- 1Department of Endocrinology and Metabology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Jinan, China
- 2Department of Endocrinology and Metabology, Shandong Provincial Qianfoshan Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 3Department of Thyroid & Breast Surgery, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Shandong University, Jinan, China
- 4Department of Thyroid & Breast Surgery, Shandong Provincial Qianfoshan Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 5Department of Endocrinology and Metabology, Qilu Hospital of Shandong University, Cheeloo College of Medicine, Shandong University, Jinan, China
Background: Hyperlipidemia has been hypothesized as a risk factor for thyroid cancer. However, the association between hypercholesterolemia and thyroid cancer is unclear, especially in Chinese population without available published data. We conducted this study to investigate the relationship between hypercholesterolemia and differentiated thyroid cancer (DTC) in Chinese population.
Methods: Three thousand seven hundred forty-eight patients were enrolled in the study, including 2,021 DTC patients and 1,727 benign subjects with benign thyroid nodules. Demographic characteristics, medical history, and clinical hematological examination were collected. Stratified analyses of association between hypercholesterolemia and risk of DTC were done. Multivariable logistic regression models were used to estimate the association between hypercholesterolemia and the risk of thyroid nodules being malignant. This study protocol was approved by the ethics committee of Shandong Provincial Qianfoshan Hospital and assigned in ClinicalTrials.gov protocol registration and results system (NCT03006289, https://clinicaltrials.gov/ct2/show/NCT03006289).
Results: The level of serum total cholesterol in patients with DTC is higher than that in benign subjects (P < 0.001). After adjusting hypercholesterolemia, age (P < 0.001), triglyceride (P = 0.003), and thyroid stimulating hormone (TSH) (P < 0.001) are found to be confounding factors. The risk of DTC in patients younger than 45 years old is 2.08 times than that of patients older than 45 years old (odds ratio = 0.48, 95% CI (0.38, 0.61), P < 0.001). A high TSH level is highly associated with the increased risk of DTC (P < 0.001). The multivariable logistic regression analysis revealed that the absence of hypercholesterolemia could reduce the risk of thyroid nodules being malignant (odds ratio = −0.75, 95% CI (−1.39, −0.12), P = 0.02). Comparing to the higher level of serum total cholesterol (>5.7 mmol/L), the closer the serum total cholesterol level is to normal (3.17–5.7 mmol/L), the less the risk of thyroid nodules being malignant is, and this difference is statistically significant (odds ratio = −0.67, 95% CI (−1.31, −0.03), P = 0.040). However, this difference is not found in the group of patients with lower level of total cholesterol (<3.17 mmol/L, odds ratio = 0.43, 95% CI (−1.22, 2.09), P = 0.068), suggesting that hypocholesterolemia is not a protective factor in the risk of thyroid nodules being malignant.
Conclusions: Hypercholesterolemia is an associated factor for risk of DTC in Chinese population.
Introduction
Thyroid cancer, the most common malignancy in endocrine system, is becoming increasingly prevalent worldwide. In China, the collection of cancer registration data in 2013 showed that the incidence of thyroid cancer in female reached 16.31 per 100,000 and became the fifth most common cancer in female (1). Thyroid cancer is divided into papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), anaplastic thyroid cancer, medullary thyroid cancer, and other rare types. Among them, PTC and FTC are called differentiated thyroid cancer (DTC) and consist of more than 90% of all thyroid cancers (2–4). For better prevention and treatment of DTC, it is particularly necessary to look for the risk factors of the increasing incidence of thyroid cancer.
Nowadays, the known risk factors for thyroid cancer include exposure of radioactive radiation, gender, age, iodine deficiency or excess, and family history of thyroid cancer (5, 6). However, these seem not to be able to fully explain the increased incidence and most of the risk factors, such as gender, age, family history of thyroid cancer, or even genetic mutation are ineluctable. An interesting finding showed that thyroid cancer has been regarded as a high-income lifestyle-associated diseases (1, 7). It was found in the United States that social economic status is strongly associated with thyroid cancer and the richer populations are more likely to develop thyroid cancer (7). Moreover, a survey conducted in China reported that the incidence of thyroid cancer in well-developed cities is four times as risky as undeveloped cities (1). Therefore, more attention has been focused on the preventable and modifiable risk factors, such as factors related to over-nutrition. It has been hypothesized that hyperlipidemia may play a role in the increased incidence of thyroid cancer (8). However, clinical studies on the relationship between hyperlipidemia and risk of thyroid cancer are lacking. Furthermore, which component of lipid plays the key role is still unknown. Therefore, this study aimed to assess the association between hyperlipidemia and DTC in Chinese population, whose diet and lifestyle are different from western population, and explore that which type of hyperlipidemia plays a key role. The influence of other classic risk factors that related to thyroid cancer on the association were also evaluated by stratified analysis.
