- Department of Oncology, The Second Xiangya Hospital, Central South University, Changsha, China
Introduction: Nasopharyngeal carcinoma (NPC) is a common malignancy in China and known prognostic factors are limited. In this study, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune inflammation index (SII), and systemic inflammation response index (SIRI) were evaluated as prognostic factors in locally advanced NPC patients.
Materials and Methods: NPC patients who received curative radiation or chemoradiation between January 2012 and December 2015 at the Second Xiangya Hospital were retrospectively reviewed, and a total of 516 patients were shortlisted. After propensity score matching (PSM), 417 patients were eventually enrolled. Laboratory and clinical data were collected from the patients’ records. Receiver operating characteristic curve analysis was used to determine the optimal cut-off value. Survival curves were analyzed using the Kaplan-Meier method. The Cox proportional hazard model was used to identify prognostic variables.
Results: After PSM, all basic characteristics between patients in the high SIRI group and low SIRI group were balanced except for sex (p=0.001) and clinical stage (p=0.036). Univariate analysis showed that NLR (p=0.001), PLR (p=0.008), SII (p=0.001), and SIRI (p<0.001) were prognostic factors for progression-free survival (PFS) and overall survival (OS). However, further multivariate Cox regression analysis showed that only SIRI was an independent predictor of PFS and OS (hazard ratio (HR):2.83; 95% confidence interval (CI): 1.561-5.131; p=0.001, HR: 5.19; 95% CI: 2.588-10.406; p<0.001), respectively.
Conclusion: Our findings indicate that SIRI might be a promising predictive indicator of locally advanced NPC patients.
Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy arising from the epithelium of the nasopharynx with a high incidence in endemic regions such as Southern China and Southeast Asia (1). More than 60% of patients are diagnosed at a locally advanced stage due to the lack of early symptoms and appropriate screening tools, and the prognosis remains poor (2). Induction chemotherapy combined with concurrent chemoradiation is the preferred treatment modality for locally advanced NPC (3). However, approximately 14% of patients relapse and 21% report metastasis (4). However, there is a persisting lack of prognostic biomarkers, and exploring prognostic factors is required for better patient stratification and treatment optimization.
There is increasing evidence that demonstrates inflammation to be one of the hallmarks of cancer, and it is closely related to cancer development (5). Activated inflammatory cells can promote cancer progression by inducing DNA damage or interfering with DNA repair systems (6). Recently, an increasing number of studies have demonstrated the value of inflammatory response biomarkers as predictive markers for prognosis in cancers. It has been shown that the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune inflammation index (SII) can predict the prognosis in various types of cancers (7–9). The systemic inflammation response index (SIRI) is a new systemic inflammatory response biomarker based on peripheral blood cells counts, and it was demonstrated to be effective in predicting the prognosis of esophagogastric junction adenocarcinoma (10), esophageal cancer (11) and cervical cancer (12). There is minimal evidence of SIRI as a prognostic marker in NPC from a previous study (13).
In this study, we aimed to evaluate the prognostic value of NLR, PLR, SII, and SIRI in a retrospective cohort of 417 locally advanced NPC patients.
Materials and Methods
Patient Selection
A cohort of patients diagnosed with NPC at the Second Xiangya Hospital, Central South University, from January 2012 to December 2015 was retrospectively analyzed. The inclusion criteria were as follows: (1) diagnosis of pathologically proven poorly differentiated nasopharyngeal squamous cell carcinoma, (2) stage III-IVa according to the 8th edition of the American Joint Committee on Cancer (AJCC) Staging System for NPC, (3) receiving radiotherapy or chemoradiotherapy in the Department of Oncology, the Second Xiangya Hospital, Central South University, with complete follow-up data available. The exclusion criteria were as follows: (1) history of chronic inflammatory diseases such as inflammatory bowel disease, (2) history of autoimmune diseases, and (3) acute infectious diseases within 4 weeks. All patients were followed up after completion of treatment at a pre-defined frequency of once every 3 months in the first 2 years, once every 6 months from the third to the fifth year, and once yearly thereafter. The last follow-up date was April 1, 2020. Relapse or metastasis of the disease was diagnosed on contrast-enhanced magnetic resonance imaging or computed tomography.
A total of 516 patients with NPC were shortlisted. To reduce patient selection bias, we used the propensity score matching (PSM) technique (14). PSM was developed using age, sex, stage, treatment mode, chemotherapy agent dose, chemotherapy agent type, etc. After PSM, 417 patients were finally included in this study. This study was conducted according to the Helsinki Declaration of 1975, revised in 2008, and approved by the ethics committee of the Second Xiangya Hospital, Central South University. And the written consent was waivered.
Data Collection
Patients’ clinical information, including that on age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS) scores, T stage, N stage, clinical stage, treatment mode, chemotherapy agent dose, and chemotherapy agent type, was collected, and routine blood results within 1 week before therapy were also collected. The NLR, PLR, SII, and SIRI were calculated using the following formula: NLR = neutrophil count/lymphocyte count, PLR = platelet count/lymphocyte count, SII = platelet count × neutrophil count/lymphocyte count, SIRI = neutrophil count × monocyte count/lymphocyte count. Overall survival (OS) was calculated from the date of diagnosis to the date of death due to any cause or to the date of the last follow-up; progression-free survival (PFS) was calculated from the date of diagnosis to the date of disease progression or death.
