- 1National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Lanzhou University Second Hospital, Lanzhou, China
- 3Fifth Medical Center of Chinese PLA General Hospital, Beijing, China
- 4Department of Surgery, Yale School of Medicine, New Haven, CT, United States
- 5Department of Environmental Health Sciences, Yale School of Public Health, New Haven, CT, United States
Background: We aimed to assess long-term survival between locally advanced proximal gastric cancer (LAPGC) patients who underwent proximal gastrectomy (PG) and those who underwent total gastrectomy (TG) to evaluate the optimal extent of resection and adjuvant therapy.
Materials and Methods: Patients diagnosed with locally advanced proximal gastric adenocarcinoma were selected from the National Cancer Data Base (2004–2015) in America. Survival analysis was performed via Kaplan-Meier and Cox proportional hazards models.
Results: A total of 4,381 eligible patients were identified, 1,243 underwent PG and 3,138 underwent TG. Patients in TG group had a poor prognosis (hazard ratio [HR] = 1.13, 95% confidence interval [CI]: 1.03–1.25) compared with those in PG group. Moreover, postoperative chemoradiation therapy was associated with improved overall survival compared to surgery alone (HR = 0.71, 95% CI: 0.53–0.97) in LAPGC patients who had PG, while preoperative chemotherapy (HR = 0.74, 95% CI: 0.59–0.92) was associated with improved survival among patients who had TG.
Conclusions: Our study suggested that LAPGC patients underwent PG experienced better long-term outcomes than those underwent TG. It also suggested that multimodality treatment of LAPGC, including preoperative chemotherapy followed by TG or postoperative chemotherapy followed by PG, should be considered to achieve better long-term outcomes.
Introduction
Gastric cancer (GC) is the fifth most common malignancy and the third leading cause of cancer-related mortality worldwide (1). While its overall incidence appears to be decreasing, there has been a dramatic rise in the incidence of proximal gastric cancer (PGC) (2). The shift in GC subsite has renewed interest in the management of PGC with a focus on the optimal extent of resection and adjuvant therapy.
Proximal gastrectomy (PG) and total gastrectomy (TG) are the most common surgical approaches for PGC. For early stage PGC, PG has been generally accepted by most surgeons for its comparable oncological radicality and safety with TG (3–5). The newly published “Japanese Gastric Cancer Treatment Guidelines 2018” also recommends that PG is suitable for early stage diseases (6). However, no consensus has been reached regarding which procedure should be selected for locally advanced PGC (LAPGC). Several studies investigated overall survival (OS) of LAPGC patients who underwent TG or PG and reached inconsistent results. Some studies (3, 5, 7–21) reported that TG and PG had similar OS, whereas other studies (22–25) showed that TG was associated with better 5-year OS than PG. Moreover, some studies even found that the prognosis of LAPGC patients undergoing PG was significantly better than those undergoing TG (9, 26). Deficiently, these published studies generally included limited number of patients ranging from 45 to 423. On the other hand, the optimum treatment strategy of neoadjuvant or adjuvant therapy targeted on LAPGC patients was not fully discussed in previous studies.
Here, we analyzed data from the American College of Surgeons (ACS) National Cancer Database (NCDB) to compare the OS of PGC patients who underwent PG to those who underwent TG, in order to determine whether the extent of resection for LAPGC affect prognosis and provides evidence for the development of guiding strategies for LAPGC patients.
Materials and Methods
Patient Population
Data were abstracted from the NCDB 2004-2015. The NCDB is a clinical oncology database sourced from hospital registry data that are collected from more than 1,500 Commission on Cancer (CoC)-accredited facilities. Eligible patients were LAPGC according to the International Classification of Diseases for Oncology codes (defined by C16.33, C16.41, C16.42, C16.52, and C16.62) and underwent definitive gastrectomy. Patients were further restricted to adenocarcinoma histology (defined by 8,140, 8,141, 8,144, 8,146, 8,147, 8,255, 8,260, 8,262, 8,310, 8,480, 8,481, 8,490, 8,510, 8,560, 8,562, and 8,570–8,576). According to the American Joint Committee on Cancer 8th (27), the locally advanced gastric cancer (LAGC) patients were defined as (1) patients treated with neoadjuvant treatment whose clinical T ≥2, N = any, M = 0, or whose T = any, N ≥1, M = 0, or (2) patients without neoadjuvant treatment whose pathological T ≥2, N = any, M = 0, or whose pathological T = any, N ≥1, M = 0. The study was exempt from the approval by the Yale Institutional Review Board as a secondary data analysis.
