- 1Department of Otolaryngology-Head & Neck Surgery, First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 2First Clinical Medical College, Guangxi Medical University, Nanning, China
- 3Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Nanning, China
The aim of the present study was to collect published studies and compare the diagnostic accuracy of different markers for nasopharyngeal carcinoma (NPC). We systematically searched PubMed/MEDLINE, EMBASE, Cochrane Library, CNKI, and Wanfang for relevant studies until April 29, 2020. The revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool was used to evaluate the methodological quality of the studies. The sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under the curve (AUC) values of the diagnostic markers were combined by a bivariate mixed effect model to compare their diagnostic accuracy. We explored heterogeneity through meta-regression. In total, 244 records from 101 articles were included, with 49,432 total study subjects (13,109 cases and 36,323 controls). EA-IgG, Zta-IgG, and Epstein–Barr virus (EBV) DNA load in non-invasive nasopharyngeal brushings (EBV-DNA brushings) have both high sensitivity and specificity, EBNA1-IgG and VCA-IgG have only high sensitivity, and EBNA1-IgA, VCA-IgA, Rta-IgG, Zta-IgA, HSP70, and serum sialic acid (SA) have only high specificity. The bivariate mixed effect model of EA-IgA had a significant threshold effect. Meta-regression analysis showed that ethnicity affected EBNA1-IgA, EBNA1-IgG, VCA-IgA, and EBV DNA load in plasma, test methods affected EBNA1-IgG, publication year affected VCA-IgA, and sample size affected Rta-IgG. There was significant publication bias for VCA-IgA and Rta-IgG (P < 0.05). EA-IgG, Zta-IgG, and EBV-DNA brushings are good diagnostic markers for NPC. The diagnostic accuracy was influenced by publication year, sample size, test methods, and ethnicity.
Introduction
Nasopharyngeal carcinoma (NPC) is an epithelial carcinoma originating from the inner layer of the mucous membrane on the superior and lateral of the nasopharynx. Compared with other malignant tumors, the incidence rate of NPC is relatively low, but its global distribution is extremely uneven (1); more than 70% of new cases are concentrated in East and Southeast Asia (2). Because of its small primary foci, NPC has no typical clinical symptoms in the early stages (stages I and II), and the tumor easily invades adjacent tissues and organs, resulting in complex and diverse clinical symptoms; thus, most patients have already reached advanced stages (stages III and IV) when they are diagnosed (3, 4). Therefore, the screening and early diagnosis of NPC are very important.
Epstein–Barr virus (EBV) is generally considered to be one of the risk factors for NPC (5–7). EBV-related antibodies have received extensive attention as diagnostic markers for NPC, and most of them maintain increased levels for an average of 38 months at the preclinical stage (3, 8, 9), which has high diagnostic predictive value. Moreover, measuring EBV-related antibodies has the characteristics of simple sample acquisition, rapid testing, convenience, and low cost (10, 11); thus, it is widely used in the screening of NPC in endemic areas. With the development of quantitative PCR (qPCR), detecting EBV-DNA load in serum, plasma, blood cells, and nasopharyngeal exfoliated cells has gradually become one of the common diagnostic methods for NPC (12–14). At the same time, some serum tumor markers, such as heat shock protein 70 (HSP70) and sialic acid (SA), play important roles in the development of NPC (15–17). Their content in serum is closely related to the progression of NPC, which makes these markers potential diagnostic markers of NPC, and more researchers have focused on this field.
Many studies have evaluated EBV-related antibodies, EBV-DNA load, and some tumor markers in the clinical diagnosis of NPC patients. However, due to different ethnicities, sample sizes, test methods, and other factors, these studies have obtained different sensitivities and specificities. To systematically evaluate the diagnostic efficacy of these markers and compare them with each other to find markers suitable for large-scale population screening and early clinical diagnosis, we conducted this meta-analysis.
Methods
Meta-Analysis
We conducted this meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the guidance of the Cochrane Handbook for Systematic Reviews of Interventions.
Search Strategy
We systematically searched PubMed/MEDLINE, EMBASE, Cochrane Library, CNKI, and Wanfang for all relevant studies up to April 29, 2020. The search language was restricted to Chinese and English, and the key words used for the search terms included the following: (“nasopharyngeal carcinoma” OR “Carcinoma, Nasopharyngeal” OR “Carcinomas, Nasopharyngeal”), (“VCA” OR “EBNA” OR “EA” OR “Zta” OR “BZLF1” OR “Rta” OR “BELF1” OR “HSP70” OR “Serum sialic acid” OR “EBV DNA”), and (“blood” OR “serum” OR “plasma” OR “blood cell” OR “leukocyte” OR “lymphocyte” OR “brush” OR “brushings”). In addition, the reference lists of relevant studies were manually searched for potential eligible studies. The search strategy is presented in Supplementary Materials.
Study Selection and Data Extraction
To find suitable diagnostic markers for large-scale population screening and early clinical diagnosis while avoiding bias due to different experimental designs, we used the following inclusion criteria: (1) retrospective studies on the evaluation of diagnostic markers in NPC, (2) NPC was confirmed by pathological examination, (3) the control group should be healthy individuals or non-NPC patients confirmed by pathological examination, (4) the samples were peripheral blood serum, plasma, blood cells, or non-invasive nasopharyngeal brushings, and (5) the articles included sufficient data to construct a 2 × 2 table, including true positive (TP), false positive (FP), false negative (FN), and true negative (TN) counts. The exclusion criteria were as follows: (1) irrelevant topics, (2) letters, comments, editorials, conference abstracts, reviews, guidelines, and case reports, and (3) non-clinical studies, such as animal or cell experiments. Two investigators independently completed the study selection and data extraction. Any disagreements were resolved by a third investigator.
Quality Assessment
Based on the recommendations of the Cochrane Collaboration, we used the revised Quality Assessment for Studies of Diagnostic Accuracy (QUADAS-2) tool to evaluate the quality of each included study. This tool assessed the bias risk and clinical applicability of the studies based on four key areas: “patient selection,” “index test,” “reference standard,” and “flow and timing.” Three investigators independently used the quality assessment tool and flowchart to evaluate the studies, and any differences were resolved by consensus.
Statistical Analysis
We performed statistical analysis and analyzed each NPC diagnostic marker separately and then compared their sensitivity, specificity, and area under the curve (AUC) values. Specifically, first, the Pearson coefficient was used to evaluate the threshold effect, and then a bivariate mixed effect model was used. We calculated the following parameters and their 95% confidence intervals (CIs): sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and AUC values of the summary receiver operating characteristic (SROC) curve. Then, a forest plot of sensitivity and specificity was drawn. We examined the forest plot and combined the Q test and I2 statistic to check the heterogeneity within studies. P < 0.05 and I2 > 50% indicated significant heterogeneity. Fagan's plot was used to test the relationship between the pre-test probability and post-test probability. Sensitivity analysis consisted of the following experiments: a graphical depiction of residual-based goodness-of-fit and a chi-squared probability plot of squared Mahalanobis distances were used for the assessment of the bivariate normality assumption, a spike plot was used to check observations that affect Cook's distance, and outliers of standardized predicted random effects were checked by scatter plots. Finally, publication bias was checked through Deek's funnel plot. P < 0.05 was considered significant. The statistical analyses were performed using Stata version 16.0 (Stata Corp, College Station, Texas, USA) and Review Manager 5.3 (The Nordic Cochrane Center, Copenhagen).
Results
Study Selection and General Characteristics
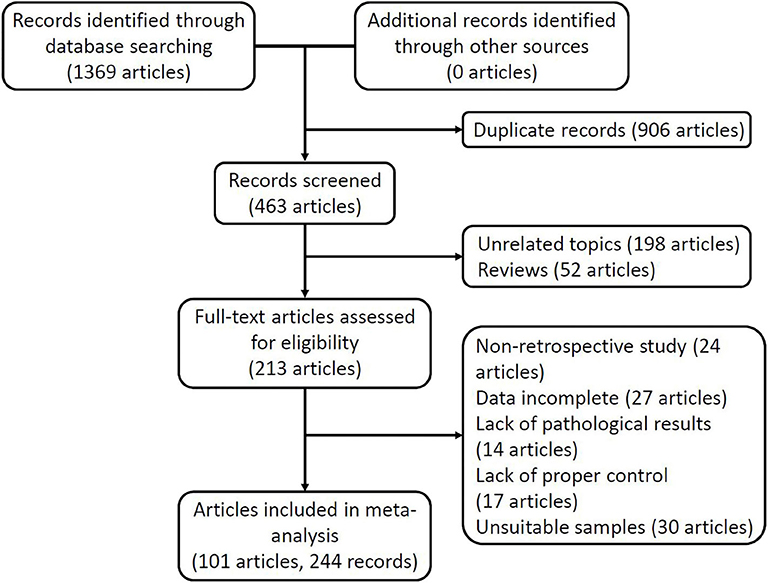
Figure 1 shows the flow of the study selection and data extraction processes. Based on the search strategy, we obtained 1,369 articles from five online electronic databases. After excluding 906 articles with duplicate data and publications, the titles and abstracts of the remaining 463 articles were checked, and 198 articles unrelated to the subject of the study and 52 review articles were excluded. We downloaded the full text of 213 articles for further review and excluded 24 non-retrospective articles, 27 articles that could not provide enough information to construct a complete 2 × 2 table, 14 articles that lacked pathological examination, 17 articles that lacked an appropriate control group, and 30 articles that did not meet the requirements of the sample. Finally, 244 records from 101 articles remained for meta-analysis, with a total sample size of 49,432 study subjects (13,109 cases and 36,323 controls). These studies were published from 1994 to 2019.
