
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 18 December 2020
Sec. Women's Cancer
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.570175
This article is part of the Research Topic Predictive, Prognostic Biomarkers and Therapeutic Targets in Breast Cancer View all 59 articles
Introduction: To investigate the prognostic and predictive effect of the American Joint Committee on Cancer (AJCC) 8th edition pathological prognostic staging system in patients with T1-2N1micM0 breast cancer who underwent mastectomy.
Methods: Data from T1-2N1micM0 breast cancer patients who underwent mastectomy from 2010–2014 were obtained from the Surveillance, Epidemiology, and End Results program. The chi-square test, binomial logistics regression, receiver-operating characteristics curve, competing-risk regression model, Cox proportional hazards regression model, and proportional hazard assumption were used for statistical analyses.
Results: We identified 4,729 patients, including 1,062 patients were received postmastectomy radiotherapy (PMRT). Stage change occurred in 88.2% of the patients, of which 84.4% were downstaged and 3.7% were upstaged. Patients with higher pathological prognostic stages were independently predicted to receive PMRT. The 5-year breast cancer-specific survival (BCSS) was 97.5, 93.7, 90.1, 86.0, and 73.5% in disease stages IA, IB, IIA, IIB, and IIIA, respectively, according to the 8th edition criteria (P < 0.001). The AJCC 8th edition demonstrated moderate discriminative ability, and it had a significantly better ability to predict the BCSS than the AJCC 7th edition criteria (P < 0.001). The multivariate prognostic analysis showed that the new pathological prognostic staging was an independent prognostic factor affecting the BCSS. The BCSS worsened with an increase in the stage. The PMRT did not affect the BCSS regardless of the pathological prognostic stage. Similar trends were found using the competing-risks regression model.
Conclusions: The 8th AJCC breast cancer pathological prognostic staging system downstaged 84.4% of patients with T1-2N1micM0 disease and the survival outcome prediction with this staging system was more accurate than the AJCC 7th edition system. Our study does not support using the prognostic stage as a guideline to escalate of PMRT.
Routine pathological assessment of the prognostic and predictive biological factors of breast cancer, including estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) status, and tumor grade, determine the treatment decision and the response of breast cancers to therapy (1–4). Recognizing this, the American Joint Committee on Cancer (AJCC) 8th edition staging system for breast cancer introduced the clinical and pathological prognostic stages by incorporating biological factors into the traditional anatomic stages (5, 6). Compared with the 7th AJCC anatomic staging system, 8th AJCC pathological prognostic stages incorporating these biological factors provide a more refined prognostication in terms of survival estimates for patients receiving appropriate multidisciplinary treatment (7–10).
With advances in surgical and histopathological techniques of breast cancer, an increased number of patients were diagnosed as having micrometastatic disease (N1mic, ≤2 mm axillary nodal metastasis; 15–20%), which were labeled as node-negative (N0) by routine histological assessment (11–13). However, the prognostic significance for this population remains controversial. Several prior studies have shown comparable disease-free survival (DFS) and/or overall survival (OS) between patients with N1mic and those with N0 disease (14, 15). Other studies have found that N1mic breast cancer does indeed confer a lower survival and that adjuvant therapy should be performed to improve survival outcomes (16–19). The DFS appears to be only slightly lower among N1mic breast cancer patients (20). Also, it remains unknown whether such patients would benefit from postmastectomy radiotherapy (PMRT). In recent years, several studies have attempted to explore the clinical value of PMRT in T1–2 (tumor size ≤5 cm) and N1mic breast cancer, but all studies have yielded negative results (21–25). It is anticipated that the biological factors in breast cancer may inform the decision to carry out PMRT for this population. In light of this, we performed this study to investigate the prognostic effect of the AJCC 8th pathological prognostic staging in T1-2N1micM0 breast cancer patients undergoing mastectomy using a large, population-based cohort. In addition, we also investigated the role of the AJCC 8th pathological prognostic staging on the decision-making of PMRT for this population.
Patients were identified from the Surveillance, Epidemiology, and End Results (SEER) database in this study. The SEER database includes cancer incidence and survival information from 18 registries and covers 28% of the United States population (26). We identified female breast cancer patients diagnosed between 2010 and 2014 and met the following criteria: T1-2N1micM0 invasive breast cancer; had undergone mastectomy with and without PMRT; available variables, including age, race/ethnicity, tumor grade, ER, PR, HER2 status, and chemotherapy administration. Patients with metastatic disease at diagnosis, those with no positive pathology diagnosis; unknown radiation status; unknown tumor grade; as well as unknown or borderline ER, PR, and HER2 status were excluded. Because the SEER database contains publicly available data for de-identified patients, Institutional Review Board approval was waived.
The following variables were extracted for analysis: age, race/ethnicity, tumor stage (T1 and T2), tumor grade (grades I, II, and III), hormone receptor status (negative, positive), HER2 status (negative, positive), chemotherapy (no, yes), and PMRT (no, yes). The tumor/node/metastasis (TNM) staging system was based on the anatomic staging according to the AJCC 7th edition staging system, and pathological prognostic staging was determined according to the AJCC 8th edition of the breast staging manual (5, 6). Grade III disease included poorly differentiated and undifferentiated histological grades.
The primary outcomes of this study were breast cancer-specific survival (BCSS) and breast cancer-specific mortality (BSCM). BCSS was estimated from the time of diagnosis of breast cancer to the time of death from breast cancer. Patients who were still alive at the last follow-up or died of other causes were excluded from the analysis. BCSM was defined as the interval from the initial diagnosis of breast cancer to the date of death from breast cancer.
Descriptive statistics were compared between patients with and without PMRT using the chi-square test. Independent predictive factors that correlated with PMRT were investigated using binomial logistics regression. BCSS curves were generated by the Kaplan-Meier method and compared using the log-rank test. A competing-risk regression model was used to estimate the cumulative incidence of BCSM. The area under the curve (AUC) was estimated using the receiver-operating characteristics (ROC) curve to investigate the discriminatory ability of 7th and 8th AJCC staging criteria to predict the BCSS. Univariate and multivariate analyses were performed using the Cox proportional hazards regression models and competing-risks regression models in the Cox model framework to determine the predictive performance of variables with respect to BCSS and BCSM, respectively. The proportional hazard assumption was tested both graphically and using the Schoenfield residual test. A two-sided P value of < 0.05 was considered statistically significant. All statistical analyses were performed by SPSS software version 22.0 (IBM Corp., Armonk, NY), Stata/SE version 14 (StataCorp, TX, USA), and R version 3.1.1 (https://www.r-project.org/).
