- 1Department of Liver Transplantation and Hepatobiliary Surgery, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Department of Liver Transplantation and Hepatobiliary Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 3Department of Breast and Thyroid Surgery, Shandong Maternity and Child Care Hospital, Jinan, China
- 4Cheeloo College of Medicine, Shandong University, Jinan, China
- 5Department of Pathology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
Background: The relationship between tumor size and survival in intrahepatic cholangiocarcinoma (ICC) is still controversial. This study aimed to evaluate the prognostic ability of tumor size for solitary ICC after resection and explore optimal cut-off values in different subgroups.
Methods: Patients with solitary ICC who underwent liver resection from the Surveillance, Epidemiology, and End Results Program and Shandong Provincial Hospital were retrospectively analyzed. Kaplan-Meier and Cox regression analysis were used to assess the prognostic ability of tumor size. The log-rank test was used to determine the optimal cut-off values, and a minimum P was regarded as the optimal one in different subgroups.
Results: Large tumor size groups had worse overall survival (OS) than small tumor size groups. Cox regression analysis suggested that tumor size was an independent prognostic factor for OS for solitary ICC after resection. Subgroup analysis showed tumor size was associated with OS for both solitary ICC with and without vascular invasion (VI). Furthermore, the optimal cut-off values for solitary ICC with and without VI were found to be 8 and 3 cm, respectively, which could divide the patients into two groups with significant differences in OS.
Conclusion: Tumor size was an independent prognostic factor for solitary ICC after resection. The existing American Joint Committee on Cancer (AJCC) staging system could be improved if the cut-off value of the T1 stage was changed to 8 cm and if the T2 stage incorporated a tumor size with a cut-off value of 3 cm. Further studies with more cases are needed to validate these findings.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most prevalent primary liver cancer after hepatocellular carcinoma (HCC), and the incidence of ICC has been increasing worldwide over the last two decades (1, 2). Liver resection is a potential curative strategy that can prolong overall survival (OS) for patients with ICC; however, the prognosis of these patients is still dismal, and recent studies suggest that the 5-year OS of patients with ICC after surgery is only approximately 30% (3). To date, many pathological features have been discovered to be associated with the prognosis of ICC, including vascular invasion (VI), tumor differentiation, tumor size, tumor number, lymph node metastasis, and surgical resection margin (1, 4).
Diameter is a crucial characteristic of solid tumors, and it has been demonstrated to be a prognostic factor for many cancers and integrated into various staging systems to guide treatment and predict prognosis (5). In the 8th American Joint Committee on Cancer (AJCC) cancer TNM staging system for ICC, tumor size is used to differentiate T1 into T1a (≤ 5 cm) and T1b (> 5 cm), while T2 is classified based on VI and tumor number, and T3 and T4 are defined based on the invasion of surrounding tissues or organs (6). The effect of tumor size on survival in ICC has been reported in many studies (7–10); however, the results in these explorations were inconsistent in terms of both the prognostic ability and the identification of optimal cut-off values. The ambiguous status of diameter as a factor in prognosis and the imprecise cut-off point for the diameter could influence the reliability of the existing staging systems and affect the selection of treatment strategy and the prediction of prognosis (7). Worse still, due to the relatively low incidence of ICC, rare studies have focused on the effect of tumor size on survival in patients with solitary ICC after surgery, which makes it difficult to evaluate the predictive efficacy of the existing TNM staging systems for ICC.
In this study, using data obtained from the Surveillance, Epidemiology, and End Results (SEER) Program, we aimed to analyze the clinicopathological characteristics among different tumor size groups and evaluate the prognostic ability of tumor size for solitary ICC after surgery. The optimal cut-off values for different subgroups were explored, and an independent patient cohort from Shandong Provincial Hospital (SDPH) was used to validate the observations in the analysis of the SEER database.
Materials and Methods
Ethics Statement
This study was approved by the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong University and was performed in accordance with the Declaration of Helsinki. Each participant provided written informed consent.
Patient Selection in SEER Set
The SEER 18 Regs Research Data (with additional treatment fields, 1975–2016 varying) were used as the data source for this study (https://seer.cancer.gov/), and SEER*Stata software (Version 8.3.5) was employed for analysis. Patients diagnosed between 1975 and 2016 were identified using the cite code C22.0 (liver) and a histological diagnosis of cholangiocarcinoma (International Classification of Diseases for Oncology, 3rd Edition [ICD-O-3] code 8,160) or the cite code C22.1 (intrahepatic bile duct) and histological code 8,140 (adenocarcinoma) or 8,160. Only patients who received surgical treatment and were diagnosed with ICC by positive histology aged between 18 and 90 years were included. Afterwards, patients diagnosed before 2002 were excluded, and those diagnosed in 2016 were also excluded because the follow-up time was less than 12 months. Patients were excluded if they had incomplete clinicopathological information, lymph node or distant metastasis, macrovascular invasion (referred to major branches of portal or hepatic vein, and vena cava), multiple tumors, and incomplete follow-up information or less than 3 months of follow-up time. Figure 1 shows the workflow of patient selection.
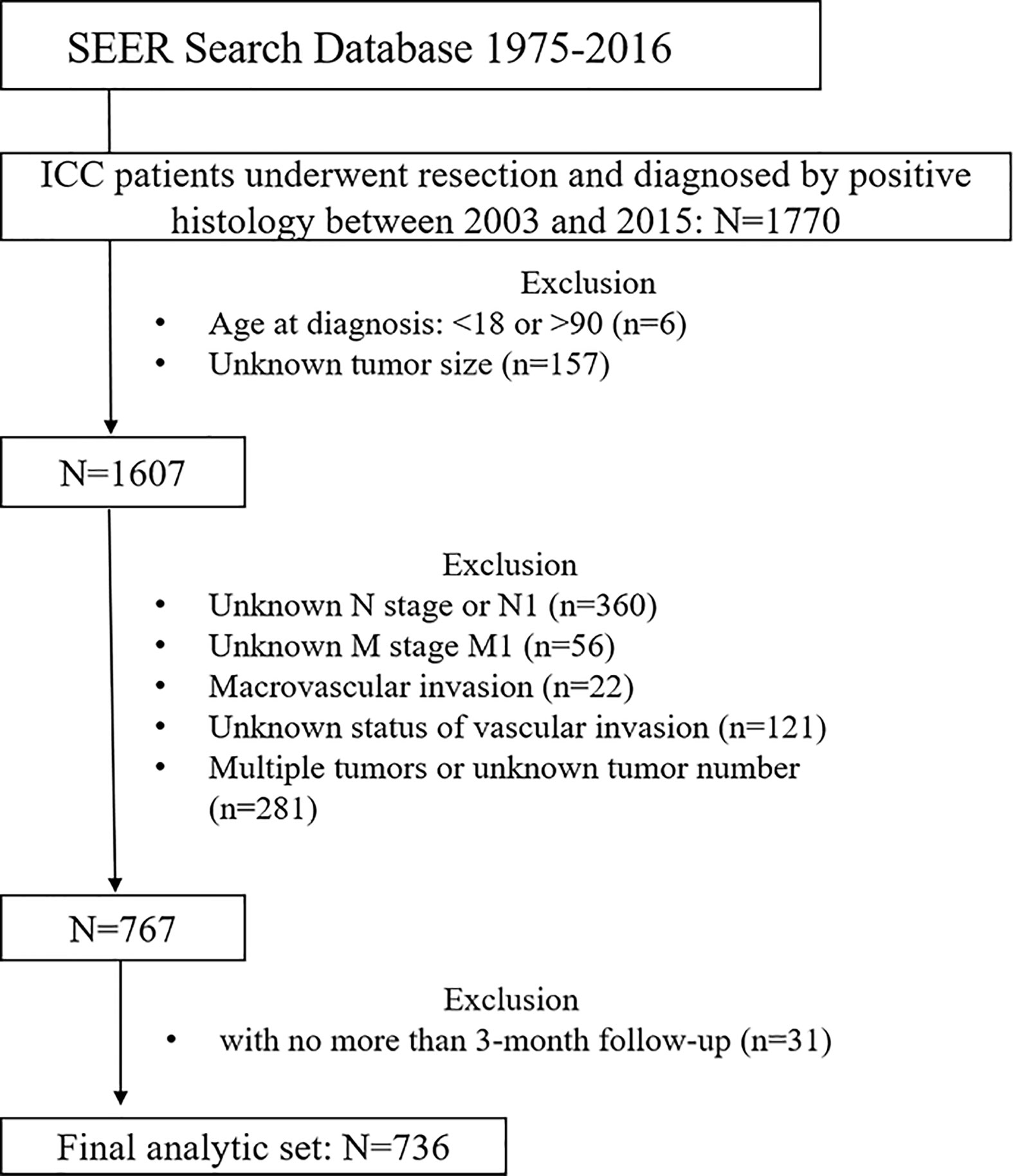
Figure 1 Workflow of patient selection. ICC, intrahepatic cholangiocarcinoma; SEER, The Surveillance, Epidemiology, and End Results Program.