Material and Methods
Study Design and Study Population
In accordance with the STROBE statement, this study with the purpose of estimating the potential relationship between hypercholesterolemia and risk of DTC was performed. Inclusion criteria for this study are as follows: (1) patients who were diagnosed with thyroid nodules by thyroid ultrasonography; (2) patients who underwent a thyroid operation at the Thyroid Surgery Department of the first affiliated hospital of Shandong First Medical University and Shandong Provincial Qianfoshan Hospital (Shandong Provincial Qianfoshan Hospital, Cheeloo College of Medicine, Shandong University) during 1 January 2013 and 30 June 2020; (3) patients who were diagnosed with PTC, FTC, or benign thyroid nodules by a surgical pathology; (4) clinical data can be collected in the electronic medical records system. Participants were excluded based on the following criteria: (1) foreigners except Chinese; (2) a history of thyroid surgery; (3) a history of radioactive iodine therapy; (4) pregnancy or breast-feeding women. Totally, 3,748 patients, diagnosed with thyroid nodules by thyroid ultrasonography and underwent a thyroidectomy, recruited in this study. Of these, 2,021 patients were diagnosed with differentiated thyroid cancer by a pathological examination. The remaining 1,727 patients were diagnosed with benign thyroid nodules. All experiments were performed in accordance with relevant guidelines and protocols. This study protocol was approved by the ethics committee of Shandong Provincial Qianfoshan Hospital and assigned in ClinicalTrials.gov protocol registration and results system (NCT03006289). No sex-based or racial/ethnic-based differences were present. All experiments were performed in accordance with relevant guidelines and protocols.
Data Collection
Demographic characteristics (sex, age, height, and body weight), history of chronic disease (diabetes, hyperlipidemia, hypertension, hyperthyroidism, hypothyroidism, Hashimoto thyroiditis), long-term medication use (hypoglycemic drugs, hypolipemic agents, hypotensive drugs, antithyroidism drug, thyroxine), and characteristics of each patient’s tumor were collected in the electronic medical records system and recorded using a standardized questionnaire. Clinical hematological examination including fasting plasma glucose (FPG), glycosylated hemoglobin (HbA1c), triglyceride (TG), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), uric acid (UA), sialic acid, free triiodothyronine (FT3), free thyroxine (FT4), thyroid stimulating hormone (TSH), thyroid peroxidase antibody (TPOAb), thyroglobulin antibody (TgAb), and thyroglobulin (Tg) were also collected in the electronic medical records system. FT3, FT4, TSH, TPOAb, TgAb, and