- Department of Breast Surgery, West China Hospital, Sichuan University, Chengdu, China
Background: For sentinel lymph node biopsy (SLNB) in patients with breast cancer, the dual tracer of blue dye and radioisotope with the 10% rule that all nodes with radioactive count of 10% or more of the hottest node ex vivo should be removed is widely accepted. However, the cut-off point of radioactivity is being questioned for possibly excessive removal of negative nodes.
Methods: To compare different percentile rules and optimize the criteria for identifying SLNs, we established a database which prospectively collected the radioactivity, status of blue dye and the pathological results of each SLN in breast cancer patients who successfully underwent SLNB with a combination of methylene blue and radioisotope.
Results: A total of 2,529 SLNs from 1,039 patients were identified from August 2010 to August 2019. 16.4% (414/2,529) positive nodes were removed at a cost of 83.6% (2115/2,529) negative nodes removed excessively. Up to 17.9% (375/2,115) negative nodes were removed as radioactively hot nodes without blue staining. By gradually increasing the threshold by each 10%, the number of negative nodes identified reduced by 18.2% (385/2,115) with only three node-positive patients (1.0%) missed to be identified using the “40% + blue” rule. In patients with ≥ 2 SLNs removed, 12.3% (238/1,942) negative nodes avoided unnecessary removal with only 0.8% (2/239) positive patients missed with the “hottest two + blue” rule.
Conclusions: Our data indicated that the “40% + blue” rule or the “hottest two + blue” rule for SLNB with the dual tracer of blue dye and radioisotope may be considered as a potential alternative rule to minimize extra nodes resected. Nonetheless, it should be validated by prospective trials with long-term follow-up.
Introduction
The sentinel lymph node (SLN) was discovered in patients with melanoma by Cabanas in 1977 and is defined as the first draining node(s) with a direct lymphatic connection to the primary tumor site (1). Since sentinel lymph node biopsy (SLNB) was first applied to breast cancer by Krag in 1993 to predict the status of axilla and guide further treatment (2), it has become the standard care of the axilla for early stage breast cancer patients with reduced arm morbidities while still offering equivalent survival compared to axillary lymph node dissection (ALND) (3). There are various tracing methods to guide surgeons to identify a sentinel node intraoperatively including blue dye, radioisotope colloid and various novel techniques such as indocyanine green optical imaging and superparamagnetic iron oxide (3). Given the lack of radioisotope and extra requirements for equipment especially in less developed areas, SLNB using single tracer, predominantly blue dye is used in a large number of institutes (4). However, the dual-tracer method combining the radioactive colloid and blue dye with a higher SLN detection rate (>90%) and a lower false negative rate (FNR) (<5%–10%) than either single tracer is constantly recommended in many guidelines such as the 2005 American Society of Clinical Oncology (ASCO) Guideline Recommendations for Sentinel Lymph Node Biopsy in Early-stage Breast Cancer and the 2011 Chinese Anti-Cancer Association (CACA) Guidelines, and is increasingly being applied in many countries and areas such as the United States, Europe, Australia and China (5–7). Most frequently, breast surgeons who use dual tracer of radioisotope and blue dye follow the “10% + blue” rule which was originally proposed by Martin and McMasters that all nodes with a radioactivity count of at least 10% of the hottest node ex vivo or blue dye staining should be removed (8).
An ideal criterion of SLN selection should minimize the number of nodes removed, without significantly sacrificing the sensitivity of the procedure. While this approach can reduce the risk of missing positive nodes with a low radioactivity count, it may result in an excessive number of nodes being removed than those identified on lymphoscintigraphy. To seek an ideal cut-off point of a hot SLN, several studies have assessed the validity of the “10% + blue” rule by comparing with other alternative node harvesting rules, including the “50% + blue” rule, the “hottest + blue” rule, and the “4 nodes” rule (9–11). In our institution, we were concerned that excessive number of negative nodes were excised by the “10% + blue” rule. The more SLNs removed, the higher the cost of the procedure for added operative time, pathological charges, medical resources, and most importantly, the long-term complications after surgeries. However, there is no study comparing the “10% + blue” rule with other alternative criteria under SLNB using radioisotope and methylene blue in China.
Herein, we performed this retrospective analysis which included a large number of breast cancer patients with a prospectively constructed SLNB database at a single institution in China. We re-evaluated the “10% + blue” rule for breast cancer patients and sought to determine whether the threshold of hot nodes could be raised and what the impact it would be on both the accuracy and the number of lymph nodes excised when a higher than 10% threshold was used to define a SLN, potentially leading to patients with positive nodes being missed.
Materials and Methods
This study was approved by our institutional review board.
Study Population
Retrospectively, we reviewed the records of breast cancer patients who underwent SLNB successfully with a combination of radioactive colloid and methylene blue at our hospital from August 2010 to August 2019. Patients who were pathologically diagnosed with invasive breast cancer were eligible. Patients who received mastectomy for ductal carcinoma in situ (DCIS) were excluded. Patients who received neoadjuvant chemotherapy were also excluded. All patients were clinically node negative (negative in ultrasound, mammography, and physical examination) and had no regional or distant metastases.
