- 1Department of Radiology and Nuclear Medicine, University Medical Center Utrecht, Utrecht, Netherlands
- 2Department of Solid Tumors, Princess Maxima Center for Pediatric Oncology, Utrecht, Netherlands
In theranostics (i.e., therapy and diagnostics) radiopharmaceuticals are used for both therapeutic and diagnostic purposes by targeting one specific tumor receptor. Biologically relevant compounds, e.g., receptor ligands or drugs, are labeled with radionuclides to form radiopharmaceuticals. The possible applications are multifold: visualization of biological processes or tumor biology in vivo, diagnosis and tumor staging, therapy planning, and treatment of specific tumors. Theranostics research is multidisciplinary and allows for the rapid translation of potential tumor targets from preclinical research to “first-in-man” clinical studies. In the last decade, the use of theranostics has seen an unprecedented value for adult cancer patients. Several radiopharmaceuticals are routinely used in clinical practice (e.g., [68Ga/177Lu]DOTATATE), and dozens are under (pre)clinical development. In contrast to these successes in adult oncology, theranostics have scarcely been developed to diagnose and treat pediatric cancers. To date, [123/131I]meta-iodobenzylguanidine ([123/131I]mIBG) is the only available and approved theranostic in pediatric oncology. mIBG targets the norepinephrine transporter, expressed by neuroblastoma tumors. For most pediatric tumors, including neuroblastoma, there is a clear need for novel and improved radiopharmaceuticals for imaging and therapy. The strategy of theranostics for pediatric oncology can be divided in (1) the improvement of existing theranostics, (2) the translation of theranostics developed in adult oncology for pediatric purposes, and (3) the development of novel theranostics for pediatric tumor-specific targets. Here, we describe the recent advances in theranostics development in pediatric oncology and shed a light on how this methodology can affect diagnosis and provide additional treatment options for these patients.
Introduction
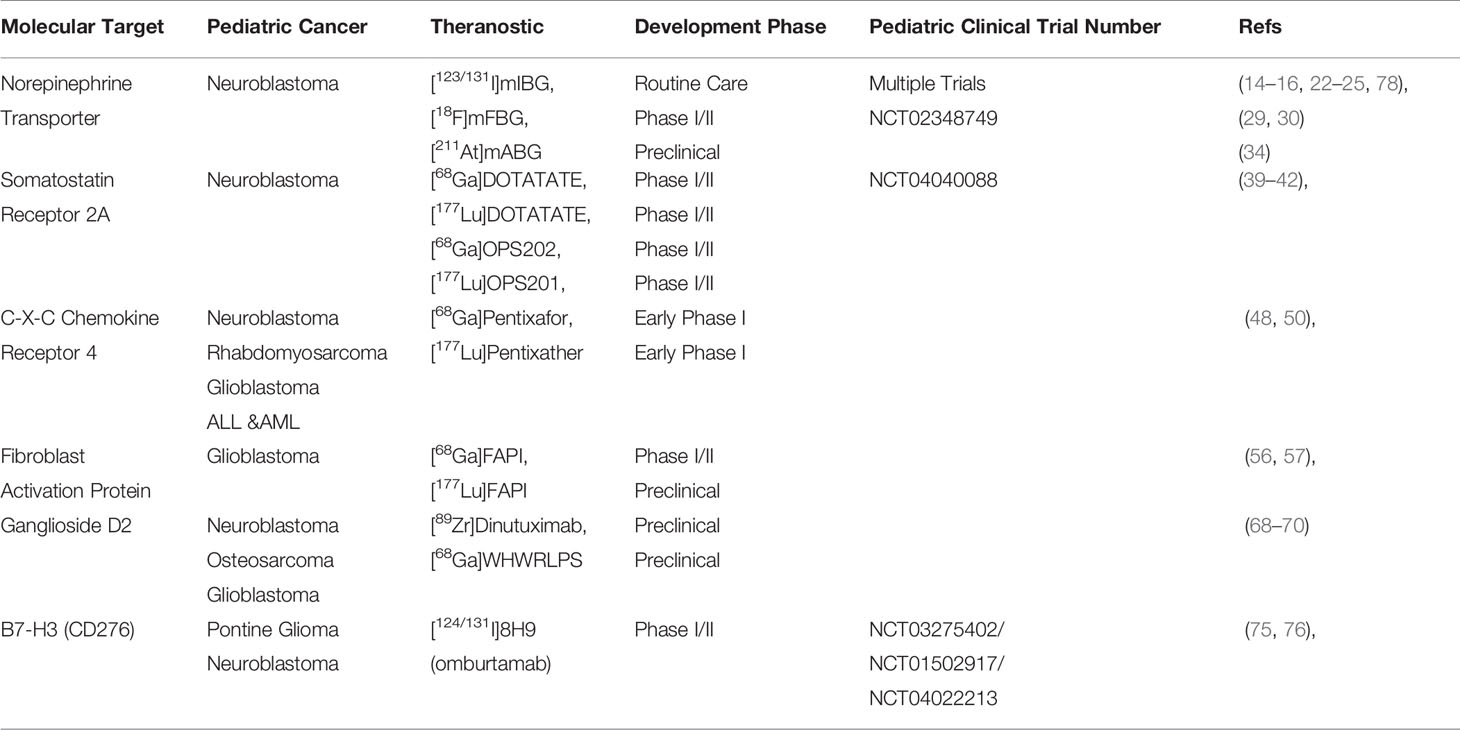
Theranostics in nuclear medicine includes the use and application of two identical or very closely related radiopharmaceuticals for therapy and diagnosis. In oncology, tumor-specific substrates, receptor ligands, or drugs can serve as lead for theranostic development when labeled with specific radionuclides for imaging or therapy (Figure 1A). As the molecular structure of both the diagnostic and therapeutic radiopharmaceuticals are identical, diagnostic images can become predictive for therapeutic response because the biological characteristics and binding potential of both are similar, irrespective of the radionuclide (2–4).