A total of 4,729 patients were included in this study. Of these patients, 88.0% (n = 4,161), 86.2% (n = 4,077), 85.3% (n = 4,036), 82.2% (n = 3,885), and 77.2% (n = 3,651) were ER-positive, invasive ductal carcinoma, HER2-negative, grade II–III, and PR-positive, respectively. Table 1 lists the patient characteristics. Moreover, 22.5% (n = 1,062) and 53.1% (n = 2,513) of the patients received PMRT and chemotherapy, respectively. A total of 2,456 (51.9%) and 2,273 (48.1%) patients were categorized into disease stages IB and IIB, respectively, according to the 7th AJCC staging system. A total of 2,571 (54.4%), 1,201 (25.4%), 373 (7.9%), 407 (8.6%), and 177 (3.7%) patients were categorized into disease stages IA, IB, IIA, IIB, and IIIA, respectively, according to the 8th AJCC pathological prognostic staging system.
Among all patients, 88.2% of patients had stage changed, of which 84.4% were downstaged and 3.7% were upstaged. In the 7th AJCC stages, 93.7% of the stage IB patients were downstaged to stage IA according to the 8th edition criteria, and there were no patients upstaged. Among patients classified as disease stage IIB according to the 7th edition classification, 74.3% of patients were downstaged, of which 11.8, 46.1, and 16.4% of patients were downstaged to disease stages IA, IB, and IIA, respectively, according to the 8th AJCC staging criteria. In addition, 177 (7.8%) patients had been upstaged to stage IIIA disease according to the 8th edition criteria.
Results of the univariate analysis showed that patients with younger age (< 50 years), higher tumor grade (grade III), larger tumor size (T2), ER-negative, PR-negative, and HER2-positive disease were more likely to receive PMRT (all P < 0.05). In addition, the percentage of patients who received PMRT increased with the staging (P < 0.001). We used two binomial logistic regression models to assess the independent predictive factors related to the receipt of PMRT (Table 2). In the first model, without including pathological prognostic staging, the results showed that younger age, invasive lobular carcinoma, grade III, T2, and PR-negative were the independent predictive factors associated with the receipt of PMRT. The new pathological prognostic staging was included in the second model, and the results showed that younger age, invasive lobular carcinoma, and higher pathological prognostic stages were independent predictors of PMRT receipt. Using stage IA as the reference, the odds ratios (ORs) for PMRT receipt in stages IB, IIA, IIB, and IIIA was 1.692 (95% confidence interval [CI] 1.433–1.997), 1.966 (95% CI 1.533–2.520), 1.967 (95% CI 1.541–2.509), and 3.727 (95% CI 2.708–5.130), respectively.
A total of 493 deaths occurred at a median follow-up of 49 months. Of these, 234 (47.5%) and 259 (52.5%) of patients died of breast cancer and other causes, respectively. The 5-year BCSS for stages IB and IIB disease in the 7th AJCC classification system were 97.0 and 90.8%, respectively (P < 0.001; Figure 1A). The 5-year BCSS were 97.5, 93.7, 90.1, 86.0, and 73.5% in stage IA, IB, IIA, IIB, and IIIA breast cancer classified according to the 8th edition criteria, respectively (P < 0.001; Figure 1C). When staged using the 7th edition AJCC system, the 5-year cumulative incidence of BCSM was 2.9% and 8.8% for stages IB and IIB, respectively (P < 0.001; Figure 2A). When staged using the 8th edition criteria, the 5-year cumulative incidence of BCSM was 2.5, 6.0, 9.7, 13.2, and 25.4% in stages IA, IB, IIA, IIB, and IIIA breast cancer, respectively (P < 0.001; Figure 2B), which were similar to the results using Kaplan-Meier analysis.
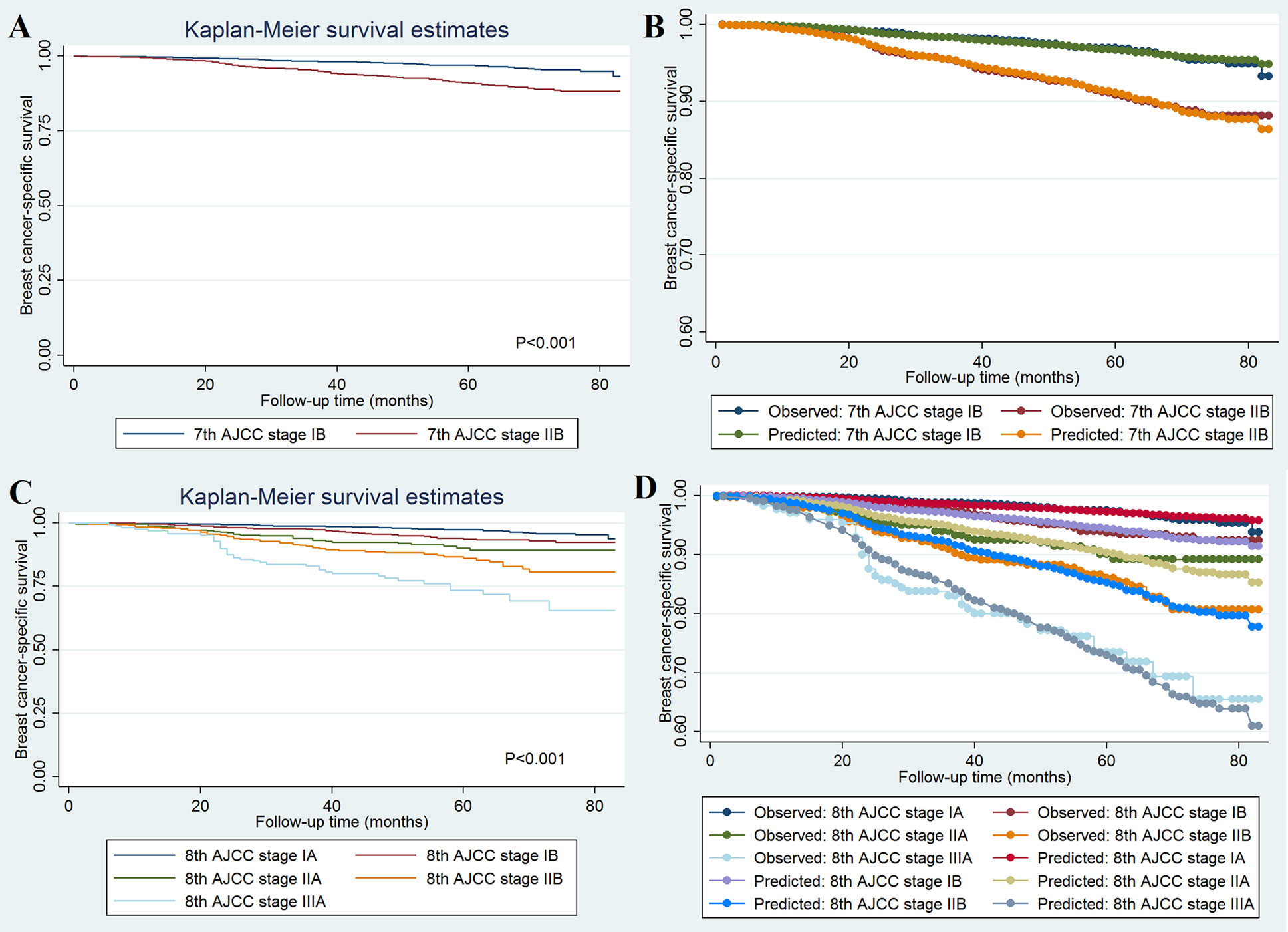
Figure 1 Breast cancer-specific survival curves and the evaluation of the proportional hazards assumption in survival analysis by the AJCC 7th edition (A, B) and 8th edition (C, D) staging systems.