Surgical Techniques for SLNB
After the informed consent was obtained from each patient, Radioisotope -99mTc (Beijing Shihong Drug Development Center; Beijing, China) was injected intradermally at tumor surface and/or at periareolar site 3 to 18 h prior to the surgery, and methylene blue (Jiangsu Jichuan Pharmaceutical Co., Ltd; Jiangsu, China) was injected intradermally/subcutaneously at tumor surface and/or at periareolar site 10 to 15 min before surgery. During surgery, a hand-held gamma probe of 99mTc (Devicor Medical Products Inc.; OH, USA) was applied to identify SLNs. Any nodes with 10% or more of the ex vivo count of the hottest node and/or any nodes with at least one blue afferent lymphatic vessels derived from the breast were removed and designated as SLNs. Suspicious lymph nodes which were firm, enlarged and palpable but not radioactive or blue stained were also removed as non-SLNs. All nodes were evaluated with intraoperative frozen sections. ALND were performed based on the result of pathological evaluation. Generally, patients with SLNs of macrometastatasis (>2 mm) received ALND. It was recommended in the guidelines of China Anti-Cancer Association in 2017 that axillary dissection can be avoided in cT1-2N0 breast cancer patients who have 1 or 2 macrometastatic SLNs and are undergoing breast-conserving therapy and whole-breast radiation (7). Starting in 2018, for patients who meet the criteria of the ACOSOG Z0011, decisions to perform ALND or not should be made with full informed consent in our institution. Patients free of metastasis and those with SLNs of isolated tumor cells avoided further ALND. For patients with SLNs of micrometastatasis (>0.2 mm, ≤ 2 mm), decisions of ALND were made jointly by patients and the surgery group. Most of nodes removed were examined by permanent sections with hematoxylin-eosin (H&E) staining and immunohistochemical (IHC) staining for breast cancer-specific antigens if no macrometastasis was identified on routine assessment.
During surgery, the radioactivity, status of blue dye staining of nodes and lymphatic vessels, and the pathological results of each SLN were prospectively recorded so that we could calculate the number of SLNs identified by different criteria of radioactivity in combination with the status of blue staining.
Statistical Analysis
In this study, we defined the rate of miss detection as the number of patients with positive nodes missed to be identified using alternative rules compared with the “10% + blue” rule divided by the total number of node-positive patients detected by the “10% + blue” rule. The chi-square test was used for categorical variables by SPSS 24 (SPSS Inc., Chicago, IL, USA). Figures were prepared by GraphPad Prism 8.0.1. Differences were considered significant at p ≤ 0.05.
Results
A total of 1,039 invasive breast cancer patients successfully performed SLNB by dual tracers with the “10% + blue” rule. The clinical and pathologic characteristics of the study population were represented in Table 1.
Results of SLNB With “10% + Blue” Rule
A total of 2,529 SLNs were identified in 1,039 patients and 16.4% (414/2,529) SLNs were positive (micrometastases or macrometastases) (Table 2). A mean of 2.4 SLNs were identified. 78.0% (810/1,039) patients had at least two SLNs identified and 6.64% patients had five or more SLNs removed (Figure 1A). 121 non-SLNs were removed for enlarged and palpable but not blue or hot, of which 38 non-SLNs were positive. In a total of 309 patients with at least one positive axillary node (micrometastases or macrometastases), 296 patients had at least one positive SLN with or without positive non-SLNs and each of the remaining 13 patients had only one positive non-SLN. We do not know how many positive lymph nodes were missed due to the lack of complementary ALND, so the probability of non-SLN metastases in patients with SLN metastases (8.4%, 25/296) in this study was lower than that in the AMAROS trial and the Z0011 trial which had approximately one-third patients with a positive non-SLN in the ALND group (12, 13). Among the 414 positive SLNs, 70.3% (291/414) had a radioactivity count of 40% or more than the hottest node and 13.3% (55/414) were blue stained with a less than 10% radiation count of the hottest node (Figure 1B). Among 2,115 negative SLNs, 1,413 nodes were blue stained while up to 1,792 were radioactively hot, leading to 17.9% (379/2,115) negative nodes being excessively excised as radioactively hot nodes. Numbers of positive and negative SLNs detected by radioactive colloid and blue dye were shown in Table S1–3, respectively.