Statistical Analyses
Patient demographics and clinical characteristics included age, gender, race, Hispanic ethnicity, insurance status, median income (calculated by the NCDB based on patient's zip code), facility location, facility type, distance (from patient's zip code to hospital reporting the case), year of diagnosis, Charlson Deyo score, tumor grade, clinical stage, pathological stage, number of lymph nodes examined, number of lymph nodes positive, scope of regional lymph node surgery, surgical margins, surgical inpatient stay, 30-day unplanned readmission after surgical discharge, and treatment. The sequence of chemotherapy was determined using the sequence of systemic therapy in relationship to surgery. Neoadjuvant therapy (NAT) was defined as preoperative therapies including chemotherapy and/or radiation therapy. Adjuvant therapy (AT) was defined as postoperative therapies including chemotherapy and/or radiation therapy. OS was defined as the interval between the date of diagnosis and the date of death or last contact.
Student's t-test for continuous variables and chi-square test for categorical variables were performed to compare patients' characteristics between PG and TG groups. Kaplan–Meier and log-rank test was used to examine OS by different treatment groups (28). Multivariate Cox regression models were employed to estimate hazard ratio (HR) and 95% confidence intervals (CI). The proportionality assumption of the cox-regression was checked by including a time-varying covariate, an interaction between the covariate and the event time. Confounding variables were selected through stepwise. Several clinically significant variables were forced into the final models although they were not statistically significant. The adjusted confounding variables included age, sex, race, Hispanic ethnicity, Charlson Deyo score, insurance status, year of diagnosis, median income, facility location, facility type, distance, tumor grade, scope of regional lymph node surgery, surgical margin, surgical inpatient stay, 30-day unplanned readmission. The adjustment for NAT and/or AT was only in the model for OS by surgical approaches. A p < 0.05 was considered statistically significant and all tests were two-sided. Sensitivity analysis was performed by excluding patients who died within 90 days after primary surgery in order to account for immortal time bias.
All analyses were conducted using SAS statistical software v9.3 (SAS Institute, Inc., Cary, NC).
Results
Cohort Characteristics
A total of 4,381 LAPGC patients with adenocarcinoma were identified, and 1,243 patients (28.37%) underwent PG and 3,138 (71.63%) underwent TG (Table 1). Approximately two thirds of the patients were aged 50–74 years at diagnosis, with a mean diagnosis age of 64.43 ± 11.57 years. Majority of the cases were male (76.69%) and white (86.56%). Compared with patients underwent TG, patients underwent PG were more likely to be older, male, and white. Patients in PG group were more likely to have a higher proportion of R0 resection (88.33 vs. 84.00%, p < 0.01) and well or moderately differentiated tumor grade, whereas patients in TG group were more likely to have a higher proportion of more than 15 nodes examined (53.98 vs. 35.32%, p < 0.01) and nodes positive (67.30 vs. 63.07%, p < 0.01). Carlson scores, surgical inpatient stay, and 30-day unplanned readmission were not significantly different between the two groups (p > 0.05).
Survival Outcomes for Patients Who Underwent PG and TG
Compared with patients in TG group, patients in PG group had longer OS as shown in Figure 1 (p = 0.0006). The median survival time was 32.99 months (95% CI: 30.06–37.03 months) for PG group and 28.19 months (95% CI: 26.64–29.57 months) for TG group. The 3- and 5-year survival rates were 47.40 and 35.94% for PG and 42.06 and 30.14% for TG, respectively). After controlling for confounding variables, patients who underwent TG had poor OS compared to patients who underwent PG (HR = 1.13, 95% CI: 1.03–1.25; p = 0.0109) (Table 2).