Quality Assessment
QUADAS-2 was used to evaluate the methodological quality of the included study, and the detailed quality evaluation form is provided in Supplementary Materials. The results showed that the bias risk mainly came from patient selection. Some studies did not explain whether patients were included in a continuous or random way, for which only “unclear” or “high risk” was given. Some studies did not include all cases. Additionally, a small number of studies did not set a threshold for testing in advance. Overall, the quality of the included studies was high.
Pooled Results
Table 1 shows the combined results of the sensitivity, specificity, PLR, NLR, DOR, AUC values, pre-test probability, and post-test probability of each diagnostic marker. Generally, when PLR is >10 and NLR is <0.1, the diagnostic marker can obtain high efficiency, and accordingly, the DOR and post-test probability will increase. It should be noted that in the effect model of EA-IgA, the threshold effect test P < 0.001, and the correlation was −0.02 after the SROC curve was fit, and the data points in the figure were checked, showing a significant “shoulder arm” shape (Figure 2A), which suggests that there may be a significant threshold effect between the included studies. When there is a significant threshold effect in the effect model, the combined results between the individual effect quantities become unstable, and more attention should be paid to the SROC curves and AUC values. No significant threshold effect was found in the effect models of the other diagnostic markers. If we define ≥85% as a high level and <75% as a low level, then the combined sensitivity and specificity of EA-IgG, Zta-IgG, and EBV-DNA brushings were at a high level, EBNA1-IgG and VCA-IgG had only combined sensitivity at a high level, EBNA1-IgA, VCA-IgA, Rta-IgG, Zta-IgA, HSP70, and SA had only combined specificity at a high level, and EBV-DNA peripheral blood cells (PMB) had both combined sensitivity and specificity at a low level. Combined with other effects, EA-IgG, EA-IgG, and EBV-DNA brushings are good diagnostic markers for NPC. The SROC curves of the diagnostic markers are listed in Figure 2 for reference, and other plots not listed can be found in Supplementary Materials.
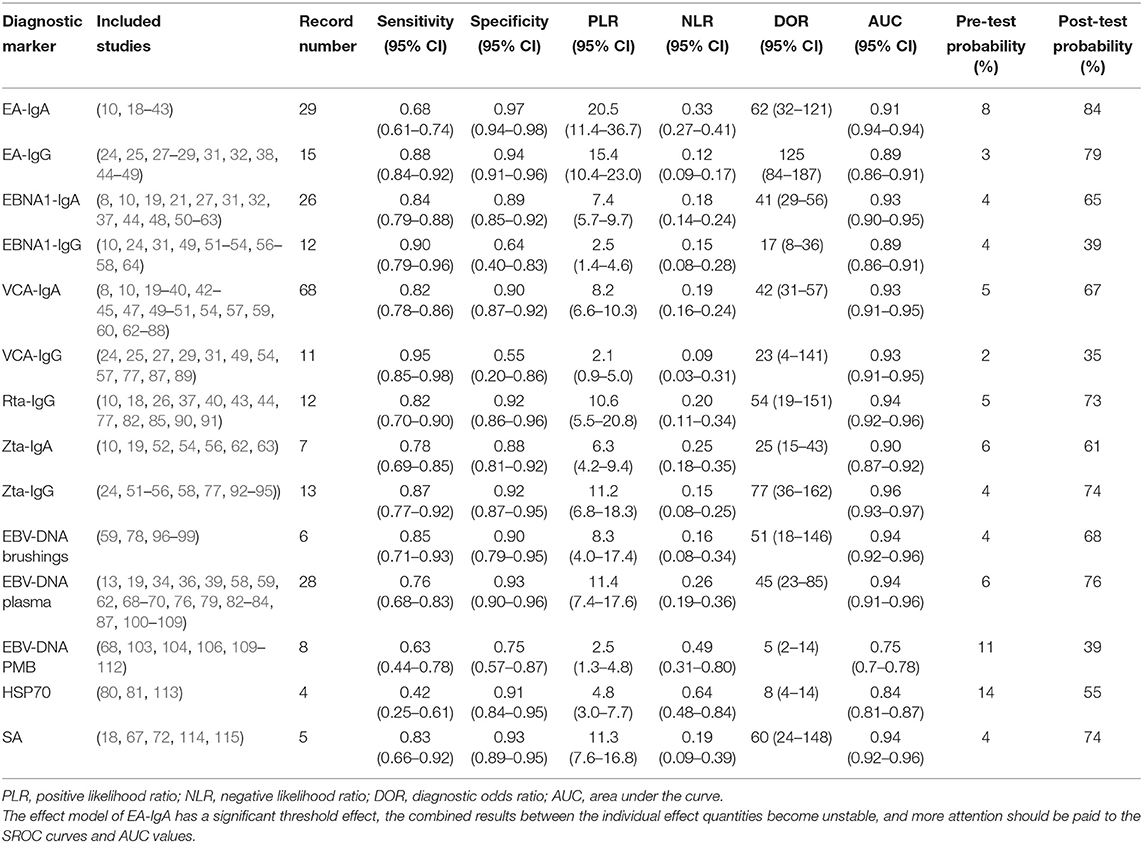
Table 1. Combined results of sensitivity, specificity, PLR, NLR, DOR, AUC values, pre-test probability, and post-test probability of each diagnostic marker.
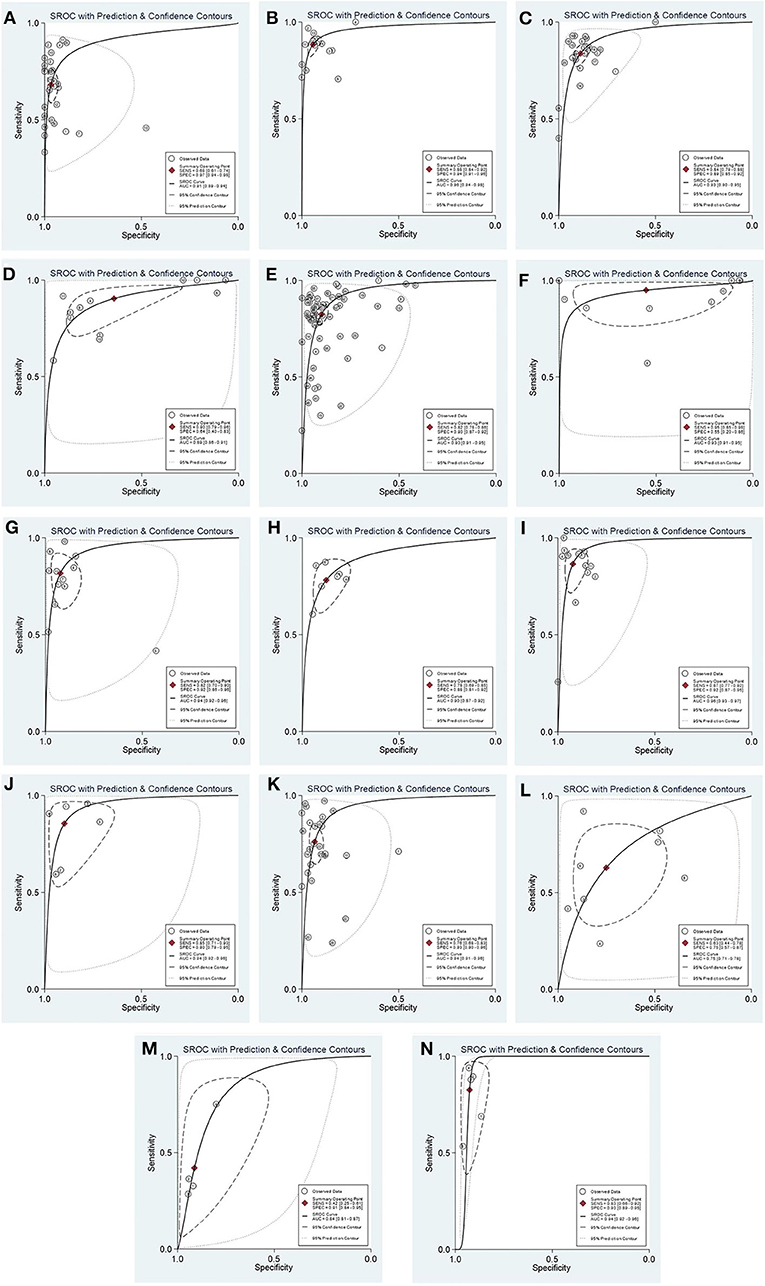
Figure 2. The SROC curves of each diagnostic marker for NPC. EA-IgA (A); EA-IgG (B); EBNA1-IgA (C); EBNA1-IgG (D); VCA-IgA (E); VCA-IgG (F); Rta-IgG (G); Zta-IgA (H); Zta-IgG (I); EBV-DNA brushings (J); EBV-DNA plasma (K); EBV-DNA PMB (L); HSP70 (M); SA (N). The curve of EA-IgA (A) showed a significant “shoulder arm” shape, suggesting that there may be a significant threshold effect between the included studies.