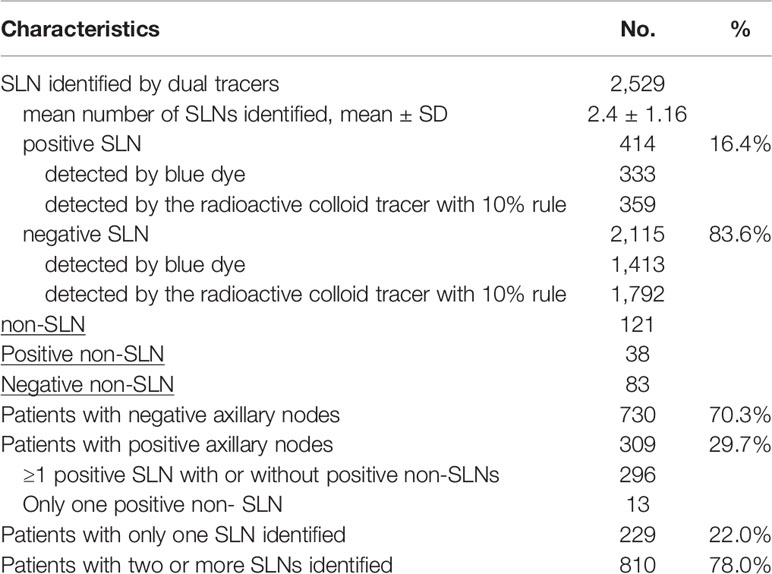
Table 2 Outcomes of the dual tracer using a combination of blue dye and radioactive colloid with the 10% criteria.

Figure 1 (A) Percentage of patients with different No. of sentinel lymph node (SLN) per patients identified by the “10% + blue” rule. (B) The radioactive count distribution of 414 positive SLNs by percentile of the hottest node. * Positive nodes with radioactive count percent <10% but blue staining.
Different Alternative Rules Compared With “10% + Blue” Rule
Different percentile rules for radioactivity were compared with the “10% + blue” rule (Table 3). As is shown in Figure 2, the balance between fewer positive nodes missed and more negative nodes reserved was between the “40% + blue” rule and the “50% + blue” rule. From the “10% rule + blue” rule to the “50% + blue” rule, the average number of SLNs identified per patients dropped from 2.43 to 2.00. Compared with the “10% + blue” rule, when the “40% + blue” rule was applied, the rate of positive SLNs increased from 14.80% to 16.58% (p>0.05) and negative SLNs decreased by 18.2% (385/2,115), resulting in a rate of miss detection of only 1.00% (3/296). If only the hottest or blue nodes were removed, seven patients with positive nodes would be undetected, resulting in a rate of miss detection was 2.7%. Characteristics of the seven patients were shown in Table S4. There was no statistically significant difference found with respect to the rate of positive SLNs and the rate of miss detection when applying the criteria anywhere from 10% to the hottest for identifying SLNs compared with the “10% + blue” rule.
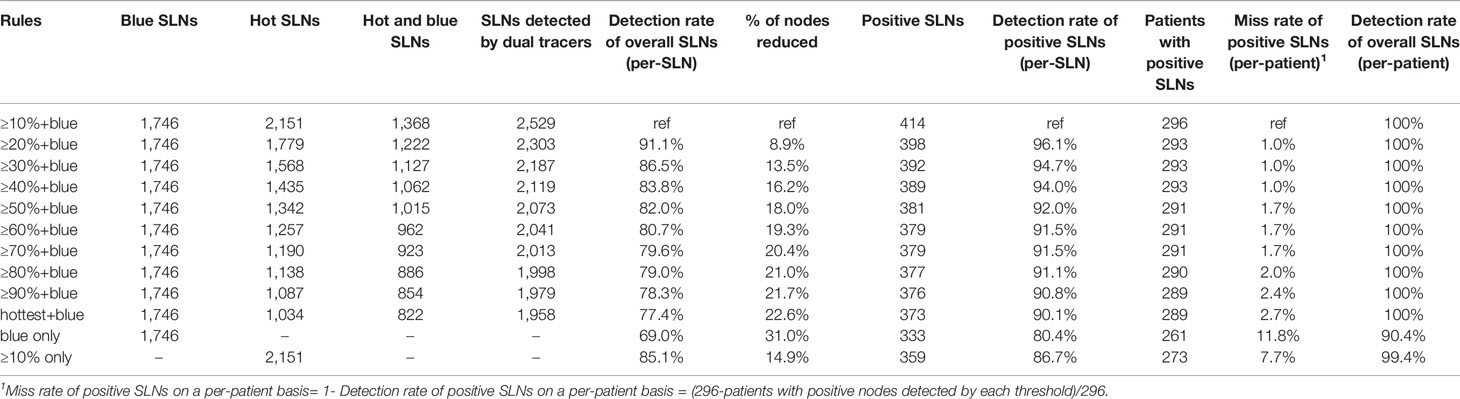
Table 3 Effect of different percentage criteria on sentinel lymph node (SLN) identification positive SLN (n=1,039).
