- 1Department of Radiotherapy, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 2Department of Respiratory Disease, Zhejiang Provincial People’s Hospital, Hangzhou, China
- 3Department of Urology, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 4Department of Ophthalmology, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 5Department of Urology, Renji Hospital, Shanghai, China
- 6Department of Radiology, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 7Department of Pathology, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 8Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
- 9The Medical Department, 3D Medicines Inc., Shanghai, China
- 10Division of Cancer, Department of Surgery and Cancer, Imperial College London, London, United Kingdom
Immune checkpoint inhibitors (ICIs) cause fewer toxicities than conventional chemotherapy. Although most of the immune-related adverse events (irAEs) are mild, reversible, and manageable, potentially severe and rare irAEs remain relevant. We present a 24-year-old man with advanced hereditary renal cancer who developed bilateral posterior uveitis and retinal detachment after systematic treatment of ICI and an anti-angiogenic drug. Axitinib and pembrolizumab were administered with a partial response and following the severe ocular irAE and systemic corticosteroid treatment was initiated. Our case indicates that ocular irAEs may occur rapidly. To the best of our knowledge, this is the first case of posterior uveitis and retinal detachment in hereditary renal cancer patients treated with ICI and anti-angiogenic drugs.
Introduction
Currently, seven ICIs have received FDA approval, targeting CTLA-4 (cytotoxic T-lymphocyte antigen 4), PD-1 (programmed death-1), or PD-L1 (programmed death ligand-1) (1–7), changing the paradigm of cancer treatment by shifting from targeting cancer itself to modulating immune cells in the tumor environment. Nivolumab, a PD-1 inhibitor, improves OS (overall survival) for patients with advanced renal cell carcinoma (RCC) following previous anti-angiogenic therapy as per data from CheckMate-025 (8). At present, pembrolizumab in combination with axitinib has become standard of care in the first line setting of advanced RCC (9, 10), which applies to all the risk groups of International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) criteria. ICI blockade reverses tumor-mediated immune suppression, which expands the production of T cell immunity (11) but its use may lead to persistent T‐cell activation and unwanted side effects (12).
IrAEs due to ICIs involve different organs including skin, gastrointestinal tract, lungs, and endocrine, musculoskeletal (13). ICIs are generally well tolerated, and irAEs are generally manageable, but irAEs may sometimes lead to treatment discontinuation or withdrawal, and rarely death (14). The overall incidence of irAEs range widely with hepatic toxicities including elevated liver enzymes (35%), endocrine toxicities such as hyperthyroidism or hypothyroidism (28%), hypophysitis (7%), and pneumonitis (7%). The mechanism of irAEs is thought due to auto-reactive T and B cells escape deletion by central tolerance (15). irAEs may also be caused by epitope spreading, which contributes to cross reactivity of self and tumor antigens (16). No prospective trials have determined strategies for managing specific irAE; therefore, clinical practice is variable.
Case Description
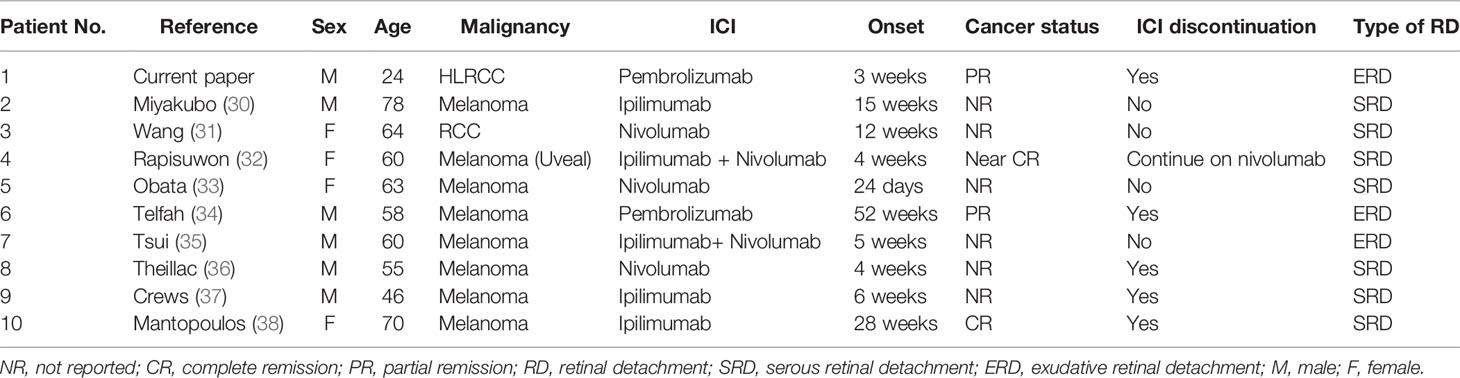
A 24-year-old male patient complained of abdominal pain in August 2018. Abdominal computed tomography (CT) and magnetic resonance imaging (MRI) scan detected a mass in his left kidney, with Mayo level IV inferior vena cava tumor thrombus (Figure 1). He had no family history and a radical nephrectomy and inferior vena cava thrombectomy were performed in September 2018. Intraoperative examination found the tumor extended to the right atrium and invaded the wall of the vena cava. A grayish-yellow mass with 11 cm × 7 cm in size with papillary arrangement, irregular nucleus, and large nucleoli and renal venous tumor thrombus formation was identified (Figure 1). Resection margins were negative, and 8 lymph nodes around the renal pedicle were negative. Histology showed was high-grade RCC, papillary type with vena cava tumor thrombus (Supplementary Figure 1). Immunohistology chemistry (IHC) staining indicated CK(pan)(+), p63(−), Napsin A(−), PAX-8(+), Vimentin (+), CK7(−), CD10(+), E-cadherin(+), CD117(−), P504S(+), TFE3(−). Pathological staging was pT3cN0M0, stage III.
