
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 27 November 2020
Sec. Head and Neck Cancer
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.565306
 Alexander M. Knops1*
Alexander M. Knops1* Andrew South2
Andrew South2 Ulrich Rodeck2
Ulrich Rodeck2 Ubaldo Martinez-Outschoorn3
Ubaldo Martinez-Outschoorn3 Larry A. Harshyne4
Larry A. Harshyne4 Jennifer Johnson3
Jennifer Johnson3 Adam J. Luginbuhl5
Adam J. Luginbuhl5 Joseph M. Curry5
Joseph M. Curry5Introduction: The progression and clinical course of head and neck squamous cell carcinoma (HNSCC) relies on complex interactions between cancer and stromal cells in the tumor microenvironment (TME). Among the most abundant of these stromal cells are cancer-associated fibroblasts (CAFs). While their contribution to tumor progression is widely acknowledged, and various CAF-targeted treatments are under development, the relationship between CAF density and the clinicopathologic course of HNSCC has not been clearly defined. Here we examine the published evidence investigating the relationship of cancer-associated fibroblasts to local recurrence and indicators of prognostic significance in HNSCC.
Methods: We conducted a meta-analysis of existing publications that compare the relationship between CAF density, local recurrence, and clinically significant pathologic criteria of disease development (T stage, nodal positivity, clinical stage, vascular invasion, perineural invasion, Ki67 expression, and differentiation). Thirteen studies met the selection criteria, providing a total study population of 926 patients. Forest plots and risk ratios were generated to illustrate overall relationships.
Results: Higher CAF density within the tumor microenvironment is associated with advanced T stage, nodal infiltration, clinical stage, vascular invasion, perineural invasion, Ki67 expression, and differentiation (p <0.05). High CAF density is also associated with increased rates of local recurrence (p <0.001).
Conclusions: Across multiple studies, increased CAF density is correlated with histopathological criteria of poor prognosis in HNSCC. These findings highlight that CAFs may play a pivotal role in HNSCC development and progression. Staining for CAFs may represent a valuable addition to current pathologic analysis and help to guide prognosis and treatment. Understanding the mechanisms by which CAFs reciprocally interact with cancer cells will be crucial for optimization of TME-focused treatment of HNSCC.
Over the last thirty years, multiple lines of evidence have illuminated the importance of the tumor microenvironment (TME) to the development of solid malignancies. Tumor cells and the components of the TME bidirectionally communicate to support neoplastic growth and expansion (1–4). Elements of the TME evolve over time and are reprogrammed to cater for the needs of a growing tumor (5, 6). These observations have changed our understanding of cancer progression and behavior. Once thought to be an isolated group of diseased cells, many malignant tumors, including Head and Neck Squamous Cell Carcinomas (HNSCC), are now regarded as a sophisticated and dynamic tissue consisting of multiple cell types (7–11). Cancer-associated fibroblasts (CAFs) are among the most abundant cells within this tissue.
During tumorigenesis, CAFs develop alongside epithelial cancer cells and adopt a distinct, activated, phenotype (12). As fibroblasts become activated, they express alpha-smooth muscle actin (α-SMA), signifying a myofibroblast phenotype and contractile function, similar to fibroblasts participating in wound healing (13, 14). In solid tumors, historically described as “wounds that do not heal”, these fibroblasts remain persistently activated as CAFs (15). CAFs modulate the TME via the secretion of autocrine and paracrine cytokines, as well as extracellular matrix components that provide scaffolds to support the growing tumor cell nests. Many of the classic “hallmarks of malignancy”, as well as emerging hallmarks such as immune evasion and metabolic compartmentalization, are influenced by the presence and functional states of CAFs (6, 16–19).
Similar to other solid cancers, the presence of CAFs in the tumor stroma of HNSCC has been associated with poor survival (20–22). It has been suggested that α-SMA staining is a better predictor for disease mortality than traditional TNM staging (23). However, other studies have not found an association with survival (24, 25).
In this meta-analysis, we sought to synthesize the existing literature to better define the relationship of CAF density on indicators of HNSCC aggressiveness and prognosis as defined by tumor stage and specific histologic findings on tumor biopsy (26, 27). By defining the significance of CAF density within a prognostic framework, utilization of CAFs in clinical medicine can be optimized.
A PubMed search using the following keywords was performed: (“Cancer Associated Fibroblasts” OR “CAFs”) AND (“squamous cell carcinoma”). Preferred Reporting Items Systematic Reviews and Meta-Analyses (PRIMSA) recommendations were followed (28, 29). Articles found from the search were selected for further analysis according to the following criteria: (1) English language; (2) human subjects; (3) squamous cell carcinoma of the head and neck; (4) measurement of CAFs using either alpha-smooth muscle actin (α-SMA) or Fibroblast Activation Protein (FAP) via immunohistochemistry; (5) available data on one or more of the following clinical and pathologic characteristics: T stage, N stage, clinical stage, vascular invasion, perineural invasion, Ki67, differentiation, and recurrence. After identification, screening, and determination of eligibility, 13 studies were included in the meta-analysis (Figure 1) (22, 24, 25, 28–38). Based on the methods of the selected studies, as well as to facilitate analysis with dichotomous variables, the pathologic markers were grouped in the following manner: advanced T stage included T3 or T4 lesions; nodal positivity included all tumors staged N1–3; clinical stages of III and IV were classified as advanced; poor differentiation was applied to only those samples truly graded as poor; vascular invasion was deemed to be positive or not positive; Ki67 staining was graded as high or low based on a cutoff percentage of positive staining (range: 26–32.4%); perineural invasion was noted to be present or not present; recurrence was either present or not present. No standard scoring system exists to assess the density of CAFs with particular samples. However, all studies included in this analysis used semi-quantitative methods, based on intensity and/or extent of positive staining, to assess and classify CAF density. The methods utilized by each study are noted in Table 1. In this analysis, samples with intermediate and high density of CAFs, as determined by the individual study, were grouped together into a high CAF group. This group was compared to a low CAF group, with weak or negative staining in the tumor samples. Correction for multiple testing was not performed. Forest Plots were generated using the Review Manager 5.3 Software (40). Plot specifications were dichotomous for data type, fixed effect for analysis method, and risk ratio for effect measure.
