- Department of Urology, Affiliated Jiang-yin Hospital of the Southeast University Medical College, Jiang-yin, China
Background and Objectives: Previous studies have demonstrated that positive surgical margins (PSMs) were independent predictive factors for biochemical and oncologic outcomes in patients with prostate cancer (PCa). This study aimed to conduct a meta-analysis to identify the predictive factors for PSMs after radical prostatectomy (RP).
Methods: We selected eligible studies via the electronic databases, such as PubMed, Web of Science, and EMBASE, from inception to December 2020. The risk factors for PSMs following RP were identified. The pooled estimates of standardized mean differences (SMDs)/odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. A fixed effect or random effect was used to pool the estimates. Subgroup analyses were performed to explore the reasons for heterogeneity.
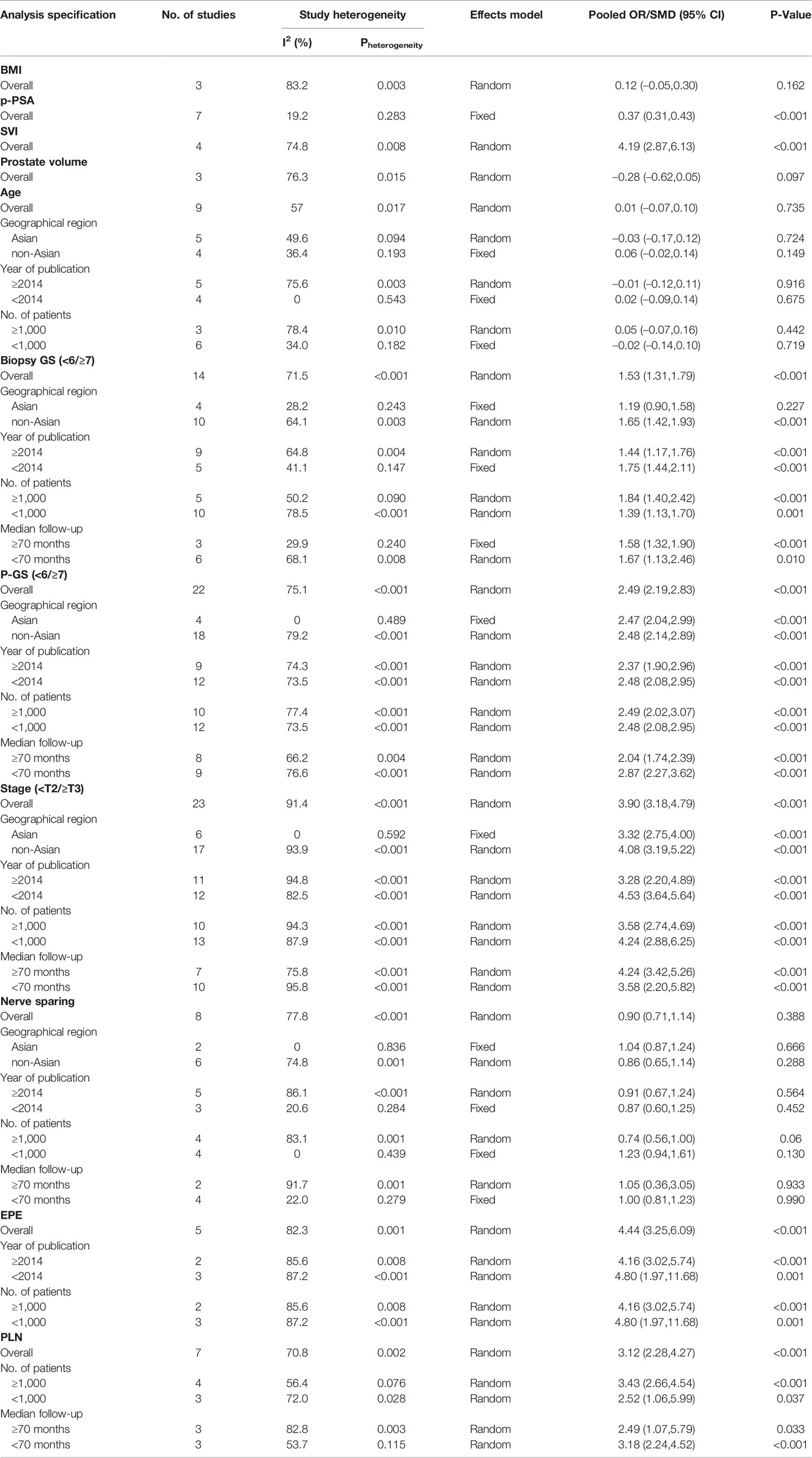
Results: Twenty-seven studies including 50,014 patients with PCa were eligible for further analysis. The results showed that PSMs were significantly associated with preoperative prostate-specific antigen (PSA) (pooled SMD = 0.37; 95% CI: 0.31–0.43; P < 0.001), biopsy Gleason Score (<6/≥7) (pooled OR = 1.53; 95% CI:1.31–1.79; P < 0.001), pathological Gleason Score (<6/≥7) (pooled OR = 2.49; 95% CI: 2.19–2.83; P < 0.001), pathological stage (<T2/≥T3) (pooled OR = 3.90; 95% CI: 3.18–4.79; P < 0.001), positive lymph node (PLN) (pooled OR = 3.12; 95% CI: 2.28–4.27; P < 0.001), extraprostatic extension (EPE) (pooled OR = 4.44; 95% CI: 3.25–6.09; P < 0.001), and seminal vesicle invasion (SVI) (pooled OR = 4.19; 95% CI: 2,87–6.13; P < 0.001). However, we found that age (pooled SMD = 0.01; 95% CI: −0.07–0.10; P = 0.735), body mass index (BMI) (pooled SMD = 0.12; 95% CI: −0.05–0.30; P = 0.162), prostate volume (pooled SMD = −0.28; 95% CI: −0.62–0.05; P = 0.097), and nerve sparing (pooled OR = 0.90; 95% CI: 0.71–1.14; P = 0.388) had no effect on PSMs after RP. Besides, the findings in this study were found to be reliable by our sensitivity and subgroup analyses.
Conclusions: Preoperative PSA, biopsy Gleason Score, pathological Gleason Score, pathological stage, positive lymph node, extraprostatic extension, and seminal vesicle invasion are independent predictors of PSMs after RP. These results may helpful for risk stratification and individualized therapy in PCa patients.
Introduction
Prostate cancer (PCa) is the most common type of newly diagnosed malignancy and a leading cause of cancer-related death in males worldwide (1). With the wide use of the prostate−specific antigen (PSA) screening test, the majority of PCa patients are diagnosed in the early stages (2). As a result, radical prostatectomy (RP) with bilateral pelvic lymph node dissection has been the gold standard for the treatment of patients with localized PCa (3). The goal of RP for PCa is complete prostate extirpation; despite favorable cancer control associated with RP, approximately 25% of all patients experience biochemical recurrence (BCR) (4). A number of factors have been found to be associated with BCR after RP, and one adverse risk factor is the presence of positive surgical margins (PSMs).
PSMs are defined as an extension of cancer cells to the inked cut surface of the RP specimen (5). Our previous findings have indicated that PSMs are significantly associated with BCR and poor survival outcome after RP (6, 7). However, none of the systematic research studies have reported about the factors that may affect the margin status of PCa after RP. Conventional parameters for risk estimation of PSMs are mainly based on factors, including preoperative PSA (p-PSA), pathological T stage, pathological Gleason Score (GS), and multiple positive biopsy cores (8–11). However, the prognostic value of these predictive factors is limited. Besides, PSMs may be affected by remnant normal tissue and inadequate surgical skill (12). Therefore, no consensus has been reported regarding the above results. Based on these considerations, a comprehensive meta-analysis and systematic review was necessary to evaluate the predictive factors for PSMs in PCa patients following RP.
Materials and Methods
Literature and Search Strategy
We carried out this meta-analysis in accordance with the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analyses statement (PRISMA) (13). A comprehensive literature search was conducted using the PubMed, Web of Science, Wanfang, and China National Knowledge Infrastructure (CNKI) databases. Search strategies were based on the combination of Medical Subject Headings (MeSH) and keywords as follows: “prostate cancer,” “radical prostatectomy,” “positive surgical margin,” “clinicopathological” and “risk factors.” The last search was conducted on December 2020. Meanwhile, to identify other eligible publications, reference lists were also screened manually. The language was restricted to English and Chinese. Because we did not perform clinical research in this study, no ethical approval was needed and all analyses were based on previously published literatures.