Statistical Analysis
SPSS was used for data analysis (version 22.0; SPSS Inc., Chicago, IL, USA). Chi-square test was used to analyze the relationship between NLR, PLR, SII, SIRI, and clinicopathological features in locally advanced NPC patients. Receiver operating characteristic (ROC) curves were used to calculate the cut-off values for NLR, PLR, SII, and SIRI. When applying the ROC curves, the optimal cut-off value was defined as the value with the maximum Youden index (15). The Kaplan–Meier method was used to calculate the survival curves. Multivariate Cox hazard regression analysis was performed on the factors that were found to be significant in the univariate analysis. A two-sided p value of <0.05 was considered statistically significant.
Results
Patient Characteristics
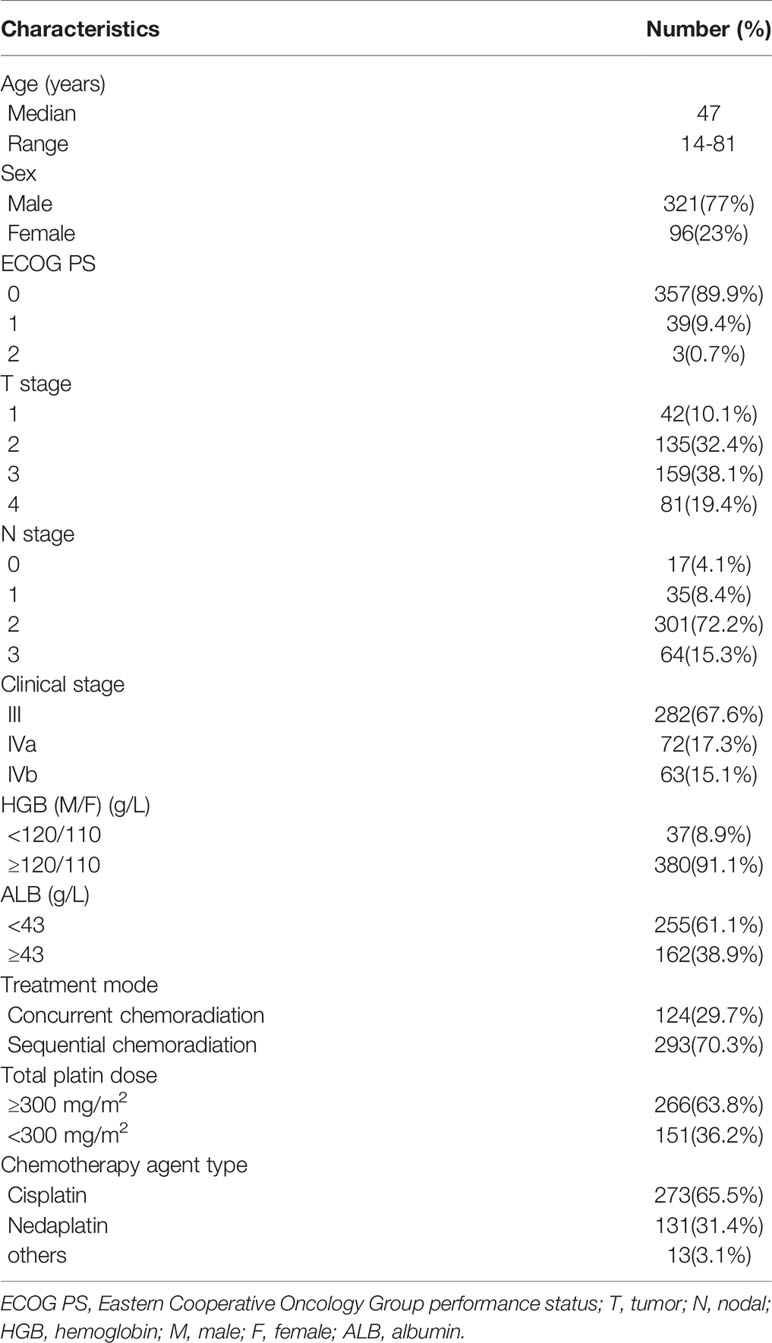
The clinicopathological features of the 417 locally advanced NPC patients are shown in Table 1. The median age was 47 years (range: 14–81 years). A total of 321 (77%) were male and 96 (23%) were female. Most patients had an ECOG score of 0 to 1 (396, 99.3%). As for the T stage, 42 (10.1%) patients were T1, 135 (32.4%) were T2, 159 (38.1%) were T3, and 81 (19.4%) were T4. For the N stage, 17 (4.1%) patients had N0, 35 (8.4%) N1, 301 (72.2%) N2, and 64 (15.3%) N3. Overall, 282 (67.6%) patients were stage III, and 135 (32.4%) were stage IVa. A total of 124 (29.7%) patients received concurrent chemoradiation, and 293 (70.3%) received sequential chemoradiation. The total dosage of cisplatin was calculated as 266 (63.8%) patients with a dosage of ≥300 mg/m2 and 151 (36.2%) with a dosage of <300 mg/m2. Among all the patients, 273 (65.5%) received cisplatin-based chemotherapy, 131 (31.4%) received nedaplatin-based chemotherapy, while 13 (3.1%) received other platin-based chemotherapy.