Characteristics of the 13 studies included in this analysis are shown in Table 1. These studies represent a population of 926 patients with surgically resected samples. Twelve studies (897 patients) used samples from oral squamous cell carcinoma, with one of the twelve limiting their analysis to advanced (T3 or T4) oral samples (31). The remaining study evaluated 29 patients with various Head and Neck SCC primary sites including sinonasal, oral, and laryngeal (34). Six studies reported clinical stage according to guidelines from the Union for International Cancer Control and American Joint Committee on Cancer, while three studies reported TNM stage without specifying which staging guidelines were utilized. All studies utilized samples from treatment-naïve patients. All thirteen studies assessed α-SMA expression to determine CAF presence.
Higher density of CAFs in the tumor microenvironment is associated with stages T3 and T4 HNSCC (Figure 2). Seven studies examined the relationship of CAF density and T stage; all seven showed higher density of CAFs was related to increased risk of high T stage tumors. The combined risk ratio of the studies was 1.82 (95%CI = 1.43–2.32). This relationship was statistically significant (p <0.001). The rate of advanced T stage in samples with high CAF density was 42.2%, compared to 28.7% of those with low CAF density.
High density of CAFs in the primary tumor TME is associated with increased rates of tumor cell dissemination to regional lymph nodes (Figure 3). The relationship of CAFs and nodal infiltration was examined in twelve of the thirteen selected studies and eleven of the studies demonstrated a positive relationship of CAF density and nodal invasion. The cumulative risk ratio of these studies was 1.82 (95%CI = 1.50–2.22, p <0.001). The average rate of nodal positivity in high CAF samples was 49.3%, compared to 29.0% of low CAF tumors.
High density of CAFs within the microenvironment correlated with advanced clinical staging of HNSCC tumors (TNM classification) (Figure 4). Nine studies examined this relationship and all nine demonstrated higher TNM classification in those tumors with high CAF density. The cumulative risk ratio was 1.82 (95%CI = 1.54–2.16, p <0.001). The average rate of advanced clinical stage was 67.8% in tumors with high CAF density. In tumors with low CAF density, the average rate was 41.1%.
Similarly, CAF density is associated with increased rates of vascular invasion (Figure 5). Two studies examined this relationship and both showed statistically significant risk ratios. The resulting risk ratio of these studies was 3.28 (95%CI = 1.52–7.09, p = 0.002). In samples that exhibited higher density of CAFs, the average rate of vascular invasion was 35.1% whereas only 9.0% of samples with low CAFs density showed vascular invasion.
Greater density of CAFs in the TME is associated with higher rates of perineural invasion (PNI) in HNSCC (Figure 6). Perineural invasion, wherein tumor cells are found to infiltrate the nerve sheath, provides a distinct form of metastatic spread in HNSCC. Two studies examined this relationship, and both showed a positive relationship between CAF density and PNI. 40.0% of samples with high CAF density demonstrated PNI, while 23.9% of samples with low CAF density had PNI upon pathologic examination. These studies had a cumulative risk ratio of 1.66 (95%CI = 1.02–2.69) which was statistically significant (p = 0.04).
Higher density of CAFs in the TME is correlated with higher levels of Ki67 expression, a measure of cell proliferation (Figure 7). Three studies assessed Ki67 levels within the tumor and all three reported a positive relationship between CAF content and Ki67 staining. Some 56.2% of the total number of samples with high CAF density showed high levels of Ki67, compared to 40.0% of the samples with low CAF density. The combined risk ratio of these studies was 1.51 (95%CI = 1.13–2.03, p = 0.006).
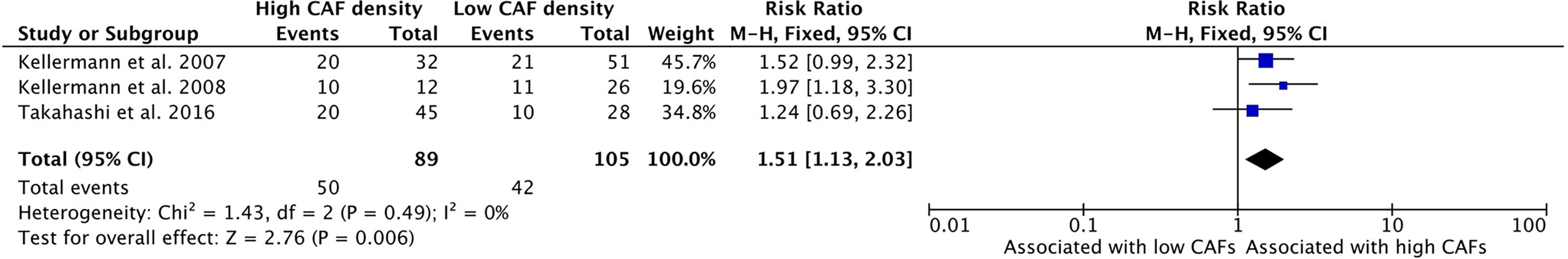
Figure 7 Increased CAF density is associated with increased cellular proliferation, as determined by Ki67 staining.