Heterogeneity, Sensitivity Analysis, and Publication Bias
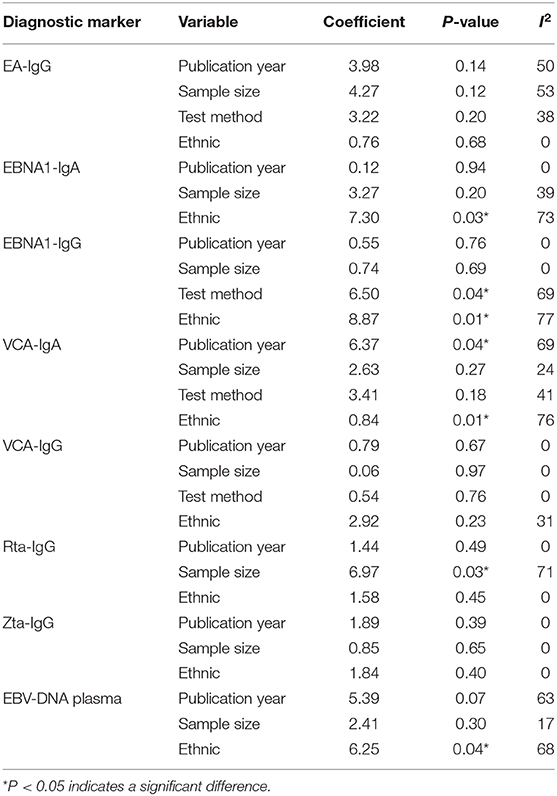
Because only the combined specificity in SA and combined sensitivity in EA-IgG and EA-IgA showed low heterogeneity, we explored the potential sources of heterogeneity. When the number of articles included in a meta-analysis is <10, meta-regression analysis is not recommended in the Cochrane Handbook for Systematic Reviews of Interventions. Additionally, EA-IgA had a significant threshold effect, so we only analyzed the sensitivity (Figure 3) and publication bias of EA-IgA, EBV-DNA brushings, EBV-DNA PMB, HSP70, and SA, whereas other diagnostic markers were additionally analyzed by meta-regression analysis. The goodness-of-fit and bivariate normality of the sensitivity analysis show the studies along the reference line, whereas the influence analysis and outlier detection show which studies have a significant impact on the effect model. Only a few studies were beyond the scope of the reference line, and the results after removing these studies one by one did not change significantly. This indicates that the effect model is stable, and few studies affect the pooling results. The meta-regression analysis mainly included the year of publication, sample size, test method, and ethnicity. The results are shown in Table 2. We found that ethnicity (Asian vs. non-Asian) has an effect on EBNA1-IgA, EBNA1-IgG, VCA-IgA, and EBV-DNA plasma, and the test method [ELISA vs. indirect fluorescent antibody (IFA)] affects EBNA1-IgG. Publication year (≥median vs. < median) affects VCA-IgA, whereas sample size (≥median vs. < median) affects Rta-IgG. The asymmetry in Deek's funnel plot showed that there was significant publication bias for VCA-IgA and Rta-IgG (P < 0.05), but no significant publication bias was found in the other effect models.
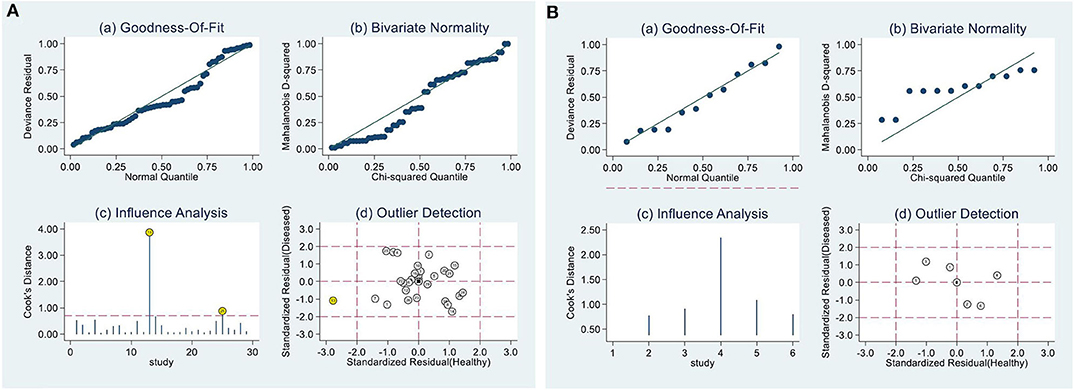
Figure 3. Sensitivity analysis. (A) Result of EA-IgG, (B) EBV-DNA brushings. (A) and (B) are composed of 4 parts, goodness-of-fit (a), bivariate normality (b), influence analysis (c) and outlier detection (d) show that only a few studies are beyond the scope of the reference line, indicating that the effect model is stable.
Discussion
By meta-analysis of published articles, we compared the diagnostic efficacy of 14 diagnostic markers for NPC. VCA-IgG had the highest combined sensitivity, EA-IgG had the highest combined specificity, and Zta-IgG had the highest combined AUC values. In general, the combined sensitivity, specificity, and diagnostic efficacy of EA-IgG, Zta-IgG, and EBV-DNA brushings are at a high level, and their sample acquisition methods are relatively simple. Additionally, the detection process is fast and inexpensive, which has high practical value in large-scale population screening and early clinical diagnosis.
At present, antibodies against EBV mainly include early antigen (EA), nuclear antigen 1 (EBNA1), viral capsid antigen (VCA), Rta protein encoded by the EBV immediate early gene BRLF1, and Zta protein encoded by the BZLF1 gene. When EBV is in incubation period, only EBNA1 can induce a strong antibody response (50). After entering the lysis cycle, Rta and Zta are encoded first, followed by EA. VCA is expressed only at the end of the EBV proliferation cycle (116), and the expression of these proteins will cause a strong antibody response in patients (10). In the preclinical stage, EBV replicates in the body of patients, and antibodies will remain at a high level, making these antibodies potential early diagnostic markers for NPC. Regarding the test method, compared with IFA, ELISA has the advantages of low cost and a standardized operation process (117, 118); moreover, the interpretation of the results is not affected by the subjective judgment of researchers, so it is easier to use in large-scale population screening.
The expression levels of different antibodies can indirectly reflect the replication of EBV, and measuring the EBV-DNA load provides a more intuitive reference. In 1999, Lo et al. (14) first proposed the use of qPCR to diagnose NPC by detecting the EBV-DNA load in plasma, and this method has good performance in disease development monitoring and prognosis prediction (119–121). Subsequently, related research on EBV-DNA load in blood cells and nasopharynx exfoliated cells was carried out successively and performed well. The BamH1-W sequence is a mature and reliable laboratory EBV-DNA detection method recommended by the WHO (122), and most primers are designed based on this sequence. With the commercialization and promotion of standardized test kits, the cost of learning and using this test method has been greatly reduced; thus, this test can be extensively applied in large-scale population screening and early clinical diagnosis.
During the occurrence and development of tumors, tumor cells express tumor antigens that are different from normal cells, and the immune system recognizes tumor antigens and produces autoantibodies (123, 124). SA is the acetylation product of neuraminic acid, an important component of cell membrane surface receptors. It has been indicated to be closely related to the occurrence, development, and metastasis of head and neck tumors (18, 125). The mechanism of abnormally increased SA may be that the glycoprotein and glycolipid on the cell membrane fall off and enter the blood circulation when the tumor cell structure is destroyed by immune cells (126). HSP70 is a highly effective inhibitor of apoptosis. Its basic expression level is very low, but it is highly expressed in a variety of tumors, such as NPC, breast cancer, and renal cell carcinoma (127, 128), and is considered an important marker of tumor occurrence and prognosis (129–131). The efficacy of serum tumor markers in the early diagnosis of NPC is attracting researchers' attention.