Selection Criteria and Data Extraction
Papers were included in this meta-analysis if they met the following criteria: (1) all patients with a diagnosis of PCa and PSMs were histopathologically confirmed; (2) treatment was limited to RP; (3) clinicopathological features were analyzed according to the surgical margins status, and all studies had a comparable study design; (4) standardized mean differences (SMDs)/odds ratios (ORs) and 95% confidence intervals (CIs) were reported in the paper or could be computed from the given data; (5) if more than one article was identified in the same cohort, the most comprehensive and largest dataset was adopted. Accordingly, studies with the following criteria were excluded: (1) case reports, review articles, editorials, and non-original articles; (2) papers not published in English and Chinese; (3) studies that did not analyze the PSMs and clinical features; (4) studies lacking sufficient data to acquire SMDs/ORs and 95% CIs. Literature search was independently performed by two investigators. Disagreement was resolved by discussion.
Data Extraction and Quality Assessment
Two researchers (BW and ZZ) assessed the titles and abstracts of the searched studies, respectively. Any disagreements were reconciled by a third researcher (JY). The following information was extracted from the included studies: publication information (first author’s last name, publication year, country of origin, and study design), patients’ characteristics (mean age, p-PSA, and follow-up time), and PCa outcomes (tumor stage, GS, and oncologic outcomes). According to the Newcastle–Ottawa quality assessment scale (NOS) (14), two researchers (HZ and YF) independently assessed the quality of each study. According to its criteria, the NOS estimates studies based on the following three parts: selection, comparability, and outcome assessment. For quality assessment, scores ranged from 0 to 9, and studies with scores of 6 or more were rated as being of high quality.
Statistical Analysis
For this meta-analysis, pooled SMDs/ORs with 95% CIs were used to describe the relationship between risk factors and PSMs. An OR >1 or SMD >0 suggested a close relationship of PSMs in patients with PCa. Heterogeneity among studies was evaluated by using Cochran’s Q test and Higgins I-squared statistic. If the I2 value was >50% or the Pheterogeneity was <0.1, it suggested a statistically significant heterogeneity in the included studies, and a random-effects (RE) model was adopted; otherwise, a fixed-effects (FE) model was used. To consider the potential reason for heterogeneity, subgroup analysis was conducted. To test the stability of the result, we performed a sensitivity analysis by excluding one study in turn. Visual inspection of asymmetry in funnel plots was carried out to assess the potential publication bias. Furthermore, we performed Egger’s tests to provide quantitative evidence of publication bias. These statistical analyses or data syntheses were calculated using STATA version 12.0 (Stata Corporation, College Station, TX, USA). All statistical tests were two sided, and P < 0.05 was considered statistically significant.
Results
Literature Search
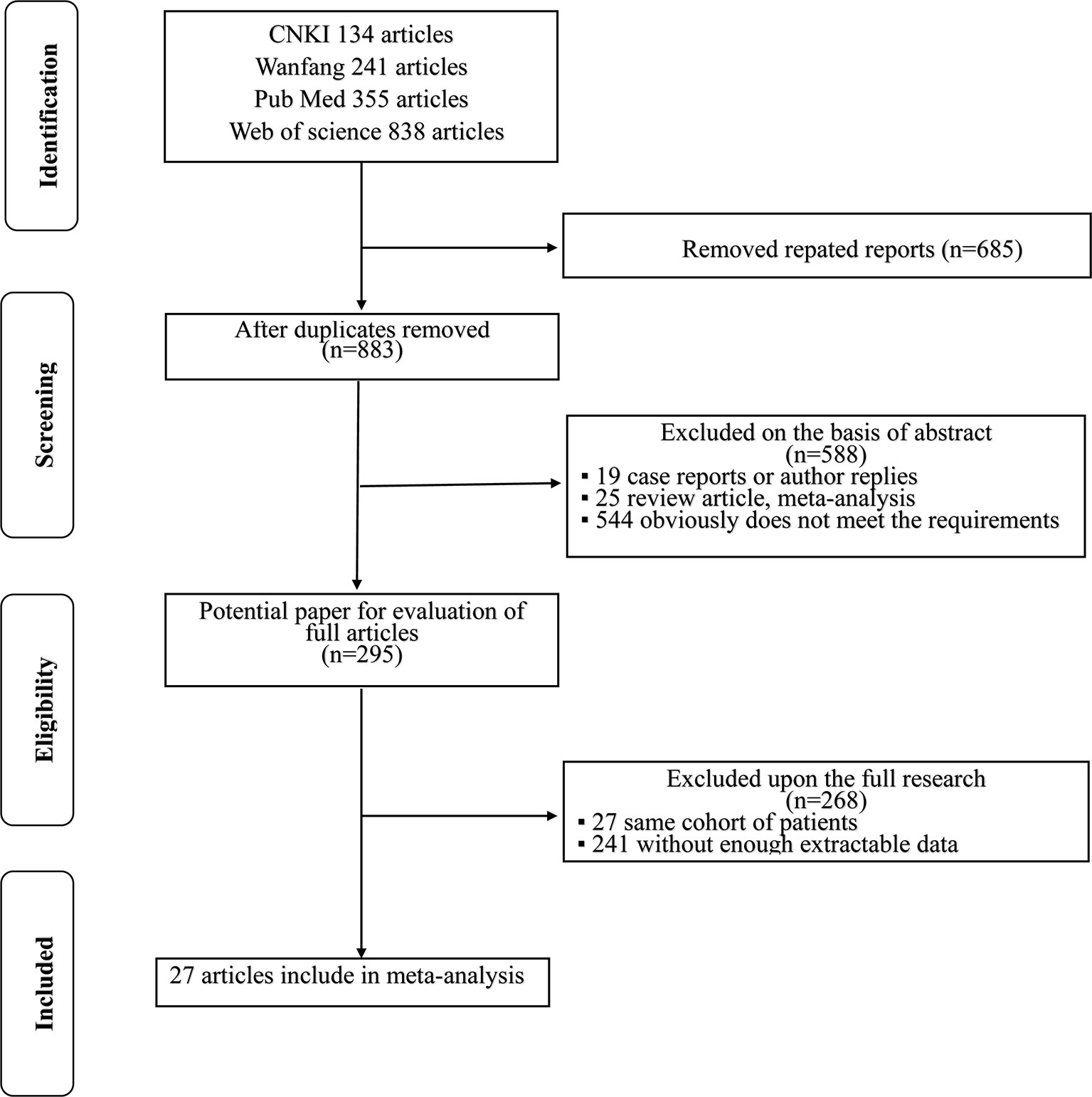
A flowchart of the literature selection process is shown in Figure 1. The initial search of electronic databases identified 1,568 records according to the search criteria; after the duplicates were removed, 883 papers remained behind. A total of 588 papers were then excluded by screening the titles and abstracts. Then, 295 full-text articles were further examined and 268 articles were excluded because 27 articles included the same cohort of patients and 241 articles lacked enough data for further research. Finally, 27 articles (8, 15–40) published between 2009 and 2020 were included in this meta-analysis.
