
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 26 October 2020
Sec. Genitourinary Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.564694
This article is part of the Research TopicEmerging Immunotherapies and Personalized Approaches in Genitourinary CancersView all 16 articles
 Tang Tang1†
Tang Tang1† Lin-ang Wang1†
Lin-ang Wang1† Peng Wang1
Peng Wang1 Dali Tong1
Dali Tong1 Gaolei Liu1
Gaolei Liu1 Jun Zhang1
Jun Zhang1 Nan Dai2
Nan Dai2 Yao Zhang1
Yao Zhang1 Gang Yuan1
Gang Yuan1 Kyla Geary3
Kyla Geary3 Dianzheng Zhang3
Dianzheng Zhang3 Qiuli Liu1*
Qiuli Liu1* Jun Jiang1*
Jun Jiang1*Background: Mutation-caused loss-of-function of factors involved in DNA damage response (DDR) is responsible for the development and progression of ~20% of prostate cancer (PCa). Some mutations can be used in cancer risk assessment and informed treatment decisions.
Methods: Target capture-based deep sequencing of 11 genes was conducted with total DNA purified from the proband’s peripheral blood. Sanger sequencing was conducted to screen potential germline mutations in the proband’s family members. Targeted sequencing of a panel of 1,021 genes was done with DNA purified from the tumor tissue.
Results: Two previously unreported germline mutations in the DDR pathway, BRCA2 (c.8474_8487delCATACCCTATACAG, p.A2825Vfs*15) and PALB2 (c.472delC, p.Q158Rfs*19) were identified in a patient with metastatic PCa. A specific therapeutic regimen including androgen deprivation therapy, locally radical radiotherapy, and systemic platinum chemotherapy worked well against his cancer. In addition, the metastatic ovarian cancer in the proband’s half-sister harboring the same BRCA2 germline mutation also responded well to platinum chemotherapy.
Conclusions: The newly identified germline mutations in DDR plays important role in PCa development. Since specific regimen worked well against this cancer, screening of DDR mutation could provide better management for patients with these mutation-mediated PCa.
Prostate cancer (PCa) is the most prevalent cancer in men and the second leading cause of cancer-related death worldwide. It has been estimated that in the United States there will be 191,930 new PCa diagnoses and 33,330 PCa-related deaths in 2020 (1). Compared with the general population, first-degree relatives of men with PCa have approximately twice the risk of developing PCa (2) and genetic mutations are responsible for ~42% of this disease (3). Genome-wide association studies have identified more than 100 common variants that account for approximately 33% of familial PCa risk (4). Multiple lines of evidence suggest that mutation in genes involved in DNA damage response (DDR) plays rather important role in cancer development and progression (5–7).
It has been estimated that the mutation of genes in DDR is responsible for at least 19% of localized PCa (6) and 23% of metastatic castration-resistant PCa (7). Proteins encoded by BRCA2, BRCA1, ATM, CHEK2, PALB2, and mismatch repair (MMR) genes including MSH2 and MSH6 play important role in DDR (7). Mutations in some of these genes have already been used for risk assessment and treatment decision-making (8). For example, germline mutation of BRCA usually confers a more aggressive PCa with higher Gleason scores (9), a higher probability of nodal involvement, distant metastasis, and shorter overall survival (10). More importantly, patients with advanced PCa harboring DDR gene mutations generally respond well to poly (ADP) ribose polymerase (PARP) inhibitors and platinum-based chemotherapy (11, 12). The U.S. Food and Drug Administration (FDA) recently approved two poly-ADP ribose polymerase (PARP) inhibitors, olaparib (11, 13), and rucaparib (14), as treatments for patients with metastatic castration resistant adenocarcinoma of the prostate harboring deleterious or suspected deleterious germline or somatic HRR gene-mutations. Therefore, the stratification of PCa patients with mutations in DDR pathway may lead to more informed therapies.
We here report a patient with metastatic PCa carrying previously unreported germline mutations in BRCA2 and PALB2, two important players in DDR. More importantly, this patient responded well to a specific therapeutic regimen including androgen deprivation therapy, locally radical radiotherapy, and systemic platinum chemotherapy. In addition, his half-sister carrying the same BRCA2 mutation with metastatic ovarian cancer responded equally well to platinum chemotherapy.
All procedures involving human participants were carried out in accordance with ethical standards of the institutional research committee at the Army Medical University in Chongqing, China and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All patients provided written, informed consent for review of their medical record and sequence of their primary and/or metastatic PCa tissue. The research was conducted with Army Medical University IRB approval.
Total DNA was extracted from either the patient’s leukocytes, primary PCa, or metastatic lung cancer tissues using the QIAamp DNA Micro Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Target capture-based deep sequencing (BGI Health, China) was conducted to screen potential mutations in a panel of 11 genes (ATM, BRCA1, BRCA2, MLH1, MLH3, MSH2, MSH3, MSH6, PALB2, PMS1, and PMS2) using DNA purified from the proband’s peripheral blood. Sanger sequencing was conducted to screen potential germline mutations in the proband’s family members. Targeted sequencing of a panel of 1021 genes (Supplementary Table S1) was done with DNA purified from the tumor tissue by Geneplus-Beijing Institute (Beijing, China).