Patient Selection in the SDPH Set
To validate the findings obtained from the analysis of the SEER program, patients with solitary ICC who received surgical treatment between May 2007 and December 2017 at SDPH were retrospectively analyzed. Patients who had incomplete clinicopathological characteristics, less than 3 months of follow-up time or incomplete follow-up information were excluded. Patients were excluded if they received adjuvant chemotherapy before surgery, were diagnosed at an age of <18 or >90 years old, had lymph node or distant metastasis or had macrovascular invasion. Tumor size was evaluated based on preoperative imaging and recorded according to the greatest dimension of axial images. Patients were postoperatively followed up using regular laboratory tests and imaging examinations at intervals of 2–3 months during the first year after surgery and 3–6 months thereafter. OS was defined as the time from the date of surgery to death or to the latest date of follow-up.
Statistical Analysis
Tumor size was treated as a categorical variable using cut-off points of 2, 5, and 7 cm, as proposed by the Liver Cancer Study Group of Japan (LCSGJ), the AJCC 8th edition staging system and a multicenter study from Eastern and Western countries (6, 7, 11), and patients were thus classified into four subgroups: Group I (0–2 cm), Group II (2–5 cm), Group III (5–7 cm), and Group IV (>7 cm). Categorical variables are displayed as numbers (n) and proportions (%), and continuous variables are displayed as the median (interquartile range, IQR). The chi-square test and Fisher’s exact test were used to compare variables among different groups. Kaplan-Meier analysis and the log-rank test were used to analyze OS. Moreover, a minimum P value method was used to determine the optimal cut-off points for tumor size, 10 cut-off points ranging from 2 to 11 cm were chosen, and the patients were divided into large and small tumor size groups using different cut-off points. Afterwards, the log-rank test was used to compare the predictive ability of OS of different tumor size thresholds, and a minimum P was regarded as the optimal one (7, 12).
The Cox proportional hazard model was applied to identify independent prognostic factors associated with OS. Variables with p <0.05 in the univariate analysis were regarded as potential risk factors and were included in the multivariate analysis. The hazard ratios (HRs) with 95% confidence intervals (95% CIs) were recorded. All statistical tests were two-sided, and p <0.05 was defined as statistical significance. SPSS version 22.0 (SPSS Inc., Chicago, IL) and R software (version 3.5.2) were employed in the statistical analysis.
Results
Demographic and Clinicopathological Characteristics of Patients With ICC
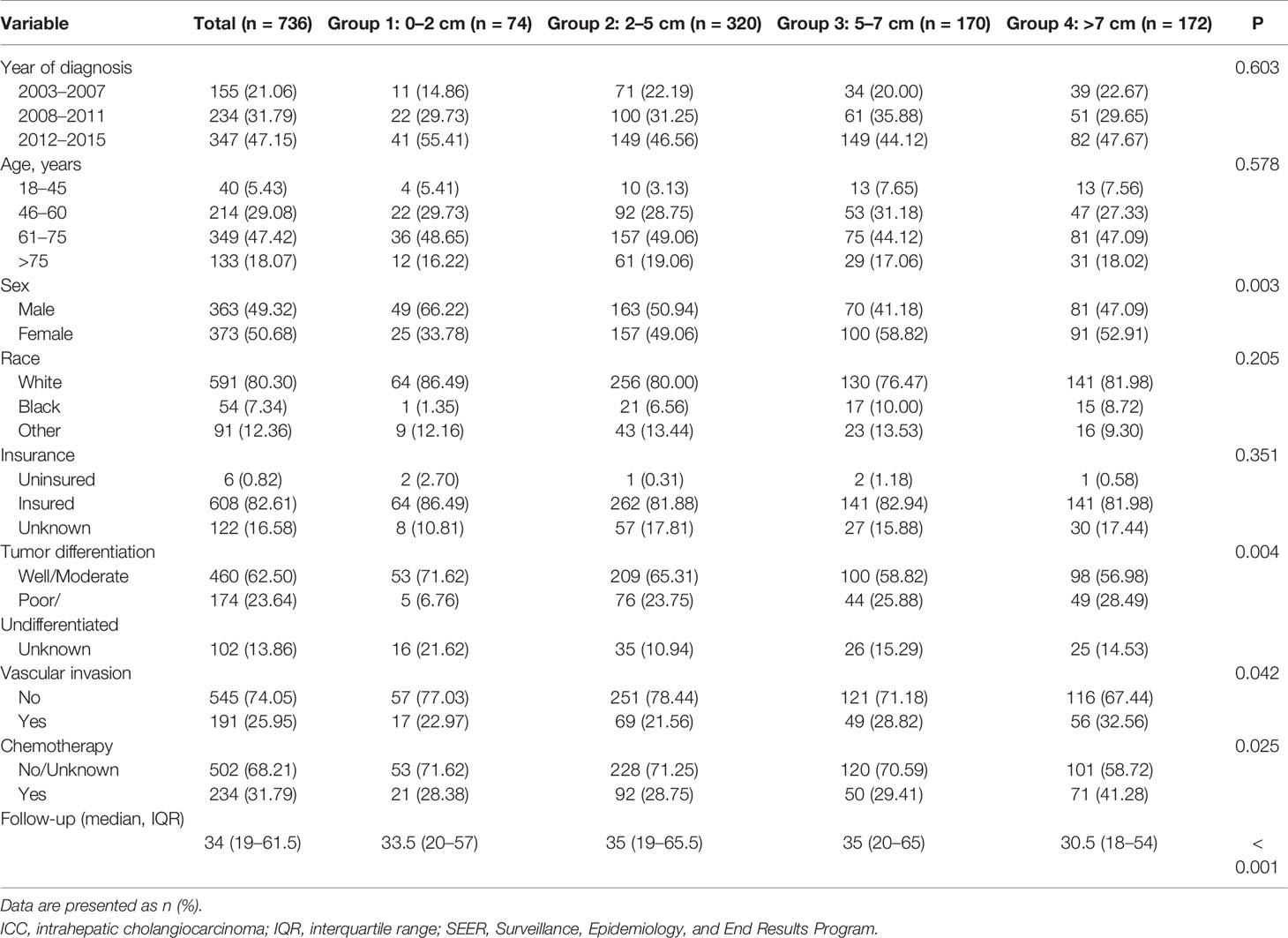
A total of 736 patients in SEER database satisfied the inclusion criteria and were included in the analysis; there were 74 (10.05%) patients in group I, 320 (43.48%) patients in group II, 170 (23.10%) patients in groups III, and 172 (23.37%) patients in group IV. A total of 155 (21.06%) patients were diagnosed with solitary ICC between 2003 and 2007, 234 (31.79%) patients between 2008 and 2011, and 347 (47.15%) patients between 2012 and 2015. Most of the patients (563 patients, 76.49%) were diagnosed between 45 and 75 years old. Three hundred sixty-three (49.32%) patients were male, and 373 (50.68%) patients were female. The median follow-up time was 34 months (IQR: 19 to 61.5 months) for all patients. Table 1 displayed the patients’ demographic and clinicopathological characteristics.