Cut-Off Values of NLR, PLR, SII, and SIRI, and Their Correlation With Clinicopathological Features
As shown in Figure 1A, the area under the curves (AUC) of NLR, PLR, SII, and SIRI were 0.623, 0.548, 0.616, and 0.656, respectively, and the optimal cut-off values were 2.50, 163.06, 488.90, and 0.86, respectively. As shown in Figure 1B, for OS, the AUCs of NLR, PLR, SII, and SIRI were 0.592, 0.552, 0.605, and 0.625, respectively, and the optimal cut-off values were 2.50, 154.04, 488.90, and 0.86, respectively.
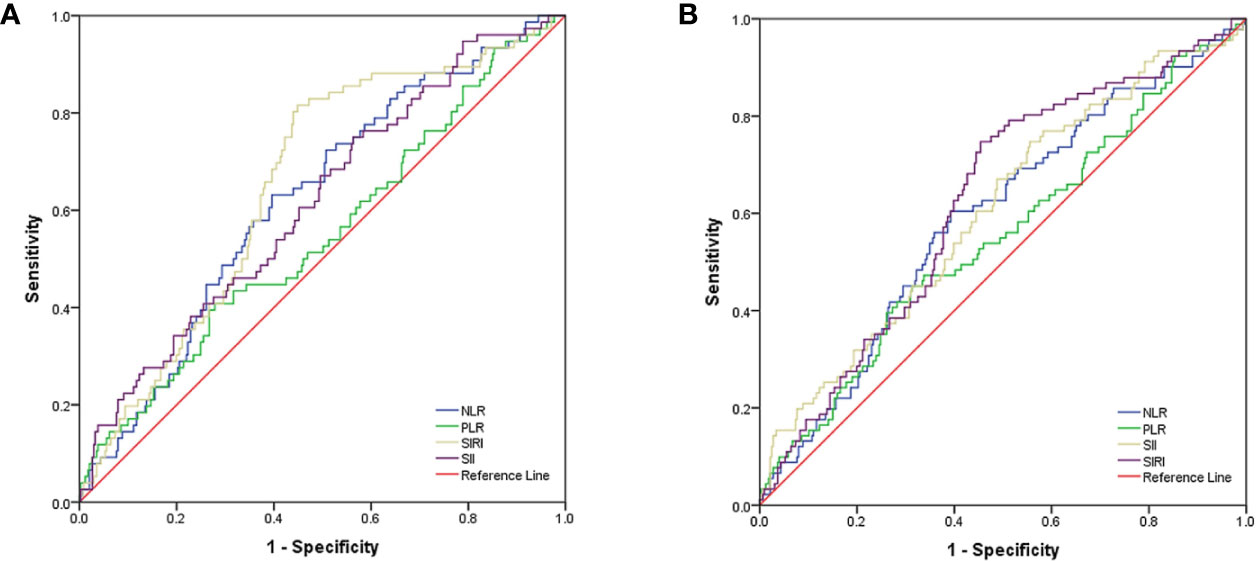
Figure 1 (A) ROC curve analysis for optimal cut-off value of NLR, PLR, SII and SIRI for PFS. (B) ROC curve analysis for optimal cut-off value of NLR, PLR, SII and SIRI for OS. ROC, receiver operating characteristic; PFS, progression free survival; OS, overall survival; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune inflammation index; SIRI, systemic inflammation response index.
Association Between NLR, PLR, SII, SIRI, and Clinicopathological Features
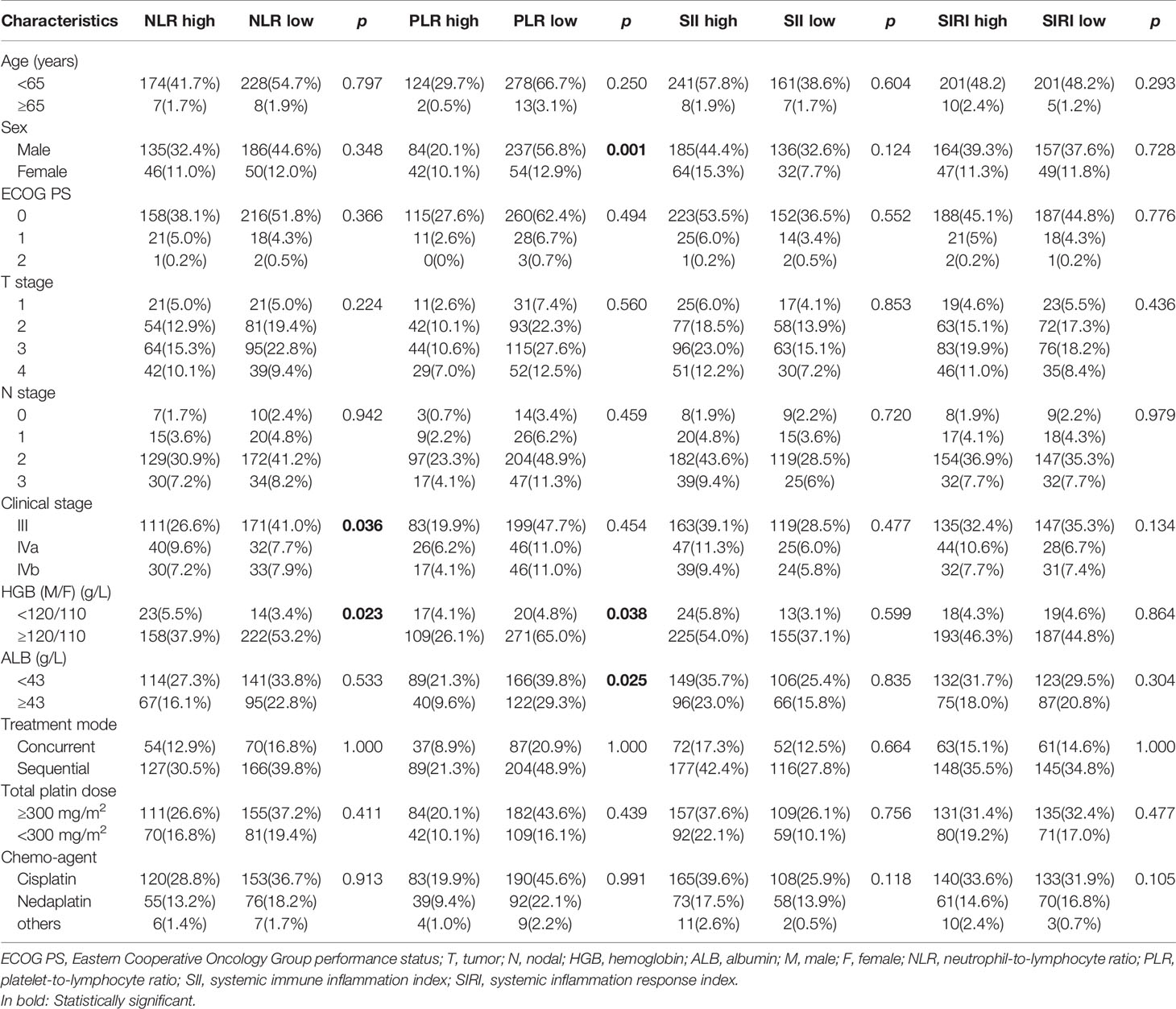