The proband is a 48-year-old Chinese male who presented to our department on July 4, 2018 after having hematuria for 2 months. Digital rectal examination found the right lobe of his prostate is hardened with irregularities. Laboratory tests showed a relatively normal level of total serum PSA (prostate-specific antigen, 3.03 ng/ml). Pelvic magnetic resonance imaging (MRI) showed a lesion (3.1 × 4.3 cm) in the peripheral zone of the right lobe of his prostate with a low-intensity signal on T2 weighted imaging (Figures 1A, upper panel). Ultrasound-guided transrectal prostate biopsies were conducted and pathological examination showed prostate adenocarcinoma in 4 of the 14 cored biopsies with an average Gleason score 4 + 4. Computed tomography (CT) chest scan also revealed two small lesions in his right lung (Figures 1B, C, upper panel). Whole body positron emission tomography (PET)-CT scan found hypermetabolic lesions in both his prostate and right side of lung, but not in the bone or other organs. Immunohistochemistry of the lesions from the right lung showed positive staining of PSA and negative staining of CDX-2 and TTF-1 (data not shown). Therefore, his diagnose was a primary PCa with lung metastasis (T2cNxM1c).
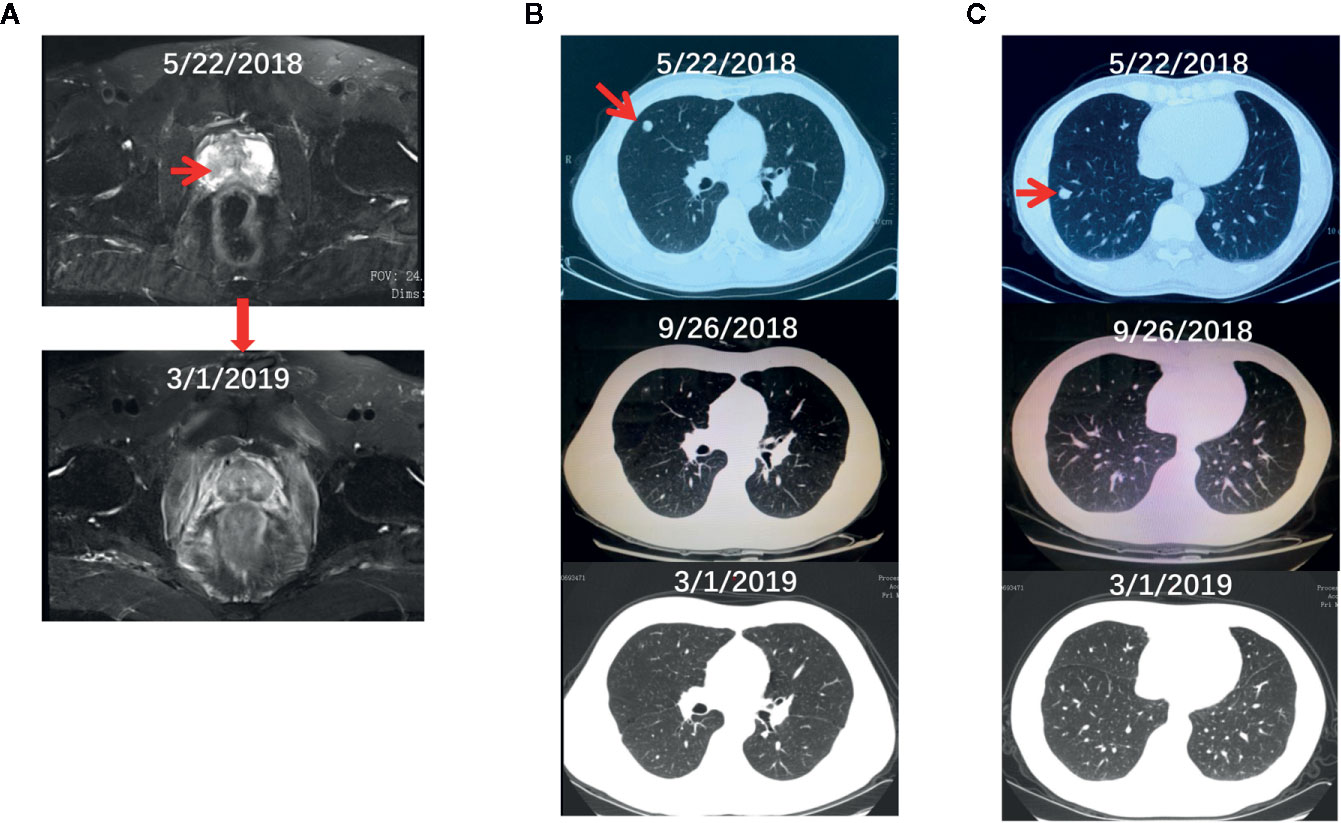
Figure 1 The radiographs of the proband before and during the treatment. (A–C) The pelvic MRI (A) and chest CT (B, C) scan of the proband before and after the systematic treatment.