Clinicopathological Characteristics Among Different Tumor Size Groups
As shown in Table 1, while the demographic traits among different tumor size groups were comparable, significant differences in clinicopathological characteristics, including sex, tumor differentiation, VI and chemotherapy (yes or No/Unknown), were found. We found that larger tumor size groups had a higher proportion of female patients (p = 0.003), had poorer tumor differentiation (p = 0.004), had a higher proportion of VI (p < 0.001), and had a higher proportion of receiving chemotherapy (p = 0.025).
Influence of Tumor Size on OS in the SEER Database
The 1-, 3-, and 5-year OS was 91.8, 71.1, and 60.0%, respectively, in group I; 91.6, 67.5, and 54.1%, respectively, in group II; 91.7, 67.8, and 50.6%, respectively, in group III; and 85.3, 58.9, and 42.7%, respectively, in group IV. Kaplan-Meier analysis showed that tumor size was associated with the prognosis of solitary ICC after surgery, and larger tumor size groups tended to have a worse prognosis (Figure 2, p = 0.032).
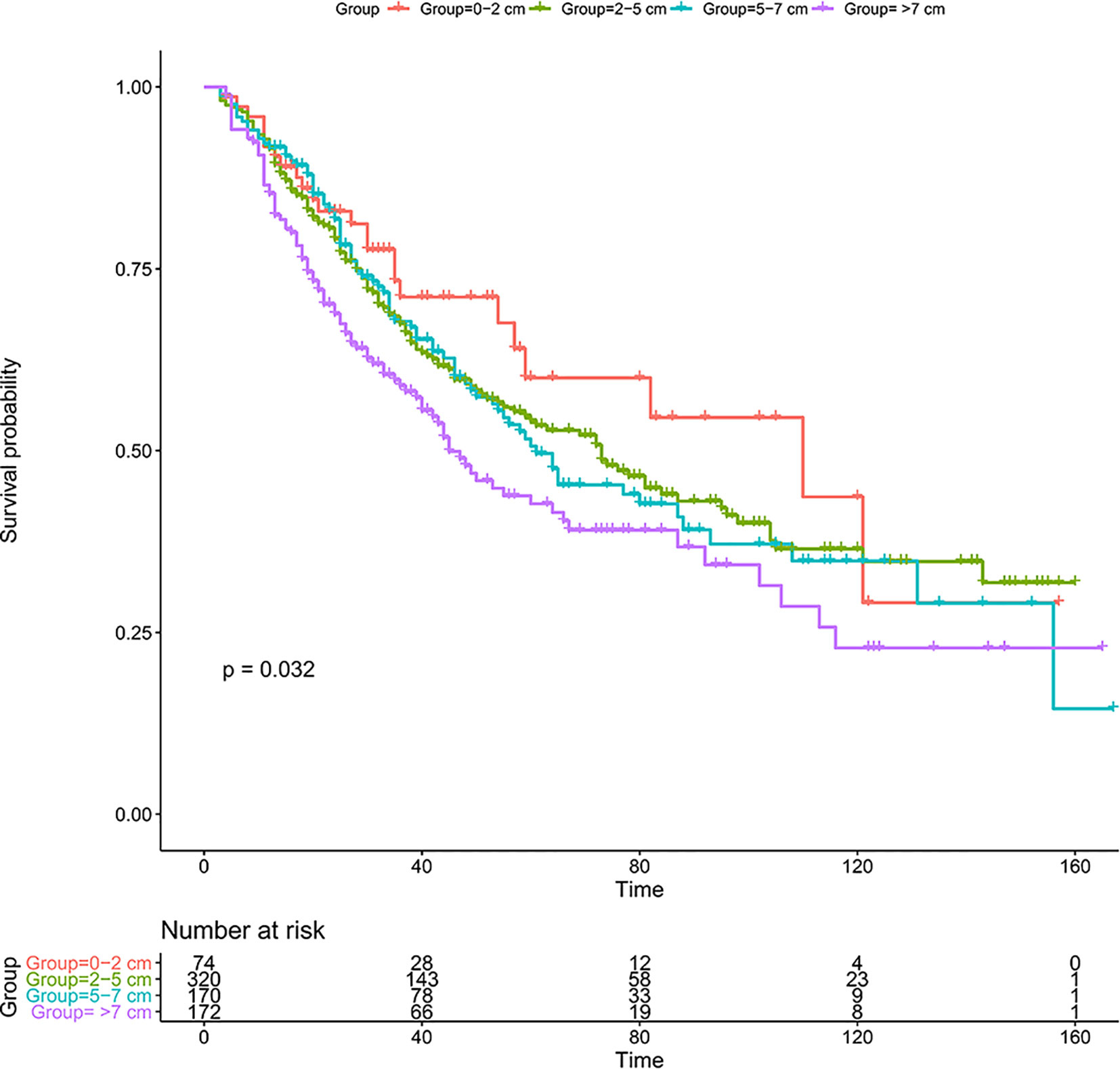
Figure 2 Kaplan-Meier analysis for solitary patients with ICC after resection in the SEER database. ICC, intrahepatic cholangiocarcinoma; SEER, The Surveillance, Epidemiology, and End Results Program.