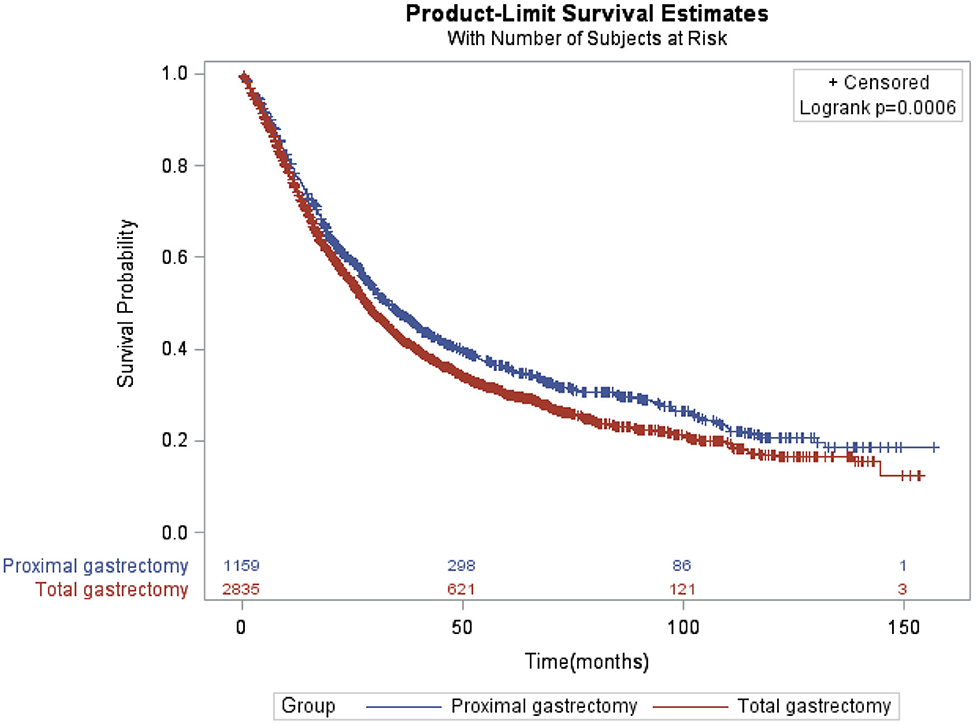
Figure 1. Kaplan-Meier of overall survival of locally advanced proximal gastric cancer patients by proximal and total gastrectomy.
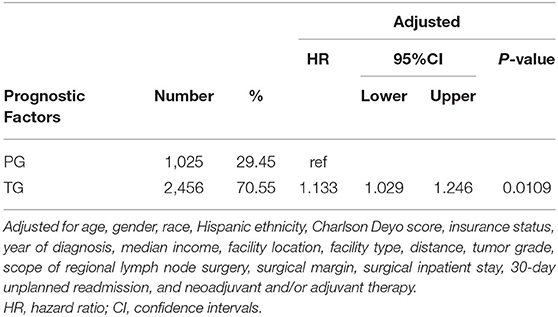
Table 2. Multivariate analysis of locally advanced proximal gastric cancer patients by surgical approach.
Survival Outcomes for Patients With Different Adjuvant Therapies in Locally Advanced Stage
Patients who received AT, NAT only, or NAT plus AT had improved OS compared with patients who underwent gastrectomy alone regardless of PG (Figure 2A, p < 0.0001) or TG (Figure 2B, p < 0.0001). The median survival time for patients who underwent PG were 23.66, 39.49, 43.83, and 54.08 months in gastrectomy alone, NAT, AT, and NAT plus AT, respectively. The corresponding median survival time for patients who underwent TG were 16.82, 34.69, 32.95, and 35.81 months, respectively. No significant differences in survival benefits between different adjuvant therapies.