Given that (i) the relative early-onset and high aggressiveness of cancer, (ii) his father died of lung cancer at the age of 70, and (iii) his half-sister suffered from metastatic ovarian cancer with severe ascites in her early 60s, we decided to screen potential germline mutations. To do so, DNA extracted from the patient’s leukocytes was used for sequencing 11 genes involved in the DDR pathway including ATM, BRCA1, BRCA2, MLH1, MLH3, MSH2, MSH3, MSH6, PALB2, PMS1, and PMS2. Two previously unreported germline mutations of BRCA2 (c.8474_8487delCATACCCTATACAG, p.A2825Vfs*15, Figure 2A) and PALB2 (c.472delC, p.Q158Rfs*19, Figure 2B) were identified. These deletions result in the expression of truncated BRCA2 and PALB2. Next, leukocyte DNA was isolated from the other 10 immediate family members of the proband and used for Sanger sequencing of the BRCA2 and PALB2 genes. The characteristics of the family members and their genetic mutations were summarized in Table 1. Based on the pedigree (Figure 2C), we postulated that the proband inherited his mutant BRCA2 allele from his father and the mutant PALB2 from his mother and therefore the proband carries a heterozygous mutation of both BRCA2 and PALB2. We then decided to screen any potential somatic mutations. Total DNA extracted from both his prostate and lung cancer tissues was used to sequence a panel of 1021 genes highly involved in PCa. In addition to the germline mutant BRCA2 and PALB2, 10 and 9 additional somatic mutations were identified in his prostate and lung lesions, respectively (Supplementary Table S2). Of note, the six mutations with the highest frequency (>10%) including PAG1 (c.702A>T, p.K234N), KDM5C (c.1869G>C, p.L623F), CDH11 (c.1048G>A, p.A350T), AFF2 (c.124_141delGATCTCTTTTCTTCAGGC, p.D42_G47del), FOXA1 (c.753_764delCAACATGTTCGA, p.M253_N256del), and HCLS1 (c.2_7dupTGTGGA, p.M1_W2dup) were identical in both the lung and the prostate lesions. These data strongly suggest that the lesions in his right lung were metastasized from his PCa.
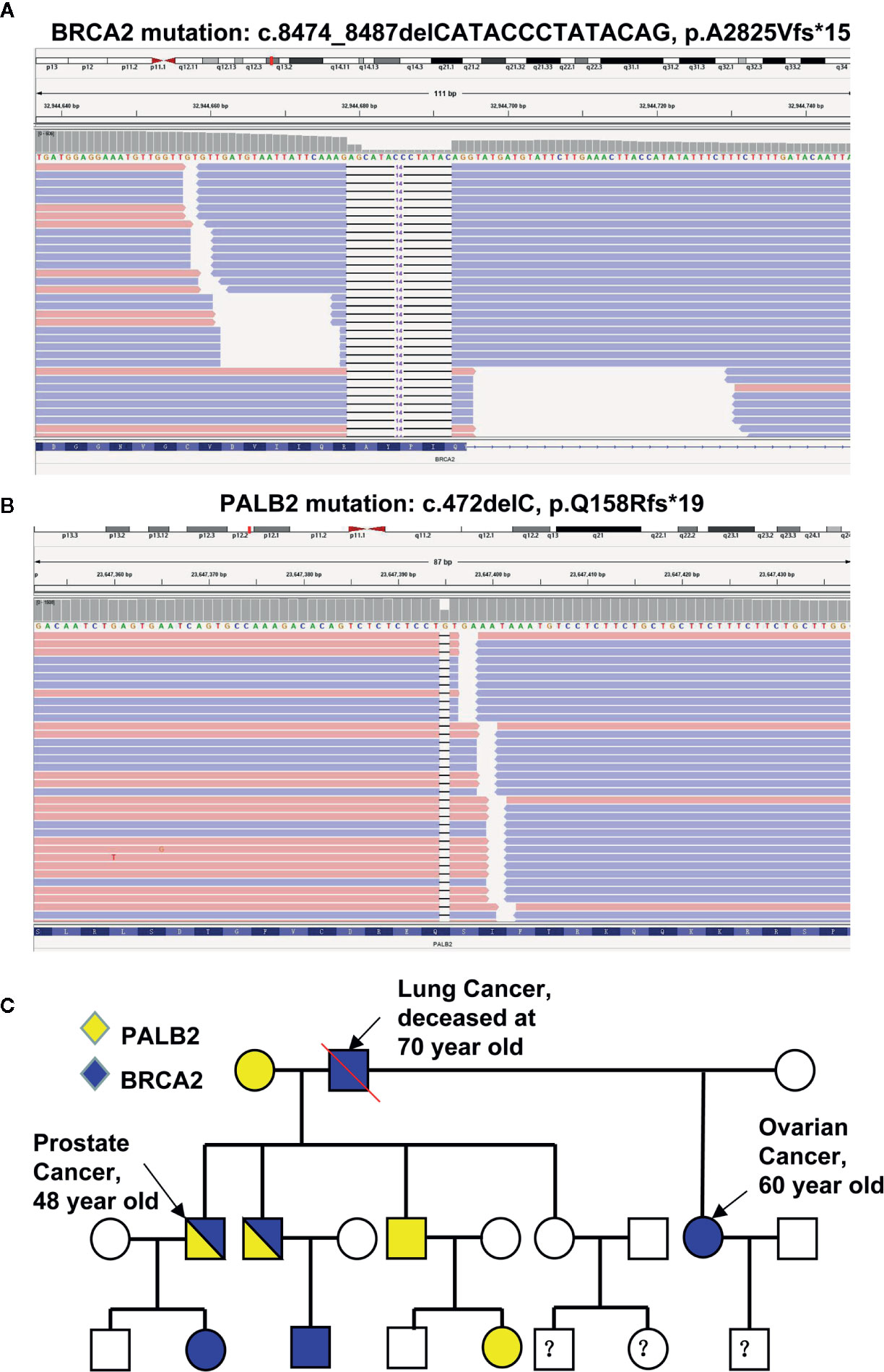
Figure 2 Identification of mutations in the proband and his family members. (A, B) Sequencing reads of BRCA2 (A) and PALB2 (B) are shown by the Integrative Genomic Viewer. (C) The pedigree of mutations.