The association between NLR, PLR, SII, SIRI, and clinicopathological features in locally advanced NPC patients is shown in Table 2. A high PLR correlated with sex (p=0.001), and clinical stage (p=0.036). However, NLR, PLR, SII, and SIRI were not significantly correlated with patient age, ECOG PS, T stage, N stage, treatment mode, chemotherapy agent dose, or chemotherapy agent type (p>0.05).
Prognostic Significance of NLR, PLR, SII, and SIRI
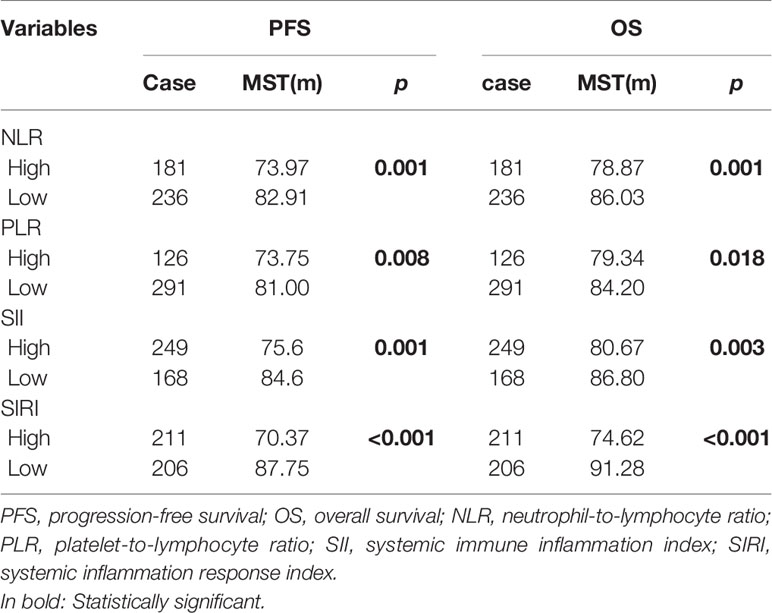
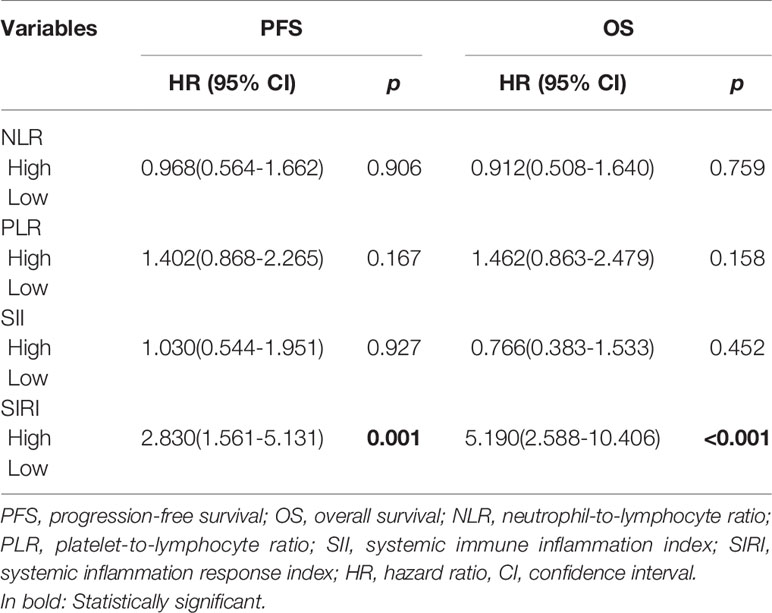
The Kaplan-Meier survival curves of PFS with regard to NLR, PLR, SII, and SIRI are shown in Figures 2A–D. NLR (p=0.001), PLR (p=0.008), SII (p=0.001), and SIRI (p<0.001) contributed to significantly unfavorable factors of PFS (Table 3). Kaplan–Meier OS curves with regard to NLR, PLR, SII, and SIRI are shown in Figures 2E–H. NLR (p=0.001), PLR (p=0.018), SII (p=0.003), and SIRI (p<0.001) were significantly correlated with unfavorable OS (Table 3). A multivariate Cox regression model including NLR, PLR, SII, and SIRI showed that SIRI was an independent predictor of PFS and OS with HR (hazard ratio) at 2.83 (95% CI 1.561-5.131, p=0.001) and 5.19 (95% CI 2.588-10.406, p <0.001), respectively (Table 4).