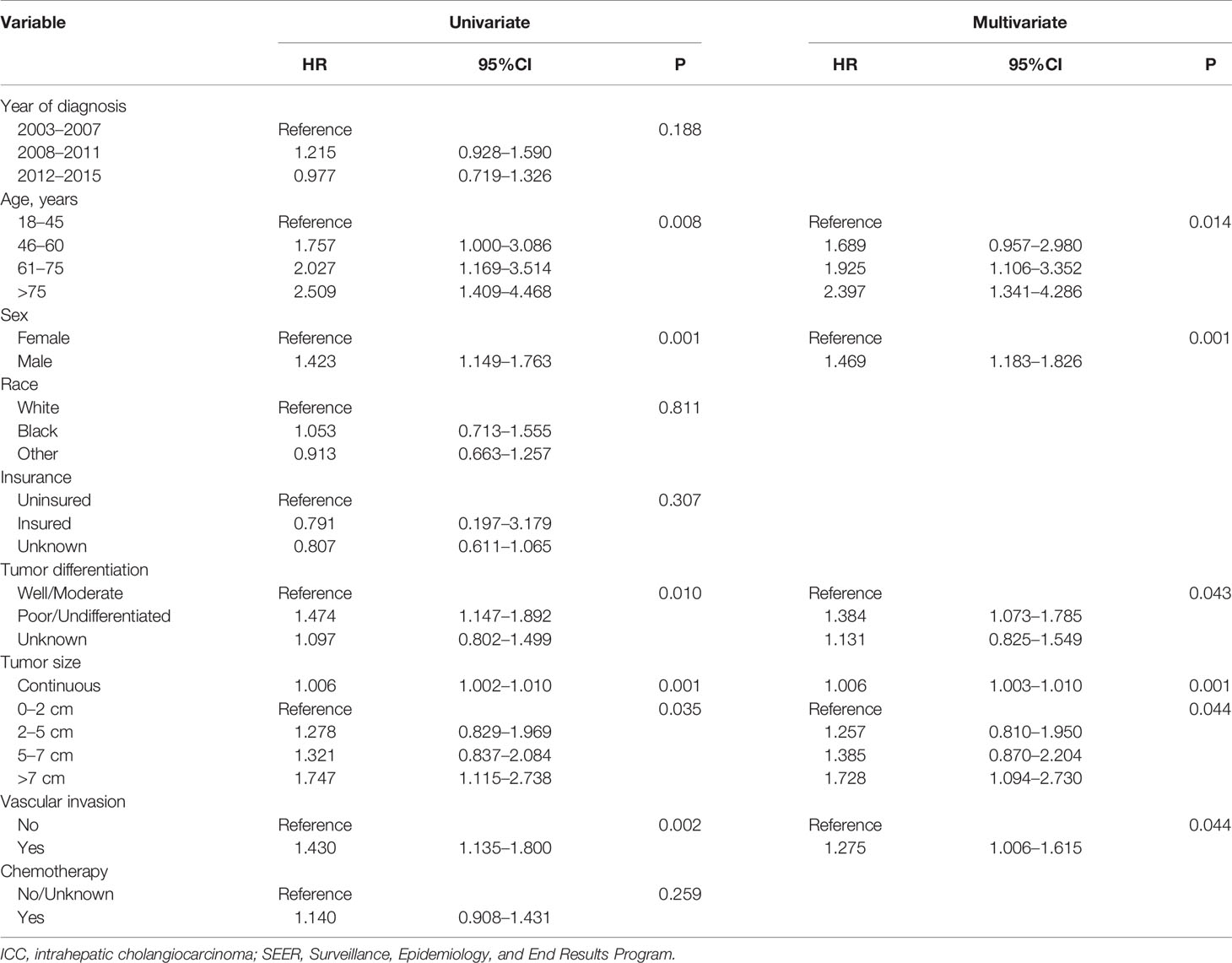
Univariate Cox regression analysis showed that age at diagnosis, sex, tumor differentiation, tumor size (treated as a continuous or categorical variable) and VI were associated with the prognosis of solitary ICC (p < 0.05, Table 2). Furthermore, multivariate analysis was conducted, and the following 5 variables were independent prognostic factors for solitary ICC after surgery: age at diagnosis (p = 0.014, 46–60: HR 1.689; 95% CI 0.957–2.980; 61–75: HR 1.925; 95% CI 1.106–3.352; >75: HR 2.397; 95% CI 1.341–4.286), sex (female: p = 0.001, HR 1.469, 95% CI 1.183–1.826), tumor differentiation (poor/undifferentiated: p = 0.043, HR 1.384, 95% CI 1.073–1.785), tumor size (continuous variable: p = 0.001, HR 1.006, 95% CI 1.003–1.010; categorical variable: p = 0.044, 2–5 cm: HR 1.257, 95% CI 0.810–1.950; 5–7 cm, HR 1.385, 95% CI 0.870–2.204; >7 cm: HR 1.728, 95% CI 1.094–2.730) and VI (Yes: p = 0.044, HR 1.275, 95% CI 1.006–1.615).
Effect of Tumor Size on OS in Subgroups Classified by VI
A total of 545 and 191 patients were classified into the solitary ICC without VI and with VI subgroups, respectively. The 1-, 3-, and 5-year OS in the different subgroups are displayed in Table S1; however, Kaplan-Meier analysis showed that tumor size was not associated with OS in both subgroups (p > 0.05, Figure S1).
Furthermore, we conducted Cox regression analysis to explore the prognostic ability of tumor size in different subgroups. As shown in Table S2, for solitary ICC without VI, although tumor size was not a prognostic factor when treated as a categorical variable (p = 0.175), it was an independent prognostic factor when regarded as a continuous variable (p < 0.001, HR 1.075, 95% CI 1.040–1.112). For solitary ICC with VI, there was not significant difference for the effect of tumor size on OS when it was treated as categorical variable (p < 0.001); however, when it was treated as a continuous variable, tumor size had an influence on OS (p = 0.055, HR 1.005, 95% CI 1.000–1.011).
Identification of Optimal Cut-Off Values in Subgroups Classified by VI
Afterwards, 10 cut-off points ranging from 2 to 11 cm were chosen, and the log-rank test was used to determine the optimal cut-off values in different groups. For solitary ICC, an optimal cut-off value of tumor size of 8 cm could divide the patients into two subgroups with 1-, 3-, and 5-year OS rates of 91.4, 67.5, and 53.3%, respectively, for 0 to 8 cm tumors and 83.5, 57.5, and 38.7%, respectively, for >8 cm tumors (Figures 3A, C). For solitary ICC without VI, an optimal cut-off value of 8 cm could divide the patients into two subgroups with 1-, 3-, and 5-year OS rates of 92.4, 69.8, and 56.3%, respectively, for 0 to 8 cm tumors, and 86.1, 56.4, and 43.6%, respectively, for >8 cm tumors (Figures 3B, D). For solitary ICC with VI, an optimal cut-off value of 3 cm could divide the patients into two subgroups with 1-, 3-, and 5-year OS rates of 90.0, 65.0, and 60.0%, respectively, for 0 to 3 cm tumors and 85.4, 58.4, and 37.5%, respectively, for >3 cm tumors (Figures 3C, E). The ROC curves also suggested that the three cut-off values had predictive efficiencies for ICC prognosis (Figure S2).
