
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 03 November 2020
Sec. Women's Cancer
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.559016
 Kelechi Njoku1,2
Kelechi Njoku1,2 Davide Chiasserini2,3
Davide Chiasserini2,3 Eleanor R. Jones1,4
Eleanor R. Jones1,4 Chloe E. Barr1,4
Chloe E. Barr1,4 Helena O’Flynn1,4
Helena O’Flynn1,4 Anthony D. Whetton2
Anthony D. Whetton2 Emma J. Crosbie1,4*
Emma J. Crosbie1,4*Endometrial cancer is the most common malignancy of the female genital tract and its incidence is rising in parallel with the mounting prevalence of obesity. Early diagnosis has great potential to improve outcomes as treatment can be curative, especially for early stage disease. Current tests and procedures for diagnosis are limited by insufficient accuracy in some and unacceptable levels of invasiveness and discomfort in others. There has, therefore, been a growing interest in the search for sensitive and specific biomarkers for endometrial cancer detection based on non-invasive sampling methodologies. Urine, the prototype non-invasive sample, is attractive for biomarker discovery as it is easily accessible and can be collected repeatedly and in quantity. Identification of urinary biomarkers for endometrial cancer detection relies on the excretion of systemic biomarkers by the kidneys or urinary contamination by biomarkers shed from the uterus. In this review, we present the current standing of the search for endometrial cancer urinary biomarkers based on cytology, genomic, transcriptomic, proteomic, and metabolomic platforms. We summarize the biomarker candidates and highlight the challenges inherent in urinary biomarker discovery. We review the various technologies with promise for biomarker detection and assess these novel approaches for endometrial cancer biomarker research.
Endometrial cancer (EC) is the most frequently diagnosed malignancy of the female genital tract and the sixth most common cancer in women globally (1, 2). The GLOBOCAN series of the International Agency for Research on Cancer reports a worldwide age-standardized incidence rate (ASR) of 8.4 per 100,000 women and mortality rate of 1.8 per 100,000 women, based on 2018 estimates from 185 countries (1). ASRs vary widely both across and within countries, from one to 30 cases per 100,000 women (2, 3). The highest incidence rates are reported in Western countries, particularly those with a high Human Development Index (HDI), where over 60% of all cases occur. Incidence rates are lowest in Sub-Saharan Africa, South Central Asia, and the Middle-East (2). In the United Kingdom, EC is the fourth most common female cancer with over 9,000 incident cases each year, and has in the past decade increased in incidence by almost 20% (4).
EC is commonly classified into two histological types based on a model that incorporates clinical, metabolic and epidemiological features (Bohkman’s dichotomous model) (5). Type I tumors are commonly low grade, estrogen driven tumors that are associated with obesity and a favorable prognosis. Type II tumors, by contrast, are high grade, estrogen independent tumors that are clinically aggressive and less strongly associated with obesity (5, 6). A molecular classification of EC by the Cancer Genome Atlas Research Network categorizes EC into four prognostically distinct subtypes: polymerase-epsilon (POLE) ultramutated, microsatellite instable, copy number low, and copy number high, and has been validated in multiple studies (7, 8).
Obesity is the strongest risk factor for EC and is estimated to be responsible for up to 40% of all EC cases (9, 10). Other EC risk factors include age, diabetes, hypertension, polycystic ovary syndrome, nulliparity, use of estrogen-only hormone replacement therapy, and tamoxifen (10, 11). Women may also have a familial predisposition to EC, in particular, those who carry a pathogenic variant in one of the DNA mismatch repair genes (Lynch syndrome) or the tumor suppressor gene-phosphatase and tensin homologue (PTEN) (Cowden syndrome) (12, 13). Over 90% of women with EC present with postmenopausal bleeding (PMB), defined as bleeding occurring at least a year after cessation of menstruation due to menopause (14). Only 5%–10% of women with PMB, however, will have EC, but the risk increases with age and in the presence of other risk factors (15). Premenopausal and perimenopausal women may present with irregular or heavy menstrual bleeding (14). Abnormal vaginal discharge, hematuria, pelvic pain, or pain during sexual intercourse are other important but less common symptoms (16).
Treatment for EC is primarily surgical with hysterectomy and bilateral salpingo-oophorectomy as standard of care worldwide (14, 15). Women with high-risk disease are offered adjuvant radiotherapy and/or chemotherapy to reduce the risk of recurrence (17, 18). Women with advanced (stages III and IV) or metastatic EC have a poor prognosis (<20% 5-year survival) and are at a higher risk of relapse compared to those diagnosed early (>90% 5-year survival) (14, 15). There are limited evidence-based treatment options available for women diagnosed at a late stage; thus, it is crucial that women are diagnosed early when treatment is able to effect cure. Early detection will also allow for radical treatments to be minimized and enable conservative management options to be offered to women of child bearing age and those with morbid obesity in whom surgery is potentially hazardous (15).
The diagnostic strategy for suspected EC has not evolved in several decades; yet, it is far from perfect. In the United Kingdom, the National Institute for Health and Care Excellence (NICE) recommends that women aged 55 and over with PMB be referred to the rapid access gynecology clinic to be seen within 2 weeks (14, 19, 20). Such a strategy could miss cases of EC, so NICE also recommends consideration of a 2-week wait referral for those aged under 55 with PMB, as well as direct access ultrasound for a select group of women aged 55 and over with unexplained vaginal discharge or frank hematuria (19, 20). Transvaginal ultrasonography (TVS) is the imaging modality of choice for the initial evaluation of suspected EC (5). Measurement of endometrial thickness (ET) using TVS is non-invasive, precise and sensitive, and is particularly useful when the endometrium is homogenous (21). Its diagnostic utility for EC is, however, limited by a low specificity, as a thickened endometrium may be caused by other pathologies including endometrial polyps, intracavitary fibroids and artefacts such as blood clots, and is seen in approximately 50% of women undergoing TVS for suspected EC (21–23). Women with a thickened endometrium therefore require further invasive tests in order to establish a diagnosis. Endometrial sampling has good accuracy for EC detection and is the gold standard for the diagnostic evaluation of women with suspected EC (5). It may, however, miss focal pathologies including EC, especially when performed blindly, as less than 50% of the endometrium is usually sampled (24). In addition, the procedure can be painful, especially in nulliparous women, and the risk of failure is high (25). Hysteroscopy with targeted biopsy is indicated following failed endometrial sampling or in cases of an irregular endometrium in the presence of risk factors, but is invasive and often plagued by operative challenges (15). In the outpatient setting, pain, cervical stenosis and sub-optimal visualization of the uterine cavity are the most frequent reasons for abandonment of procedure (14). Rarely, life threatening complications such as uterine perforation and cervical laceration occur (26).
While invasive testing is necessary for a tissue diagnosis, most women with PMB have a benign explanation for their bleeding. Currently, thousands of women with PMB undergo hysteroscopy and/or endometrial biopsy, invasive tests that are unpleasant, sometimes technically challenging, and painful or extremely painful for 30%–40% of women (15, 26, 27). Restricting invasive testing to women with sinister underlying pathology would save many thousands of women per year in every developed country in the world from invasive tests they do not need. A novel EC detection tool that could triage women for invasive testing or quick reassurance would transform clinical pathways for EC.