Features of the Included Studies
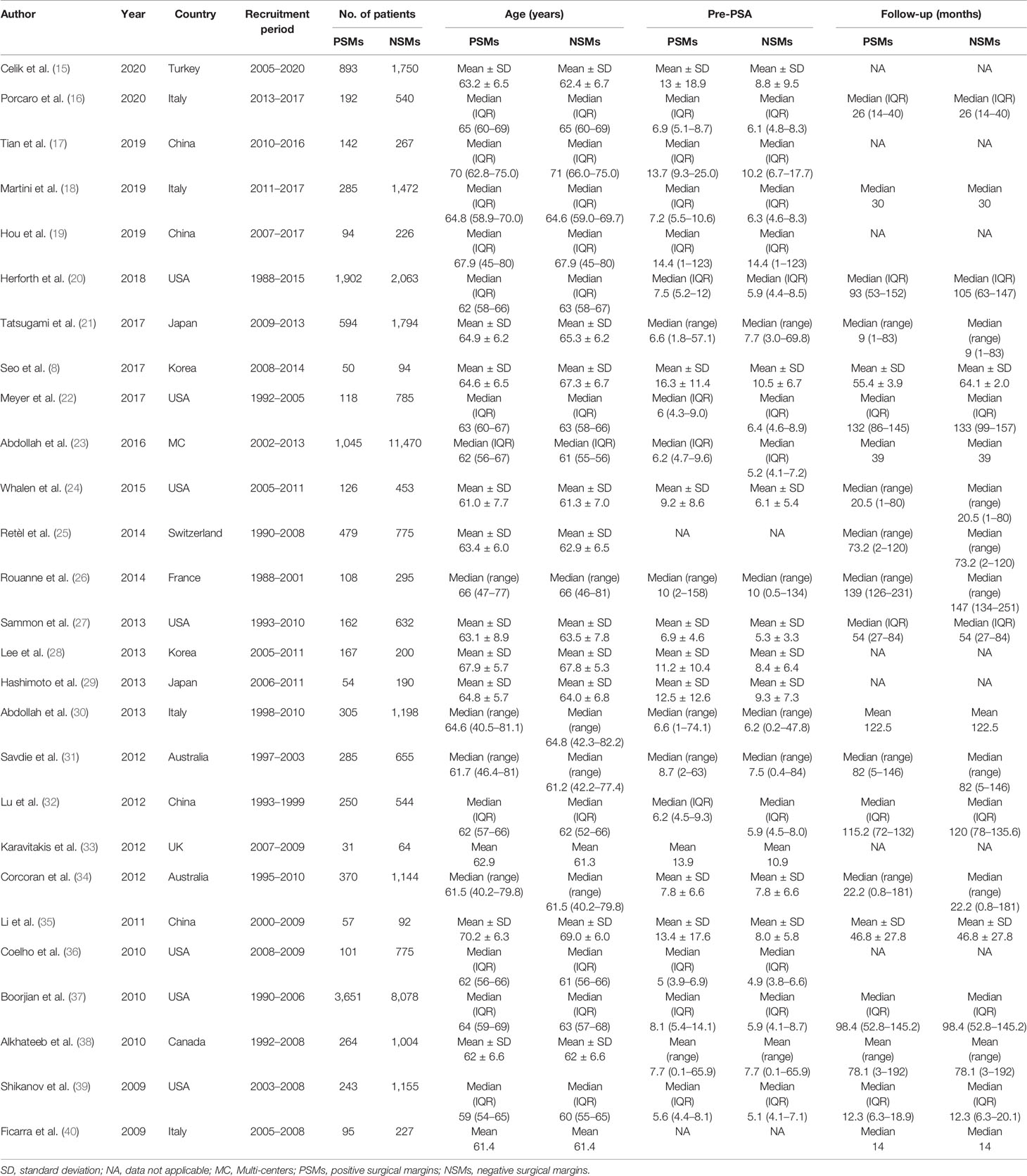
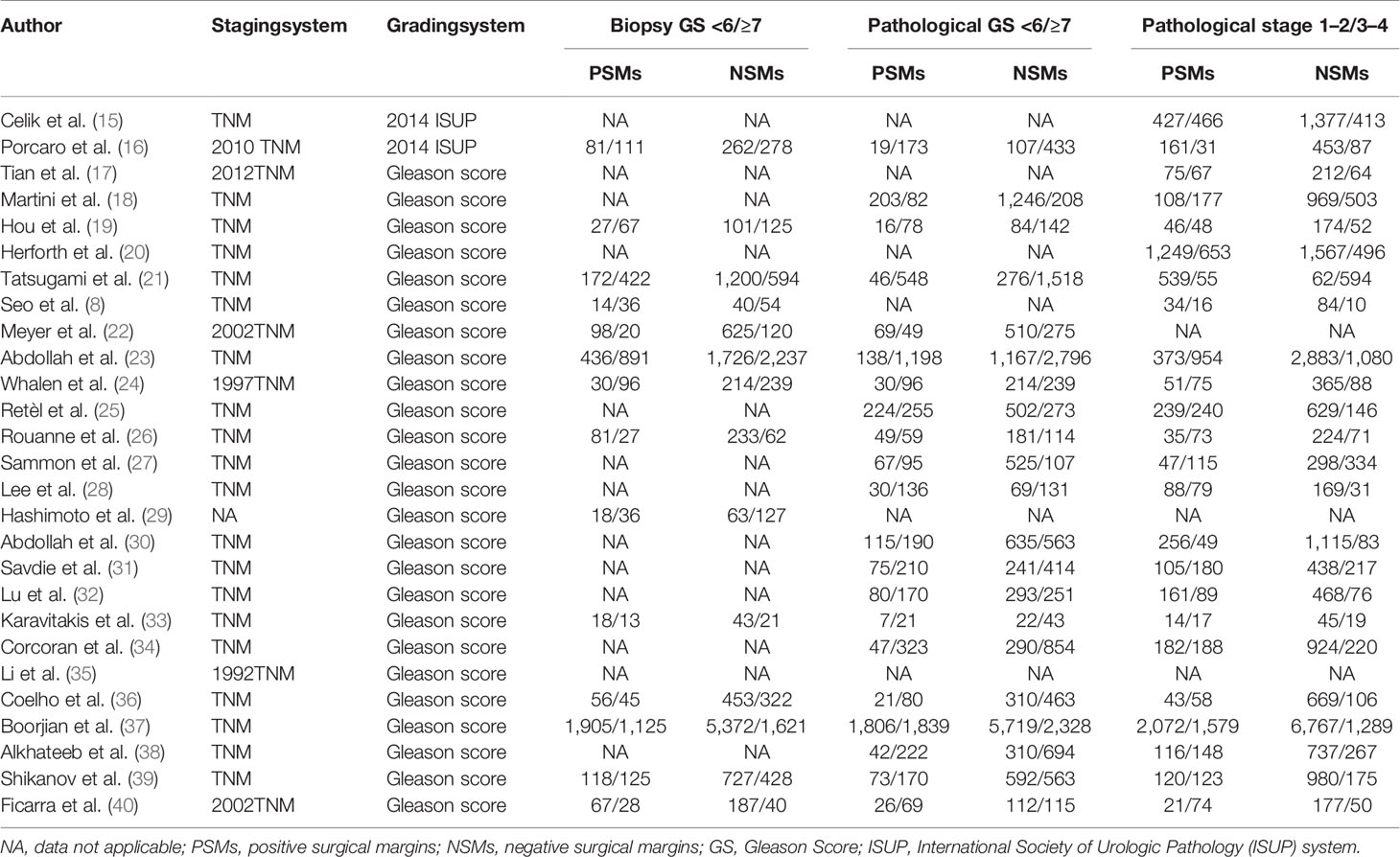
Summary of the major characteristics of these studies is shown in Table 1 and Table 2. All studies had a retrospective study design. The sample size ranged from 144 to 12,515, and a total of 50,014 patients were included. A total of 12,093 PCa patients with PSMs were included in our study, which accounted for 24.2% of all patients. Geographically, eight studies were conducted in Asia, eight in North America, eight in Europe, two in Australia, and one in multi-center locations. All patients had received RP as primary treatment for PCa. According to the NOS quality assessment, all studies included in this study were categorized as being of high quality (Supplementary Table S1).
Meta-Analysis
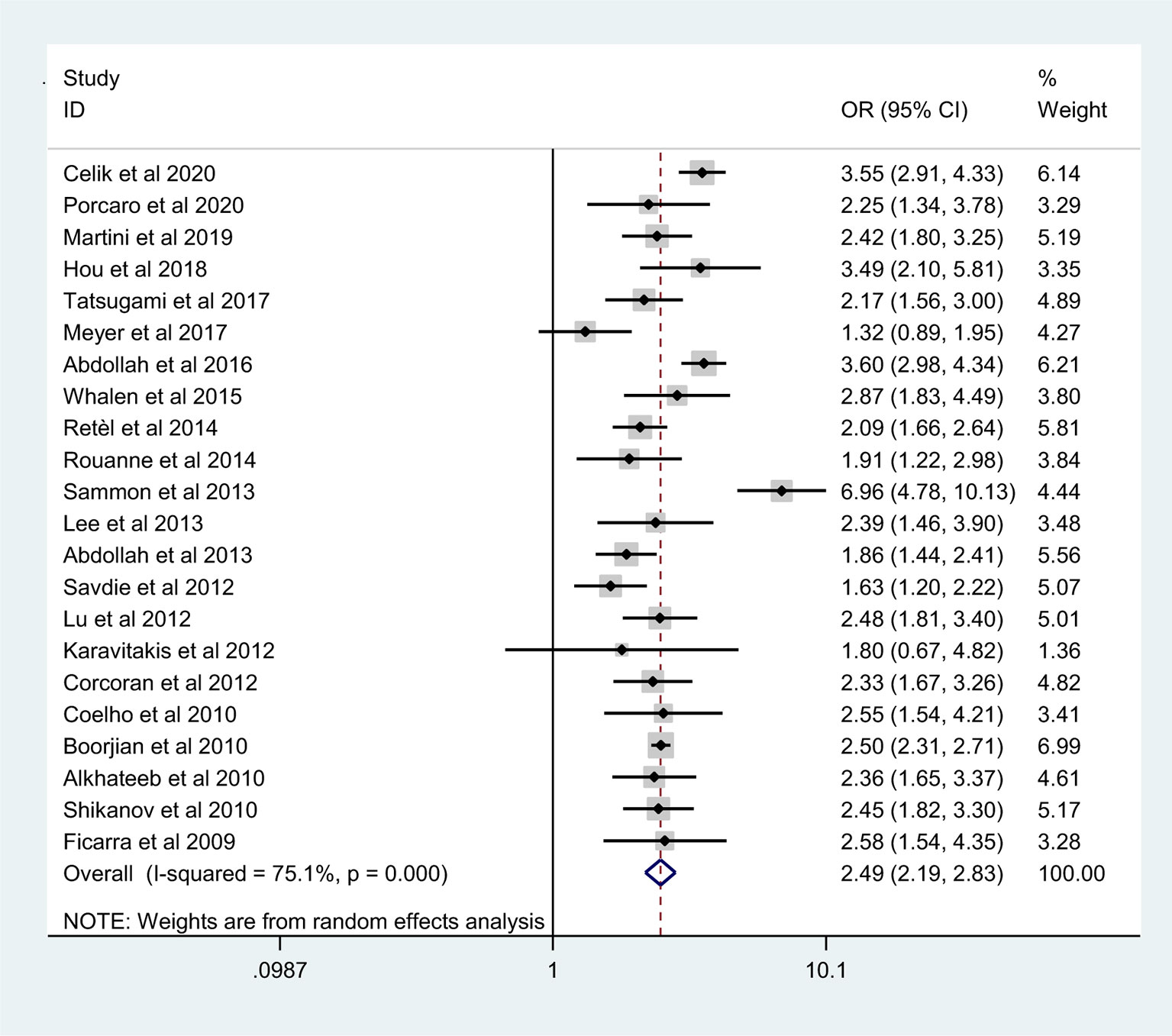