High CAF density within the TME is further associated with poor differentiation of HNSCC (Figure 8). Ten of the thirteen selected studies determined the relationship between these characteristics; nine of them demonstrated a positive relationship. The average rate of poor differentiation in high CAF density tumors was 19.3%, while low CAF density tumors displayed poor differentiation 13.6% of the time. The resulting risk ratio was 1.90 (95%CI = 1.35–2.69, p <0.001).
High CAF density within the TME is associated with increased rates of local recurrence of HNSCC (Figure 9). Six studies examined this relationship with five studies reporting higher recurrence rates in high CAF density tumors. The cumulative risk ratio was 1.84 (95%CI = 1.35–2.53, P <0.001). The average rate of local recurrence was 31.8% in high CAF density samples, compared to 22.7% in low CAF density samples.
HNSCC, for which mortality remains high despite recent advances in malignancy treatment, demonstrates a remarkable quantity of CAFs, up to 80% of tumor volume in later stage tumors (41, 42). The existing literature has suggested that CAFs negatively affect prognostic characteristics in HNSCC but has not produced a uniform conclusion, with some studies failing to show an association and others claiming a strong positive association (20–25). Two recent meta-analyses have shown that high CAF density is associated with worse overall survival in oral SCC (20, 43). This paper sought to clarify the relationship between CAFs, recurrence, and clinicopathologic markers of significance in HNSCC, including 13 independent studies for a study population of over 900 patients. To our knowledge, this is the first quantitative meta-analysis to examine these relationships. Taken together, the present study shows that, in HNSCC, CAFs are associated with poor prognostic characteristics and increased rates of local recurrence.
This analysis demonstrated that high density of CAFs is associated with higher clinical stage, including T stage and TNM stage (Figures 2 and 4). These characteristics were reported by most of the published studies and demonstrated strongly positive risk ratios. Although some publications did not demonstrate a statistically significant relationship, the relationship with T stage and TNM stage was uniform among the studies. This may be attributable to the aforementioned CAF burden in late stage HNSCC, wherein CAFs accumulate and contribute to high proportions of tumor volume.
The presence of high density of CAFs is associated with common modes of tumor spread and metastasis, including perineural invasion, vascular invasion, and lymph node metastasis (Figures 3, 5 and 6). These insidious findings are indicative of a propensity for metastasis and often suggest the need for more aggressive treatment. The relationship between lymph node metastasis and CAF density was not uniform in the literature, with one study showing an inverse, although non-significant, relationship (32). This meta-analysis revealed a strong positive association, with a cumulative risk ratio of 1.82. Vascular invasion and PNI were the least reported finding among the literature, with only two studies remarking on their association with CAFs. Both studies that investigated vascular invasion showed a statistically significant effect of CAFs on vascular involvement. This was reflected in the analysis, with vascular invasion demonstrating the highest risk ratio of all prognostic indicators (3.28), exceeding the 95% confidence interval for all other pathologic variables. Both studies that assessed the relationship with PNI demonstrated increased rates in tumors with high CAF density, however neither demonstrated independent statistical significance between high and low CAF groups. In total, this study suggests that CAFs play a mechanistic role in invasion and correlates with the presence of various forms of cancer spread.
Similarly, high density of CAFs in tumor samples was significantly associated with aggressive histologic features, including poor differentiation and high Ki67 expression (Figures 7 and 8). Most studies reported a positive association between CAFs and histologic findings of poor prognosis; however, few studies met statistical significance individually. The relationship between differentiation and CAF density was particularly uncertain in this literature, with only one published study showing a significant relationship (25). However, when summed together, the cumulative literature demonstrated a statistically significant association. The strength of the risk ratios for both poor differentiation and high Ki67 expression were high (1.45 and 1.51, respectively), illustrating a clear association of CAFs with tumor aggressiveness and behavior.
This study showed a significant association of high CAF density with rates of local recurrence (Figure 9). Within the literature, this relationship had not been clearly demonstrated, with only two of six studies demonstrating a statistically significant association (32, 37). Our analysis showed a strongly positive risk ratio (1.84), suggesting that CAF density is a clinically meaningful indicator of disease course and outcome.
These findings strongly support the hypothesis that the density of CAFs in HNSCC is associated with multiple poor prognostic factors. Similarly, we demonstrate that high density of CAFs is associated with increased rates of local recurrence. This evidence provides a strong rationale to further investigate the functional relationship of CAFs with carcinoma cells in the tumor microenvironment. Furthermore, this work suggests that staining for CAFs is an indicator of clinical outcomes and may be a valuable addition to existing prognostic strategies.
Immunohistochemical staining for α-SMA was the most commonly utilized method for detecting CAFs in the microenvironment. This allowed for a more homogenous study population and facilitated the comparison between different studies. Other markers for assessing the presence of CAFs have also been suggested in the literature. Higashino et al. performed both FAP and α-SMA staining on esophageal SCC samples and found different staining patterns between the two populations (44). Differences in staining between these markers, and other proposed CAF identifiers, may represent differing origins or functional subtypes of CAFs. The findings in this meta-analysis may be most applicable for α-SMA+ CAF subtypes. Further studies to investigate the differences between these subtypes are needed.
Knowledge of CAFs allows for better understanding of tumorigenesis and increases the feasibility of CAF-directed treatment. Bidirectional interaction between CAFs and tumor cells has been demonstrated in multiple studies and may contribute to the increased tumor aggression and dissemination shown in this meta-analysis.