The treatment regimen for the proband is shown in Figure 3A. Based on the recommendation from the NCCN guideline for M1 castration-naive PCa (15), primary ADT (androgen deprivation therapy) with Goserelin (10.8mg sc 1/3months) started on the date of diagnosis and continued for the rest of the treatment. Between June 29, 2018, and November 20, 2018, six cycles of chemotherapy with the combination of nedaplatin (130 mg, formula: 75 mg/m2 × body surface area) and docetaxel (130 mg, formula: 75 mg/m2 × body surface area) were administrated. His body surface area was about 1.75 m2 based on the formula 0.0061 × height (167 cm) + 0.0124 × weight (60 kg)-0.0099. In addition, EBRT (External Beam Radiation Therapy; 66 Gy/30 F, 2.2 Gy/F) was applied to the local primary PCa between June 4th and June 25th. Only mild adverse events such as slightly reduced leucocyte count were seen during the whole regimen. Workup to evaluate the treatment included the levels of serum PSA and testosterone (Supplementary Table S3), bone imaging, Chest CT and pelvic MRI without contrast. Figure 3B showed that the level of serum testosterone decreased to castrated level 4 months after the beginning of ADT. The levels of serum TPSA became and remained nearly undetectable 5 months after the start of ADT. The concentration of TPSA was 0.01 ng/ml 15 months after the start of the treatment. Pelvic MRI (March 2, 2019) revealed that the prostate volume shrank markedly and the tumor was barely detectable (Figure 1A, bottom panel) 10 months after the start of ADT. Chest CT scans conducted on September 26, 2018 (Figures 1B, C, middle panel) and March 1, 2019 (Figures 1B, C, bottom panel) and PET-CT examination on October 3, 2019 (data not shown) found that the lesions in his right lung disappeared completely. ADT has been continued without any sign of disease progression up to the preparation of this report. In addition and according to the recommendation of the NCCN guideline (16), we have also treated his half-sister’s metastatic ovarian cancer with platinum-based chemotherapy. After six cycles of chemotherapy, her general vital signs including appetite and physical energy improved greatly. More importantly, the ascites disappeared, and the tumors in her ovaries and abdominal cavity did not progress as of the preparation of this report.
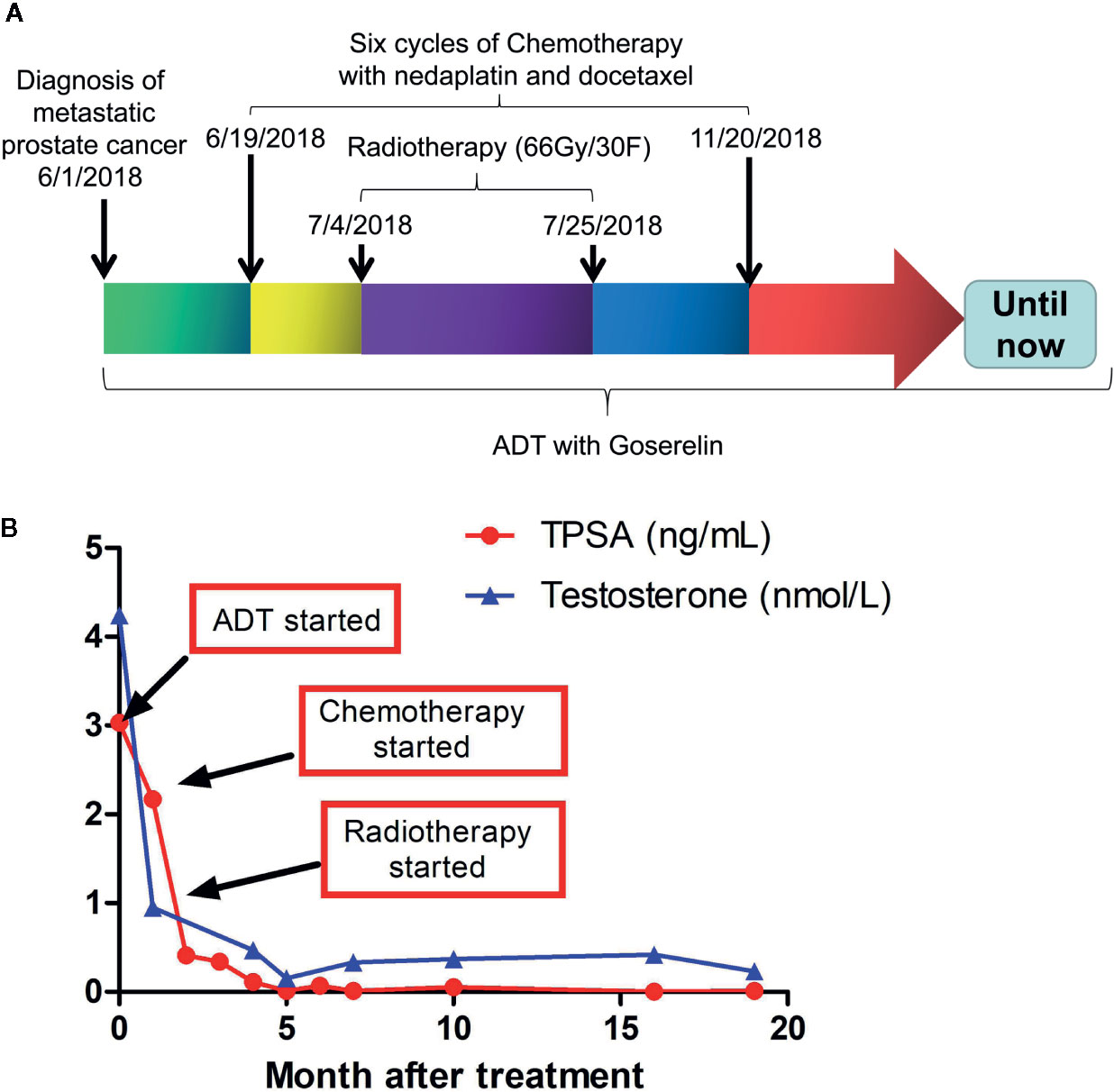
Figure 3 Treatment regimens and laboratory test results during and after the treatment. (A) therapeutic schedules for the proband. (B) Serum levels of TPSAand testosterone in the proband during and after treatment. TPSA, total prostate-specific antigen.