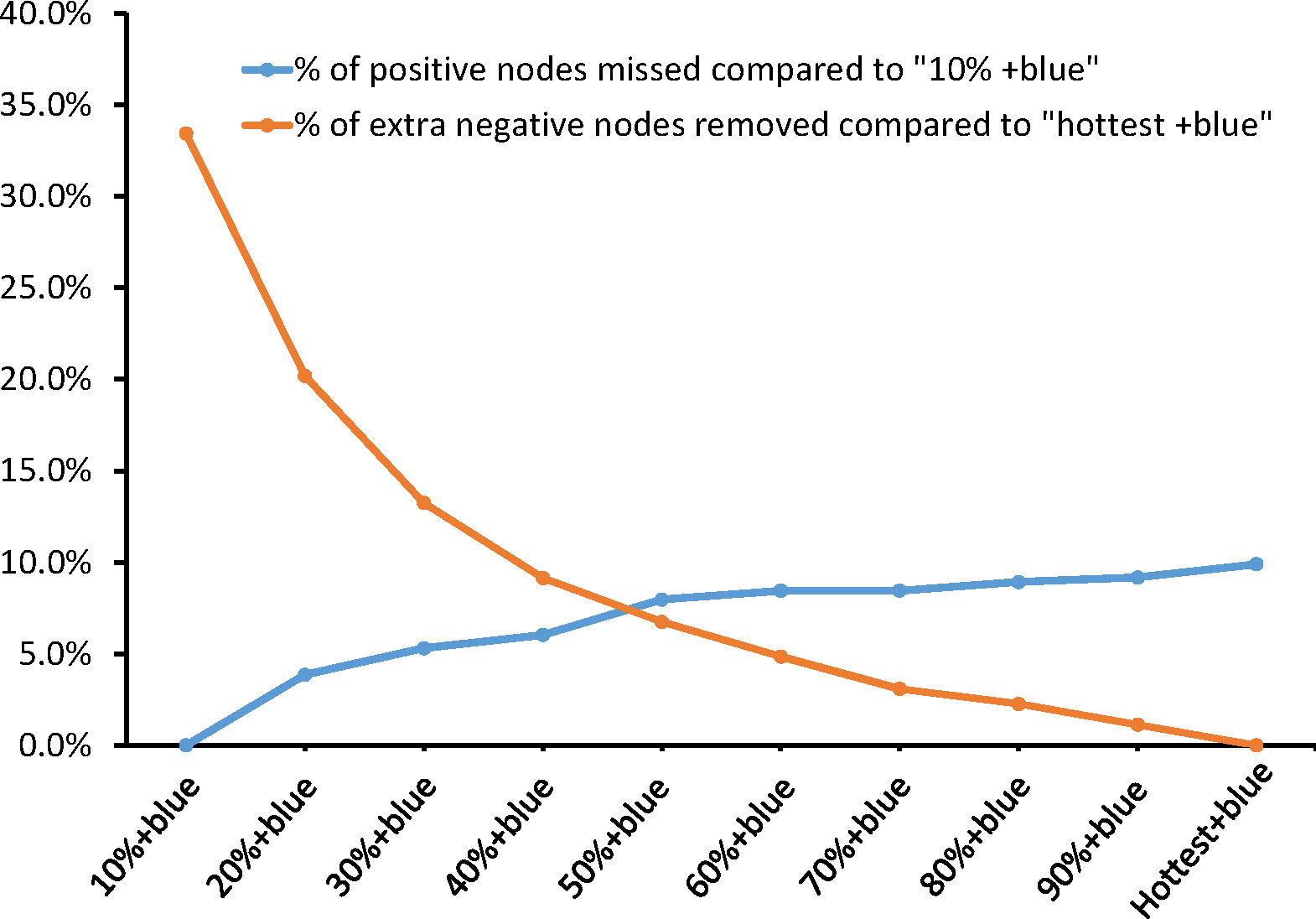
Figure 2 Positive nodes missed and extra negative removed by combing different percentage rules with blue dye.
Finally, we assessed the “hottest two + blue” rule in 810 patients with at least two SLNs identified by the “10% + blue” rule in this study. The outcomes were presented in Table 4. Compared to the “10% + blue” rule, 23 positive nodes were undetected causing 0.84% (2/239) patients with positive nodes missed whereas 12.26% (238/1,942) negative nodes were reserved. Of note, among the 23 positive nodes missed to be identified, 3 nodes were from two node-positive patients who would have been missed to be detected using the “hottest two + blue” rule, and other 20 nodes were from 20 node-positive patients who could have been identified using the “hottest two + blue” rule.
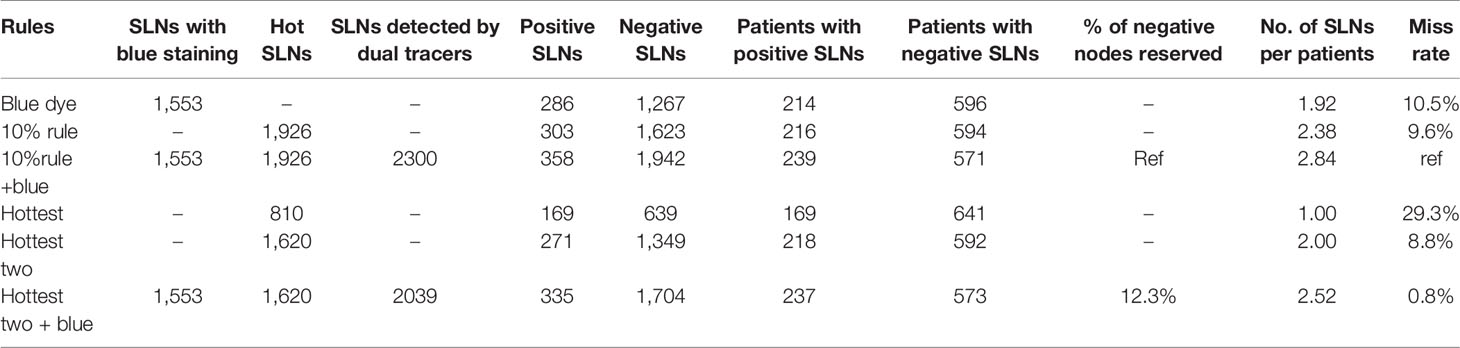
Table 4 Effect of different criteria on sentinel lymph node (SLN) identification in patients with two or more SLNs removed (n=810).