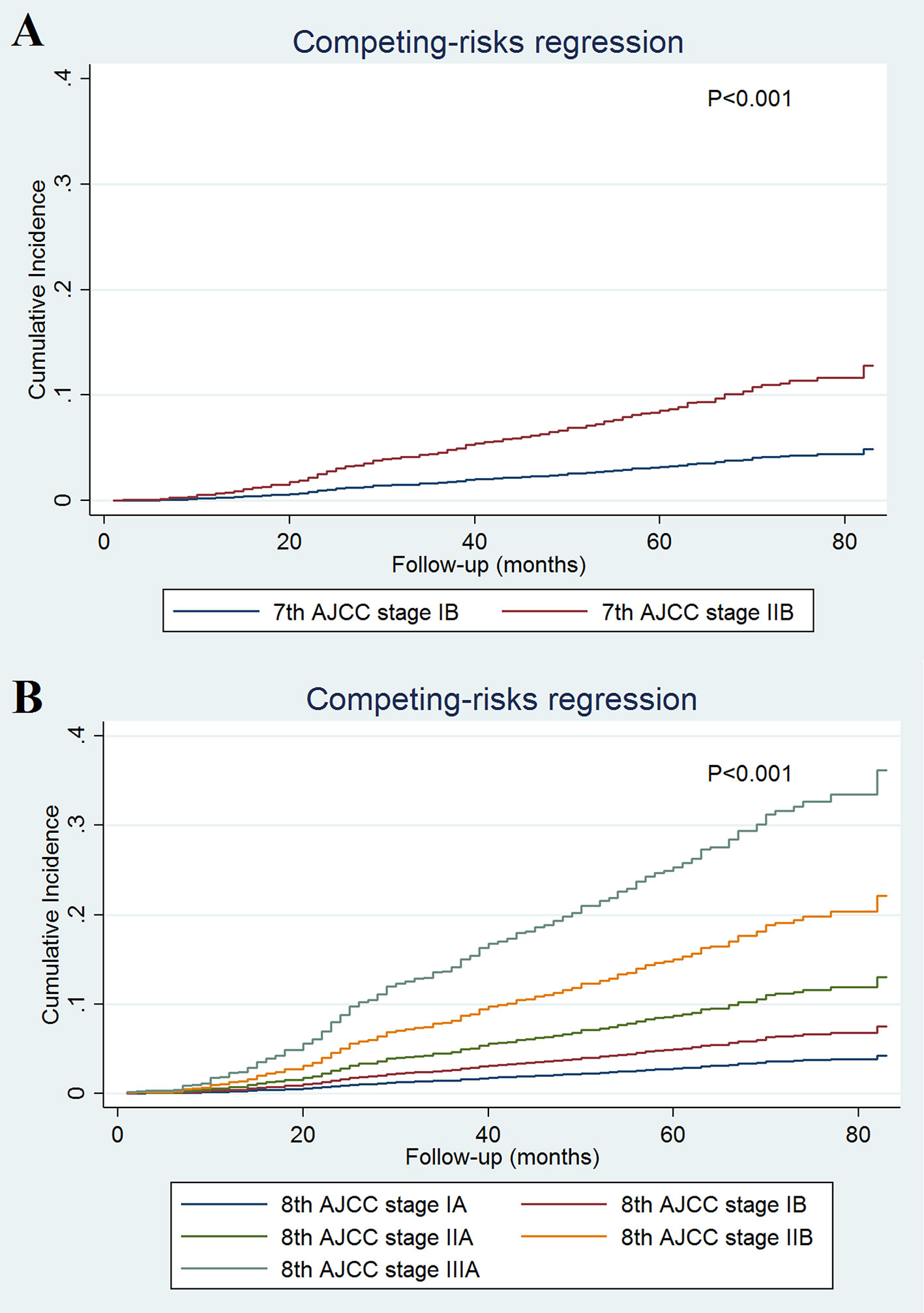
Figure 2 Cumulative incidence of breast cancer-specific mortality by the AJCC 7th (A) and 8th (B) edition staging systems.
Univariate and multivariate analyses were performed to determine the prognostic performance of variables with respect to BCSS and BCSM, respectively. As more than half of the deaths did not occur from breast cancer, we used two multivariate prognostic models, including the Cox regression model and the competing-risks regression model to assess the independent risk factors influencing patient survival. Univariate analysis showed that the prognostic staging was associated with patient survival (Table 3). The results of both multivariate prognostic models also revealed that pathological prognostic staging was an independent prognostic factor of patient survival. As the stage increased, the BCSS decreased (Cox regression model) and the risk of BCSM increased (competing-risks regression model) (Table 3). In addition, age was an independent risk factor affecting BCSM. However, PMRT was not found to affect patient survival in either model. Similarly, chemotherapy also did not affect patient survival.