Figure 1 (A) Theranostics concept explained. A tumor-specific ligand can be used for both imaging and therapy, dependent on the nuclide of choice. PET images before/after therapy in a prostate cancer patient diagnosed and treated with [68Ga/177Lu]PSMA. PET image adapted from SNMMI image of the year 2018 by Hofman et al.; (B) left, [123I]mIBG SPECT image of a neuroblastoma patient with lesions indicated with black arrows; right, [18F]mFBG PET image of the same patient showing greater contrast and additional lesions that were not observed with [123I]mIBG. Image adapted from Pandit-Taskar et al. (1); (C) left, [123I]mIBG SPECT image of a neuroblastoma patient with only vague tumor uptake; right, [68Ga]DOTATOC PET image of the same patient showing SSTR-2A expression, greater contrast and additional lesions. Patient is treated with [177Lu]DOTATATE with an additional survival of 24 months (unpublished data, UMC Utrecht).
For diagnosis, positron emission tomography (PET) is a nuclear imaging technique that enables the visualization and quantification of molecules equipped with positron emitting radionuclides. The most used radionuclide for imaging is Fluorine-18 (18F) in the form of [18F]FDG. [18F]FDG PET visualizes increased carbohydrate uptake in tissue, e.g., tumor tissue, and is important for diagnosis, staging and treatment monitoring. For PET tracer development, any molecule that displays tumor-specific targeting can be used, including small molecules, peptides or biologicals. Radionuclides used for PET tracer development are, among others, Carbon-11 (11C) and Fluorine-18 (18F) facilitating small molecule labeling, Gallium-68 (68Ga) for peptide radiolabeling, and Copper-64 (64Cu) or Zirconium-89 (89Zr) for the labeling of monoclonal antibodies (mAbs) and other biologicals. PET imaging enables studying the distribution and kinetics of labeled molecules and the biochemical and physiological processes. Molecular imaging by means of PET can, thus, facilitate and guide cancer treatment in many ways (5, 6). Currently, PET is the most sensitive technique for nuclear imaging; it requires nanomolar amounts of the radiopharmaceutical for imaging. These nanomolar amounts will not induce pharmacological effects, hold minimal risks for toxicity, and are described as the micro-dosing concept. Micro-dosing allows for fast translation of novel PET tracers into clinical trials in small “first-in-man” or phase 0 studies, when produced under good manufacturing practice (GMP). Single photon emission computed tomography (SPECT) is an alternative nuclear imaging technique and enables the visualization of γ-emitting radionuclides and was the basis for early theranostics development, where, among others, the different radionuclides of iodine were used for imaging (e.g., Iodine-123 (123I) and Iodine-131 (131I)).
Therapeutic radiopharmaceuticals for treatment of cancer are predominantly labeled with β-emitting radionuclides. The radionuclide 131I, Lutetium-177 (177Lu) and Yttrium-90 (90Y) are frequently used for this purpose. The emitted β-particles travel 1–12 mm through tissue upon decay while losing energy and causing cytotoxic damage to the cell to induce apoptosis. Alternatively and more recently, α-emitting radionuclides, e.g., Astatine-211 (211At) or Actinium-225 (225Ac) were explored for therapy (7–9). The high energy deposition and a limited range of the α-particles in tissue (0.005–0.11 mm) result in very strong cytotoxic and therapeutic effects. Nowadays, α-emitting radionuclides become more widely available, research toward the development of therapeutic radiopharmaceuticals with these radionuclides is emerging, and first-in-man studies are expected in the near future.
Successful theranostics have been developed for somatostatin receptor positive neuroendocrine tumors with [68Ga/177Lu]DOTATATE and prostate-specific membrane antigen (PSMA) positive prostate cancer patients as prime examples (10–13). Currently, for childhood cancers and more specifically norepinephrine transporter (NET) positive neuroblastoma tumors, [123/131I]meta-iodobenzylguanidine ([123/131I]mIBG) is the only available theranostic to date (14–16). Despite the proven value of theranostics in adult oncology, its potential was minimally explored for childhood cancer and is still at its infant stage. However, many opportunities and applications present themselves. In this review, we discuss different strategies for theranostics development for childhood cancer and divided these into (1) the existing theranostics and improvement thereof, (2) theranostics developed for adult oncology and translation thereof for childhood cancer, and (3) the development of novel theranostics for specific pediatric tumor targets. By describing the recent advances in theranostics research we discuss how it can affect diagnosis and therapy for childhood cancer in the future.
Current Theranostics in Pediatric Oncology
[123/131I]mIBG is the only theranostic currently available for routine clinical use to image and treat neuroblastoma tumors that express the norepinephrine transporter (NET). mIBG is a structural analog of the neurotransmitter norepinephrine and is actively transported into the tumor by NET. Inside the cell, mIBG is stored in the cytoplasm, mitochondria, and in vesicular monoamine transporter (VMAT)-coated and neurosecretory vesicles (17–21). [123I]mIBG SPECT imaging is currently the standard of care to diagnose primary tumors and distant metastases in neuroblastoma and for staging and disease response evaluation after treatment. In total, approximately 95% of neuroblastoma tumors are [123I]MIBG avid. The remaining 5% of tumors are either well-differentiated ganglioblastoma or very undifferentiated neuroblastoma with little or no NET transporter expression. Although [123I]mIBG SPECT has a high specificity and sensitivity, it also has disadvantages being poor image resolution, long scanning times, and iodine-driven thyroid toxicity. Accompanied by imaging, [131I]mIBG initially showed therapeutic effectiveness in bulky tumors (22). Subsequently, it was shown that [131I]mIBG was feasible and effective in the first treatment of high-risk neuroblastoma patients (23). However, two systematic reviews failed to show a survival advantage for [131I]mIBG treated patients (24, 25). In two studies [131I]mIBG was combined with busulfan and melphalan followed by autologous stem cell rescue. For both, acceptable toxicity in highly pretreated patients and encouraging responses were observed. This has led to the implementation of this combination for ultra-high-risk patients who failed to respond adequately during induction treatment for high-risk neuroblastoma. The current European SIOPEN VERITAS study explores the role of [131I]mIBG in combination with topotecan and stem cell rescue followed by another high-dose consolidation with Buslfan and Melphalan and a second stem cell rescue. The aim is to increase the survival of these ultra-high-risk patients. In conclusion, [131I]mIBG treatment is still under investigation and its definitive role has not been determined. In addition to the discussion on therapeutic response, patients receiving [131I]mIBG also suffer from iodine uptake in the thyroid and increased risk for long-term thyroid dysfunction or secundary thyroid cancer. Last, after [131I]mIBG administration, patients need to live in isolation for 5–7 days and strict precepts for 2–3 weeks. Despite the value of [123/131I]mIBG as a theranostic, both imaging and therapy have serious disadvantages and limitations that steer the research toward novel approaches.