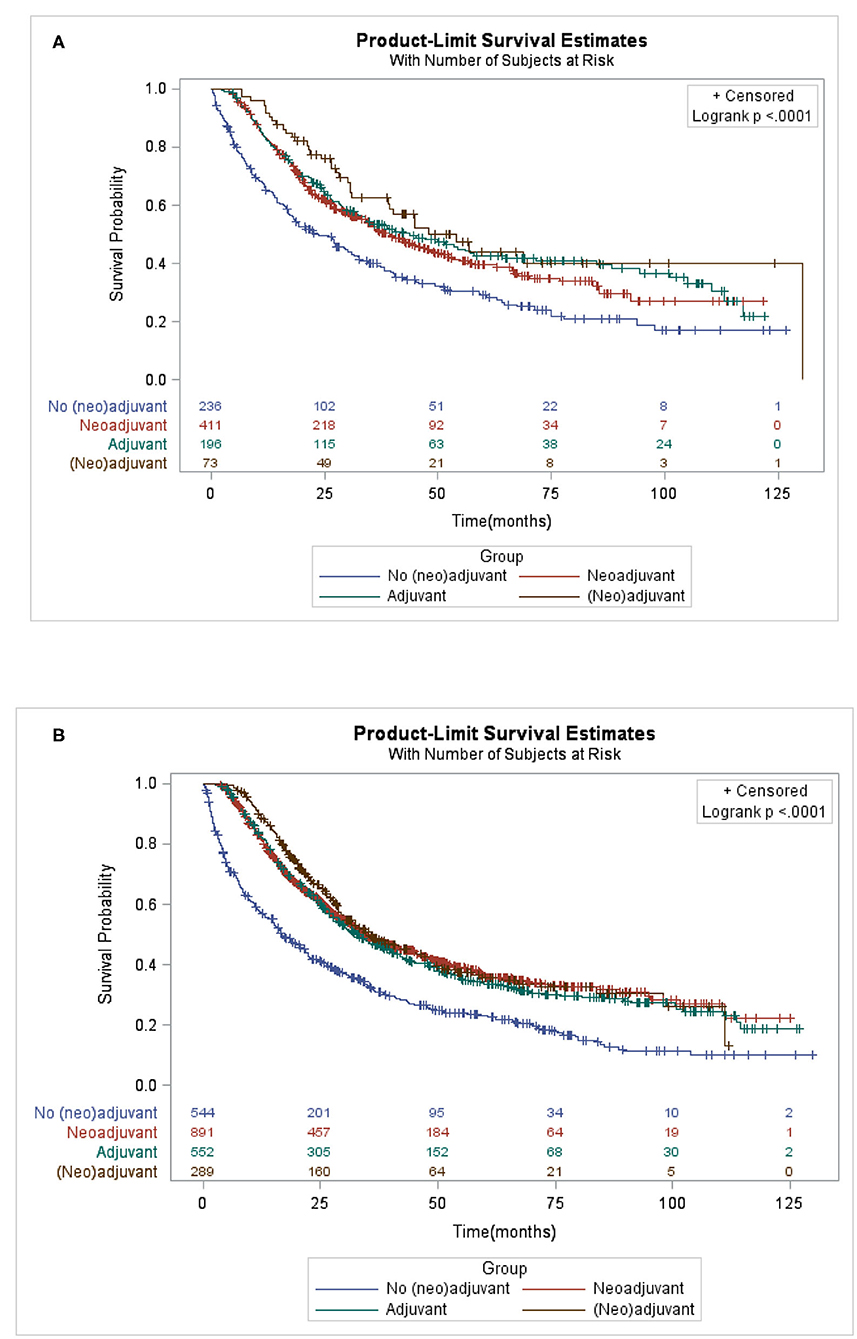
Figure 2. Kaplan-Meier of overall survival of locally advanced proximal gastric cancer patients who underwent (A) proximal gastrectomy (B) or total gastrectomy by different adjuvant therapy.
After controlling for confounding variables (Table 3), only AT was associated with improved OS compared to surgery alone in PG group (HR = 0.70, 95% CI: 0.52–0.92; p = 0.0114). However, there was no significant survival benefit for various adjuvant therapies among patients who underwent TG.

Table 3. Multivariate analysis for locally advanced proximal gastric cancer patients by neoadjuvant and adjuvant therapy.
We further analyzed the data by detailed therapies (Table 4), among patients who underwent PG, postoperative CT plus RT (HR = 0.71, 95% CI: 0.53–0.97; p = 0.0316) was associated with improved survival compared to surgery alone. Among patients who underwent TG, only preoperative CT (HR = 0.74, 95% CI: 0.59–0.92; p = 0.0078) was associated with improved survival compared to surgery alone.

Table 4. Multivariate analysis for locally advanced proximal gastric cancer patients with detailed neoadjuvant and adjuvant therapy.
Discussion
Our study suggested an improved survival benefit of PG compared to TG among patients diagnosed with LAPGC. In contrast to early studies that have reported no differences in survival outcomes between the two surgical procedures (3, 5, 7–21) or a better survival was associated with TG procedure (22–25). Varying patients' characteristics in different study populations might account for the inconsistent results. A meta-analysis (22) reported no difference in survival between TG and PG groups among LAPGC patients, which was inconsistent with our study results. A possible explanation was that patients in TG group had a higher proportion (58.92%) of poorer tumor grade in our cohort whereas the patients in early studies (9–12, 14) had a lower proportion (ranging from 27.5 to 52%) of poorer tumor grade. Another possible explanation was that confounding factors, such as adjuvant or neoadjuvant therapies were not controlled in early studies.