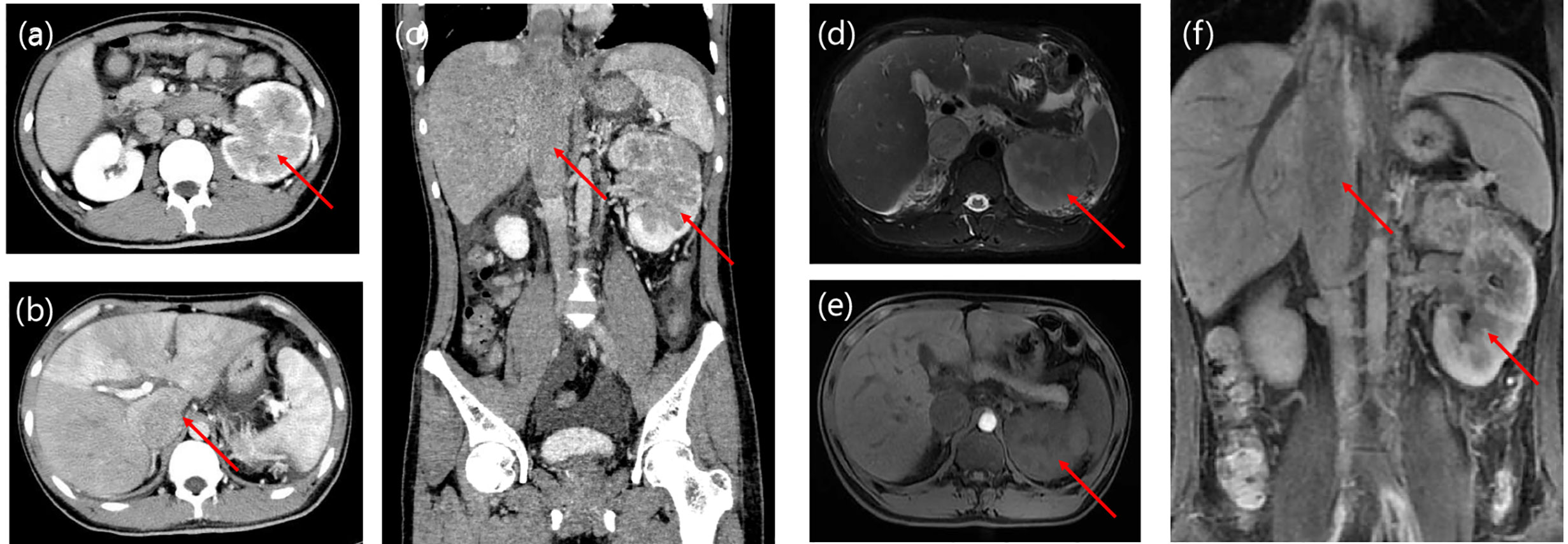
Figure 1 Enhanced CT (A–C) and MRI (D–F) imaging. Significant abnormal findings of left kidney and tumor thrombus of vena cava were noted (arrow).
Genomic profiling of DNA extracted from tumor specimen and peripheral white cell was performed through next-generation sequencing (NGS). NGS results revealed that TMB (tumor mutation burden) value was 4.03 mutants/Mb and the microsatellite state was stable. IHC indicated that PD-L1 Tumor Proportion Score (TPS) was 25% (Supplementary Figure 1).
Germline mutations of his peripheral blood revealed one FH (Fumarate hydratase) gene germline mutation p.G346Vfs*11 was identified, which has been confirmed as a pathogenic mutation. His tumor sections were stained with anti-FH and anti-2-succinocysteine (2SC) antibodies to confirm the diagnosis (Supplementary Figure 1). His final diagnosis was hereditary leiomyomatosis and renal cell cancer (HLRCC), an unusual and highly aggressive form of renal carcinoma. Furthermore, a CYP2C19 p.P227P mutation was detected, which is reported to contribute to a minor (10%) pathway of axitinib metabolism (17).
Sunitinib (50-mg QD orally) was used as an adjuvant treatment after surgery. Abdominal enhanced CT in May 2019 suggested metastatic lesions in the liver and right adrenal gland (Figure 3). Axitinib (5-mg BID orally) was used as first-line treatment. Response evaluation by B-ultrasound after 1 month confirmed progression of disease (Supplementary Figure 2). Based on the results of KEYNOTE-426 which demonstrated axitinib in combination with pembrolizumab conferred a survival benefit for patients with advanced RCC in the first line setting (18), subsequent treatment with this was given from June 2019 but dosing of pembrolizumab was 100 mg every 3 weeks due to economic considerations. Response evaluation after one dose of pembrolizumab showed a partial response, with shrinkage of hepatic and right adrenal lesions, and response persisted for 5 months (Supplementary Figure 1). However, the patient developed vision deterioration within 3 weeks of the first dose of pembrolizumab, but he did not inform the doctor. In January 2020, his vision deteriorated quickly, and he was referred to an ophthalmologist.