Although the origin of CAFs in the TME have yet to be clearly defined, the current consensus is that local tissue fibroblasts are activated to become CAFs, under the influence of tumor-derived paracrine factors and cytokines (28, 45, 46). Once activated, CAFs give rise to physical and chemical changes within the microenvironment that facilitate enhanced growth and progression as shown in this data. Through secretion of cytokines like transforming growth factor-beta (TGF-β), CAFs induce a desmoplastic response in the surrounding environment, synthesizing collagen which stiffens the tissue and alters homeostasis (47). The mechanical stress of desmoplasia collapses nearby blood vessels to promote hypoxia, resulting in more aggressive cancer phenotypes. Recurrent malignancy after treatment with radiation exemplifies this phenotype. Orchestrated by CAFs, these recurrences are often exquisitely desmoplastic with extensive collagen cross linking and loss of microvascular structure (48, 49). This modified environment is hypothesized to provide a physical barrier to immune surveillance, enabling unabated tumor growth (50).
Metabolic coupling between CAFs and HNSCC cells further drives tumor development. CAFs demonstrate a “reverse Warburg” effect wherein oxidative stress to tumor cells induces glycolytic behavior and autophagy in nearby CAFs. Catabolites produced in this glycolytic process are then shuttled to tumor cells via monocarboxylate transporters (MCTs), allowing for mitochondria-rich tumor cells with high rates of Oxidative Phosphorylation (42, 51). These changes serve to “feed” anabolic tumors cells (52, 53). The findings in this meta-analysis, linking higher CAF density with larger, and more aggressive, primary tumors may reflect this symbiosis.
As noted above, CAFs possess the capacity to directly and indirectly alter the host immune response. CAFs in HNSCC can modulate the function and suppress proliferation of T cells, contributing to the immunosuppressive environment in HNSCC lesions (54, 55). Cytokines secreted by CAFs reportedly promote the attraction of tumor-associated macrophages and skew their differentiation state towards the M2 subtype, which is linked to poor prognosis and outcomes in HNSCC (36, 56, 57). The presence of CAFs may allow tumors to evade immune surveillance and weaken immune response, providing a permissive environment for tumor growth and proliferation.
This meta-analysis demonstrated a strong association between CAF density and tumor invasion. The impact of CAFs on invasion is multifaceted, involving changes to both the TME and cancer cells themselves. It is well documented that CAFs serve to remodel the extracellular matrix and promote “tracks” for invading tumor cells (58). Tumor migration and invasion is supported by CAFs through the secretion of matrix-dissolving proteases including matrix metalloproteins (MMPs) and growth factors including TGF-β (59, 60). Local invasion facilitates dissemination to lymph nodes and neurons, as demonstrated by the increased rates of lymph node positivity and PNI shown in this meta-analysis. Secretion of TGF-β by CAFs may additionally support migration and invasion of tumor cells through robust effects on the epithelial-to-mesenchymal transition (EMT) of HNSCC and enhanced metastatic potential (61).
Prognostication and clinical stratification remain a difficult task in HNSCC. Recent staging modifications by the AJCC have expanded to incorporate extra-nodal extension and depth of invasion into their algorithm (62). However, patients with identical staging may have disparate outcomes. Histopathology may provide valuable data to be incorporated into clinical decision making. Marsh et al. found α-SMA to be the most significant prognostic indicator of all clinical, pathologic, and molecular features (23). Our findings, and recent studies correlating high CAF density with overall survival, support further consideration of CAF utilization within existing prognostic algorithms (20, 43).
The prognostic qualities of CAFs may not be applicable to precancerous lesions. Vered et al. showed a “burst” of CAFs that coincided with the appearance of tongue carcinoma, but not in hyperplastic or dysplastic lesions (63). A study by Etemad-Moghadam et al. demonstrated no α-SMA staining in either normal oral epithelium or oral dysplasia, but all samples of oral carcinoma contained CAFs, to varying degrees (64). This differs from the paradigm seen in other cancer types including colon, breast, and cervical (65–67), wherein α-SMA positive myofibroblasts are present in non-invasive lesions and are thought to promote the progression to carcinoma. Similarly, HPV-positive HNSCC has a unique pathogenesis with different CAF stimulation and activation patterns than those found in HPV-negative disease (68, 69). Further investigation into HPV-associated CAF quantities and function will help to clarify this interaction and guide future treatment.
Tumors with invasive phenotypes or poor differentiation often have a high stromal burden, consisting of large CAF populations. It is important to determine whether differences in prognosis associated with CAF density could be attributable to intrinsic tumor characteristics. To this end, Sun et al. cultured identical SCC lines with either normal fibroblasts or CAFs, demonstrating increased invasion and migration in cell lines co-cultured with CAFs compared to normal fibroblasts (35). Similar findings have been seen in breast cancer experiments (70). These in vitro studies offer some insight into the deterministic impact of CAF; however, continued research is necessary to understand how specific CAF and tumor cell interactions result in the prognostic effects shown here.
As the specific impact of CAFs in HNSCC has been better elucidated, CAF-targeted treatment have emerged. For example, inhibitors of Fibroblast Activation Protein (FAP), have shown promising preclinical results in reducing the progression of tumors and promoting anti-tumor immune response (71–73). Similarly, CAF-derived cytokines, like HGF and TGF-β, have been targeted to inhibit tumor aggressiveness and slow resistance to traditional chemotherapy (74, 75). A better understanding of the functional relationship of CAFs and malignant cells is likely to guide identification of additional therapeutic targets in HNSCC.
Further complicating our understanding, CAFs show remarkable heterogeneity and plasticity. Despite underlying commonalities, CAFs within a single tumor may arise via different pathways, have different phenotypes, and exhibit functionally different effects on the tumor cells and environment (75–77). This is exemplified by the different expression patterns seen in different methods of CAF identification (44). As recent research has shown, CAF function can be altered by environmental factors (19). Many believe that CAFs, like tumor-associated macrophages, may have either pro-tumoral or anti-tumoral effects depending on subtype or activation (78). This may in part explain why complete α-SMA depletion in mouse models resulted in aggressive pancreatic tumors and reduced survival (79).
