
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 11 February 2021
Sec. Thoracic Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.569715
This article is part of the Research TopicIssues and Challenges in NSCLC ImmunotherapyView all 26 articles
 Daniel Morgensztern1*
Daniel Morgensztern1* Manuel Cobo Dols2
Manuel Cobo Dols2 Santiago Ponce Aix3
Santiago Ponce Aix3 Pieter E. Postmus4
Pieter E. Postmus4 Jaafar Bennouna5
Jaafar Bennouna5 Jürgen R. Fischer6
Jürgen R. Fischer6 Oscar Juan-Vidal7
Oscar Juan-Vidal7 David J. Stewart8
David J. Stewart8 Andrea Ardizzoni9
Andrea Ardizzoni9 Rafia Bhore10
Rafia Bhore10 Marianne Wolfsteiner11
Marianne Wolfsteiner11 Martin Reck12
Martin Reck12 Denis Talbot13
Denis Talbot13 Ramaswamy Govindan1
Ramaswamy Govindan1 Teng Jin Ong10 on behalf of the ABOUND.2L+ investigators
Teng Jin Ong10 on behalf of the ABOUND.2L+ investigatorsBackground: The standard therapy for advanced stage non-small cell lung cancer (NSCLC) with no actionable gene alterations is a platinum-based chemotherapy doublet and immune checkpoint blocker (ICB), either concurrently or sequentially, followed by docetaxel at the time of tumor progression. However, more effective treatments are needed. We evaluated the nab-paclitaxel and durvalumab combination in patients with previously treated advanced stage NSCLC.
Methods: Patients with advanced stage NSCLC previously treated with one line of platinum-based doublet with or without an ICB and no activating EGFR mutations or ALK translocations received nab-paclitaxel 100 mg/m2 (days 1 and 8) plus durvalumab 1,125 mg (day 15) every 21 days. The primary endpoint was progression-free survival (PFS). Key secondary endpoints included overall survival (OS) and safety.
Results: Between February 2016 and December 2016, 79 patients were enrolled. The median age was 63 years. Most patients were males (68.4%), had non-squamous histology (69.6%), and had no prior ICB treatment (88.6%). The median PFS was 4.5 months; median OS was 10.1 months. A post hoc analysis of survival by prior ICB treatment revealed a median PFS and OS of 4.4 and 9.9 months, respectively, in ICB-naive patients and 6.9 months and not estimable, respectively, in patients previously treated with ICB. The most common treatment-emergent adverse events were asthenia (46.2%) and diarrhea (34.6%); four treatment-related deaths (5.1%) occurred.
Conclusions: The nab-paclitaxel and durvalumab combination is feasible and demonstrated antitumor activity without new safety signals. Additional studies using taxanes and ICB in patients with previously treated NSCLC are warranted.
Clinical Trial Registration: ClinicalTrials.gov registration (NCT02250326).
EudraCT number: 2014-001105-41
The standard initial therapy for patients with advanced stage non-small cell lung cancer (NSCLC) and no actionable gene alterations includes platinum-based chemotherapy doublet and immune checkpoint blockers (ICB) either sequentially or concurrently (1, 2). For patients previously treated with chemotherapy alone, monoclonal antibodies against programmed death-1 (PD-1) or its ligand (PD-L1) are associated with improved overall survival (OS) when compared to docetaxel in the second-line setting, although prolonged benefit is observed only in a small percentage of patients (3–6). For those already treated with both chemotherapy and ICB, docetaxel with or without ramucirumab remains the standard option (7). Response rates and survival, however, remain poor for the majority of patients treated with second-line ICB monotherapy or docetaxel, indicating the need for new treatment options.
Nanoparticle albumin-bound paclitaxel (nab-paclitaxel), a cremophor-free formulation that can be administered without dexamethasone premedication (8), is one of the recommended drugs for locally advanced or metastatic NSCLC in combination with carboplatin, with or without pembrolizumab, in the first-line setting for patients who are not candidates for curative surgery or radiation therapy (2, 9). Single-agent nab-paclitaxel has been associated with promising results in previously treated patients with metastatic NSCLC (10, 11) and better tolerability compared with docetaxel in a randomized clinical trial for patients with metastatic breast cancer (12).