In addition, an increasing number of studies have pointed out that microRNAs, lncRNAs, and circRNAs are closely related to the physiological and pathological processes of NPC (132–134). Although these noncoding RNAs do not have the function of translating and coding proteins, they can inhibit the translation or degradation of target mRNAs through complete or incomplete complementary pairing, regulating downstream protein expression or signaling pathways; thus, they participate in the process of cell proliferation and differentiation. The abnormal expression of specific non-coding RNAs plays an important role in the occurrence and development of NPC and may become potential diagnostic markers.
In the screening and early diagnosis of NPC, it is important to reduce the missed diagnosis rate as much as possible and control the misdiagnosis rate within the acceptable range. Some prospective and retrospective studies have indicated that a combination of multiple diagnostic markers can achieve high sensitivity compared with using only a single diagnostic marker (10, 19–21, 44). When the patient obtains a positive result in screening or early diagnosis, further imaging and pathological examination and frequent follow-up visits can accurately determine whether the patient has NPC. Therefore, exploring the best combination of diagnostic markers will be the focus of future NPC screening and early diagnosis research.
There are some limitations in this study. First, the global distribution of NPC is uneven. Most of the articles we included were from Asia; thus, whether our results are applicable in non-NPC endemic areas needs to be further confirmed. Second, most of the case groups in the study included NPC patients in different stages, and the level of diagnostic markers among them may be different. Third, the control group was not entirely composed of healthy people and included some patients with non-NPC head and neck diseases, which may have a certain impact on the actual results. Fourth, other diseases caused by EBV (including infectious mononucleosis, Burkitt's lymphoma, etc.) may also lead to increased EBV-related antibodies, and the results of EBV-DNA load are FPs. Finally, our results showed heterogeneity. We conducted a meta-regression analysis of the publication year, sample size, test method, and ethnicity, but these items cannot fully explain the source of heterogeneity, and further research is needed.
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.
Author Contributions
YF and WX: conceptualization and validation. YF, WX, and GH: methodology and literature search. YF: software. YF and RK: writing—original draft preparation. LL, MX, and AT: writing—review and editing. XY: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of China (grant number 81760188).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01779/full#supplementary-material
References
1. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. (2019) 394:64–80. doi: 10.1016/S0140-6736(19)30956-0
2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136:E359–86. doi: 10.1002/ijc.29210
3. Cao SM, Liu Z, Jia WH, Huang QH, Liu Q, Guo X, et al. Fluctuations of epstein-barr virus serological antibodies and risk for nasopharyngeal carcinoma: a prospective screening study with a 20-year follow-up. PloS ONE. (2011) 6:e19100. doi: 10.1371/journal.pone.0019100
4. Leong YH, Soon YY, Lee KM, Wong LC, Tham IWK, Ho FCH. Long-term outcomes after reirradiation in nasopharyngeal carcinoma with intensity-modulated radiotherapy: a meta-analysis. Head Neck. (2018) 40:622–31. doi: 10.1002/hed.24993
5. Sarmiento MP, Mejia MB. Preliminary assessment of nasopharyngeal carcinoma incidence in the philippines: a second look at published data from four centers. Chin J Cancer. (2014) 33:159–64. doi: 10.5732/cjc.013.10010
6. Jia WH, Collins A, Zeng YX, Feng BJ, Yu XJ, Huang LX, et al. Complex segregation analysis of nasopharyngeal carcinoma in guangdong, China: evidence for a multifactorial mode of inheritance (complex segregation analysis of NPC in China). Eur J Hum Genet. (2005) 13:248–52. doi: 10.1038/sj.ejhg.5201305
7. Chien YC, Chen JY, Liu MY, Yang HI, Hsu MM, Chen CJ, et al. Serologic markers of epstein-barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med. (2001) 345:1877–82. doi: 10.1056/NEJMoa011610
8. Gao R, Wang L, Liu Q, Zhang LF, Ye YF, Xie SH, et al. Evaluation of seven recombinant VCA-IgA ELISA kits for the diagnosis of nasopharyngeal carcinoma in China: a case-control trial. BMJ Open. (2017) 7:e013211. doi: 10.1136/bmjopen-2016-013211
9. Chen MR, Liu MY, Hsu SM, Fong CC, Chen CJ, Chen IH, et al. Use of bacterially expressed EBNA-1 protein cloned from a nasopharyngeal carcinoma (NPC) biopsy as a screening test for NPC patients. J Med Virol. (2001) 64:51–7. doi: 10.1002/jmv.1017
10. Liu Y, Huang Q, Liu W, Liu Q, Jia W, Chang E, et al. Establishment of VCA and EBNA1 IgA-based combination by enzyme-linked immunosorbent assay as preferred screening method for nasopharyngeal carcinoma: a two-stage design with a preliminary performance study and a mass screening in southern China. Int J Cancer. (2012) 131:406–16. doi: 10.1002/ijc.26380
11. Liu Z, Ji MF, Huang QH, Fang F, Liu Q, Jia WH, et al. Two epstein-Barr virus-related serologic antibody tests in nasopharyngeal carcinoma screening: results from the initial phase of a cluster randomized controlled trial in Southern China. Am J Epidemiol. (2013) 177:242–50. doi: 10.1093/aje/kws404
12. Shotelersuk K, Khorprasert C, Sakdikul S, Pornthanakasem W, Voravud N, Mutirangura A. Epstein-Barr virus DNA in serum/plasma as a tumor marker for nasopharyngeal cancer. Clin Cancer Res. (2000) 6:1046–51.
13. Hsiao JR, Jin YT, Tsai ST. Detection of cell free epstein-barr virus DNA in sera from patients with nasopharyngeal carcinoma. Cancer. (2002) 94:723–9. doi: 10.1002/cncr.10251
14. Lo YM, Chan LY, Lo KW, Leung SF, Zhang J, Chan AT, et al. Quantitative analysis of cell-free epstein-barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. (1999) 59:1188−91.
15. Rucksaken R, Pairojkul C, Pinlaor P, Khuntikeo N, Roytrakul S, Selmi C, et al. Plasma autoantibodies against heat shock protein 70, enolase 1 and ribonuclease/angiogenin inhibitor 1 as potential biomarkers for cholangiocarcinoma. PloS ONE. (2014) 9:e103259. doi: 10.1371/journal.pone.0103259
16. Xu YW, Peng YH, Chen B, Wu ZY, Wu JY, Shen JH, et al. Autoantibodies as potential biomarkers for the early detection of esophageal squamous cell carcinoma. Am J Gastroenterol. (2014) 109:36–45. doi: 10.1038/ajg.2013.384
17. Wongkham S, Bhudhisawasdi V, Chau-in S, Boonla C, Muisuk K, Kongkham S, et al. Clinical significance of serum total sialic acid in cholangiocarcinoma. Clin Chim Acta. (2003) 327:139–47. doi: 10.1016/S0009-8981(02)00371-6
18. Xia C, Zhu K, Zheng G. Expression of EBV antibody EA-IgA, Rta-IgG and VCA-IgA and SA in serum and the implication of combined assay in nasopharyngeal carcinoma diagnosis. Int J Clin Exp Pathol. (2015) 8:16104–10.
19. Chan KH, Gu YL, Ng F, Ng PS, Seto WH, Sham JS, et al. EBV specific antibody-based and DNA-based assays in serologic diagnosis of nasopharyngeal carcinoma. Int J Cancer. (2003) 105:706–9. doi: 10.1002/ijc.11130
20. Chen H, Chen S, Lu J, Wang X, Li J, Li L, et al. Multiparametric detection of antibodies against different EBV antigens to predict risk for nasopharyngeal carcinoma in a high-risk population of china. Cancer Prevent Res. (2017) 10:542–50. doi: 10.1158/1940-6207.CAPR-17-0035
21. Coghill AE, Hsu WL, Pfeiffer RM, Juwana H, Yu KJ, Lou PJ, et al. Epstein-Barr virus serology as a potential screening marker for nasopharyngeal carcinoma among high-risk individuals from multiplex families in Taiwan. Cancer Epidemiol Biomarkers Prevent. (2014) 23:1213–9. doi: 10.1158/1055-9965.EPI-13-1262
22. Zou ZM, Cai WM, Cao Y, Liu YY, Xu GZ, Hu YH. EB virus antibody evaluation in the diagnosis and differential diagnosis of nasopharyngeal carcinoma: a double-blind study of 200 cases. J Med Res. (1995) 02:16–20.