The ideal detection tool is simple, non-invasive, accurate and cost effective. It should be able to identify women with EC at the earliest possible stages while re-assuring the large majority who do not have EC (28). “What simple, non-invasive, painless, cost-effective, and convenient tests can be used to detect cancer early?” ranked first in the top ten research priorities for early cancer detection by the James Lind Alliance partnership representing patients, carers, and clinician groups (29). A similar study exploring unmet research needs in EC, found “which women with abnormal bleeding require urgent specialist referral and which can be safely reassured” to be second most important priority (30). Based on these gap analyses, studies exploring EC detection using non-invasive samples such as urine are urgently needed (28).
A biomarker is defined by the National Cancer Institute as a “biological substance in body fluids or tissues that is indicative of a normal or abnormal process or of a condition or disease” (31). Biomarkers can be proteins and peptides (e.g., an enzyme or receptor), nucleic acids (e.g., DNA, microRNA), antibodies or metabolites. Biomarkers can have single or multiple components, for example, individual proteins (e.g., CA-125) or genomic, proteomic or metabolomic signatures (32).
Multiple approaches have been employed in the search for cancer diagnostic biomarkers. A classic approach is to select potential markers based on tumor biology. More recently, however, with the advent of new technologies including next-generation sequencing and mass spectrometry (MS), an objective and pragmatic approach to biomarker identification using biofluids has come to the fore (32). Potential diagnostic biomarkers must overcome several hurdles before they can be used in the clinical setting: discovery, validation, and verification (32, 33). Importantly, the performance of any novel test needs to be evaluated in terms of its analytical performance, clinical validity and clinical utility (Table 1) (34). Analytical performance refers to the accuracy with which a particular characteristic of interest can be identified by a given laboratory test. An ideal biomarker assay should not only be accurate but also reproducible within and between laboratories. The accuracy with which a test identifies a patient’s clinical status such as the presence of EC (clinical validity) and the risks and benefits resulting from the test use (clinical utility) are other test properties that must be considered. Clinical validity is described in terms of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) (Table 1) (34, 35). Safety, acceptability, fit in the diagnostic pathway, and cost effectiveness inform clinical utility and must be taken into consideration when evaluating a novel diagnostic test prior to translation into routine clinical settings (Table 1) (35, 36).
Urine is the prototype non-invasive sample and is a useful biological fluid for biomarker discovery due to its accessibility and potential for repeated samples and unlimited volumes. Its collection is cheap and is usually without side effects or complications (37). Thus, it fits the description of an ideal biomarker source for EC detection (Table 1). A wide variety of substances with potential to serve as EC biomarkers can be found in urine and include endogenous metabolites, genetic products such as tumor DNA, peptides/proteins, malignant cells and secreted organelles such as extracellular vesicles (38–40). The exploration of each of these targets for biomarker identification requires the use of specialized techniques based on platforms such as cytology/single cell technology, spectroscopy, genomics, transcriptomics, proteomics, and metabolomics (33, 41, 42).
Identification of urinary biomarkers for endometrial cancer detection relies on the excretion of systemic biomarkers by the kidneys or urinary contamination with biomarkers shed from the uterus (28, 43). Systemic proteins and metabolites excreted in urine originate from several organs including the uterus and find their way into the proximal tubules by escaping total reclamation by the renal filtration barrier (44). Proteins and peptides excreted in urine are less complex and more stable compared to plasma proteins, thus conferring an advantage for biomarker discovery (37).
The anatomical continuity between the upper and lower genital tracts provides an opportunity for non-invasive sampling of uterine derived proteins and malignant cells (45). Previous studies have shown that endometrial tumor debris passes through the cervix and into the vagina, from where it can be collected with soft brushes or tampons (46–48). The proximity of the urethra to the vagina also allows for the potential contamination of self-collected urine by naturally shed tumor material. While renally excreted biomarkers may be difficult to measure due to the low abundance of tumor-derived molecules in the circulation, especially in early stage EC, for uterine shed biomarkers, it is the consistency and reliability with which these contaminate urinary samples that limits their clinical utility, especially in asymptomatic women.
Techniques for cancer diagnosis based on urine analysis have evolved over time from the microscopic assessment of urinary sediments to the comprehensive examination of urinary analytes, made possible by recent advances in high-throughput technologies (42). Endometrial cancer cells may be identified in urine, especially in women with bleeding symptoms, by the microscopic assessment of urine (cytology) (39) or by the use of single cell technology (49). Urinary cell-free tumor DNA, on the other hand, may be renally excreted or may result from the breakdown of malignant cells contaminating urinary flow. Tumor DNA characterization, including an assessment of DNA concentrations, the presence of mutations and methylation status in urine has great potential to yield relevant biomarkers and needs further exploration. Urinary proteins and micro-RNA closely mirror the dynamic state of cells and are viable sources of EC biomarkers (28). Metabolites, on the other hand, are the most proximal of the omics markers and best reflect a cell systems physiological phenotype (42). Figure 1 summarizes the various sources of urinary biomarkers for EC detection and the technologies with promise for EC urinary biomarker research.
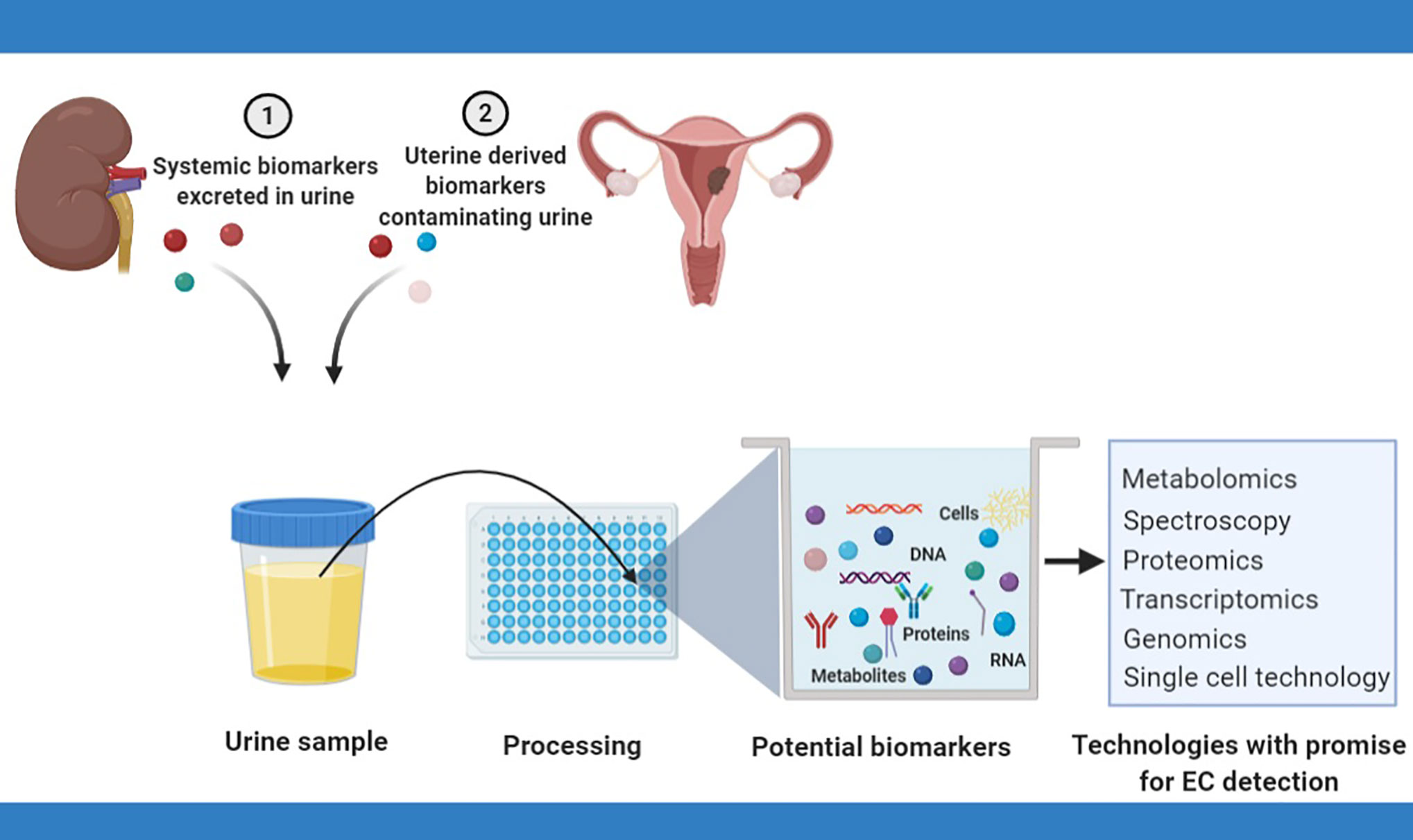
Figure 1 Urinary biomarkers for endometrial cancer detection rely on the renal excretion of systemic biomarkers or the contamination of urinary flow by naturally shed uterine biomarkers. Several techniques have potential for EC biomarker discovery and include cytology, spectroscopy, metabolomics, transcriptomics, and proteomics.