Neoadjuvant therapy or adjuvant therapy have been developed over the last decades as part of a multimodality treatment in order to improve survival for LAPGC patients with gastrectomy (29). However, no consensus has been reached regarding the optimal choice. Our study revealed a better prognosis for PGC patients given AT compared to gastrectomy alone only in PG group, but no significant survival benefits of AT in TG group. Previous studies have reported improved patient survival with the addition of AT or NAT compared to surgery alone (30, 31). Surgery does not always result in complete resection, which is likely to cause recurrence and metastasis and influence the long-term outcomes (22). NAT is expected to improve the resection rate and long-term follow-up results by reducing the size of the primary lesion and controlling lymph node metastasis and micrometastasis (32). Therefor NAT has been recommended for PGC patients with advanced clinical stage. AT controls residual tumor cells following curative resection, and therefore improve the long-term follow-up results (31).
The increased use of preoperative CT for patients with PGC was a dominant trend among patients with locally advanced disease (33). Preoperative CT has several advantages, including a greater likelihood of completing treatment, a rapid improvement in tumor-related symptoms, the potential to downstage tumors, and the ability to assess response to preoperative therapy (33). In our study, survival benefit of preoperative CT only was shown for LAPGC patients who underwent TG but not those who underwent PG. The reasons for this are currently unclear and warrant further investigation. Postoperative CT or RT is delivered with an intention to reduce recurrence by controlling residual tumor cells following curative resection. Recent advances in postoperative CT have achieved considerable tumor regression in many cases of gastric cancer (6). In our study, we also observed postoperative CT with or without RT showed OS benefit only for LAPGC patients who underwent PG.
Study limitations should be considered when interpreting the study results. Although ~70% of all newly diagnosed cases of cancer in the United States were reported to the NCDB, the NCDB collects data only from Commission on Cancer–accredited hospitals which might affect the generalizability of the study results. Some treatment-related information was unavailable, including recurrence time, metastasis time, treatment intent, specific chemotherapy regimens, completion of prescribed treatment schedules, and toxicities of the received therapies. Information concerning more granular endpoints including disease specific survival, recurrence, and postoperative complications were also not available. In addition, the numbers of patients in certain specific neoadjuvant and adjuvant therapy groups were too small, which provided limited power to evaluate their effects on OS. Despite these limitations, the NCDB provides a large sample size, making this study one of the largest studies to assess long-term survival between LAPGC patients who underwent PG and TG.
In conclusion, long-term outcome disparities exist between LAPGC patients who underwent TG and those who underwent PG in the United States. Patients treated with PG had better OS than those who underwent TG, suggesting PG might be an optimal extent of resection for LAPGC patients. The study also suggested that LAPGC should be treated with multimodality treatment approach, including preoperative CT followed by TG or postoperative CT followed by PG. The findings in our study need to be verified in randomized controlled clinical trials.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