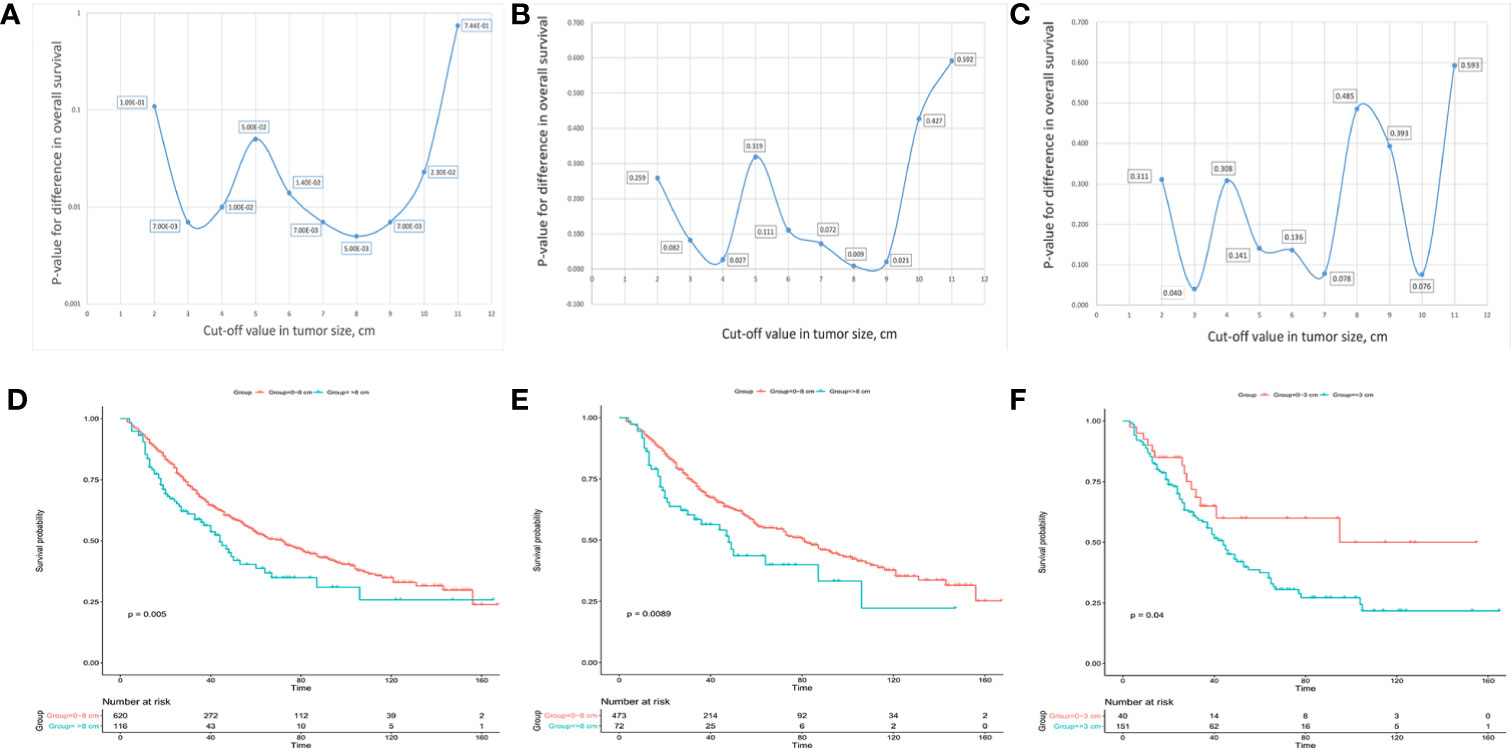
Figure 3 Charts for different cut-off points with corresponding p values and Kaplan-Meier analysis for the optimal cut-off points in solitary ICC (A, D), solitary ICC without VI (B, E) and solitary ICC with VI (C, F).
Validating the Prognostic Ability of Tumor Size in the SDPH Set
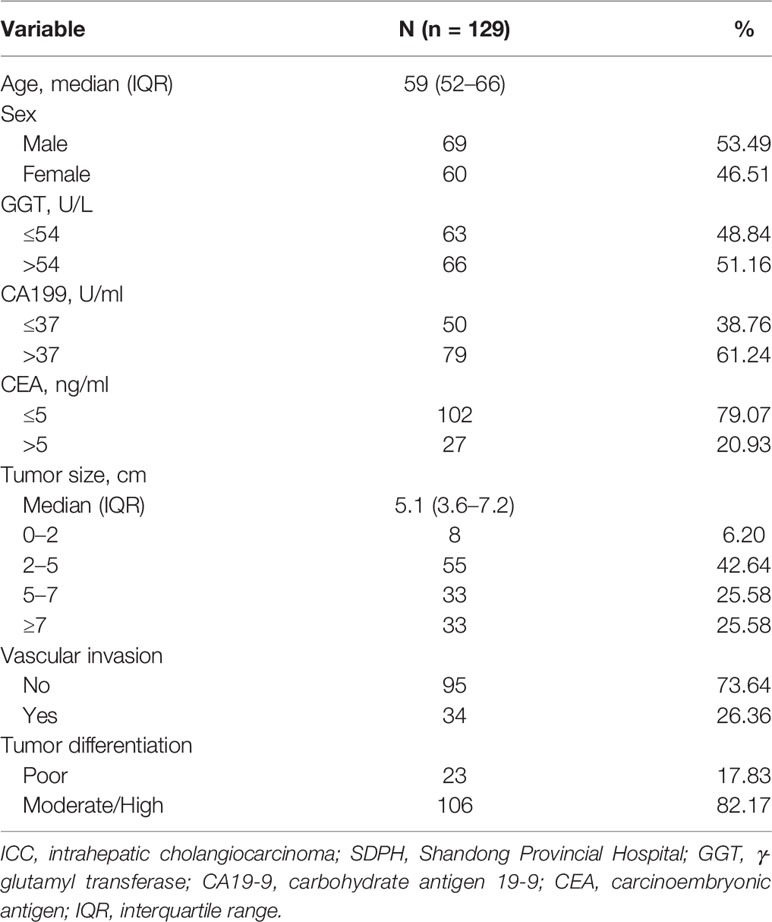
To validate the findings in the SEER set, a total of 129 eligible patients with solitary ICC from the SDPH set were analyzed as the external validation dataset. The demographic and clinicopathological characteristics of the patients are shown in Table 3.