Metobolomics has been employed to analyze urine for EC biomarkers (see Table 2). This is because it systematically identifies and quantifies metabolic products from cells, tissues or biofluids (65). By enabling the analysis of the downstream products of genomic, transcriptomic and proteomic processes, metabolomics closely mirrors a systems phenotype and effectively summarizes the effects of other “omics” technologies (42). Metabolic profiling can either be targeted or untargeted. While targeted approaches deal with the measurement of a pre-defined select group of metabolites, untargeted approaches aim to comprehensively analyze all measurable products in a given sample with no prior assumptions (42, 66, 67). The hypothesis driven nature of targeted studies lends to a high level of precision and accuracy, in contrast to untargeted approaches which are prone to false positives (42). Targeted approaches are thus often employed to validate findings obtained from untargeted studies. Two platforms are commonly used in metabolomic biomarker research: MS and nuclear magnetic resonance (NMR) spectroscopy (33, 68).
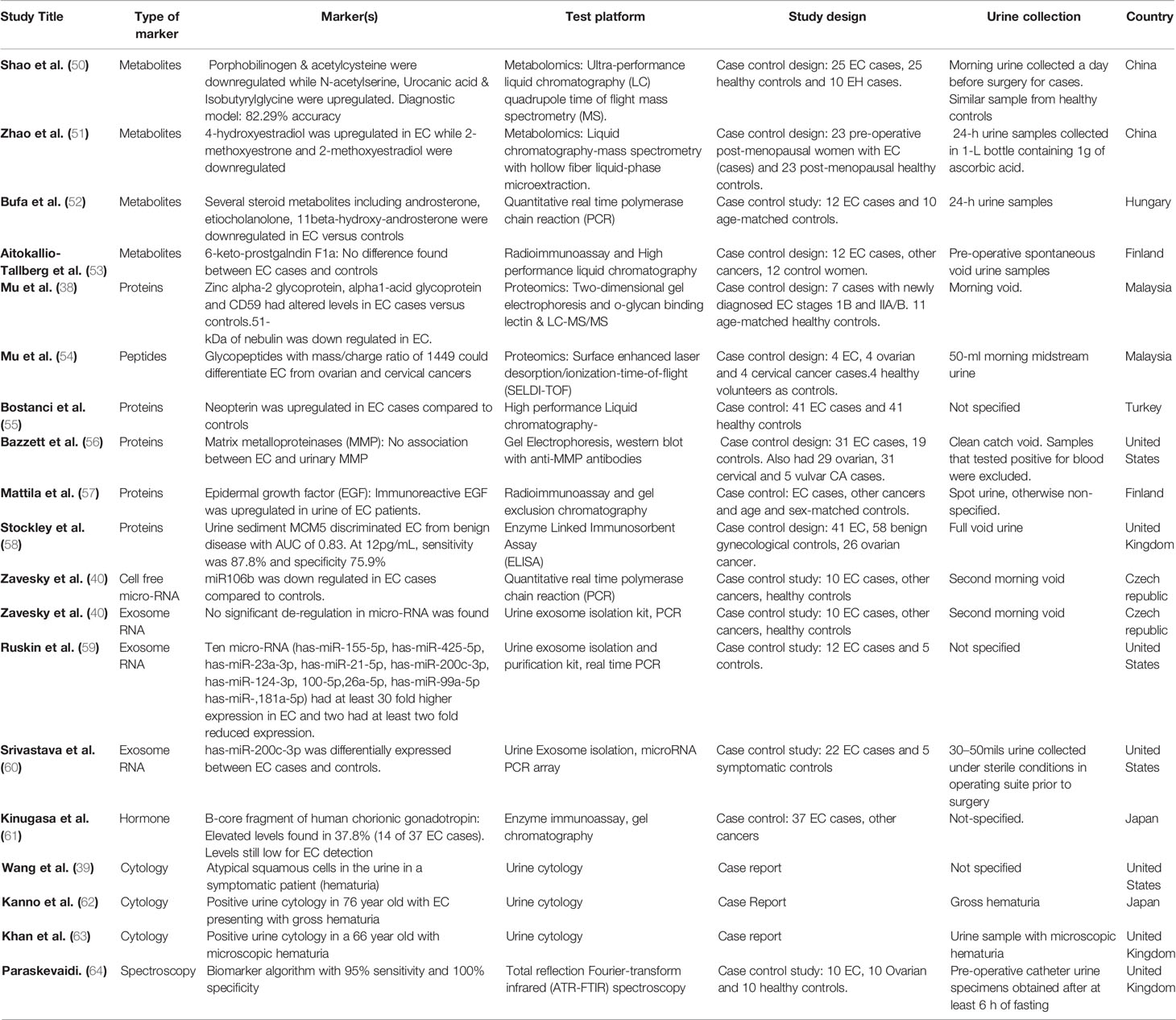
Table 2 Study characteristics and diagnostic accuracies of potential urinary biomarkers for EC detection.
Multiple studies have sought to identify possible urinary metabolites with potential for EC detection (50, 51, 53). Amino acid, lipid and hormonal metabolites have all been suggested to have potential as EC diagnostic biomarkers. Shao and colleagues, using ultra-performance liquid chromatography quadrupole time-of-flight MS (UPLC-Q-TOF/MS) on urinary specimens from 25 EC cases and 25 controls identified a set of five metabolites as possible biomarkers for EC detection: porphobilinogen, acetylcysteine, N-acetyserine, urocanic acid, and isobutyrylglycine (50). Of these five, porphobilinogen and acetylcysteine were downregulated in EC while N-acetyserine, urocanic acid and isobutyrylglycine were upregulated (50). A predictive model based on these five biomarker candidates using the partial least squares-discriminant analysis was able to distinguish EC from endometrial hyperplasia (EH) (n = 10) and healthy controls. While porphobilinogen and acetylcysteine discriminated between EC and the merged group of EH and healthy controls, there was no significant difference between EH and healthy controls (50). None of these biomarker candidates have been independently validated and further studies are needed to elucidate their role in EC tumorigenesis. Some urinary metabolites have also been reported as being able to discriminate between EC (n = 40) and benign ovarian tumors (n = 62). These include 3-dehydroquinic acid, 3-indolelactic acid, S-reticuline, selenocystathionine, 1-(1Z-hexadecenyl)-sn-glycero-3-3-phosphate, N-acetylneuraminic acid, 3-sialyl-N-acetyllactosamine and 3-sialylactose (42).