A slit‐lamp examination revealed no kerato-precipitates. Tyndall effect was observed and anterior chamber (AC) cells were not obvious in both eyes. His best corrected visual acuity (BCVA) was 0.4 in the both eyes. The intraocular pressure was 13 mmHg in the right eye and 11 mmHg in the left eye. Dilated fundus examination indicated vitreous floaters were not obvious. Large areas of depigmentation were observed in retina, and local pigmentation were noticed (Figure 2). Posterior and inferior retinal detachment was observed. Ultrasound examination detected minimal echogenicity in the vitreous and posterior and inferior retinal detachment. Fluorescein (FFA) angiography showed dotted hyperfluorescence in early phase and a little leakage in the maculae. Optical coherence tomography (OCT) revealed neuroepithelial detachment with spotty to patchy high signal, and the choroid is thickened significantly.
Figure 2 (A, B) Bilateral ultrasound images showing posterior retinal detachment in both eyes. (C, D) Fundus photographs showing large areas of depigmentation in retina. (E–H) Fluorescein fundus angiography (FFA) reveals early hypofluorescence of left (E) and right (G) side and late hyperfluorescence of left (F) and right (H) side in the optic disc area. (I, J) Optical coherence tomography of the left (I) and right (J) eye shows pockets of subretinal fluid.
The diagnosis was posterior uveitis with bilateral retinal detachment. Immune-related tests and infection-related tests were also performed to rule out uveitis caused by infection or tumor metastasis. The complete blood count (CBC) results were normal and his erythrocyte sedimentation rate (ESR) was increased. Serologic investigations revealed negative for bacterial infection, rheumatoid factor, and tuberculosis. Serum cytokine release was tested, including IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, and IL-17A. Among these, IL-6 and IL-17A were increased (Table 1). From the time of occurrence, the ocular adverse events may due to immune checkpoint inhibitor. Based on the CTCAE (Common Terminology Criteria for Adverse Events; version 5.0), his ocular irAE was categorized as grade 3. He was treated with intravenous methylprednisolone 60 mg (1 mg/kg) systematically, followed by oral methylprednisolone. Treatment with topical prednisolone acetate (1%) every 2 h was initiated in the both eyes. Follow-ups of OCT is shown in Figure 3. Eye MRI at January 2020 also confirmed a thickening of posterior walls (Figure 3). His endocrine results indicated hypothyroidism, which was considered as an irAE, and levothyroxine tablets were commenced.
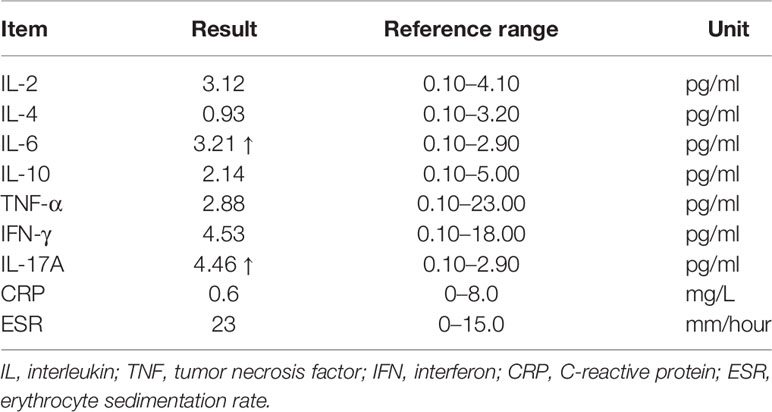
Table 1 Circulating cytokine level after diagnosis of posterior uveitis with bilateral retinal detachment.
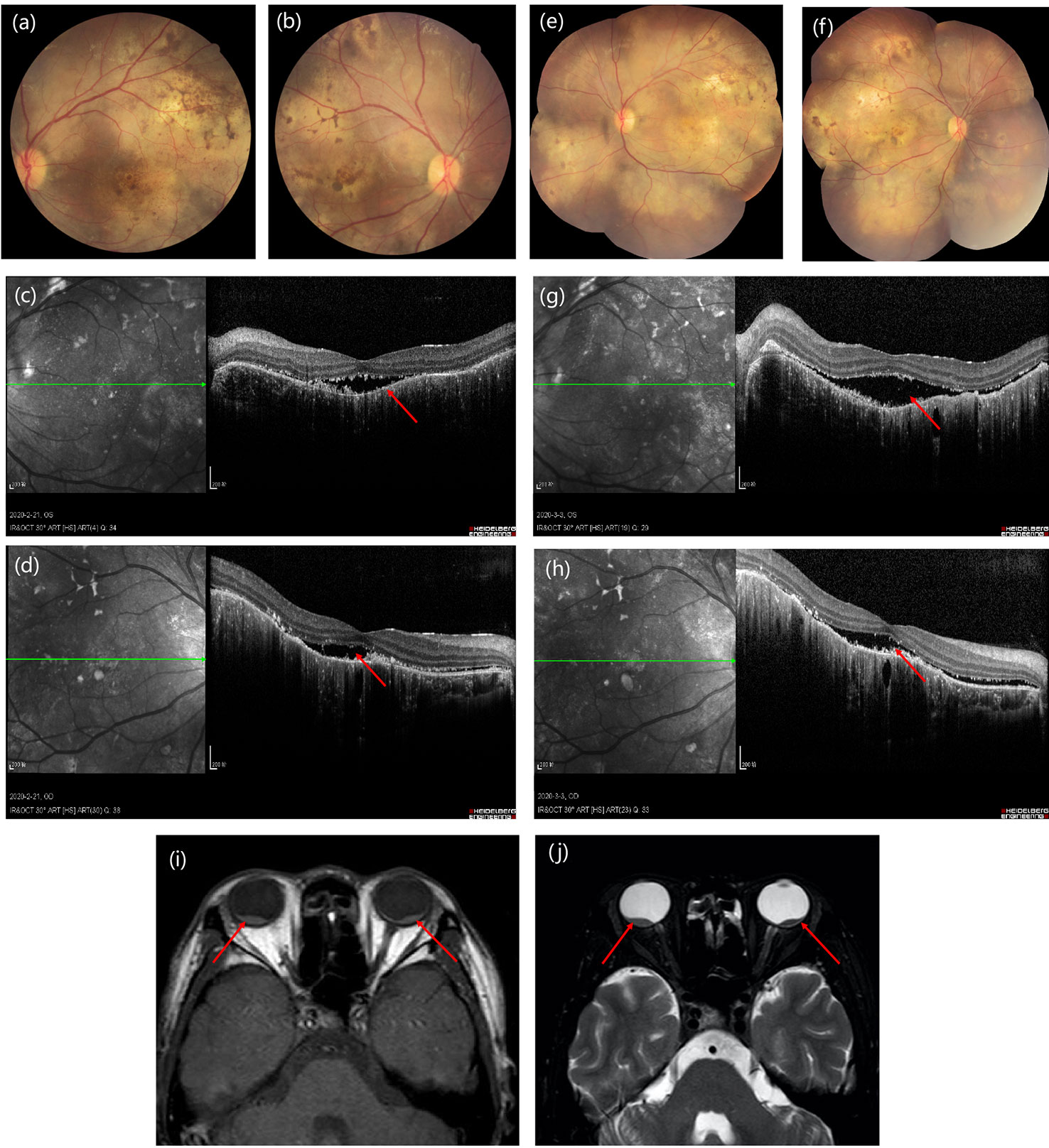
Figure 3 Time course of images of the presentation in fundus examination (A, B) and optical coherence tomography (C, D) at 3-week follow-up to fundus examination (E, F) and optical coherence tomography (G, H) at 6-week follow-up. (I, J) Eye MRI shows bilateral retinal detachment of optic nerve.