Tg were tested by electrochemical luminescence method. The rest laboratory testing were tested by Roche automatic analyzer. Body mass index (BMI) was determined by dividing weight in kilograms by height meters squared (kg/m2) and it was collected to as far back as 2 years prior to the thyroidectomy and as recently as the most recently. All the laboratory testing was tested before the thyroid surgery. Hypercholesterolemia was defined by a fasting serum total cholesterol exceeds the normal limit (total cholesterol >5.7 mmol/L, or a history of hypercholesterolemia).
Statistical Analysis
Continuous variables with normally distribution were presented as means and standard deviations (SD) and assessed by the Mann-Whitney test. Continuous variables with none normally distribution was presented as median and inter-quartile range and assessed by the Z test. Categorical variables were presented as percentages and frequencies, and the chi-square test was used for categorical variables. The association of hypercholesterolemia and risk of DTC was assessed with the use of multivariable logistic regression models. For further analysis, serum total cholesterol was included as a continuous variable in the model. We also calculated the unadjusted and adjusted odds ratio (OR) with 95% CI. Multivariable logistic regression model for age, sex, systolic blood pressure (SBP), TG, FPG, sialic acid, UA and TSH, TPOAb, TgAb, and BMI were adjusted in different models. We conducted the stratified analyses according to age, sex, UA, plasma sialic acid, TG, FPG, TPOAb, TgAb, TSH, and hypertension [both traditional and new guide (9)]. The statistical analysis was performed by Statistical Package for Social Sciences Version 19.0 software (SPSS Inc., Chicago, IL, USA) and two-side P values less than 0.05 indicate statistical significance.
Results
Characteristics of the Subjects
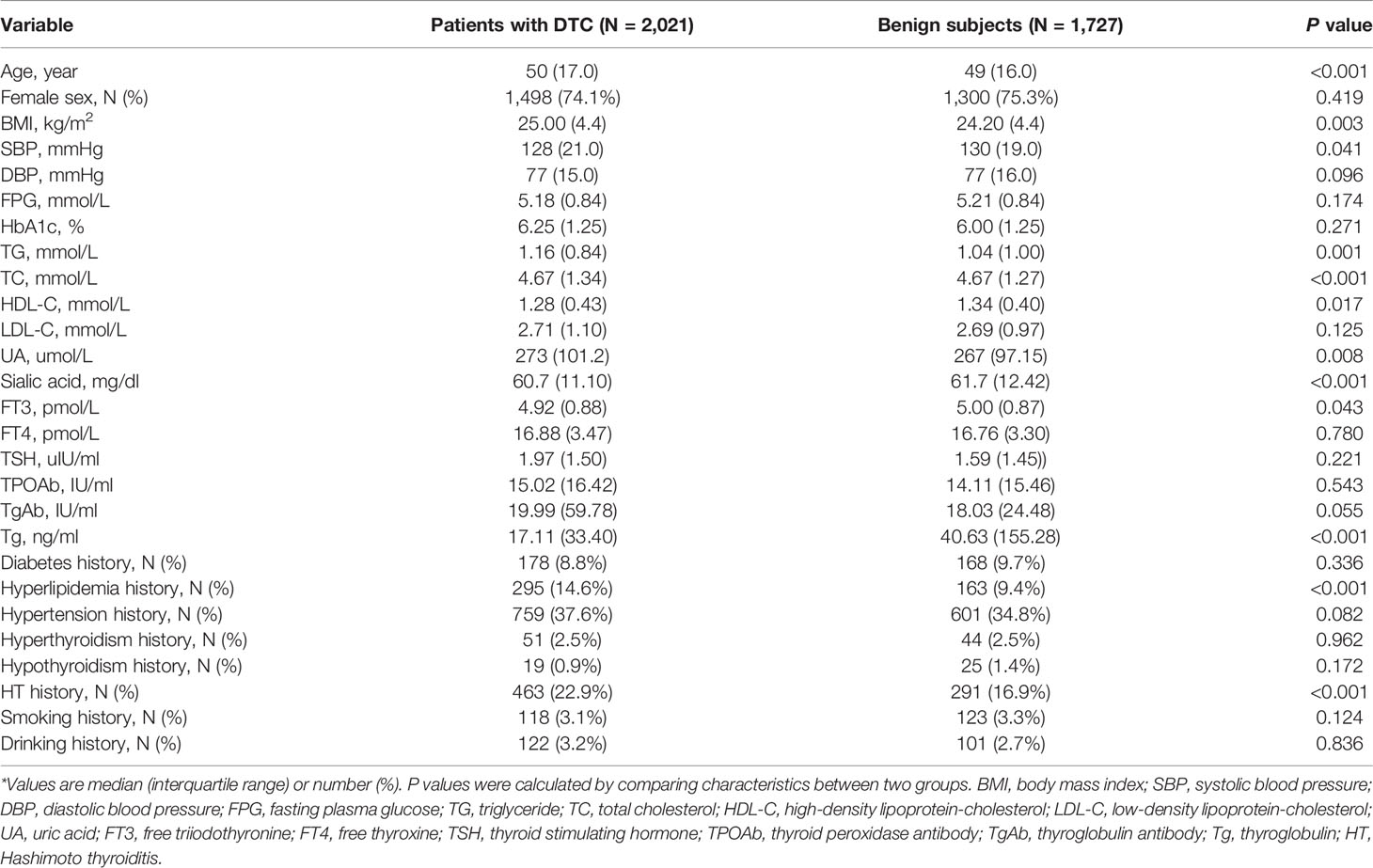
There were 2,021 DTC patients and 1,727 subjects diagnosed with benign thyroid nodules enrolled in this study. Table 1 shows the main characteristics of each group as well as their anthropometric measurements. There were statistically significant differences in age (P < 0.001), BMI (P = 0.003), SBP (P = 0.041), TG (P = 0.001), TC (P < 0.001), HDL-C (P = 0.017), UA (P = 0.008), sialic acid (P < 0.001), FT3 (P = 0.043), Tg (P < 0.001), hyperlipidemia history and HT history between the DTC group and benign group. Patients with DTC were younger than the benign subjects (mean age, 47.96 vs. 53.93 years, P < 0.001). The BMI in DTC group was higher than that in benign group (mean BMI, 25.18 vs. 24.63 kg/m2), and the difference was statistically significant (P = 0.003). Additionally, patients with DTC had a significantly higher level of serum lipid, including TG (P = 0.001), TC (P < 0.001) and HDL-C (P = 0.017), than that in patients with benign thyroid nodules. Twenty-one patients in DTC group and 17 patients in benign group took lipid-lowering therapy.