YL: conceptualization, methodology, and writing–review and editing. LZ: conceptualization, methodology, and writing–review. XW: writing–review and editing. SK: conceptualization, investigation, resources, and writing–review and editing. YC: conceptualization, methodology, funding acquisition, and writing–review and editing. YZ: conceptualization, methodology, investigation, resources, supervision, and writing–review and editing. ST, FL, and ST: conceptualization, methodology, and writing first draft. FL: conceptualization, methodology, data analysis, and writing–review and editing. All authors: contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from National Key R&D Program of China (Grant Nos. 2017YFC0908300 and 2016YFC1302500).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Borch K, Jonsson B, Tarpila E, Franzen T, Berglund J, Kullman E, et al. Changing pattern of histological type, location, stage and outcome of surgical treatment of gastric carcinoma. Br J Surg. (2000) 87:618–26. doi: 10.1046/j.1365-2168.2000.01425.x
3. Ikeguchi M, Kader AK, Takaya S, Fukumoto Y, Osaki T, Saito H, et al. Prognosis of patients with gastric cancer who underwent proximal gastrectomy. Int Surg. (2012) 97:275–9. doi: 10.9738/cc150.1
4. Nozaki I, Hato S, Kobatake T, Ohta K, Kubo Y, Kurita A. Long-term outcome after proximal gastrectomy with jejunal 1nterposition for gastric cancer compared with total gastrectomy. World J Surg. (2013) 37:558–64. doi: 10.1007/s00268-012-1894-4
5. Ichikawa D, Komatsu S, Kubota T, Okamoto K, Shiozaki A, Fujiwara H, et al. Long-term outcomes of patients who underwent limited proximal gastrectomy. Gastric Cancer. (2014) 17:141–5. doi: 10.1007/s10120-013-0257-7
6. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018. (5th edition). Gastric Cancer. (2020) (Suppl. 1):1–21. doi: 10.1007/s10120-020-01042-y
7. Shiraishi N, Adachi Y, Kitano S, Kakisako K, 1nomata M, Yasuda K. Clinical outcome of proximal versus total gastrectomy for proximal gastric cancer. World J Surg. (2002) 26:1150. doi: 10.1007/s00268-002-6369-6
8. Jakl RJ, Miholic J, Koller R, Markis E, Wolner E. Prognostic factors in adenocarcinoma of the cardia. Am J Surg. (1995) 169:316–9. doi: 10.1016/S0002-9610(99)80166-4
9. Sugoor P, Shah S, Dusane R, Desouza A, Goel M, Shrikhande SV. Proximal gastrectomy versus total gastrectomy for proximal third gastric cancer: total gastrectomy is not always necessary. Langenbecks Arch Surg. (2016) 401:1–11. doi: 10.1007/s00423-016-1422-3
10. An JY, Youn HG, Choi MG, Noh JH, Sohn TS, Kim S. The difficult choice between total and proximal gastrectomy in proximal early gastric cancer. Am J Surg. (2008) 196:587–91. doi: 10.1016/j.amjsurg.2007.09.040
11. Harrison LE, Karpeh MS, Brennan MF. Total gastrectomy is not necessary for proximal gastric cancer. Surgery. (1998) 123:127. doi: 10.1016/S0039-6060(98)70248-X
12. Ooki A, Yamashita K, Kikuchi S, Sakuramoto S, Katada N, Hutawatari N, et al. Clinical sign if cance of total gastrectomy for proximal gastric cancer. Anticancer Res. (2008) 28:2875. doi: 10.1097/CAD.0b013e32830c2380
13. Ahn SH, Lee JH, Park DJ, Kim HH. Comparative study of clinical outcomes between laparoscopy-assisted proximal gastrectomy (LAPG) and laparoscopy-assisted total gastrectomy (LATG) for proximal gastric cancer. Gastric Cancer. (2013) 16:282–9. doi: 10.1007/s10120-012-0178-x
14. Chen S, Li J, Liu H, Zeng J, Yang G, Wang J, et al. Esophagogastrostomy plus gastrojejunostomy: a novel reconstruction procedure after curative resection for proximal gastric cancer. J Gastrointest Surg. (2014) 18:497–504. doi: 10.1007/s11605-013-2391-2
15. Jung DH, Lee Y, Kim DW, Park YS, Ahn SH, Park DJ, et al. Laparoscopic proximal gastrectomy with double tract reconstruction is superior to laparoscopic total gastrectomy for proxima I early gastric cancer. Surg Endoscop. (2017) 31:3961–9. doi: 10.1007/s00464-017-5429-9
16. Chang HY, Sohn BH, Han WK, Pae WK. Long-term results of proximal and total gastrectomy for adenocarcinoma of the upper third of the stomach. Cancer Res Treat. (2004) 36:50–5. doi: 10.4143/crt.2004.36.1.50
17. Son MW, Kim YJ, Jeong GA, Cho GS, Lee MS. Long-term outcomes of proximal gastrectomy versus total gastrectomy for upper-third gastric cancer. J Gastric Cancer. (2014) 14:246–51. doi: 10.5230/jgc.2014.14.4.246