23. Low WK, Leong JL, Goh YH, Fong KW. Diagnostic value of Epstein-Barr Viral Serology in nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. (2000) 123:505–7. doi: 10.1067/mhn.2000.108201
24. Dardari R, Khyatti M, Benider A, Jouhadi H, Kahlain A, Cochet C, et al. Antibodies to the epstein-Barr virus transactivator protein (ZEBRA) as a valuable biomarker in young patients with nasopharyngeal carcinoma. Int J Cancer. (2000) 86:71–5. doi: 10.1002/(SICI)1097-0215(20000401)86:1<71::AID-IJC11>3.0.CO;2-1
25. Hsu MM, Hsu WC, Sheen TS, Kao CL. Specific IgA antibodies to recombinant early and nuclear antigens of Epstein-Barr virus in nasopharyngeal carcinoma. Clin Otolaryngol Allied Sci. (2001) 26:334–8. doi: 10.1046/j.1365-2273.2001.00489.x
26. Feng P, Chan SH, Soo MYR, Liu D, Guan M, Ren EC, et al. Antibody response to epstein-Barr virus Rta protein in patients with nasopharyngeal carcinoma a new serologic parameter for diagnosis. Cancer. (2001) 92:1872–80. doi: 10.1002/1097-0142(20011001)92:7<1872::AID-CNCR1704>3.0.CO;2-N
27. Dardari R, Hinderer W, Lang D, Benider A, El Gueddari B, Joab I, et al. Antibody responses to recombinant epstein-Barr virus antigens in nasopharyngeal carcinoma patients: complementary test of ZEBRA protein and early antigens p54 and p138. J Clin Microbiol. (2001) 39:3164–70. doi: 10.1128/JCM.39.9.3164-3170.2001
28. Zhang CQ, Zong YS, Huang BZ, Xu Y, Ye YZ, Feng KT, et al. Enhancing the efficiency of epstein-Barr viral serologic test in the diagnosis of nasopharyngeal carcinoma. Chinesejournalofoncology. (2002) 24:35602e. doi: 10.3760/j.issn:0253-3766.2002.04.013
29. Wong MM, Lye MS, Cheng HM, Sam CK. Epstein-Barr virus serology in the diagnosis of nasopharyngeal carcinoma. Asian Pacific J Allergy Immunol. (2005) 23:65–7.
30. Tang JW, Rohwader E, Chu IM, Tsang RK, Steinhagen K, Yeung AC, et al. Evaluation of epstein-Barr virus antigen-based immunoassays for serological diagnosis of nasopharyngeal carcinoma. J Clin Virol. (2007) 40:284–8. doi: 10.1016/j.jcv.2007.09.006
31. Paramita DK, Fachiroh J, Haryana SM, Middeldorp JM. Evaluation of commercial EBV recombLine assay for diagnosis of nasopharyngeal carcinoma. J Clin Virol. (2008) 42:343–52. doi: 10.1016/j.jcv.2008.03.006
32. Gu AD, Mo HY, Xie YB, Peng RJ, Bei JX, Peng J, et al. Evaluation of a multianalyte profiling assay and an enzyme-linked immunosorbent assay for serological examination of epstein-barr virus-specific antibody responses in diagnosis of nasopharyngeal carcinoma. Clin Vaccine Immunol. (2008) 15:1684–8. doi: 10.1128/CVI.00135-08
33. Zhan SB, Zhong JM, Mai ZP, Ye SQ, Zhou L, Zeng Y, et al. Improve onserological diagonsis method of nasopharynegal carcinoma. Chinese J Exp Clin Virol. (2009) 23:65009. doi: 10.3760/cma.j.issn.1003-9279.2009.01.023
34. Luo YL, Ou GP, Chi PD, Liang YN, Liu YH, Huang MY. Combined determination of Epstein-Barr virus-related antibodies and antigens for diagnosis of nasopharyngeal carcinoma. Chinese J Cancer. (2009) 28:96–9. doi: 10.3321/j.issn:1000-467X.2009.01.019
35. Chen Y, Liu GY, Yao K, Xu JJ, Lu SQ, Cheng R, et al. Quantitative assay of Epstein-Barr virus specific VCA-IgA and EA-IgA antibodies for diagnosing nasopharyngeal carcinoma. Acta Univ Med Nanjing. (2009) 29:1638–638. doi: 10.7655/j.issn.1007-4368.2009.08.029
36. Tan YJ, Su XK, Cui JH, Qu WH, Xue XY. Comparison of detection of serum VCA-IgA, EA-IgA and EBV-DNA in nasopharygeal carcinoma patients. Chongqing Medical. (2010) 39:70310i 6. doi: 10.3969/j.issn.1671-8348.2010.06.030
37. Cai YL, Zheng YM, Wang W, Wei Y, Sheng XX, Cheng JR, et al. Combined detection of Epstein-Barr virus antibodies for serodiagnosis of nasopharyngeal carcinoma. J South Med Univ. (2010) 30:2746–8.
38. Zhou Y, Zhu H. Serum epstein-barr virus antibody test for clinical diagnosis of nasopharyngeal carcinoma. J Fourth Mil Med Univ. (2009) 30:1102.
39. Chen H, Luo YL, Zhang L, Tian LZ, Feng ZT, Liu WL. EA-D p45-IgG as a potential biomarker for nasopharyngeal carcinoma diagnosis. Asian Pacific J Cancer Prev. (2013) 14:7433–8. doi: 10.7314/APJCP.2013.14.12.7433
40. Zhang XL, Zhou JL, Cao YP. The value of detection of three anti-Epstein-Barr viral antibodies for nasopharyngeal carcinoma diagnosis. Chinese J Lab Med. (2015) 38:111–4. doi: 10.4103/0366-6999.147829
41. Akbar A, Fachiroh J, Paramita DK. IgA responses against epstein-barr virus early antigen (EBV-EA) peptides as potential candidates of nasopharyngeal carcinoma detection marker. BMC Proceedings. (2016) 10 (1 Suppl. 1). doi: 10.1186/s12919-016-0001-5
42. Zheng XH, Lu LX, Cui C, Chen MY, Li XZ, Jia WH. Epstein-Barr virus mir-bart1-5p detection via nasopharyngeal brush sampling is effective for diagnosing nasopharyngeal carcinoma. Oncotarget. (2016) 7:4972–80. doi: 10.18632/oncotarget.6649
43. Yi XH, Lai HC, Liu JZ, Lin SC, Li C, Chen XQ, et al. The combined interpretation scheme including VCA-IgA, EA-IgAand Rta-IgG in the diagnosis of nasopharyngeal carcinoma. J Clin Otorhinolaryngol Head Neck Surg. (2018) 32:1740–74. doi: 10.13201/j.issn.1001-1781.2018.22.014
44. Ai P, Wang T, Zhang H, Wang Y, Song C, Zhang L, et al. Determination of antibodies directed at EBV proteins expressed in both latent and lytic cycles in nasopharyngeal carcinoma. Oral Oncol. (2012) 49:326–31. doi: 10.1016/j.oraloncology.2012.10.001
45. Zhang CQ, Xiao XB, Li JL, Huang BZ, Feng KT, Ye YZ, et al. The significance of detection for EBV lgG/EA Antibody by ELISA in nasopharyngeal carcinoma (NPC) serologic diagnosis. Chinese J Cancer. (1998) 44-5+8.
46. Xiao XB, Zhang CQ, Huang TB, Zhang F, Li JL, Feng KT, et al. Application of epstein-barr virus early antigen IgG antibody in nasopharyngeal carcinoma screening. Chinese J Otorhinolaryngol. (2001) 36:309. doi: 10.3760/j.issn:1673-0860.2001.04.023
47. Huang JY. The significance of EB virus IgG EA antibody in serological diagnosis of nasopharyngeal carcinoma by ELISA. Chinese J Immunol. (2002) 18:142.
48. Zhang CQ, Zong YS, Shun Y, Zhang Y, Lin SX, Ye YZ, et al. Value of EBNA1-IgA and EA-IgG in serological diagnosis of nasopharyngeal carcinoma. Chinese J Oncol. (2004) 26:482–4. doi: 10.3760/j.issn:0253-3766.2004.08.011
49. Fei XY, Xu YH, Li T. Application of epstein-barr virus antibody detection in diagnosis of nasopharyngeal carcinoma and infectious mononucleosis. Acta Univ Med Anhui. (2018) 53:27519. doi: 10.19405/j.cnki.issn1000-1492.2018.02.024
50. Fachiroh J, Paramita DK, Hariwiyanto B, Harijadi A, Dahlia HL, Indrasari SR, et al. Single-assay combination of epstein-barr virus (EBV) EBNA1- and viral capsid antigen-p18-derived synthetic peptides for measuring anti-EBV immunoglobulin G (IgG) and IgA antibody levels in sera from nasopharyngeal carcinoma patients: options for field screening. J Clin Microbiol. (2006) 44:1459–67. doi: 10.1128/JCM.44.4.1459-1467.2006
51. Cheng WM, Chan KH, Chen HL, Luo RX, Ng SP, Luk W, et al. Assessing the risk of nasopharyngeal carcinoma on the basis of EBV antibody spectrum. Int J Cancer. (2002) 97:489–92. doi: 10.1002/ijc.1641
52. Gu YL, Zhang CQ, Wu ZB, Zong YS, Liang YJ, Cheng YL. Study on sero-diagnosis of nasopharyngeal carcinoma using a dual antibody test against recombinant epstein-barr virus antigens. Chinese J Cancer. (2003) 22:90306.
