- 1Carilion Clinic, Section of Neurosurgery, Roanoke, VA, United States
- 2Virginia Tech Carilion School of Medicine, Roanoke, VA, United States
- 3Virginia Tech School of Neuroscience, Blacksburg, VA, United States
Treatment of brain metastases often includes surgical resection, chemotherapeutics and radiotherapy. Given the difficulty in obtaining therapeutic levels of medications within the immune-privileged central nervous system, chemotherapy as a stand-alone treatment modality for brain metastases is an uncommon option. However, there is a growing body of evidence to suggest that immunomodulatory agents can induce a robust immune response in the central nervous system. Here, we describe a 68-year old male who presented with radiographic evidence of new and enlarging lung nodules with mediastinal adenopathy. Lung biopsy was consistent with adenocarcinoma. Immunohistochemical staining demonstrated high expression of programmed cell death protein 1 with a tumor proportion score of 100%. Surveillance magnetic resonance imaging of the brain demonstrated a single enhancing 11 × 7 × 12 mm lesion along the mesial surface of the right frontal lobe. The patient deferred surgical resection as well as stereotactic radiosurgery but agreed to treatment with pembrolizumab. Repeat magnetic resonance imaging at 3-months after initiation of treatment demonstrated complete radiographic resolution of the brain lesion. To our knowledge, this is one of only a few reports in the current literature to document complete resolution of non-small cell lung cancer brain metastasis with pembrolizumab alone. We discuss the emerging literature regarding the efficacy of pembrolizumab in the treatment of brain metastases, central nervous system penetration, and emerging new treatment paradigms involving novel immunotherapy agents.
Background
Brain metastases are a common finding in non-small cell lung cancer (NSCLC) occurring in ~16–44% of patients (1–4). Improvement in systemic disease control and widespread availability of imaging has likely contributed to the increasing incidence of brain metastases (5, 6). While the advancement of immunotherapy has begun to become an integral part of the treatment paradigm for NSCLC, brain metastases continues to be managed with surgical resection and radiotherapy (7). Treatment of a single metastatic intracranial lesion often involves surgical resection in the appropriate patient population with accessible lesions followed by stereotactic radiosurgery (SRS). Historically, whole brain radiation therapy (WBRT) was the standard treatment of choice for the management of metastatic disease dissemination of the brain parenchyma with the aim in managing both the visible metastases as well as disease at the cellular level (8). However, due to the consequential cognitive decline experienced by many patients with WBRT, the field has moved away from WBRT and toward local control with SRS (9, 10). In patients deemed poor surgical candidates or lesions that are either deep seated or in eloquent areas, SRS alone has become a reasonable and reliable treatment option replacing WBRT in many patient subsets (8, 11).
While chemotherapy or immunotherapy are effective and necessary in the treatment and control of primary NSCLC, the literature depicting pembrolizumab as stand-alone treatment modality for NSCLC brain metastases is sparse (12–17). Limited studies have demonstrated control of brain metastases when used with WBRT and after failure of other treatment regimens (12, 13). As the use of immune checkpoint inhibitors have become more widespread, research has demonstrated some activity against brain metastases; however, given the lack of response by many patients, the effectiveness of immunotherapy in the treatment of intracranial metastatic disease has yet to be fully elucidated (14–17). Here, we describe a patient with newly diagnosed NSCLC who desired to follow his brain metastasis with serial imaging after initiating pembrolizumab immunotherapy. Imaging to date has demonstrated a complete radiographic response. To our knowledge, this is one of only a few reports in the current literature to document complete resolution of NSCLC brain metastasis with pembrolizumab alone.
Case Presentation
A 68-year old male, with a past medical history remarkable for an 88 pack-year smoking history and chronic obstructive pulmonary disease on supplemental oxygen, underwent an annual lung cancer screening with a computed tomography (CT) scan of the chest ordered by his primary care physician. CT demonstrated an increase in previously identified lung nodules as well as five new nodules, fluid within the right lung and ipsilateral mediastinal adenopathy. Subsequent bronchoscopy and lymph node biopsies demonstrated pulmonary adenocarcinoma that was EGFR negative, ALK negative, ROS1 negative, BRAF wild type, and highly positive for programmed cell death protein 1 (PD-1) (>50%) with a tumor proportion score of 100%. Surveillance magnetic resonance imaging (MRI) of the brain (1 mm cuts) demonstrated a single, avidly enhancing 11 × 7 × 12 mm lesion along the mesial surface of the right frontal lobe within the cingulate sulcus with surrounding vasogenic edema (Figures 1A,B). As such, his primary disease was considered stage IV (T0N2M1b). He was started on pembrolizumab immunotherapy by medical oncology and referred to neurosurgery for further treatment considerations of his single metastatic intracranial lesion.