Durvalumab is a human IgG1 monoclonal antibody against PD-L1, approved as consolidation therapy after chemoradiation in patients with stage III NSCLC (13). In patients with advanced stage NSCLC, single-agent durvalumab is associated with similar activity and safety profiles when compared with other ICBs (14).
Based on both preclinical (15) and clinical (16–18) studies demonstrating a benefit from concurrent chemotherapy and ICB in NSCLC, we postulated that the same principles may apply to patients treated with nab-paclitaxel after progression on platinum-based chemotherapy with or without ICB.
ABOUND.2L+ was a randomized clinical trial comparing nab-paclitaxel alone or in combination with CC-486, an oral formulation of azacitidine (19). The study showed no benefit from the addition of azacitidine to nab-paclitaxel in the randomized cohorts of the study, although single-agent nab-paclitaxel was associated with a tolerable safety profile and promising outcomes, including response rates, median progression-free survival (PFS), and median OS of 16.3%, 4.2, and 17.0 months, respectively. Here we present the results of the third arm of the study evaluating the combination of nab-paclitaxel and durvalumab, which was non-randomized and added as an amendment.
Eligible patients were 18 years of age or older and had histologically or cytologically confirmed advanced stage NSCLC, radiologically documented measurable disease by Response Evaluation Criteria In Solid Tumors (RECIST) 1.1, an Eastern Cooperative Oncology Group performance status 0 or 1, adequate hematologic, renal, and hepatic function, and no other current active malignancy requiring anticancer therapy. One prior line of platinum-based chemotherapy regimen for metastatic or recurrent disease was allowed, with the exception of taxanes, which were allowed only if used in the adjuvant setting more than 12 months prior to enrollment into the trial. Prior use of ICBs, either as a component of the first-line therapy or in the second line, was allowed. Key exclusion criteria included known activating EGFR mutations or ALK translocations, peripheral neuropathy grade 2 or higher by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0, active or prior documented autoimmune or inflammatory disorder, use of systemic immunosuppressive therapy within 14 days from starting durvalumab except for corticosteroids, at doses up to 10 mg per day of prednisone or its equivalent, and brain metastases unless asymptomatic and clinically stable for at least 8 weeks following completion of therapy.
The study was approved by the institutional review board or independent ethics committee at participating sites and conducted in accordance with the principles of good clinical practice and the Declaration of Helsinki. All patients provided written informed consent prior to treatment initiation.
This was an open-label phase II study. Initially, patients were randomized 1:1 to receive nab-paclitaxel 100 mg/m2 on days 8 and 15 plus CC-486 200 mg on days 1 to 14 or nab-paclitaxel 100 mg/m2 alone on days 1 and 8 of each 21-day cycle (19). After enrollment for the nab-paclitaxel alone and nab-paclitaxel plus CC-486 arms was completed, the protocol was amended to include a third arm, which enrolled patients with advanced stage non-squamous or squamous NSCLC and one prior platinum-based chemotherapy. Patients were assigned to this arm and received nab-paclitaxel 100 mg/m2 infused over 30 min on days 1 and 8 plus durvalumab 1,125 mg infused over 1 h on day 15, with the cycles repeating every 21 days. Hence, randomization did not occur between the nab-paclitaxel plus durvalumab and nab-paclitaxel alone arms. Treatment was continued until documented tumor progression, unacceptable toxicity, withdrawal of consent, lost to follow-up, or death.
The primary endpoint was the duration of PFS in the intent-to-treat (ITT) population, defined as the time from date of treatment initiation to the date of disease progression, based on investigator assessment using RECIST version 1.1, or death from any cause. Secondary endpoints included OS, defined as the time between the first treatment and death from any cause, overall response rate (ORR), disease control rate (DCR), and safety. Imaging studies with computer tomography scans were performed at baseline and every 42 days until treatment discontinuation. All patients who received at least one treatment dose underwent safety analysis, with documentation of treatment-emergent adverse events (TEAEs) graded based on NCI-CTCAE version 4.0.
The median PFS and median OS were estimated using the Kaplan-Meier estimates with corresponding two-sided 95% confidence intervals (CI). The sample size estimation was based on the expected median PFS of 4.25 months for nab-paclitaxel plus durvalumab and 2.5 months for nab-paclitaxel alone based on historical data with docetaxel alone (7, 20, 21).