An 18F-labeled analog of [123I]mIBG, [18F]meta-fluorobenzylguanidine ([18F]mFBG]) has long been proposed as a possible PET alternative for the imaging of NET-positive neuroblastoma tumors (26). The radionuclide 18F is a cyclotron produced β+-emitter with a short range in vivo, resulting in a high image quality. Furthermore, PET-CT (or PET-MRI) images can be analyzed quantitatively for tracer distribution. 18F-labeled radiopharmaceuticals are, therefore, ideal for high-resolution diagnosis, faster acquisition, and low radiation burden. Until recently, however, the production of [18F]mFBG has been challenging. It requires a nucleophilic aromatic substitution of an electron-rich molecule (27, 28). Recent advances and novel radiofluorination reactions now give access to the production and clinical translation of [18F]mFBG (1, 29).
Pandit-Taskar et al. reported the first clinical results with [18F]mFBG, described a biodistribution and dosimetry study in neuroblastoma patients, and compared the results with [123I]mIBG. In all five neuroblastoma patients, [18F]mFBG scored better than [123I]mIBG with respect to lesion counts, improved image quality, and the absence of any thyroid uptake (Figure 1B). These encouraging results gave rise to additional and more extensive clinical testing of [18F]mFBG as an alternative to [123I]mIBG as the current gold standard (Table 1) (30, 31).
In addition to improved imaging, research is now focused on the development of an improved alternative for [131I]mIBG therapy. 131I is a β–-emitter with a t1/2 of 8.04 days. Furthermore, when [131I]mIBG is metabolized and 131I is released, it will accumulate in the thyroid. Therefore, the thyroid is blocked as a preventive action by administration of excess iodine to avoid undesired effects. As an alternative for 131I, 211At has been explored. 211At is an α-emitter with a t1/2 of 7.2 h and a range of 0.005–0.11 mm in tissue. These physical properties cause very strong cytotoxic and therapeutic effects. Furthermore, 211At does not accumulate in the thyroid and potentially will not cause any undesired damage (32, 33), As 211At has benefits over 131I, [211At]meta-astatobenzylguanidine ([211At]mABG) was reported as an alternative for [131I]mIBG for the treatment of NET positive tumors. To date, [211At]mABG has only been evaluated in preclinical models on PC12 xenografted mice (Table 1). [211At]mABG showed a dose-dependent tumor regression and increased survival compared to the control animals. It should, however, be noted that a high dose of [211At]mABG caused the death of the animals. Therefore, the toxicity profile and maximum tolerated dose of [211At]mABG needs to be assessed and compared to [131I]mIBG. An important additional note is the availability of 211At to produce [211At]mABG, which may become a practical concern. 211At can only be produced by high-energy cyclotrons, of which a few are installed worldwide, and thereby the access is limited (34).
From Adult Oncology to Pediatric Oncology
Theranostics available in routine clinical care are a rich source of potential theranostic candidates in pediatric oncology.
The somatostatin receptor (SSTR) family is one of the first discovered and most successful targets identified for which theranostics were developed. To date, 5 subtypes of SSTR (i.e., SSTR-1, 2A, 3, 4, and 5) are characterized. In particular, SSTR-2A is important with high expression levels for neuroendocrine tumors. It is involved in secretion, proliferation, and the induction of apoptosis (35). For pediatric cancers, SSTR-2A expression was reported for neuroblastoma tumors by Alexander et al. as well as for neuro-oncological malignancies (e.g., glioblastomas and medulloblastomas) (36–38). Analogs of somatostatin, the natural ligand of SSTR-2A, have successfully been developed to inhibit neuroendocrine tumor growth. Radiolabeling of these compounds led to the development of [68Ga]DOTATATE as a PET tracer and received FDA approval in 2016 (Table 1). In 2018, [177Lu]DOTATATE (Lutathera, AAA/Novartis) was approved as a therapeutic agent to treat SSTR-2A positive tumors. As DOTATATE is an SSTR-2A agonist, it stimulates the receptors, potentially causing undesired tumor growth. To circumvent these agonistic effects, the theranostics pair [68Ga]OPS202/[177Lu]OPS201 (Ipsen) was developed as an SSTR-2A antagonist and is currently in Phase I/II trials (Table 1) (10, 11, 39, 40). Because SSTR-2A expression was also validated for neuroblastomas and neuro-oncological malignancies and with several theranostics available, a straightforward translation to pediatric oncology is feasible. Small-scale experimental pilot studies were reported for these pediatric cancers with [68Ga]DOTATATE, and results are encouraging (41). This warrants further clinical studies on imaging and treating SSTR-2A positive pediatric cancers with these theranostics in the near future (Figure 1C) (42).