We report in this study a PCa patient carries previously unreported germline mutation of BRCA2 and PALB2 and the same BRCA2 mutation was found in his half-sister who suffered from metastatic ovarian cancer. In addition, the PCa in the proband has also metastasized to his right lung. The DNA sequencing of the proband’s family members showed that the proband’s brother carries the same BRCA2 and PALB2 mutations. In addition, six individuals carry either the mutant BRCA2 (three individuals) or PALB2 (three individuals) in his first- and second-degree relatives (Figure 2C). The proband underwent ADT and platinum-based chemotherapy as well as local radiation for his PCa, and his half-sister with metastatic ovarian cancer was treated with standard platinum-based chemotherapy. Both patients responded extremely well to the regimens, although the follow-up duration is relatively short. Particularly, the proband was found with an undetectable level of PSA, barely detectable PCa, and totally disappeared lung metastases after the systemic treatments.
Germline mutation in genes involved in Lynch syndrome (MSH2, MSH6, and MLH1) and those in homologous recombination (BRCA1, BRCA2, ATM, PALB2, and CHEK2) increase not only the incidence but also aggressiveness of multiple cancer types including prostate, breast, and ovarian cancer. Cancers with these mutations usually also have poorer outcomes (17, 18). Consistent with the findings that mutations of genes in the DDR pathway such as BRCA2, PALB2, and ATM play important roles in PCa (19), we report a PCa patient with BRCA2 and PALB2 double mutations. Germline BRCA2 mutations were found in 5.35% of PCa patients from Caucasian (4) and 6.3% from Chinese population (20). BRCA2 is a protein comprised of 3418 amino acid residues and functions as a scaffold to form a multiprotein complex with Rad51, BRCA1. This complex acts as a caretaker of genome integrity by enabling HR (homologous recombination)-based double-strand DNA break repair and intra-S phase DNA damage checkpoint control. The germline mutant BRCA2 (c.8474_8487delCATACCCTATACAG, p.A2825Vfs*15) identified in this research encodes a truncated protein with 2840 amino acid and lack the 578 residuals at its C-terminus. Since the function of BRCA2 is severely affected when the 110 residuals at its C-terminus are lost (21), the truncated BRCA2 identified in the current report likely encode a loss-of-function BRCA2. Previous studies have demonstrated that loss of heterozygosity (LOH) occurred in most of BRCA carriers, including 100% ovarian cancer with germline BRCA1 mutation (22) and 67% PCa with germline BRCA2 mutation (23). Therefore, we evaluated the LOH of BRCA2 and PALB2 in our case by using allele frequency comparisons (22). Based on the HE staining of the biopsy specimens, we estimated the percentage of tumor cells in the biopsy specimens and found that ~80% of the lung biopsy tissue is composed of tumor cells. However, only about 50% of the prostate biopsy sample is tumor cells. We have also noticed that the variant allele frequencies (VAF) of the mutation PAG1 (c.702A>T) in the lung and prostate biopsy samples were 73.8% and 48.2% (Supplementary Table S2), respectively, proportional to the abovementioned tumor cell percentage, which indicating that the mutation was homozygous. Since the VAF of BRCA2 in lung and prostate biopsy tissues are very close to that of PAG1 in these tissues, we inclined to conclude that BRCA2 mutation in both lung and PCa cells is homozygous resulted from loss-of-heterozygosity in both cancers. On the other hand, since the VAF of PALB2 is about 50% of PAG1’s VAF in both tissues, we conclude that loss-of-heterozygosity did not happen to PALB2 gene in these cancer cells. However, due to lacking any experimental evidence, we were unable to unequivocally conclude that either or both of them are driver genes in PCa development. Nevertheless, based on their roles in DNA damage repair especially additional 10 and 9 somatic mutations were found in prostate and lung lesions, respectively (Supplementary Table S2), we propose that these mutations play important roles in PCa initiation/progression.
Multiple lines of evidence indicate that PCa with germline BRCA2 mutations is more aggressive than those with sporadic mutations (17, 18). In addition, double mutations of members in the DDR pathway not only confer an early onset but also a more aggressive phenotype of the tumors (24, 25). The proband also carries a germline mutation in PALB2 (c.472delC, p.Q158Rfs*19), another member of the DDR pathway. It has reported that the mutant PALB2 (c.1592delT) encodes a loss-of-function PALB2 (26), which is unable to mediate BRCA2 nuclear localization/accumulation and led to HR/DSBR (double strand break repair) deficiency (27). Since the truncated peptide expressed in the proband is even shorter than the previously reported PALB2 expressed from the PALB2 (c.1592delT), it is conceivable that the DDR pathway in the proband with co-mutation of BRCA2 and PALB2 would be affected more severely than those with either one alone. Since the PCa of the proband is encapsulated (T2c) and the level of TPSA (3.03 ng/ml) is not elevated, the tumor in his prostate is likely to be still at its early stage. However, given that (i) his lung cancer is positive of PSA and negative of both CDX-2 and TTF-1, (ii) the extremely high similarity between the mutations found in the lung and prostate lesions with the top six mutations identical, he was diagnosed as a primary PCa with lung metastases, an extremely rare case. Since the PCa has already metastasized to the lung but not the lymph node nor the bone although these organs are the most common metastatic sites of PCa (28), we speculated that PCa with germline co-mutation of BRCA2 and PALB2 might be more aggressive. Therefore, his brother carrying the same double mutations could also have a higher risk of developing an advanced PCa.