Single cell technologies, such as RNA sequencing (scRNA-seq), have recently provided the ability to assess the transcriptional and phenotypical heterogeneity of CAFs. These approaches offer the ability to unmask specific cellular subtypes via their transcriptome, providing greater resolution than α-SMA staining. ScRNA-seq research in breast and colon cancer have shown clear delineations between CAF sub-populations (80, 81). Projects that seek to define these subtypes in HNSCC and their prognostic and mechanistic effects are necessary, both for understanding of tumorigenesis and optimization of therapy.
While this meta-analysis demonstrated strong associations between CAF density and indicators of poor prognosis of HNSCC, it was limited in the following ways. First, literature that does not demonstrate significant relationships are far less likely to be published. Because meta-analyses rely on published literature, these findings could be missing unpublished findings that did not meet statistical significance. Additionally, the initial literature search contained multiple studies that examined this relationship but did not present data in a manner amenable to compilation. Thus, we did not include these studies in the statistical analysis. This study demonstrates a correlation between CAF density and prognostic indicators; to determine causality, further investigation would need to be undertaken.
Finally, and as discussed above, the heterogeneity of CAF populations, even within a single tumor, should not be underestimated. Distinct CAF subtypes have been suggested in HNSCC and each subpopulation may hold distinct prognostic significance (76). While this study utilized a broad and encompassing categorization of CAFs, certain subpopulations may have stronger clinical implications.
CAFs play pivotal roles within the microenvironment of various solid malignancies including HNSCC by aiding tumor invasion, immune suppression, and satisfying the metabolic requirements of rapidly growing tumors (82, 83). This meta-analysis reveals that in HNSCC, the presence of CAFs is associated with poor clinicopathologic features. Samples with high CAF density also demonstrate increased rates of local recurrence (Figure 10). Understanding the precise mechanisms, and functional relationship of CAFs with tumor cells will be crucial for optimizing HNSCC treatment. To this end, the functional and structural differences between CAF subtypes and normal fibroblasts need to be more clearly elucidated. The findings support the growing interest in CAFs and their potential to be utilized for clinical stratification and decision-making.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
JC designed the project. AK performed the literature review, selected papers, collected the data, utilized RevMan to analyze the findings and generate forest plots, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, et al. Role of tumor microenvironment in tumorigenesis. J Cancer (2017) 8(5):761–73. doi: 10.7150/jca.17648
2. Li H, Fan X, Houghton J. Tumor microenvironment: The role of the tumor stroma in cancer. J Cell Biochem (2007) 101(4):805–15. doi: 10.1002/jcb.21159
3. Hanahan D, Coussens LM. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell (2012) 21(3):309–22. doi: 10.1016/j.ccr.2012.02.022
4. Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med (2011) 17(3):320–9. doi: 10.1038/nm.2328
5. Franco OE, Shaw AK, Strand DW, Hayward SW. Cancer Associated Fibroblasts in Cancer Pathogenesis. Semin Cell Dev Biol (2010) 21(1):33–9. doi: 10.1016/j.semcdb.2009.10.010
6. Markwell SM, Weed SA. Tumor and Stromal-Based Contributions to Head and Neck Squamous Cell Carcinoma Invasion. Cancers (2015) 7(1):382–406. doi: 10.3390/cancers7010382
7. Curry JM, Sprandio J, Cognetti D, Luginbuhl A, Bar-ad V, Pribitkin E, et al. Tumor Microenvironment in Head and Neck Squamous Cell Carcinoma. Semin Oncol (2014) 41(2):217–34. doi: 10.1053/j.seminoncol.2014.03.003
8. Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer (2007) 7(2):139–47. doi: 10.1038/nrc2067
9. Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer (2006) 6(5):392–401. doi: 10.1038/nrc1877
10. Wang M, Wu C, Guo Y, Cao X, Zheng W, Fan G-K. The primary growth of laryngeal squamous cell carcinoma cells in vitro is effectively supported by paired cancer-associated fibroblasts alone. Tumour Biol: J Int Soc Oncodevelopmental Biol Med (2017) 39(5):1010428317705512. doi: 10.1177/1010428317705512
11. Plzák J, Bouček J, Bandúrová V, Kolář M, Hradilová M, Szabo P, et al. The Head and Neck Squamous Cell Carcinoma Microenvironment as a Potential Target for Cancer Therapy. Cancers (2019) 11(4):440. doi: 10.3390/cancers11040440
12. Bu L, Baba H, Yoshida N, Miyake K, Yasuda T, Uchihara T, et al. Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment. Oncogene (2019) 38(25):4887–901. doi: 10.1038/s41388-019-0765-y
13. Kawashiri S, Tanaka A, Noguchi N, Hase T, Nakaya H, Ohara T, et al. Significance of stromal desmoplasia and myofibroblast appearance at the invasive front in squamous cell carcinoma of the oral cavity. Head Neck (2009) 31(10):1346–53. doi: 10.1002/hed.21097
14. Tripathi M, Billet S, Bhowmick NA. Understanding the role of stromal fibroblasts in cancer progression. Cell Adhesion Migration (2012) 6(3):231–5. doi: 10.4161/cam.20419
15. Dvorak HF. Tumors: Wounds That Do Not Heal. New Engl J Med (1986) 315(26):1650–9. doi: 10.1056/NEJM198612253152606
16. Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell (2000) 100(1):57–70. doi: 10.1016/S0092-8674(00)81683-9
17. Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
18. Pietras K, Östman A. Hallmarks of cancer: Interactions with the tumor stroma. Exp Cell Res (2010) 316(8):1324–31. doi: 10.1016/j.yexcr.2010.02.045
19. Domingo-Vidal M, Whitaker-Menezes D, Martos-Rus C, Tassone P, Snyder CM, Tuluc M, et al. Cigarette Smoke Induces Metabolic Reprogramming of the Tumor Stroma in Head and Neck Squamous Cell Carcinoma. Mol Cancer Res: MCR (2019) 17(9):1893–909. doi: 10.1158/1541-7786.MCR-18-1191
20. Dourado MR, Guerra ENS, Salo T, Lambert DW, Coletta RD. Prognostic value of the immunohistochemical detection of cancer-associated fibroblasts in oral cancer: A systematic review and meta-analysis. J Oral Pathol Med (2018) 47(5):443–53. doi: 10.1111/jop.12623
21. Bello IO, Vered M, Dayan D, Dobriyan A, Yahalom R, Alanen K, et al. Cancer-associated fibroblasts, a parameter of the tumor microenvironment, overcomes carcinoma-associated parameters in the prognosis of patients with mobile tongue cancer. Oral Oncol (2011) 47(1):33–8. doi: 10.1016/j.oraloncology.2010.10.013
22. Ding L, Zhang Z, Shang D, Cheng J, Yuan H, Wu Y, et al. α-Smooth muscle actin-positive myofibroblasts, in association with epithelial–mesenchymal transition and lymphogenesis, is a critical prognostic parameter in patients with oral tongue squamous cell carcinoma. J Oral Pathol Med (2014) 43(5):335–43. doi: 10.1111/jop.12143
23. Marsh D, Suchak K, Moutasim KA, Vallath S, Hopper C, Jerjes W, et al. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol (2011) 223(4):470–81. doi: 10.1002/path.2830
24. Fujii N, Shomori K, Shiomi T, Nakabayashi M, Takeda C, Ryoke K, et al. Cancer-associated fibroblasts and CD163-positive macrophages in oral squamous cell carcinoma: Their clinicopathological and prognostic significance. J Oral Pathol Med (2012) 41(6):444–51. doi: 10.1111/j.1600-0714.2012.01127.x
25. Akrish SJ, Rachmiel A, Sabo E, Vered M, Ben-Izhak O. Cancer-associated fibroblasts are an infrequent finding in the microenvironment of proliferative verrucous leukoplakia-associated squamous cell carcinoma. J Oral Pathol Med (2017) 46(5):353–8. doi: 10.1111/jop.12503
26. Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and neck cancers—Major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA: A Cancer J Clin (2017) 67(2):122–37. doi: 10.3322/caac.21389
27. Abbas SA, Saeed J, Tariq MU, Baksh AR, Hashmi S. Clinicopathological prognostic factors of oral squamous cell carcinoma: An experience of a tertiary care hospital. JPMA J Pakistan Med Assoc (2018) 68(7):1115–9.
28. Kellermann MG, Sobral LM, Silva SD, Zecchin KG, Graner E, Lopes MA, et al. Myofibroblasts in the stroma of oral squamous cell carcinoma are associated with poor prognosis. Histopathology (2007) 51(6):849–53. doi: 10.1111/j.1365-2559.2007.02873.x
29. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ (2009) 339:b2700. doi: 10.1136/bmj.b2700
30. Kellermann MG, Sobral LM, da Silva SD, Zecchin KG, Graner E, Lopes MA, et al. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: Induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral Oncol (2008) 44(5):509–17. doi: 10.1016/j.oraloncology.2007.07.001
31. Liang L, Luo H, He Q, You Y, Fan Y, Liang J. Investigation of cancer-associated fibroblasts and p62 expression in oral cancer before and after chemotherapy. J Cranio-Maxillo-Facial Surg: Off Publ Eur Assoc Cranio-Maxillo-Facial Surg (2018) 46(4):605–10. doi: 10.1016/j.jcms.2017.12.016
32. Lin N-N, Wang P, Zhao D, Zhang F-J, Yang K, Chen R. Significance of oral cancer-associated fibroblasts in angiogenesis, lymphangiogenesis, and tumor invasion in oral squamous cell carcinoma. J Oral Pathol Med (2017) 46(1):21–30. doi: 10.1111/jop.12452
33. Luksic I, Suton P, Manojlovic S, Virag M, Petrovecki M, Macan D. Significance of myofibroblast appearance in squamous cell carcinoma of the oral cavity on the occurrence of occult regional metastases, distant metastases, and survival. Int J Oral Maxillofacial Surg (2015) 44(9):1075–80. doi: 10.1016/j.ijom.2015.05.009
34. Ramos-Vega V, Venegas Rojas B, Donoso Torres W. Immunohistochemical analysis of cancer-associated fibroblasts and podoplanin in head and neck cancer. Med Oral Patol Oral Y Cirugia Bucal (2020) 25(2):e268–76. doi: 10.4317/medoral.23335
35. Sun L-P, Xu K, Cui J, Yuan D-Y, Zou B, Li J, et al. Cancer-associated fibroblast-derived exosomal miR-382-5p promotes the migration and invasion of oral squamous cell carcinoma. Oncol Rep (2019) 42(4):1319–28. doi: 10.3892/or.2019.7255
36. Takahashi H, Sakakura K, Kudo T, Toyoda M, Kaira K, Oyama T, et al. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget (2016) 8(5):8633–47. doi: 10.18632/oncotarget.14374
37. Wang Q, Zhang Y, Zhu L, Pan L, Yu M, Shen W, et al. Heat shock factor 1 in cancer-associated fibroblasts is a potential prognostic factor and drives progression of oral squamous cell carcinoma. Cancer Sci (2019) 110(5):1790–803. doi: 10.1111/cas.13991
38. Zhang Z, Tao D, Zhang P, Liu X, Zhang Y, Cheng J, et al. Hyaluronan synthase 2 expressed by cancer-associated fibroblasts promotes oral cancer invasion. J Exp Clin Cancer Res: CR (2016) 35:181. doi: 10.1186/s13046-016-0458-0
39. Cheng Y, Wang K, Ma W, Zhang X, Song Y, Wang J, et al. Cancer-associated fibroblasts are associated with poor prognosis in esophageal squamous cell carcinoma after surgery. Int J Clin Exp Med (2015) 8(2):1896–903. doi: 10.1371/journal.pone.0159947