Hypercholesterolemia and Serum Total Cholesterol
Hypercholesterolemia was defined as serum total cholesterol levels higher than 5.7 mmol/L or patients who had been diagnosed as hypercholesterolemia before and were being treated with lipid-lowering drugs. Serum total cholesterol was detected in 253 (12.5%) of the 2,021 DTC patients and in 638 (36.9%) of the 1,727 benign subjects. The mean serum total cholesterol level in the DTC group was significantly higher than that in the benign group (4.79 ± VS. 4.69 mmol/L, P < 0.001, Figure 1).
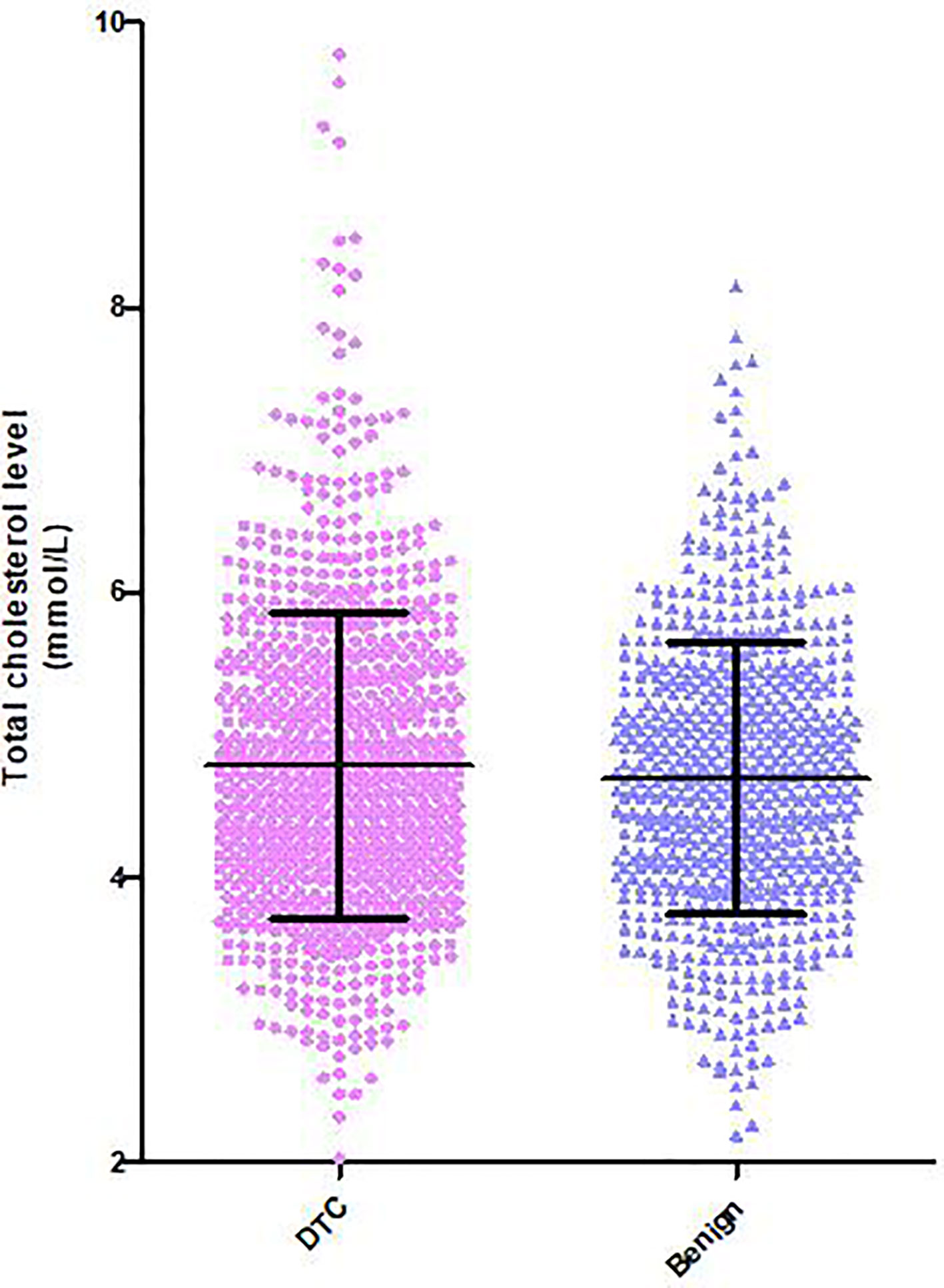
Figure 1 Distribution of serum total cholesterol levels in patients with DTC and benign thyroid nodules. Serum total cholesterol in the DTC group was significantly higher than that in patients without thyroid carcinoma.
Relationship Between Hypercholesterolemia and Risk of DTC
The stratified analysis of the association between hypercholesterolemia and the risk of DTC were done and showed in Table 2. The result of stratified analysis showed that after adjusting hypercholesterolemia, age (P < 0.001), TG (P = 0.003) and TSH (P < 0.001) are found to be confounding factors. The risk of DTC in patients younger than 45 years old is 2.08 times than that of patients older than 45 years old [OR = 0.48, 95% CI (0.38, 0.61), P < 0.001]. Furthermore, a statistical association between hypercholesterolemia and risk of DTC was revealed in patients with TG higher than 1.39 mmol/L [OR = 1.52, 95% CI (1.17, 1.97), P = 0.002]. A high TSH level is highly associated with the increased risk of DTC both when TSH is grouped by trisected and if TSH is higher than 2 uIU/ml (P < 0.001). The risk of DTC was 53% elevated in patients with TSH ≥2.00 uIU/ml than patients with TSH <2.00 uIU/ml [OR = 1.53; 95% CI (1.22, 1.90), P < 0.001].
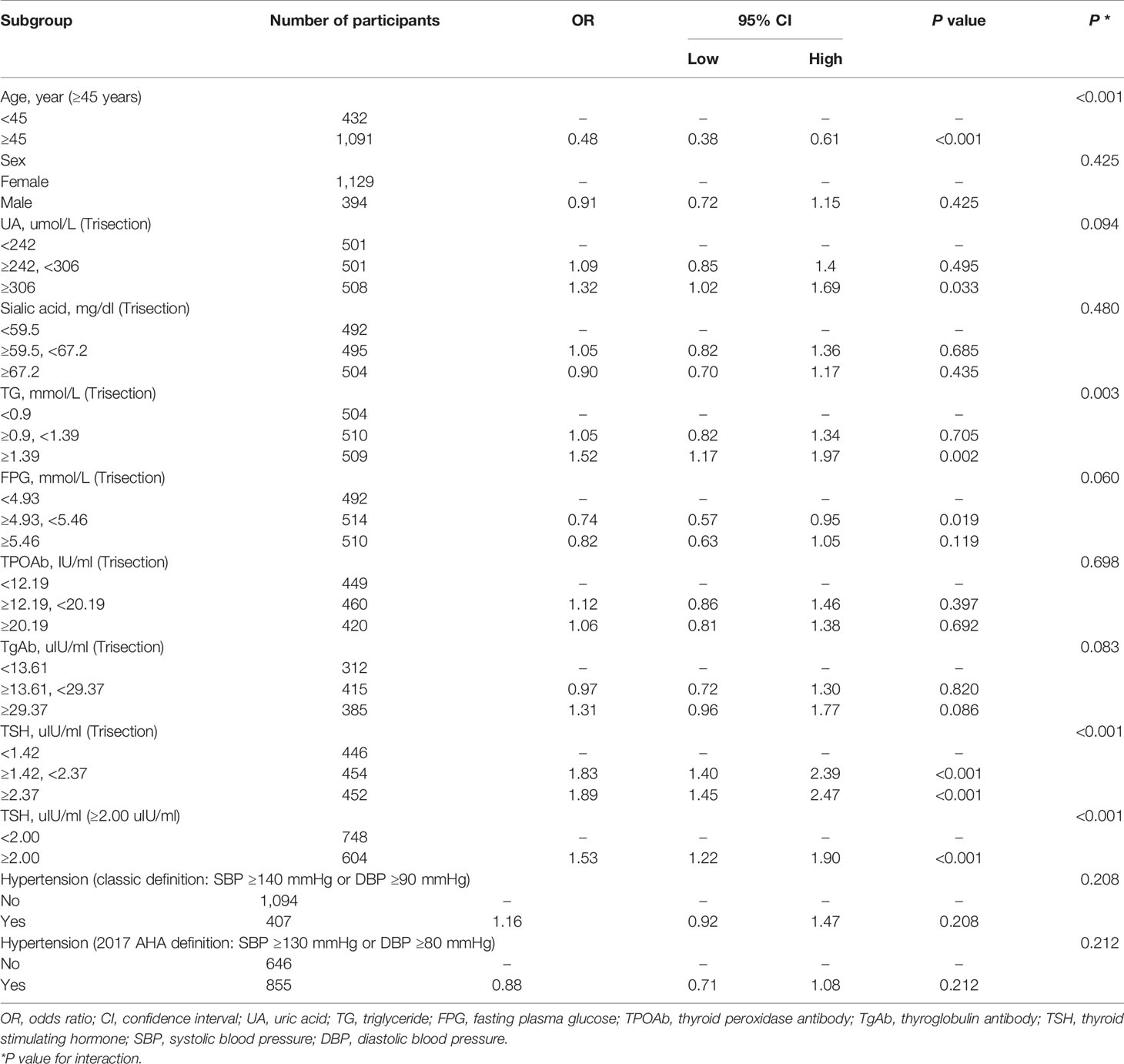
Table 2 Association between hypercholesterolemia and risk of DTC according to baseline characteristics.
Multivariable logistic regression analysis evaluating the association between serum total cholesterol and risk of DTC was shown in Table 3. The multivariate logistic regression analysis showed a significantly association between hypercholesterolemia and risk of DTC in whole participants [OR = −0.36; 95% CI (−0.64, −0.081), P=0.012]. After adjusting for a variety of possible confounders (Model I was adjusted for age, sex, SBP, TG, FPG, sialic acid, UA, and TSH. Model II was further adjusted for TPOAb, TgAb, Tg, and BMI), the correlation still remained that the absence