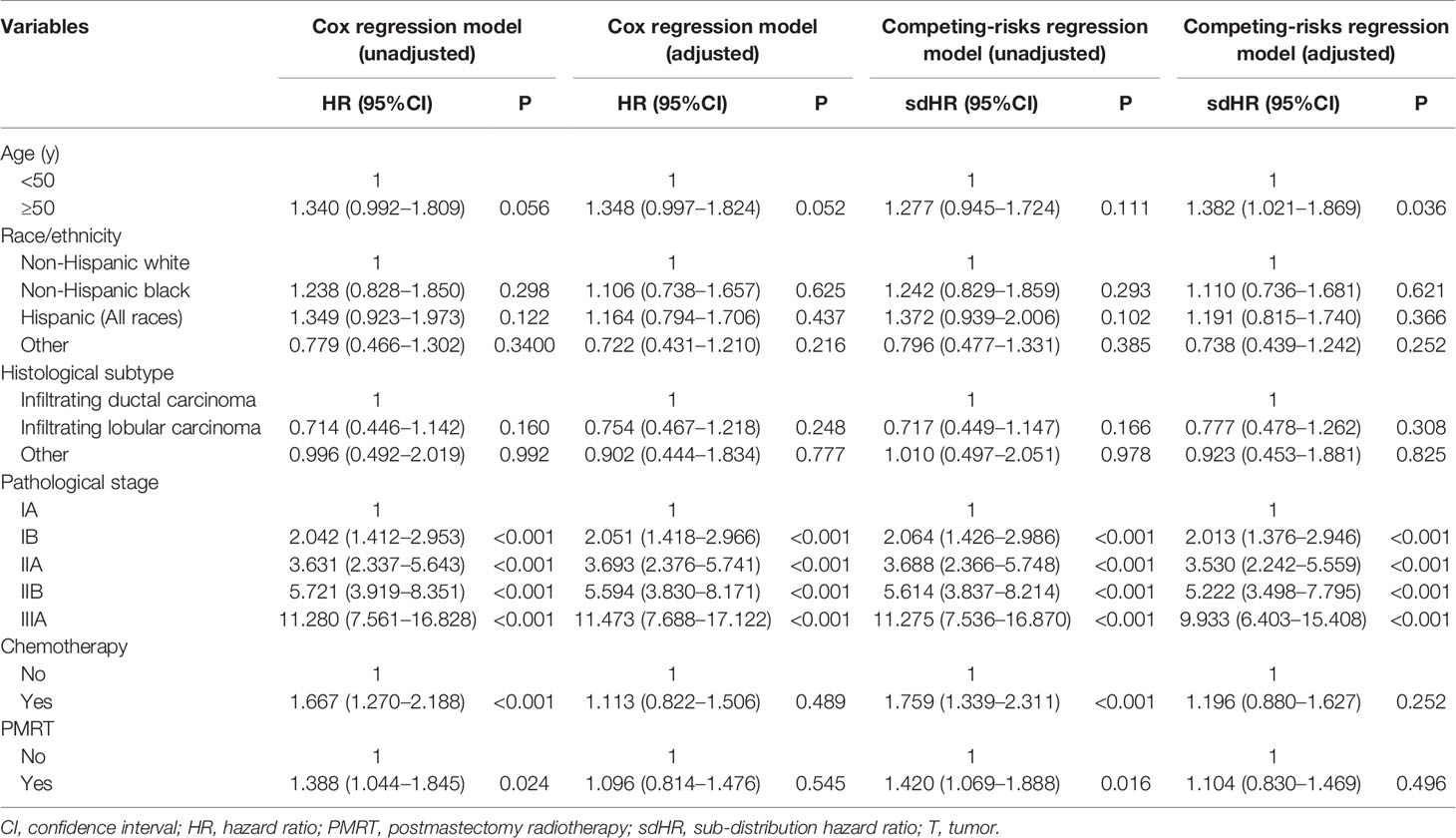
Table 3 Univariate and multivariate prognostic analysis using the Cox regression model and competing-risks regression model.
The effect of 7th (Figure 1B) and 8th (Figure 1D) AJCC staging on BCSS met the proportional hazard assumption, which indicated that the constant hazard ratios from the Cox model were reliable. The ROC was assessed using BCSS as the dependent variable, and the 8th edition AJCC staging system demonstrated moderate discriminative ability [AUC = 0.711, standard error (SE) = 0.018, 95% CI 0.698–0.724], which was significantly better than the 7th edition AJCC staging system in predicting the BCSS (AUC = 0.625, SE = 0.015, 95% CI 0.611–0.639; P < 0.001).
As patients with increasing stages were at a higher risk of breast cancer-related death, we further evaluated the value of PMRT in patients with different pathological prognostic stages. Univariate analysis using the Kaplan-Meier method (Figure 3) and competing-risk regression model (Figure 4) did not find any association between BCSS survival and PMRT in different pathological prognostic stages. The details of univariate Cox regression analysis and competing-risks regression analysis were showed in Table 4. After adjusting for age, race/ethnicity, histology, and chemotherapy, PMRT receipt was also not associated with better BCSS or lower BCSM compared to no PMRT, regardless of the pathological prognostic stage (Table 4).
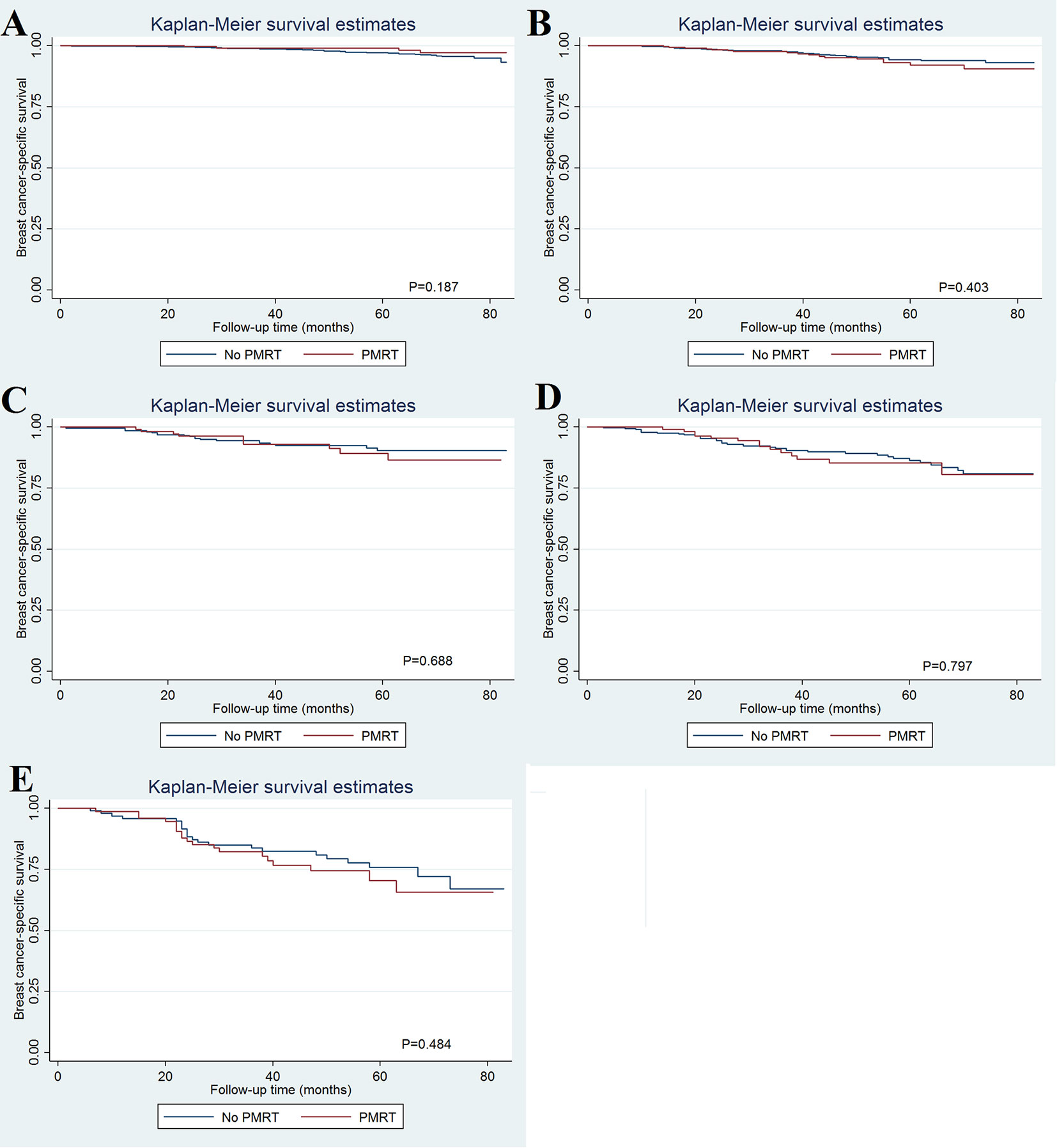
Figure 3 Effect of postmastectomy radiotherapy on breast cancer-specific survival by different pathological prognostic stages (A, stage IA; B, stage IB; C, stage IIA; D, stage IIB; E, stage IIIA).