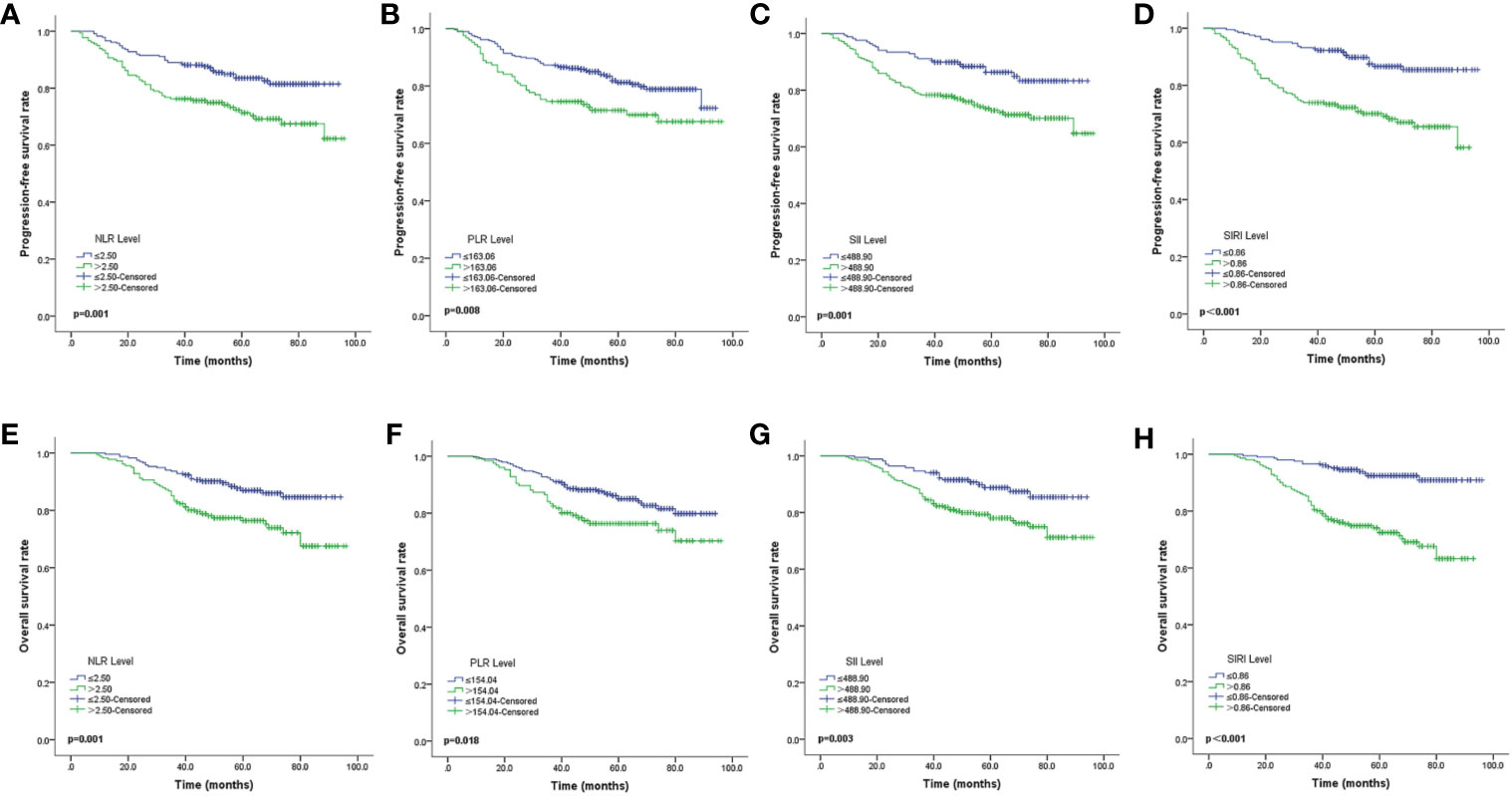
Figure 2 Kaplan-Meier survival curves of locally advanced NPC patients (A–D). Kaplan-Meier curves for PFS according to NLR (A), PLR (B), SII (C) and SIRI (D) (E–H). Kaplan-Meier curves for OS according to NLR (E), PLR (F), SII (G) and SIRI (H). PFS, progression-free survival; OS, overall survival; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune inflammation index; SIRI, systemic inflammation response index.