of hypercholesterolemia could reduce the risk of thyroid nodules being malignant [Model I: OR = −0.54, 95% CI (−0.87, −0.21), P = 0.001; Model II: OR = −0.75, 95% CI (−1.39, −0.12), P = 0.02]. Thus, patients with hypercholesterolemia had a 1.33-fold (model II: 1/0.75) higher risk of DTC than those without hypercholesterolemia. Patients who took a lipid-lowering therapy were not enrolled to analysis in the following subgroup analysis. Serum total cholesterol is divided into three groups: low (<3.17 mmol/L), normal (3.17–5.7 mmol/L), and high (>5.7 mmol/L) by the normal reference range. Comparing to the high group (>5.7 mmol/L), the closer the serum total cholesterol level is to normal (3.17–5.7 mmol/L), the less risk of thyroid nodules being malignant, and this difference is statistically significant in model II [OR = −0.67, 95% CI (−1.31, −0.03), P = 0.040]. However, this difference is not found in the group of patients with lower level of total cholesterol [<3.17 mmol/L, OR = 0.43, 95% CI (−1.22, 2.09), P = 0.068], suggesting that hypocholesterolemia is not a protective factor in the risk of thyroid nodules being malignant.
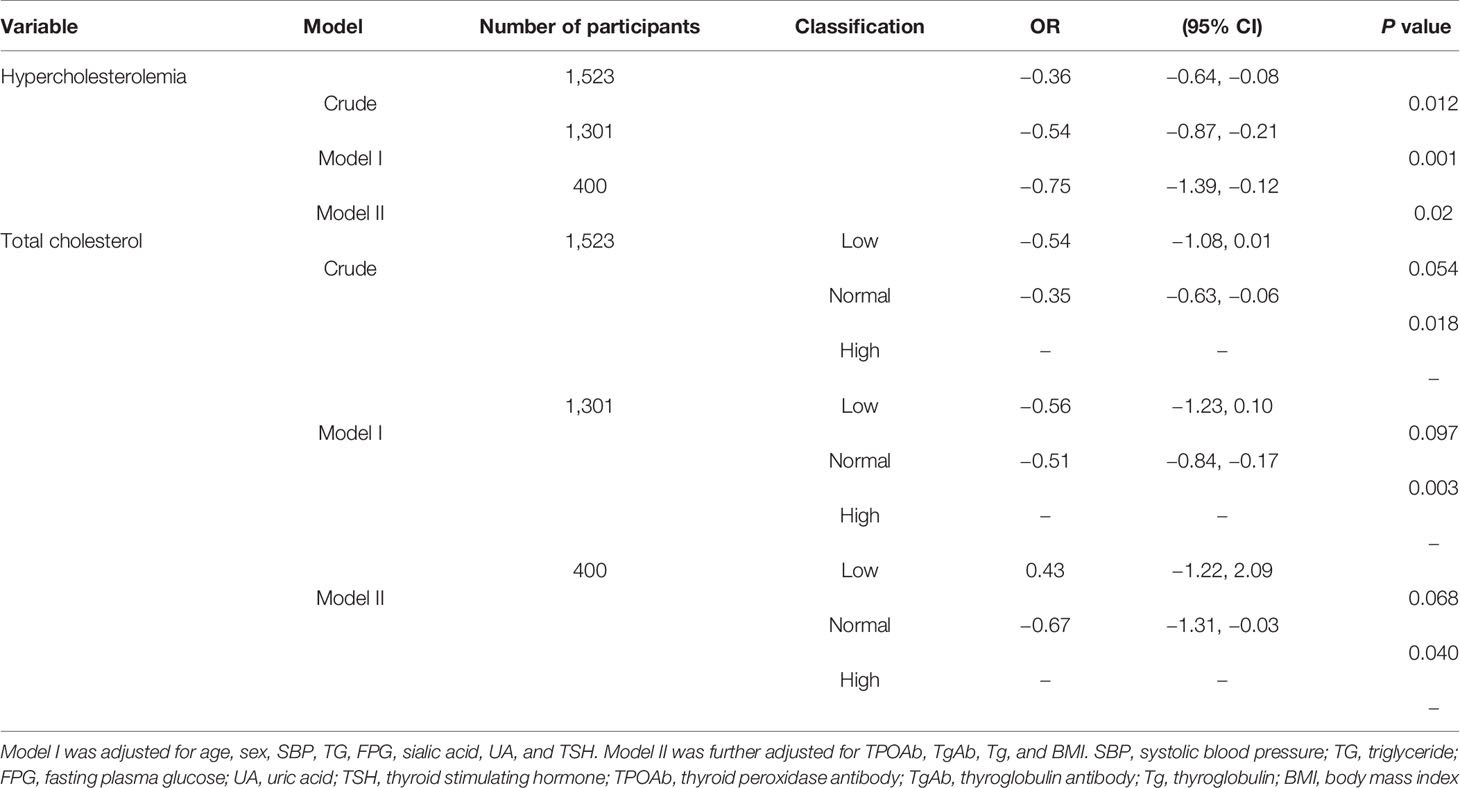
Table 3 Multivariable logistic regression analysis evaluating the association between serum total cholesterol and risk of DTC.
Discussion
In this study, we found that serum total cholesterol levels were significantly associated with risk of DTC in Chinese population. Subjects with hypercholesterolemia had a 1.33-fold higher risk of DTC than those without hypercholesterolemia. However, lower level of serum total cholesterol cannot be a protective factor in the risk of thyroid nodules being malignant.
Cholesterol, an essential component of cellular membrane and the precursor of bile acid and steroid hormones, is essential for cell proliferation. Moreover, it has been reported that cholesterol may induce an increase of tumor angiogenesis and proliferation, and a decreased apoptosis of tumor cells (10). Both increased intake and accelerated synthesis of cholesterol were found in the proliferation of malignant tumor cells (11, 12). Increasing studies indicated that high level of cholesterol is associated with various kinds of tumors, such as breast cancer (13, 14), gastric cancer (15), colorectal cancer (16, 17), prostate cancer (18, 19), and renal cancer (20). Cholesterol is the key component of lipid rafts in lipid biomolecules and it can affect the transduction of signal pathway by regulating the structure of lipid rafts. Recently, some studies indicated that lipid raft which was rich in cholesterol took part in the signal pathway of growth factor receptor in tumor cells and abnormal highly cholesterol could break the balance of lipid and protein, thus inducing an abnormal pathological signal and causing the carcinogenesis.