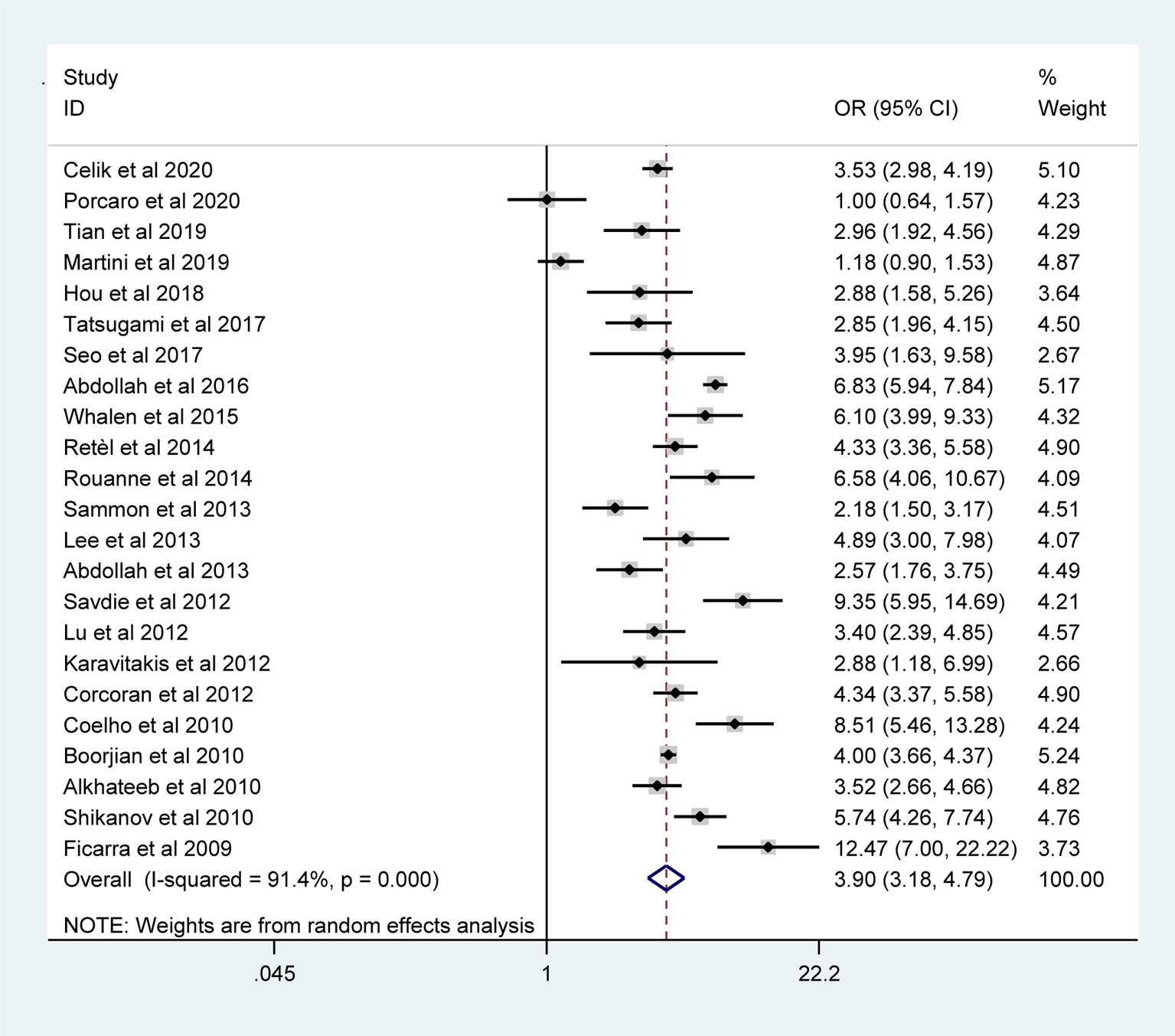
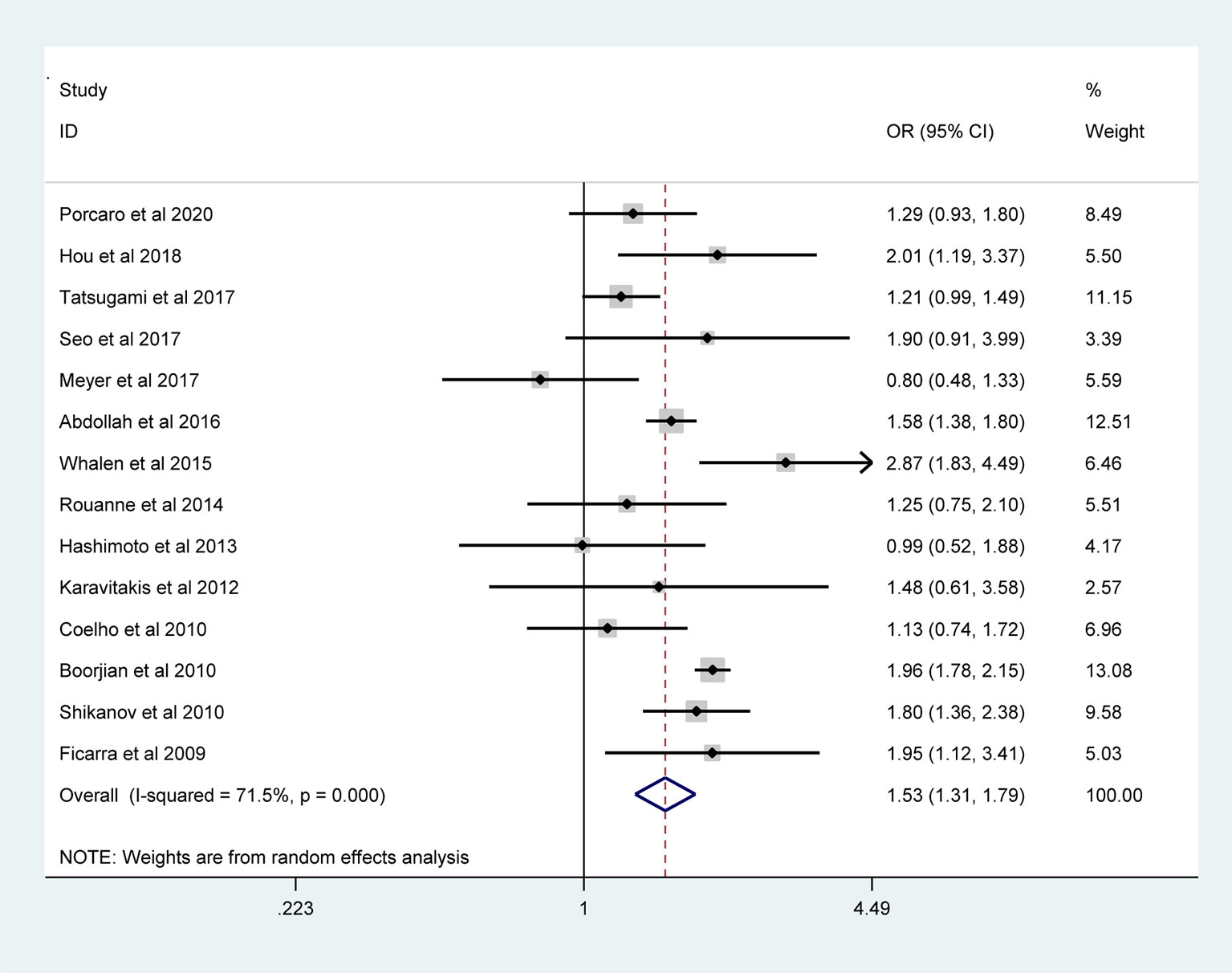
The pooled results from the included studies indicated that PSMs were associated with pathological GS (<6/≥7) (RE model, pooled OR = 2.49; 95% CI: 2.19–2.83; P < 0.001, Figure 2), pathological stage (<T2/≥T3) (RE model, pooled OR = 3.90; 95% CI: 3.18–4.79; P < 0.001, Figure 3), biopsy GS (<6/≥7) (RE model, pooled OR = 1.53; 95% CI: 1.31–1.79; P < 0.001, Figure 4), p-PSA (FE model, pooled SMD = 0.37; 95% CI: 0.31–0.43; P < 0.001, Figure 5A), positive lymph node (PLN) (RE model, pooled OR = 3.12; 95% CI: 2.28–4.27; P < 0.001, Figure 5B), extraprostatic extension (EPE) (RE model, pooled OR = 4.44; 95% CI: 3.25–6.09; P < 0.001, Figure 5C), and seminal vesicle invasion (SVI) (RE model, pooled OR = 4.19; 95% CI: 2.87–6.13; P < 0.001, Figure 5D).