Discussion
NPC is a malignancy with ethnic and geographical distribution preferences (16). Most patients have locally advanced disease with poor prognosis at time of diagnosis. Therefore, there is an urgent need to identify new prognostic factors for NPC patients. In the present study, we evaluated the prognostic value of inflammatory response biomarkers, NLR, PLR, SII, and SIRI in a retrospective cohort of 417 locally advanced NPC patients. The results showed that high NLR, PLR, SII, and SIRI contributed to significantly unfavorable PFS and OS. Furthermore, SIRI was an independent predictor of PFS and OS.
Recent studies have found that inflammation is one of the hallmarks of cancer and is involved in promoting cancer processes. Inflammation associated with cancer development is triggered by a variety of blood immune cells, including neutrophils, macrophages, dendritic cells, and T and B lymphocytes (17). Increasing evidence has shown that systemic immune response markers are promising prognostic biomarkers for various cancers. The combined indicators of neutrophils, lymphocytes, monocytes, and platelets, such as NLR (18, 19), PLR (20), SII (21, 22) and SIRI (23, 24), have been reported to be predictive of cancer prognosis. SIRI is a new systemic inflammatory response biomarker based on neutrophil, monocyte, and lymphocyte counts. Neutrophils aid cancer cells to escape immune surveillance (25). Tumor-associated macrophages, which can promote cancer development and migration, are derived from peripheral monocytes (26). Lymphocytes are major mediators of host anti-cancer immunity. Reduction of peripheral lymphocytes impairs the host’s anticancer immunity and accelerates the progression of cancers. SIRI combines the above cell types and reflects the tumor microenvironment.
The prognostic value of NLR, PLR, and SII has been reported in many types of cancers, including NPC. Pan et al. (27) conducted a retrospective study in stage II NPC patients and found that NLR was an independent prognostic biomarker in stage II NPC patients; NLR≥2.92 was associated with poorer 5-year OS (84.3% vs. 97.4%, p=0.001). Another study in NPC patients without distant metastasis reported that NLR≥ 2.28 and PLR≥174 were significantly associated with a shorter OS (p<0.05), and NLR≥2.28 was associated with a shorter PFS (p<0.05) (28). For NPC patients receiving intensity-modulated radiotherapy, Oei et al. (29) reported that SII was an independent prognostic factor for OS (p=0.003), PFS (p=0.002), and distant metastasis-free survival (p=0.002). Our results were consistent with the above findings, indicating that NLR, PLR, and SII may act as prognostic biomarkers for locally advanced NPC.
SIRI is a novel systemic inflammatory response biomarker that is valuable for predicting the prognosis of cancers. Qi Q et al. (30) conducted a cohort study of patients with advanced pancreatic carcinoma who received palliative chemotherapy, and found that SIRI≥1.8 had a shorter time to progression (HR: 2.348; 95% CI: 1.559-3.535; p=0.003) and shorter OS (HR: 2.789; 95% CI: 1.897-4.121; p <0.001). In patients with head and neck squamous cell carcinomas, research showed that compared with SIRI<1.1, patients with SIRI between 1.10 and 2.80 had a 1.92 times higher risk of disease-specific death (95% CI: 1.33‐2.82; p=0.001), and patients with SIRI >2.80 had a 2.89 times higher risk (95% CI: 1.86‐4.42; p=0.0001), SIRI was an independent predictor of disease-specific survival (31). SIRI was also investigated in patients with NPC. In a cohort of 285 patients with NPC, ROC curves showed that the AUC obtained with SIRI was superior to the AUC of other parameters such as NLR or PLR, and SII (p <0.001) were significantly associated with PFS and OS (13). These results were consistent with the results of our study, indicating that SIRI acts as a superior prognostic factor for locally advanced NPC patients.
The present study has some inherent limitations. First, it was a single-center retrospective study with a comparatively small sample size, and no validation group was used to confirm the results. Second, the inflammatory response biomarkers NLR, PLR, SII, and SIRI could be affected by various factors such as inflammation and infection, which may have biased the results. Third, the prognosis of patients may be affected by other factors, including EBV-DNA (32), LDH (33), CRP (34), and D-dimer (35). However, in the present study, due to incomplete data, these factors were not considered for evaluation, and this may have biased the results. Finally, the cut-off value for SIRI varied in prior studies, and the optimal cut-off value still needs further investigation. Thus, the results should be interpreted with caution.
In summary, the present study showed that SIRI was an independent predictor of PFS and OS in locally advanced NPC patients. It is a convenient and easy-to-use biomarker that may help in better patient stratification and treatment optimization. Early intervention for system inflammation may be a promising strategy to improve the prognosis of locally advanced NPC patients, warranting further investigation.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of the Second Xiangya Hospital, Central South University. Written informed consent to participate in this study was waivered by the ethics committee.