53. Cheng WM, Ji MF, Li XL, Yang JL, Zong YS. Screening out nasopharyngeal carcinoma by two- stage ELISA for EB virus. Chinese J Immunol. (2003) 19:834−34
54. Hu WW, Zong YS, Li FP, Li GM, Zhong BL, Zhang M, et al. Comparison of 6 antibody assays detecting epstein-barr virus for serodiagnosis of nasopharyngeal carcinoma. Chinese J Clin Oncol. (2006) 33:795−95. doi: 10.3969/j.issn.1000-8179.2006.14.005
55. Cheng WM, Ji MF, Li XL, Su NH, Yang JL. Analysis of serum levels of antibodies against EBV EBNA1 and EBZta in individuals in a nasopharyngeal carcinoma clan who have non-nasopharyngeal carcinoma. Chinese J Clin Oncol. (2007) 34:1238–238. doi: 10.3969/j.issn.1000-8179.2007.21.011
56. Liang YJ, Zong YS, Gu YL, Zhang Y, Feng YF, Liu YD, et al. Application of enzyme-linked immunosorbent assay to the serological diagnosis of nasopharyngeal carcinoma. J Pract Med. (2008) 24:3055–8. doi: 10.1128/CVI.00425-08
57. Ayadi W, Karray-Hakim H, Feki L, Khabir A, Boudawara T, Ghorbel A, et al. IgA antibodies against the Epstein-Barr nuclear antigen1 as a valuable biomarker for the diagnosis of nasopharyngeal carcinoma in Tunisian patients. J Med Virol. (2009) 81:1412–21. doi: 10.1002/jmv.21532
58. Jiang SQ, Liu Q. Logistic applicant of logistic registration in combination with multiple diagnostic tests for auxiliary diagnosis of nasopharyngeal carcinoma. Chinese J Cancer. (2009) 28:213–6. doi: 10.3321/j.issn:1000-467X.2009.02.020
59. Adham M, Greijer AE, Verkuijlen SAWM, Juwana H, Fleig S, Rachmadi L, et al. Epstein-barr virus DNA load in nasopharyngeal brushings and whole blood in nasopharyngeal carcinoma patients before and after treatment. Clin Cancer Res. (2013) 19:2175–86. doi: 10.1158/1078-0432.CCR-12-2897
60. Hu B, Chen ZC, Zhou WY, Wu XP, Liang ZC, Li LE. Application of prokaryotic expression of EBNA1 gene of epstein-barr virus detection of nasopharyngeal carcinoma. Chinese J Cancer Prev Treat. (2014) 21:1244–7. doi: 10.16073/j.cnki.cjcpt.2014.16.004
61. Coghill AE, Bu W, Nguyen H, Hsu WL, Yu KJ, Lou PJ, et al. High levels of antibody that neutralize B-cell infection of epstein-barr virus and that bind EBV gp350 are associated with a lower risk of nasopharyngeal carcinoma. Clin Cancer Res. (2016) 22:3451–7. doi: 10.1158/1078-0432.CCR-15-2299
62. Yu X, Ji MF, Cheng WM, Huang YL, Li FG. Assessment of EBV antibodies and EBV-DNA in the diagnosis and stages of nasopharyngeal carcinoma. Chinese J Clin Oncol. (2016) 43:65016n. doi: 10.3969/j.issn.1000-8179.2016.15.393
63. Gu XM, Li YY, Lv R, Huang JS, Zhou XJ, Du GY. The correlation between serum homocysteine and EB virus three antibodies in nasopharyngeal carcinoma and the evaluation of its diagnosis performance. Chinese J Health Lab Technol. (2016) 26:231−3.
64. Cheng WM, Chen GX, Chen HL, Luo RX, Wu ZB, Lu YS, et al. Assessment of nasopharyngeal carcinoma risk by EB virus antibody profile. Chinese J Oncol. (2002) 24:56103.
65. Xiao YK, Zhang J, Lu YR, Lin P. Value of detection of serum Epstein-Berr virus IgA/VCA by immunoenzyme technique in the diagnosis of nasopharyngeal carcinoma. Prac J Cancer. (1995) 24:371–3.
66. Liu MY, Shih YY, Chou SP, Chen CJ, Sheen TS, Yang CS, et al. Antibody against the Epstein-Barr virus BHRF1 protein, a homologue of Bcl-2, in patients with nasopharyngeal carcinoma. J Med Virol. (1998) 56:179–85. doi: 10.1002/(SICI)1096-9071(199811)56:3<179::AID-JMV1>3.0.CO;2-4
67. Zou YC, Li GL, Zhang ZS. Discussion on combined detection of serum tumor markers for nasopharyngeal carcinoma. Chinese J Clin Oncol. (2001) 28:22501n. doi: 10.3969/j.issn.1000-8179.2001.03.020
68. Mai S, Zong Y, Zhang M, Zhong B, Lin S. Detection of epstein-Barr virus DNA in plasma/serum: a useful serological indicator for diagnosis of nasopharyngeal carcinoma. Chinese Med J. (2002) 115:1895–7. doi: 10.3760/cma.j.issn.0366-6999.2002.12.128
69. Shao JY, Li YH, Gao HY, Wu QL, Cui NJ, Zhang L, et al. Comparison of plasma epstein-barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer. (2004) 100:1162–70. doi: 10.1002/cncr.20099
70. Leung SF, Tam JS, Chan AT, Zee B, Chan LY, Huang DP, et al. Improved accuracy of detection of nasopharyngeal carcinoma by combined application of circulating epstein-barr virus DNA and anti-Epstein-Barr viral capsid antigen IgA antibody. Clin Chem. (2004) 50:339–45. doi: 10.1373/clinchem.2003.022426
71. Huang LS, Huang WC, Chen YH. The value of CYFRA21-1, TSGF, EB-VCA-IgA detection in the diagnosis of nasopharyngeal carcinoma. J Guangxi Med Univ. (2005) 22:23304. doi: 10.3969/j.issn.1005-930X.2005.02.027
72. Jiang LN, Dai LC, He JF, Chen YW, Ma ZH. Significance of detection of serum sialic acid and Epstein-Barr virus VCA-IgA in diagnosis and monitoring radiotherapy effectiveness in nasopharyngeal carcinoma patients. Chinese J Exp Clin Virol. (2006) 20:30–2. doi: 10.3760/cma.j.issn.1003-9279.2006.02.010
73. Huang LS, Zhu B, Chen YH, Zhao HL. Significance of combined detection of serum tumor markers in the diagnosis of nasopharyngeal carcinoma. Lab Med. (2006) 21:472–4. doi: 10.3969/j.issn.1673-8640.2006.05.011
74. Zhu HQ, Xiong H, Alhosam J, Zhou LQ, Zhang P, Huang HX, et al. Expression of LMP-1 in nasopharyngeal cancer tissue and its relation with peripheral blood EBV-IgA/ VCA. Acta Med Univ Sci Technol Huazhong. (2008) 37:5098ho. doi: 10.3870/j.issn.1672-0741.2008.04.025
75. Zeng X, Wang CL. Clinical analysis of serum EBV-VCA-IgA in NPC patients. China J Modern Med. (2010) 20:879–81, 85. doi: 10.3971/j.issn.1000-8578.2013.04.014
76. Baizig NM, Morand P, Seigneurin JM, Boussen H, Fourati A, Gritli S, et al. Complementary determination of Epstein-Barr virus DNA load and serum markers for nasopharyngeal carcinoma screening and early detection in individuals at risk in Tunisia. Eur Arch Otorhinolaryngol. (2012) 269:1005–11. doi: 10.1007/s00405-011-1717-5
77. Li XH, Meng YL, Zhao LJ, Huang CL, Huang GL, Qi GZ. Epstein-barr virus antibodies response for zhuang npc patients of different ages. Cancer Res Prev Treat. (2013) 40:37713re