In the randomized part of the trial, it was estimated that a total of 160 patients would be needed to observe 120 PFS events, which would have provided 80% power to detect a hazard ratio of 0.60 using a one-sided test at the 2.5% level of significance. After enrollment in the nab-paclitaxel plus CC-486 and nab-paclitaxel monotherapy arms was completed (each arm had reached a total of approximately 80 patients), all patients were assigned to the nab-paclitaxel plus durvalumab arm until approximately 80 patients were enrolled in that arm. The statistical assumptions used were identical to the nab-paclitaxel plus CC-486 arm. An interim analysis for PFS comparing the nab-paclitaxel plus durvalumab and nab-paclitaxel monotherapy arms was conducted when approximately 30 PFS events were observed in the nab-paclitaxel plus durvalumab combination arm.
Between September 2016 and December 2016, 99 patients were screened and 79 were enrolled into the study (Figure 1). The median age was 63 years (range 29–84 years); most patient were males (68.4%) and had non-squamous histology (69.6%) and no prior use of ICB (88.6%) (Table 1). Prior chemotherapies included a platinum (97.5%), pemetrexed (50.6%), vinorelbine (25.3%), and gemcitabine (26.6%). The median duration of prior platinum plus pemetrexed (39 patients) was 10.4 weeks (range 1.6–43.4 weeks). In total, nine patients (11.4%) received prior ICB, which was their most immediate prior line of therapy. Among these nine patients, six received nivolumab (66.7%), two received pembrolizumab (22.2%), and one received avelumab (11.1%), with the latter in the first-line setting. Of these nine patients, eight (88.9%) received prior ICB monotherapy. The remaining patient (11.1%) received prior combination therapy with ICB and carboplatin. One patient did not receive the study treatment. In total, 63 patients (80.8%) discontinued treatment due to progressive disease (36 [46.2%]), death (12 [15.4%]), patient withdrawal (5 [6.4%]), clinical progression (4 [5.1%]), adverse events (4 [5.1%]), and other reasons (2 [2.6%]). The adverse events (AEs) leading to nab-paclitaxel and durvalumab discontinuation were pneumonitis, urinary tract infection, and Pneumocystis jirovecii pneumonia (one patient each) and increased white blood cell count, abnormal liver function, localized edema, and peripheral edema (one patient). The median follow-up for survival was 12.9 months.
For the primary analysis of investigator-assessed PFS in the ITT population, 56 patients (70.9%) had progressive disease (PD) or died. The median PFS was 4.5 months (95% CI, 3.5–5.9 months), with an estimated PFS rate at 12 months of 25.7% (95% CI, 16.3–36.2%; Figure 2A).
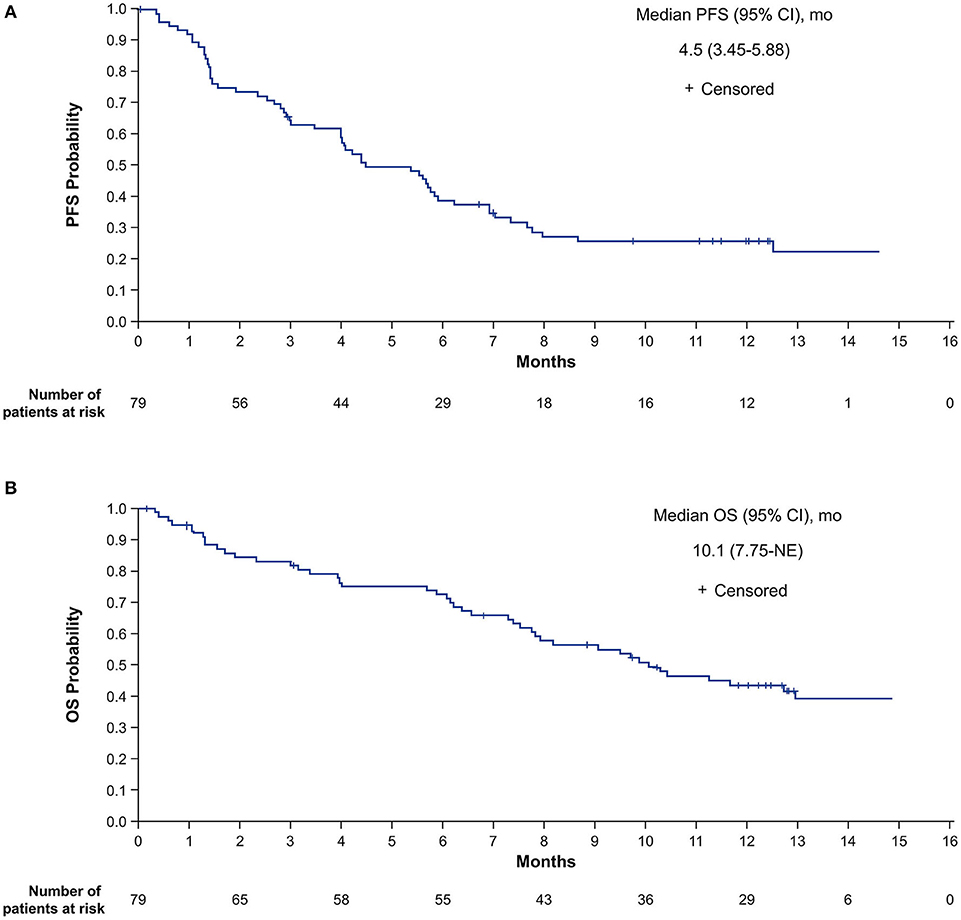
Figure 2. Investigator-assessed PFS (A) and OS (B) in the intent-to-treat population. NE, not estimable; OS, overall survival; PFS, progression-free survival.