Visceral metastases are commonly associated with advanced castration-resistant PCa, prolonged treatment, and neuroendocrine PCa (29, 30). Primary PCa with solitary lung metastasis has rarely been reported with a low incidence of 0.2% (31) and we report a PCa patient with solitary distant metastases to the lung who was found with germline co-mutation of BRCA2 and PALB2. A recent study (32) has shown that mHSPC (metastatic Hormone-Sensitive Prostate Cancer) with lung-only metastases might be an unique molecular and clinical subgroup with significant enrichment for MMR (25%) and HDR (25%) mutations. Of note, two patients with solitary lung metastases were found with germline mutations of BRCA2. Moreover, that study demonstrated that these patients showed favorable clinical outcomes to first-line ADT treatments. Although ADT is the gold standard for patients with metastatic PCa, a universally accepted regimen for these kinds of patients is lacking. Based on the report that (i) mCRPC in patients with biallelic mutant BRCA2 responded well to platinum chemotherapy (33) and (ii) localized PCa with mutant BRCA can be treated with radiotherapy effectively (34, 35), we carefully crafted a regimen tailored to the proband (Figure 3A) and the patient responded to the treatment well (Figure 3B). In addition, his half-sister with metastatic ovarian cancer and the same germline mutation of BRCA2 also responded to platinum-based chemotherapies particularly well. Since cells with HR-deficiencies cannot repair DNA-damage efficiently, the cancer cells in the patients reported here would be more prone to chemo- and/or radiotherapy-mediated cancer cell apoptosis (36–38). We do acknowledge that an 18-month follow-up for PCa is too short to make any solid conclusion for the long-term effect of this regimen. The finding reported here are the first of its kind. We believe that longer follow-up and further research on a larger cohort of patients with these mutations will undoubtedly provide a more solid conclusion for the long-term effect of our therapeutic regimen. In addition, we could not simply attribute the current therapeutic effect to platinum chemotherapy, because ADT and docetaxel might also result in favorable outcomes during such short follow-up (32). Even so, the therapeutic effect of platinum chemotherapy on this patient should be highlighted. Just as Mark M. Pomerantz et al. showed that carboplatin-based chemotherapy could render better prognosis in patients with BRCA2 germline mutations than those without (39), although BRCA2 mutations are associated with more aggressive PCa. Given that PARP inhibitor olaparib and rucaparib can improve progression-free survival for mCRPC patients with mutations in DNA-repair genes (11), olaparib and rucaparib could be an alternative treatment for patients with BRCA2 and/or PALB2 mutations. TOPARP-A (11) and TOPARP-B (13) trails have revealed that olaparib, an orally bioavailable inhibitor of the catalytic activity of PARP1 and PARP2, has antitumor activity against metastatic castration-resistant PCa with specific DDR gene aberrations. More recently, the phase II TRITON2 study has found that the PARP inhibitor rucaparib has antitumor activity in mCRPC patients with a deleterious BRCA alteration (14) and specific non-BRCA DDR gene (e.g., PALB2) alteration (40). In addition, additional clinical trials are in progress for talazoparib, velinarib and niraparib (41).
In summary, we identified two previously unreported germline mutations in the DNA double-strand repair pathway, BRCA2 and PALB2 in a PCa patient with solitary lung metastasis but without bone lesion (T2cNxM1c). Since there is no consensus treatment for these patients, we designed a therapeutic regimen including androgen deprivation therapy, systemic platinum chemotherapy and locally radical radiotherapy specifically tailored to his prostate tumor and the patient responded well. These findings support the guidelines and consensus statements from international clinical organizations (42, 43) that recommend DDR mutation screening for the management of particular PCa patients. More importantly, these newly identified mutations in DDR are associated with PCa and can serve as the base for designing personalized treatment.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Army Medical University IRB. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Conception/design: JJ and QL. Provision of study material or patients: TT and L-aW. Collection and/or assembly of data: TT, L-aW, PW, DT, GL, and GY. Data analysis and interpretation: JZ, YZ, ND, and JJ. Manuscript writing and revising: TT, L-aW, QL, KG, DZ, and JJ. All authors contributed to the article and approved the submitted version.
This work was supported by University Research Project of Army Medical University (2017XYY07, JJ).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank all the patients and their families in this study for their collaboration.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.564694/full#supplementary-material
DDR, DNA damage response; PCa, Prostate cancer; MMR, mismatch repair; PARP, poly (ADP) ribose polymerase; PSA, prostate-specific antigen; MRI, magnetic resonance imaging; CT, Computed tomography; PET, Positron emission tomography; ADT, androgen deprivation therapy; EBRT, External Beam Radiation Therapy; HR, homologous recombination; DSBR, double strand break repair.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
2. Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Institute (1994) 86(21):1600–8. doi: 10.1093/jnci/86.21.1600
3. Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. New Engl J Med (2000) 343(2):78–85. doi: 10.1056/NEJM200007133430201
4. Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. New Engl J Med (2016) 375(5):443–53. doi: 10.1056/NEJMoa1603144
5. Beltran H, Yelensky R, Frampton GM, Park K, Downing SR, MacDonald TY, et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur Urol (2013) 63(5):920–6. doi: 10.1016/j.eururo.2012.08.053
6. Cancer Genome Atlas Research N. The Molecular Taxonomy of Primary Prostate Cancer. Cell (2015) 163(4):1011–25. doi: 10.1016/j.cell.2015.10.025
7. Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell (2015) 162(2):454. doi: 10.1016/j.cell.2015.06.053
8. Pritchard CC. Molecular insights into the germline for prostate cancer initiation, progression, and aggressiveness. Can J Urol (2019) 26(5S2):24–6.