18. Hosoda K, Yamashita K, Katada N, Moriya H, Mieno H, Shibata T, et al. Potential benefts of laparoscopy-assisted proximal gastrectomy with esophagogastrostomy for cT1 upper-third gastric cancer. Surg Endoscop. (2016) 30:3426–36. doi: 10.1007/s00464-015-4625-8
19. lsobe T, Hashimoto K, Kizaki J, Matono S, Murakami N, Kinugasa T, et al. Reconstruction methods and complications in proximal gastrectomy for gastric cancer, and a comparison with total gastrectomy. Kurume Med J. (2014) 61:23–9. doi: 10.2739/kurumemedj.MS64003
20. Zhou Y, Pen J, Sheng Y, Liu H, Fan Z. Surgical treatment effects in cancer of the cardia and esophagogastric junction. Oncol Trans Med. (2007) 6:220–1. doi: 10.1007/s10330-006-0040-x
21. Rosa F, Quero G, Fiorillo C, Bissolati M, Cipollari C, Rausei S, et al. Total vs proximal gastrectomy for adenocarcinoma of the upper third of the stomach: a propensity-score-matched analysis of a multicenter western experience (On behalf of the Italian Research Group for Gastric Cancer-GIRCG). Gastric Cancer. (2018) 21:845–52. doi: 10.1007/s10120-018-0804-3
22. Ying KM, Chen Z, Dang CX, Sun MC, Van GR, Kan BH, et al. Clinicopathology and survival in patients with gastroesophageal Ref hx after radical surgery of proximal gastric cancer. Dig Dis Sci. (2018) 63:1035–42. doi: 10.1007/s10620-018-4960-4
23. Zhao D, Xu H, Li K, Sun Z. Prognostic factors for patients after curative resection for proximal gastric cancer. J Huazhong Univ Sci Technol. (2010) 30:530–5. doi: 10.1007/s11596-010-0463-z
24. Kim JH, Park SS, Kim J, Boo YJ, Kim SJ, Mok YJ, et al. Surgical outcomes for gastric cancer in the upper third of the stomach. World J Surg. (2006) 30:1870–6. doi: 10.1007/s00268-005-0703-8
25. Huang H, Wang W, Chen Z, Jin J J, Long Z W, Cai H, et al. Prognostic factors and survival in patients with gastric stump cancer. World J Gastroenterol. (2015) 21:1865–71. doi: 10.3748/wjg.v21.i6.1865
26. Yura M, Yoshikawa T, Otsuki S, Yamagata Y, Morita S, Katai H, et al. Oncological safety of proximal gastrectomy for T2/13 proximal gastric cancer. Gastric Cancer. (2019) 22:1029–35. doi: 10.1007/s10120-019-00938-8
27. Amin MB, Edge SB, Greene FL, Brookland RK, Washingtin MK, Gershenwald JF, et al. AJCC Cancer Staging Manual, 8th Edition. New York, NY: Springer (2017).
28. Qiu P, Jun S. A two-stage procedure for comparing hazard rate functions. J R Statist Soc. (2008). 70:191–208. doi: 10.2307/20203818
29. Ajani JA, Bentrem DJ, Besh S, D'Amico TA, Das P, Denlinger C, et al. Gastric Cancer, version 2.2013. J Natl Compre Cancer Network. (2013) 11:531–46. doi: 10.6004/jnccn.2013.0070
30. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. (2002) 345:725–30. doi: 10.1056/NEJMoa010187
31. Cunningham D, Allum WH, Stenning Sp' Thompson IN, Velde CHV, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. (2008) 355:11. doi: 10.1056/NEJMoa055531
32. Das M. Neoadjuvant chemotherapy: survival beneft in gastric cancer. Lancet. Oncol. (2017) 18:e307. doi: 10.1016/S1470-2045(17)30321-2
Keywords: national cancer data base, locally advanced proximal gastric cancer, proximal gastrectomy, total gastrectomy, long-term survival
Citation: Tang S, Liu F, Li Y, Zhao L, Wang X, Khan SA, Chen Y and Zhang Y (2020) Treatment Selection and Survival Outcomes in Locally Advanced Proximal Gastric Cancer: A National Cancer Data Base Analysis. Front. Oncol. 10:537051. doi: 10.3389/fonc.2020.537051
Received: 25 March 2020; Accepted: 26 August 2020;
Published: 25 September 2020.
Edited by:
Guangwen Cao, Second Military Medical University, ChinaReviewed by:
Zhihua Yin, China Medical University, ChinaAlejandra Castanon, King's College London, United Kingdom
Copyright © 2020 Tang, Liu, Li, Zhao, Wang, Khan, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingtai Chen, eWluZ3RhaS5jaGVuQGhvdG1haWwuY29t; Yawei Zhang, eWF3ZWkuemhhbmdAeWFsZS5lZHU=
†These authors have contributed equally to this work
 Song Tang
Song Tang Fangfang Liu3†
Fangfang Liu3† Sajid A. Khan
Sajid A. Khan Yingtai Chen
Yingtai Chen Yawei Zhang
Yawei Zhang