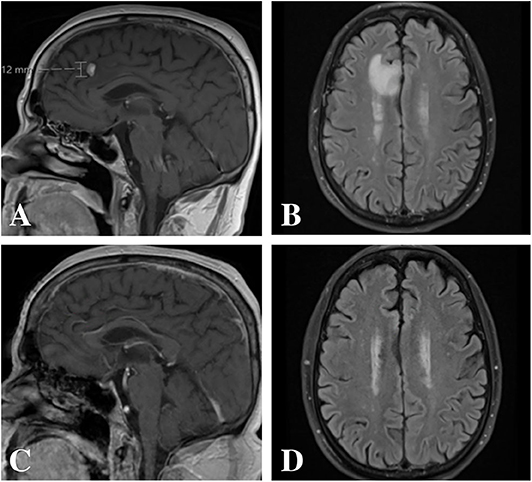
Figure 1. Magnetic resonance imaging pre- and post-treatment. (A,B) Pre-treatment imaging demonstrated an avidly enhancing 11 × 7 × 12 mm lesion along the mesial surface of the right frontal lobe within the cingulate sulcus with surrounding vasogenic edema. (C,D) Post-treatment imaging with complete radiographic resolution of the lesion and associated vasogenic edema.
Risks and benefits of treating and withholding treatment for the single metastatic intracranial lesion were discussed at length with the patient. Given the patient's poor pulmonary status and the location of the brain metastasis, we recommended SRS. However, the patient refused treatment other than pembrolizumab but was amenable to considering intervention should the lesion progress on follow-up imaging. Four cycles of pembrolizumab (200 mg every 3 weeks) were planned with follow up MRI thereafter to revisit treatment options based upon imaging findings. Repeat MRI (1 mm cuts) 3 months after initiating pembrolizumab demonstrated complete radiographic resolution of the intracranial lesion and associated vasogenic edema without any new interim findings (Figures 1C,D). Follow-up positron-emission tomography 9 months after starting treatment demonstrated no focal accumulations within the brain parenchyma but with persistent yet decreased uptake within the right upper lobe, two areas of new uptake and interval resolution of mediastinal and hilar lymph node activity. The patient completed ten cycles of pembrolizumab without any reported clinical sequelae throughout his treatment course. At 1-year follow up after initial diagnosis, the patient was doing well with his pulmonary function at baseline.
Discussion
Metastatic brain tumors continue to be the leading cause of central nervous system malignancy in adults with NSCLC accounting for more than 30–60% of all cases (18). The development of immunotherapies, specifically those targeted at immune checkpoints, has led to significant progress in the field of medical oncology, especially for patients with advanced NSCLC. However, there is limited data on the permeability of pembrolizumab and other immune checkpoint inhibitors at the blood-brain barrier. Traditional chemotherapy agents, including platinum analogs, paclitaxel, docetaxel, gemcitabine and other anti-cancer drugs used to treat adenocarcinoma, have poor penetration and duration into brain tumor tissues at sufficient concentrations to cause significant anti-tumor effects (19–23). Immunotherapy for the treatment of NSCLC, including agents such as pembrolizumab, nivolumab, atezolizumab, and durvalumab, are fairly novel agents used in the treatment of metastatic NSCLC. Central nervous system penetration and efficacy have yet to been substantiated, though there are reports that suggest potential activity and therapeutic responses (14–16).