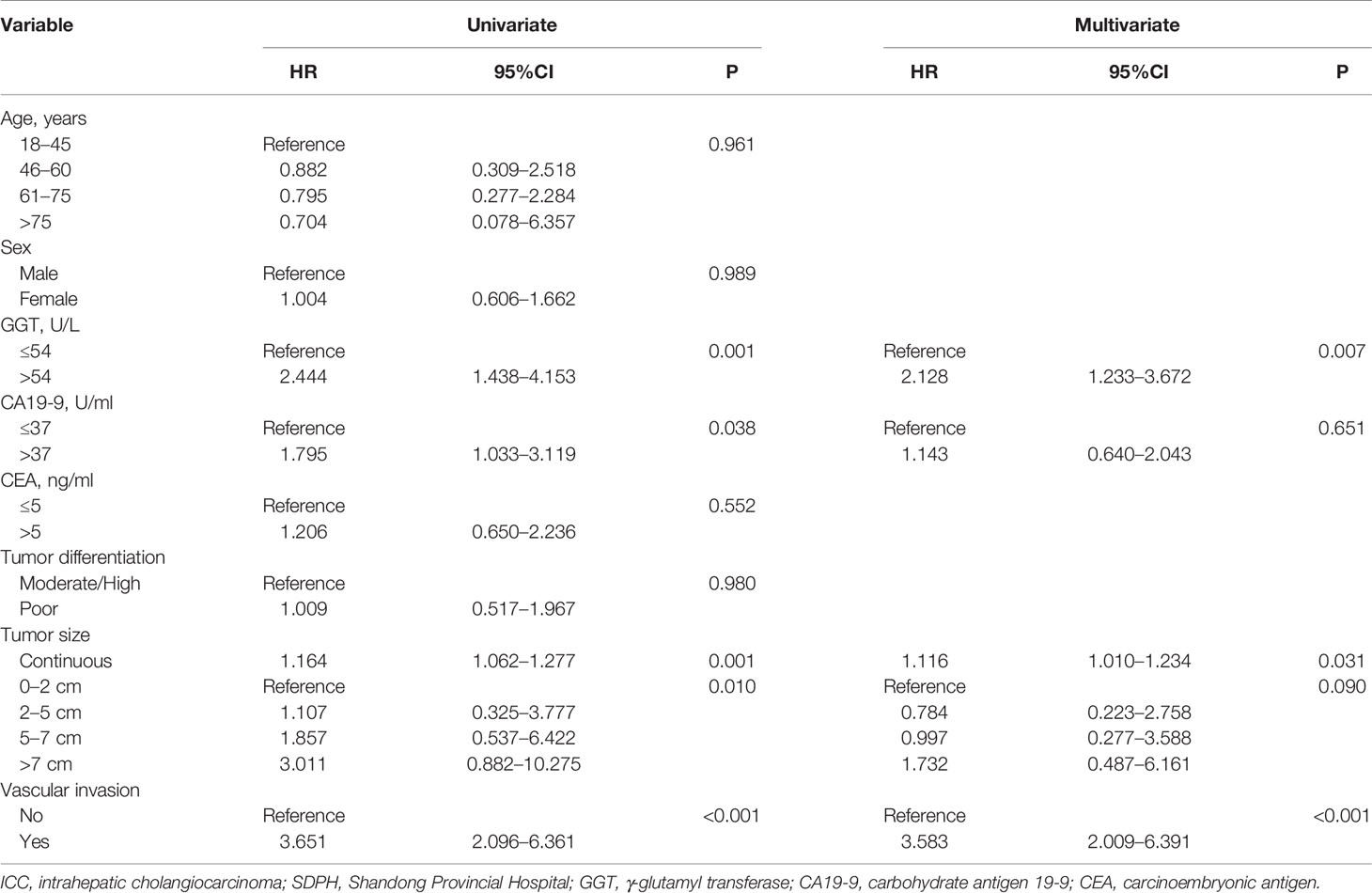
We first studied the prognostic ability of tumor size in the SDPH set. Kaplan-Meier analysis showed that tumor size was associated with OS for patients with ICC (p = 0.0072, Figure 4A). Moreover, Cox analysis suggested that tumor size was an independent prognostic factor for OS when it was treated as a continuous variable (p = 0.031, HR 1.116, 95% CI 1.010–1.234), while no significant difference was found when it was treated as a categorical variable (p = 0.090) (Table 4). Afterwards, we tested the optimal cut-off values in the SDPH set. Kaplan-Meier analysis showed that patients with 0–8 cm tumors had better OS than those with >8 cm tumors in the solitary ICC (p = 0.0028, Figure 4B) and solitary ICC without VI subgroups (p = 0.027, Figure 4C). In the Cox regression analysis, we discovered that tumor size was an independent prognostic factor for OS in the solitary ICC (p = 0.015, HR 2.115, 95% CI 1.153–3.879) and solitary ICC without VI subgroups (p = 0.032, HR 2.369, 95% CI 1.075–5.221) when it was treated as a categorical variable, and the cut-off value was 8 cm (Table S3). However, for solitary ICC with VI, since there were only three patients with a tumor size ≤3 cm, we failed to evaluate the efficacy of cut-off value of 3 cm for the prediction of OS.
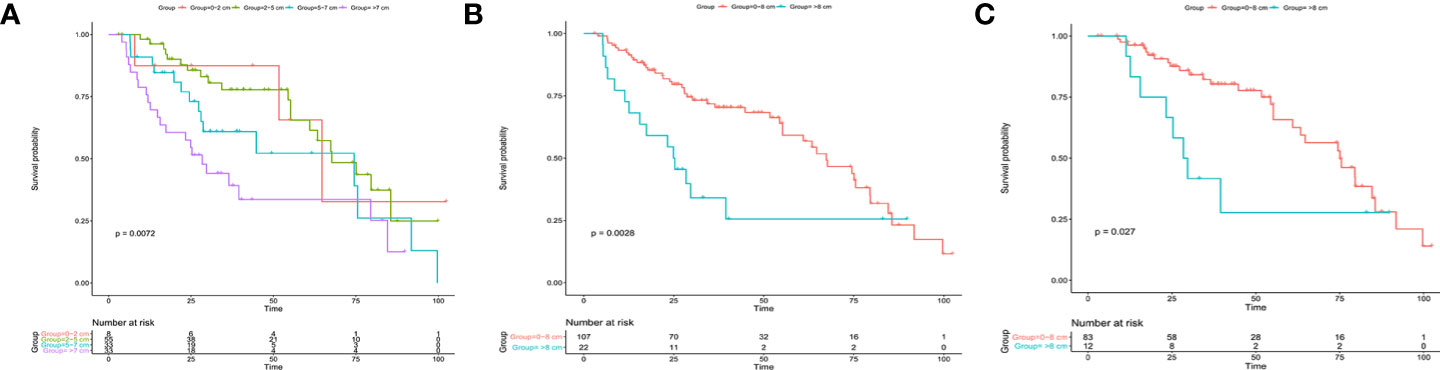
Figure 4 Kaplan-Meier analysis for patients with solitary ICC after resection in the SDPH set. (A) Solitary ICC with cut-off values of 2, 5, and 7 cm; (B) solitary ICC with a cut-off value 8 cm; (C) solitary ICC without VI with a cut-off value of 8 cm. ICC, intrahepatic cholangiocarcinoma; SDPH, Shandong Provincial Hospital; VI, vascular invasion.
Discussion
Although it has been integrated into the latest AJCC staging system, the roles of tumor size in ICC prognosis, both its prognostic ability and optimal cut-off value, are still controversial (7, 10, 11, 13). In this study, using data collected from the SEER registry, we classified solitary ICC into 4 groups based on tumor size and found that a larger tumor size was associated with various malignant variables. Afterwards, we discovered that larger tumor size groups were associated with poorer OS and that tumor size was an independent prognostic factor for solitary ICC. We further explore the prognostic ability of tumor size in different subgroups stratified by VI, and Cox regression analysis showed it was an independent prognostic factor. The log-rank test found that 8, 8 and 3 cm were the optimal cut-off values for solitary ICC, solitary ICC without VI and solitary ICC with VI, respectively. Finally, a patient cohort containing 129 cases from our center was used to validate the findings in the analysis of the SEER program, and tumor size gave similar results.