9. Mitra A, Fisher C, Foster CS, Jameson C, Barbachanno Y, Bartlett J, et al. Prostate cancer in male BRCA1 and BRCA2 mutation carriers has a more aggressive phenotype. Br J Cancer (2008) 98(2):502–7. doi: 10.1038/sj.bjc.6604132
10. Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol: Off J Am Soc Clin Oncol (2013) 31(14):1748–57. doi: 10.1200/JCO.2012.43.1882
11. Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. New Engl J Med (2015) 373(18):1697–708. doi: 10.1056/NEJMoa1506859
12. Banks P, Xu W, Murphy D, James P, Sandhu S. Relevance of DNA damage repair in the management of prostate cancer. Curr Prob Cancer (2017) 41(4):287–301. doi: 10.1016/j.currproblcancer.2017.06.001
13. Mateo J, Porta N, Bianchini D, McGovern U, Elliott T, Jones R, et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol (2020) 21(1):162–74. doi: 10.1016/S1470-2045(19)30684-9
14. Abida W, Patnaik A, Campbell D, Shapiro J, Bryce AH, McDermott R, et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol: Off J Am Soc Clin Oncol (2020) JCO2001035. doi: 10.1200/JCO.20.01035
15. Mohler JL, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, Dorff T, et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw: JNCCN (2019) 17(5):479–505. doi: 10.6004/jnccn.2019.0023
16. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. NCCN Guidelines Insights: Ovarian Cancer, Version 1.2019. J Natl Compr Cancer Netw: JNCCN (2019) 17(8):896–909. doi: 10.6004/jnccn.2019.0039
17. Castro E, Goh C, Leongamornlert D, Saunders E, Tymrakiewicz M, Dadaev T, et al. Effect of BRCA Mutations on Metastatic Relapse and Cause-specific Survival After Radical Treatment for Localised Prostate Cancer. Eur Urol (2015) 68(2):186–93. doi: 10.1016/j.eururo.2014.10.022
18. Taylor RA, Fraser M, Livingstone J, Espiritu SM, Thorne H, Huang V, et al. Germline BRCA2 mutations drive prostate cancers with distinct evolutionary trajectories. Nat Commun (2017) 8:13671. doi: 10.1038/ncomms13671
19. Lang SH, Swift SL, White H, Misso K, Kleijnen J, Quek RGW. A systematic review of the prevalence of DNA damage response gene mutations in prostate cancer. Int J Oncol (2019) 55(3):597–616. doi: 10.3892/ijo.2019.4842
20. Wei Y, Wu J, Gu W, Qin X, Dai B, Lin G, et al. Germline DNA Repair Gene Mutation Landscape in Chinese Prostate Cancer Patients. Eur Urol (2019) 76(3):280–3. doi: 10.1016/j.eururo.2019.06.004
21. Sugano K, Nakamura S, Ando J, Takayama S, Kamata H, Sekiguchi I, et al. Cross-sectional analysis of germline BRCA1 and BRCA2 mutations in Japanese patients suspected to have hereditary breast/ovarian cancer. Cancer Sci (2008) 99(10):1967–76. doi: 10.1111/j.1349-7006.2008.00944.x
22. Kanchi KL, Johnson KJ, Lu C, McLellan MD, Leiserson MD, Wendl MC, et al. Integrated analysis of germline and somatic variants in ovarian cancer. Nat Commun (2014) 5:3156. doi: 10.1038/ncomms4156
23. Castro E, Jugurnauth-Little S, Karlsson Q, Al-Shahrour F, Pineiro-Yanez E, Van de Poll F, et al. High burden of copy number alterations and c-MYC amplification in prostate cancer from BRCA2 germline mutation carriers. Ann Oncol: Off J Eur Soc Med Oncol (2015) 26(11):2293–300. doi: 10.1093/annonc/mdv356
24. Andres R, Menao S, Arruebo M, Quilez E, Cardiel MJ. Double heterozygous mutation in the BRCA1 and ATM genes involved in development of primary metachronous tumours: a case report. Breast Cancer Res Treat (2019) 177(3):767–70. doi: 10.1007/s10549-019-05343-4
25. Heidemann S, Fischer C, Engel C, Fischer B, Harder L, Schlegelberger B, et al. Double heterozygosity for mutations in BRCA1 and BRCA2 in German breast cancer patients: implications on test strategies and clinical management. Breast Cancer Res Treat (2012) 134(3):1229–39. doi: 10.1007/s10549-012-2050-4
26. Erkko H, Xia B, Nikkila J, Schleutker J, Syrjakoski K, Mannermaa A, et al. A recurrent mutation in PALB2 in Finnish cancer families. Nature (2007) 446(7133):316–9. doi: 10.1038/nature05609
27. Xia B, Sheng Q, Nakanishi K, Ohashi A, Wu J, Christ N, et al. Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol Cell (2006) 22(6):719–29. doi: 10.1016/j.molcel.2006.05.022
28. Gandaglia G, Abdollah F, Schiffmann J, Trudeau V, Shariat SF, Kim SP, et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate (2014) 74(2):210–6. doi: 10.1002/pros.22742