Pembrolizumab is a highly selective humanized monoclonal IgG4-kappa isotope antibody that is directed against PD-1 (24). PD-1 is located on T lymphocytes and pro-B lymphocytes and interacts with two ligands: programmed cell death ligand 1 (PD-L1) and ligand 2. T-cell function becomes inhibited when PD-L1 binds PD-1 (25). As such, pembrolizumab inhibits formation of the PD-1: PD-L1 complex, which allows for an improved T-cell response to tumor cells (25). As the KEYNOTE-001 clinical trial demonstrated, 23.2% of NSCLC patients not treated previously with chemotherapy and 15.5% of previously treated patients were alive after 5 years with pembrolizumab monotherapy (7). This is a significant improvement to a historical 5-year survival of 5.5% prior to immunotherapy (7). However, the question as to whether immunotherapy can be a stand-alone treatment for single brain metastases with the goal of avoiding invasive surgical procedures and radiation has yet to be answered. There are few case reports and one prospective trial of complete resolution of metastatic brain lesions in NSCLC, though many of these patients also received some type of additional therapy including WBRT, SRS or surgical resection (14–16, 22, 26, 27). Research is sparse in detailing the effectiveness of immunomodulatory agents in treatment of NSCLC brain metastases in the absence of previous radiation, though recent reports have begun to highlight this area (1, 14–16).
Pembrolizumab may be beneficial for stage IV NSCLC with brain metastasis as a monotherapy without the use of radiotherapy. To date, there is only one prospective study by Goldberg et al. investigating the efficacy of pembrolizumab in patients with NSCLC or melanoma with untreated brain metastases measuring 5–20 mm (15). The study consisted of two cohorts: cohort one was for patients with PD-L1 expression of at least 1% and cohort two was for patients with PD-L1 <1%. Eleven of 37 patients (29.7%) in cohort one exhibited a brain metastasis response on subsequent radiographic imaging. Specifically, seven patients exhibited a partial response on subsequent imaging whereas four patients exhibited complete radiographic resolution at a median follow up time of 1.8 months after the initiation of treatment. Moreover, none of the five patients in cohort two demonstrated a response. Interestingly, median overall survival did not differ significantly between patients with PD-L1 expression of at least 1% vs. PD-L1 expression <1% (15).
The resolution of intracranial lesions on follow-up imaging suggests permeability of the blood-brain barrier to pembrolizumab, though numerous questions remain to be answered regarding which patients may have a propensity to respond, and which imaging, clinical findings, or biomarkers may correlate with central nervous system efficacy (1). Accurate prediction of which patients with NSCLC brain metastases will respond to immunotherapy may help prevent or at least delay unnecessary invasive therapies. While some studies suggest that NSCLC patients with high tumor PD-1 expression show better response rates and longer survival with immunotherapy, this does not necessarily translate to the response rate of NSCLC brain metastases (28). The heterogeneity of PD-1 receptor status of tumor cells as well as potential variations in the immunophenotypes of the receptors between the primary lesion compared to the metastatic lesions make generalizations difficult (1, 29). Though receptor status of the brain metastases is inevitably of interest, other research has focused on the PD-1 status of the tumor infiltrating lymphocytes (TIL) in metastatic brain lesions. Higher TIL scores in metastatic brain lesions have correlated to improved progression free survival; however, other studies indicate that TIL scores of the metastatic brain lesion may not correlate with the primary lesion (17, 30). Moreover, the variability of currently available assays makes definitive analysis, and, thus, clinical correlations difficult (31).
Conclusions
As the median survival of NSCLC continues to improve with increasingly efficacious therapies, addressing secondary anticipated effects of longer survival times such as an increasing rate of brain metastases will become a more demanding issue. Patients choosing immunotherapy and forgoing more invasive modalities, such as surgical resection or radiation treatment, for asymptomatic, incidental lesions may become a challenge that needs further investigation and substantiation. Delaying surgical or radiation treatment with the hope that immunotherapy will successfully treat the metastatic process is only a feasible concept if research can accurately identify likely responders. A non-aggressive approach toward an aggressive lesion may lead to increased growth of the lesion and associated vasogenic edema that is often confounded by neurologic symptomatology, seizures, cognitive decline, and deterioration in the patient's performance status (32). Certainly, there is a direct correlation between performance status and clinical outcomes (33). Indeed, an improved understanding of immunotherapy and its effectiveness in the central nervous system may lead to better treatment paradigms for metastatic brain cancer and, ultimately, improved clinical outcomes.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The patient described in this report provided written informed consent for its publication.