Another theranostic candidate target that was extensively explored in adult oncology is the C-X-C chemokine receptor 4 (CXCR4). The expression levels of CXCR4 and its natural ligand, CXCL12, are correlated to tumor development and metastasis and were validated for breast cancer, prostate cancer, lung cancer, colorectal cancer, and primary brain tumors (43). By immunohistochemical staining, CXCR4 expression was also demonstrated for neuroblastomas, rhabdomyosarcomas, glioblastomas, and hematological malignancies (44–47). To date, several CXCR4-targeting drugs are under (pre)clinical development, e.g., Ulocuplumab, PRX177561, AMD3100, and Plerixafor, which demonstrates that CXCR4 targeting is clinically feasible and relevant. For theranostic development, the PET tracer and cyclic-pentapeptide [68Ga]Pentixafor (Scintomics) is currently the most advanced and under investigation in multiple Phase I clinical trials (Table 1) (48). Labeling of pentixafor with 177Lu or 90Y to obtain the therapeutic counterpart of the diagnostic led to a strongly decreased affinity for the target receptor. This affinity could be restored after small molecular adaptations to the pentixafor scaffold and resulted in the successful development of [177Lu]Pentixather (Table 1) (43, 49, 50). [68Ga]Pentixafor and [177Lu]Pentixather are candidates for clinical trials in pediatric patients as well as CXCR4 is reported for these tumors.
A target that recently received much attention is the fibroblast activation protein α (FAP) (51). FAP is a serine protease that is selectively expressed in the stromal fibroblasts of the tumor, which is often observed for breast cancer, colon cancer, and pancreatic cancers (52). FAP expression is observed in glioblastomas and can be a valuable theranostic target for pediatric cancers. FAP-specific inhibitors (FAPI) have been developed based on quinoline scaffolds. For diagnostic purposes, promising results were obtain after radiolabeling with 68Ga (53, 54). In particular, [68Ga]FAPI-04, -21, and -46 resulted in high-contrast images, and as a proof-of-concept, 28 different tumor types were visualized with [68Ga]FAPI-04 (55, 56). All FAPI compounds allow radiolabeling with 177Lu too to obtain the corresponding therapeutic radiopharmaceutical. Preclinical studies with [177Lu]FAPI-21 and -46 in tumor-bearing mice gave promising results (Table 1) (57). As FAP is also expressed by glioblastomas, these theranostics have potential for the diagnosis and treatment of pediatric cancers.
Monoclonal antibodies (mAbs) and mAb-fragments had unprecedented impact on the treatment of cancer patients. However, clinical benefit is usually only achieved in a percentage of the patient population. The application of 89Zr-labeled mAbs as ImmunoPET tracers has become increasingly important to visualize these compounds in vivo and assess the distribution, kinetics, and the biochemical and physiological behaviour (4, 58). Nowadays, more than 75 clinical trials are ongoing with 89Zr-labeled mAbs and the radiolabeling can be achieved via generic methods (59, 60). Despite the clinical impact of ImmunoPET with [89Zr]mAbs for adult oncology and other indications, ImmunoPET with available [89Zr]mAbs has barely been explored for pediatric cancers. The only reported application of ImmunoPET was [89Zr]bevacizumab in diffuse intrinsic pontine glioma to study vascular endothelial growth factor (VEGF) excretion and the potential to treat these patients with bevacizumab (61). Though ImmunoPET in pediatric cancer patients is not common, it should be anticipated that this methodology can also have an impact for these patients in the future.
Specific Theranostic Targets in Pediatric Oncology
Pediatric cancers have a distinct biological profile with unique molecular targets that are not expressed in adult cancers. These targets embody unique opportunities for the diagnosis and treatment of pediatric cancers, but due to small patient populations, it remains a challenge to identify them and develop theranostics against these targets.
A target of interest for theranostic development is ganglioside D2 (GD2). GD2 is a glycosphingolipid and selective cellular marker that is expressed by neuroblastomas, osteosarcomas, and glioblastomas (62, 63). Though its exact function is still not fully understood, it is assumed that it plays a crucial role in cell adhesion, migration, and tumor metastasis. Dinutuximab (Unituxin®, United Therapeutics) is FDA approved, and Dinutuximab beta (Qarziba®, EUSA Pharma) is EMA approved for the treatment of GD2-positive neuroblastoma tumors (64, 65). Despite increased survival rates from 46% to 66%, for high-risk neuroblastoma patients, 30% of the patients will relapse independent of the GD2 expression levels (Table 1) (66). Several radiopharmaceuticals have been developed to image GD2-positive tumors. 64Cu-labeled hu14.18K322A showed clear accumulation and retention in preclinical osteosarcoma models, and [89Zr]dinutuximab was mentioned as a PET tracer in meeting abstracts (67–69). In addition to radiolabeled mAbs, Müller et al. reported on the development of [68Ga]DOTA- WHWRLPS heptapeptide and demonstrated its accumulation in neuroblastoma xenografted mice (70). Though encouraging, clinical translation of these radiopharmaceuticals has yet to be achieved.
More recently, B7-H3 (CD276) has become a validated pediatric cancer target for immunotherapy in pontine gliomas and neuroblastomas (71, 72). To date, two mAbs were developed, Enoblituzumab (MacroGenics) and Omburtamab (Y-mAbs), to treat B7-H3 positive tumors (73, 74) Based on these immunotherapeutics, attempts at the development of theranostics are reported. Especially with 8H9 (i.e., Omburtamab) multiple clinical trials are ongoing. The theranostics pair [124/131I]8H9 is investigated for B7-H3 positive pontine glioma tumors and a modest survival benefit was reported (Table 1) (75, 76). As specific brain tumors (e.g., gliomas) express B7-H3, it is important that passage and delivery of the radiopharmaceutical across the blood–brain barrier is achieved. As such, radiolabeled [124/131I]Omburtamab is ideal to investigate drug targeting in these patients. As B7-H3 is acknowledged as a pan-tumor target, theranostics targeting B7-H3 might become of general importance for childhood cancer.
Considerations and Requirements for Nuclear Medicine in Childhood Cancer
The application of theranostics for the diagnosis and treatment of childhood cancers is in its infancy. With the availability of radiopharmaceuticals and theranostics for the various adult cancers, there is a lot of potential to translate and directly apply these for childhood cancers. Clinically available SPECT/PET tracers and therapeutic radiopharmaceuticals can directly be applied for pediatric cancers when the target is present and validated for the respective tumor type. Examples include SSTR-2A, CXCR4, and FAP-positive tumors. As childhood cancers have unique target expression profiles, with GD2 and B7-H3 as examples, novel theranostics can be developed for these yet unexplored targets. Unique target-finding programs are in place to unveil novel childhood cancer-specific biological features for which theranostics can be developed. A critical note and challenge is that target expression of cancers in general cannot always be directly correlated to positive imaging and treatment results. Preclinical research programs are, therefore, required to validate target expression and the potential of the target against which to developed theranostics.