29. Pezaro C, Omlin A, Lorente D, Rodrigues DN, Ferraldeschi R, Bianchini D, et al. Visceral disease in castration-resistant prostate cancer. Eur Urol (2014) 65(2):270–3. doi: 10.1016/j.eururo.2013.10.055
30. Flechon A, Pouessel D, Ferlay C, Perol D, Beuzeboc P, Gravis G, et al. Phase II study of carboplatin and etoposide in patients with anaplastic progressive metastatic castration-resistant prostate cancer (mCRPC) with or without neuroendocrine differentiation: results of the French Genito-Urinary Tumor Group (GETUG) P01 trial. Ann Oncol: Off J Eur Soc Med Oncol (2011) 22(11):2476–81. doi: 10.1093/annonc/mdr004
31. Fabozzi SJ, Schellhammer PF. el-Mahdi AM. Pulmonary metastases from prostate cancer. Cancer (1995) 75(11):2706–9. doi: 10.1002/1097-0142(19950601)75:11<2706::aid-cncr2820751111>3.0.co;2-y
32. Shenderov E, Isaacsson Velho P, Awan AH, Wang H, Mirkheshti N, Lotan TL, et al. Genomic and clinical characterization of pulmonary-only metastatic prostate cancer: A unique molecular subtype. Prostate (2019) 79(13):1572–9. doi: 10.1002/pros.23881
33. Cheng HH, Pritchard CC, Boyd T, Nelson PS, Montgomery B. Biallelic Inactivation of BRCA2 in Platinum-sensitive Metastatic Castration-resistant Prostate Cancer. Eur Urol (2016) 69(6):992–5. doi: 10.1016/j.eururo.2015.11.022
34. Liu Q, Tong D, Liu G, Yi Y, Xu J, Yang X, et al. A novel BRCA2 mutation in prostate cancer sensitive to combined radiotherapy and androgen deprivation therapy. Cancer Biol Ther (2018) 19(8):669–75. doi: 10.1080/15384047.2018.1451278
35. Bratt O, Loman N. Clinical Management of Prostate Cancer in Men with BRCA Mutations. Eur Urol (2015) 68(2):194–5. doi: 10.1016/j.eururo.2014.11.005
36. Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature (2005) 434(7035):917–21. doi: 10.1038/nature03445
37. Tan DS, Rothermundt C, Thomas K, Bancroft E, Eeles R, Shanley S, et al. “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol: Off J Am Soc Clin Oncol (2008) 26(34):5530–6. doi: 10.1200/JCO.2008.16.1703
38. Tutt AN, Lord CJ, McCabe N, Farmer H, Turner N, Martin NM, et al. Exploiting the DNA repair defect in BRCA mutant cells in the design of new therapeutic strategies for cancer. Cold Spring Harbor Symp Quantitative Biol (2005) 70:139–48. doi: 10.1101/sqb.2005.70.012
39. Pomerantz MM, Spisak S, Jia L, Cronin AM, Csabai I, Ledet E, et al. The association between germline BRCA2 variants and sensitivity to platinum-based chemotherapy among men with metastatic prostate cancer. Cancer (2017) 123(18):3532–9. doi: 10.1002/cncr.30808
40. Abida W, Campbell D, Patnaik A, Shapiro JD, Sautois B, Vogelzang NJ, et al. Non-BRCA DNA Damage Repair Gene Alterations and Response to the PARP Inhibitor Rucaparib in Metastatic Castration-Resistant Prostate Cancer: Analysis From the Phase II TRITON2 Study. Clin Cancer Res: Off J Am Assoc Cancer Res (2020) 26(11):2487–96. doi: 10.1158/1078-0432.CCR-20-0394
41. Bryce AH, Sartor O, de Bono J. DNA Repair and Prostate Cancer: A Field Ripe for Harvest. Eur Urol (2020) 78(4):486–8. doi: 10.1016/j.eururo.2020.06.020
42. Giri VN, Knudsen KE, Kelly WK, Abida W, Andriole GL, Bangma CH, et al. Role of Genetic Testing for Inherited Prostate Cancer Risk: Philadelphia Prostate Cancer Consensus Conference 2017. J Clin Oncol: Off J Am Soc Clin Oncol (2018) 36(4):414–24. doi: 10.1200/JCO.2017.74.1173
Keywords: BRCA2, PALB2, prostate cancer, platinum-based chemotherapy, radiotherapy, case report
Citation: Tang T, Wang L-a, Wang P, Tong D, Liu G, Zhang J, Dai N, Zhang Y, Yuan G, Geary K, Zhang D, Liu Q and Jiang J (2020) Case Report: Co-Existence of BRCA2 and PALB2 Germline Mutations in Familial Prostate Cancer With Solitary Lung Metastasis. Front. Oncol. 10:564694. doi: 10.3389/fonc.2020.564694
Received: 22 May 2020; Accepted: 29 September 2020;
Published: 26 October 2020.
Edited by:
Michal Mego, Campus Bio-Medico University, ItalyReviewed by:
Emmanuel S. Antonarakis, Sidney Kimmel Cancer Center, United StatesCopyright © 2020 Tang, Wang, Wang, Tong, Liu, Zhang, Dai, Zhang, Yuan, Geary, Zhang, Liu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Jiang, amlhbmdqdW5fNjRAMTYzLmNvbQ==; Qiuli Liu, bGl1cWl1bGk5MDA4MjdAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.