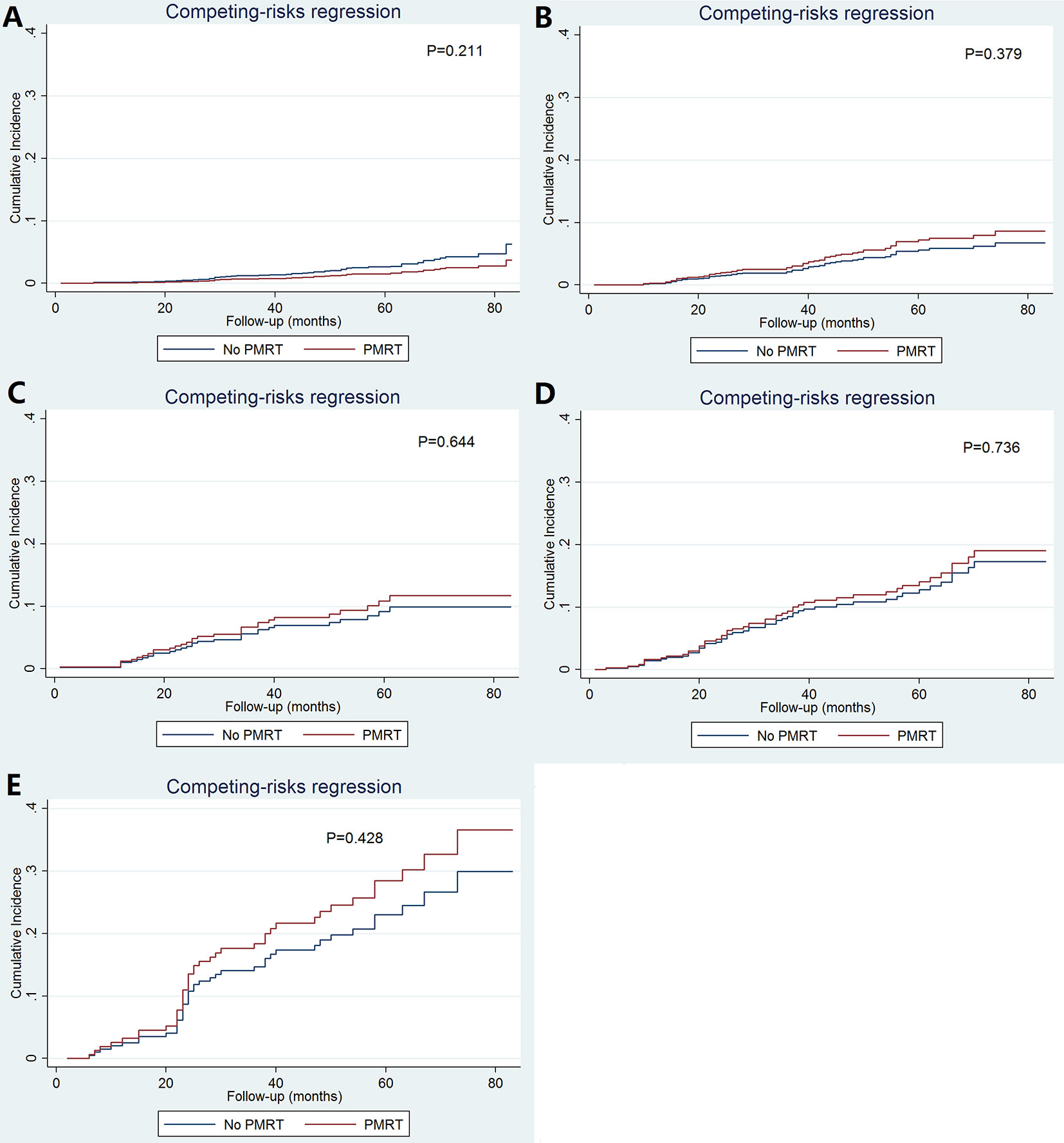
Figure 4 Cumulative incidence of breast cancer-specific mortality with and without postmastectomy radiotherapy according to different pathological prognostic stages (A, stage IA; B, stage IB; C, stage IIA; D, stage IIB; E, stage IIIA).
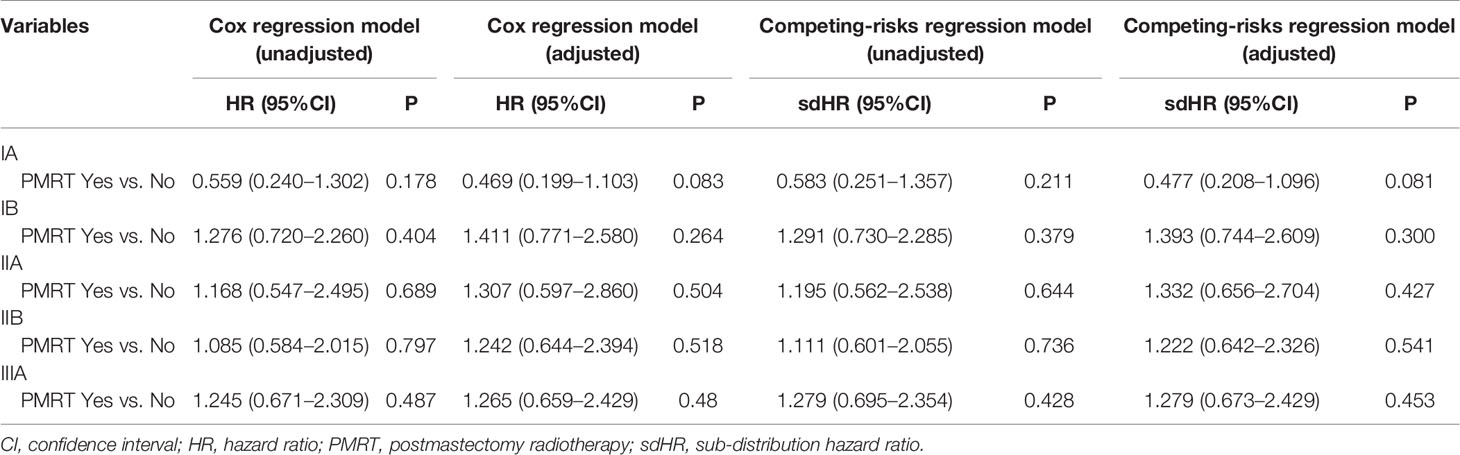
Table 4 Univariate and multivariate prognostic analysis using the Cox regression model and competing-risks regression model by pathological prognostic stages.