78. Zheng XH, Lu LX, Li XZ, Jia WH. Quantification of epstein-barr virus DNA load in nasopharyngeal brushing samples in the diagnosis of nasopharyngeal carcinoma in southern China. Cancer Sci. (2015) 106:1196–201. doi: 10.1111/cas.12718
79. Yang X, Dai W, Kwong DLW, Szeto CYY, Wong EHW, Ng WT, et al. Epigenetic markers for noninvasive early detection of nasopharyngeal carcinoma by methylation-sensitive high resolution melting. Int J Cancer. (2015) 136:E127–E35. doi: 10.1002/ijc.29192
80. Peng YH, Xu YW, Huang LS, Zhai TT, Dai LH, Qiu SQ, et al. Autoantibody signatures combined with epstein-barr virus capsid antigen-IgA as a biomarker panel for the detection of nasopharyngeal carcinoma. Cancer Prev Res. (2015) 8:729–36. doi: 10.1158/1940-6207.CAPR-14-0397
81. Peng YH, Chen WZ, Huang LS, Fang YS, Xu YY. Combination of antibodies against HSP70 and viral capsid antigen immunoglobulin for improved detection of nasopharyngeal carcinoma. Chinese J Cancer Prev Treat. (2015) 22:1198–201, 206. doi: 10.16073/j.cnki.cjcpt.2015.15.009
82. Li Y, Wang K, Yin SK, Zheng HL, Min DL. Expression of epstein-Barr virus antibodies EA-IgG, Rta-IgG, and VCA-IgA in nasopharyngeal carcinoma and their use in a combined diagnostic assay. Genet Mol Res. (2016) 15. doi: 10.4238/gmr.15017368
83. Li RC, Du Y, Zeng QY, Tang LQ, Zhang H, Li Y, et al. Epstein-Barr virus glycoprotein gH/gL antibodies complement IgA-viral capsid antigen for diagnosis of nasopharyngeal carcinoma. Oncotarget. (2016) 7:16372–83. doi: 10.18632/oncotarget.7688
84. Li RC, Du Y, Zeng QY, Tang LQ, Zhang H, Li Y, et al. Antibodies against epstein-barr virus glycoprotein gp42 for the diagnosis of nasopharyngeal carcinoma. Clin Lab. (2016) 62:553–61. doi: 10.7754/Clin.Lab.2015.150723
85. Wu CY, Qiu MH, Zeng XL, Du MY, Yao M, Chen YX. VCA-IgA and Rta-IgG joint detection diagnosis and effectiveness of nasopharyngeal carcinoma. Chinese J Lab Med. (2016) 39:609–12. doi: 10.3760/cma.j.issn.1009-9158.2016.08.012
86. Cai Y, Song Y, Cen D, Zhang C, Mao S, Ye X, et al. Novel ELISA for serodiagnosis of nasopharyngeal carcinoma based on a B cell epitope of epstein-barr virus latent membrane protein 2. Oncol Lett. (2018) 16:4372–8. doi: 10.3892/ol.2018.9216
87. Gurtsevitch VE, Senyuta NB, Ignatova AV, Lomaya MV, Kondratova VN, Pavlovskaya AI, et al. Epstein-Barr virus biomarkers for nasopharyngeal carcinoma in non-endemic regions. J Gen Virol. (2017) 98:2118–27. doi: 10.1099/jgv.0.000889
88. Liu L, Zuo L, Yang J, Xin S, Zhang J, Zhou J, et al. Exosomal cyclophilin A as a novel noninvasive biomarker for epstein-barr virus associated nasopharyngeal carcinoma. Cancer Med. (2019) 8:3142–51. doi: 10.1002/cam4.2185
89. Abdulamir AS, Hafidh RR, Abu Bakar F, Abbas K. Novel epstein-barr virus immunoglobulin G-based approach for the specific detection of nasopharyngeal carcinoma. Am J Otolaryngol. (2010) 31:410–7. doi: 10.1016/j.amjoto.2009.06.006
90. Feng P, Ren EC, Liu D, Chan SH, Hu H. Expression of epstein-barr virus lytic gene BRLF1 in nasopharyngeal carcinoma: potential use in diagnosis. J Gen Virol. (2000) 81:2417–23. doi: 10.1099/0022-1317-81-10-2417
91. Zheng YM, Cai YL, Cheng JR, Li J, Mo YK, Gao JQ, et al. Evaluation of detection of Epstein-Barr virus Rta/IgG in nasopharyngeal carcinoma. Chinese J Exp Clin Virol. (2009) 23:28597. doi: 10.3760/cma.j.issn.1003-9279.2009.04.015
92. Li D, Zeng Y. Detection of IgG/ZEBRA antibodies in sera from patients with nasopharyngeal carcinoma by ELISA methods. Chinese J Virol. (1994) 01:78–80. doi: 10.13242/j.cnki.bingduxuebao.000836
93. Cao SM, Huang TB, Jian SY, Liu Q. The relationship between eb virus ZEBRA/IGG change regulation and nasopharyngeal carcinoma. Cancer Res Prev Treat. (1999) 26:382–4.
94. Zhang XM, Zhong JM, Tang MZ, Zhang XG, Liao J, Zheng YM, et al. Comparison of IgA/VCA, IgA/EA, IgG/EA in immunoenzyme methods and ZEBRA ELISA in early diagnosis of nasopharyngeal carcinoma. Chinese J Exp Clin Virol. (2006) 20:263−63. doi: 10.3760/cma.j.issn.1003-9279.2006.03.020
95. Yi X, Wu YY, Xie Y, Kang M, Tang AZ. Antibody response to recombinant epstein-barr virus Zta protein in patients with nasopharyngeal carcinoma. J Guangxi Med Univ. (2007) 24:365–7. doi: 10.3969/j.issn.1005-930X.2007.03.012
96. Kerekhanjanarong V, Sitawarin S, Sakdikul S, Saengpanich S, Chindavijak S, Supiyaphun P, et al. Telomerase assay and nested polymerase chain reaction from nasopharyngeal swabs for early noninvasive detection of nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. (2000) 123:624–9. doi: 10.1067/mhn.2000.109368
97. Stevens SJ, Verkuijlen SA, Hariwiyanto B, Harijadi Paramita DK, Fachiroh J, et al. Noninvasive diagnosis of nasopharyngeal carcinoma: nasopharyngeal brushings reveal high epstein-barr virus DNA load and carcinoma-specific viral BARF1 mRNA. Int J Cancer. (2006) 119:608–14. doi: 10.1002/ijc.21914
98. Sun ZL, Xiao CQ, Jiang W, Feng XR, Liu YY. Comparison of Epstein-Barr virus in nasopharynx tissue and desquamatine cell for diagnosis of nasopharyngeal carcinoma. J Clin Otorhinolaryngol. (2006) 20:153–4. doi: 10.3969/j.issn.1001-1781.2006.04.004
99. Deng YL, Huang XY, Liu Y, Zhang XB, Cui DY.Detection and significance of EB virus DNA in nasopharyngeal secretions of patients with nasopharyngeal carcinoma. Guangdong Med J. (2008) 29:1163–5. doi: 10.3969/j.issn.1001-9448.2008.07.039
100. Cao SM, Gao JS, Liu XD, Hong MH, Xiao XB, Chen FJ, et al. The value of fluorescence quantitative PCR in detection of plasma epstein-barr virus DNA in the diagnosis of nasopharyngeal carcinoma. Chinese J Cancer. (2002) 21:328−2. doi: 10.3969/j.issn.1000-467X.2002.03.026
101. Yuan H, Yang BB. Quantitative analysis of plasma Epstein-Barr virus DNA in patients with nasopharyngeal carcinoma. J Prac Oncol. (2002) 17:27–9. doi: 10.3969/j.issn.1001-1692.2002.01.010
102. Krishna SM, James S, Kattoor J, Balaram P. Serum EBV DNA as a biomarker in primary nasopharyngeal carcinoma of Indian origin. Japanese J Clin Oncol. (2004) 34:307–11. doi: 10.1093/jjco/hyh055
103. Shao JY, Zhang Y, Li YH, Gao HY, Feng HX, Wu QL, et al. Comparison of epstein-barr virus DNA level in plasma, peripheral blood cell and tumor tissue in nasopharyngeal carcinoma. Anticancer Res. (2004) 24:4059–66.