40. Review Manager (RevMan). Computer program. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration (2014).
41. Ansems M, Span PN. The tumor microenvironment and radiotherapy response; a central role for cancer-associated fibroblasts. Clin Trans Radiat Oncol (2020) 22:90–7. doi: 10.1016/j.ctro.2020.04.001
42. Kumar D, New J, Vishwakarma V, Joshi R, Enders J, Lin F, et al. Cancer-Associated Fibroblasts Drive Glycolysis in a Targetable Signaling Loop Implicated in Head and Neck Squamous Cell Carcinoma Progression. Cancer Res (2018) 78(14):3769–82. doi: 10.1158/0008-5472.CAN-17-1076
43. Graizel D, Zlotogorski-Hurvitz A, Tsesis I, Rosen E, Kedem R, Vered M. Oral cancer-associated fibroblasts predict poor survival: Systematic review and meta-analysis. Oral Dis (2019) 26:733–44. doi: 10.1111/odi.13140
44. Higashino N, Koma Y, Hosono M, Takase N, Okamoto M, Kodaira H, et al. Fibroblast activation protein-positive fibroblasts promote tumor progression through secretion of CCL2 and interleukin-6 in esophageal squamous cell carcinoma. Lab Invest (2019) 99(6):777–92. doi: 10.1038/s41374-018-0185-6
45. Lewis MP, Lygoe KA, Nystrom ML, Anderson WP, Speight PM, Marshall JF, et al. Tumour-derived TGF-β1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br J Cancer (2004) 90(4):822–32. doi: 10.1038/sj.bjc.6601611
46. Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer (2020) 20(3):174–86. doi: 10.1038/s41568-019-0238-1
47. Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg G II, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell (2005) 8(3):241–54. doi: 10.1016/j.ccr.2005.08.010
48. Pandya JA, Srikant N, Boaz K, Manaktala N, Kapila SN, Yinti SR. Post-radiation changes in oral tissues—An analysis of cancer irradiation cases. South Asian J Cancer (2014) 3(3):159–62. doi: 10.4103/2278-330X.136785
49. Barker HE, Paget JTE, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat Rev Cancer (2015) 15(7):409–25. doi: 10.1038/nrc3958
50. Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng (2014) 16:321–46. doi: 10.1146/annurev-bioeng-071813-105259
51. Curry JM, Tuluc M, Whitaker-Menezes D, Ames JA, Anantharaman A, Butera A, et al. Cancer metabolism, stemness and tumor recurrence. Cell Cycle (2013) 12(9):1371–84. doi: 10.4161/cc.24092
52. Martinez-Outschoorn UE, Lisanti MP, Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol (2014) 25:47–60. doi: 10.1016/j.semcancer.2014.01.005
53. Guido C, Whitaker-Menezes D, Capparelli C, Balliet R, Lin Z, Pestell, et al. Metabolic reprogramming of cancer-associated fibroblasts by TGF-β drives tumor growth: Connecting TGF-β signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle (Georgetown Tex) (2012) 11(16):3019–35. doi: 10.4161/cc.21384
54. Takahashi H, Sakakura K, Kawabata-Iwakawa R, Rokudai S, Toyoda M, Nishiyama M, et al. Immunosuppressive activity of cancer-associated fibroblasts in head and neck squamous cell carcinoma. Cancer Immunol Immunother: CII (2015) 64(11):1407–17. doi: 10.1007/s00262-015-1742-0
55. Tong CCL, Kao J, Sikora AG. Recognizing and reversing the immunosuppressive tumor microenvironment of head and neck cancer. Immunol Res (2012) 54(1):266–74. doi: 10.1007/s12026-012-8306-6
56. Kumar AT, Knops A, Swendseid B, Martinez-Outschoom U, Harshyne L, Philp N, et al. Prognostic Significance of Tumor-Associated Macrophage Content in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Front Oncol (2019) 9:656. doi: 10.3389/fonc.2019.00656
57. Troiano G, Caponio VCA, Adipietro I, Tepedino M, Santoro R, Laino L, et al. Prognostic significance of CD68+ and CD163+ tumor associated macrophages in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol (2019) 93:66–75. doi: 10.1016/j.oraloncology.2019.04.019
58. Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol (2007) 9(12):1392–400. doi: 10.1038/ncb1658
59. Fullár A, Kovalszky I, Bitsche M, Romani A, Schartinger VH, Sprinzl GM, et al. Tumor cell and carcinoma-associated fibroblast interaction regulates matrix metalloproteinases and their inhibitors in oral squamous cell carcinoma. Exp Cell Res (2012) 318(13):1517–27. doi: 10.1016/j.yexcr.2012.03.023
60. Koontongkaew S, Amornphimoltham P, Monthanpisut P, Saensuk T, Leelakriangsak M. Fibroblasts and extracellular matrix differently modulate MMP activation by primary and metastatic head and neck cancer cells. Med Oncol (Northwood London England) (2012) 29(2):690–703. doi: 10.1007/s12032-011-9871-6
61. Yu Y, Xiao C-H, Tan L-D, Wang Q-S, Li X-Q, Feng Y-M. Cancer-associated fibroblasts induce epithelial–mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br J Cancer (2014) 110(3):724–32. doi: 10.1038/bjc.2013.768
62. Lydiatt W, O’Sullivan B, Patel S. Major Changes in Head and Neck Staging for 2018. Am Soc Clin Oncol Educ Book (2018) 38:505–14. doi: 10.1200/EDBK_199697