For the OS analysis in the ITT population, 44 patients (55.7%) had died. The median OS was 10.1 months (95% CI, 7.8 months-not estimable [NE]), with estimated survival at 12 months of 43.8% (95% CI, 32.3–54.7%; Figure 2B).
The ORR was 27.8% (95% CI, 18.3–39.1%), with complete response (CR) in one patient (1.3%) and partial response (PR) in 21 patients (26.6%). The DCR was 70.9% (95% CI, 59.6–80.6%), with 34 patients (43.0%) achieving stable disease (SD).
Due to the heterogeneity of the patient population, a post hoc analysis was performed to evaluate outcomes according to prior ICB treatment and histology in the 78 patients with known histology. The median PFS was 4.4 months (95% CI, 2.96–5.68 months) in ICB-naive patients and 6.9 months (95% CI, 1.38 months-NE) in patients previously treated with ICB (Figure 3A). Among ICB-naive patients, the median PFS was 5.6 months (95% CI, 1.3–7.8 months) in those with squamous histology and 4.1 months (2.7–5.7 months) in those with non-squamous histology, with corresponding 12-month PFS of 27.1% (95% CI, 9.0–49.2%) and 20.9% (95% CI, 10.6–33.6%), respectively (Figure 3B). The median OS was 9.9 months (95% CI, 7.52–12.94 months) in ICB-naive patients and NE in those previously treated with ICB (Figure 3C). Among ICB-naive patients, the median OS for squamous and non-squamous histologies was 8.9 months (95% CI, 2.99 months-NE) and 10.3 months (95% CI, 6.57 months-NE), respectively (Figure 3D).
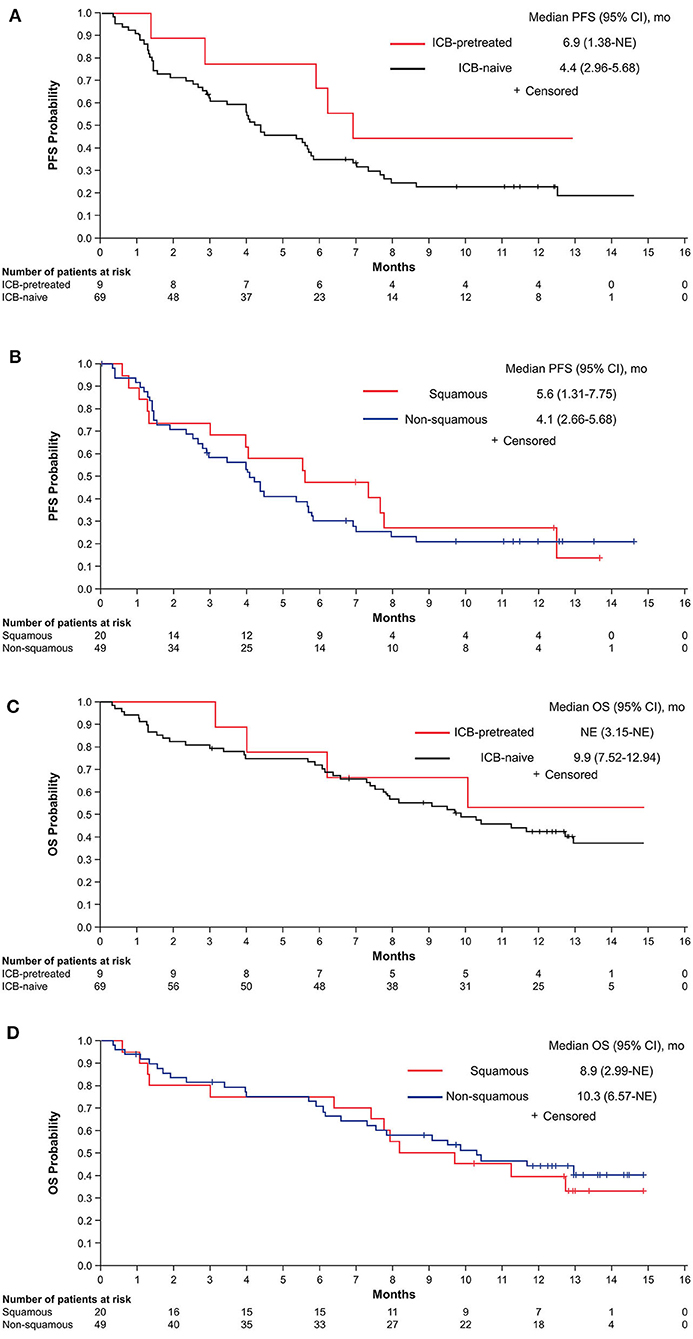
Figure 3. PFS by ICB treatment status (A) and in ICB-naive patients according to histology (B) and OS by ICB treatment status (C) and in ICB-naive patients according to histology (D). ICB, immune checkpoint blocker; NE, not estimable; OS, overall survival; PFS, progression-free survival.