Author Contributions
TH developed the idea for the study, analyzed and interpreted the patient data, and revised the manuscript. YF collected the patient data and was a major contributor in writing the draft of manuscript. SW, WZ, YH, JAM, and PL collected and cured the data. XL and CH interpreted the data and supervised the research. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Natural Science Foundation of Hunan Province (2020JJ5807).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Chow JC, Ngan RK, Cheung KM, Cho WC. Immunotherapeutic approaches in nasopharyngeal carcinoma. Expert Opin Biol Ther (2019) 19(11):1165–72. doi: 10.1080/14712598.2019.1650910
2. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N Engl J Med (2019) 381(12):1124–35. doi: 10.1056/NEJMoa1905287
3. Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, Sun Y, et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: Long-term results of phase 3 randomized controlled trial. Int J Cancer (2019) 145(1):295–305. doi: 10.1002/ijc.32099
4. Lee AWM, Ng WT, Chan JYW, Corry J, Mäkitie A, Mendenhall WM, et al. Management of locally recurrent nasopharyngeal carcinoma. Cancer Treat Rev (2019) 79:101890. doi: 10.1016/j.ctrv.2019.101890
5. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol (2014) 15(11):e493–503. doi: 10.1016/s1470-2045(14)70263-3
6. Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity (2019) 51(1):27–41. doi: 10.1016/j.immuni.2019.06.025
7. Hu C, Bai Y, Li J, Zhang G, Yang L, Bi C, et al. Prognostic value of systemic inflammatory factors NLR, LMR, PLR and LDH in penile cancer. BMC Urol (2020) 20(1):57. doi: 10.1186/s12894-020-00628-z
8. Szudy-Szczyrek A, Mlak R, Mielnik M, Szczyrek M, Nowaczynska A, Homa-Mlak I, et al. Prognostic value of pretreatment neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in multiple myeloma patients treated with thalidomide-based regimen. Ann Hematol (2020) Online ahead of print. doi: 10.1007/s00277-020-04092-5
9. Zhang Y, Liu X, Xu M, Chen K, Li S, Guan G. Prognostic value of pretreatment systemic inflammatory markers in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy. Sci Rep (2020) 10(1):8017. doi: 10.1038/s41598-020-64684-z
10. Chen Y, Jin M, Shao Y, Xu G. Prognostic Value of the Systemic Inflammation Response Index in Patients with Adenocarcinoma of the Oesophagogastric Junction: A Propensity Score-Matched Analysis. Dis Markers (2019) 2019:1–9. doi: 10.1155/2019/4659048
11. Geng Y, Zhu D, Wu C, Wu J, Wang Q, Li R, et al. A novel systemic inflammation response index (SIRI) for predicting postoperative survival of patients with esophageal squamous cell carcinoma. Int Immunopharmacol (2018) 65:503–10. doi: 10.1016/j.intimp.2018.10.002
12. Chao B, Ju X, Zhang L, Xu X, Zhao Y. A Novel Prognostic Marker Systemic Inflammation Response Index (SIRI) for Operable Cervical Cancer Patients. Front Oncol (2020) 10:766:766. doi: 10.3389/fonc.2020.00766
13. Chen Y, Jiang W, Xi D, Chen J, Xu G, Yin W, et al. Development and validation of nomogram based on SIRI for predicting the clinical outcome in patients with nasopharyngeal carcinomas. J Invest Med (2019) 67(3):691–8. doi: 10.1136/jim-2018-000801
14. Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med (2014) 33(7):1242–58. doi: 10.1002/sim.5984
15. Bantis LE, Nakas CT, Reiser B. Construction of confidence intervals for the maximum of the Youden index and the corresponding cutoff point of a continuous biomarker. Biometrical J Biometrische Z (2019) 61(1):138–56. doi: 10.1002/bimj.201700107
16. Zhan Y, Fan S. Multiple Mechanisms Involving in Radioresistance of Nasopharyngeal Carcinoma. J Cancer (2020) 11(14):4193–204. doi: 10.7150/jca.39354
17. Karki R, Man SM, Kanneganti T-D. Inflammasomes and Cancer. Cancer Immunol Res (2017) 5(2):94–9. doi: 10.1158/2326-6066.cir-16-0269
18. Yu Y, Wang H, Yan A, Wang H, Li X, Liu J, et al. Pretreatment neutrophil to lymphocyte ratio in determining the prognosis of head and neck cancer: a meta-analysis. BMC Cancer (2018) 18(1):383. doi: 10.1186/s12885-018-4230-z
19. Nomelini RS, Carrijo Chiovato AF, Abdulmassih FBF, da Silva RC, Tavares-Murta BM, Murta EFC. Neutrophil-to-lymphocyte ratio and platelet count as prognostic factors in ovarian malignancies. J Cancer Res Ther (2019) 15(6):1226–30. doi: 10.4103/jcrt.JCRT_304_17