Author Contributions
EM, KF, AK, and JC: provided substantial contributions to the conception and design of the manuscript, contributed to manuscript revision, read, and approved the submitted version, agree to be accountable for all aspects of the work ensuring that questions related to the accuracy or integrity of any part of the work are investigated and resolved. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
NSCLC, non-small cell lung cancer; SRS, stereotactic radiosurgery; WBRT, whole brain radiation therapy; CT, computed tomography; MRI, magnetic resonance imaging; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; TIL, tumor infiltrating lymphocytes.
References
1. Wang S, Hu C, Xie F, Liu Y. Use of programmed death receptor-1 and/or programmed death ligand 1 inhibitors for the treatment of brain metastasis of lung cancer. Onco Targets Ther. (2020) 13:667–83. doi: 10.2147/OTT.S235714
2. Lim JH, Um SW. The risk factors for brain metastases in patients with non-small cell lung cancer. Ann Transl Med. (2018) 6(Suppl 1):S66. doi: 10.21037/atm.2018.10.27
3. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. (2004) 22:2865–72. doi: 10.1200/JCO.2004.12.149
4. Schouten LJ, Rutten J, Huveneers HAM, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. (2002) 94:2698–05. doi: 10.1002/cncr.10541
5. Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Gonçalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. (2012) 32:4655–62.
6. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Niksic M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
7. Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 Study. J Clin Oncol. (2019) 37:2518–27. doi: 10.1200/JCO.19.00934
8. Shinde A, Akhavan D, Sedrak M, Glaser S, Amini A. Shifting paradigms: whole brain radiation therapy vs. stereotactic radiosurgery for brain metastases. CNS Oncol. (2019) 8:CNS27. doi: 10.2217/cns-2018-0016
9. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. (2009) 10:1037–44. doi: 10.1016/S1470-2045(09)70263-3
10. Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs. stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. (2006) 295:2483–91. doi: 10.1001/jama.295.21.2483
11. Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. (2017) 18:1049–60. doi: 10.1016/S1470-2045(17)30441-2
12. Welsh JW, Komaki R, Amini A, Munsell MF, Unger W, Allen PK, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. (2013) 31:895–902. doi: 10.1200/JCO.2011.40.1174
13. Falchook GS, Naing A, Hong DS, Zinner R, Fu S, Piha-Paul SA, et al. Dual EGFR inhibition in combination with anti-VEGF treatment: a phase I clinical trial in non-small cell lung cancer. Oncotarget. (2013) 4:118–27. doi: 10.18632/oncotarget.763
14. Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. (2016) 17:976–83. doi: 10.1016/S1470-2045(16)30053-5
15. Goldberg SB, Schalper KA, Gettinger SN, Mahajan A, Herbst RS, Chiang AC, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancel Oncol. (2020) 21:655–3. doi: 10.1016/S1470-2045(20)30111-X
16. Di M, Zhang L. Pembrolizumab for non-small cell lung cancer with central nervous system metastases: a two-case report. Thorac Cancer. (2019) 10:381–5. doi: 10.1111/1759-7714.12963
17. Kim R, Keam B, Kim S, Kim M, Kim SH, Kim JW, et al. Differences in tumor microenvironments between primary lung tumors and brain metastases in lung cancer patients: therapeutic implications for immune checkpoint inhibitors. BMC Cancer. (2019) 19:19. doi: 10.1186/s12885-018-5214-8
18. Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G. Epidemiology of metastatic brain tumors. Neurosurg Clin N Am. (2011) 22:1–6. doi: 10.1016/j.nec.2010.08.007
19. Jacobs S, McCully CL, Murphy RF, Bacher J, Balis FM, Fox E. Extracellular fluid concentrations of cisplatin, carboplatin, and oxaliplatin in brain, muscle, and blood measured using microdialysis in nonhuman primates. Cancer Chemother Pharmacol. (2010) 65:817–24. doi: 10.1007/s00280-009-1085-7
20. Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhanel M, Spruss T, et al. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest. (2002) 110:1309–18. doi: 10.1172/JCI0215451