LDL-C and HDL-C are the main forms of cholesterol that exist in the blood and nearly 75% of cholesterol is present in the form of LDL-C. It has been reported that higher levels of LDL-C and TG were considered as the risk factor of cancer (21). A higher level of LDL-C might be associated with hematological malignant tumor (22) and a lower level of HDL-C (≤20 mg/dl) could lead to a 6.5-fold increased risk of cancer (23). Notably, LDL-C has been confirmed to be able to induce a proliferation and metastasis of breast cancer by activating ErbB2 pathway and reducing the expression of adhesion molecule (14). In addition, another potential mechanism was that increased level of LDL-C and LDL-C/HDL-C contributed to the metastasis of lymph node. HDL-C has an anti-tumor efficiency by inhibiting O2, H2O2, and other hydroxyl radicals (24). Moreover, HDL-C was supposed to inhibit the proliferation and growth of tumor by inhibiting the expression of tumor necrosis factor-α and interleukin-6, whereas it induced the apoptosis of tumor cells by promoting the expression of interleukin-10 (25). Interestingly, the expression of LDL-C receptor in tissues of breast cancer was higher than that in normal tissue, which might indicate that LDL-C was active in breast cancer cells, which might be one of the mechanisms (26).
As the incidence of thyroid cancer has increased dramatically worldwide, a large number of clinical studies and mechanistic experiments are devoted to find the potential risk factors that caused such change (27–32). However, if there is any association between hyperlipidemia and thyroid cancer remains a question. Our research focused on a large sample size of population with thyroid nodule(s) and each patient has undergone thyroid nodule surgery with the pathological confirmation. Secondly, a comprehensive clinical data is collected in the current research, including thyroid function, thyroid related antibodies, and even detailed disease history. Thirdly, not only both male and female data but also a wide range of different age groups from 12 to 86 years old, were included. Thus the founding of our study can be extended throughout both sex and to a relatively wider population. Unfortunately, there are some limitations in our study: (1) Not all of the 3,748 patients have the results of lipid measurements or other parameters that shorten the sample size, which might influence the power of estimation. (2) Body weight was collected as far back as 2 years prior to the thyroid surgery and as recently as the most recent that might cause the recall bias which could not be avoidable. These limitations should be considered in the further studies.