A successfully developed theranostic that shows potential in preclinical studies warrants clinical translation. To achieve that, the theranostic needs to be produced under GMP to guarantee product quality and patient safety (77). Clinical translation of developed theranostics is relatively straightforward as procedures and GMP production facilities are widely available.
Conclusion
Theranostics have unprecedented value to diagnose and treat cancers. Many novel theranostics are under development and expected to enter clinical trials and care in the near future. For the diagnosis and treatment of childhood cancers, theranostics research is still in its infancy, but following the path of adult oncology, its value is promising. They are expected to become additional and valuable tools to diagnose and treat childhood cancers.
Author Contributions
AP drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Sponsored by UMC Utrecht and Princess Máxima Center for Pediatric Oncology, Netherlands.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Rotstein B, Wang L, Liu R, Patteson J, Kwan EE, Vasdev N, et al. Mechanistic Studies and Radiofluorination of Structurally Diverse Pharmaceuticals with Spirocyclic Iodonium(III) Ylides. Chem Sci (2016) 7:4407–17. doi: 10.1039/C6SC00197A
2. Langbein T, Weber W, Eiber M. Future of Theranostics: An Outlook on Precision Oncology in Nuclear Medicine. J Nucl Med (2019) 60:13S–9S. doi: 10.2967/jnumed.118.220566
3. Turner JH. Recent advances in theranostics and challenges for the future. Br J Radiol (2018) 91:1091. doi: 10.1259/bjr.20170893
4. Rösch F, Herzog H, Qaim SM. The Beginning and Development of the Theranostic Approach in Nuclear Medicine, as Exemplified by the Radionuclide Pair 86Y and 90Y. Pharmaceuticals (Basel) (2017) 10:56. doi: 10.3390/ph10020056
5. Van Dongen GAMS, Poot AJ, Vugts DJ. PET imaging with radiolabeled antibodies and tyrosine kinase inhibitors: immuno-PET and TKI-PET. Tumour Biol (2012) 33:607–15. doi: 10.1007/s13277-012-0316-4
6. Slobbe P, Poot AJ, Windhorst AD, Van Dongen GAMS. PET imaging with small-molecule tyrosine kinase inhibitors: TKI-PET. Drug Discovery Today (2012) 17:1175–87. doi: 10.1016/j.drudis.2012.06.016
7. Navalkissoor S, Grossman A. Targeted Alpha Particle Therapy for Neuroendocrine Tumours: The Next Generation of Peptide Receptor Radionuclide Therapy. Neuroendocrinology (2019) 108:256–64. doi: 10.1159/000494760
8. Guérard F, Gestin J-F, Brechbiel MW. Production of [211At]-Astatinated Radiopharmaceuticals and Applications in Targeted α-Particle Therapy. Cancer Biother Radiopharm (2013) 28:1–20. doi: 10.1089/cbr.2012.1292
9. Morgenstern A, Apostolidis C, Kratochwil C, Sathekge M, Krolicki L, Bruchertseifer F. An Overview of Targeted Alpha Therapy with 225Actinium and 213Bismuth. Curr Radiopharm (2018) 11:200–8. doi: 10.2174/1874471011666180502104524
10. Sanli Y, Garg I, Kandathil A, Kendi T, Baladron Zanetti MJ, Kuyumcu S, et al. Neuroendocrine Tumor Diagnosis and Management: 68Ga-DOTATATE PET/CT. Am J Roentgenol (2018) 2:267–77. doi: 10.2214/AJR.18.19881
11. Mittra ES. Neuroendocrine Tumor Therapy: 177Lu-DOTATATE. Am J Roentgenol (2018) 2:278–85. doi: 10.2214/AJR.18.19953
12. Rahbar K, Afshar-Oromieh A, Jadvar H, Ahmadzadehfar H. PSMA Theranostics: Current Status and Future Directions. Mol Imaging (2018) 17. doi: 10.1177/1536012118776068
13. Ahmadzadehfar H, Rahbar K, Essler M, Biersack HJ. PSMA-Based Theranostics: A Step-by-Step Practical Approach to Diagnosis and Therapy for mCRPC Patients. Semin Nucl Med (2019) 50:98–109. doi: 10.1053/j.semnuclmed.2019.07.003
14. Matthay KK, Shulkin B, Laderstein R, Michon J, Giammarile F, Lewington V, et al. Criteria for evaluation of disease extent by 123I-metaiodobenzylguanidine scans in neuroblastoma: a report for the International Neuroblastoma Risk Group (INRG) Task Force. Br J Cancer (2010) 102:1319–26. doi: 10.1038/sj.bjc.6605621
15. Matthay KK, Panina C, Huberty J, Price D, Glidden DV, Tang HR, et al. Correlation of tumor and whole-body dosimetry with tumor response and toxicity in refractory neuroblastoma treated with 131I-MIBG. J Nucl Med (2001) 42:1713–21.
16. Kraal KCJM, Tytgat GAM, Van Eck-Smit BLF, Kam B, Caron HN, Van Noesel MM. Upfront treatment of high-risk neuroblastoma with a combination of 131I-MIBG and Topotecan. Pediatr Blood Cancer (2015) 62:1886–91. doi: 10.1002/pbc.25580
17. Vaidyanathan G. Meta-iodobenzylguanidine and analogues: chemistry and biology. Q J Nucl Med Mol Imaging (2008) 52:351–68.
18. Glowniak JV, Kilty JE, Amara SG, Hoffman BJ, Turner FE. Evaluation of metaiodobenzylguanidine uptake by the norepinephrine, dopamine and serotonin transporters. J Nucl Med (1993) 34:1140–6.