The median percentage change from baseline in sum of diameters of target lesions was −17.3% (range −100.0 to +65.4%) for ICB-naive patients and −21.4% (range −76.2 to +28.1%) for those previously treated with ICB (Figure 4). Among ICB-naive patients, one achieved CR (1.4%), 17 achieved PR (24.6%), and 30 had SD (43.5%) for a DCR of 69.6%. Of the remaining patients, 10 had PD (14.5%) and 11 (15.9%) had no post-treatment response assessment. Among patients previously treated with ICB, four achieved PR (44.4%), four achieved SD (44.4%), and one had PD (11.1%).
The median number of cycles and treatment duration were 7 (range 1–21) and 24.4 weeks (range 1.4–66.1 weeks), respectively. The median cumulative doses of nab-paclitaxel and durvalumab were 1,250 mg/m2 and 6,750 mg, respectively. Dose reductions for nab-paclitaxel due to toxicity occurred in 11 patients (14.1%); per protocol, durvalumab dose reductions were not allowed. Dose delays of nab-paclitaxel and durvalumab occurred in 39 patients (50%) and 24 patients (30.8%), respectively.
All patients developed at least one TEAE, with grade 3 or 4 TEAEs occurring in 43 patients (55.1%) (Table 2). The most common TEAEs of any grade were asthenia (46.2%), diarrhea (34.6%), and decreased appetite (33.3%), while the most common grade 3 or 4 TEAEs were asthenia (12.8%), dyspnea (7.7%), and pneumonia (7.7%). Peripheral neuropathy was seen in 29 patients (37.2%), of which 3 (3.8%) were grade 3 or 4.
Immune-related TEAEs of grade 3 or 4 were observed in seven patients (9.0%). The grade 3 or 4 immune-related TEAEs were diarrhea (1 [1.3%]), rash (2 [2.6%]), pneumonitis (1 [1.3%]), and adrenal insufficiency (3 [3.8%]). Among the nine patients who received prior ICB, immune-related TEAEs of grade 3 or 4 were observed in two patients (22.2%). The grade 3 or 4 immune-related TEAEs were adrenal insufficiency and rash (1 patient [11.1%] each). Other AEs of interest included grade 1 or 2 dermatitis (10.3%) and thyroid dysfunction (hypothyroidism, 6.4%; hyperthyroidism, 2.6%; thyroiditis, 1.3%).
Overall, four patients (5.1%) experienced a grade 5 TEAE suspected to be treatment related. The specific grade 5 treatment-related TEAEs were pneumonitis, pulmonary hemorrhage, Pneumocystis jirovecii pneumonia, and clinical deterioration.