Zhao and colleagues investigated endogenous estrogen metabolites as biomarker candidates for endometrial cancer diagnosis using urine samples from 23 EC cases and 23 post-menopausal healthy controls (51). While 4-hydroxyestradiol (4-OHE2) was up-regulated in EC, 2-methoxyestrone (2-MeOE1) and 2-methoxyestradiol (2-MeOE2) were down-regulated. Twenty-four-hour urinary 17β-estradiol (E2) was also found to be elevated in EC cases (51). E2 is linked to endometrial carcinogenesis through the activation of P13K/AKT and MAPK signaling pathways. 4-OHE2, on the other hand, has been linked with EC tumorigenesis through the upregulation of CYP1B1 (51). 2-MEOE1, an estrone analogue of 2-MeOE2, exhibits anti-proliferative and pro-apoptotic properties, in keeping with the finding of its down-regulation in EC (51, 69). While hormonal imbalance from adipose derived unopposed estrogen is the most established biological pathway implicated in obesity driven endometrial carcinogenesis, the finding of endogenous estrogen metabolites in urine is not necessarily diagnostic of EC (70). They do, however, provide unique insights in endometrial cancer urinary biomarker discovery and may, in combination with other biomarker candidates, be used to improve the accuracy of an EC urinary biomarker panel. Other approaches that have been tried include the use of attenuated total reflection Fourier-transform infrared (ATR-FTIR) spectroscopy by Paraskevaidi and colleagues, who analyzed urinary specimens form 10 EC cases, 10 ovarian cases and 10 healthy controls (64). They were able to develop a biomarker algorithm with 95% sensitivity and 100% specificity for EC detection that is yet to be externally validated (64). Several other studies (52, 53, 61) have explored metabolites for EC detection in urine, however, none have yet been translated into routine clinical use.
A number of issues need to be addressed when developing robust urine-based metabolite biomarker discovery protocols (Table 3). First, is the variability of exogenous sources of urinary metabolites. While the endogenous metabolic process is prone to individual biological variations, there is greater variability in the metabolic products resulting from exogenous substances such as water, drugs and food and this can significantly impact on study findings (Table 1). As such, it is important to identify these potential confounding variables and control for them (Table 3). The collection of urine after an over-night fast, for instance, can control for diet and is encouraged (67). Seasonal variations in dietary and other lifestyle factors such as levels of physical activity can be minimized by ensuring that urine samples are collected at a specific time of the year and not all year round (42). Strategies often used to control for drug effects include sample collection before any medications are used on the day, asking study participants to temporarily withhold use of medications where feasible and excluding specific drug metabolites during analysis (42). Levels of several urinary metabolites also exhibit a circadian rhythm (76). As such, standard operating procedures should be applied, especially with regards to time of sample collection (28). With obesity as the strongest risk factor for EC (70), urinary metabolic markers of adiposity are likely to systematically differ between EC cases and controls and should be controlled for (74). Such markers may be used in combination with other biomarker candidates to improve their diagnostic accuracy and address the issues shown in Table 1 with respect to false positive results.
Protein and peptide profiling are important tools for biomarker discovery and have been explored in various studies seeking to identify urinary biomarkers for EC detection. Proteomics systematically characterizes all of the proteins within a cell and in combination with computational analyses and machine learning techniques is able to identify biomarkers that differ between cohorts (28). Using 2-dimensionalgel electrophoresis (2-DE) and O-Glycan binding lectin analysis by LC-MS/MS, Mu and colleagues demonstrated altered levels of Zinc alpha-2 glycoprotein (ZAG), alpha-1 acid glycoprotein (AAG) and CD59 in the urine specimens of EC cases compared to healthy controls. Nebulin, on the other hand, was found to be downregulated (38). These biomarkers, though promising, are limited by their lack of specificity for EC and are yet to be validated. Importantly, 2-DE is a time consuming technique with limited sample throughput and narrow depth of proteome analysis. Bostanci and colleagues used high performance liquid chromatography to compare urine samples from 41 EC cases and 41 healthy controls and found neopterin to be differentially expressed (55). Neopterin, a marker of cellular immune activation, was also able to differentiate early from late stage disease, suggesting a potential prognostic use especially if it is found to be a specific marker of EC and not an inflammatory response marker. The positive predictive value of neopterin is poor, however, as elevated levels may also be found in autoimmune conditions, viral and bacterial infections and other malignant states (55). In the study by Stockley and colleagues, urine sediment MCM5 discriminated EC cases (n = 41) from benign gynecological disease (n = 58) with an AUC of 0.83. At a 12pg/ml cut-off value, a sensitivity of 87.8% was reported at 75.9% specificity for EC exclusion (58). The finding of MCM5, a marker of cellular proliferation, in the urine sediments of EC cases, is most likely reflective of the contamination of urine by uterine shed cellular components/proteins, rather than the renal excretion of systemic proteins. However, this finding is yet to be externally validated and further studies are needed. The upregulation of MCM5 has been reported in several other cancers, including bladder and cervical cancers, thus limiting its utility as a specific marker for EC detection.
A number of proteins have been suggested as potential biomarkers for EC detection based on proteomic analysis of blood (28). Although none have been translated into routine clinical practice due mainly to their sub-optimal accuracy and lack of robust validation, the results are encouraging, especially with the development of multi-marker panels and integration of clinical, genomic, transcriptomic and metabolomic data. There is, however, insufficient evidence to suggest that the urinary equivalents of the blood-based EC biomarker candidates are as promising. As an example, serum epididymis protein 4 (HE4), a useful biomarker in the management of ovarian cancer has been reported as a potential biomarker for EC detection (77–79). While multiple studies suggest a potential utility of urine HE4 in discriminating ovarian cancer cases from the general population, there is a lack of evidence to suggest a similar utility for EC detection (37). Similarly, Matrix Metalloproteinases (MMP) have been reported as blood based EC biomarker candidates (28). However, Bazzett and colleagues, using gel electrophoresis on urine samples from 31 endometrial cancer cases and 19 controls found insufficient evidence to recommend urinary MMP for EC detection (56). Mattilla and colleagues, on the other hand, when investigating urinary epidermal growth factor concentrations in various human malignancies found an up-regulation of immunoreactive EGF in the urine samples of EC cases (57). EGFR is a receptor tyrosine kinase that is over expressed in several human malignancies including EC where a 46%–67% expression rate has been reported. Further studies are needed to validate its potential utility for EC detection.
Urinary glycosylated peptides have also been suggested as potential EC biomarkers. Using SELDI-TOF in an N-glycopeptide profiling of urine samples from patients with EC, ovarian and cervical cancers, Mu et al. demonstrated that urinary glycopeptide with mass/charge ratio 1449 was able to differentiate EC from ovarian and cervical cancers (54). This was however based on a small number of subjects and it was unclear whether the study was sufficiently powered for biomarker identification. Certainly, SELDI-TOF offers little in terms of biomarker characterization compared to more recent technologies.
The most important consideration in EC urinary protein biomarker research is the wide variability in urinary protein concentrations due to factors such as age, genetics, physical activity status, drugs and diet among others (Table 3) (37, 73). Hypertension, for instance, is a risk factor for EC and the prevalence of hypertension, and by extension, the use of anti-hypertensives, is likely to systematically differ between cohorts of women with and without EC (44). Strategies to deal with such confounding variables are summarized in Table 3.