19. Gaze MN, Huxham IM, Mairs RJ, Barrett A. Intracellular localization of metaiodobenzyl guanidine in human neuroblastoma cells by electron spectroscopic imaging. Int J Cancer (1991) 47:875–80. doi: 10.1002/ijc.2910470615
20. Smets LA, Janssen M, Metwally E, Loesberg C. Extragranular storage of the neuron blocking agent meta-iodobenzylguanidine (MIBG) in human neuroblastoma cells. Biochem Pharmacol (1990) 39:1959–64. doi: 10.1016/0006-2952(90)90615-R
21. Smets LA, Janssen M, Rutgers M, Ritzen K, Buttenhuis C. Pharmacokinetics and intracellular distribution of the tumor-targeted radiopharmaceutical m-iodo-benzylguanidine in SK-N-SH neuroblastoma and PC-12 pheochromocytoma cells. Int J Cancer (1991) 48:609–15. doi: 10.1002/ijc.2910480421
22. Hoefnagel CA, De Kraker J, Valdes Olmos RA, Voûte PA. 131I-MIBG as a first-line treatment in high-risk neuroblastoma patients. Nucl Med Commun (1994) 9:712–7. doi: 10.1097/00006231-199409000-00008
23. Kraal KCJM, Bleeker GM, Van Eck-Smit BLF, van Eijkelenburg NKA, Berthold F, Van Noesel MM, et al. Feasibility, toxicity and response of upfront metaiodobenzylguanidine therapy therapy followed by German Pediatric Oncology Group Neuroblastoma 2004 protocol in newly diagnosed stage 4 neuroblastoma patients. Eur J Cancer (2017) 76:188–96. doi: 10.1016/j.ejca.2016.12.013
24. Kraal KCJM, Van Dalen EC, Tytgat GAM, Van Eck-Smit BLF. Iodine-131-meta-iodobenzylguanidine therapy for patients with newly diagnosed high-risk neuroblastoma. Cochrane Database Syst Rev (2017) 4:CD010349. doi: 10.1002/14651858.CD010349.pub2
25. Wilson JS, Gains JE, Moroz V, Wheatley K, Gaze MN. A systematic review of 131I-meta iodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur J Cancer (2014) 50:801–5. doi: 10.1016/j.ejca.2013.11.016
26. Garg PK, Garg S, Zalutsky MR. Synthesis and preliminary evaluation of para- and meta-[18F]fluorobenzylguanidine. Nucl Med Biol (1994) 21:97–103. doi: 10.1016/0969-8051(94)90135-x
27. Cole EL, Stewart MN, Littich R, Hoareau R, Scott PJ. Radiosyntheses using fluorine-18: the art and science of late stage fluorination. Curr Top Med Chem (2014) 14:875–900. doi: 10.2174/1568026614666140202205035
28. Van der Born D, Pees A, Poot AJ, Orru RVA, Windhorst AD, Vugts DJ. Fluorine-18 labelled building blocks for PET tracer synthesis. Chem Soc Rev (2017) 46:4709–73. doi: 10.1039/c6cs00492j
29. Tredwell M, Preshlock SM, Taylor NJ, Gruber S, Huiban M, Passchier J, et al. A General Copper Mediated Nucleophilic 18F Fluorination of Arenes. Angew Chem Int Ed (2014) 53:7751–5. doi: 10.1002/anie.201404436
30. Pandit-Taskar N, Zanzonico P, Staton KD, Carrasquillo JA, Reidy-Lagunes D, Lyashchenko S, et al. Biodistribution and dosimetry of 18F-Meta Fluorobenzyl Guanidine (MFBG): A first-in-human PET-CT imaging study of patients with neuroendocrine malignancies. J Nucl Med (2018) 59:147–53. doi: 10.2967/jnumed.117.193169
31. Zhang H, Huang R, Cheung N-KV, Guo H, Zanzonico PB, Thaler HT, et al. Imaging the norepinephrine transporter in neuroblastoma: a comparison of [18F]-MFBG and 123I-MIBG. Clin Cancer Res (2014) 20:2182–91. doi: 10.1158/1078-0432.CCR-13-1153
32. Zalutsky MR, Pruszynski M. Astatine-211: Production and Availability. Curr Radiopharm (2011) 4:177–85. doi: 10.2174/1874471011104030177
33. Vaidyanethan G, Zalutsky MR. Applications of 211At and 223Ra in Targeted Alpha-Particle Radiotherapy. Curr Radiopharm (2011) 4:283–94. doi: 10.2174/1874471011104040283
34. Ohshima Y, Sudo H, Watanabe S, Nagatsu K, Tsuji AB, Sakashita T, et al. Antitumor effects of radionuclide treatment using α-emitting meta-211At-astato-benzylguanidine in a PC12 pheochromocytoma model. Eur J Nucl Med Mol Imaging (2018) 45:999–1010. doi: 10.1007/s00259-017-3919-6
35. Mizutani G, Nakahishi Y, Watanabe N, Honma T, Obana Y, Seki T, et al. Expression of Somatostatin Receptor (SSTR) Subtypes (SSTR-1, 2A, 3, 4 and 5) in Neuroendocrine Tumors Using Real-time RT-PCR Method and Immunohistochemistry. Acta Histochem Cytomchem (2012) l45:167–76. doi: 10.1267/ahc.12006
36. Alexander N, Marrano P, Thorner P, Naranjo A, Van Ryn C, Martinez D, et al. Prevalence and clinical correlations of somatostatin receptor-2 (SSTR2) expression in neuroblastoma. J Pediatr Hematol Oncol (2019) 41:222–7. doi: 10.1097/MPH.0000000000001326
37. Kiviniemi A, Gardberg M, Kivinen K, Posti JP, Vourinen V, Sipilä J, et al. Somatostatin receptor 2A in gliomas: Association with oligodendrogliomas and favourable outcome. Oncotarget (2017) 8:49123–32. doi: 10.18632/oncotarget.17097
38. Guyotat J, Champier J, Pierre GS, Jouvet A, Bret P, Brisson C, et al. Differential Expression of Somatostatin Receptors in Medulloblastoma. J Neuro-Oncol (2001) 51:93–103. doi: 10.1023/a:1010624702443
39. Chen S, Cheung W, Leung YL, Cheng K, Wong KN, Wong YH, et al. 177Lu-DOTATATE radionuclide therapy for pediatric patients with relapsed high-risk neuroblastoma negative on 131I-MIBG imaging - a pilot study. J Nucl Med (2018) 59:Suppl 1–307.