63. Vered M, Allon I, Buchner A, Dayan D. Stromal myofibroblasts and malignant transformation in a 4NQO rat tongue carcinogenesis model. Oral Oncol (2007) 43(10):999–1006. doi: 10.1016/j.oraloncology.2006.11.007
64. Etemad-Moghadam S, Khalili M, Tirgary F, Alaeddini M. Evaluation of myofibroblasts in oral epithelial dysplasia and squamous cell carcinoma. J Oral Pathol Med (2009) 38(8):639–43. doi: 10.1111/j.1600-0714.2009.00768.x
65. Sappino A-P, Skalli O, Jackson B, Schürch W, Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer (1988) 41(5):707–12. doi: 10.1002/ijc.2910410512
66. Martin M, Pujuguet P, Martin F. Role of Stromal Myofibroblasts Infiltrating Colon Cancer in Tumor Invasion. Pathol Res Pract (1996) 192(7):712–7. doi: 10.1016/S0344-0338(96)80093-8
67. Cintorino M, Marco EBD, Leoncini P, Tripodi SA, Ramaekers FC, Sappino AP, et al. Expression of α-sooth-muscle actin in stromal cells of the uterine cervix during epithlial neoplastic changes. Int J Cancer (1991) 47(6):843–6. doi: 10.1002/ijc.2910470609
68. Bolt R, Foran B, Murdoch C, Lambert DW, Thomas S, Hunter KD. HPV-negative, but not HPV-positive, oropharyngeal carcinomas induce fibroblasts to support tumour invasion through micro-environmental release of HGF and IL-6. Carcinogenesis (2017) 39(2):170–9. doi: 10.1093/carcin/bgx130
69. Barros MR, de Melo CML, Barros M. L. C. M. G. R., de Cássia Pereira de Lima R, de Freitas AC, Venuti A. Activities of stromal and immune cells in HPV-related cancers. J Exp Clin Cancer Res (2018) 37(1):137. doi: 10.1186/s13046-018-0802-7
70. Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal Fibroblasts Present in Invasive Human Breast Carcinomas Promote Tumor Growth and Angiogenesis through Elevated SDF-1/CXCL12 Secretion. Cell (2005) 121(3):335–48. doi: 10.1016/j.cell.2005.02.034
71. Teichgräber V, Monasterio C, Chaitanya K, Boger R, Gordon K, Dieterle T, et al. Specific inhibition of fibroblast activation protein (FAP)-alpha prevents tumor progression in vitro. Adv Med Sci (2015) 60(2):264–72. doi: 10.1016/j.advms.2015.04.006
72. Togo S, Polanska UM, Horimoto Y, Orimo A. Carcinoma-Associated Fibroblasts Are a Promising Therapeutic Target. Cancers (2013) 5(1):149–69. doi: 10.3390/cancers5010149
73. Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Sci (N Y NY) (2010) 330(6005):827–30. doi: 10.1126/science.1195300
74. Chan JSK, Sng MK, Teo ZQ, Chong HC, Twang JS, Tan NS. Targeting nuclear receptors in cancer-associated fibroblasts as concurrent therapy to inhibit development of chemoresistant tumors. Oncogene (2018) 37(2):160–73. doi: 10.1038/onc.2017.319
75. Liu T, Han C, Wang S, Fang P, Ma Z, Xu L, et al. Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. J Hematol Oncol (2019) 12:86. doi: 10.1186/s13045-019-0770-1
76. Puram SV, Tirosh I, Parikh AS, Patel AP, Yizhak K, Gillespie S, et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell (2017) 171(7):1611–1624.e24. doi: 10.1016/j.cell.2017.10.044
77. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer (2016) 16(9):582–98. doi: 10.1038/nrc.2016.73
78. Öhlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med (2014) 211(8):1503–23. doi: 10.1084/jem.20140692
79. Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu C-C, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell (2014) 25(6):719–34. doi: 10.1016/j.ccr.2014.04.005
80. Bartoschek M, Oskolkov N, Bocci M, Lövrot J, Larsson C, Sommarin M, et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat Commun (2018) 9(1):1–13. doi: 10.1038/s41467-018-07582-3
81. Li H, Courtois ET, Sengupta D, Tan Y, Chen KH, Goh JJL, et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat Genet (2017) 49(5):708–18. doi: 10.1038/ng.3818
82. Leef G, Thomas SM. Molecular Communication between Tumor-Associated Fibroblasts and Head and Neck Squamous Cell Carcinoma. Oral Oncol (2013) 49(5):381–6. doi: 10.1016/j.oraloncology.2012.12.014
Keywords: cancer-associated fibroblasts, CAF, tumor microenvironment, myofibroblast, alpha-smooth muscle actin, prognosis, head and neck squamous cell carcinoma
Citation: Knops AM, South A, Rodeck U, Martinez-Outschoorn U, Harshyne LA, Johnson J, Luginbuhl AJ and Curry JM (2020) Cancer-Associated Fibroblast Density, Prognostic Characteristics, and Recurrence in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Front. Oncol. 10:565306. doi: 10.3389/fonc.2020.565306
Received: 24 May 2020; Accepted: 27 October 2020;
Published: 27 November 2020.
Edited by:
Makoto Tahara, National Cancer Center Hospital East, JapanReviewed by:
Sjoukje F. Oosting, University Medical Center Groningen, NetherlandsCopyright © 2020 Knops, South, Rodeck, Martinez-Outschoorn, Harshyne, Johnson, Luginbuhl and Curry. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander M. Knops, YWxleGFuZGVyLmtub3BzQGplZmZlcnNvbi5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.