Our multidisciplinary team decided to permanently discontinue his use of pembrolizumab. At follow-up in February 2020, progressive disease in his adrenal, hepatic and bones were observed (Supplementary Figure 3). There was no indication of metastatic lesions in his eyes. Third-line systemic therapy of bevacizumab and erlotinib was initiated, and the patient responded well to the combination therapy. The timeline of his diagnosis, treatment and onset of irAE were shown in Supplementary Figure 4.
Discussion
Ocular adverse events are rare, occurring in less than 1% of patients treated with ICIs (19). A systematic review and meta-analysis of ocular toxicities due to ICIs reported that the most common ocular toxicities included uveitis and dry eyes. Pooled analysis for OR (odds ratio) of all-grade immune-related ocular toxicities is 3.40 (95% CI: 1.32–8.71) (20). Due to the inherent characteristics of cohort studies, the prevalence of rare AEs such as ocular toxicities is somewhat underestimated, so the real incidence might be higher than expected (21). A disproportionality analysis of U.S. Food and Drug Administration (FDA) database FAERS demonstrated the reported odds ratios (RORs) of ICIs. Among approved ICIs, nivolumab ranks the highest of ocular adverse events associated with ICI (ROR = 10.54, 95% CI 7.30 - 15.22) (22).
The precise causal relationship in the scenario of combination therapy is difficult to make a decisive conclusion. As previously reported, targeted therapy and other anti-cancer drugs may also cause ocular toxicities (23). The association of oral anti-VEGF drugs with retinal detachments, while rare, is plausible. But the association of oral anti-VEGF drugs with post uveitis and retinal detachment are unclassified although this suggests a “signal” requiring further study (24). Currently, various anti-angiogenic drugs, such as anti-VEGF antibody (ranibizumab), VEGF trap (aflibercept), and multikinase tyrosine kinase inhibitor (axitinib) are currently used to treat corneal neovascularization. Sunitinib malate at the concentration of 12.5 mg/ml caused no toxicity, but 25 mg/ml caused retinal changes suggesting toxicity in the in vivo model (25). There is only an observational case report of impaired retinal circulation of axitinib in treating patient with RCC (26).
We searched FAERS database of spontaneous reports of uveitis and retinal detachment of ICIs using web search tool (https://www.pharmapendium.com/home), using the generic drug name “atezolizumab, avelumab, cemiplimab, durvalumab, ipilimumab, nivolumab, and pembrolizumab”, AND “uveitis”, AND “retinal detachment” up to February 20, 2020. A total of 184 uveitis following ICIs were reported in the database, among which eight cases with uveitis and retinal detachment were identified, including five patients who received nivolumab and three patients with pembrolizumab. We also searched reported cases with sunitinib and axitinib, and there were no cases of uveitis with retinal detachment. The onset time of vision change occurs 3 weeks after initiation of pembrolizumab of this patient, therefore, we suspected the ocular toxicity of this patient were primarily due to pembrolizumab. A retrospective study reported the average onset time of ocular immune-related adverse events (irAEs) from initiation of ICI measured 15.7 weeks (27), but cases also reported ocular irAE onset within 20 days of ICI use (28, 29).