40. Nicolas GP, Beykan S, Bouterfa H, Kaufmann J, Bauman A, Lassmann M, et al. Safety, Biodistribution, and Radiation Dosimetry of 68Ga-OPS202 in Patients with Gastroenteropancreatic Neuroendocrine Tumors: A Prospective Phase I Imaging Study. J Nucl Med (2018) 59:909–14. doi: 10.2967/jnumed.117.199737
41. Nicolas GP, Mansi R, McDougall L, Kaufman J, Bouterfa H, Wild D, et al. Biodistribution, Pharmacokinetics, and Dosimetry of 177Lu-, 90Y-, and 111In-Labeled Somatostatin Receptor Antagonist OPS201 in Comparison to the Agonist 177Lu-DOTATATE: The Mass Effect. J Nucl Med (2017) 58:1435–41. doi: 10.2967/jnumed.117.191684
42. search term: Neuroblastoma, SSTR2, DOTATATE (2020). Available at: www.clinicaltrials.gov (Accessed May 25, 2020).
43. Kircher M, Herhaus P, Schottelius M, Buck AK, Werner RA, Wester H-J, et al. CXCR4-directed theranostics in oncology and inflammation. Ann Nucl Med (2018) 32:503–11. doi: 10.1007/s12149-018-1290-8
44. Russell HV, Hicks J, Okcu MF, Nuchtern JG. CXCR4 expression in neuroblastoma primary tumors is associated with clinical presentation of bone and bone marrow metastases. J Pediatr Surg (2004) 39:1506–11. doi: 10.1016/j.jpedsurg.2004.06.019
45. Miyoshi K, Kohashi K, Fushimi F, Yamamoto H, Kishimoto J, Taguchi T, et al. Close correlation between CXCR4 and VEGF expression and frequent CXCR7 expression in rhabdomyosarcoma. Hum Pathol (2014) 45:1900–9. doi: 10.1016/j.humpath.2014.05.012
46. Eckert F, Schilbach K, Klumpp L, Bardoscia L, Sezgin EC, Schwab M, et al. Potential Role of CXCR4 Targeting in the Context of Radiotherapy and Immunotherapy of Cancer. Front Immunol (2018) 9:3018:3018. doi: 10.3389/fimmu.2018.03018
47. Peled A, Klein S, Beider K, Burger JA, Abraham M. Role of CXCL12 and CXCR4 in the pathogenesis of hematological malignancies. Cytokine (2018) 109:11–6. doi: 10.1016/j.cyto.2018.02.020
48. search term: Pentixafor, CXCR4 (2020). Available at: www.clinicaltrials.gov (Accessed June 8, 2020).
49. Cooper TM, Sison EAR, Baker SD, Li L, Ahmed A, Trippett T, et al. A phase 1 study of the CXCR4 antagonist plerixafor in combination with high-dose cytarabine and etoposide in children with relapsed or refractory acute leukemias or myelodysplastic syndrome: A Pediatric Oncology Experimental Therapeutics Investigators’ Consortium study (POE 10-03). Pediatr Blood Cancer (2017) 64. doi: 10.1002/pbc.26414
50. Herrmann K, Schottelius M, Lapa C, Osl T, Poschenrieder A, Hänscheid H, et al. First-in-Human Experience of CXCR4-Directed Endoradiotherapy with 177Lu- and 90Y-Labeled Pentixather in Advanced-Stage Multiple Myeloma with Extensive Intra- and Extramedullary Disease. J Nucl Med (2016) 57:248–51. doi: 10.2967/jnumed.115.167361
51. Busek P, Mateu R, Zubal M, Kotackova L, Sedo A. Targeting Fibroblast Activation Protein in Cancer - Prospects and Caveats. Front Biosci (Landmark Ed) (2018) 23:1933–68. doi: 10.2741/4682
52. Puré E, Blomberg R. Pro-tumorigenic roles of fibroblast activation protein in cancer: back to the basics. Oncogene (2018) 37:4343–57. doi: 10.1038/s41388-018-0275-3
53. Busek P, Balaziova E, Matrasova I, Hilser M, Tomas R, Syrucek M, et al. Fibroblast Activation Protein Alpha Is Expressed by Transformed and Stromal Cells and Is Associated With Mesenchymal Features in Glioblastoma. Tumour Biol (2016) 37:13961–71. doi: 10.1007/s13277-016-5274-9
54. Jansen K, Heirbaur L, Cheng JD, Joossens J, Ryabtsova O, Cos P, et al. Selective Inhibitors of Fibroblast Activation Protein (FAP) with a (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine Scaffold. ACS Med Chem Lett (2013) 4:491–6. doi: 10.1021/ml300410d
55. Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, et al. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J Nucl Med (2018) 59:1415–22. doi: 10.2967/jnumed.118.210443
56. Giesel FL, Kratochwil C, Lindner T, Marschalek MM, Lehnert W, Debus J, et al. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J Nucl Med (2018) 60:386–92. doi: 10.2967/jnumed.118.215913
57. Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J Nucl Med (2019) 60:801–5. doi: 10.2967/jnumed.119.227967
58. Loktev A, Lindner T, Burger A-M, Altmann A, Giesel F, Kratochwil C, et al. Development of Fibroblast Activation Protein-Targeted Radiotracers With Improved Tumor Retention. J Nucl Med (2019) 60:1421–9. doi: 10.2967/jnumed.118.224469
59. Jauw YWS, Menke-van der Houven van Oordt CW, Hoekstra OS, Hendrikse NH, Vugts DJ, Zijlstra JM, et al. Immuno-Positron Emission Tomography with Zirconium-89-Labeled Monoclonal Antibodies in Oncology: What Can We Learn from Initial Clinical Trials? Front Pharmacol (2016) 7:131. doi: 10.3389/fphar.2016.00131
60. Heskamp S, Raavé R, Boerman O, Rijpkema M, Goncalves V, Denat F. 89Zr-Immuno-Positron Emission Tomography in Oncology: State-of-the-Art 89Zr Radiochemistry. Bioconjug Chem (2017) 28:2211–23. doi: 10.1021/acs.bioconjchem.7b00325
61. 89Zr, Zirconium-89 (2020). Available at: www.clinicaltrials.gov (Accessed June 8, 2020).