Discussion
During the last decades, the concept of the treatment strategy for breast cancer has shifted from maximum tolerated therapy to minimum effective therapy. With the improvement of imaging examination and the popularization of screening, breast cancers diagnosed at early stage have strongly increased (14–17). In non-surgical area, improvements in multimodal therapy, including advances in modern radiotherapy technology, optimization of chemotherapy, and anti-HER2 therapy regimens, novel endocrine agents, and target drugs, as well as clinical utility of immunotherapy, could further diminish breast cancer mortality and contribute to increase chances for cure in 70%–80% patients with early breast cancer (18, 19). In large clinical trials such as the AMAROS and the ACOSOG Z0011, the residual tumor burden from limited metsastatic nodes may be further reduced, resulting in an extremely low recurrence rate (<2%) (12, 13, 20). With extended survival, the quality of life is becoming more important. The dual tracer combining radioisotope and blue dye remains the mainstream in the current clinical routine, especially in institutions where materials and equipment for new tracing method are not available. Exploring optimized criteria based on the dual-tracer method is more conducive to improve the quality of life for a wide range of patients. Therefore, in this study, we merely focused on the dual tracer method of radioactive colloid and methylene blue, rather than other new techniques for SLNB such as indocyaninegreen.
Although SLNB is associated with improved quality of life and reduced arm morbidities without compromising the survival in patients with early stage breast cancer compared to ALND (21, 22), a considerable number of patients undergoing SLNB still suffer from arm and shoulder impairments. Prevalence of lymphedema one year after SLNB ranges between 3% and 17% and for pain, prevalence between 3.3% and 56.6% have been reported in SLN-negative breast cancer patients (23–25). Some studies reported that a greater number of nodes removed, especially more than ten nodes dissected, was associated with an increased risk of lymphedema in ALND patients (26–28) although existing studies failed to find this association in SLNB patients (24, 29, 30). However, the observation that the arm morbidity occurs in a certain proportion of patients who received SLNB leads us to worry that a larger number of SLNs dissected may contribute to a higher risk of arm morbidity. In this study, 16.4% nodes were harvested for metastases at an expense of 83.6% negative nodes removed excessively. Furthermore, up to 17.9% negative nodes were removed as radioactively hot nodes without blue staining. Besides, 6.64% patients had five or more SLNs removed, which may weaken the advantage of SLNB as a less invasive procedure. The more SLNs removed, the higher the cost of the procedure for added time during surgery and increased pathological charges. When no metastases are detected by routine H&E, more in-depth histologic evaluation such as IHC will be applied to detect (micro-)metastases, making the procedure more expensive than routine histology (31, 32).
Is there a more reasonable guide for identifying SLNs with less unnecessary nodes removed for breast cancer? To our knowledge, several previous retrospective studies compared the dual tracer using 10% rule with various blue dye and a few studies attempted to seek alternative methods. Nagao et al. assessed the “10% + blue” rule and the “4 node” rule by involving 302 patients with Tis-T3 breast cancer who underwent SLNB with a combination of radioisotope and indigo carmine blue dye and concluded that terminating SLNB at the first three SLNs identified all node positive patients with a low false negative rate (FNR) and rate of complication (9). In a study of 475 patients with T1-2 breast cancer who underwent SLNB with a combination of radioisotope (10% rule) and blue dye (lymphazurin or methylene), Dutta et al. indicated that no more than 4 SLNs should be removed because all patients with positive nodes were identified within the first 4 SLNs removed (10). Liu et al. studied 332 patients with T1-T3 breast cancer who underwent SLNB and showed that using the “40%” rule as the criteria for removal of SLN resulted in a 10.3% FNR and “10%” rule resulted in a 6.4% FNR; however, surgeons selectively used lymphazurin blue so the radioisotope was generally used alone in the study (11). Another large retrospective study involving 6519 patients with T1-T3 breast cancer who underwent SLNB with a combination of radioisotope and isosulfan blue dye performed by Chung et al. reported that the “10% +blue” rule was a reliable guideline but they didn’t determine other potential percentile cut-off of hot nodes (33). We first built the model by gradually increasing the percentile threshold of radioactivity count in a large prospectively collected database of breast cancer patients who performed SLNB by dual tracers of methylene blue and radioisotope in China. Our data demonstrated that compared with the “10% + blue” rule, the number of nodes identified would reduce by 16.2% at a cost of only three positive patients being missed (1.0%) when the “40% +blue” rule was used. Similarly, in patients with at least two SLNs removed, 12.3% negative nodes were able to avoid being removed unnecessarily with only 0.8% positive patients missed by the “hottest two + blue” criteria. The potential 16.2% and 12.3% reduction in nodes that need pathological examination may offer a considerable cost-effectiveness benefit of the procedure. Our result revealed that replacing the “10% +blue” rule with the “40% +blue” rule or the “hottest two + blue” rule can be considered as a potential alternative model to minimize extra negative nodes removed without significantly increasing the number of node positive patients missed.