The traditional anatomic TNM staging system might not be enough to predict the prognosis and make treatment decisions in breast cancer. The AJCC 8th edition staging system, which includes various biomarkers, could better reflect the prognostic information and selection of therapy for breast cancer (5, 6). In this study, we verified the prognostic effect and predicted the survival benefits of PMRT using the new pathological prognostic system in T1-2N1micM0 breast cancer. Our results indicated that the AJCC 8th edition staging system could refine the prognostic information for this population, but PMRT was not associated with better BCSS regardless of the pathological prognostic stages.
Regarding the 7th AJCC staging system, T1-2N1micM0 was represented by disease stages IB and IIB. We sought to determine the probability of patients staged with the 7th edition criteria to be restaged according to the new pathological prognostic stages. Several population-based and larger cohort studies with stages I–III patients have shown that 36.6–53.2% of patients were restaged from the 7th anatomic stages to the 8th pathological prognostic stages, of which 19.4–29.7% were downstaged and 6.8–31.2% were upstaged (8, 9, 27). In our study, 88.2% of patients were restaged, including 84.4% and 3.7% of patients were assigned to more and less favorable stages (downstaged and upstaged), respectively. Differences in the distributions of biological factors between stage N1mic and other nodal status may be a possible explanation for the higher percentage of patients who were downstaged in our study. In this study, 88.0% of patients were ER-positive, which was similar to the results from Bae et al., who found that there was a significantly higher percentage of ER-positive (87.4% vs.75.9–81.3%) and PR-positive (75.7% vs. 62.4–73.0%) diseases among patients with N1mic breast cancer than patients with N0 and N1a breast cancers (28).
The 8th AJCC pathological prognostic stages were the first time incorporated biological factors, including ER, PR, HER2, and tumor grade into the staging classification system. Several recent studies have confirmed that the new staging system is more reliable than the AJCC 7th edition system for accurately predicting the survival outcome of breast cancer patients (7–10). Similarly, our study also revealed that the 8th AJCC pathological prognostic staging system provided a better distinguish of survival outcomes compared with the 7th AJCC staging system, suggesting that the 8th edition stages also hold true when adjusted by T1-2N1micM0 breast cancer. When comparing the rates of BCSS and BCSM, the application of the AJCC 8th edition staging system resulted in an incremental reduction in BCSS and an increase in BCSM for each stage increase. The superior fit of the AJCC 8th edition staging system makes it a useful tool to discuss survival for this population. Our findings support the concept that biological factors rather than lower nodal burden are the primary driver of survival in N1mic breast cancer.
Breast cancer is predominantly a disease of aging, and increased age has a direct effect on non-breast cancer mortality. In our study, of the 493 death events, 47.5% of the deaths were from breast cancer, while 52.5% were non-cancer mortality or deaths from other cancers, which was similar to the findings reported in previous studies (29, 30). Therefore, in addition to the Cox regression model, we used the competing-risks regression model to avoid overestimation of the risk of BCSM (31), while similar results were found between the Cox regression model and the competing-risks regression model.
There is a paucity of prospective studies to answer the question regarding the effect of PMRT in T1-2N1micM0 breast cancer. In our study, patients with adverse prognostic factors such as younger age, grade III, larger tumor size, and PR-negative status were more like to receive PMRT. Regarding the pathological prognostic stages, patients with advanced stages were more likely to receive PMRT, suggesting that biological factors were also essential indicators to support the decision to administer PMRT. However, whether PMRT had an impact on the survival of T1-2N1micM0 patients remains controversial. The current guidelines of breast cancer recommend strong consideration of PMRT for patients with T1-2N1 (one to three positive axillary nodes) breast cancer after mastectomy, but whether or not N1mic contributes to the positive lymph node count is uncertain (1). Several studies have attempted to answer this question. Mamtani et al. studied 141 N1mic patients who received mastectomy, most of them received appropriate multidisciplinary treatment, including chemotherapy (95%), anti-HER2 targeted therapy (92% of patients with HER2 positive), and endocrine therapy (96% of patients with ER-positive), and they reported that PMRT was not associated with lower locoregional recurrence (LRR) rate (21). Another large cohort study from the MD Anderson Cancer Center (MDACC) found no difference in the LRR rate among N1mic patients with and without PMRT (22). The National Cancer Database included 14019 T1-2N1micM0 patients who underwent mastectomy, and the probability of PMRT receipt (18.5%) and chemotherapy receipt (59.4%) was similar to those reported in our study (23). The results showed that PMRT conferred no benefit to the OS regardless of patient age, hormone receptor status, and tumor grade (23). Moreover, another study by Patel et al. included 5,878 patients from the SEER database, 20% of whom were treated with PMRT. The results showed that PMRT was not associated with better BCSS and OS (24). Finally, in a multicentric cohort study investigated French patients with N0-1mic breast cancer, more than half of them were treated with PMRT. The results indicated that PMRT was not related to improvement in the survival outcomes irrespective of the number of associated recurrence risk factors (25). In our study, 22.5% of patients received PMRT, and PMRT administration did not lead to any significant effect on the BCSS. Thus, none of the abovementioned studies support the significant effects of PMRT in the LRR, BCSS, and OS of N1mic patients.