The median PFS of 4.5 months exceeded the pre-specified threshold, and the response rate of 27.8% is higher than previously described in patients treated with either docetaxel or ICB monotherapy (3–6).
There are increasing data suggesting that the efficacy of conventional chemotherapy drugs relies not only on their cytotoxic effects, but also on the ability to stimulate the immune system. In the case of paclitaxel, there are many postulated mechanisms for its immunostimulatory effects in addition to tumor debulking in case of effective cytotoxic activity, with reduction of the systemic immunosuppression caused by malignant cells. Paclitaxel induces immunogenic cell death through increased chromosomal content, which causes endoplasmic stress response and calreticulin exposure, stimulates toll-like receptor 4 increasing T cell priming by dendritic cells, and depletes myeloid derived suppressor cells (22, 23). Paclitaxel may also increase the antigenicity of cancer cells by stimulating their production of interferon-β, leading to increasing MHC class I expression (24, 25). Another mechanism is the sensitization to cytotoxic T lymphocytes by upregulating mannose-6-phosphate receptors on tumor cells, which increases the permeability of the membrane to granzyme B, leading to cancer cell death independent from perforin (26). Since nab-paclitaxel does not require the use of premedication with corticosteroids, it may be a better partner for combination with ICB when compared with other taxanes since, at least in patients treated with single-agent ICB, use of corticosteroids at doses of 10 mg or higher has been associated with worse outcomes compared with no use within 30 days (27).
There are limited data on the combination of taxanes and ICB without platinum in patients with previously treated NSCLC. In a small phase Ib study evaluating the combination of chemotherapy and nivolumab 10 mg/kg every 3 weeks, there were six patients previously treated with platinum-doublets who were enrolled into the docetaxel arm (28). One patient (16.5%) responded to the treatment, and the median PFS was 3.1 months. All patients developed grade 3 or 4 AEs, which were mostly hematologic.
In our study, the combination of nab-paclitaxel plus durvalumab was generally well-tolerated; however, the 4 grade 5 treatment-related TEAEs were an unexpected finding. Patients in the nab-paclitaxel plus durvalumab arm received more treatment cycles and a greater cumulative dose of nab-paclitaxel compared with those who received nab-paclitaxel with or without CC-486 in the randomized portion of this trial (19). Therefore, it is reasonable to speculate that the grade 5 treatment-related TEAEs were due, at least in part, to a greater treatment exposure with second-line combination therapy. Although there were no grade 5 AEs reported with second-line pembrolizumab plus docetaxel in the phase II PROLUNG trial (29), that study accrued patients considerably younger than those treated with nab-paclitaxel plus durvalumab in the current study (mean, 50.1 vs. 62.7 years).
Our study has several limitations. The durvalumab arm started enrollment after the completion of the randomized nab-paclitaxel with or without CC-486, precluding a more reliable comparison to single-agent nab-paclitaxel, and the increased use of pembrolizumab or atezolizumab in the first-line setting decreased the number of ICB-naive patients eligible for the nab-paclitaxel plus durvalumab combination in the clinical setting (16–18). Furthermore, we did not collect data on PD-L1 status of the tumors or genetic biomarkers, which are known predictors for response to ICBs in previously untreated patients (5, 30), although the role is not clear in patients with resistance to ICBs.
Nevertheless, despite these limitations, our study provides the initial data on the use of nab-paclitaxel plus durvalumab after progression on ICB, a setting with increased relevance since the trial was designed. Despite the multiple ongoing studies evaluating combinations involving antibodies against PD-1 or PD-L1 with other immunostimulatory antibodies, antiangiogenic agents and targeted drugs (31–33), none has an established role in NSCLC patients previously treated with ICBs. Although the number of patients previously treated with ICB in our study was small, the preliminary results are promising, with all but one patient achieving tumor control and a prolonged benefit observed in four of the nine patients.
Since there are limited data on the efficacy of docetaxel after tumor progression on ICB and we cannot clearly separate the effects of nab-paclitaxel and durvalumab, this question could only be addressed in a randomized clinical trial comparing a taxane, either docetaxel or nab-paclitaxel, alone or in combination with an ICB.
The datasets presented in this article are not readily available. Data requests may be submitted to Celgene, A Bristol Myers Squibb Company, at: https://vivli.org/ourmember/celgene/ and must include a description of the research proposal.
Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
All authors satisfied the following criteria: contributed to the conception or design of the research or the acquisition, analysis, or interpretation of data for the research, drafted the manuscript or critically revised it for important intellectual content, gave final approval of the version to be published, agreed to be accountable for all aspects of the research in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This work was supported by Bristol Myers Squibb Company, Princeton, New Jersey. The sponsor was involved in the design of the study as well as in the collection, analysis, and interpretation of the data. The sponsor agreed to the decision to submit the article for publication.
DM has been an advisory board member for AbbVie, Bristol Myers Squibb, PharmaMar, Takeda, Gilead, and Boehringer Ingelheim. PEP has been an advisory board member for AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene (a Bristol Myers Squibb Company), Clovis, Janssen, MSD, and Roche. JB has been an advisory board member for Amgen, AstraZeneca, Bristol Myers Squibb, Novartis, and Roche. OJ-V has been an advisory board member for AstraZeneca, AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Merck Sharp & Dohme, and Roche/Genentech. DS has served as an advisory for Roche Canada; he has received grant support from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene (a Bristol Myers Squibb Company), and Novartis. AA has received honoraria from Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, and MSD. RB and TJO are employees of and hold stock in Bristol Myers Squibb Company. MW is contracted for medical review by Celgene (a Bristol Myers Squibb Company) and was employed by Pharmaceutical Research Associates Inc. (PRA) Health Sciences. MR served as a consultant or advisory for AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene (a Bristol Myers Squibb Company), Lilly, Merck Sharp & Dohme, Pfizer, and Roche. DT has been an advisory board member and received honorarium from Celgene (a Bristol Myers Squibb Company). RG serves in an advisory role for AbbVie, AstraZeneca, Baxalta, Boehringer Ingelheim, Celgene (a Bristol Myers Squibb Company), Merck, MSK, Pfizer, and Roche; he serves as a consultant for AbbVie, ARAID, Astellas, Bristol Myers Squibb, Genentech, and INC Research; he has received honoraria from AbbVie. JF was employed by the company Lungenklinik Löwenstein gGmbH.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank the patients who participated in the study and their families. In addition, the authors thank the investigators, nurses, and research staff across the participating centers.
1. Ettinger DS, Aisner DL, Wood DE, Akerley W, Bauman J, Chang JY, et al. NCCN guidelines insights: non-small cell lung cancer, version 5.2018. J Natl Compr Canc Netw. (2018) 16:807–21. doi: 10.6004/jnccn.2018.0062
2. Brahmer JR, Govindan R, Anders RA, Antonia SJ, Sagorsky S, Davies MJ, et al. The Society for immunotherapy of Cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J Immunother Cancer. (2018) 6:75. doi: 10.1186/s40425-018-0382-2
3. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
4. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
5. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. (2016) 387:1540–50. doi: 10.1016/S0140-6736(15)01281-7
6. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. (2017) 389:255–65. doi: 10.1016/S0140-6736(16)32517-X
7. Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. (2014) 384:665–73. doi: 10.1016/S0140-6736(14)60845-X
8. Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. (2002) 8:1038–44.