Cholesterol metabolism plays an important role in the occurrence of tumor development, invasion, and metastasis. Nowadays, there were many studies regarding the relationship between cholesterol metabolism and tumors, but limited studies paid attention to the thyroid cancer. Notably, this is the first clinical study demonstrating that hypercholesterolemia is an associated factor for risk of DTC in Chinese population. Furthermore, the mechanism of cholesterol and its components on the complex pathophysiological processes of these malignant tumors still unknown, and that call for further investigations. Based on the previous research and current study, we speculate that cholesterol may play a role in the malignancy risk of thyroid nodule.
Conclusions
In conclusion, hypercholesterolemia is an associated factor for risk of DTC in Chinese population. A prospective and randomized controlled trial of cholesterol lowering therapy and further basic researches on the mechanism of hypercholesterolemia and risk of DTC are needed to confirm the results.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary materials.
Ethics Statement
The studies involving human participants were reviewed and approved by Shandong Provincial Qianfoshan Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin, or by the participants themselves if they were of legal consenting age.
Author Contributions
JZ, LL, and JD conceived of and designed the study. JZ, YT, JY, HG, RZ, and HW collected and analyzed the data. JZ wrote the paper. LL and JD supervised the whole study and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Projects of Medicine and Health Science Technology Development Program in Shandong Province (grant number 2016WS0499, 2014WS0103), National Natural Science Foundation of China Grants (grant number 81570742, 81670757, 81770822) Shandong Provincial Natural Science Foundation of China Grants (grant number ZR2016HQ26, ZR2019PH025), and Grant for the development of science and technology of Jinan City (grant number 201602172). They support the study design; the collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the article for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully acknowledge the valuable cooperation of the colleague from the department of Thyroid & Breast Surgery, Shandong Provincial Qianfoshan Hospital.
References
1. Zheng R, Zeng H, Zhang S, Chen W. Estimates of cancer incidence and mortality in China, 2013. Chin J Cancer (2017) 36:66. doi: 10.1186/s40880-017-0234-3
2. Davies L, Morris LG, Haymart M, Chen AY, Goldenberg D, Morris J, et al. American association of clinical endocrinologists and American college of endocrinology disease state clinical review: The increasing incidence of thyroid cancer. Endocr Pract (2015) 21:686–96. doi: 10.4158/EP14466.DSCR
3. Tuttle RM, Haddad RI, Ball DW, Byrd D, Dickson P, Duh QY, et al. Thyroid carcinoma, version 2.2014. J Natl Compr Canc Netw (2014) 12:1671–80. doi: 10.6004/jnccn.2014.0169
4. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin (2011) 61:69–90. doi: 10.3322/caac.20107
6. Nagataki S, Nystrom E. Epidemiology and primary prevention of thyroid cancer. Thyroid (2002) 12:889–96. doi: 10.1089/105072502761016511
7. Boscoe FP, Henry KA, Sherman RL, Johnson CJ. The relationship between cancer incidence, stage and poverty in the United States. Int J Cancer (2016) 139:607–12. doi: 10.1002/ijc.30087
8. Hung SH, Lin HC, Chung SD. Statin use and thyroid cancer: a population-based case-control study. Clin Endocrinol (Oxf) (2015) 83:111–6. doi: 10.1111/cen.12570
9. Carey RM, Whelton PK. ACC/AHA Hypertension Guideline Writing Committee. Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension Guideline. Ann Intern Med (2018) 168:351–8. doi: 10.7326/M17-3203
10. Cruz P, Torres C, Ramírez ME, Epuñán MJ, Valladares LE, Sierralta WD. Proliferation of human mammary cancer cells exposed to 27-hydroxycholesterol. Exp Ther Med (2010) 1:531–6. doi: 10.3892/etm_00000084
11. Kim JH, Lee J, Jung SY, Kim J. Dietary Factors and Female Breast Cancer Risk: A Prospective Cohort Study. Nutrients (2017) 9:pii: E1331. doi: 10.3390/nu9121331
12. Lin X, Liu L, Fu Y, Gao J, He Y, Wu Y, et al. Dietary Cholesterol Intake and Risk of Lung Cancer: A Meta-Analysis. Nutrients (2018) 10:pii: E185. doi: 10.3390/nu10020185
13. Agnoli C, Grioni S, Sieri S, Sacerdote C, Ricceri F, Tumino R, et al. Metabolic syndrome and breast cancer risk: a case-cohort study nested in a multicentre italian cohort. PloS One (2015) 10:e0128891. doi: 10.1371/journal.pone.0128891
14. Rodrigues Dos Santos C, Fonseca I, Dias S, Mendes de Almeida JC. Plasma level of LDL-cholesterol at diagnosis is a predictor factor of breast tumor progression. BMC Cancer (2014) 14:132. doi: 10.1186/1471-2407-14-132