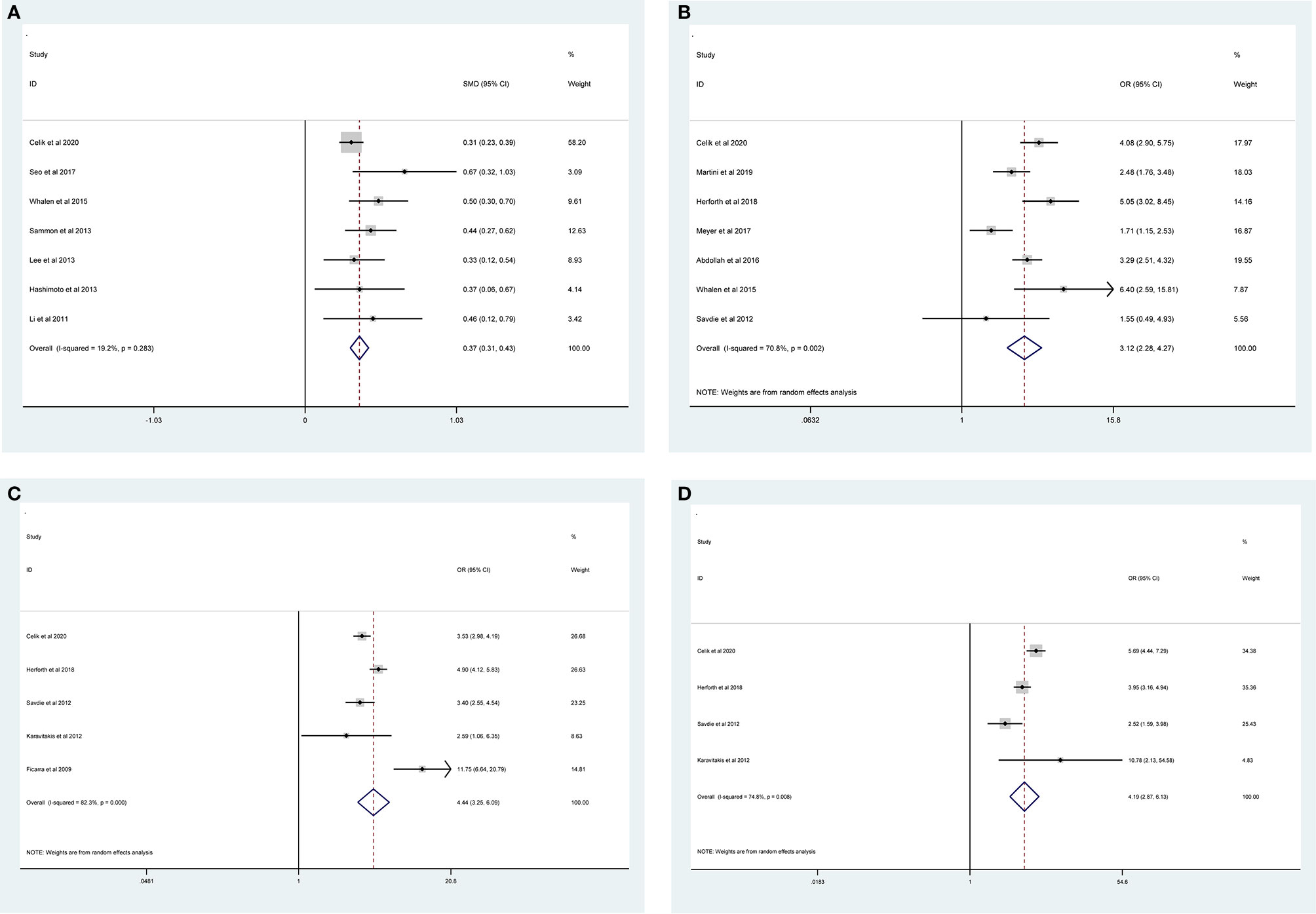
Figure 5 Forest plots of studies evaluating the prognostic factors for p-PSA (A), PLN (B), EPE (C), and SVI (D) with PSMs risk.
The results of meta-analysis of PSMs showed that no significant associations were found between PSMs and age (RE model, pooled SMD = 0.01; 95% CI: −0.07–0.10; P = 0.735, Figure 6A), nerve sparing (RE model, pooled OR = 0.90; 95% CI: 0.71–1.14; P = 0.388, Figure 6B), body mass index (BMI) (RE model, pooled SMD = 0.12; 95% CI: −0.05–0.30; P = 0.162, Figure 6C), and prostate volume (RE model, pooled SMD = −0.28; 95% CI: −0.62–0.05; P = 0.097, Figure 6D).
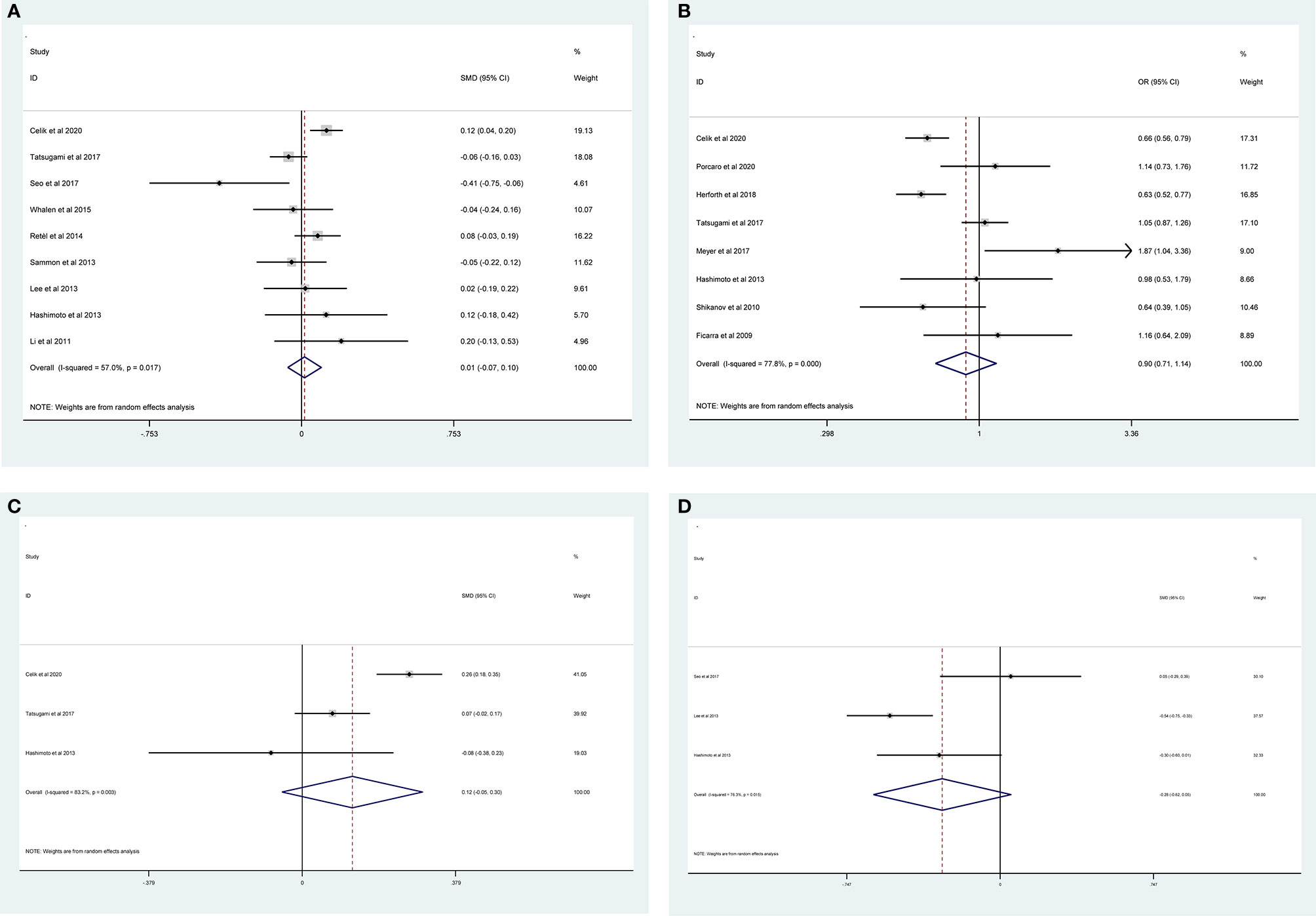
Figure 6 Forest plots of studies evaluating the association of PSMs and clinicopathological features in PCa patients. Age (A), nerve sparing (B), BMI (C), and prostate volume (D).