MicroRNAs are small noncoding RNAs with post-translational regulatory functions in a wide variety of cellular processes including tumorigenesis (80, 81). They have emerged as viable biomarker candidates for cancer diagnosis and have been explored in various studies using tissue specimens and cell lines. In recent times, research focus has shifted to identifying microRNAs in minimally invasive samples such as plasma/serum (82). Available evidence suggests that cell free micro-RNAs may be detected in a wide variety of non-invasive bodily fluids including urine (40). The diagnostic potential of urinary cell free microRNAs has been explored, particularly in upper urinary tract and bladder urothelial cancers. Zavesky and colleagues, using quantitative real-time PCR, investigated the expression of candidate cell-free urinary microRNAs in endometrial and ovarian cancer patients and reported miR106b to be downregulated in EC cases compared to controls (40).
Urinary extracellular vesicles have also been explored as possible sources of microRNA for EC diagnosis. Using exosome RNA, Zavesky and colleagues found no significant de-regulations in microRNA expressions in EC versus controls (40). Ruskin et al., on the other hand, isolated urinary exosomes from 12 EC cases and 5 controls and reported 10 microRNAs to have at least 30 fold higher expression in EC while two microRNAs had at least a two-fold reduced expression (59). While further studies are very much needed to validate these biomarker candidates, the results offer cautious optimism as three of the overexpressed microRNAs had PTEN, Notch, Vascular Endothelial Growth Factor (VEGF), Protein Kinase B (AKT), and Programmed Cell Death 4 (PDCD4) as molecular targets. PTEN, a tumor suppressor gene that negatively regulates PI3-AKT signaling pathway has been implicated in endometrial carcinogenesis (83), as has Notch, a regulator of cellular proliferation, differentiation and apoptosis (84). Angiogenesis has been shown to play an important role in the growth of EC (85) while PDCD4 expression has been linked to tumor grade in endometrioid EC (86). Srivastava and colleagues studied 81 microRNAs from urine derived exosomes from patients with and without EC, 57 of which were amplified in qPCR and reported has-miR-200c-3p to be differentially expressed (60). miR-200c is a tumor suppressor microRNA that prevents the epithelial to mesenchymal transition of cancer cells and has been found to be dysregulated in many cancers (87). Its utility for EC detection is thus limited by its low specificity. While microRNA profiling of endometrial cancer is yet to be fully characterized, there is evidence in support of the differential expression of miR-200 family of microRNAs in endometrial cancer tissues in comparison to normal endometrium (60). Urinary miR-200c3p is thus a promising non-invasive EC biomarker but needs validation (as part of a microRNA panel) prior to translation into routine clinical practice. Other microRNA candidates and the estimates of their differential expression are summarized in Table 2.
Identification of malignant cells in urine is a potential EC diagnostic strategy that needs exploration, especially in symptomatic women. Bladder cancer can be detected by microscopic evaluation of exfoliated urothelial tumor cells (88, 89). Wang and colleagues report on the role of urine cytology in detecting EC following the presentation of an 82 year old woman with hematuria (39). The possibility of finding endometrial malignant cells in urine is dependent on its contamination by EC cells shed during postmenopausal bleeding since the local infiltration of the bladder by metastatic disease is a late and rare event in EC (63). A papillary structuring of malignant clusters of cells in urine has been reported in both transitional cell carcinoma of the bladder and serous endometrial cancer (63). Positive urine cytology in women with hematuria, in the absence of a demonstrable urinary tract malignancy, should therefore raise the suspicion of a possible uterine cancer. Intermittent tumor shedding may not lend itself to reliable biomarker detection, particularly in women without bleeding symptoms; however, very little research has been done (28). In the UK, the NICE recommends a direct access ultrasound scan to assess for EC in women aged 55 and over who present with visible hematuria with either low hemoglobin levels, thrombocytosis or high blood glucose levels (20). Urine cytology may offer further evidence for a likely endometrial malignancy, especially if it is shown to out-perform TVS in terms of accuracy. Cytology is, however, limited by its dependency on the experience of the cytologist which may vary widely. Platforms such as single cell technology have shown great promise in identifying circulating tumor cells in bodily fluids but are limited by costs and experimental time (49). Flow cytometry is another technology that has been explored in identifying cancer cells in urine (90). Further studies are needed to assess the accuracy of tumor cell identification in the urine of symptomatic women with EC, especially those who present with hematuria (28).
A urine-based biomarker is ideal for EC detection and relies on the renal excretion of systemic biomarkers or urinary contamination with biomarkers shed from the uterus. Urinary metabolites, proteins and micro-RNA have all been reported as possible EC biomarker candidates. Currently, there is insufficient evidence to support their use due to lack of robust validation. Most of the studies exploring urine for EC diagnosis have been small pilot studies and larger studies are needed. While systemic biomarkers excreted in urine are somewhat limited by the low abundance of cancer related signals in the circulation, especially in early stage EC, contaminating biomarkers in the urine from the uterus may be unreliably shed, especially in asymptomatic women. Molecular analysis of urinary specimens from symptomatic women with EC has great potential to yield clinically relevant non-invasive biomarkers for EC detection. Spectroscopy and tumor cell identification by cytology or single cell technology are other diagnostic strategies with promise that need further exploration, especially in symptomatic women. The use of artificial intelligence to combine “signals” from several modalities or those found using a specific technique will benefit the discovery and validation process. Several factors including age, diet, level of physical activity, medication use, menopausal status, and seasonal effects must be taken into consideration when designing studies for urinary EC biomarker discovery.
Conceptualization: KN. Writing—original draft preparation: KN. Writing—review and editing: KN, DC, ERJ, HO’F, CEB, EJC, and ADW Visualization: KN, DC, ADW, and EJC. Supervision: DC, ADW, and EJC. Funding acquisition: ADW and EJC. All authors contributed to the article and approved the submitted version.
KN is supported by a Cancer Research UK Manchester Cancer Research Centre Clinical Research Fellowship (C147/A25254) and the Wellcome Trust Manchester Translational Informatics Training Scheme. HO’F is supported by the National Institute of Health Research Doctoral Research Fellowship (DRF-2018-11-ST2-054). ERJ is supported by a grant from the JP Moulton Charitable Foundation. EJC and ADW are supported by NIHR Manchester Biomedical Research Centre (IS-BRC-1215-20007). The Medical Research Council (MR/M008959/1) and CRUK Manchester Major Centre award (C147/A25254) supports work in Whetton’s Lab. CEB is funded on a Manchester University NHS Foundation Trust Clinical Research Fellowship.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978-2013. J Natl Cancer Institute (2018) 110(4):354–61. doi: 10.1093/jnci/djx214
3. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer (2015) 136(5):E359–86. doi: 10.1002/ijc.29210
4. CRUK. Cancer Research United Kingdom: Uterine Cancer Incidence Statistics. (2020). Available online at: www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer/incidence, February 2020.
5. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol (1983) 15(1):10–7. doi: 10.1016/0090-8258(83)90111-7
6. Suarez AA, Felix AS, Cohn DE. Bokhman redux: endometrial cancer “types” in the 21st century. Gynecol Oncol (2017) 144(2):243–9. doi: 10.1016/j.ygyno.2016.12.010
7. Stelloo E, Bosse T, Nout RA, MacKay HJ, Church DN, Nijman HW, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Modern Pathol (2015) 28(6):836. doi: 10.1038/modpathol.2015.43
8. Talhouk A, McConechy MK, Leung S, Yang W, Lum A, Senz J, et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer (2017) 123(5):802–13. doi: 10.1002/cncr.30496
9. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet (2008) 371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X
10. MacKintosh ML, Crosbie EJ. Prevention Strategies in Endometrial Carcinoma. Curr Oncol Rep (2018) 20(12):101. doi: 10.1007/s11912-018-0747-1
11. Njoku K, Abiola J, Russell J, Crosbie EJ. Endometrial Cancer Prevention in High Risk Women. Best Pract Res Clin Obstet Gynaecol (2019) 65:66–78. doi: 10.1016/j.bpobgyn.2019.12.005
12. Ryan N, Glaire M, Blake D, Cabrera-Dandy M, Evans D, Crosbie E. The proportion of endometrial cancers associated with Lynch syndrome: a systematic review of the literature and meta-analysis. Genet Med (2019) 21(10):2167–80. doi: 10.1038/s41436-019-0536-8
13. Gammon A, Jasperson K, Champine M. Genetic basis of Cowden syndrome and its implications for clinical practice and risk management. Appl Clin Genet (2016) 9:83. doi: 10.2147/TACG.S41947
14. Sundar S, Balega J, Crosbie E, Drake A, Edmondson R, Fotopoulou C, et al. BGCS uterine cancer guidelines: recommendations for practice. Eur J Obstet Gynecol Reprod Biol (2017) 213:71–97. doi: 10.1016/j.ejogrb.2017.04.015
15. Colombo N, Creutzberg C, Amant F, Bosse T, Gonzalez-Martin A, Ledermann J, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-up. Int J Gynecol Cancer (2016) 26(1):2–30. doi: 10.1097/IGC.0000000000000609
16. Funston G, O’Flynn H, Ryan NA, Hamilton W, Crosbie EJ. Recognizing Gynecological Cancer in Primary Care: Risk Factors, Red Flags, and Referrals. Adv Ther (2018) 35(4):577–89. doi: 10.1007/s12325-018-0683-3
17. Wortman B, Creutzberg C, Putter H, Jürgenliemk-Schulz I-M, Jobsen J, Lutgens L, et al. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: improving patient selection for adjuvant therapy. Br J Cancer (2018) 119(9):1067–74. doi: 10.1038/s41416-018-0310-8
18. de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol (2018) 19(3):295–309. doi: 10.1016/S1470-2045(18)30079-2
19. NICE . Gynaecological cancers, recognition and referral. Available online at:https://cks.nice.org.uk/topics/gynaecological-cancers-recognition-referral. Last accessed 27/10/2020.
20. Hamilton W, Hajioff S, Graham J, Schmidt-Hansen M. Suspected cancer (part 2—adults): reference tables from updated NICE guidance. BMJ (2015) 350:h3044. doi: 10.1136/bmj.h3044
21. Schramm A, Ebner F, Bauer E, Janni W, Friebe-Hoffmann U, Pellegrino M, et al. Value of endometrial thickness assessed by transvaginal ultrasound for the prediction of endometrial cancer in patients with postmenopausal bleeding. Arch Gynecol Obstet (2017) 296(2):319–26. doi: 10.1007/s00404-017-4439-0
22. Wong ASW, Lao TTH, Cheung CW, Yeung SW, Fan HL, Ng PS, et al. Reappraisal of endometrial thickness for the detection of endometrial cancer in postmenopausal bleeding: A retrospective cohort study. BJOG: Int J Obstet Gynaecol (2016) 123(3):439–46. doi: 10.1111/1471-0528.13342
23. Tabor A, Watt HC, Wald NJ. Endometrial thickness as a test for endometrial cancer in women with postmenopausal vaginal bleeding. Obstet Gynecol (2002) 99(4):663–70. doi: 10.1097/00006250-200204000-00029
24. Dijkhuizen FPH, Mol BW, Brölmann HA, Heintz APM. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer (2000) 89(8):1765–72. doi: 10.1002/1097-0142(20001015)89:8<1765::AID-CNCR17>3.0.CO;2-F
25. Lipscomb GH, Lopatine SM, Stovall TG, Ling FW. A randomized comparison of the Pipelle, Accurette, and Explora endometrial sampling devices. Am J Obstet Gynecol (1994) 170(2):591–4. doi: 10.1016/S0002-9378(94)70234-9
26. Jansen FW, Vredevoogd CB, Van Ulzen K, Hermans J, Trimbos JB, Trimbos-Kemper TC. Complications of hysteroscopy: a prospective, multicenter study. Obstet Gynecol (2000) 96(2):266–70. doi: 10.1097/00006250-200008000-00021
27. Marabini A, Stefanetti M, Del Vecchio C, Bovicelli L. Acceptability and pain of outpatient hysteroscopy. J Am Assoc Gynecol Laparoscopists (2000) 7(1):71–5. doi: 10.1016/S1074-3804(00)80012-2
28. Njoku K, Chiasserini D, Whetton AD, Crosbie EJ. Proteomic Biomarkers for the Detection of Endometrial Cancer. Cancers (2019) 11(10):1572. doi: 10.3390/cancers11101572
29. Badrick E, Cresswell K, Ellis P, Crosbie P, Hall PS, O’Flynn H, et al. Top ten research priorities for detecting cancer early. Lancet Public Health (2019) 41(22):e551. doi: 10.1016/S2468-2667(19)30185-9
30. Wan YL, Beverley-Stevenson R, Carlisle D, Clarke S, Edmondson RJ, Glover S, et al. Working together to shape the endometrial cancer research agenda: the top ten unanswered research questions. Gynecol Oncol (2016) 143(2):287–93. doi: 10.1016/j.ygyno.2016.08.333
31. Henry NL, Hayes DF. Cancer biomarkers. Mol Oncol (2012) 6(2):140–6. doi: 10.1016/j.molonc.2012.01.010
32. Manne U, Srivastava R-G, Srivastava S. Keynote review: Recent advances in biomarkers for cancer diagnosis and treatment. Drug Discovery Today (2005) 10(14):965–76. doi: 10.1016/S1359-6446(05)03487-2
33. Banach P, Suchy W, Derezinski P, Matysiak J, Kokot ZJ, Nowak-Markwitz E. Mass spectrometry as a tool for biomarkers searching in gynecological oncology. Biomed Pharmacother (2017) 92:836–42. doi: 10.1016/j.biopha.2017.05.146
34. Burke W. Genetic tests: clinical validity and clinical utility. Curr Protoc Hum Genet (2014) 81:9.15.1–8. doi: 10.1002/0471142905.hg0915s81
35. Banno K, Kisu I, Yanokura M, Tsuji K, Masuda K, Ueki A, et al. Biomarkers in endometrial cancer: possible clinical applications. Oncol Lett (2012) 3(6):1175–80. doi: 10.3892/ol.2012.654
36. Pauker SG, Kassirer JP. Therapeutic decision making: a cost-benefit analysis. New Engl J Med (1975) 293(5):229–34. doi: 10.1056/NEJM197507312930505
37. Grayson K, Gregory E, Khan G, Guinn B-A. Urine Biomarkers for the Early Detection of Ovarian Cancer–Are We There Yet? Biomarkers Cancer (2019) 11:1179299X19830977. doi: 10.1177/1179299X19830977
38. Mu AK-W, Lim B-K, Hashim OH, Shuib AS. Detection of differential levels of proteins in the urine of patients with endometrial cancer: Analysis using two-dimensional gel electrophoresis and O-glycan binding lectin. Int J Mol Sci (2012) 13(8):9489–501. doi: 10.3390/ijms13089489
39. Wang Y, Otis CN, Florence RR. Atypical squamous cells in the urine revealing endometrioid adenocarcinoma of the endometrium with squamous cell differentiation: A case report. Diagn Cytopathol (2015) 43(1):49–52. doi: 10.1002/dc.23118
40. Záveský L, Jandáková E, Turyna R, Langmeierová L, Weinberger V, Drábková LZ, et al. Evaluation of cell-free urine microRNAs expression for the use in diagnosis of ovarian and endometrial cancers. A pilot study. Pathol Oncol Res (2015) 21(4):1027–35. doi: 10.1007/s12253-015-9914-y
41. Ari Ş, Arikan M. Next-generation sequencing: advantages, disadvantages, and future. Plant Omics: Trends and Applications. Springer, Berlin (2016) p. 109–35.