20. Bardash Y, Olson C, Herman W, Khaymovich J, Costantino P, Tham T. Platelet-Lymphocyte Ratio as a Predictor of Prognosis in Head and Neck Cancer: A Systematic Review and Meta-Analysis. Oncol Res Treat (2019) 42(12):665–77. doi: 10.1159/000502750
21. Zhang W, Wang R, Ma W, Wu Y, Maskey N, Guo Y, et al. Systemic immune-inflammation index predicts prognosis of bladder cancer patients after radical cystectomy. Ann Trans Med (2019) 7(18):431. doi: 10.21037/atm.2019.09.02
22. Zhang K, Hua YQ, Wang D, Chen LY, Wu CJ, Chen Z, et al. Systemic immune-inflammation index predicts prognosis of patients with advanced pancreatic cancer. J Transl Med (2019) 17(1):30. doi: 10.1186/s12967-019-1782-x
23. Chen L, Kong X, Wang Z, Wang X, Fang Y, Wang J. Pretreatment Systemic Inflammation Response Index in Patients with Breast Cancer Treated with Neoadjuvant Chemotherapy as a Useful Prognostic Indicator. Cancer Manag Res (2020) 12:1543–67. doi: 10.2147/cmar.s235519
24. Chen Y, Jin M, Shao Y, Xu G. Prognostic Value of the Systemic Inflammation Response Index in Patients with Adenocarcinoma of the Oesophagogastric Junction: A Propensity Score-Matched Analysis. Dis Markers (2019) 2019:4659048. doi: 10.1155/2019/4659048
25. Nicolás-Ávila J, Adrover JM, Hidalgo A. Neutrophils in Homeostasis, Immunity, and Cancer. Immunity (2017) 46(1):15–28. doi: 10.1016/j.immuni.2016.12.012
26. Salmaninejad A, Valilou SF, Soltani A, Ahmadi S, Abarghan YJ, Rosengren RJ, et al. Tumor-associated macrophages: role in cancer development and therapeutic implications. Cell Oncol (Dordr) (2019) 42(5):591–608. doi: 10.1007/s13402-019-00453-z
27. Pan XB, Huang ST, Zhu XD. Neutrophil-to-lymphocyte ratio predicts the prognosis of stage II nasopharyngeal carcinoma. Cancer Manag Res (2019) 11:8269–75. doi: 10.2147/cmar.s213264
28. Lu A, Li H, Zheng Y, Tang M, Li J, Wu H, et al. Prognostic Significance of Neutrophil to Lymphocyte Ratio, Lymphocyte to Monocyte Ratio, and Platelet to Lymphocyte Ratio in Patients with Nasopharyngeal Carcinoma. Biomed Res Int (2017) 2017:3047802. doi: 10.1155/2017/3047802
29. Oei RW, Ye L, Kong F, Du C, Zhai R, Xu T, et al. Prognostic value of inflammation-based prognostic index in patients with nasopharyngeal carcinoma: a propensity score matching study. Cancer Manag Res (2018) 10:2785–97. doi: 10.2147/cmar.s171239
30. Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer (2016) 122(14):2158–67. doi: 10.1002/cncr.30057
31. Valero C, Pardo L, Sansa A, Garcia Lorenzo J, López M, Quer M, et al. Prognostic capacity of Systemic Inflammation Response Index (SIRI) in patients with head and neck squamous cell carcinoma. Head Neck (2020) 42(2):336–43. doi: 10.1002/hed.26010
32. Nie M, Sun P, Chen C, Bi X, Wang Y, Yang H, et al. Albumin-to-Alkaline Phosphatase Ratio: A Novel Prognostic Index of Overall Survival in Cisplatin-based Chemotherapy-treated Patients with Metastatic Nasopharyngeal Carcinoma. J Cancer (2017) 8(5):809–15. doi: 10.7150/jca.17536
33. Tang SQ, Xu C, Wang XS, Tang LL, Li WF, Chen L, et al. Induction versus adjuvant chemotherapy combined with concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: A propensity score-matched analysis. Oral Oncol (2020) 105:104686. doi: 10.1016/j.oraloncology.2020.104686
34. Gao N, Yang RN, Meng Z, Wang WH. The prognostic value of C-reactive protein/albumin ratio in nasopharyngeal carcinoma: a meta-analysis. Biosci Rep (2018) 38(6):BSR20180686. doi: 10.1042/BSR20180686
Keywords: nasopharyngeal carcinoma, locally advanced, prognosis, survival, systemic inflammation response index
Citation: Feng Y, Zhang N, Wang S, Zou W, He Y, Ma JA, Liu P, Liu X, Hu C and Hou T (2020) Systemic Inflammation Response Index Is a Predictor of Poor Survival in Locally Advanced Nasopharyngeal Carcinoma: A Propensity Score Matching Study. Front. Oncol. 10:575417. doi: 10.3389/fonc.2020.575417
Received: 04 August 2020; Accepted: 29 October 2020;
Published: 10 December 2020.
Edited by:
Simona Gurzu, University of Medicine and Pharmacy of Târgu Mureş, RomaniaReviewed by:
Honggang Ren, Vanderbilt University, United StatesManidhar Reddy Lekkala, University of Rochester, United States
Copyright © 2020 Feng, Zhang, Wang, Zou, He, Ma, Liu, Liu, Hu and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Hou, aG91dGFvQGNzdS5lZHUuY24=
 Yuhua Feng
Yuhua Feng Na Zhang
Na Zhang Wen Zou
Wen Zou Ping Liu
Ping Liu Chunhong Hu
Chunhong Hu Tao Hou
Tao Hou