Subgroup Analysis
Considering that there was no significant heterogeneity in p-PSA and the number of studies that evaluated BMI, SVI, and prostate volume was relatively small, we only conducted subgroup analysis for biopsy GS, pathological GS, pathological stage, PLN, EPE, age, and nerve sparing (Table 3). Subgroup analyses were conducted according to the geographical region (Asian vs. non-Asian), year of publication (≥2014 vs. <2014), number of patients (≥1,000 vs. <1,000), and median follow-up (≥70 months vs. <70 months). The results of subgroup analysis were roughly the same as overall results. Besides, the heterogeneity decreased significantly in some subgroup analyses, such as geographical region in Asian, year of publication <2014, and number of patients <1,000 cases.
Sensitivity Analysis
To validate the reliability of our results, sensitivity analysis was performed. As shown in Supplementary Figure S1, the combined ORs for biopsy GS ranged from 1.47 (95% CI: 1.25 –1.72) to 1.58 (95% CI: 1.37–1.85) (Supplementary Figure S1A), the combined ORs for pathological GS ranged from 2.39 (95% CI: 2.14–2.67) to 2.56 (95% CI: 2.26–2.90) (Supplementary Figure S1B), the combined ORs for pathological stage ranged from 3.73 (95% CI: 3.04–4.58) to 4.15 (95% CI: 3.47–4.96) (Supplementary Figure S1C), the combined ORs for PLN ranged from 2.88 (95% CI: 2.08–4.00) to 3.51 (95% CI: 2.67–4.79) (Supplementary Figure S1D), the combined ORs for nerve sparing ranged from 0.83 (95% CI: 0.66–1.04) to 0.97 (95% CI: 0.74–1.27) (Supplementary Figure S1E), and the combined ORs for EPE ranged from 3.84 (95% CI: 3.05–4.85) to 4.68 (95% CI: 3.36–6.53) (Supplementary Figure S1F). The pooled SMD for p-PSA ranged from 0.36 (95% CI: 0.29–0.42) to 0.44 (95% CI: 0.35–0.54) (Supplementary Figure S2A), and the pooled SMD for age ranged from −0.01 (95% CI: −0.09–0.07) to 0.03 (95% CI: −0.05–0.12) (Supplementary Figure S2B). These data suggested that the results were statistically robust. Because the number of included studies for BMI, EPE, SVI, and prostate volume were small, the sensitivity analysis was not valuable.
Publication Bias
The shape of funnel plots did not reveal any evidence of asymmetry (Figure 7). The statistical results of Egger’s test still did not show any publication bias for biopsy GS (p- Egger = 0.277, Figure 7A), pathological GS (p- Egger = 0.945, Figure 7B), pathological stage (p- Egger = 0.830, Figure 7C), PLN (p- Egger = 0.605, Figure 7D), EPE (p- Egger = 0.513, Figure 7E), SVI (p- Egger = 0.797, Figure 7F), age (p- Egger = 0.431, Figure 7G), and nerve sparing (p- Egger = 0.197, Figure 7H). However, a minimal publication bias existed in p-PSA (p- Egger = 0.047). As the number of studies on prostate volume and BMI was limited, the publication bias was not assessed.
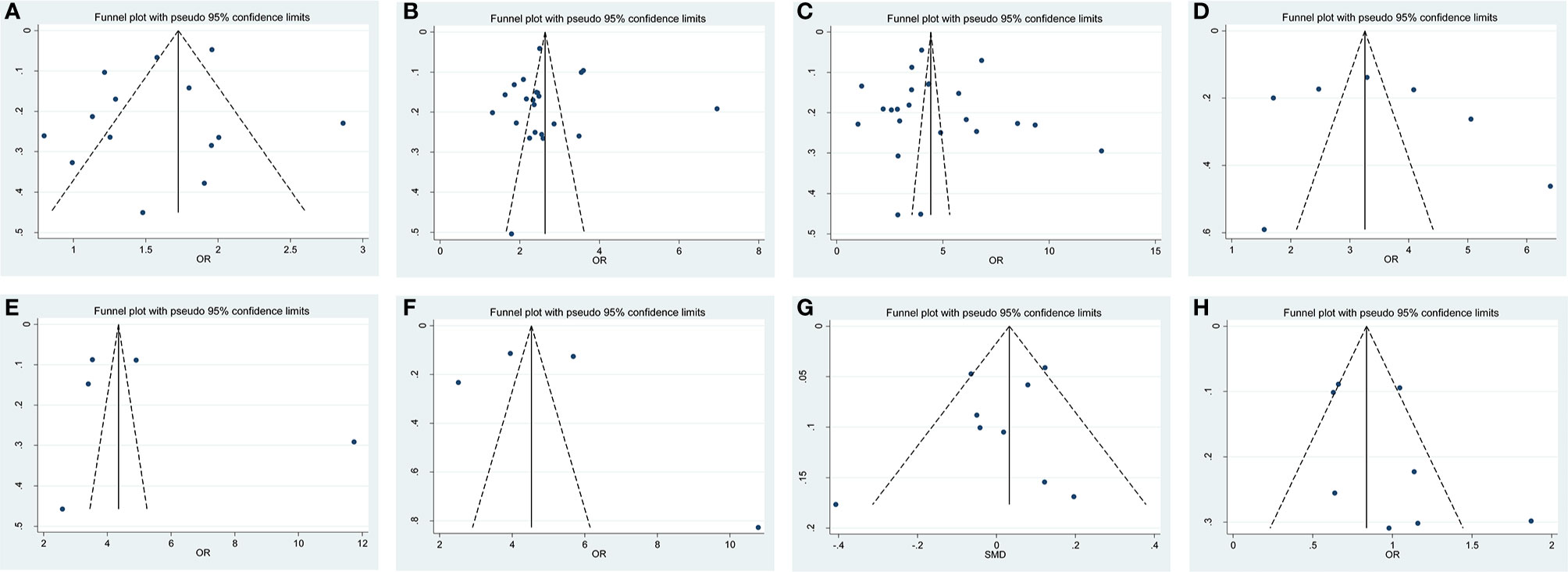
Figure 7 Funnel plot and Begg test for publication bias. (A) biopsy Gleason Score, (B) pathological GS, (C) pathological stage, (D) PLN, (E) EPE, (F) SVI, (G) age and (H) nerve sparing.
Discussion
PSMs are unfavorable pathological features, which suggest incomplete tumor resection and confer poorer cancer control after RP (38). It was reported that PSMs were present in 11–38% of patients treated by RP and patients with PSMs have a higher risk of BCR compared to those with negative surgical margins (NSMs) (41). A multi-institutional review in 2009 conducted by Yossepowitch et al. (42) concluded that PSMs in RP specimens may be considered as an adverse outcome following RP. Consistent with these findings, our recent studies (6, 7) demonstrated the adverse effect of PSMs on both BCR and cancer-specific survival through a systematic review and meta-analysis. However, not all patients with PSMs have poor tumor outcomes, and some patients with localized PCa will show tumor progression even in the no-PSMs cases.
PSMs are factors that may be modified by the surgical technique. It seems that surgeon’s experience plays an important role in the decrease in the incidence of PSMs (43). Considerable efforts have been devoted to identifying factors, such as p-PSA (44), positive biopsy cores (10), and clinical stage (36), which can predict PSMs and clinical outcome following RP. The conclusion of several published studies indicated that several unfavorable pathological features may be associated with PSMs. However, inconsistent results have also been demonstrated in the published studies. Besides, for patients with adverse features of PSMs, prediction parameters that are currently available for PSMs may not reliable.
A retrospective study conducted by Boorjian et al. (37) found that increased p-PSA and BMI, higher pathological stage/GS, and greater tumor volume were significantly associated with the risk of PSMs. Likewise, Ficarra et al. (40) found an association between PSMs and biopsy GS, pathologic stage and GS, and EPE; however, no correlation was found between PSMs and p-PSA. Hashimoto et al. (29) found that only PSA density and prostate volume were independent predictors of PSMs after robot-assisted RP based on the data from 244 Japanese patients. Moreover, Yuksel et al. (45) considered the number of positive biopsies, pathologic stage and GS, SVI, and EPE as predictive factors for PSMs after robot-assisted RP. Meanwhile, no correlation was found with p-PSA, biopsy GS, and PLN. The inconsistent results from the above studies may due to small sample size, single-center design, and inhomogeneous population.