42. Dinges SS, Hohm A, Vandergrift LA, Nowak J, Habbel P, Kaltashov IA, et al. Cancer metabolomic markers in urine: evidence, techniques and recommendations. Nat Rev Urol (2019) 16(6):339–62. doi: 10.1038/s41585-019-0185-3
43. Cottrell JS. Protein identification using MS/MS data. J Proteomics (2011) 74(10):1842–51. doi: 10.1016/j.jprot.2011.05.014
44. Jing J, Gao Y. Urine biomarkers in the early stages of diseases: current status and perspective. Discovery Med (2018) 25(136):57–65.
45. Costas L, Frias-Gomez J, Guardiola M, Benavente Y, Pineda M, Pavón MÁ, et al. New perspectives on screening and early detection of endometrial cancer. Int J Cancer (2019) 145(12):3194–06. doi: 10.1002/ijc.32514
46. Bakkum-Gamez JN, Wentzensen N, Maurer MJ, Hawthorne KM, Voss JS, Kroneman TN, et al. Detection of endometrial cancer via molecular analysis of DNA collected with vaginal tampons. Gynecol Oncol (2015) 137(1):14–22. doi: 10.1016/j.ygyno.2015.01.552
47. Fiegl H, Gattringer C, Widschwendter A, Schneitter A, Ramoni A, Sarlay D, et al. Methylated DNA collected by tampons—a new tool to detect endometrial cancer. Cancer Epidemiol Prev Biomarkers (2004) 13(5):882–8.
48. Heng S, Stephens AN, Jobling TW, Nie G. Measuring PC activity in endocervical swab may provide a simple and non-invasive method to detect endometrial cancer in post-menopausal women. Oncotarget (2016) 7(29):46573. doi: 10.18632/oncotarget.10287
49. Liang S-B, Fu L-W. Application of single-cell technology in cancer research. Biotechnol Adv (2017) 35(4):443–9. doi: 10.1016/j.biotechadv.2017.04.001
50. Shao X, Wang K, Liu X, Gu C, Zhang P, Xie J, et al. Screening and verifying endometrial carcinoma diagnostic biomarkers based on a urine metabolomic profiling study using UPLC-Q-TOF/MS. Clinica Chimica Acta (2016) 463:200–6. doi: 10.1016/j.cca.2016.10.027
51. Zhao H, Jiang Y, Liu Y, Yun C, Li L. Endogenous estrogen metabolites as biomarkers for endometrial cancer via a novel method of liquid chromatography-mass spectrometry with hollow fiber liquid-phase microextraction. Hormone Metab Res (2015) 47(02):158–64. doi: 10.1055/s-0034-1371865
52. Bufa A, Bíró I, Poór V, Molnár G, Kovács KA, Felinger A, et al. Altered urinary profiles of endogenous steroids in postmenopausal women with adenocarcinoma endometrii. Gynecol Endocrinol (2010) 26(1):10–5. doi: 10.1080/09513590903159581
53. Aitokallio-Tallberg A, Viinikka L, Ylikorkala O. Urinary 6-keto-prostaglandin F1a in patients with gynaecological tumours. Cancer Lett (1987) 34(2):201–6. doi: 10.1016/0304-3835(87)90011-5
54. Mu AK-W, Lim B-K, Aminudin N, Hashim OH, Shuib AS. Application of SELDI-TOF in N-glycopeptides profiling of the urine from patients with endometrial, ovarian and cervical cancer. Arch Physiol Biochem (2016) 122(3):111–6. doi: 10.3109/13813455.2016.1151441
55. Bostanci EI, Dikmen AU, Girgin G, Gungor T, Baydar T, Danisman AN. A new diagnostic and prognostic marker in endometrial cancer: Neopterin. Int J Gynecol Cancer (2017) 27(4):754–8. doi: 10.1097/IGC.0000000000000952
56. Bazzett LB, Magnus M, Taylor DD, Gercel-Taylor C. Urinary matrix metalloproteinases as a potential screening test for gynecologic malignancies. Gynecol Oncol (2003) 90(2):435–42. doi: 10.1016/S0090-8258(03)00334-2
57. Mattila A, Saario I, Viinikka L, Ylikorkala O, Perheentupa J. Urinary epidermal growth factor concentrations in various human malignancies. Br J Cancer (1988) 57(2):139. doi: 10.1038/bjc.1988.29
58. Stockley J, Akhand R, Kennedy A, Nyberg C, Crosbie EJ, Edmondson RJ. Detection of MCM5 as a novel non-invasive aid for the diagnosis of endometrial and ovarian tumours. BMC Cancer (2020) 21(1):1000. doi: 10.1186/s12885-020-07468-y
59. Ruskin R, Srivastava A, McMeekin D, Ramesh R, Moxley K. Liquid Gold: Urinary exosomes as a potential source of biomarkers in endometrial cancer. Gynecol Oncol (2016) 143(1):207. doi: 10.1016/j.ygyno.2016.08.272
60. Srivastava A, Moxley K, Ruskin R, Dhanasekaran DN, Zhao YD. Ramesh R. A non-invasive liquid biopsy screening of urine-derived exosomes for miRNAs as biomarkers in endometrial cancer patients. AAPS J (2018) 20(5):82. doi: 10.1208/s12248-018-0220-y
61. Kinugasa M, Nishimura R, Koizumi T, Morisue K, Higashida T, Natazuka T, et al. Combination Assay of Urinary β-Core Fragment of Human Chorionic Gonadotropin with Serum Tumor Markers in Gynecologic Cancers. Japanese J Cancer Res (1995) 86(8):783–9. doi: 10.1111/j.1349-7006.1995.tb02469.x
62. Kanno T, Ito M, Kawase N, Taki Y, Hosaka N. A case of corpus cancer presenting with positive urine cytology. Hinyokika kiyo Acta Urol Japonica (2002) 48(8):479–81.