We also searched published literature regarding ICI-related retinal detachment, identifying nine cases with retinal detachment in patient receiving ICIs (Table 2) (30–38). Among them, most of the cases reported were anti-CTLA-4 antibodies. Anti-CTLA-4 agents have a higher function by enhancing T-cell priming, while blockade of PD-1/L1 pathway resulting in re-invigorating CD8 T-cell responses (39). IrAEs are more common in patients treated with CTLA-4 blockades than PD-1/L1 inhibitors, reflecting their different roles in immune regulation (40).
Timely detection of ocular symptoms and subsequent treatment are vital to the outcome of irAEs. Most of the symptoms are not typical, and are easily confounded by underlying co-morbidities, so mild manifestations of ocular toxicities are commonly overlooked. For example, visual deterioration may have different etiologies such as myopia and cataract, etc. Differentiating ocular irAE from other causes has important implications for optimal treatment, including infection, tumor metastasis to eye, and other possible reasons. Patients complaining of eye symptoms while receiving ICI should be seen by an ophthalmologist (21). Therefore, as the occurrence of ocular toxicities, other causes must always be ruled out. A high level of suspicion is needed to monitor and treat ocular toxicities, while in real-world setting, great variation exists. Apart from hypothyroidism and few other possible etiologies during ICI treatment, agreement among observers was poor (41).
In order to rule out the possibility of cancer-related retinopathy, infection and tumor metastasis, observation of ocular findings using multiple imaging analysis can provide insights into possible etiology of the condition. Whole-body PET/CT can be useful for clinical evaluation of patients with uveal metastases (42). The eyes are considered as an immune privileged site, but ocular irAEs can involve any component of the ocular apparatus (43). Patients experiencing ocular adverse events (AEs) may present with any of the following symptoms: blurred/distorted vision, blind spots, change in color vision, photophobia, tenderness/pain, eyelid swelling, and proptosis. The most common adverse effects include dry eyes, conjunctivitis, uveitis, and myasthenia gravis (19). Referring to an ophthalmologist is suggested if the symptoms occur. It is recommended that patients should receive ocular examination including visual acuity in each eye, color vision, pupil size, shape, and reactivity, red reflex, and fundoscopic examination (44). During evaluation of vision, sight-threatening effects are ocular myasthenia, corneal punctate epithelial erosions, subconjunctival hemorrhage, corneal perforation, uveitis, hypotony maculopathy, cystoid macular edema, and serous retinal detachment (45). Here, the irAE involves bilateral posterior and retina. Certain parts of the eye are unable to regenerate after destructive inflammation, such as neural retina (46).
There are several guidelines regarding the management of irAEs, including those published by ESMO, SITC, and ASCO/NCCN (47–49). However, the treatment of rare irAEs such as ocular toxicity is not fully clear due to lack of adequate evidence (50). NCCN guidelines recommend the use of systemic corticosteroids and tropical steroids as initial treatment (47). If the ocular irAE is grade 1~2, it is possible to continue prior ICI balancing the risk/benefit ratio for each individual patient. The time to resolution of irAEs depends on different organs involved (51). In our case report, this patient received steroids for more than a month, while his vision remained stable. In response to the acute inflammatory phase, many cytokines are continuously secreted, notably IL (interleukin)-1, IL-6, and human tumor necrosis factor α (TNFα). An assessment of baseline cytokine level assessment before ICI therapy followed by repeated measurements in case of irAE might be useful.
There are some other issues that require addressing. Firstly, the irAEs in different underlying malignancies may also vary. A systemic review compared the likelihood of irAEs in different tumor types, including RCC, melanoma, and non-small cell carcinoma (NSCLC) (52). HLRCC is a type of familial renal cancers which is driven by FH gene mutation, which is the most aggressive tumor among the familial renal cancer syndromes (53). Most of HLRCCs do not express PD-L1 (54), while in our case the expression of PD-L1 of tumor cell was 25%. There were not enough evidences to support whether this special subtype of RCC would have any link to this rare irAE.
Secondly, combinations of ICI with other drugs such as ICI, chemotherapy or anti-angiogenic drug may lead to higher incidences of irAEs (55, 56). Combination therapy with PD-1 inhibitors and chemotherapy have similar side effects to those of chemotherapy alone; however, combination therapy with CTLA-4 and PD-1 inhibitors may lead to more severe AEs than those with monotherapy (57).
Thirdly, the addition of ICI on the basis of axitinib has led to shrinkage of his hepatic and right adrenal metastatic lesions, while he developed serious ocular toxicities. Literature suggests irAE onset is predictive of anti-PD-(L)1 antibody response across a variety of solid tumors (58–60). However, the relationship of efficacy and toxicity in the ICI evokes debate regarding the “survivor bias” phenomenon (61).
In conclusion, this is the first report of posterior uveitis and retinal detachment with severe vision deterioration in a HLRCC patient receiving ICI and anti-angiogenic drug. The use of ICIs in medical oncology continues to rise significantly; therefore, the number of patients with rare irAEs will increase. There is a need for routinely querying patients during their treatment regarding the occurrence of unexpected adverse events. This case highlights the monitoring and timely detection of rare irAEs in RCC patients receiving ICIs.