We further analyzed the differences in patient survival with and without PMRT across different stages as patient survival significantly differed between the two staging systems used. However, although the 5-year BCSM in stages IIB and IIIA reached 13.2 and 25.4%, respectively, PMRT did not improve survival in these stages. The purpose of PMRT was to reduce the LRR and to improve survival (32). The insignificant improvement of survival in different pathological prognostic subgroups may be related to the extremely low LRR rate. The SEER database does not record LRR information. However, several previous studies have investigated the LRR rate in T1-2N1micM0 patients. Mamtani et al. showed that only 3.5% of 141 patients who did not receive PMRT developed LRR (21). The results from the MDACC also showed a 10-year LRR of 3.8% in patients who underwent sentinel lymph node biopsy alone with no PMRT (22). The results from a French multicentric cohort study also showed only 1% of 5-year LRR in patients with N0-1mic breast cancer (25). Similarly, Bazan et al. reported low event rates in N1mic patients after mastectomy, and the 6-year LRR and distant metastasis rates were 0 and 5.8%, respectively, and the LRR and distant metastasis rates showed no significant association with systemic therapy (33). However, we should note that the small number of N1mic patients enrolled in the abovementioned studies. Thus, it was difficult to evaluate the risk of LRR based on biological factors. In our previous study, we performed multigene panel testing based on the 21-gene recurrence score and found that PMRT did not improve the survival of patients with T1-2N1micM0, but only ER-positive and HER2-negative patients were included in this study (34). Appropriate identification of patients with excellent or inferior outcomes is key to identifying patients who may be offered de-escalating and escalating treatment strategies, respectively. Therefore, in the future, more studies are needed to explore the impact of new pathological prognostic staging on decision-making of PMRT in this population.
This is the first study to validate the prognostic effect and determine the survival benefit of PMRT in T1-2N1micM0 breast cancer according to the 8th AJCC pathological prognostic staging system. Although we included a large population-based cohort of patients, our study is limited by its retrospective nature and the potential for selection bias. Second, the SEER database does not include complete details on the specifics of the systemic treatments administered. However, the survival trends by the 8th AJCC pathological prognostic staging system observed in our study indicated that most of the patients included in the analysis should also receive appropriate multidisciplinary treatment. Third, the follow-up time in our study was relatively short, which may impact the prognostic and predictive effect of the pathological prognostic stages for this favorable cohort. Furthermore, our findings are not generalizable to the populations of low- and middle-income countries, wherein routine testing of molecular markers and anti-HER2-targeted therapy may not be available. Finally, the SEER database lacks information on locoregional and distant recurrence data, which has a defined correlation with PMRT in this population.
In conclusion, the 8th AJCC breast cancer pathological prognostic staging system downstaged 84.4% of patients with T1-2N1micM0 disease and could provide more accurate predictions for the survival outcome compared to the AJCC 7th edition staging system. However, our study does not support the use of pathological prognostic staging as a guideline to offer PMRT. Thus, long-term follow-up studies are necessary to further study the role of PMRT in this population.
Publicly available datasets were analyzed in this study. This data can be found here: www.seer.cancer.gov.
JS, C-LL, FC, Z-HY, and S-GW are lead authors who participated in the manuscript drafting, table/figure creation, and manuscript revision. S-GW and Z-HY aided in the data collection. JW, JL, PZ, and LH are senior authors who aided in drafting the manuscript and manuscript revision. Z-YH and S-GW are the corresponding authors who initially developed the concept and drafted and revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was partly supported by the National Natural Science Foundation of China (No. 81872459), the Commission Young and Middle-aged Talents Training Project of Fujian Health Commission (No. 2019-ZQNB-25), and the Natural Science Foundation of Guangdong Province (Nos. 2018A030313666 and 2017A030310422).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. NCCN. NCCN Clinical Practice Guidelines in Oncology V.2.2019(2019). Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (Accessed Access AUG 21, 2019).
2. Chavez-MacGregor M, Mittendorf EA, Clarke CA, Lichtensztajn DY, Hunt KK, Giordano SH. Incorporating Tumor Characteristics to the American Joint Committee on Cancer Breast Cancer Staging System. Oncologist (2017) 22(11):1292–300. doi: 10.1634/theoncologist.2017-0116
3. Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, et al. Tailoring therapies–improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol (2015) 26(8):1533–46. doi: 10.1093/annonc/mdv221
4. Krop I, Ismaila N, Stearns V. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early-Stage Invasive Breast Cancer: American Society of Clinical Oncology Clinical Practice Focused Update Guideline Summary. J Oncol Pract (2017) 13(11):763–6. doi: 10.1200/JOP.2017.024646
5. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al AJCC cancer staging manual. New York: Springer International Publishing (2018).
6. Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin (2017) 67(4):290–303. doi: 10.3322/caac.21393
7. Lee SB, Sohn G, Kim J, Chung IY, Lee JW, Kim HJ, et al. A retrospective prognostic evaluation analysis using the 8th edition of the American Joint Committee on Cancer staging system for breast cancer. Breast Cancer Res Treat (2018) 169(2):257–66. doi: 10.1007/s10549-018-4682-5
8. Plichta JK, Ren Y, Thomas SM, Greenup RA, Fayanju OM, Rosenberger LH, et al. Implications for Breast Cancer Restaging Based on the 8th Edition AJCC Staging Manual. Ann Surg (2020) 271(1):169–76. doi: 10.1097/SLA.0000000000003071
9. Kim I, Choi HJ, Ryu JM, Lee SK, Yu JH, Kim SW, et al. Prognostic Validation of the American Joint Committee on Cancer 8th Staging System in 24,014 Korean Patients with Breast Cancer. J Breast Cancer (2018) 21(2):173–81. doi: 10.4048/jbc.2018.21.2.173