63. Khan F, Fernando A, Khan S, Ismail B, Cole R. Positive urine cytology with localised uterine malignancy. Br J Med Surg Urol (2011) 4(5):210–2. doi: 10.1016/j.bjmsu.2010.11.001
64. Paraskevaidi M, Morais CL, Lima KM, Ashton KM, Stringfellow HF, Martin-Hirsch PL, et al. Potential of mid-infrared spectroscopy as a non-invasive diagnostic test in urine for endometrial or ovarian cancer. Analyst (2018) 143(13):3156–63. doi: 10.1039/C8AN00027A
65. Altmae S, Esteban FJ, Stavreus-Evers A, Simon C, Giudice L, Lessey BA, et al. Guidelines for the design, analysis and interpretation of ‘omics’ data: Focus on human endometrium. Hum Reprod Update (2014) 20(1):12–28. doi: 10.1093/humupd/dmt048
66. Njoku K, Sutton CJ, Whetton AD, Crosbie EJ. Metabolomic Biomarkers for Detection, Prognosis and Identifying Recurrence in Endometrial Cancer. Metabolites (2020) Jul 3110(8):314. doi: 10.3390/metabo10080314
67. Zhang T, Wu X, Ke C, Yin M, Li Z, Fan L, et al. Identification of potential biomarkers for ovarian cancer by urinary metabolomic profiling. J Proteome Res (2012) 12(1):505–12. doi: 10.1021/pr3009572
68. Kruk J, Doskocz M, Jodłowska E, Zacharzewska A, Łakomiec J, Czaja K, et al. NMR techniques in metabolomic studies: A quick overview on examples of utilization. Appl Magnetic Resonance (2017) 48(1):1–21. doi: 10.1007/s00723-016-0846-9
69. Maran A, Gorny G, Oursler MJ, Zhang M, Shogren K, Yaszemski MJ, et al. 2-methoxyestradiol inhibits differentiation and is cytotoxic to osteoclasts. J Cell Biochem (2006) 99(2):425–34. doi: 10.1002/jcb.20924
70. Mackintosh M, Crosbie E. Obesity-driven endometrial cancer: is weight loss the answer? BJOG: Int J Obstet Gynaecol (2013) 120(7):791–4. doi: 10.1111/1471-0528.12106
71. Chak CM, Lacruz ME, Adam J, Brandmaier S, Covic M, Huang J, et al. Ageing Investigation Using Two-Time-Point Metabolomics Data from KORA and CARLA Studies. Metabolites (2019) 9(3):44. doi: 10.3390/metabo9030044
72. Kitson SJ, Evans DG, Crosbie EJ. Identifying High-Risk Women for Endometrial Cancer Prevention Strategies: Proposal of an Endometrial Cancer Risk Prediction Model. Cancer Prev Res (2017) 10(1):1–13. doi: 10.1158/1940-6207.CAPR-16-0224
73. Li X, Zhao M, Li M, Jia L, Gao Y. Effects of three commonly-used diuretics on the urinary proteome. Genomics Proteomics Bioinf (2014) 12(3):120–6. doi: 10.1016/j.gpb.2013.12.002
74. Elliott P, Posma JM, Chan Q, Garcia-Perez I, Wijeyesekera A, Bictash M, et al. Urinary metabolic signatures of human adiposity. Sci Trans Med (2015) 7(285):285ra62–ra62. doi: 10.1126/scitranslmed.aaa5680
75. Yuan J-M, Gao Y-T, Murphy SE, Carmella SG, Wang R, Zhong Y, et al. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res (2011) 71(21):6749–57. doi: 10.1158/0008-5472.CAN-11-0209
76. Giskeødegård GF, Davies SK, Revell VL, Keun H, Skene DJ. Diurnal rhythms in the human urine metabolome during sleep and total sleep deprivation. Sci Rep (2015) 5:14843. doi: 10.1038/srep14843
77. Angioli R, Plotti F, Capriglione S, Montera R, Damiani P, Ricciardi R, et al. The role of novel biomarker HE4 in endometrial cancer: a case control prospective study. Tumor Biol (2013) 34(1):571–6. doi: 10.1007/s13277-012-0583-0
78. Chen Y, Ren YL, Li N, Yi XF, Wang HY. Serum human epididymis protein 4 vs. carbohydrate antigen 125 and their combination for endometrial cancer diagnosis: A meta-Analysis. Eur Rev Med Pharmacol Sci (2016) 20(10):1974–85.
79. Moore RG, Brown AK, Miller MC, Badgwell D, Lu Z, Allard WJ, et al. Utility of a novel serum tumor biomarker HE4 in patients with endometrioid adenocarcinoma of the uterus. Gynecol Oncol (2008) 110(2):196–201. doi: 10.1016/j.ygyno.2008.04.002
80. Ardekani AM, Akhondi MM, Sadeghi MR. Application of genomic and proteomic technologies to early detection of cancer. Arch Iranian Med (2008) 11(4):427–34.
81. Creighton CJ, Benham AL, Zhu H, Khan MF, Reid JG, Nagaraja AK, et al. Discovery of novel microRNAs in female reproductive tract using next generation sequencing. PloS One (2010) 5(3):e9637. doi: 10.1371/journal.pone.0009637
82. Jia W, Wu Y, Zhang Q, Gao G, Zhang C, Xiang Y. Identification of four serum microRNAs from a genome−wide serum microRNA expression profile as potential non−invasive biomarkers for endometrioid endometrial cancer. Oncol Lett (2013) 6(1):261–7. doi: 10.3892/ol.2013.1338
83. Risinger JI, Hayes K, Maxwell GL, Carney ME, Dodge RK, Barrett JC, et al. PTEN mutation in endometrial cancers is associated with favorable clinical and pathologic characteristics. Clin Cancer Res (1998) 4(12)3005–10.
84. Jonusiene V, Sasnauskiene A, Lachej N, Kanopiene D, Dabkeviciene D, Sasnauskiene S, et al. Down-regulated expression of Notch signaling molecules in human endometrial cancer. Med Oncol (2013) 30(1):438. doi: 10.1007/s12032-012-0438-y
85. Zanotti L, Bignotti E, Calza S, Bandiera E, Ruggeri G, Galli C, et al. Human epididymis protein 4 as a serum marker for diagnosis of endometrial carcinoma and prediction of clinical outcome. Clin Chem Lab Med (2012) 50(12):2189–98. doi: 10.1515/cclm-2011-0757
86. Liu Y, Sun H, Mao H, Gao M, Tan X, Li Y, et al. Expression of tumor suppressor programmed cell death 4 in endometrioid endometrial carcinomas and clinicopathological significance. Oncol Lett (2018) 15(6):9369–76. doi: 10.3892/ol.2018.8517
87. Adam L, Zhong M, Choi W, Qi W, Nicoloso M, Arora A, et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res (2009) 15(16):5060–72. doi: 10.1158/1078-0432.CCR-08-2245
88. Sullivan PS, Chan JB, Levin MR, Rao J. Urine cytology and adjunct markers for detection and surveillance of bladder cancer. Am J Trans Res (2010) 2(4):412.
89. Nemoto R, Kato T, Harada M, Shibata K, Kano M. Mass screening for urinary tract cancer with urine cytology. J Cancer Res Clin Oncol (1982) 104(1-2):155–9. doi: 10.1007/BF00402063
Keywords: urine, early detection, diagnostic biomarkers, endometrial cancer (EC), non-invasive (urine)
Citation: Njoku K, Chiasserini D, Jones ER, Barr CE, O’Flynn H, Whetton AD and Crosbie EJ (2020) Urinary Biomarkers and Their Potential for the Non-Invasive Detection of Endometrial Cancer. Front. Oncol. 10:559016. doi: 10.3389/fonc.2020.559016
Received: 04 May 2020; Accepted: 12 October 2020;
Published: 03 November 2020.
Edited by:
Shannon Neville Westin, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Thangesweran Ayakannu, University of Liverpool, United KingdomCopyright © 2020 Njoku, Chiasserini, Jones, Barr, O’Flynn, Whetton and Crosbie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emma J. Crosbie, ZW1tYS5jcm9zYmllQG1hbmNoZXN0ZXIuYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.