Ethics Statement
The patient provided his written informed consent for the publication of images or data included in this article.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This study was partially supported by Natural Science Foundation of Zhejiang Province, China (Grant number: LY19H160041).
Conflict of Interest
SQC was employed by the company 3D Medicines Inc. JS is editor-in-chief of Oncogene. JS has sat on a number of scientific advisory boards, including Benevolent AI, and consults with Lansdowne partners and Vitruvian; he sits on the Board of Directors for BB Biotech Healthcare Trust and chairs Xerion Healthcare.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.549168/full#supplementary-material
Supplementary Figure 1 | Histological and genetic assessment of tumor. (a) Histopathologic findings of renal papillary carcinoma (Hematoxylin and Eosin, 100×). Large atypical cells with marked nucleus proliferate diffusely. (b) PD-L1 staining of the specimen (100×). (c) IHC confirmed loss of staining for FH (100×). (d) Positive staining of 2-succinocysteine (100×). (e) TMB value was 4.03 mutants/Mb. (f) Microsatellite state was stable.
Supplementary Figure 2 | Radiological mages of tumor evaluation. (a) Hepatic metastatic lesion of CT scan on May 2019 (arrow). (b) Right adrenal metastatic lesion of CT scan on May 2019 (arrow). (c, d) Ultrasound confirmed hepatic and right adrenal lesions on June 2019 (arrow). (e, f) Abdominal MRI indicated shrinkage of hepatic lesion and right adrenal metastasis (arrow) on July 2019. (g, h) Abdominal MRI indicated remission of right adrenal metastasis on November 2019 (arrow).
Supplementary Figure 3 | A positron emission tomography/computed tomography scan identified increased fluorodeoxyglucose uptake in the (a) liver, (b) right adrenal gland, (c, d) nerve roots and (e, f) vertebrae (arrow).
Supplementary Figure 4 | Clinical course of the patient.
References
1. Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med (2010) 363(8):711–23. doi: 10.1056/NEJMoa1003466
2. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
3. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774
4. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (2016) 387(10031):1909–20. doi: 10.1016/S0140-6736(16)00561-4
5. Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol (2016) 17(10):1374–85. doi: 10.1016/S1470-2045(16)30364-3
6. Powles T, Huddart RA, Elliott T, Sarker SJ, Ackerman C, Jones R, et al. Double-Blind, Randomized Trial That Compared Maintenance Lapatinib Versus Placebo After First-Line Chemotherapy in Patients With Human Epidermal Growth Factor Receptor 1/2-Positive Metastatic Bladder Cancer. J Clin Oncol (2017) 35(1):48–55. doi: 10.1200/JCO.2015.66.3468
7. Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med (2018) 379(4):341–51. doi: 10.1056/NEJMoa1805131
8. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med (2015) 373(19):1803–13. doi: 10.1056/NEJMoa1510665
9. Flippot R, Escudier B, Albiges L. Immune Checkpoint Inhibitors: Toward New Paradigms in Renal Cell Carcinoma. Drugs (2018) 78(14):1443–57. doi: 10.1007/s40265-018-0970-y
10. Albiges L, Powles T, Staehler M, Bensalah K, Giles RH, Hora M, et al. Updated European Association of Urology Guidelines on Renal Cell Carcinoma: Immune Checkpoint Inhibition Is the New Backbone in First-line Treatment of Metastatic Clear-cell Renal Cell Carcinoma. Eur Urol (2019) 76(2):151–6. doi: 10.1016/j.eururo.2019.05.022
11. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity (2013) 39(1):1–10. doi: 10.1016/j.immuni.2013.07.012
12. Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med (2018) 378(2):158–68. doi: 10.1056/NEJMra1703481
13. Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol (2016) 27(4):559–74. doi: 10.1093/annonc/mdv623
14. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol (2018) 4(12):1721–8. doi: 10.1001/jamaoncol.2018.3923
15. Theofilopoulos AN, Kono DH, Baccala R. The multiple pathways to autoimmunity. Nat Immunol (2017) 18(7):716–24. doi: 10.1038/ni.3731
16. June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med (2017) 23(5):540–7. doi: 10.1038/nm.4321
17. Zientek MA, Goosen TC, Tseng E, Lin J, Bauman JN, Walker GS, et al. In Vitro Kinetic Characterization of Axitinib Metabolism. Drug Metab Dispos (2016) 44(1):102–14. doi: 10.1124/dmd.115.065615
18. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med (2019) 380(12):1116–27. doi: 10.1056/NEJMoa1816714
19. Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA. Checkpoint Inhibitor Immune Therapy: Systemic Indications and Ophthalmic Side Effects. Retina (2018) 38(6):1063–78. doi: 10.1097/IAE.0000000000002181