21. ten Tije AJ, Loos WJ, Zhao M, Baker SD, Enting RH, van der Meulen H, et al. Limited cerebrospinal fluid penetration of docetaxel. Anticancer Drugs. (2004) 15:715–8. doi: 10.1097/01.cad.0000136882.19552.8f
22. Kerr JZ, Berg SL, Dauser R, Nuchtern J, Egorin MJ, McGuffey L, et al. Plasma and cerebrospinal fluid pharmacokinetics of gemcitabine after intravenous administration in nonhuman primates. Cancer Chemother Pharmacol. (2001) 47:411–4. doi: 10.1007/s002800000253
23. Jacus MO, Daryani VM, Harstead KE, Patel YT, Throm SL, Stewart CF. Pharmacokinetic properties of anticancer agents for the treatment of central nervous system tumors: update of the literature. Clin Pharmacokinet. (2016) 55:297–311. doi: 10.1007/s40262-015-0319-6
24. McDermott J, Jimeno A. Pembrolizumab: PD-1 inhibition as a therapeutic strategy in cancer. Drugs Today. (2015) 51:7–20. doi: 10.1358/dot.2015.51.1.2250387
25. Jia L, Zhang Q, Zhang R. PD-1/PD-L1 pathway blockade works as an effective and practical therapy for cancer immunotherapy. Cancer Biol Med. (2018) 15:116–23. doi: 10.20892/j.issn.2095-3941.2017.0086
26. Lee KA, Cioni M, Robson A, Bataille V. Metastatic porocarcinoma achieving complete radiological and clinical response with pembrolizumab. BMJ Case Rep. (2019) 12:e228917. doi: 10.1136/bcr-2018-228917
27. Uprety D, Arjyal L, Vallatharasu Y, Bista A, Wittchow RJ, Marinier DE. Durable response after 2 doses of pembrolizumab in a patient with non-small-cell lung cancer with an isolated brain metastasis. Clin Lung Cancer. (2019) 20:e552–4. doi: 10.1016/j.cllc.2019.06.002
28. Incorvaia L, Fanale D, Badalamenti G, Barraco N, Bono M, Corsini LR, et al. Programmed death ligand 1 (PD-L1) as a predictive biomarker for pembrolizumab therapy in patients with advanced non-small-cell lung cancer (NSCLC). Adv Ther. (2019) 36:2600–17. doi: 10.1007/s12325-019-01057-7
29. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. (2017) 377:1919–29. doi: 10.1056/NEJMoa1709937
30. Campbell AM, Cai WL, Burkhardt D, Gettinger SN, Goldberg SB, Amodio M, et al. Final results of a phase II prospective trial evaluating the combination of stereotactic body radiotherapy (SBRT) with concurrent pembrolizumab in patients with metastatic non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. (2019) 105:s36–7. doi: 10.1016/j.ijrobp.2019.06.453
31. McLaughlin J, Han G, Schalper KA, Carvajal-Hausdorf D, Pelekanou V, Rehman J, et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol. (2016) 2:46–54. doi: 10.1001/jamaoncol.2015.3638
32. Cagney DN, Martin AM, Catalano PJ, Redig AJ, Lin NU, Lee EQ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol. (2017) 19:1511–21. doi: 10.1093/neuonc/nox077
33. Agboola O, Benoit B, Cross P, Da Silva V, Esche B, Lesiuk H, et al. Prognostic factors derived from recursive partition analysis (RPA) of Radiation Therapy Oncology Group (RTOG) brain metastases trials applied to surgically resected and irradiated brain metastatic cases. Int J Radiat Oncol Biol Phys. (1998) 42:155–9. doi: 10.1016/S0360-3016(98)00198-9
Keywords: pembrolizumab, immunotherapy, non-small cell lung cancer, central nervous system metastases, brain metastases (BM)
Citation: Marvin EA, Furrow KL, Kar A and Cuoco JA (2020) Response of Pembrolizumab Alone for Non-small Cell Lung Cancer With Brain Metastasis: A Case Report and Literature Review. Front. Oncol. 10:577159. doi: 10.3389/fonc.2020.577159
Received: 07 July 2020; Accepted: 18 September 2020;
Published: 26 October 2020.
Edited by:
German Torres, New York Institute of Technology, United StatesReviewed by:
Zhiyuan Xu, University of Virginia, United StatesKawaljit Kaur, University of California, Los Angeles, United States
Copyright © 2020 Marvin, Furrow, Kar and Cuoco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joshua A. Cuoco, amFjdW9jb0BjYXJpbGlvbmNsaW5pYy5vcmc=
 Eric A. Marvin
Eric A. Marvin Kimberley L. Furrow
Kimberley L. Furrow Ayesha Kar
Ayesha Kar Joshua A. Cuoco
Joshua A. Cuoco