The main concern for patients with SLNB is the impact of missed nodes on locoregional recurrence and survival. In the NSABP B-06 trial which was designed to determine whether SLNB achieve an equivalent survival and regional control as ALND, breast cancer patients with negative SLNs were randomly assigned 1:1 to ALND or SLNB alone. It reported that each group had less than 1% regional node recurrences as first events by eight years (ALND group vs SLNB group: 8/1,975 vs 12/2,011, p=0.22) with 9.8% FNR in the ALND group (34). The Milan trial also showed that 2 patients in the SLNB group developed axillary recurrence with 8 patients estimated to have occult axillary involvement (35). Besides, in the AMAROS trial and the Z0011 trial, the axillary recurrence was extremely low (<1%) in the SLNB group with an estimated one-third residual lesions (12, 13). In our study, only 0.29% (3/1,039) node-positive patients were missed when we changed the “10% +blue” rule to the “40% +blue” rule and 0.25% (2/810) when we replaced the “10% +blue” rule with the “hottest two + blue” rule. In the era of subsequent effective and complete adjuvant therapy, the residual lesions may be further reduced. We therefore would expect to see an extremely low recurrence rate.
Some limitations of this study should be mentioned. First, in this retrospective study, ALND was not performed in patients with negative SLNs because of ethical issues. A small number of SLN-positive patients chose to avoid further ALND starting in 2018, which was a bit behind the clinical trials and guidelines. Therefore, the actual sensitivity, specificity, accuracy and FNR of SLNB were unlikely to be calculated. What we were most concerned about was the FNR of alternatives to the “10% + blue” rule. Thus we defined the term “the rate of miss detection” similar to Liu and Murphy (36, 37) and no statistically significance was found anywhere from the “10% +blue” rule to the “hottest + blue” rule. Besides, in our institution, to ensure a low FNR within 5%, at least 40 cases were required for the learning curve for SLNB before surgeons could contribute to this database so that our conclusion could not be affected by the shortcoming of unknowing true FNR. Second, patients with micrometastatic SLNs were offered observation or ALND with full informed consent, which was somewhat behind the latest guidelines and the IBCSG 23-01 trial which indicated that ALND should be avoided in SLN-micrometastatic patients receiving breast-conserving surgery (38). Besides, we didn’t group patients prospectively and the study was a single monocentric experience without confirmation in an external dataset, so we did not know the local control of patients undergoing SLNB with different criteria. Though the effect of missing positive patients on survival was not expected to be great according previous literature as discussed above, the results of this study should be validated by multiple-center prospective studies with long-term follow up for prognosis.
Conclusions
Our data demonstrated that the “40% + blue” rule or the “hottest two + blue” rule can be considered as a potential alternative model to minimize extra negative nodes removed without significantly increasing the number of node-positive patients missed. The results should be further validated in prospective clinical trials with long-term follow up.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Westchina hospital, Sichuan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Study conception and design: QL. Data collection: ZD, FL, YX, QYL, JC, and HZ. Data analysis: LX and JY. Data interpretation: QL, ZD, LX, and JY. Manuscript preparation: LX and JY. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study is supported by the funding from the National Natural Science Foundation of China (81902686); China Postdoctoral Science Foundation Funded Project (2019M663511); the Post-Doctor Research Project, West China Hospital, Sichuan University (2019HXBH046); the Program of the Science and Technology Bureau of Sichuan (2020YJ0293, 2019YFH0146 and 2020YFS0199); and Chengdu Science and Technology Bureau (2019-YF05-01082-SN).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.588067/full#supplementary-material
References
1. Cabanas RM. An approach for the treatment of penile carcinoma. Cancer (1977) 39(2):456–66. doi: 10.1002/1097-0142(197702)39:2<456::AID-CNCR2820390214>3.0.CO;2-I