Diameter is an important characteristic of solid tumors and has been shown to be associated with OS in various solid cancers, such as hepatocellular carcinoma and non-small cell lung cancer (14, 15). The prognostic ability of tumor size has also been discussed widely in ICC. In a previous population-based cohort study, Nathan et al. (10) retrospectively analyzed 598 patients with ICC from SEER database between 1998 and 2004, proposed a staging system and proved that tumor size was not a prognostic factor for OS. In evaluating the predictive ability of the AJCC 7th edition staging system, Li et al. (16) reported that tumor size was not associated with OS and RFS for patients with ICC. Several studies also suggested that tumor size might not influence the prognosis of patients with ICC after surgery (17–19). As a result, tumor size was not integrated into the previous AJCC staging systems (6). However, most recently, the prognostic ability of tumor size for OS has been demonstrated in several large cohort studies (20). In a multicenter study containing 514 patients from Eastern and Western countries, Hyder et al. (11) demonstrated that tumor size was an independent prognostic factor for OS in ICC and constructed a prognostic nomogram. Wang et al. (4) also obtained similar conclusions in a study containing 367 patients with ICC. Consequently, tumor size was included in the latest AJCC staging system and was used to predict prognosis for patients with ICC. However, the optimal cut-off value for tumor size to predict prognosis is still controversial (6, 7, 11), and since rare studies have focused on the prognostic ability of tumor size in solitary ICC, it is difficult to conclude more concrete results for these issues.
In our study, by retrospectively analyzing patients with solitary ICC from the SEER database between 2003 and 2015, we found that tumor size was an independent prognostic factor for solitary ICC after surgery. We then further explored the prognostic ability of tumor size in different subgroups classified by VI. Consistent with the AJCC TNM staging system, for solitary ICC without VI, we found that tumor size was associated with OS; however, we discovered that 8 cm was the optimal cut-off value instead of 5 cm. Interestingly, many other studies have also proposed a larger than 5 cm cut-off point for evaluating the prognostic ability of tumor size for ICC. For instance, in a multi-institutional database study, Spolverato et al. (21) proved that patients with ICC with a tumor size >7 cm had poorer recurrence-free survival and OS, which was similar to the findings of Sahara’s studies (11, 22). Hyder et al. (11) also found that 7 cm was the best cut-off point to assess the effect of diameter on the HR of mortality. For patients with solitary ICC with VI who were classified in the T2 stage according to the latest AJCC staging system, we also found that tumor size was a prognostic factor and that 3 cm was the optimal cut-off value. However, due to the limited number of study cases, we failed to evaluate this finding in the external set. A small cut-off value for ICC has also been discussed in several previous studies (7, 8). In a recent study, Ruzzenente et al. (23) found that patients with ICC with tumors ≤3 cm had better OS than those with a larger tumor size. Based on our findings, for the AJCC TNM staging system, we proposed that there should be a change in the cut-off value for T1, tumor size should be incorporated into T2 stage, and 3 cm could be an appropriate cut-off value.
Our results also suggested that solitary ICC was a heterogeneous group, and larger tumor size was associated with malignant pathological factors, including worse tumor differentiation (20, 22) and VI (20, 24). It was believed that a larger tumor size was associated with various malignant variables (25–28). The prognostic ability of these variables has also been reported in ICC (4, 11, 22), and tumor size has been integrated into several risk systems to predict the presence of malignant pathological factors and OS (24, 29, 30). However, many studies have reported that the negative effect of tumor size on OS was largely due to the association between tumor size and malignant variables instead of tumor size itself (10, 31). In this study, using multivariate Cox regression analysis, we still found that tumor size was an independent prognostic factor for solitary ICC. Based on these findings, we hypothesized that tumor size reflected ICC progression and might be used to predict the presence of certain malignant pathological factors and OS.
This study was performed using a large number of patients with solitary ICC, and an independent external patient cohort was employed for the validation of related findings. However, limitations still exist. First, this was a retrospective study, and the findings should be further validated in other institutions with more patients in a prospective style. Second, due to the small number of patients in external set, we failed to evaluate 3 cm as the optimal cut-off value for solitary ICC with VI; thus, further studies with more patients should be conducted to evaluate this issue. Third, several crucial prognostic factors, such as carbohydrate antigen 19-9 (CA19-9) levels, postoperative complications and treatment strategy (R resection, surgical options), were unavailable in SEER database, which could influence the analysis in our study.
Conclusions
In conclusion, this study proved that tumor size was associated with several malignant clinicopathological features and was an independent prognostic factor for solitary ICC after resection. The current 8th AJCC staging system might be improved by changing the cut-off value in T1 stage from 5 to 8 cm and incorporating tumor size into the T2 stage with a cut-off value of 3 cm.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JK, JL, and JW contributed to the conception and design of the study. XL, YC, and JC collected patients’ details and extracted data. XL, CL, and JC analyzed the data. JK and YC drafted the manuscript. JL, JW, and CL contributed with a critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81373172, 81770646) and Shandong Provincial Natural Science Foundation, China (No. ZR2014HP065).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.559911/full#supplementary-material
Supplementary Figure 1 | Kaplan-Meier analysis for solitary ICC after resection in different subgroups from the SEER database. (A) Solitary ICC without VI; (B) solitary ICC with VI. ICC, intrahepatic cholangiocarcinoma; SEER, The Surveillance, Epidemiology, and End Results Program; VI, vascular invasion.
Supplementary Figure 2 | ROC curves analysis for the predictive efficiency of tumor size for ICC prognosis at 1, 3, and 5 years in different subgroups. (A) Solitary ICC; (B) Solitary ICC without VI; (C) solitary ICC with VI. ICC, intrahepatic cholangiocarcinoma; SEER, The Surveillance, Epidemiology, and End Results Program; VI, vascular invasion.