10. Weiss A, Chavez-MacGregor M, Lichtensztajn DY, Yi M, Tadros A, Hortobagyi GN, et al. Validation Study of the American Joint Committee on Cancer Eighth Edition Prognostic Stage Compared With the Anatomic Stage in Breast Cancer. JAMA Oncol (2018) 4(2):203–9. doi: 10.1001/jamaoncol.2017.4298
11. Maaskant AJ, van de Poll-Franse LV, Voogd AC, Coebergh JW, Tutein Nolthenius-Puylaert MC, Nieuwenhuijzen GA. Stage migration due to introduction of the sentinel node procedure: a population-based study. Breast Cancer Res Treat (2009) 113(1):173–9. doi: 10.1007/s10549-008-9913-8
12. van der Heiden-van der Loo M, Bezemer PD, Hennipman A, Siesling S, van Diest PJ, Bongers V, et al. Introduction of sentinel node biopsy and stage migration of breast cancer. Eur J Surg Oncol (2006) 32(7):710–4. doi: 10.1016/j.ejso.2006.04.001
13. Salhab M, Patani N, Mokbel K. Sentinel lymph node micrometastasis in human breast cancer: an update. Surg Oncol (2011) 20(4):e195–206. doi: 10.1016/j.suronc.2011.06.006
14. Mittendorf EA, Ballman KV, McCall LM, Yi M, Sahin AA, Bedrosian I, et al. Evaluation of the stage IB designation of the American Joint Committee on Cancer staging system in breast cancer. J Clin Oncol (2015) 33(10):1119–27. doi: 10.1200/JCO.2014.57.2958
15. Montagna E, Viale G, Rotmensz N, Maisonneuve P, Galimberti V, Luini A, et al. Minimal axillary lymph node involvement in breast cancer has different prognostic implications according to the staging procedure. Breast Cancer Res Treat (2009) 118(2):385–94. doi: 10.1007/s10549-009-0446-6
16. de Boer M, van Dijck JA, Bult P, Borm GF, Tjan-Heijnen VC. Breast cancer prognosis and occult lymph node metastases, isolated tumor cells, and micrometastases. J Natl Cancer Inst (2010) 102(6):410–25. doi: 10.1093/jnci/djq008
17. Weaver DL, Ashikaga T, Krag DN, Skelly JM, Anderson SJ, Harlow SP, et al. Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med (2011) 364(5):412–21. doi: 10.1056/NEJMoa1008108
18. Iqbal J, Ginsburg O, Giannakeas V, Rochon PA, Semple JL, Narod SA. The impact of nodal micrometastasis on mortality among women with early-stage breast cancer. Breast Cancer Res Treat (2017) 161(1):103–15. doi: 10.1007/s10549-016-4015-5
19. de Boer M, van Deurzen CH, van Dijck JA, Borm GF, van Diest PJ, Adang EM, et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med (2009) 361(7):653–63. doi: 10.1056/NEJMoa0904832
20. Dutta SW, Volaric A, Morgan JT, Chinn Z, Atkins KA, Janowski EM. Pathologic Evaluation and Prognostic Implications of Nodal Micrometastases in Breast Cancer. Semin Radiat Oncol (2019) 29(2):102–10. doi: 10.1016/j.semradonc.2018.11.001
21. Mamtani A, Patil S, Stempel M, Morrow M. Axillary Micrometastases and Isolated Tumor Cells Are Not an Indication for Post-mastectomy Radiotherapy in Stage 1 and 2 Breast Cancer. Ann Surg Oncol (2017) 24(8):2182–8. doi: 10.1245/s10434-017-5866-7
22. FitzSullivan E, Bassett RL, Kuerer HM, Mittendorf EA, Yi M, Hunt KK, et al. Outcomes of Sentinel Lymph Node-Positive Breast Cancer Patients Treated with Mastectomy Without Axillary Therapy. Ann Surg Oncol (2017) 24(3):652–9. doi: 10.1245/s10434-016-5605-5
23. Wu SP, Tam M, Shaikh F, Lee A, Chun J, Schnabel F, et al. Post-mastectomy Radiation Therapy in Breast Cancer Patients with Nodal Micrometastases. Ann Surg Oncol (2018) 25(9):2620–31. doi: 10.1245/s10434-018-6632-1
24. Patel MA, Li C, Aronson J, Howie C, Maraboyina S, Prabhu AV, et al. The Effect of Post Mastectomy Radiation Therapy on Survival in Breast Cancer Patients with N1mic Disease. Breast (2020) 51:50–6. doi: 10.1016/j.breast.2020.02.009
25. Forissier V, Tallet A, Cohen M, Classe JM, Reyal F, Chopin N, et al. Is post-mastectomy radiation therapy contributive in pN0-1mi breast cancer patients? Results of a French multi-centric cohort. Eur J Cancer (2017) 87:47–57. doi: 10.1016/j.ejca.2017.10.004
26. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 Sub (1975-2016 varying) - Linked To County Attributes - Total U.S., 1969-2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission.
27. Shao N, Xie C, Shi Y, Ye R, Long J, Shi H, et al. Comparison of the 7th and 8th edition of American Joint Committee on Cancer (AJCC) staging systems for breast cancer patients: a Surveillance, Epidemiology and End Results (SEER) Analysis. Cancer Manag Res (2019) 11:1433–42. doi: 10.2147/CMAR.S185212
28. Bae HW, Yoon KH, Kim JH, Lim SM, Kim JY, Park HS, et al. Impact of Micrometastatic Axillary Nodes on Survival of Breast Cancer Patients with Tumors ≤2 cm. World J Surg (2018) 42(12):3969–78. doi: 10.1007/s00268-018-4725-4
29. Derks MGM, Bastiaannet E, van de Water W, de Glas NA, Seynaeve C, Putter H, et al. Impact of age on breast cancer mortality and competing causes of death at 10 years follow-up in the adjuvant TEAM trial. Eur J Cancer (2018) 99:1–8. doi: 10.1016/j.ejca.2018.04.009
30. Wasif N, Neville M, Gray R, Cronin P, Pockaj BA. Competing Risk of Death in Elderly Patients with Newly Diagnosed Stage I Breast Cancer. J Am Coll Surg (2019) 229(1):30–6.e1. doi: 10.1016/j.jamcollsurg.2019.03.013
31. de Glas NA, Kiderlen M, Vandenbroucke JP, de Craen AJ, Portielje JE, van de Velde CJ, et al. Performing Survival Analyses in the Presence of Competing Risks: A Clinical Example in Older Breast Cancer Patients. J Natl Cancer Inst (2015) 108(5):djv366. doi: 10.1093/jnci/djv366
32. Recht A, Comen EA, Fine RE, Fleming GF, Hardenbergh PH, Ho AY, et al. Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. Pract Radiat Oncol (2016) 6(6):e219–34. doi: 10.1016/j.prro.2016.08.009
33. Bazan JG, Majithia L, Quick AM, Wobb JL, Terando AM, Agnese DM, et al. Heterogeneity in Outcomes of Pathologic T1-2N1 Breast Cancer After Mastectomy: Looking Beyond Locoregional Failure Rates. Ann Surg Oncol (2018) 25(8):2288–95. doi: 10.1245/s10434-018-6565-8
Keywords: breast cancer, nodal micrometastasis, AJCC staging, mastectomy, radiotherapy
Citation: Shi J, Lian C-L, Chi F, Zhou P, Lei J, Hua L, Wang J, He Z-Y and Wu S-G (2020) Prognostic and Predictive Value of the American Joint Committee on Cancer Pathological Prognostic Staging System in Nodal Micrometastatic Breast Cancer. Front. Oncol. 10:570175. doi: 10.3389/fonc.2020.570175
Received: 06 June 2020; Accepted: 16 November 2020;
Published: 18 December 2020.
Edited by:
Shengtao Zhou, Sichuan University, ChinaReviewed by:
Qi-Jun Wu, ShengJing Hospital of China Medical University, ChinaCopyright © 2020 Shi, Lian, Chi, Zhou, Lei, Hua, Wang, He and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen-Yu He, aGV6aHlAc3lzdWNjLm9yZy5jbg==; San-Gang Wu, dW5vd3UxMjM0NUBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.