104. Zhang Y, Gao HY, Feng HX, Deng L, Huang MY, Hu B, et al. Quantitative analysis of epstein-Barr virus DNA in plasma and peripheral blood cells in patients with nasopharyngeal carcinoma. Natl Med J China. (2004) 84:982–6. doi: 10.3760/j:issn:0376-2491.2004.12.005
105. Yang X, Goldstein AM, Chen CJ, Rabkin CS, Chen JY, Cheng YJ, et al. Distribution of Epstein-Barr viral load in serum of individuals from nasopharyngeal carcinoma high-risk families in Taiwan. Int J Cancer. (2006) 118:780–4. doi: 10.1002/ijc.21396
106. Chen SP, Lin SX, Zhong BL, Liang YJ, Mai SJ, Zong YS. Relationship between detection of EB virus DNA and tumor cell apoptosis in patients with nasopharyngeal carcinoma. Guangdong Med J. (2008) 29:1823–8. doi: 10.3969/j.issn.1001-9448.2008.11.024
107. Chai SJ, Pua KC, Saleh A, Yap YY, Lim PVH, Subramaniam SK, et al. Clinical significance of plasma epstein-barr virus DNA loads in a large cohort of Malaysian patients with nasopharyngeal carcinoma. J Clin Virol. (2012) 55:34–9. doi: 10.1016/j.jcv.2012.05.017
108. Yip TTC, Kwok HYY, You SSY, Kwong DLW, Fong AHW, Cheng WW, et al. A new circulating plasma/serum Epstein-Barr virus (EBV) DNA test by locked nucleic acid (LNA) PCR technique with enhanced sensitivity in diagnosis & monitoring of patients suffering from nasopharyngeal carcinoma (NPC) and other EBV associated malignancies. Cancer Res. (2016) 76 (14 Suppl.). doi: 10.1158/1538-7445.AM2016-3153
109. Wang H, Wei XQ, Yang KY. Role of combining EBNA assay and Bamh1-W assay in detection of EBV DNA loads in NPC. J Prac Med. (2017) 33:2918–22. doi: 10.3969/j.issn.1006-5725.2017.17.029
110. Wang H, Deng L, Chen SH, Wang SQ, Cao Y. Real-time quantitative PCR assay of Epstein-Barr virus in whole blood cells of nasopharyngeal carcinoma patients. Acta Biol Exp Sinica. (2003) 36:32–6. doi: 10.3321/j.issn:1673-520X.2003.01.006
111. Fang P, Li XH, Sha Q, Zhang KL, Zhou Q. Using PCR method to study the EB virus DNA in NPC. J Clin Otorhinolaryngol. (2004) 18:5994hin. doi: 10.3969/j.issn.1001-1781.2004.10.009
112. Tang XL, Tian SD. Correlation of Epstein-Barr viral load in outward blood lymphocyte and diagnosis and stages of nasopharyngeal carcinoma. China J Modern Med. (2006) 16:3412–4. doi: 10.3969/j.issn.1005-8982.2006.22.015
113. Yi B, Zhou HQ, Yao R, Xiao ZQ. Diagnostic value of 4 kinds of serum autoantibodies in nasopharyngeal carcinoma. Chinese J Lab Med. (2010) 33:747–51. doi: 10.3760/cma.j.issn.1009-9158.2010.08.008
114. Zhang HQ, Zhou ZB, Fan L, Jing F, Fei Y, Li XH, et al. Dynamic observation on serum sialic acid in nasopharyngeal carcinoma patients. Chinese J Otorhinolaryngol Head Neck Surg. (2005) (06) :411-414. Available online at: http://rs.yiigle.com/CN11533020054006/54860.htm
115. Huang JS, Gu XM, Li YY, Du GY. Application of serum homocysteine and sialic acid in the diagnosis of nasopharyngeal carcinoma. Lab Med Clin. (2016) 13:3456–57+3460. doi: 10.3969/j.issn.1672-9455.2016.24.008
116. Zhang G, Li Z, Zhou Q. Utility of serum EB Virus zta antibody in the diagnostic of nasopharyngeal carcinoma: evidences from 2,126 cases and 15,644 controls. Front Oncol. (2019) 9:1391. doi: 10.3389/fonc.2019.01391
117. Gan YY, Fones-Tan A, Chan SH. Molecular diagnosis of nasopharyngeal carcinoma: a review. Ann Acad Med. (1996) 25:71–4.
118. Dardari R, Khyatti M, Benider A, Jouhadi H, Kahlain A, Cochet C, et al. Antibodies to the epstein-barr virus transactivator protein (ZEBRA) as a valuable biomarker in young patients with nasopharyngeal carcinoma. Int J Cancer. (2000) 86:71–5. doi: 10.1002/(SICI)1097-0215(20000401)86:1<71::AID-IJC11>3.0.CO;2-1
119. Vo JH, Nei WL, Hu M, Phyo WM, Wang F, Fong KW, et al. Comparison of circulating tumour cells and circulating cell-free epstein-barr virus DNA in patients with nasopharyngeal carcinoma undergoing radiotherapy. Sci Rep. (2016) 6:13. doi: 10.1038/s41598-016-0006-3
120. Chan KC, Leung SF, Yeung SW, Chan AT, Lo YM. Quantitative analysis of the transrenal excretion of circulating EBV DNA in nasopharyngeal carcinoma patients. Clin Cancer Res. (2008) 14:4809–13. doi: 10.1158/1078-0432.CCR-08-1112
121. Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS, et al. Quantification of plasma epstein-barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. (2004) 350:2461–70. doi: 10.1056/NEJMoa032260
122. Abeynayake J, Johnson R, Libiran P, Sahoo MK, Cao H, Bowen R, et al. Commutability of the epstein-barr virus who international standard across two quantitative PCR methods. J Clin Microbiol. (2014) 52:3802–4. doi: 10.1128/JCM.01676-14
123. Stockert E, Jager E, Chen YT, Scanlan MJ, Gout I, Karbach J, et al. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. (1998) 187:1349–54. doi: 10.1084/jem.187.8.1349
124. Scanlan MJ, Welt S, Gordon CM, Chen YT, Gure AO, Stockert E, et al. Cancer-related serological recognition of human colon cancer: identification of potential diagnostic and immunotherapeutic targets. Cancer Res. (2002) 62:4041–7.
125. Kimura Y, Fujieda S, Takabayashi T, Tanaka T, Sugimoto C, Saito H. Conventional tumor markers are prognostic indicators in patients with head and neck squamous cell carcinoma. Cancer letters. (2000) 155:163–8. doi: 10.1016/S0304-3835(00)0423-7
126. Kokoglu E, Suer S, Ozyurt E, Siyahhan A, Sonmez H. Plasma fibronectin and sialic acid levels in various types of human brain tumors. Cancer Biochemistry Biophys. (1995) 15:35–40.
127. Ramp U, Mahotka C, Heikaus S, Shibata T, Grimm MO, Willers R, et al. Expression of heat shock protein 70 in renal cell carcinoma and its relation to tumor progression and prognosis. Histol Histopathol. (2007) 22:1099–107. doi: 10.14670/hh-22.1099
128. Kalogeraki A, Giannikaki E, Tzardi M, Kafousi M, Ieromonachou P, Dariviannaki K, et al. Correlation of heat shock protein (HSP70) expression with cell proliferation (MIB1), estrogen receptors (ER) and clinicopathological variables in invasive ductal breast carcinomas. J Exp Clin Cancer Res. (2007) 26:367–8.
129. Van Molle W, Van Roy M, Van Bogaert T, Dejager L, Van Lint P, Vanlaere I, et al. Protection of zinc against tumor necrosis factor induced lethal inflammation depends on heat shock protein 70 and allows safe antitumor therapy. Cancer Res. (2007) 67:7301–7. doi: 10.1158/0008-5472.CAN-06-4010
130. Afanasyeva EA, Komarova EY, Larsson LG, Bahram F, Margulis BA, Guzhova IV. Drug-induced Myc-mediated apoptosis of cancer cells is inhibited by stress protein Hsp70. Int J Cancer. (2007) 121:2615–21. doi: 10.1002/ijc.22974
131. Lin JF, Xu J, Tian HY, Gao X, Chen QX, Gu Q, et al. Identification of candidate prostate cancer biomarkers in prostate needle biopsy specimens using proteomic analysis. Int J Cancer. (2007) 121:2596–605. doi: 10.1002/ijc.23016
132. Zhou DN, Ye CS, Yang QQ, Deng YF. Integrated analysis of transcriptome profiling predicts potential lncRNA and circRNA targets in human nasopharyngeal carcinoma. Oncol Lett. (2020) 19:3123–36. doi: 10.3892/ol.2020.11412
133. Ma DD, Yuan LL, Lin LQ. LncRNA HOTAIR contributes to the tumorigenesis of nasopharyngeal carcinoma via up-regulating FASN. Eur Rev Med Pharmacol Sci. (2017) 21:5143−52. doi: 10.26355/eurrev_201711_13831
Keywords: nasopharyngeal carcinoma, EB virus, diagnostic, screening, meta-analysis
Citation: Feng Y, Xia W, He G, Ke R, Liu L, Xie M, Tang A and Yi X (2020) Accuracy Evaluation and Comparison of 14 Diagnostic Markers for Nasopharyngeal Carcinoma: A Meta-Analysis. Front. Oncol. 10:1779. doi: 10.3389/fonc.2020.01779
Received: 22 May 2020; Accepted: 11 August 2020;
Published: 18 September 2020.
Edited by:
Aviram Mizrachi, Rabin Medical Center, IsraelReviewed by:
Stefania Staibano, University of Naples Federico II, ItalyNimrod Amitai, Rabin Medical Center, Israel
Copyright © 2020 Feng, Xia, He, Ke, Liu, Xie, Tang and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Yi, MTkwMDk4NjY0QHFxLmNvbQ==
†These authors have contributed equally to this work
 Yiwei Feng
Yiwei Feng Wei Xia
Wei Xia Guangyao He1
Guangyao He1