To the best of our knowledge, none of the studies have systematically addressed the preoperative predictive factors for PSMs after RP. In the present study, we identified 27 studies involving 50,014 patients, and the rate of PSMs was 24.2%, which is comparable to that in previous reports. The meta-analysis showed that p-PSA, biopsy GS (<6/≥7), pathological GS (<6/≥7), pathological stage (<T2/≥T3), PLN, EPE, and SVI had a statistically significant association with PSMs. Moreover, the pooled OR/SMD of the results suggested that age, BMI, prostate volume, and nerve sparing were not independent prognostic factors for PSMs in patients after RP. Subgroup analyses revealed a similar result despite different geographical regions, publication years, sample sizes, and median follow-ups. Further, sensitivity analysis and publication bias test were also performed, and the overall results showed that our data were stable and reliable.
This is the first comprehensive study to investigate the pathological features of PSMs and predictive factors for PSMs in patients treated with RP, and the results of this analysis are meaningful. The two strengths of this study are as follows: First, a large sample size of PCa patients from different geographic areas was included, and the findings of our study were more robust than those of an individual study. Second, a summary OR/SMD was conducted to compare the difference between PSMs and NSMs in PCa patients categorized by several confounders. Therefore, our findings could provide solid evidence for prognostic factors in PCa patients with PSMs.
Nevertheless, the present study has some limitations that should be acknowledged. First, all the studies were retrospectively performed, which made our research more susceptible to recall or selection bias. Second, a substantial heterogeneity was detected, while sensitivity analysis and subgroup analysis failed to identify the potential heterogeneity. Third, this study was limited to articles published in English and Chinese, which might have contributed to selection bias. As known, articles with positive results are more likely to be published. Therefore, this article also had a certain publication bias. Fourth, the number of included studies was limited in terms of publication bias and subgroup and sensitivity analyses, which could have led to unpersuasive conclusions. Therefore, more studies are required, which can provide more detailed individual high-quality data.
Conclusion
The meta-analysis demonstrates that p-PSA, biopsy GS, pathological GS, pathological stage, PLN, EPE, and SVI were independent factors predicting PSMs after RP, and a combination of these factors might be useful for predicting PSMs in PCa patients undergoing RP. Considering the limitations of the present analysis, it is necessary to conduct more large-scale and well-designed studies to validate our results in the future.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
LZ conceptualized the study. BW, ZZ, and JY performed the literatue search. HZ and YF analyzed the data. HZ wrote the original draft. LZ wrote, reviewed, and edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript has been released as a pre-print at research square, Lijin Zhang et al.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.539592/full#supplementary-material
Supplementary Figure 1 | Sensitivity analysis (pooled ORs) of the association between the predictive factors and PSMs risk. (A) biopsy GS; (B) pathological GS; (C) pathological stage; (D) PLN, and (E) nerve sparing.
Supplementary Figure 2 | Sensitivity analysis (pooled SMDs) of the association between the predictive factors and PSMs risk. (A) p-PSA; (B) age.
Abbreviations
PCa, renal cell cancer; PSMs, positive surgical margins; NSMs, negative surgical margins; RP, radical prostatectomy; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; NOS, Newcastle Ottawa scale; ORs, odds ratios; SMD, standard mean differences; CIs, corresponding confidence intervals; p-PSA, preoperative PSA; GS, Gleason Score; PLN, positive lymph node; EPE, extraprostatic extension; SVI, seminal vesicle invasion; BMI, body mass index; RE, random-effects; FE, fixed-effects.
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: Cancer J Clin (2017) 67(1):7–30. doi: 10.3322/caac.21387
2. Ilic D, Djulbegovic M, Jung JH, Hwang EC, Zhou Q, Cleves A, et al. Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ (Clinical Res ed) (2018) 362:k3519. doi: 10.1136/bmj.k3519
3. Matulay JT, DeCastro GJ. Radical Prostatectomy for High-risk Localized or Node-Positive Prostate Cancer: Removing the Primary. Curr Urol Rep (2017) 18(7):53. doi: 10.1007/s11934-017-0703-x
4. Novara G, Ficarra V, Mocellin S, Ahlering TE, Carroll PR, Graefen M, et al. Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur Urol (2012) 62(3):382–404. doi: 10.1016/j.eururo.2012.05.047
5. Keller EX, Bachofner J, Britschgi AJ, Saba K, Mortezavi A, Kaufmann B, et al. Prognostic value of unifocal and multifocal positive surgicalmargins in a large series of robot-assisted radical prostatectomy for prostatecancer. World J Urol (2019) 37(9):1837–44. doi: 10.1007/s00345-018-2578-y
6. Zhang L, Wu B, Zha Z, Zhao H, Yuan J, Jiang Y, et al. Surgical margin status and its impact on prostate cancer prognosis after radical prostatectomy: a meta-analysis. World J Urol (2018) 36(11):1803–15. doi: 10.1007/s00345-018-2333-4
7. Zhang L, Wu B, Zha Z, Zhao H, Jiang Y, Yuan J. Positive surgical margin is associated with biochemical recurrence risk following radical prostatectomy: a meta-analysis from high-quality retrospective cohort studies. World J Surg Oncol (2018) 16(1):124. doi: 10.1186/s12957-018-1433-3
8. Seo WI, Kang PM, Yoon JH, Kim W, Chung JI. Correlation between postoperative prostate-specific antigen and biochemical recurrence in positive surgical margin patients: Single surgeon series. Prostate Int (2017) 5(2):53–8. doi: 10.1016/j.prnil.2017.02.002
9. Lallas CD, Fashola Y, Den RB, Gelpi-Hammerschmidt F, Calvaresi AE, McCue P, et al. Predictors of positive surgical margins after radical prostatectomy at a single institution: preoperative and pathologic factors, and the impact of surgeon variability and technique on incidence and location. Can J Urol (2014) 21(5):7479–86.
10. Tuliao PH, Koo KC, Komninos C, Chang CH, Choi YD, Chung BH, et al. Number of positive preoperative biopsy cores is a predictor of positive surgical margins (PSM) in small prostates after robot-assisted radical prostatectomy (RARP). BJU Int (2015) 116(6):897–904. doi: 10.1111/bju.12888
11. Ouzzane A, Rozet F, Salas RS, Galiano M, Barret E, Prapotnich D, et al. Positive surgical margins after minimally invasive radical prostatectomy in patients with pT2 and pT3a disease could be considered pathological upstaging. BJU Int (2014) 113(4):586–91. doi: 10.1111/bju.12249
12. Eastham JA, Kattan MW, Riedel E, Begg CB, Wheeler TM, Gerigk C, et al. Variations among individual surgeons in the rate of positive surgical margins in radical prostatectomy specimens. J Urol (2003) 170(6 Pt 1):2292–5. doi: 10.1097/01.ju.0000091100.83725.51
13. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine (2009) 6(7):e1000100. doi: 10.1371/journal.pmed.1000100
14. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
15. Çelik S, Aslan G, Sözen S, Özen H, Akdoğan B, Baltaci S, et al. Factors Affecting Surgical Margin Positivity after Radical Prostatectomy in the Turkish Population: A Multicenter Study of the Urooncology Association. Urologia Internationalis (2020) 104(9-10):724–30. doi: 10.1159/000507268
16. Porcaro AB, Sebben M, Corsi P, Tafuri A, Processali T, Pirozzi M, et al. Risk factors of positive surgical margins after robot-assisted radical prostatectomy in high-volume center: results in 732 cases. J Robotic Surgery (2020) 14(1):167–75. doi: 10.1007/s11701-019-00954-x
17. Tian XJ, Wang ZL, Li G, Cao SJ, Cui HR, Li ZH, et al. Development and validation of a preoperative nomogram for predicting positive surgical margins after laparoscopic radical prostatectomy. Chin Med J (2019) 132(8):928–34. doi: 10.1097/CM9.0000000000000161
