–505. doi: 10.1016/j.breast.2010.05.014
4. Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst (2010) 102(3):170–8. doi: 10.1093/jnci/djp482
5. Liu J, Sun D, Chen L, Fang Z, Song W, Guo D, et al. Radiomics Analysis of Dynamic Contrast-Enhanced Magnetic Resonance Imaging for the Prediction of Sentinel Lymph Node Metastasis in Breast Cancer. Front Oncol (2019) 9:980. doi: 10.3389/fonc.2019.00980
6. James TA, Palis B, McCabe R, Pardo JA, Alapati A, Ukandu O, et al. Evaluating the role of sentinel lymph node biopsy in patients with DCIS treated with breast conserving surgery. Am J Surg (2020) 220(3): 654–9. doi: 10.1016/j.amjsurg.2020.01.014
7. Holm-Rasmussen EV, Jensen MB, Balslev E, Kroman N, Tvedskov TF. The use of sentinel lymph node biopsy in the treatment of breast ductal carcinoma in situ: A Danish population-based study. Eur J Cancer (2017) 87:1–9. doi: 10.1016/j.ejca.2017.09.037
8. van Roozendaal LM, Goorts B, Klinkert M, Keymeulen K, De Vries B, Strobbe LJA, et al. Sentinel lymph node biopsy can be omitted in DCIS patients treated with breast conserving therapy. Breast Cancer Res Treat (2016) 156(3):517–25. doi: 10.1007/s10549-016-3783-2
9. Nicholson S, Hanby A, Clements K, Kearins O, Lawrence G, Dodwell D, et al. Variations in the management of the axilla in screen-detected ductal carcinoma in situ: evidence from the UK NHS breast screening programme audit of screen detected DCIS. Eur J Surg Oncol (2015) 41(1):86–93. doi: 10.1016/j.ejso.2014.09.003
10. Coromilas EJ, Wright JD, Huang Y, Feldman S, Neugut AI, Chen L, et al. The Influence of Hospital and Surgeon Factors on the Prevalence of Axillary Lymph Node Evaluation in Ductal Carcinoma In Situ. JAMA Oncol (2015) 1(3):323–32. doi: 10.1001/jamaoncol.2015.0389
11. Watanabe Y, Anan K, Saimura M, Koga K, Fujino M, Mine, et al. Upstaging to invasive ductal carcinoma after mastectomy for ductal carcinoma in situ: predictive factors and role of sentinel lymph node biopsy. Breast Cancer (2018) 25(6):663–70. doi: 10.1007/s12282-018-0871-7
12. Sorrentino L, Sartani A, Bossi D, Amadori R, Nebuloni M, Truffi M, et al. Sentinel node biopsy in ductal carcinoma in situ of the breast: Never justified? Breast J (2018) 24(3):325–33. doi: 10.1111/tbj.12928
13. Heymans C, van Bastelaar J, Visschers RGJ, Vissers YLJ. Sentinel Node Procedure Obsolete in Lumpectomy for Ductal Carcinoma In Situ. Clin Breast Cancer (2017) 17(3):e87–93. doi: 10.1016/j.clbc.2016.10.002
14. Kotani H, Yoshimura A, Adachi Y, Ishiguro J, Hisada T, Ichikawa M, et al. Sentinel lymph node biopsy is not necessary in patients diagnosed with ductal carcinoma in situ of the breast by stereotactic vacuum-assisted biopsy. Breast Cancer (2016) 23(2):190–4. doi: 10.1007/s12282-014-0546-y
15. Han JS, Molberg KH, Sarode V. Predictors of invasion and axillary lymph node metastasis in patients with a core biopsy diagnosis of ductal carcinoma in situ: an analysis of 255 cases. Breast J (2011) 17(3):223–9. doi: 10.1111/j.1524-4741.2011.01069.x
16. Prendeville S, Ryan C, Feeley L, O'Connell F, Browne TJ, O'Sullivan MJ, et al. Sentinel lymph node biopsy is not warranted following a core needle biopsy diagnosis of ductal carcinoma in situ (DCIS) of the breast. Breast (2015) 24(3):197–200. doi: 10.1016/j.breast.2015.01.004
17. Francis AM, Haugen CE, Grimes LM, Crow JR, Yi M, Mittendorf EA, et al. Is Sentinel Lymph Node Dissection Warranted for Patients with a Diagnosis of Ductal Carcinoma In Situ? Ann Surg Oncol (2015) 22(13):4270–9. doi: 10.1245/s10434-015-4547-7
18. Lazzaretti MG, Ponti A, Mano MP, Barca A, Casella D, Frigerio A, et al. Reducing harms from treatment. Sixteen years of surgery of the axilla for screen-detected breast cancers in Italy. Breast (2018) 42:15–22. doi: 10.1016/j.breast.2018.08.001
19. Yoo TK, Kim SJ, Lee J, Lee SB, Lee SJ, Park HY, et al. A N0 Predicting Model for Sentinel Lymph Node Biopsy Omission in Early Breast Cancer Upstaged From Ductal Carcinoma in Situ. Clin Breast Cancer (2020) 20(3):e281–9. doi: 10.1016/j.clbc.2019.11.011
20. Zhang K, Zhu Q, Sheng D, Li J, Chang C. A New Model Incorporating Axillary Ultrasound After Neoadjuvant Chemotherapy to Predict Non-Sentinel Lymph Node Metastasis in Invasive Breast Cancer. Cancer Manag Res (2020) 12:965–72. doi: 10.2147/CMAR.S239921
21. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol (2013) 31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984
22. Holm-Rasmussen EV, Jensen MB, Balslev E, Kroman N, Tvedskov TF. Risk factors of sentinel and non-sentinel lymph node metastases in patients with ductal carcinoma in situ of the breast: A nationwide study. Breast (2018) 42:128–32. doi: 10.1016/j.breast.2018.09.004
23. Bevilacqua JL, Kattan MW, Fey JV, Cody HS,3, Borgen PI, Van Zee KJ. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J Clin Oncol (2007) 25(24):3670–9. doi: 10.1200/JCO.2006.08.8013
24. Qiu SQ, Zeng HC, Zhang F, Chen C, Huang WH, Pleijhuis RG, et al. A nomogram to predict the probability of axillary lymph node metastasis in early breast cancer patients with positive axillary ultrasound. Sci Rep (2016) 6:21196. doi: 10.1038/srep21196
Keywords: breast carcinoma, ductal carcinoma in situ, core needle biopsy, sentinel lymph node, axillary surgery
Citation: Zhang K, Qian L, Zhu Q and Chang C (2020) Prediction of Sentinel Lymph Node Metastasis in Breast Ductal Carcinoma In Situ Diagnosed by Preoperative Core Needle Biopsy. Front. Oncol. 10:590686. doi: 10.3389/fonc.2020.590686
Received: 03 August 2020; Accepted: 14 October 2020;
Published: 10 November 2020.
Edited by:
Gianluca Franceschini, Catholic University of the Sacred Heart, ItalyReviewed by:
Armando Orlandi, Agostino Gemelli University Polyclinic, ItalyAlejandro Martin Sanchez, Fondazione Policlinico Universitario A. Gemelli IRCCS, Italy
Copyright © 2020 Zhang, Qian, Zhu and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cai Chang, Y2hhbmdjNjJAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Kai Zhang
Kai Zhang Lang Qian†
Lang Qian†