15. Ghahremanfard F, Mirmohammadkhani M, Shahnazari B, Gholami G, Mehdizadeh J. The valuable role of measuring serum lipid profile in cancer progression. Oman Med J (2015) 30:353–7. doi: 10.5001/omj.2015.71
16. Gorin A, Gabitova L, Astsaturov I. Regulation of cholesterol biosynthesis and cancer signaling. Curr Opin Pharmacol (2012) 12:710–6. doi: 10.1016/j.coph.2012.06.011
17. Wertheim BC, Smith JW, Fang C, Alberts DS, Lance P, Thompson PA. Risk modification of colorectal adenoma by CYP7A1 polymorphisms and the role of bile acid metabolism in carcinogenesis. Cancer Prev Res (Phila) (2012) 5:197–204. doi: 10.1158/1940-6207.CAPR-11-0320
18. Kitahara CM, Berrington de González A, Freedman ND, Huxley R, Mok Y, Jee SH, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol (2011) 29:1592–8. doi: 10.1200/JCO.2010.31.5200
19. Moon H, Ruelcke JE, Choi E, Sharpe LJ, Nassar ZD, Bielefeldt-Ohmann H, et al. Diet-induced hypercholesterolemia promotes androgen-independent prostate cancer metastasis via IQGAP1 and caveolin-1. Oncotarget (2015) 6:7438–53. doi: 10.18632/oncotarget.3476
20. de Martino M, Leitner CV, Seemann C, Hofbauer SL, Lucca I, Haitel A, et al. Preoperative serum cholesterol is an independent prognostic factor for patients with renal cell carcinoma (RCC). BJU Int (2015) 115:397–404. doi: 10.1111/bju.12767
21. Wuermli L, Joerger M, Henz S, Schmid HP, Riesen WF, Thomas G, et al. Hypertriglyceridemia as a possible risk factor for prostate cancer. Prostate Cancer Prostatic Dis (2005) 8:316–20. doi: 10.1038/sj.pcan.4500834
22. Shor R, Wainstein J, Oz D, Boaz M, Matas Z, Fux A, et al. Low serum LDL cholesterol levels and the risk of fever, sepsis, and malignancy. Ann Clin Lab Sci (2007) 37:343–8.
23. Shor R, Wainstein J, Oz D, Boaz M, Matas Z, Fux A, et al. Low HDL levels and the risk of death, sepsis and malignancy. Clin Res Cardiol (2008) 97:227–33. doi: 10.1007/s00392-007-0611-z
24. Liu CS, Hsu HS, Li CI, Jan CI, Li TC, Lin WY, et al. Central obesity and atherogenic dyslipidemia in metabolic syndrome are associated with increased risk for colorectal adenoma in a Chinese population. BMC Gastroenterol (2010) 10:51. doi: 10.1186/1471-230X-10-51
25. Wolk A, Larsson SC, Johansson JE, Ekman P. Long-term fatty fish consumption and renal cell carcinoma incidence in women. JAMA (2006) 296:1371–6. doi: 10.1001/jama.296.11.1371
26. Pires LA, Hegg R, Freitas FR, Tavares ER, Almeida CP, Baracat EC, et al. Effect of neoadjuvant chemotherapy on low-density lipoprotein (LDL) receptor and LDL receptor-related protein 1 (LRP-1) receptor in locally advanced breast cancer. Braz J Med Biol Res (2012) 45:557–64. doi: 10.1590/S0100-879X2012007500068
27. Schmid D, Ricci C, Behrens G, Leitzmann MF. Adiposity and risk of thyroid cancer: a systematic review and meta-analysis. Obes Rev (2015) 16:1042–54. doi: 10.1111/obr.12321
28. Choi JS, Kim EK, Moon HJ, Kwak JY. Higher body mass index may be a predictor of extrathyroidal extension in patients with papillary thyroid microcarcinoma. Endocrine (2015) 48:264–71. doi: 10.1007/s12020-014-0293-z
29. Jing Z, Hou X, Liu Y, Yan S, Wang R, Zhao S, et al. Association between height and thyroid cancer risk: a meta-analysis of prospective cohort studies. Int J Cancer (2015) 137:1484–90. doi: 10.1002/ijc.29487
30. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body Fatness and Cancer–Viewpoint of the IARC Working Group. N Engl J Med (2016) 375:794–8. doi: 10.1056/NEJMsr1606602
31. Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, et al. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev (2011) 20:464–72. doi: 10.1158/1055-9965.EPI-10-1220
Keywords: differentiated thyroid cancer, thyroid nodule, hypercholesterolemia, total cholesterol, clinical study
Citation: Zhao J, Tian Y, Yao J, Gu H, Zhang R, Wang H, Liao L and Dong J (2021) Hypercholesterolemia Is an Associated Factor for Risk of Differentiated Thyroid Cancer in Chinese Population. Front. Oncol. 10:508126. doi: 10.3389/fonc.2020.508126
Received: 29 October 2019; Accepted: 10 December 2020;
Published: 28 January 2021.
Edited by:
An Jin Zhang, Shanghai University of Medicine and Health Sciences, ChinaReviewed by:
Shu Wang, Shanghai Jiao Tong University School of Medicine, ChinaMingzhao Xing, Johns Hopkins University, United States
Copyright © 2021 Zhao, Tian, Yao, Gu, Zhang, Wang, Liao and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Liao, bGlhb2xpbkBzZHUuZWR1LmNu; Jianjun Dong, Y3djX2xsQHNkdS5lZHUuY24=
 Junyu Zhao
Junyu Zhao Yutian Tian
Yutian Tian Jinming Yao
Jinming Yao He Gu
He Gu Rui Zhang
Rui Zhang Huanjun Wang
Huanjun Wang Lin Liao
Lin Liao Jianjun Dong5*
Jianjun Dong5*