References
1. Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, et al. Surgery for cholangiocarcinoma. Liver Int (2019) 39(Supp. 1):143–55. doi: 10.1111/liv.14089
2. Zhang H, Yang T, Wu M, Shen F. Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management. Cancer Lett (2016) 379:198–205. doi: 10.1016/j.canlet.2015.09.008
3. Lin XH, Luo JC. The risk factors and prognostic factors of intrahepatic cholangiocarcinoma. J Chin Med Assoc (2017) 80:121–2. doi: 10.1016/j.jcma.2016.12.001
4. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol (2013) 31:1188–95. doi: 10.1200/JCO.2012.41.5984
5. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin (2017) 67:93–9. doi: 10.3322/caac.21388
6. Kim Y, Moris DP, Zhang XF, Bagante F, Spolverato G, Schmidt C, et al. Evaluation of the 8th edition American Joint Commission on Cancer (AJCC) staging system for patients with intrahepatic cholangiocarcinoma: A surveillance, epidemiology, and end results (SEER) analysis. J Surg Oncol (2017) 116:643–50. doi: 10.1002/jso.24720
7. Sakamoto Y, Kokudo N, Matsuyama Y, Sakamoto M, Izumi N, Kadoya M, et al. Proposal of a new staging system for intrahepatic cholangiocarcinoma: Analysis of surgical patients from a nationwide survey of the Liver Cancer Study Group of Japan. Cancer Am Cancer Soc (2016) 122:61–70. doi: 10.1002/cncr.29686
8. Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg (2003) 10:288–91. doi: 10.1007/s00534-002-0732-8
9. Okabayashi T, Yamamoto J, Kosuge T, Shimada K, Yamasaki S, Takayama T, et al. A new staging system for mass-forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer Am Cancer Soc (2001) 92:2374–83. doi: 10.1002/1097-0142(20011101)92:9<2374::aid-cncr1585>3.0.co;2-l
10. Nathan H, Aloia TA, Vauthey J, Abdalla EK, Zhu AX, Schulick RD, et al. A Proposed Staging System for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol (2009) 16:14–22. doi: 10.1245/s10434-008-0180-z
11. Hyder O, Marques H, Pulitano C, Marsh JW, Alexandrescu S, Bauer TW, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: An Eastern and Western experience. JAMA Surg (2014) 149:432–8. doi: 10.1001/jamasurg.2013.5168
12. Groot VP, Gemenetzis G, Blair AB, Rivero-Soto RJ, Yu J, Javed AA, et al. Defining and Predicting Early Recurrence in 957 Patients with Resected Pancreatic Ductal Adenocarcinoma. Ann Surg (2019) 269:1154–62. doi: 10.1097/SLA.0000000000002734
13. Yamada T, Nakanishi Y, Okamura K, Tsuchikawa T, Nakamura T, Noji T, et al. Impact of serum carbohydrate antigen 19-9 level on prognosis and prediction of lymph node metastasis in patients with intrahepatic cholangiocarcinoma. J Gastroenterol Hepatol (2018). doi: 10.1111/jgh.14124
14. Wu G, Wu J, Wang B, Zhu X, Shi X, Ding Y. Importance of tumor size at diagnosis as a prognostic factor forhepatocellular carcinoma survival: a population-based study. Cancer Manag Res (2018) 10:4401–10. doi: 10.2147/CMAR.S177663
15. Gulack BC, Yang CF, Speicher PJ, Meza JM, Gu L, Wang X, et al. The impact of tumor size on the association of the extent of lymph node resection and survival in clinical stage I non-small cell lung cancer. Lung Cancer (2015) 90:554–60. doi: 10.1016/j.lungcan.2015.10.011
16. Li T, Qin LX, Zhou J, Sun HC, Qiu SJ, Ye QH, et al. Staging, prognostic factors and adjuvant therapy of intrahepatic cholangiocarcinoma after curative resection. Liver Int (2014) 34:953–60. doi: 10.1111/liv.12364
17. Nakagawa T, Kamiyama T, Kurauchi N, Matsushita M, Nakanishi K, Kamachi H, et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg (2005) 29:728–33. doi: 10.1007/s00268-005-7761-9
18. Uenishi T, Yamazaki O, Yamamoto T, Hirohashi K, Tanaka H, Tanaka S, et al. Serosal invasion in TNM staging of mass-forming intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg (2005) 12:479–83. doi: 10.1007/s00534-005-1026-8
19. Orimo T, Kamiyama T, Mitsuhashi T, Kamachi H, Yokoo H, Wakayama K, et al. Impact of tumor localization on the outcomes of surgery for an intrahepatic cholangiocarcinoma. J Gastroenterol (2018) 53:1206–15. doi: 10.1007/s00535-018-1469-8
20. Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and Prognosis for Patients with Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg (2014) 149:565–74. doi: 10.1001/jamasurg.2013.5137
21. Spolverato G, Kim Y, Alexandrescu S, Popescu I, Marques HP, Aldrighetti L, et al. Is Hepatic Resection for Large or Multifocal Intrahepatic Cholangiocarcinoma Justified? Results from a Multi-Institutional Collaboration. Ann Surg Oncol (2015) 22:2218–25. doi: 10.1245/s10434-014-4223-3
22. Sahara K, Tsilimigras DI, Mehta R, Bagante F, Guglielmi A, Aldrighetti L, et al. A novel online prognostic tool to predict long-term survival after liver resection for intrahepatic cholangiocarcinoma: The “metro-ticket” paradigm. J Surg Oncol (2019) 120:223–30. doi: 10.1002/jso.25480
23. Ruzzenente A, Conci S, Viganò L, Ercolani G, Manfreda S, Bagante F, et al. Role of Lymph Node Dissection in Small (≤ 3 cm) Intrahepatic Cholangiocarcinoma. J Gastrointest Surg (2019) 23:1122–9. doi: 10.1007/s11605-019-04108-0
24. Spolverato G, Ejaz A, Kim Y, Sotiropoulos GC, Pau A, Alexandrescu S, et al. Tumor size predicts vascular invasion and histologic grade among patients undergoing resection of intrahepatic cholangiocarcinoma. J Gastrointest Surg (2014) 18:1284–91. doi: 10.1007/s11605-014-2533-1
25. Goh BK, Teo JY, Chan CY, Lee SY, Jeyaraj P, Cheow PC, et al. Importance of tumor size as a prognostic factor after partial liver resection for solitary hepatocellular carcinoma: Implications on the current AJCC staging system. J Surg Oncol (2016) 113:89–93. doi: 10.1002/jso.24099
26. Liang Y, Liu L, Xie X, Xia L, Meng J, Xu R, et al. Tumor Size Improves the Accuracy of the Prognostic Prediction of Lymph Node-Negative Gastric Cancer. J Surg Res (2019) 240:89–96. doi: 10.1016/j.jss.2019.02.037
27. Ytre-Hauge S, Husby JA, Magnussen IJ, Werner HM, Salvesen OO, Bjorge L, et al. Preoperative tumor size at MRI predicts deep myometrial invasion, lymph node metastases, and patient outcome in endometrial carcinomas. Int J Gynecol Cancer (2015) 25:459–66. doi: 10.1097/IGC.0000000000000367
28. Bhindi B, Thompson RH, Lohse CM, Mason RJ, Frank I, Costello BA, et al. The Probability of Aggressive Versus Indolent Histology Based on Renal Tumor Size: Implications for Surveillance and Treatment. Eur Urol (2018) 74:489–97. doi: 10.1016/j.eururo.2018.06.003
29. Jeong S, Cheng Q, Huang L, Wang J, Sha M, Tong Y, et al. Risk stratification system to predict recurrence of intrahepatic cholangiocarcinoma after hepatic resection. BMC Cancer (2017) 17:464. doi: 10.1186/s12885-017-3464-5
30. Sasaki K, Margonis GA, Andreatos N, Bagante F, Weiss M, Barbon C, et al. Preoperative Risk Score and Prediction of Long-Term Outcomes after Hepatectomy for Intrahepatic Cholangiocarcinoma. J Am Coll Surg (2018) 226:393–403. doi: 10.1016/j.jamcollsurg.2017.12.011
Keywords: intrahepatic cholangiocarcinoma, tumor size, solitary, resection, overall survival, SEER
Citation: Kong J, Cao Y, Chai J, Liu X, Lin C, Wang J and Liu J (2021) Effect of Tumor Size on Long-Term Survival After Resection for Solitary Intrahepatic Cholangiocarcinoma. Front. Oncol. 10:559911. doi: 10.3389/fonc.2020.559911
Received: 07 May 2020; Accepted: 01 December 2020;
Published: 21 January 2021.
Edited by:
Mark Girgis, University of California, Los Angeles, United StatesReviewed by:
David Holroyd, University of Glasgow, United KingdomBenedetto Ielpo, Parc de Salut Mar, Spain
Copyright © 2021 Kong, Cao, Chai, Liu, Lin, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Liu, ZHJfbGl1anVuMTk2N0AxMjYuY29t; Jianping Wang, ZHJfanB3YW5nQDEyNi5jb20=
†These authors have contributed equally to this work
 Junjie Kong
Junjie Kong Yukun Cao1,2†
Yukun Cao1,2†