2. Krag DN, Weaver DL, Alex JC, Fairbank JT. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol (1993) 2(6):335–9; discussion 40. doi: 10.1016/0960-7404(93)90064-6
3. Qiu SQ, Zhang GJ, Jansen L, de Vries J, Schroder CP, de Vries EGE, et al. Evolution in sentinel lymph node biopsy in breast cancer. Crit Rev Oncol (2018) 123:83–94. doi: 10.1016/j.critrevonc.2017.09.010
4. Arrington AK, Kruper L, Vito C, Yim J, Kim J, Chen SL. Rural and urban disparities in the evolution of sentinel lymph node utilization in breast cancer. Am J Surg (2013) 206(5):674–81. doi: 10.1016/j.amjsurg.2013.07.007
5. Lyman GH, Giuliano AE, Somerfield MR, Benson AB, 3rd, Bodurka DC, Burstein HJ, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol (2005) 23(30):7703–20. doi: 10.1200/JCO.2005.08.001
6. Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, Giuliano AE. Sentinel Lymph Node Biopsy for Patients With Early-Stage Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol (2017) 35(5):561–4. doi: 10.1200/JCO.2016.71.0947
7. Association CA-C. Guideline and standard for the diagnosis and treatment of breast cancer by Chinese Anti-Cancer Association. China Oncol (2017) 27(9):75. doi: 10.1016/S1470-2045(20)30466-6
8. Martin RC,2, Edwards MJ, Wong SL, Tuttle TM, Carlson DJ, Brown CM, et al. Practical guidelines for optimal gamma probe detection of sentinel lymph nodes in breast cancer: results of a multi-institutional study. For the University of Louisville Breast Cancer Study Group. Surgery (2000) 128(2):139–44. doi: 10.1067/msy.2000.108064
9. Nagao T, Kinoshita T, Hojo T, Kurihara H, Tsuda H. Sentinel lymph node biopsy using indigo carmine blue dye and the validity of ‘10% rule’ and ‘4 nodes rule’. Breast (Edinburgh Scotland) (2012) 21(4):455–8. doi: 10.1016/j.breast.2011.10.011
10. Dutta R, Kluftinger A, MacLeod M, Kindrachuk G, Baliski C. Revisiting the “10% rule” in breast cancer sentinel lymph node biopsy: an approach to minimize the number of sentinel lymph nodes removed. Am J Surg (2012) 203(5):623–7. doi: 10.1016/j.amjsurg.2012.01.010
11. Liu LC, Lang JE, Jenkins T, Lu Y, Ewing CA, Hwang SE, et al. Is it necessary to harvest additional lymph nodes after resection of the most radioactive sentinel lymph node in breast cancer? J Am Coll Surg (2008) 207(6):853–8. doi: 10.1016/j.jamcollsurg.2008.08.008
12. Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol (2014) 15(12):1303–10. doi: 10.1016/S1470-2045(14)70460-7
13. Giuliano AE, Ballman K, McCall L, Beitsch P, Whitworth PW, Blumencranz P, et al. Locoregional Recurrence After Sentinel Lymph Node Dissection With or Without Axillary Dissection in Patients With Sentinel Lymph Node Metastases: Long-term Follow-up From the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 Randomized Trial. Ann Surg (2016) 264(3):413–20. doi: 10.1097/SLA.0000000000001863
14. Romanoff A, Constant TH, Johnson KM, Guadiamos MC, Vega AMB, Zunt J, et al. Association of Previous Clinical Breast Examination With Reduced Delays and Earlier-Stage Breast Cancer Diagnosis Among Women in Peru. JAMA Oncol (2017) 3(11):1563–7. doi: 10.1001/jamaoncol.2017.1023
15. Cho N, Han W, Han BK, Bae MS, Ko ES, Nam SJ, et al. Breast Cancer Screening With Mammography Plus Ultrasonography or Magnetic Resonance Imaging in Women 50 Years or Younger at Diagnosis and Treated With Breast Conservation Therapy. JAMA Oncol (2017) 3(11):1495–502. doi: 10.1001/jamaoncol.2017.1256
16. de Glas NA, de Craen AJ, Bastiaannet E, Op ‘t Land EG, Kiderlen M, van de Water W, et al. Effect of implementation of the mass breast cancer screening programme in older women in the Netherlands: population based study. BMJ (2014) 349:g5410. doi: 10.1136/bmj.g5410
17. Chen X, He Y, Wang J, Huo L, Fan Z, Li J, et al. Feasibility of using negative ultrasonography results of axillary lymph nodes to predict sentinel lymph node metastasis in breast cancer patients. Cancer Med (2018) 7(7):3066–72. doi: 10.1002/cam4.1606
18. Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Primers (2019) 5(1):66. doi: 10.1038/s41572-019-0111-2
19. Huang NS, Si J, Yang BL, Quan CL, Chen JJ, Wu J. Trends and clinicopathological predictors of axillary evaluation in ductal carcinoma in situ patients treated with breast-conserving therapy. Cancer Med (2018) 7(1):56–63. doi: 10.1002/cam4.1252
20. Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol (2013) 14(4):297–305. doi: 10.1016/S1470-2045(13)70035-4
21. Ashikaga T, Krag DN, Land SR, Julian TB, Anderson SJ, Brown AM, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol (2010) 102(2):111–8. doi: 10.1002/jso.21535
22. Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst (2006) 98(9):599–609. doi: 10.1093/jnci/djj158
23. Verbelen H, Gebruers N, Eeckhout FM, Verlinden K, Tjalma W. Shoulder and arm morbidity in sentinel node-negative breast cancer patients: a systematic review. Breast Cancer Res Treat (2014) 144(1):21–31. doi: 10.1007/s10549-014-2846-5
24. De Groef A, Van Kampen M, Tieto E, Schonweger P, Christiaens MR, Neven P, et al. Arm lymphoedema and upper limb impairments in sentinel node-negative breast cancer patients: A one year follow-up study. Breast (Edinburgh Scotland) (2016) 29:102–8. doi: 10.1016/j.breast.2016.07.021
25. Verbelen H, Tjalma W, Meirte J, Gebruers N. Long-term morbidity after a negative sentinel node in breast cancer patients. Eur J Cancer Care (Engl) (2019) 28(5):e13077. doi: 10.1111/ecc.13077
26. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol (2013) 14(6):500–15. doi: 10.1016/S1470-2045(13)70076-7
27. Hack TF, Kwan WB, Thomas-Maclean RL, Towers A, Miedema B, Tilley A, et al. Predictors of arm morbidity following breast cancer surgery. Psychooncology (2010) 19(11):1205–12. doi: 10.1002/pon.1685
28. Meeske KA, Sullivan-Halley J, Smith AW, McTiernan A, Baumgartner KB, Harlan LC, et al. Risk factors for arm lymphedema following breast cancer diagnosis in Black women and White women. Breast Cancer Res Treat (2009) 113(2):383–91. doi: 10.1007/s10549-008-9940-5
29. Goldberg JI, Wiechmann LI, Riedel ER, Morrow M, Van Zee KJ. Morbidity of sentinel node biopsy in breast cancer: the relationship between the number of excised lymph nodes and lymphedema. Ann Surg Oncol (2010) 17(12):3278–86. doi: 10.1245/s10434-010-1155-4
30. Boughey JC, Hoskin TL, Cheville AL, Miller J, Loprinzi MD, Thomsen KM, et al. Risk factors associated with breast lymphedema. Ann Surg Oncol (2014) 21(4):1202–8. doi: 10.1245/s10434-013-3408-5
31. Jeruss JS, Hunt KK, Xing Y, Krishnamurthy S, Meric-Bernstam F, Cantor SB, et al. Is intraoperative touch imprint cytology of sentinel lymph nodes in patients with breast cancer cost effective? Cancer (2006) 107(10):2328–36. doi: 10.1002/cncr.22275
32. Agnese DM, Abdessalam SF, Burak WE Jr., Magro CM, Pozderac RV, Walker MJ. Cost-effectiveness of sentinel lymph node biopsy in thin melanomas. Surgery (2003) 134(4):542–7; discussion 7-8. doi: 10.1016/S0039-6060(03)00275-7
33. Chung A, Yu J, Stempel M, Patil S, Cody H, Montgomery L. Is the “10% rule” equally valid for all subsets of sentinel-node-positive breast cancer patients? Ann Surg Oncol (2008) 15(10):2728–33. doi: 10.1245/s10434-008-0050-8
34. Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol (2010) 11(10):927–33. doi: 10.1016/S1470-2045(10)70207-2
35. Veronesi U, Viale G, Paganelli G, Zurrida S, Luini A, Galimberti V, et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg (2010) 251(4):595–600. doi: 10.1097/SLA.0b013e3181c0e92a
36. Liu LC, Parrett BM, Jenkins T, Lee W, Morita E, Treseler P, et al. Selective sentinel lymph node dissection for melanoma: importance of harvesting nodes with lower radioactive counts without the need for blue dye. Ann Surg Oncol (2011) 18(10):2919–24. doi: 10.1245/s10434-011-1689-0
37. Murphy AD, Britten A, Powell B. Hot or not? The 10% rule in sentinel lymph node biopsy for malignant melanoma revisited. J Plast Reconstr Aesthet Surg (2014) 67(3):316–9. doi: 10.1016/j.bjps.2013.11.008
38. Galimberti V, Cole BF, Viale G, Veronesi P, Vicini E, Intra M, et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol (2018) 19(10):1385–93. doi: 10.1016/S1470-2045(18)30380-2
Keywords: breast cancer, sentinel lymph node biopsy, radioisotope, methylene blue, 10% rule
Citation: Xu L, Yang J, Du Z, Liang F, Xie Y, Long Q, Chen J, Zeng H and Lv Q (2020) Redefining Criteria to Ensure Adequate Sentinel Lymph Node Biopsy With Dual Tracer for Breast Cancer. Front. Oncol. 10:588067. doi: 10.3389/fonc.2020.588067
Received: 28 July 2020; Accepted: 06 November 2020;
Published: 03 December 2020.
Edited by:
Gianluca Franceschini, Catholic University of the Sacred Heart, ItalyReviewed by:
Tomoharu Sugie, Kansai Medical University Hospital, JapanArmando Orlandi, Agostino Gemelli University Polyclinic, Italy
Alba Di Leone, A. Gemelli University Hospital Foundation, Italy
Copyright © 2020 Xu, Yang, Du, Liang, Xie, Long, Chen, Zeng and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Lv, bHZxaW5nd2VzdGNoaW5hQDE2My5jb20=
†These authors share first authorship
 Li Xu
Li Xu Jiqiao Yang
Jiqiao Yang Zhenggui Du
Zhenggui Du