20. Abdel-Rahman O, Oweira H, Petrausch U, Helbling D, Schmidt J, Mannhart M, et al. Immune-related ocular toxicities in solid tumor patients treated with immune checkpoint inhibitors: a systematic review. Expert Rev Anticancer Ther (2017) 17(4):387–94. doi: 10.1080/14737140.2017.1296765
21. Bitton K, Michot JM, Barreau E, Lambotte O, Haigh O, Marabelle A, et al. Prevalence and Clinical Patterns of Ocular Complications Associated With Anti-PD-1/PD-L1 Anticancer Immunotherapy. Am J Ophthalmol (2019) 202:109–17. doi: 10.1016/j.ajo.2019.02.012
22. Fang T, Maberley DA, Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol (2019) 31(3):319–22. doi: 10.1016/j.joco.2019.05.002
23. Ho WL, Wong H, Yau T. The ophthalmological complications of targeted agents in cancer therapy: what do we need to know as ophthalmologists? Acta Ophthalmol (2013) 91(7):604–9. doi: 10.1111/j.1755-3768.2012.02518.x
24. Fraunfelder FT, Fraunfelder FW. Oral Anti-Vascular Endothelial Growth Factor Drugs and Ocular Adverse Events. J Ocul Pharmacol Ther (2018) 34(6):432–5. doi: 10.1089/jop.2018.0019
25. Dib E, Maia M, Lima Ade S, de Paula Fiod Costa E, de Moraes-Filho MN, Rodrigues EB, et al. In vivo, in vitro toxicity and in vitro angiogenic inhibition of sunitinib malate. Curr Eye Res (2012) 37(7):567–74. doi: 10.3109/02713683.2011.635916
26. Kimura M, Kusuhara S, Tagami M, Nakamura M. Impaired Retinal Circulation during Axitinib Treatment for Metastatic Renal Cell Carcinoma. Case Rep Ophthalmol (2019) 10(1):5–10. doi: 10.1159/000496197
27. Noble CW, Gangaputra SS, Thompson IA, Yuan A, Apolo AB, Lee JM, et al. Ocular Adverse Events following Use of Immune Checkpoint Inhibitors for Metastatic Malignancies. Ocul Immunol Inflamm (2020) 28(6):854–59. doi: 10.1080/09273948.2019.1583347
28. Sengul Samanci N, Ozan T, Celik E, Demirelli FH. Optic Neuritis Related to Atezolizumab Treatment in a Patient With Metastatic Non-Small-Cell Lung Cancer. JCO Oncol Pract (2020) 16(2):96–8. doi: 10.1200/JOP.19.00438
29. Emens LA, Davis SL, Oliver SCN, Lieu CH, Reddy A, Solomon S, et al. Association of Cancer Immunotherapy With Acute Macular Neuroretinopathy and Diffuse Retinal Venulitis. JAMA Ophthalmol (2019) 137(1):96–100. doi: 10.1001/jamaophthalmol.2018.5191
30. Miyakubo T, Mukai R, Nakamura K, Matsumoto H. Akiyama H. A Case Of Ipilimumab-Induced Unusual Serous Retinal Detachment In Bilateral Eyes. Int Med Case Rep J (2019) 12:355–61. doi: 10.2147/IMCRJ.S225173
31. Wang W, Lam WC, Chen L. Recurrent grade 4 panuveitis with serous retinal detachment related to nivolumab treatment in a patient with metastatic renal cell carcinoma. Cancer Immunol Immunother (2019) 68(1):85–95. doi: 10.1007/s00262-018-2260-7
32. Rapisuwon S, Izar B, Batenchuk C, Avila A, Mei S, Sorger P, et al. Exceptional response and multisystem autoimmune-like toxicities associated with the same T cell clone in a patient with uveal melanoma treated with immune checkpoint inhibitors. J Immunother Cancer (2019) 7(1):61. doi: 10.1186/s40425-019-0533-0
33. Obata S, Saishin Y, Teramura K, Ohji M. Vogt-Koyanagi-Harada Disease-Like Uveitis during Nivolumab (Anti-PD-1 Antibody) Treatment for Metastatic Cutaneous Malignant Melanoma. Case Rep Ophthalmol (2019) 10(1):67–74. doi: 10.1159/000496682
34. Telfah M, Whittaker TJ G. CD. Vision loss with pembrolizumab treatment: A report of two cases. J Oncol Pharm Pract (2019) 25(6):1540–6. doi: 10.1177/1078155219841683
35. Tsui E, Madu A, Belinsky I, Yannuzzi LA, Freund KB, Modi YS. Combination Ipilimumab and Nivolumab for Metastatic Melanoma Associated With Ciliochoroidal Effusion and Exudative Retinal Detachment. JAMA Ophthalmol (2017) 135(12):1455–7. doi: 10.1001/jamaophthalmol.2017.4872
36. Theillac C, Straub M, Breton AL, Thomas L, Dalle S. Bilateral uveitis and macular edema induced by Nivolumab: a case report. BMC Ophthalmol (2017) 17(1):227. doi: 10.1186/s12886-017-0611-3
37. Crews J, Agarwal A, Jack L, Xu D, Do DV, Nguyen QD. Ipilimumab-Associated Retinopathy. Ophthal Surg Lasers Imaging Retina (2015) 46(6):658–60. doi: 10.3928/23258160-20150610-10
38. Mantopoulos D, Kendra KL, Letson AD, Cebulla CM. Bilateral Choroidopathy and Serous Retinal Detachments During Ipilimumab Treatment for Cutaneous Melanoma. JAMA Ophthalmol (2015) 133(8):965–7. doi: 10.1001/jamaophthalmol.2015.1128
39. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature (2017) 541(7637):321–30. doi: 10.1038/nature21349
40. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer (2016) 54:139–48. doi: 10.1016/j.ejca.2015.11.016