9. Hanna N, Johnson D, Temin S, Baker S Jr, Brahmer J, Ellis PM, et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. (2017) 35:3484–515. doi: 10.1200/JCO.2017.74.6065
10. Sakata S, Saeki S, Okamoto I, Otsubo K, Komiya K, Morinaga R, et al. Phase II trial of weekly nab-paclitaxel for previously treated advanced non-small cell lung cancer: Kumamoto thoracic oncology study group (KTOSG) trial 1301. Lung Cancer. (2016) 99:41–5. doi: 10.1016/j.lungcan.2016.06.009
11. Tanaka H, Taima K, Morimoto T, Tanaka Y, Itoga M, Nakamura K, et al. A single-arm phase II study of nab-paclitaxel for patients with chemorefractory non-small cell lung cancer. BMC Cancer. (2017) 17:683. doi: 10.1186/s12885-017-3684-8
12. Gradishar WJ, Krasnojon D, Cheporov S, Makhson AN, Manikhas GM, Clawson A, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. (2009) 27:3611–9. doi: 10.1200/JCO.2008.18.5397
13. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. (2018) 379:2342–50. doi: 10.1056/NEJMoa1809697
14. Garassino MC, Cho BC, Kim JH, Mazieres J, Vansteenkiste J, Lena H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. (2018) 19:521–36. doi: 10.1016/S1470-2045(18)30144-X
15. Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. (2008) 8:59–73. doi: 10.1038/nri2216
16. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
17. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. (2018) 379:2040–51. doi: 10.1056/NEJMoa1810865
18. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. (2018) 378:2288–301. doi: 10.1056/NEJMoa1716948
19. Morgensztern D, Cobo M, Ponce Aix S, Postmus PE, Lewanski CR, Bennouna J, et al. ABOUND.2L+: a randomized phase 2 study of nanoparticle albumin-bound paclitaxel with or without CC-486 as second-line treatment for advanced nonsquamous non-small cell lung cancer NSCLC). Cancer. (2018) 124:4667–75. doi: 10.1002/cncr.31779
20. Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. (2000) 18:2095–103. doi: 10.1200/JCO.2000.18.10.2095
21. Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. (2004) 22:1589–97. doi: 10.1200/JCO.2004.08.163
22. Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. (2015) 28:690–714. doi: 10.1016/j.ccell.2015.10.012
23. Pfannenstiel LW, Lam SS, Emens LA, Jaffee EM, Armstrong TD. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell Immunol. (2010) 263:79–87. doi: 10.1016/j.cellimm.2010.03.001
24. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. (2013) 39:74–88. doi: 10.1016/j.immuni.2013.06.014
25. Wan S, Pestka S, Jubin RG, Lyu YL, Tsai YC, Liu LF. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS One. (2012) 7:e32542. doi: 10.1371/journal.pone.0032542
26. Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. (2010) 120:1111–24. doi: 10.1172/JCI40269
27. Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. (2018) 36:2872–78. doi: 10.1200/JCO.2018.79.0006
28. Kanda S, Goto K, Shiraishi H, Kubo E, Tanaka A, Utsumi H, et al. Safety and efficacy of nivolumab and standard chemotherapy drug combination in patients with advanced non-small-cell lung cancer: a four arms phase Ib study. Ann Oncol. (2016) 27:2242–50. doi: 10.1093/annonc/mdw416
29. Arrieta O, Barron F, Ramirez-Tirado LA, Zatarain-Barron ZL, Cardona AF, Diaz-Garcia D, et al. Efficacy and safety of pembrolizumab plus docetaxel vs docetaxel alone in patients with previously treated advanced non-small cell lung cancer: the PROLUNG phase 2 randomized clinical trial. JAMA Oncol. (2020) 6:856–64. doi: 10.1001/jamaoncol.2020.0409
30. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
31. Melero I, Berman DM, Aznar MA, Korman AJ, Perez Gracia JL, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. (2015) 15:457–72. doi: 10.1038/nrc3973
32. Ott PA, Hodi FS, Kaufman HL, Wigginton JM, Wolchok JD. Combination immunotherapy: a road map. J Immunother Cancer. (2017) 5:16. doi: 10.1186/s40425-017-0218-5
Keywords: advanced stage non-small cell lung cancer, durvalumab, immune checkpoint blocker plus chemotherapy, nab-paclitaxel, second-line therapy
Citation: Morgensztern D, Dols MC, Ponce Aix S, Postmus PE, Bennouna J, Fischer JR, Juan-Vidal O, Stewart DJ, Ardizzoni A, Bhore R, Wolfsteiner M, Reck M, Talbot D, Govindan R and Ong TJ (2021) nab-Paclitaxel Plus Durvalumab in Patients With Previously Treated Advanced Stage Non-small Cell Lung Cancer (ABOUND.2L+). Front. Oncol. 10:569715. doi: 10.3389/fonc.2020.569715
Received: 04 June 2020; Accepted: 14 September 2020;
Published: 11 February 2021.
Edited by:
Qing Zhou, Guangdong Provincial People's Hospital Lung Cancer Institute, ChinaReviewed by:
Vincent Lam, Johns Hopkins University, United StatesCopyright © 2021 Morgensztern, Dols, Ponce Aix, Postmus, Bennouna, Fischer, Juan-Vidal, Stewart, Ardizzoni, Bhore, Wolfsteiner, Reck, Talbot, Govindan and Ong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Morgensztern, ZGFuaWVsbW9yZ2Vuc3p0ZXJuQHd1c3RsLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.