18. Martini A, Gandaglia G, Fossati N, Scuderi S, Bravi CA, Mazzone E, et al. Defining Clinically Meaningful Positive Surgical Margins in PatientsUndergoing Radical Prostatectomy for Localised Prostate Cancer. Eur UrolOncol (2019) S2588-9311(19)30039-2. doi: 10.1016/j.euo.2019.03.006
19. Hou H, Jiang X, Liu M, Diao T, Wang J. The characteristics and independent associated factors of positive surgical margin after radical prostatectomy. Chin J Urol (2018) 39(10):740–4. doi: 10.3760/cma.j.issn.1000-6702.2018.10.004
20. Herforth C, Stroup SP. Radical prostatectomy and the effect of close surgical margins: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. BJU Int (2018) 122: (4):592–8. doi: 10.1111/bju.14178
21. Tatsugami K, Yoshioka K, Shiroki R, Eto M, Yoshino Y, Tozawa K, et al. Reality of nerve sparing and surgical margins in surgeons’ early experience with robot-assisted radical prostatectomy in Japan. Int J Urol (2017) 24(3):191–6. doi: 10.1111/iju.13281
22. Meyer CP, Hansen J, Boehm K, Tilki D, Abdollah F, Trinh QD, et al. Tumor volume improves the long-term prediction of biochemical recurrence-free survival after radical prostatectomy for localized prostate cancer with positive surgical margins. World J Urol Feb (2017) 35(2):199–206. doi: 10.1007/s00345-016-1861-z
23. Abdollah F, Moschini M, Sood A, Sammon J, Dalela D, Hsu L, et al. When Should a Positive Surgical Margin Ring a Bell? An Analysis of a Multi-Institutional Robot-Assisted Laparoscopic Radical Prostatectomy Database. J Endourol (2016) 30(2):201–7. doi: 10.1089/end.2015.0465
24. Whalen MJ, Shapiro EY, Rothberg MB, Turk AT, Woldu SL, Roy Choudhury A, et al. Close surgical margins after radical prostatectomy mimic biochemical recurrence rates of positive margins. Urologic Oncol (2015) 33(11):494.e499–e414. doi: 10.1016/j.urolonc.2015.07.005
25. Retel VP, Bouchardy C, Usel M, Neyroud-Caspar I, Schmidlin F, Wirth G, et al. Determinants and effects of positive surgical margins after prostatectomy on prostate cancer mortality: a population-based study. BMC Urol (5) 201414:86. doi: 10.1186/1471-2490-14-86
26. Rouanne M, Rode J, Campeggi A, Allory Y, Vordos D, Hoznek A, et al. Long-term impact of positive surgical margins on biochemical recurrence after radical prostatectomy: ten years of follow-up. Scand J Urol (2014) 48(2):131–7. doi: 10.3109/21681805.2013.813067
27. Sammon JD, Trinh QD, Sukumar S, Ravi P, Friedman A, Sun M, et al. Risk factors for biochemical recurrence following radical perineal prostatectomy in a large contemporary series: a detailed assessment of margin extent and location. Urologic Oncol (2013) 31(8):1470–6. doi: 10.1016/j.urolonc.2012.03.013
28. Lee JW, Ryu JH, Kim YB, Yang SO, Lee JK, Jung TY. Do positive surgical margins predict biochemical recurrence in all patients without adjuvant therapy after radical prostatectomy? Korean J Urol (2013) 54(8):510–5. doi: 10.4111/kju.2013.54.8.510
29. Hashimoto T, Yoshioka K, Gondo T, Takeuchi H, Nakagami Y, Nakashima J, et al. Predictors for positive surgical margins after robot-assisted radical prostatectomy: a single surgeon’s series in Japan. Int J Urol (2013) 20(9):873–8. doi: 10.1111/iju.12081
30. Abdollah F, Sun M, Suardi N, Gallina A, Capitanio U, Bianchi M, et al. Presence of positive surgical margin in patients with organ-confined prostate cancer equals to extracapsular extension negative surgical margin. A plea for TNM staging system reclassification. Urologic Oncol (2013) 31(8):1497–503. doi: 10.1016/j.urolonc.2012.04.013
31. Savdie R, Horvath LG, Benito RP, Rasiah KK, Haynes AM, Chatfield M, et al. High Gleason grade carcinoma at a positive surgical margin predicts biochemical failure after radical prostatectomy and may guide adjuvant radiotherapy. BJU Int (2012) 109(12):1794–800. doi: 10.1111/j.1464-410X.2011.10572.x
32. Lu J, Wirth GJ, Wu S, Chen J, Dahl DM, Olumi AF, et al. A close surgical margin after radical prostatectomy is an independent predictor of recurrence. J Urol (2012) 188(1):91–7. doi: 10.1016/j.juro.2012.02.2565
33. Karavitakis M, Ahmed HU, Abel PD, Hazell S, Winkler MH. Margin status after laparoscopic radical prostatectomy and the index lesion: implications for preoperative evaluation of tumor focality in prostate cancer. J Endourol (2012) 26(5):503–8. doi: 10.1089/end.2011.0345
34. Corcoran NM, Hovens CM, Metcalfe C, Hong MK, Pedersen J, Casey RG, et al. Positive surgical margins are a risk factor for significant biochemical recurrence only in intermediate-risk disease. BJU Int (2012) 110(6):821–7. doi: 10.1111/j.1464-410X.2011.10868.x
35. Li K, Li H, Yang Y, Ian LH, Pun WH, Ho SF. Risk factors of positive surgical margin and biochemical recurrence of patients treated with radical prostatectomy: a single-center 10-year report. Chin Med J (2011) 124(7):1001–5.
36. Coelho RF, Chauhan S, Orvieto MA, Palmer KJ, Rocco B, Patel VR. Predictive factors for positive surgical margins and their locations after robot-assisted laparoscopic radical prostatectomy. Eur Urol (2010) 57(6):1022–9. doi: 10.1016/j.eururo.2010.01.040
37. Boorjian SA, Karnes RJ, Crispen PL, Carlson RE, Rangel LJ, Bergstralh EJ, et al. The impact of positive surgical margins on mortality following radical prostatectomy during the prostate specific antigen era. J Urol (2010) 183(3):1003–9. doi: 10.1016/j.juro.2009.11.039
38. Alkhateeb S, Alibhai S, Fleshner N, Finelli A, Jewett M, Zlotta A, et al. Impact of positive surgical margins after radical prostatectomy differs by disease risk group. J Urol (2010) 183(1):145–50. doi: 10.1016/j.juro.2009.08.132
39. Shikanov S, Song J, Royce C, Al-Ahmadie H, Zorn K, Steinberg G, et al. Length of positive surgical margin after radical prostatectomy as a predictor of biochemical recurrence. J Urol (2009) 182(1):139–44. doi: 10.1016/j.juro.2009.02.139
40. Ficarra V, Novara G, Secco S, D'Elia C, Boscolo-Berto R, Gardiman M, et al. Predictors of positive surgical margins after laparoscopic robot assisted radical prostatectomy. J Urol (2009) 182(6):2682–8. doi: 10.1016/j.juro.2009.08.037
41. Preisser F, Mazzone E, Knipper S, Nazzani S, Bandini M, Shariat SF, et al. Rates of Positive Surgical Margins and Their Effect on Cancer-specific Mortality at Radical Prostatectomy for Patients With Clinically Localized Prostate Cancer. Clin Genitourinary Cancer (2019) 17(1):e130–9. doi: 10.1016/j.clgc.2018.09.024
42. Yossepowitch O, Bjartell A, Eastham JA, Graefen M, Guillonneau BD, Karakiewicz PI, et al. Positive surgical margins in radical prostatectomy: outlining the problem and its long-term consequences. Eur Urol (2009) 55(1):87–99. doi: 10.1016/j.eururo.2008.09.051
43. Sooriakumaran P, John M, Wiklund P, Lee D, Nilsson A, Tewari AK. Learning curve for robotic assisted laparoscopic prostatectomy: a multi-institutional study of 3794 patients. Minerva Urologica e Nefrologica = Ital J Urol Nephrol (2011) 63(3):191–8.
44. Choo MS, Cho SY, Jeong CW, Lee SB, Ku JH, Hong SK, et al. Predictors of positive surgical margins and their location in Korean men undergoing radical prostatectomy. Int J Urol (2014) 21(9):894–8. doi: 10.1111/iju.12465
45. Yuksel M, Karamik K, Anil H, Islamoglu E, Ates M, Savas M. Factors affecting surgical margin positivity in robotic assisted radical prostatectomy. Archivio Italiano di Urol Androl Organo Ufficiale [di] Societa Italiana di Ecografia Urologica e Nefrologica (2017) 89(1):71–4. doi: 10.4081/aiua.2017.1.71
Keywords: prostate cancer, radical prostatectomy, positive surgical margins, risk factors, meta-analysis
Citation: Zhang L, Zhao H, Wu B, Zha Z, Yuan J and Feng Y (2021) Predictive Factors for Positive Surgical Margins in Patients With Prostate Cancer After Radical Prostatectomy: A Systematic Review and Meta-Analysis. Front. Oncol. 10:539592. doi: 10.3389/fonc.2020.539592
Received: 01 March 2020; Accepted: 22 December 2020;
Published: 08 February 2021.
Edited by:
Ian Pearce, Manchester University NHS Foundation Trust (MFT), United KingdomReviewed by:
Luca Mazzarella, European Institute of Oncology (IEO), ItalyXiangming Cheng, Peking Union Medical College Hospital (CAMS), China
Copyright © 2021 Zhang, Zhao, Wu, Zha, Yuan and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijin Zhang, c3R6bGo5MTM3Mjk1NTNAMTYzLmNvbQ==
 Lijin Zhang
Lijin Zhang Hu Zhao
Hu Zhao Bin Wu
Bin Wu Jun Yuan
Jun Yuan Yejun Feng
Yejun Feng