41. Hsiehchen D, Watters MK, Lu R, Xie Y, Gerber DE. Variation in the Assessment of Immune-Related Adverse Event Occurrence, Grade, and Timing in Patients Receiving Immune Checkpoint Inhibitors. JAMA Netw Open (2019) 2(9):e1911519. doi: 10.1001/jamanetworkopen.2019.11519
42. Patel P, Finger PT. Whole-body 18F FDG positron emission tomography/computed tomography evaluation of patients with uveal metastasis. Am J Ophthalmol (2012) 153(4):661–8. doi: 10.1016/j.ajo.2011.09.028
43. Benhar I, London A, Schwartz M. The privileged immunity of immune privileged organs: the case of the eye. Front Immunol (2012) 3:296. doi: 10.3389/fimmu.2012.00296
44. Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J Natl Compr Canc Netw (2020) 18(3):230–41. doi: 10.6004/jnccn.2020.0012
45. Onodera H, Sasaki S, Otake S, Tomohiro M, Shibuya K, Nomura M. General considerations in ocular toxicity risk assessment from the toxicologists’ viewpoints. J Toxicol Sci (2015) 40(3):295–307. doi: 10.2131/jts.40.295
46. Keino H, Horie S, Sugita S. Immune Privilege and Eye-Derived T-Regulatory Cells. J Immunol Res (2018) 2018:1679197. doi: 10.1155/2018/1679197
47. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol (2018) 36(17):1714–68. doi: 10.1200/JCO.2017.77.6385
48. Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer (2017) 5(1):95. doi: 10.1186/s40425-017-0300-z
49. Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2017) 28(suppl_4):iv119–42. doi: 10.1093/annonc/mdx225
50. Schoenfeld SR, Aronow ME, Leaf RK, Dougan M, Reynolds KL. Diagnosis and Management of Rare Immune-Related Adverse Events. Oncologist (2020) 25(1):6–14. doi: 10.1634/theoncologist.2019-0083
51. Eigentler TK, Hassel JC, Berking C, Aberle J, Bachmann O, Grunwald V, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev (2016) 45:7–18. doi: 10.1016/j.ctrv.2016.02.003
52. Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol (2017) 28(10):2377–85. doi: 10.1093/annonc/mdx286
53. Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet (2002) 30(4):406–10. doi: 10.1038/ng849
54. Alaghehbandan R, Stehlik J, Trpkov K, Magi-Galluzzi C, Condom Mundo E, Pane Foix M, et al. Programmed death-1 (PD-1) receptor/PD-1 ligand (PD-L1) expression in fumarate hydratase-deficient renal cell carcinoma. Ann Diagn Pathol (2017) 29:17–22. doi: 10.1016/j.anndiagpath.2017.04.007
55. Winer A, Bodor JN, Borghaei H. Identifying and managing the adverse effects of immune checkpoint blockade. J Thorac Dis (2018) 10(Suppl 3):S480–9. doi: 10.21037/jtd.2018.01.111
56. Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol (2016) 13(8):473–86. doi: 10.1038/nrclinonc.2016.58
57. Chamoto K, Hatae R, Honjo T. Current issues and perspectives in PD-1 blockade cancer immunotherapy. Int J Clin Oncol (2020) 25(5):790–800. doi: 10.1007/s10147-019-01588-7
58. Fujii T, Colen RR, Bilen MA, Hess KR, Hajjar J, Suarez-Almazor ME, et al. Incidence of immune-related adverse events and its association with treatment outcomes: the MD Anderson Cancer Center experience. Invest New Drugs (2018) 36(4):638–46. doi: 10.1007/s10637-017-0534-0
59. Akamatsu H, Murakami E, Oyanagi J, Shibaki R, Kaki T, Takase E, et al. Immune-Related Adverse Events by Immune Checkpoint Inhibitors Significantly Predict Durable Efficacy Even in Responders with Advanced Non-Small Cell Lung Cancer. Oncologist (2020) 25(4):e679-83. doi: 10.1634/theoncologist.2019-0299
60. Das S, Ciombor KK, Haraldsdottir S, Pumpalova Y, Sahin IH, Pineda G, et al. Immune-Related Adverse Events and Immune Checkpoint Inhibitor Efficacy in Patients with Gastrointestinal Cancer with Food and Drug Administration-Approved Indications for Immunotherapy. Oncologist (2020) 25(8):669–79. doi: 10.1634/theoncologist.2019-0637
Keywords: retinal detachment, immune checkpoint inhibitor, pembrolizumab, immunotherapy, anti-angiogenesis
Citation: Peng L, Mao Q-Q, Jiang B, Zhang J, Zhao Y-L, Teng X-D, Yang J-S, Xia Y, Chen S-Q, Stebbing J and Jiang H (2020) Bilateral Posterior Uveitis and Retinal Detachment During Immunotherapy: A Case Report and Literature Review. Front. Oncol. 10:549168. doi: 10.3389/fonc.2020.549168
Received: 07 May 2020; Accepted: 29 September 2020;
Published: 09 November 2020.
Edited by:
Fabio Grizzi, Humanitas Research Hospital, ItalyReviewed by:
Kerry Reynolds, Harvard Medical School, United StatesTakeshi Yuasa, Japanese Foundation For Cancer Research, Japan
Copyright © 2020 Peng, Mao, Jiang, Zhang, Zhao, Teng, Yang, Xia, Chen, Stebbing and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai Jiang, c2hpajEzNkB6anUuZWR1LmNu
†These authors have contributed equally to this work
 Ling Peng
Ling Peng Qi-Qi Mao3†
Qi-Qi Mao3† Shi-Qing Chen
Shi-Qing Chen