62. Jansen MH, Veldhuijzen van Zanten SEM, Van Vuurden DG, Huisman MC, Vugts DJ, Hoekstra O, et al. Molecular Drug Imaging: 89Zr-Bevacizumab PET in Children with Diffuse Intrinsic Pontine Glioma. J Nucl Med (2017) 58:711–6. doi: 10.2967/jnumed.116.180216
63. Sait S, Modak S. Anti-GD2 immunotherapy for neuroblastoma. Expert Rev Anticanc (2017) 17:889–904. doi: 10.1080/14737140.2017.1364995
64. Hung J-T, Yu A. Chapter-4: GD2-targeted immunotherapy of neuroblastoma. In: Neuroblastoma, Molecular Mechanisms and Therapeutic Interventions. Cambridge, United States: Academic Press (2019). p. 63–78. doi: 10.1016/B978-0-12-812005-7.00004-7
65. Hoy S. Dinutuximab, a review in high-risk neuroblastoma. Target Oncol (2016) 11:247–53. doi: 10.1007/s11523-016-0420-2
66. Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med (2010) 363:1324–34. doi: 10.1056/NEJMoa0911123
67. Terzic T, Cordeau M, Herblot S, Teira P, Cournoyer S, Beaunoyer M, et al. Expression of Disialoganglioside (GD2) in Neuroblastic Tumors: A Prognostic Value for Patients Treated With Anti-GD2 Immunotherapy. Ped Dev Path (2018) 21:355–62. doi: 10.1177/1093526617723972
68. Butch ER, Mead PE, Diaz VA, Tillman H, Stewart E, Mishra JK, et al. Positron Emission Tomography Detects In Vivo Expression of Disialoganglioside GD2 in Mouse Models of Primary and Metastatic Osteosarcoma. Cancer Res (2019) 79:3112–24. doi: 10.1158/0008-5472.CAN-18-3340
69. Butch E, Mishra J, Diaz VA, Vavere A, Snyder S. Selective detection of GD2-positive pediatric solid tumors using 89Zr-Dinutuximab PET to facilitate anti-GD2 immunotherapy. J Nucl Med (2018) 59:suppl 1–170.
70. Müller J, Reichel R, Vogt S, Sauerwein W, Brandau W, Eggert A, et al. Identification and Tumour-Binding Properties of a Peptide with High Affinity to the Disialoganglioside GD2. PloS One (2016) 11:e0163648. doi: 10.1371/journal.pone.0163648
71. Castellanos JR, Purvis IJ, Labak CM, Guda MR, Tsung AJ, Velpula KK, et al. B7-H3 role in the immune landscape of cancer. Am J Clin Exp Immunol (2017) 6:66–75.
72. Flem-Karlsen K, Fodstad O, Tan M, Nunes-Xavier CE. B7-H3 in Cancer – Beyond Immune Regulation. Trends Cancer (2018) 4:401–4. doi: 10.1016/j.trecan.2018.03.010
73. Powderly J, Cote G, Flaherty K, Szmulewitz RZ, Ribas A, Weber J, et al. Interim results of an ongoing Phase I, dose escalation study of MGA271 (Fc-optimized humanized anti-B7-H3 monoclonal antibody) in patients with refractory B7-H3-expressing neoplasms or neoplasms whose vasculature expresses B7-H3. J Immunother Cancer (2015) 3:Suppl 2-O8. doi: 10.1186/2051-1426-3-S2-O8
74. Modak S, Kramer K, Gultekin SH, Guo HF, Cheung N-KV. Monoclonal Antibody 8H9 Targets a Novel Cell Surface Antigen Expressed by a Wide Spectrum of Human Solid Tumors. Cancer Res (2001) 61:4048–54.
75. Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol (2010) 97:409–18. doi: 10.1007/s11060-009-0038-7
76. Luther N, Zhou Z, Zanzonico P, Cheung NK, Humm J, Edgar MA, et al. The potential of theragnostic ¹²3I-8H9 convection-enhanced delivery in diffuse intrinsic pontine glioma. Neuro Oncol (2014) 6:800–6. doi: 10.1093/neuonc/not298
77. European Pharmacopoea 10th edition (2020). Available at: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition (Accessed June 8, 2020).
Keywords: theranostics, childhood cancer, radiopharmaceuticals, nuclear imaging (e.g. PET, SPECT), nuclear therapy
Citation: Poot AJ, Lam MGEH and van Noesel MM (2020) The Current Status and Future Potential of Theranostics to Diagnose and Treat Childhood Cancer. Front. Oncol. 10:578286. doi: 10.3389/fonc.2020.578286
Received: 30 June 2020; Accepted: 09 October 2020;
Published: 19 November 2020.
Edited by:
Hua Tan, University of Texas Health Science Center at Houston, United StatesReviewed by:
Jaume Mora, Hospital Sant Joan de Déu Barcelona, SpainYong-mi Kim, Children’s Hospital of Los Angeles, United States
Copyright © 2020 Poot, Lam and van Noesel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Max M. van Noesel, bS5tLnZhbm5vZXNlbEBwcmluc2VzbWF4aW1hY2VudHJ1bS5ubA==
 Alex J. Poot
Alex J. Poot Marnix G. E. H. Lam1
Marnix G. E. H. Lam1